An Environmentally Benign Solvent for the Cationic Polymerization of Low Ceiling Temperature Polyaldehydes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical Recycling to Monomer for an Ideal, Circular Polymer Economy. Nat. Rev. Mater. 2020, 5, 501–516. [Google Scholar] [CrossRef]
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and Biological Catalysis for Plastics Recycling and Upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Clark, R.A.; Shaver, M.P. Depolymerization within a Circular Plastics System. Chem. Rev. 2024, 124, 2617–2650, Erratum in Chem. Rev. 2024, 124, 8823. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, E.M.; Lopez Hernandez, H.; Feinberg, E.C.; Yourdkhani, M.; Zen, E.K.; Mejia, E.B.; Sottos, N.R.; Moore, J.S.; White, S.R. Fully Recyclable Metastable Polymers and Composites. Chem. Mater. 2018, 31, 398–406. [Google Scholar] [CrossRef]
- Diesendruck, C.E.; Peterson, G.I.; Kulik, H.J.; Kaitz, J.A.; Mar, B.D.; May, P.A.; White, S.R.; Martínez, T.J.; Boydston, A.J.; Moore, J.S. Mechanically Triggered Heterolytic Unzipping of a Low-Ceiling-Temperature Polymer. Nat. Chem. 2014, 6, 623–628. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Chemically Recyclable Polymers: A Circular Economy Approach to Sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Yardley, R.E.; Kenaree, A.R.; Gillies, E.R. Triggering Depolymerization: Progress and Opportunities for Self-Immolative Polymers. Macromolecules 2019, 52, 6342–6360. [Google Scholar] [CrossRef]
- Seo, W.; Phillips, S.T. Patterned Plastics That Change Physical Structure in Response to Applied Chemical Signals. J. Am. Chem. Soc. 2010, 132, 9234–9235. [Google Scholar] [CrossRef]
- Peterson, G.I.; Larsen, M.B.; Boydston, A.J. Controlled Depolymerization: Stimuli-Responsive Self-Immolative Polymers. Macromolecules 2012, 45, 7317–7328. [Google Scholar] [CrossRef]
- Wang, F.; Diesendruck, C.E. Polyphthalaldehyde: Synthesis, Derivatives, and Applications. Macromol. Rapid Commun. 2018, 39, 1700519. [Google Scholar] [CrossRef]
- Schwartz, J.M.; Phillips, O.; Engler, A.; Sutlief, A.; Lee, J.; Kohl, P.A. Stable, High-Molecular-Weight Poly(Phthalaldehyde). J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1166–1172. [Google Scholar] [CrossRef]
- Kaitz, J.A.; Diesendruck, C.E.; Moore, J.S. End Group Characterization of Poly(Phthalaldehyde): Surprising Discovery of a Reversible, Cationic Macrocyclization Mechanism. J. Am. Chem. Soc. 2013, 135, 12755–12761. [Google Scholar] [CrossRef]
- Aso, C.; Tagami, S.; Kunitake, T. Polymerization of Aromatic Aldehydes. II. Cationic Cyclopolymerization of Phthalaldehyde. J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 497–511. [Google Scholar] [CrossRef]
- Hossaini, R.; Chipperfield, M.P.; Montzka, S.A.; Leeson, A.A.; Dhomse, S.S.; Pyle, J.A. The Increasing Threat to Stratospheric Ozone from Dichloromethane. Nat. Commun. 2017, 8, 15962. [Google Scholar] [CrossRef]
- Schlosser, P.M.; Bale, A.S.; Gibbons, C.F.; Wilkins, A.; Cooper, G.S. Human Health Effects of Dichloromethane: Key Findings and Scientific Issues. Environ. Health Perspect. 2015, 123, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.; Von Burg, R. Methylene Chloride. J. Appl. Toxicol. 1995, 15, 329–335. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic Parameters for Solvents of Interest in Green Chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- de Juan, A.; Fonrodona, G.; Casassas, E. Solvent Classification Based on Solvatochromic Parameters: A Comparison with the Snyder Approach. TrAC Trends Anal. Chem. 1997, 16, 52–62. [Google Scholar] [CrossRef]
- DiLauro, A.M.; Robbins, J.S.; Phillips, S.T. Reproducible and Scalable Synthesis of End-Cap-Functionalized Depolymerizable Poly(Phthalaldehydes). Macromolecules 2013, 46, 2963–2968. [Google Scholar] [CrossRef]
- Lingier, S.; Nevejans, S.; Espeel, P.; De Wildeman, S.; Du Prez, F.E. High Molecular Weight Poly(Cycloacetals) towards Processable Polymer Materials. Polymer 2016, 103, 98–103. [Google Scholar] [CrossRef]
- Feinberg, E.C.; Hernandez, H.L.; Plantz, C.L.; Mejia, E.B.; Sottos, N.R.; White, S.R.; Moore, J.S. Cyclic Poly(Phthalaldehyde): Thermoforming a Bulk Transient Material. ACS Macro Lett. 2018, 7, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.; Kohl, P.A. Kinetic Investigation on the Cationic Polymerization of O-Phthalaldehyde: Understanding Ring-Expansion Polymerization. Macromolecules 2020, 53, 1543–1549. [Google Scholar] [CrossRef]
- Busfield, W.K.; McEwen, I.J. The Cationic Polymerization of Fluoral—Ii Kinetics and Mechanism of the Polymerization Initiated by Boron Trifluoride Etherate in Dichloromethane Solution. Eur. Polym. J. 1973, 9, 1127–1141. [Google Scholar] [CrossRef]
- Yasuda, H.; Tani, H. Stereospecific Polymerization of O-Phthalaldehyde. Macromolecules 1973, 6, 303–304. [Google Scholar] [CrossRef]
- Schwartz, J.M.; Gourdin, G.; Phillips, O.; Engler, A.; Lee, J.; Abdulkadir, N.R.; Miller, R.C.; Sutlief, A.; Kohl, P.A. Cationic Polymerization of High-Molecular-Weight Phthalaldehyde-Butanal Copolymer. J. Appl. Polym. Sci. 2019, 136, 46921. [Google Scholar] [CrossRef]
- Engler, A.; Phillips, O.; Miller, R.C.; Tobin, C.; Kohl, P.A. Cationic Copolymerization of o-Phthalaldehyde and Functional Aliphatic Aldehydes. Macromolecules 2019, 52, 4020–4029. [Google Scholar] [CrossRef]
- Vogl, O. Cationic Polymerization of Aldehydes. Die Makromol. Chem. 1974, 175, 1281–1308. [Google Scholar] [CrossRef]
- Schwartz, J.M.; Engler, A.; Phillips, O.; Lee, J.; Kohl, P.A. Determination of Ceiling Temperature and Thermodynamic Properties of Low Ceiling Temperature Polyaldehydes. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 221–228. [Google Scholar] [CrossRef]
- Lutz, J.P.; Davydovich, O.; Hannigan, M.D.; Moore, J.S.; Zimmerman, P.M.; McNeil, A.J. Functionalized and Degradable Polyphthalaldehyde Derivatives. J. Am. Chem. Soc. 2019, 141, 14544–14548. [Google Scholar] [CrossRef]
- Cooper, A.I. Polymer Synthesis and Processing Using Supercritical Carbon Dioxide. J. Mater. Chem. 2000, 10, 207–234. [Google Scholar] [CrossRef]
- Kendall, J.L.; Canelas, D.A.; Young, J.L.; DeSimone, J.M. Polymerizations in Supercritical Carbon Dioxide. Chem. Rev. 1999, 99, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Scaiano, J.C.; Encinas, M.V.; George, M.V. Photochemistry of O-Phthalaldehyde. J. Chem. Soc. Perkin Trans. 2 1980, 724–730. [Google Scholar] [CrossRef]
- Hayashi, H.; Tachi, H.; Suyama, K. Synthesis and Photo-Degradation of Polyphthalaldehydes with Oxime Ether Terminals. J. Photopolym. Sci. Technol. 2020, 33, 269–278. [Google Scholar] [CrossRef]
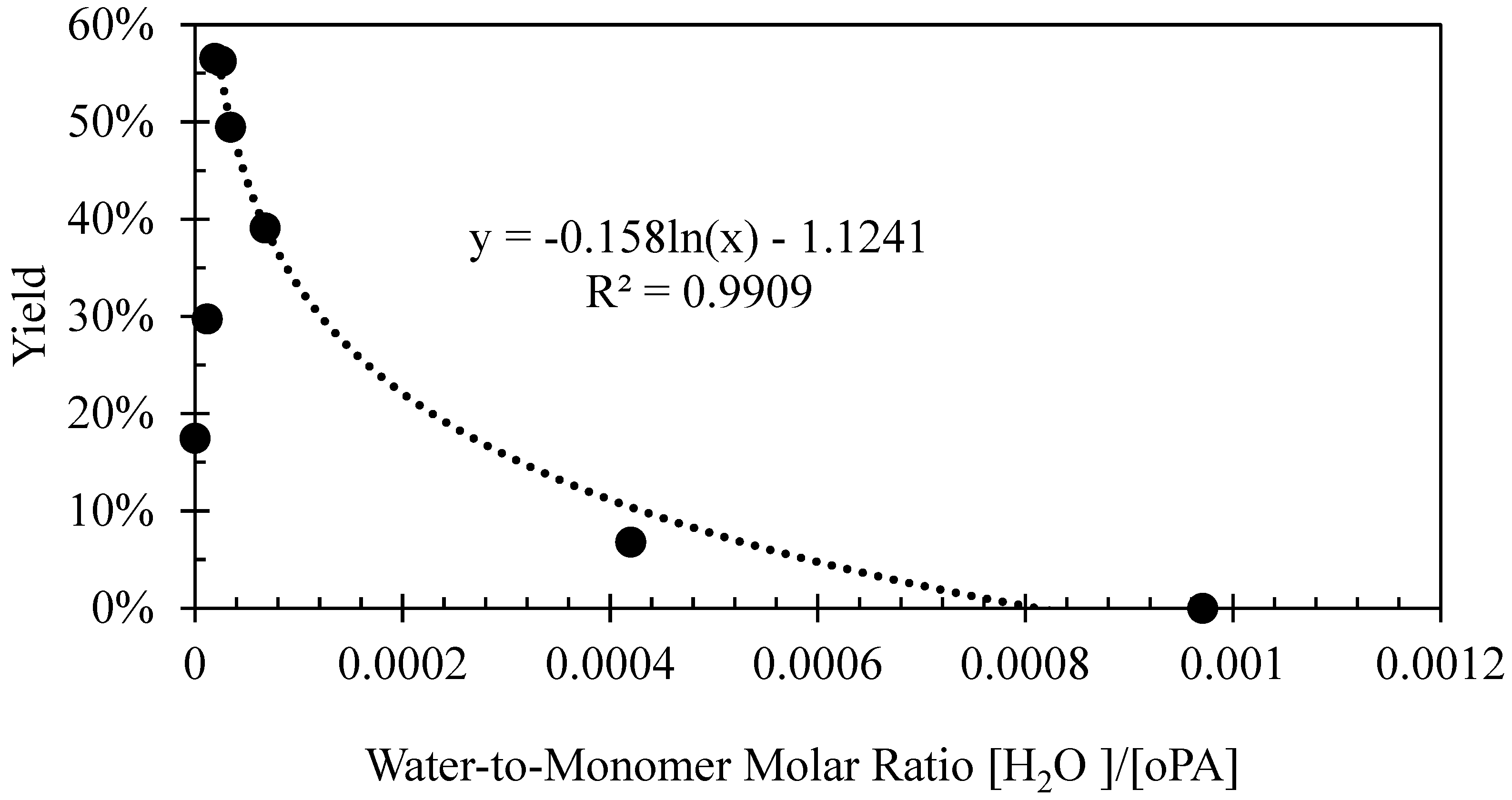
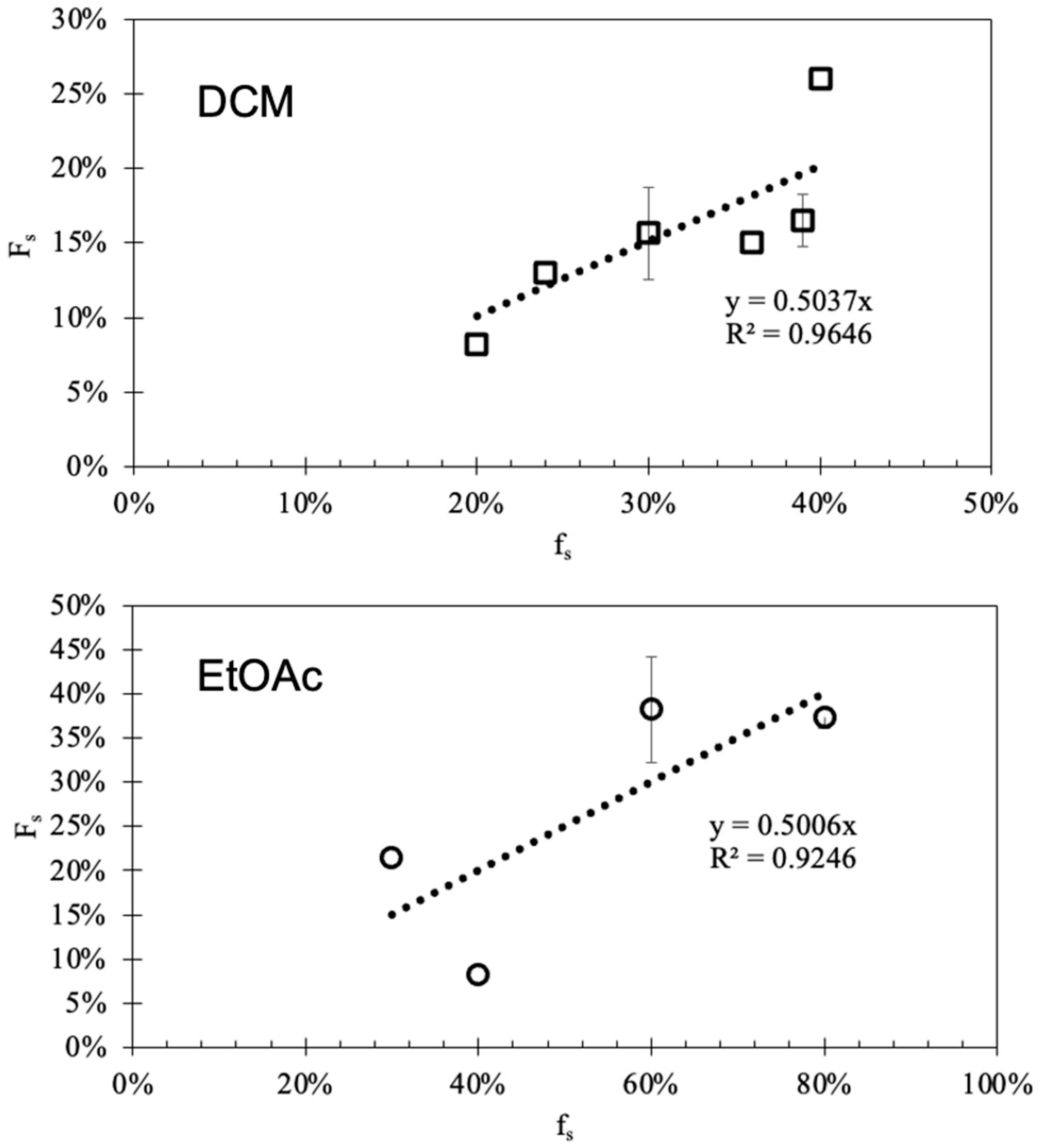
| Solvent | Solvatomagnetic β | Maximum Yield |
|---|---|---|
| DCM | 0.0 | 98% |
| Tol | 0.11 | 45% |
| Valeronitrile | 0.46 | None |
| Acetone | 0.49 | None |
| MeOAc | 0.51 | 38% |
| EtOAc | 0.52 | 98% |
| THF | 0.58 | None |
| nBuOAc | 0.58 | None |
| Diethyl ether | 0.59 | None |
| PrOAc | 0.6 | None |
| CPME | 0.63 | None |
| mTHF | 0.66 | None |
| MTBE | 0.67 | None |
| iBuOAc | - | None |
| DMI | - | None |
| iAmOAc | - | None |
| Quench Method | Stability (45 °C Oven) |
|---|---|
| MeOH Precipitation | 60 days |
| MeOH + Pyridine Wash | 41 days |
| MeOH Wash | 86 days |
| CsF | 28 days |
| DCM | EtOAc | |||||
|---|---|---|---|---|---|---|
| Catalyst | Yield | Mn (kg/mol) | Ð | Yield | Mn (kg/mol) | Ð |
| BF3 | 87% | 148 | 1.55 | 92% | 74.6 | 1.67 |
| [Al2Cl7−] | 63% | 61.4 | 1.58 | 96% | 30.0 | 1.72 |
| Solvent System | Ethanal Content | Mn (kg/mol) | Yield |
|---|---|---|---|
| DCM | 26 mol% | 64.0 | 38% |
| EtOAc | 26 mol% | 10.4 | 41% |
| Ethanal | 25 mol% | 3.8 | 5.2% |
| Solvent | Yield | Mn (kDa) | Ɖ |
|---|---|---|---|
| DCM (Parr) | 75.1% | 91.7 | 1.35 |
| DCM + CO2 | 67.5% | 51.0 | 1.39 |
| Liquid CO2 | Slight | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez Ninantay, J.C.; Engler, A.C.; Schwartz, J.M.; Kohl, P.A. An Environmentally Benign Solvent for the Cationic Polymerization of Low Ceiling Temperature Polyaldehydes. Polymers 2025, 17, 3210. https://doi.org/10.3390/polym17233210
Lopez Ninantay JC, Engler AC, Schwartz JM, Kohl PA. An Environmentally Benign Solvent for the Cationic Polymerization of Low Ceiling Temperature Polyaldehydes. Polymers. 2025; 17(23):3210. https://doi.org/10.3390/polym17233210
Chicago/Turabian StyleLopez Ninantay, Jose C., Anthony C. Engler, Jared M. Schwartz, and Paul A. Kohl. 2025. "An Environmentally Benign Solvent for the Cationic Polymerization of Low Ceiling Temperature Polyaldehydes" Polymers 17, no. 23: 3210. https://doi.org/10.3390/polym17233210
APA StyleLopez Ninantay, J. C., Engler, A. C., Schwartz, J. M., & Kohl, P. A. (2025). An Environmentally Benign Solvent for the Cationic Polymerization of Low Ceiling Temperature Polyaldehydes. Polymers, 17(23), 3210. https://doi.org/10.3390/polym17233210






