Novel Superelastic Polyesters Based on 2,5-Furandicarboxylic Acid for Potential Use in Ophthalmic Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Homopolymers
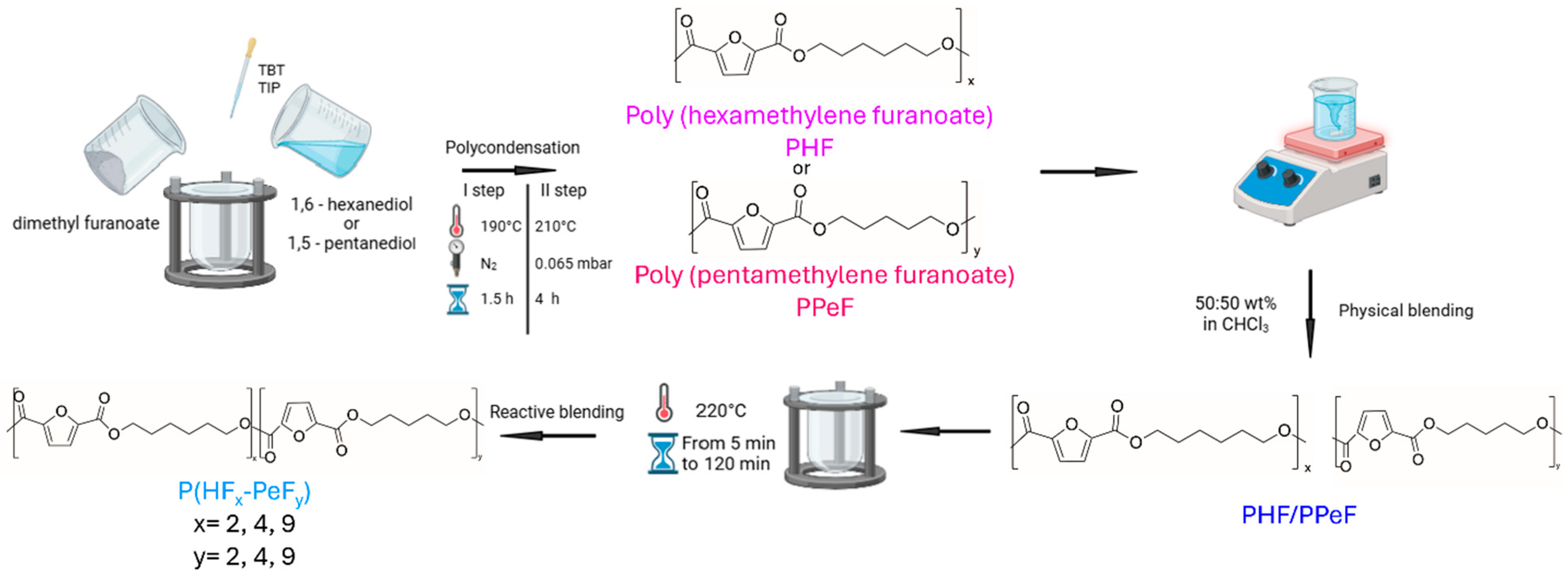
2.3. Blend Preparation
2.4. Copolymer Synthesis
2.5. Film Preparation
2.6. Molecular Characterisation
2.7. Thermal Analysis
2.8. Structural and Morphological Characterisation
2.9. Water Contact Angle Measurements
2.10. Mechanical Characterisation
2.11. Hydrolytic Degradation Tests
2.12. In Vitro Citotoxicity Evaluation
3. Results and Discussion
3.1. Molecular Characterisation
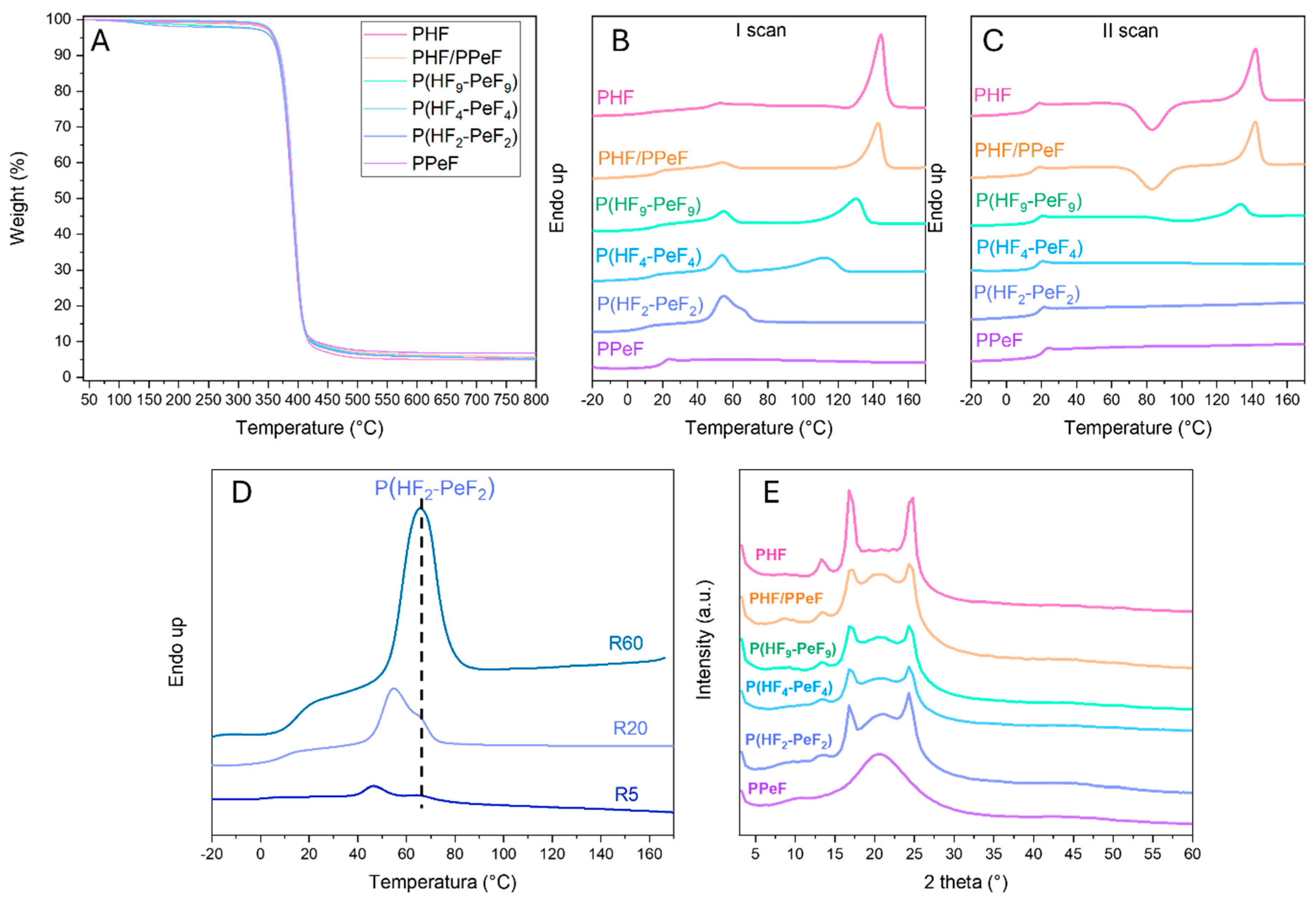
3.2. Thermal Characterisation
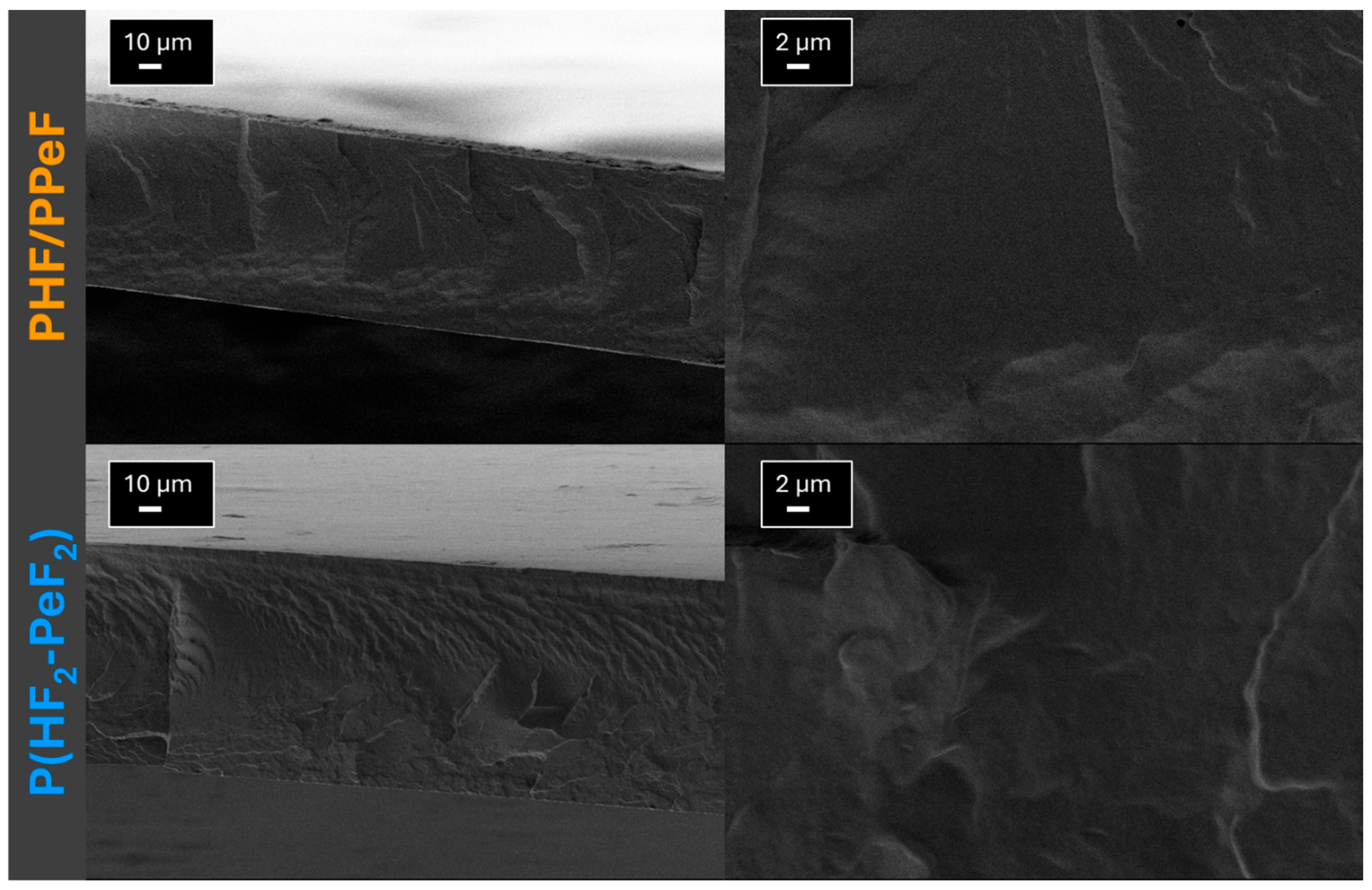
3.3. Structural and Morphological Characterisation
3.4. Water Contact Angle Measurements
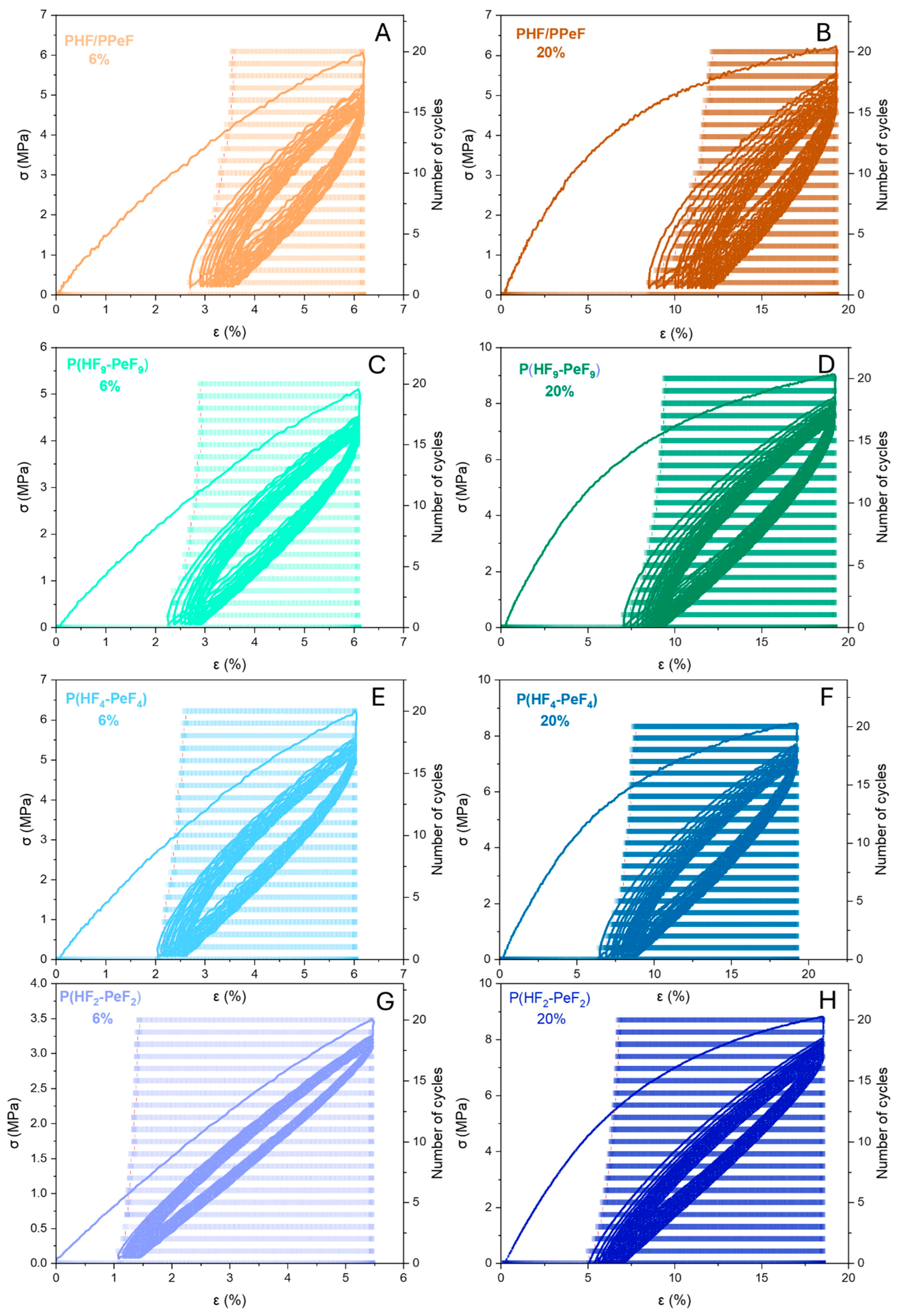
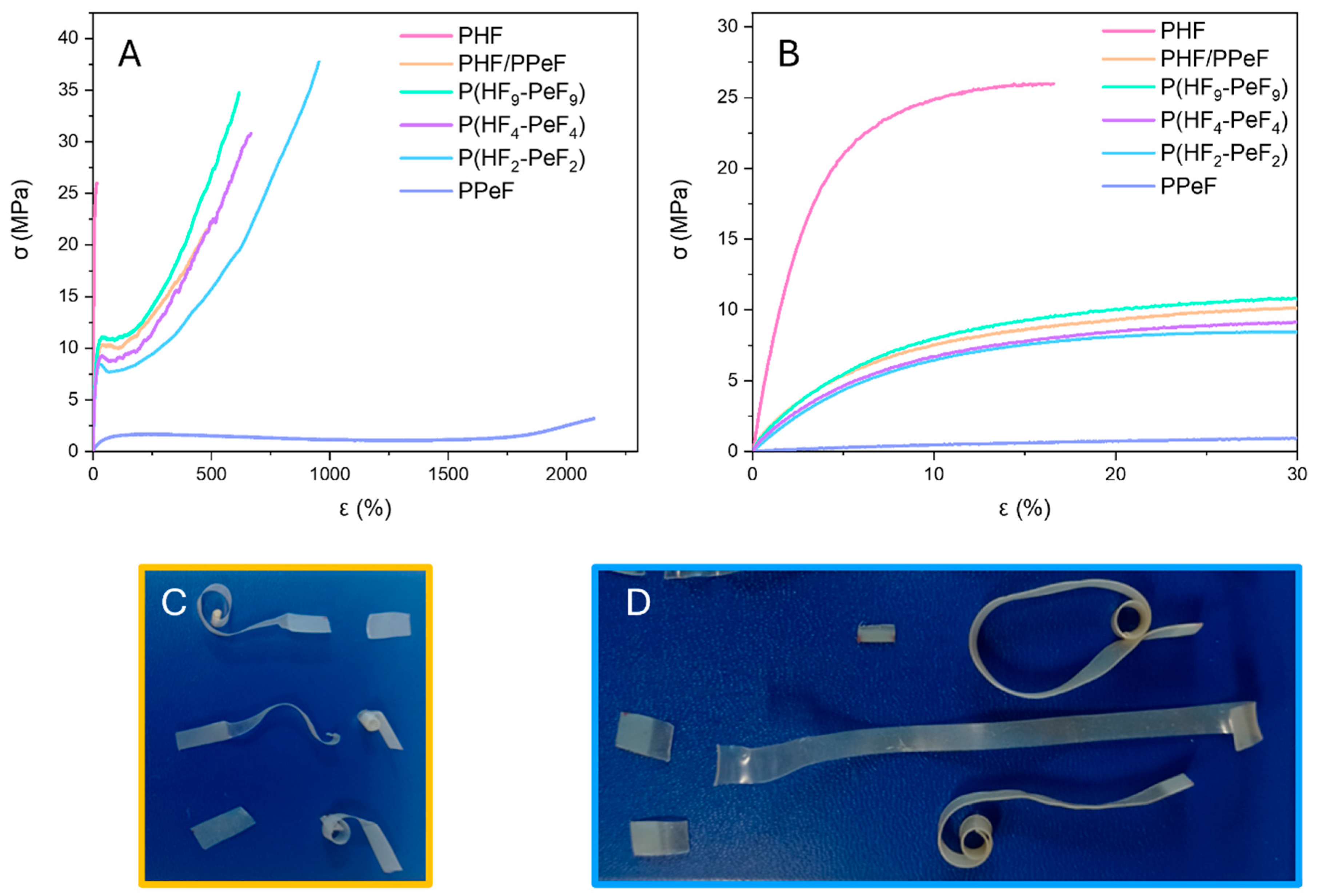
3.5. Mechanical Characterisation
3.6. Hydrolytic Degradation Tests
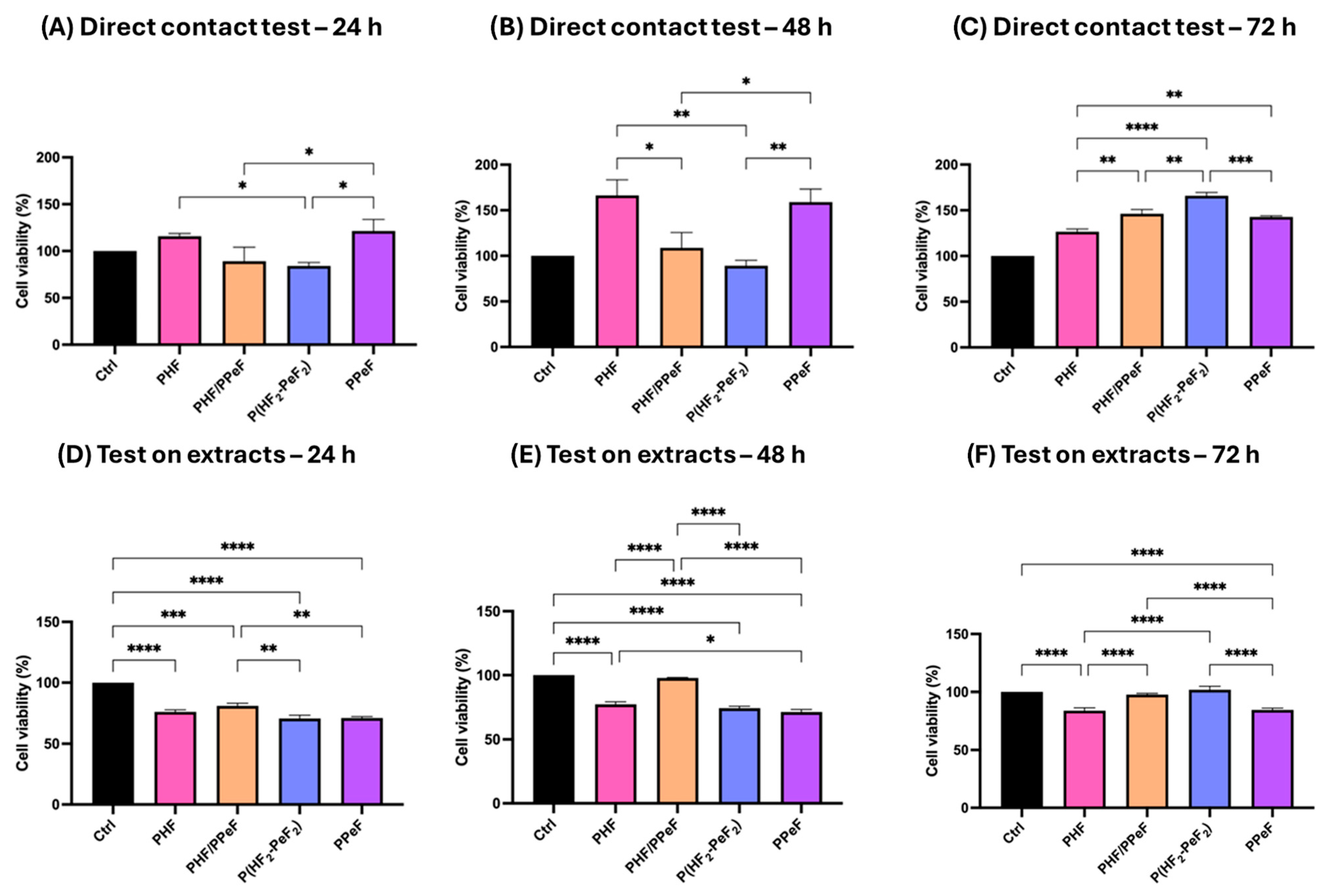
3.7. Citotoxicity Tests
3.7.1. Direct Contact Test
3.7.2. Extract Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Otsuka, K.; Ren, X. Recent Developments in the Research of Shape Memory Alloys. Intermetallics 1999, 7, 511–528. [Google Scholar] [CrossRef]
- Huang, W.M.; Ding, Z.; Wang, C.C.; Wei, J.; Zhao, Y.; Purnawali, H. Shape Memory Materials. Mater. Today 2010, 13, 54–61. [Google Scholar] [CrossRef]
- Smith, C.A.; Christie, L.M.; Cooperman, S.R.; Hyer, C.F. Modified Lapidus Procedure with a Nitinol Staple and Two Screw Construct Technique. J. Foot Ankle Surg. 2025, 64, 279–284. [Google Scholar] [CrossRef] [PubMed]
- McNaney, J.M.; Imbeni, V.; Jung, Y.; Papadopoulos, P.; Ritchie, R.O. An Experimental Study of the Superelastic Effect in a Shape-Memory Nitinol Alloy under Biaxial Loading. Mech. Mater. 2003, 35, 969–986. [Google Scholar] [CrossRef]
- Hunt, K.J. Nitinol Staples in Foot and Ankle Surgery: Panacea or the Next Bubble? Foot Ankle Int. 2024, 45, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Molnárová, O.; Šittner, P. Effect of Microstructure on Fatigue of Superelastic NiTi Wires. Int. J. Fatigue 2021, 152, 106400. [Google Scholar] [CrossRef]
- Ganesan, S.; Pandey, S.; Krishnasamy, S.; Thiagmani, S.M.K. Prediction of Thermal Cycling Behaviour of Ni-Rich NiTi SMA Using Empirical and Artificial Neural Network Modelling. Discov. Mater. 2025, 5, 48. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Xiao, F.; Hou, R.; Lin, Z.; Cai, X.; Zuo, S.; Zhou, Y.; Hua, S.; Chen, Y.; et al. Braided NiTi Alloys Microfilaments with Near-Linear Responses: Toward Flexible High-Pressure Sensors. J. Mater. Sci. Technol. 2025, 229, 269–278. [Google Scholar] [CrossRef]
- De Aragon, J.S.M.; Villada, J.R.; Ruiz-Moreno, J.M. Ocular Biocompatibility of a Nitinol Capsular Tension Ring (CTR). EuroBiotech J. 2022, 6, 167–173. [Google Scholar] [CrossRef]
- Horner, K.J.; Fiala, K.C.; Summerhays, B.; Schweser, K.M. Clinical and Radiographic Outcomes of Nitinol Compression Staples for Midfoot and Chopart Arthrodesis. J. Foot Ankle Surg. 2024, 63, 717–723. [Google Scholar] [CrossRef]
- Wever, D.J.; Veldhuizen, A.G.; Sanders, M.M.; Schakenraad, J.M.; Van Horn, J.R. Cytotoxic, Allergic and Genotoxic Activity of a Nickel-Titanium Alloy. Biomaterials 1997, 18, 1115–1120. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; Li, C.; Xu, K. Deformation Twin Enhances the Recoverable Strain up to 14% with Excellent Stability in NiTi Shape Memory Alloy. J. Mater. Sci. Technol. 2025, 238, 250–265. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, W.; Yang, W.; Song, J.; Hu, W.; Wang, K. Polyetheretherketone Biomaterials and Their Current Progress, Modification-Based Biomedical Applications and Future Challenges. Mater. Des. 2025, 252, 113716. [Google Scholar] [CrossRef]
- Nagaraja, S.; Pelton, A.R. Corrosion Resistance of a Nitinol Ocular Microstent: Implications on Biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, H.; Wu, Z.; Wu, Y.; Zhou, X.; Ji, D. Advances in the Application of Smart Materials in the Treatment of Ophthalmic Diseases. Biomaterials 2025, 321, 123316. [Google Scholar] [CrossRef]
- Yi, Y.; An, H.W.; Wang, H. Intelligent biomaterialomics: Molecular design, manufacturing, and biomedical applications. Adv. Mater. 2024, 36, 2305099. [Google Scholar] [CrossRef]
- Dallaev, R. Smart and Biodegradable Polymers in Tissue Engineering and Interventional Devices: A Brief Review. Polymers 2025, 17, 1976. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable Contact Lens Biosensors for Continuous Glucose Monitoring Using Smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Kim, S.; Lee, G.; Jeon, C.; Han, H.H.; Kim, S.; Mok, J.W.; Joo, C.; Shin, S.; Sim, J.; Myung, D.; et al. Bimetallic Nanocatalysts Immobilized in Nanoporous Hydrogels for Long-Term Robust Continuous Glucose Monitoring of Smart Contact Lens. Adv. Mater. 2022, 34, 2110536. [Google Scholar] [CrossRef]
- Shen, Y.; Liang, L.; Zhang, S.; Huang, D.; Zhang, J.; Xu, S.; Liang, C.; Xu, W. Organelle-Targeting Surface-Enhanced Raman Scattering (SERS) Nanosensors for Subcellular pH Sensing. Nanoscale 2018, 10, 1622–1630. [Google Scholar] [CrossRef]
- Atiq Ur Rehman, M.; Bastan, F.E.; Haider, B.; Boccaccini, A.R. Electrophoretic Deposition of PEEK/Bioactive Glass Composite Coatings for Orthopedic Implants: A Design of Experiments (DoE) Study. Mater. Des. 2017, 130, 223–230. [Google Scholar] [CrossRef]
- Wu, K.Y.; Khan, S.; Liao, Z.; Marchand, M.; Tran, S.D. Biopolymeric Innovations in Ophthalmic Surgery: Enhancing Devices and Drug Delivery Systems. Polymers 2024, 16, 1717. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Wang, J.; Zhang, X.; Jia, Z.; Jiang, Y.; Liu, X. Recent Progress on Bio-Based Polyesters Derived from 2,5-Furandicarbonxylic Acid (FDCA). Polymers 2022, 14, 625. [Google Scholar] [CrossRef] [PubMed]
- Karayilan, M.; Clamen, L.; Becker, M.L. Polymeric Materials for Eye Surface and Intraocular Applications. Biomacromolecules 2021, 22, 223–261. [Google Scholar] [CrossRef]
- Xia, D.; Chen, J.; Zhang, Z.; Dong, M. Emerging Polymeric Biomaterials and Manufacturing Techniques in Regenerative Medicine. Aggregate 2022, 3, e176. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Chen, S.; Ling, P.; Kuss, M.A.; Duan, B.; Wu, S. Advances, Challenges, and Prospects for Surgical Suture Materials. Acta Biomater. 2023, 168, 78–112. [Google Scholar] [CrossRef]
- Legnani, C.; Ventura, A. Synthetic Grafts for Anterior Cruciate Ligament Reconstructive Surgery. Med. Eng. Phys. 2023, 117, 103992. [Google Scholar] [CrossRef]
- Bondi, E.; Restivo, E.; Soccio, M.; Guidotti, G.; Bloise, N.; Motta, I.; Gazzano, M.; Ruggeri, M.; Fassina, L.; Visai, L.; et al. Design and Characterization of Aromatic Copolyesters Containing Furan and Isophthalic Rings with Suitable Properties for Vascular Tissue Engineering. Int. J. Mol. Sci. 2025, 26, 6470. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, M.; Wu, Y.; Li, L.; Wang, Z.; Wang, R.; Zhou, G. Development of High-Molecular-Weight Fully Renewable Biopolyesters Based on Oxabicyclic Diacid and 2,5-Furandicarboxylic Acid: Promising as Packaging and Medical Materials. ACS Sustain. Chem. Eng. 2021, 9, 6799–6809. [Google Scholar] [CrossRef]
- Kang, H.; Miao, X.; Li, J.; Li, D.; Fang, Q. Synthesis and Characterization of Biobased Thermoplastic Polyester Elastomers Containing Poly(Butylene 2,5-Furandicarboxylate). RSC Adv. 2021, 11, 14932–14940. [Google Scholar] [CrossRef]
- Wei, S.; Liu, L.; Duan, Y.; Chen, X.; Tian, B.; Yuan, P.; Lv, S.; Zhang, Y. Advances in the Research of 2,5-Furandicarboxylic Acid (FDCA)-Based Polymers. Eur. Polym. J. 2025, 241, 114364. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Kong, Z.; Wang, K.; Ying, W.B.; Wang, J.; Zhu, J. Bio-Based Poly(Butylene Furandicarboxylate)-b-Poly(Ethylene Glycol) Copolymers: The Effect of Poly(Ethylene Glycol) Molecular Weight on Thermal Properties and Hydrolysis Degradation Behavior. Adv. Ind. Eng. Polym. Res. 2019, 2, 167–177. [Google Scholar] [CrossRef]
- Sahu, P.; Sharma, L.; Dawsey, T.; Gupta, R.K. Fully Biobased High-Molecular-Weight Polyester with Impressive Elasticity, Thermo-Mechanical Properties, and Enzymatic Biodegradability: Replacing Terephthalate. Macromolecules 2024, 57, 9302–9314. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New Poly(Pentylene Furanoate) and Poly(Heptylene Furanoate) Sustainable Polyesters from Diols with Odd Methylene Groups. Mater. Lett. 2016, 178, 64–67. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.-C.; Gutiérrez-Fernández, E.; Ezquerra, T.A.; Siracusa, V.; Munari, A.; Lotti, N. Evidence of a 2D-Ordered Structure in Biobased Poly(Pentamethylene Furanoate) Responsible for Its Outstanding Barrier and Mechanical Properties. ACS Sustain. Chem. Eng. 2019, 7, 17863–17871. [Google Scholar] [CrossRef]
- Guidotti, G.; Palumbo, A.; Soccio, M.; Gazzano, M.; Salatelli, E.; Siracusa, V.M.; Lotti, N. Fully Bio-Based Blends of Poly (Pentamethylene Furanoate) and Poly (Hexamethylene Furanoate) for Sustainable and Flexible Packaging. Polymers 2024, 16, 2342. [Google Scholar] [CrossRef]
- Manfroni, M.; Coatti, A.; Soccio, M.; Siracusa, V.; Boanini, E.; Salatelli, E.; Lotti, N. Eco-design of biobased poly(butylene succinate-b-pentamethylene 2,5-furanoate) copolymers with optimized mechanical, thermal and barrier properties for flexible food-packaging. Eur. Polym. J. 2025, 225, 113728. [Google Scholar] [CrossRef]
- Bianchi, E.; Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Salatelli, E.; Lotti, N. Biobased and Compostable Multiblock Copolymer of Poly(l-lactic acid) Containing 2,5-Furandicarboxylic Acid for Sustainable Food Packaging: The Role of Parent Homopolymers in the Composting Kinetics and Mechanism. Biomacromolecules 2023, 24, 2356–2368. [Google Scholar] [CrossRef]
- Siracusa, C.; Quartinello, F.; Soccio, M.; Manfroni, M.; Lotti, N.; Dorigato, A.; Guebitz, G.M.; Pellis, A. On the Selective Enzymatic Recycling of Poly(pentamethylene 2,5-furanoate)/Poly(lactic acid) Blends and Multiblock Copolymers. ACS Sustain. Chem. Eng. 2023, 11, 9751–9760. [Google Scholar] [CrossRef]
- Lin, H.; Li, W.; Ou, Q.; Liu, J.; Wei, Z.; Wang, Z.; Shi, D.; Lei, W.; Zhang, L. Design and Characterization of High Gas Barrier and Fully Biobased Poly(1,5-pentylene succinate-co-itaconate-co-furanoate) Elastomers. ACS Sustain. Chem. Eng. 2024, 12, 5640–5650. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Zubkiewicz, A.; Szymczyk, A.; Piesowicz, E.; Rozwadowski, Z.; Goracy, K. Biobased Thermoplastic Elastomers: Structure-Property Relationship of Poly(hexamethylene 2,5-furanodicarboxylate)-Block-Poly(tetrahydrofuran) Copolymers Prepared by Melt Polycondensation. Polymers 2021, 13, 397. [Google Scholar] [CrossRef]
- Feng, S.; Jiang, Z.; Qiu, Z. Synthesis, Thermal Behavior, and Mechanical Properties of Fully Biobased Poly(Hexamethylene 2,5-Furandicarboxylate-Co-Sebacate) Copolyesters. Polymers 2023, 15, 85. [Google Scholar] [CrossRef]
- Feng, S.; Qiu, Z. Synthesis and property of two biobased poly(hexamethylene 2,5-furandicarboxylate) copolyesters with slight difference in the chemical structure in comonomers. Eur. Polym. J. 2024, 214, 113160. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Z.; Qiu, Z. Synthesis and properties of poly(hexamethylene 2,5-furandicarboxylate-co-adipate) copolyesters. Eur. Polym. J. 2021, 161, 110860. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Tests for In Vitro Cytotoxicity, Definitive. ISO: Geneva, Switzerland, 2025.
- Morales-Huerta, J.C.; Martínez De Ilarduya, A.; Muñoz-Guerra, S. Sustainable Aromatic Copolyesters via Ring Opening Polymerization: Poly(Butylene 2,5-Furandicarboxylate-Co-Terephthalate)s. ACS Sustain. Chem. Eng. 2016, 4, 4965–4973. [Google Scholar] [CrossRef]
- Kasmi, N.; Wahbi, M.; Papadopoulos, L.; Terzopoulou, Z.; Guigo, N.; Sbirrazzuoli, N.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and Characterization of Two New Biobased Poly(Pentylene 2,5-Furandicarboxylate-Co-Caprolactone) and Poly(Hexamethylene 2,5-Furandicarboxylate-Co-Caprolactone) Copolyesters with Enhanced Enzymatic Hydrolysis Properties. Polym. Degrad. Stab. 2019, 160, 242–263. [Google Scholar] [CrossRef]
- Chen, S.; Zou, R.; Li, L.; Shang, J.; Lin, S.; Lan, J. Preparation of Biobased Poly(Propylene 2,5-furandicarboxylate) Fibers: Mechanical, Thermal and Hydrolytic Degradation Properties. J. Appl. Polym. Sci. 2021, 138, app50345. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Qi, Z.; He, L.; Peng, L. Progress in the Synthesis and Properties of 2,5-Furan Dicarboxylate Based Polyesters. BioResources 2020, 15, 4502–4527. [Google Scholar] [CrossRef]
- Fredi, G.; Rigotti, D.; Bikiaris, D.N.; Dorigato, A. Tuning Thermo-Mechanical Properties of Poly(Lactic Acid) Films through Blending with Bioderived Poly(Alkylene Furanoate)s with Different Alkyl Chain Length for Sustainable Packaging. Polymer 2021, 218, 123527. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Irska, I.; Miadlicki, P.; Walkowiak, K.; Rozwadowski, Z.; Paszkiewicz, S. Structure, Thermal and Mechanical Properties of Copoly(Ester Amide)s Based on 2,5-furandicarboxylic Acid. J. Mater. Sci. 2021, 56, 19296–19309. [Google Scholar] [CrossRef]
- Müller, A.J.; Arnal, M.L. Thermal Fractionation of Polymers. Prog. Polym. Sci. 2005, 30, 559–603. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Bikiaris, D.N.; Papageorgiou, G.Z. Crystallization and Polymorphism of Poly(Ethylene Furanoate). Cryst. Growth Des. 2015, 15, 5505–5512. [Google Scholar] [CrossRef]
- Wang, D.; Liu, B.; Niu, D.; Yang, W.; Zhang, X.; Xu, P.; Ma, P. Strong, Ductile and Transparent Biaxially Oriented Poly(Lactic Acid) with Low Content of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) by Temperature-Gradient Stretching: The Role of Mesophase. Compos. Commun. 2025, 55, 102311. [Google Scholar] [CrossRef]
- Ge, W.; Huang, W.; Zhang, X.; Wei, L.; Wang, P.; Chen, P. Studies on Molecular Origin of Polylactide Mesophase and Its Thermal Stability. Polymer 2025, 319, 128025. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Papageorgiou, M.; Bikiaris, D.N. Evaluation of Polyesters from Renewable Resources as Alternatives to the Current Fossil-Based Polymers. Phase Transitions of Poly(Butylene 2,5-Furan-Dicarboxylate). Polymer 2014, 55, 3846–3858. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, M.; Wu, B.; Li, H.; Li, Y. In Situ Following Oriented Crystallization of Pre-Stretched Poly(Ethylene 2,5-Furandicarboxylate) Under Post Heating. Polymers 2025, 17, 1508. [Google Scholar] [CrossRef]
- Martínez-Tong, D.E.; Soccio, M.; Robles-Hernández, B.; Guidotti, G.; Gazzano, M.; Lotti, N.; Alegria, A. Evidence of Nanostructure Development from the Molecular Dynamics of Poly(Pentamethylene 2,5-Furanoate). Macromolecules 2020, 53, 10526–10537. [Google Scholar] [CrossRef]
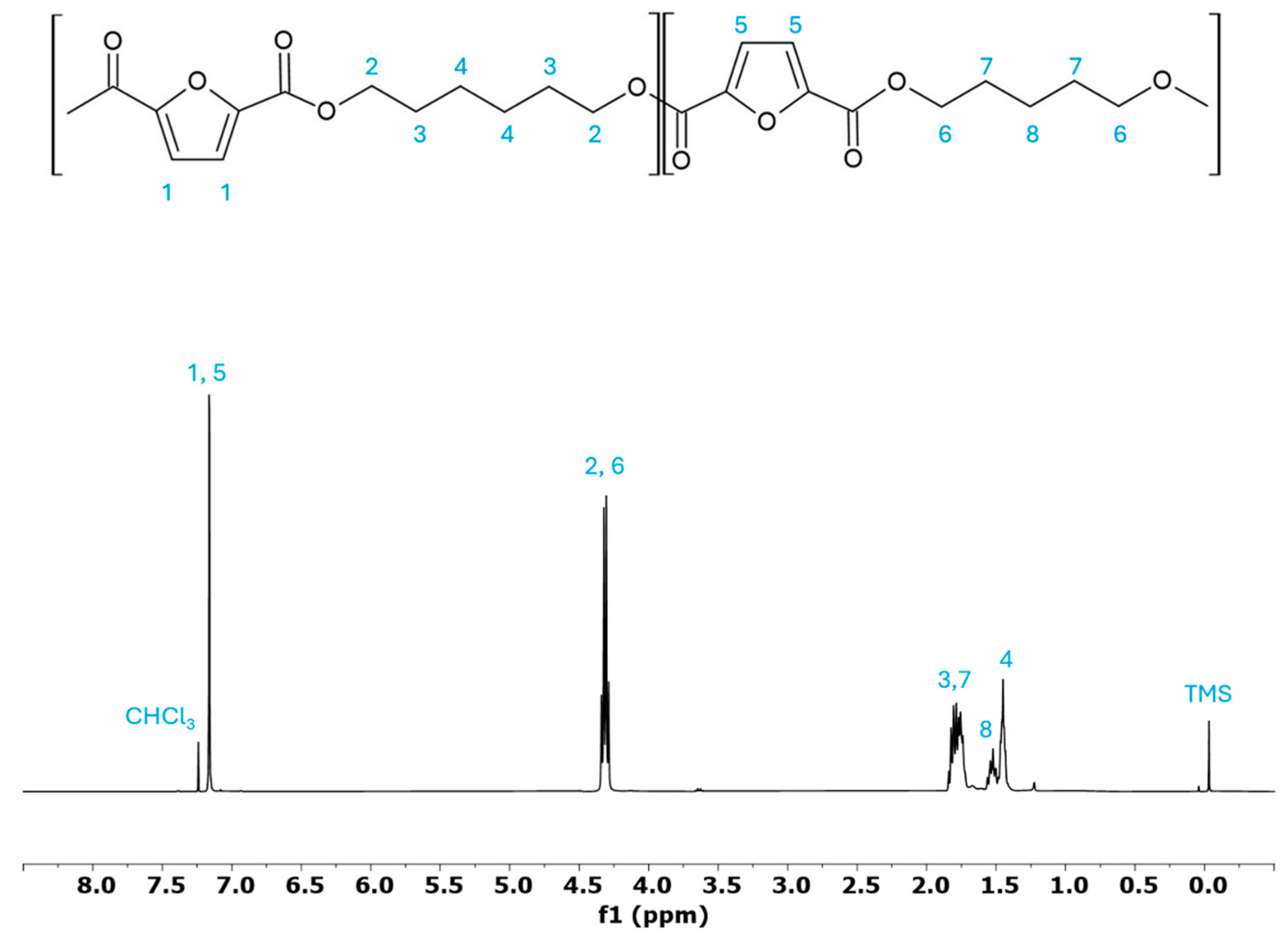
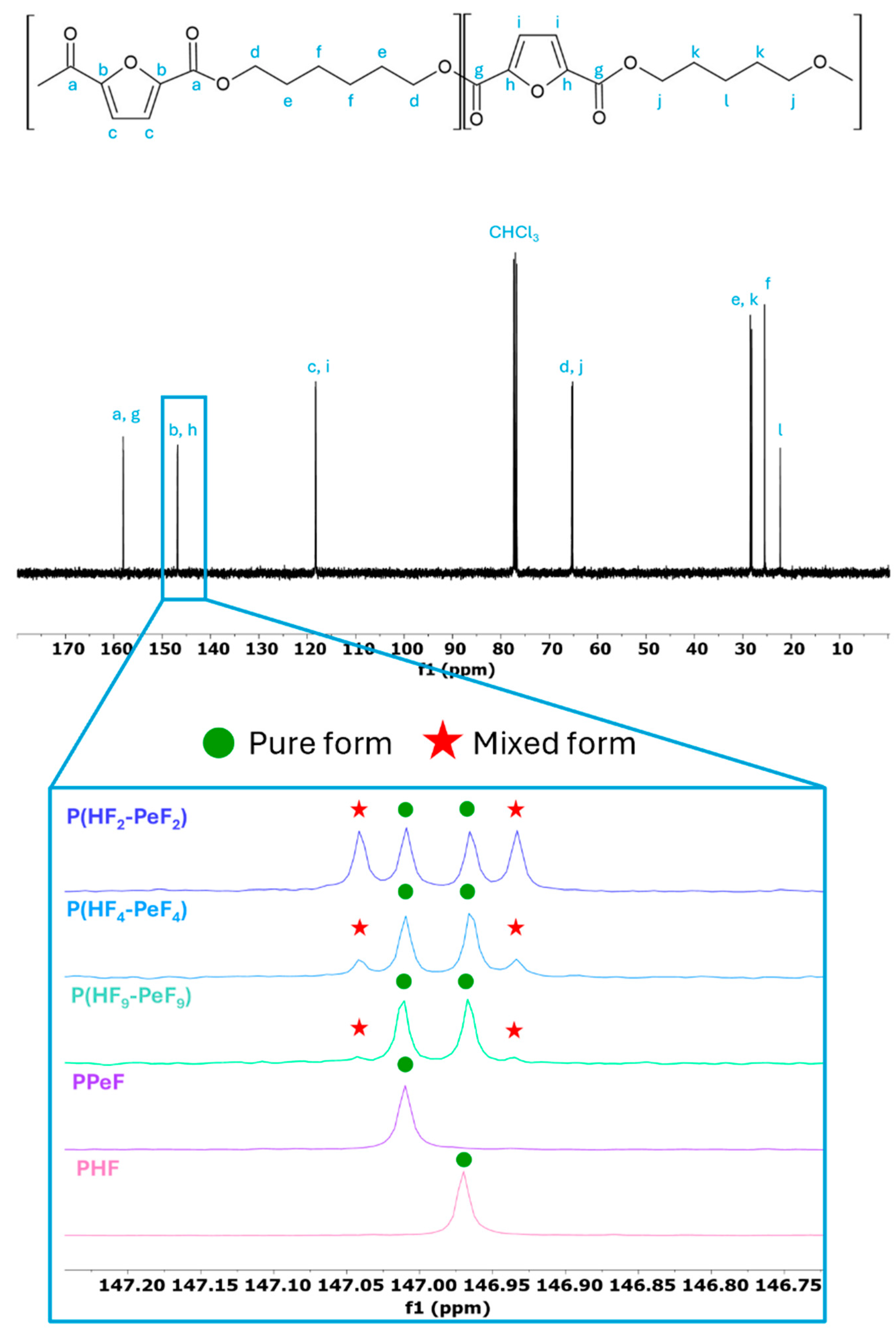
| 13C-NMR | GPC | ||||
|---|---|---|---|---|---|
| LH-F | LPe-F | R | Mn (g/mol) | Đ | |
| PHF | - | - | - | 43,900 | 1.7 |
| P(HF9–PeF9) | 9 | 9 | 0.22 | 46,000 | 1.7 |
| P(HF4–PeF4) | 4 | 4 | 0.5 | 49,700 | 1.5 |
| P(HF2–PeF2) | 2 | 2 | 1 | 69,400 | 1.6 |
| PPeF | - | - | - | 52,600 | 1.4 |
| DSC | TGA | WAXS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I Scan | II Scan | ||||||||||||
| Tg (°C) | ΔCp (J/g*°C) | Tm (°C) | ΔHm (J/g) | Tg (°C) | ΔCp (J/g*°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Tonset (°C) | Tmax (°C) | Xc (°C) | |
| PHF | n.d. | n.d. | 53 144 | 1.4 38 | 17 | 0.258 | 60 | 23 | 144 | 35 | 375 | 393 | 37 ± 4 |
| PHF/PPeF | 17 | 0.433 | 54 143 | 3.6 26 | 17 | 0.567 | 83 | 25 | 144 | 25 | 370 | 390 | 20 ± 4 |
| P(HF9–PeF9) | 17 | 0.203 | 54 131 | 6.3 20 | 17 | 0.382 | 101 | 7 | 133 | 7 | 369 | 390 | 19 ± 4 |
| P(HF4–PeF4) | 18 | 0.305 | 54 108 | 9 13 | 18 | 0.347 | - | - | - | - | 373 | 390 | 16 ± 3 |
| P(HF2–PeF2) | 18 | 0.433 | 55 63 | 17 4 | 18 | 0.481 | - | - | - | - | 373 | 391 | 11 ± 3 |
| PPeF | 18 | 0.464 | - | - | 18 | 0.579 | - | - | - | - | 370 | 391 | 0 |
| E (MPa) | σb (MPa) | εb (%) | WCA (°) | |
|---|---|---|---|---|
| PHF | 674 ± 46 | 25 ± 1 | 17 ± 2 | 100 ± 2 |
| PHF/PPeF | 184 ± 34 | 21 ± 5 | 479 ± 65 | 98 ± 2 |
| P(HF9–PeF9) | 166 ± 14 | 31 ± 2 | 612 ± 57 | 97 ± 2 |
| P(HF4–PeF4) | 182 ± 41 | 32 ± 5 | 666 ± 71 | 97 ± 3 |
| P(HF2–PeF2) | 111 ± 12 | 42 ± 5 | 950 ± 93 | 99 ± 2 |
| PPeF | 60 ± 13 | 2.5 ± 0.7 | 2025 ± 183 | 97 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, A.; Astolfi, G.; Guidotti, G.; Soccio, M.; Boanini, E.; Versura, P.; Lotti, N. Novel Superelastic Polyesters Based on 2,5-Furandicarboxylic Acid for Potential Use in Ophthalmic Surgery. Polymers 2025, 17, 3220. https://doi.org/10.3390/polym17233220
Palumbo A, Astolfi G, Guidotti G, Soccio M, Boanini E, Versura P, Lotti N. Novel Superelastic Polyesters Based on 2,5-Furandicarboxylic Acid for Potential Use in Ophthalmic Surgery. Polymers. 2025; 17(23):3220. https://doi.org/10.3390/polym17233220
Chicago/Turabian StylePalumbo, Arianna, Gloria Astolfi, Giulia Guidotti, Michelina Soccio, Elisa Boanini, Piera Versura, and Nadia Lotti. 2025. "Novel Superelastic Polyesters Based on 2,5-Furandicarboxylic Acid for Potential Use in Ophthalmic Surgery" Polymers 17, no. 23: 3220. https://doi.org/10.3390/polym17233220
APA StylePalumbo, A., Astolfi, G., Guidotti, G., Soccio, M., Boanini, E., Versura, P., & Lotti, N. (2025). Novel Superelastic Polyesters Based on 2,5-Furandicarboxylic Acid for Potential Use in Ophthalmic Surgery. Polymers, 17(23), 3220. https://doi.org/10.3390/polym17233220











