Recent Insights into the Research of (Bio)Active Additives for Advanced Polymer Materials
Abstract
1. Introduction
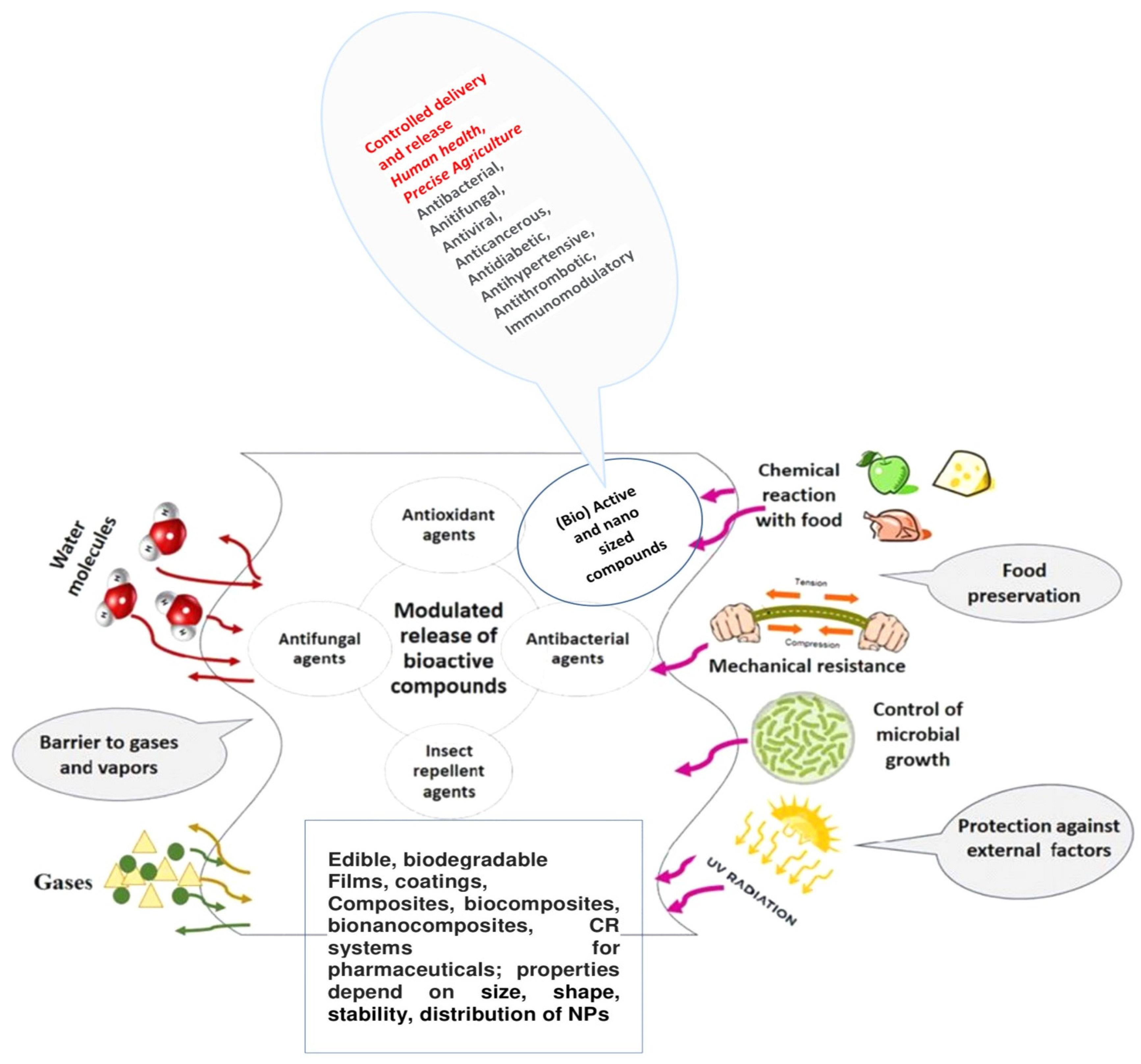
2. Types of (Bio)Active Additives/Ingredients
| Antimicrobials/Antifungals | Antioxidants/Stabilizers | Oxygen Scavengers | Ethylene or Oxygen Scavengers | Carbon Dioxide Scavengers | Moisture Scavengers | Flavor Emitters | Colorants | Nanoscale Compounds |
|---|---|---|---|---|---|---|---|---|
|
|
|
| Emitters
Scavengers
|
|
|
| Inorganic NPs
|
2.1. Natural Active Additives
2.1.1. Phytochemicals
2.1.2. Natural Extracts
2.1.3. Essential Oils (EOs)
2.1.4. Composition Properties/Activities Relationship
| Active Compounds | Activity and Application | Refs |
|---|---|---|
| Terpenes and terpenoids | Protect plants from predators or lure the pollinators. Carvone is used in anti-infective therapy; thymol, carvacrol, eugenol, and menthol are good alternatives to synthetic fungicides in the food industries due to their potent and wide antifungal activity, protecting agricultural crops from microbial infections. Therapeutic effects in neurodegenerative and cardiovascular diseases, cancer, diabetes, and aging processes. Terpenoid derivatives exhibit antimicrobial activity; composites demonstrated long-term antioxidant release activity. The antioxidative biocomposites are used in active protective packaging of both food (fruits) and cosmetic products. | [151,152,153,154,155,156,157,158,159] |
| Phenols and polyphenols (vanillin, gallic acid, eugenol, ferulic acid, carvacol, thymol) | Antibacterial activity, in vitro and in vivo animal tests demonstrated that they have antioxidant, anticancer, antibacterial, and antidiabetic capacity and health-promoting properties. | [152,153,154,160,161,162,163,164,165,166,167,168,169,170,171] |
| Flavonoids: flavonols, flavan-3-ols (catechins), flavones, and isoflavones, anthocyanins, with different heterocyclic structures | Strong antimicrobial action against a wide range of pathogenic microorganisms, Robust antioxidant and biological activities; useful in the pharmaceutical and health care industries. | [172,173] |
| Anthocyanins | Natural water-soluble colorants/pigments applied as safe food colorants; multifunctional properties such as excellent antioxidant, antimicrobial, and pH-sensitive properties; useful as pH colorimetric film indicators; potential health-promoting properties as antidiabetic and anticancer activities, and in cardiovascular and neuroprotective prevention. | [25,174,175,176,177,178,179,180] |
| Sulfur-containing compounds (allicin, ajoene, dialkenyl, dialkyl sulphides, S-allyl cysteine and S-allyl-mercapto cysteine, and isothiocyanates) | Antibacterial action against both Gram-positive and Gram-negative bacteria. | [181] |
| Coumarins | Chemical defense against predators, pathogens, and insects. Strong antibacterial action against S. aureus, Salmonella typhi, Enterobacter cloacae, and Enterobacter aerogenes. Potential therapeutic agents against several human diseases, anti-inflammatory, anticoagulant, antibacterial, antifungal, antiviral, antihypertensive, antituberculous, anticonvulsant, antiadipogenic, anticancer, and antihyperglycemic. Pharmacological activities, vasodilator, estrogenic, anticoagulant, analgesic, sedative and hypnotic, hypothermic, antihelminthic, antioxidant, and dermal photosensitizing activity, and neuroprotective actions. | [182,183] |
| Tannins | Water-soluble polyphenolic biomolecules. Applications as coagulants, adhesives, flotation agents, tanners, dyes, additives, or biomolecules | [184,185] |
| Lignins | Antimicrobial agents in packaging and textiles, antioxidants, adhesives, biosurfactants, reinforcing agents, compatibilizers, hydrogels, adhesives, anticorrosion agents, carbon fibers or carbon black, phenolic resins, flame retardants, foam composites, new biomedical materials, and cosmetics. | [186,187] |
| Alpha-tocopherol | Vitamin E subclasses. A type of lipid is a naturally occurring antioxidant. | [188,189,190] |
| Carotenoids (>600): β-carotene, lutein, astaxanthin, bixin, norbixin, capsanthin, lycopene, canthaxanthin, β-Apo-8-carotenal, zeaxanthin, β-apo-8-carotenal-ester, etc.) | Preclinical, clinical, and epidemiological studies showed their efficacy on cancer, obesity, type 2 diabetes, cardiovascular diseases, osteoporosis, neurodegenerative disease, mental health, eye, and skin health. | [191,192,193] |
| Alkaloids (piperine, berberine, quinoline, reserpine, sanguinarine, tomatidine, chanoclavine, squalamine) | A broad spectrum of antimicrobial activity and diverse bioactivities, such as bacteriostatic and bactericidal effects, are interesting for the food, pharmaceutical (drugs for therapeutic applications), and cosmetic industries. | [194,195] |
| Antimicrobial peptides | Active against a broad spectrum of microorganisms (bacteria, viruses, fungi, and protozoa). | [196,197] |
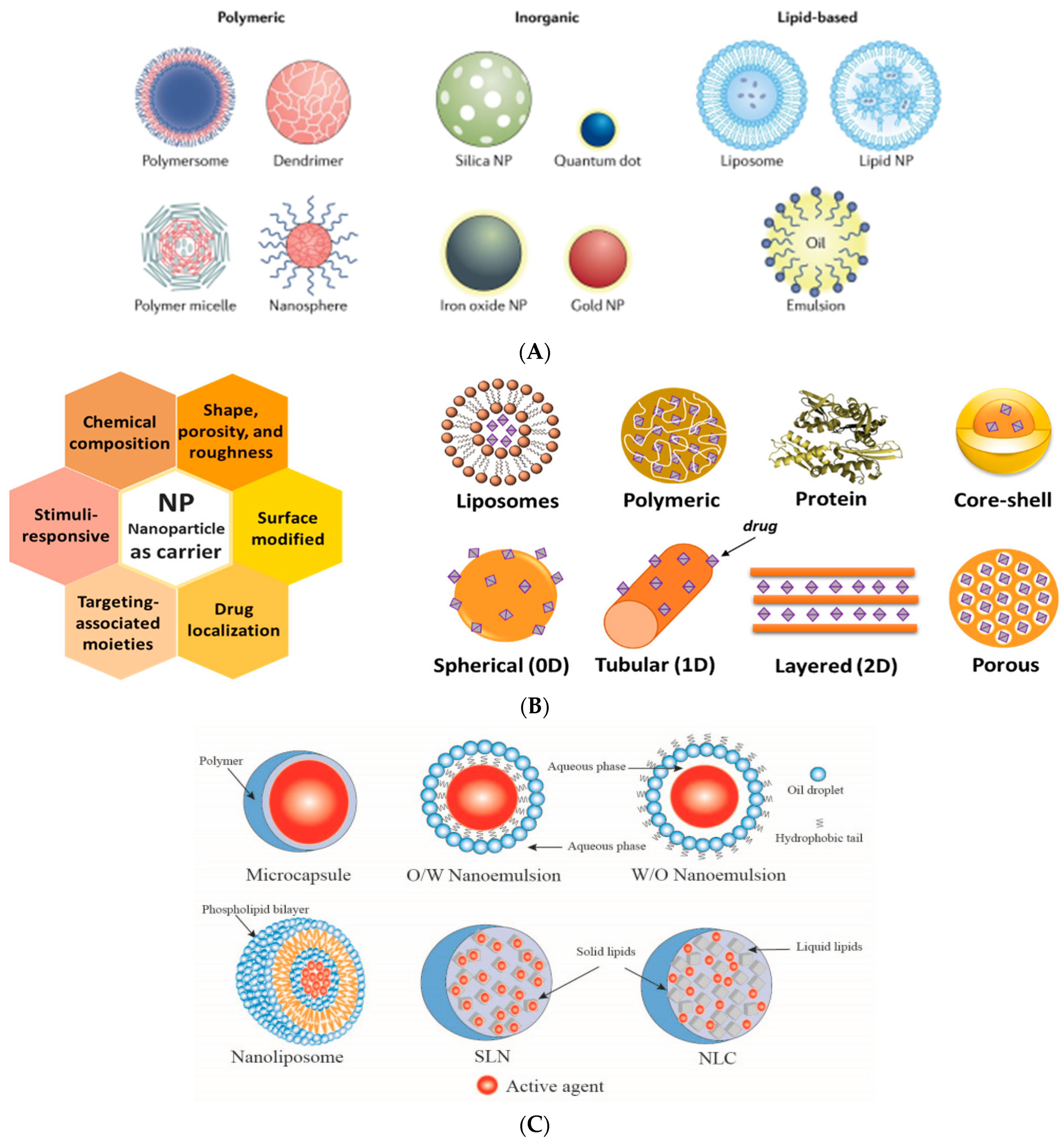
2.2. Nanoscale (Bio)Active Additives
- (I)
- The inorganic active additives are nanoparticles (NPs) made from inorganic substances as: (i) metallic, such as silver, gold, or copper; (ii) metal oxides, such as ZnO, TiO2, SiO2, copper oxides (CuO, Cu2O), MgO, Al2O3, and iron oxides (e.g., Fe3O4, α-Fe2O3 hematite, γFe2O3 maghemite, FeMnO3); (iii) nanocarbon-based, including graphene, graphene oxide, carbon nanotubes (CNTs), fullerene, and quantum dots; (iv) nanoclays (mesoporous silica, nanoparticles of layered mineral silicates such as montmorillonite (MMT), cloisite, bentonite, kaolinite, hectorite, halloysite, laptonite, sepiolite, and organically modified nanoclays, etc. [204]).
- (II)
- The organic NPs are: polymeric nanocapsules, micelles, liposomes, dendrimers, and nanobiopolymers such as cellulose nanowhiskers or nanofibers [205], starch nanocrystals, chitosan NPs and nanofibers, lignin NPs, DNA nanoparticles, and recently developed [206] two-component biopolymer NPs as sustainable lignin/chitosan. These are effective food coatings, promoting eco-friendly packaging, reducing waste quantity, and offering advanced bio-based solutions for food technology [207], etc.
3. Sources for (Bio)Active Compounds and Biopolymers
3.1. Agro-Food By-Products and Aquiculture Waste
3.2. Residual Materials from Cereals
3.3. Residual Materials from the Meat Industry
3.4. Residual Materials from the Dairy Industry
3.5. Residual Materials from Algae and Marine Biowaste
3.6. Other Resources
4. Developments in Extraction and Processing Methods to Assure the Quality/Activities of the Active Additives
4.1. Extraction
| Method | Advantages | Limitations |
|---|---|---|
| Conventional methods | Conventional methods are still used to extract fragrance and aroma oils from plants due to their simplicity | Disadvantages associated with conventional solvent extraction techniques include prolonged extraction time, substantial amounts of solvents, and many extraction steps Thermolabile phytochemicals decompose or degrade during heating |
| Maceration | Simple extraction method, filtration or decantation applied to thermolabile AC |
|
| Digestion | Slight warming in the extraction process (35 to 40 °C) |
|
| Infusion and percolation | Easily soluble constituents; phenolic compounds were extracted from fruits, phenols, and flavonoids in boiling water for 10 min-infusion; a water–alcohol solvent mixture; aqueous HCl; extra efficient extraction |
|
| Soxhlet extraction |
|
|
| Modern techniques | ||
| High solvent temperature and pressure mark the ASE operations’ favorable conditions; better recovery of lipophilic and hydrophilic phytochemicals |
|
| Supercritical fluid extraction (SFE) |
| The highest phenolic acid content yield was achieved with high temperature, whereas lower temperatures afforded more efficiency in extracting high yields of flavonoids In some cases, Soxhlet extraction is preferred |
| Microwave-assisted extraction (MAE): incorporate microwaves and solvents during the extraction process |
| The viscosity of the solvent affects the extraction process, and also the solvent and plant material dielectric susceptibility affect the energy used in MAE |
| Ultrasound-assisted extraction (UAE) | Ultrasound-assisted extraction is an environmentally friendly, green, and cheap technique compared to conventional techniques, recovering green extracts in concentrated form without residual solvents, impurities, or defects
|
|
| Supercritical fluid extraction: carbon dioxide |
|
|
| Enzyme-assisted extraction:
|
|
|
| Pressurized hot water extraction |
|
|
4.2. Processing Methods and Encapsulation
4.2.1. Emulsion-Based Systems
4.2.2. Nanoemulsions
- (a)
- Emulsifiers of different provenances, such as plant-based (e.g., vicilin, whey protein isolate, soy or pea protein); polysaccharides, such as gum arabic or modified starch, pectin (sugar beet pectin), plant mucilages, octenyl succinic anhydride-modified starch; phospholipids, such as soy or sunflower lecithin; and natural (like quillaja saponin) or synthetic (Tween and Spans). Pea, lentil, and faba bean proteins have been used as effective emulsifiers for the formulation of 10 wt% algae nanoemulsions obtained by microfluidization. The best efficacy was shown by the lentil protein in terms of providing resistance to environmental stresses such as pH, ionic strength, and temperature changes, while the sesame protein is an effective emulsifier for forming fish oil nanoemulsions.
- (b)
- Surfactants (sugar esters, polyoxyethylene) reduce the interfacial tension by electrostatic/steric stabilization.
- (c)
- Various stabilizers, including ripening inhibitors (as corn oil, sunflower oil, medium-chain triglyceride oil), The accepted mechanism of stabilization in Pickering emulsions considers the formation of a steric barrier by solid particles adsorbing at the oil–water interface, particles being able to irreversibly attach to the oil–water interface, leading to a more efficient stabilization than surfactant adsorption.
- (d)
- Texture modifiers as thickening and gelling agents (carboxymethyl cellulose, sodium alginate, or pectin) to thicken or gel the aqueous phase, thereby inhibiting droplet movement. Thickening agents increase the viscosity of aqueous solutions, whereas gelling agents provide semi-solid characteristics, forming a 3D network of crosslinked molecules.
- (e)
- Weighting agents that inhibit gravitational separation, i.e., creaming and sedimentation, are hydrophobic substances such as brominated vegetable oil, ester gum, damar gum, and sucrose acetate isobutyrate.
- (f)
- Interfacial stabilizers are important components for electrostatic deposition methods with modified polysaccharides (as chitosan hydrochloride electrostatic deposition coats oil droplets with polysaccharides), increasing the steric and electrostatic repulsion between them, inhibiting aggregation. Thymol nanoemulsions’ stability was increased by whey protein-coated oil droplets and chitosan hydrochloride [293].
- (g)
- Ripening Inhibitors: Some antimicrobial nanoemulsions, especially those containing pure, polar, water-soluble EOs, are highly unstable due to a phenomenon known as Oswald ripening, by which small oil molecules form larger oil droplets, resulting in an increase in the mean droplet size over time, leading to creaming or coalescence, making the nanoemulsion unstable. By adding hydrophobic substances as a ripening inhibitor, like corn oil, sunflower oil, or medium-chain triglyceride oil, this instability is avoided for an optimum concentration. These little droplets help to increase the droplet’s surface area, which improves the functional qualities of the encapsulated component.
- (h)
- Pickering emulsion utilizes solid particles as stabilizers, which accumulate at the interface between two immiscible liquids (typically as oil and water phases) and stabilize droplets against coalescence. Inorganic particles, including silica, clay, and hydroxyapatite, and also some organic particles, can effectively serve as Pickering emulsifiers [273,294]. Multiple Pickering emulsions (MPEs) can be excellent carriers of bioactive compounds, offering a robust background to design MPEs with functional and nutritional benefits. MPEs offer the following advantages: (i) reduced coalescence, bringing higher stability to emulsions; (ii) useful characteristics of many solid particles, such as conductivity, responsiveness, porosity, etc.; (iii) lower toxicity of some food-grade solid particles, leading to higher safety for in vivo usage.
- (i)
- Antioxidants (as quercetin or curcumin) reduce oxidation in nanoemulsions.
4.2.3. Micro and/or Nanoencapsulation
- -
- Extended shelf life.
- -
- Improved stability during processing and in the final product.
- -
- Change in the structure from liquid to solid.
- -
- Liquidity, dispersibility, and dosage accuracy in the final product are improved.
- -
- Controlled release of aroma compounds, prolonging exposure to odor or taste; masking of taste and odor.
- -
- Protection from external factors, separation of chemically unstable and highly volatile substances from environmental factors, protection from UV radiation, and degradation reactions under heat, oxidation, and dehydration.
- -
- Improved safety by reducing the flammability of volatile substances [298].
- (a)
- Physical-mechanical processes (e.g., spray drying, dripping, fluid bed coating, spray cooling/chilling, extrusion, emulsification and freeze drying, supercritical fluid or anti-solvent drying, co-crystallization, lyophilization, pan-coating, spinning discs deposition onto substrate, electrospinning, and copigmentation). Spray drying converts a nanoemulsion into a fine powder, which is easy to handle, store, and transport and is much more stable. Nanoemulsions in a fluid state are turned into powders having high encapsulation efficiencies (95–98%).
- (b)
- Chemical processes (e.g., liposomes, coacervation or molecular inclusion in cyclodextrins, complexation, yeast encapsulation, emulsions, emulsion polymerization, interfacial polymerization, interfacial crosslinking), ionic gelation.
- (c)
- Physico-chemical methods such as coacervation, sol–gel synthesis method, organic phase separation, solvent evaporation, layer-by-layer adsorption, and liposome entrapment.
4.2.4. Protein–Polysaccharide Nanocomplexes
4.2.5. Electrospinning/Electrospraying
5. Green Synthesis of the Nanosized Active Additives
6. Controlled/Targeting Release of (Bio)Active Agents
| No | Type of CR Mechanism | Some Characteristics and Dependence on Main Factors |
|---|---|---|
| 1. | Dissolution | Easiest to occur; thermodynamically compatible |
| 2. | Diffusion | The concentration gradient is one main factor influencing behavior |
| 3. | Solvent-activated | Accompanied by swelling; chewing gum |
| 4. | Osmotically-controlled | Similar to solvent-activated, the length of channels |
| 5. | Barrier properties | Permeability; wall thickness |
| 6. | pH-controlled | Targeted delivery; GIT delivery |
| 7. | Temperature-activated | Glassy and rubbery; shrink/swelling |
| 8. | Pressure-activated | Fragments; force fractured release |
| 9. | Melting-activated | Physical state changes: Melting point |
| 10. | Receptor delivery | Targeted release; ligand/receptor |
| 11. | Erosion-controlled | Degradation is prevalent; no transport |
| 12. | Enzyme-controlled | High sensitivity; smart carriers |
| 13. | Nanosystem- controlled | Size and shape, microstructure, molecular interaction, density, environment, rigidity/flexibility, surface chemistry of the nanotextures, bacteria specificity (e.g., Gram-positive and Gram-negative), motility, etc. |
- (I)
- The mathematical definition of dissolution is based on the following equation:
- (II)
- For the diffusion mechanism, defined by the mass flux (J) as the mass amount (M) passing through a surface (S) during the time (t), expressed by Equation (3),
7. Some Considerations on the Mechanisms of Antimicrobial, Antifungal and Antiviral Activities
7.1. Mechanism of the Antimicrobial Activity of the Bioactive Additives
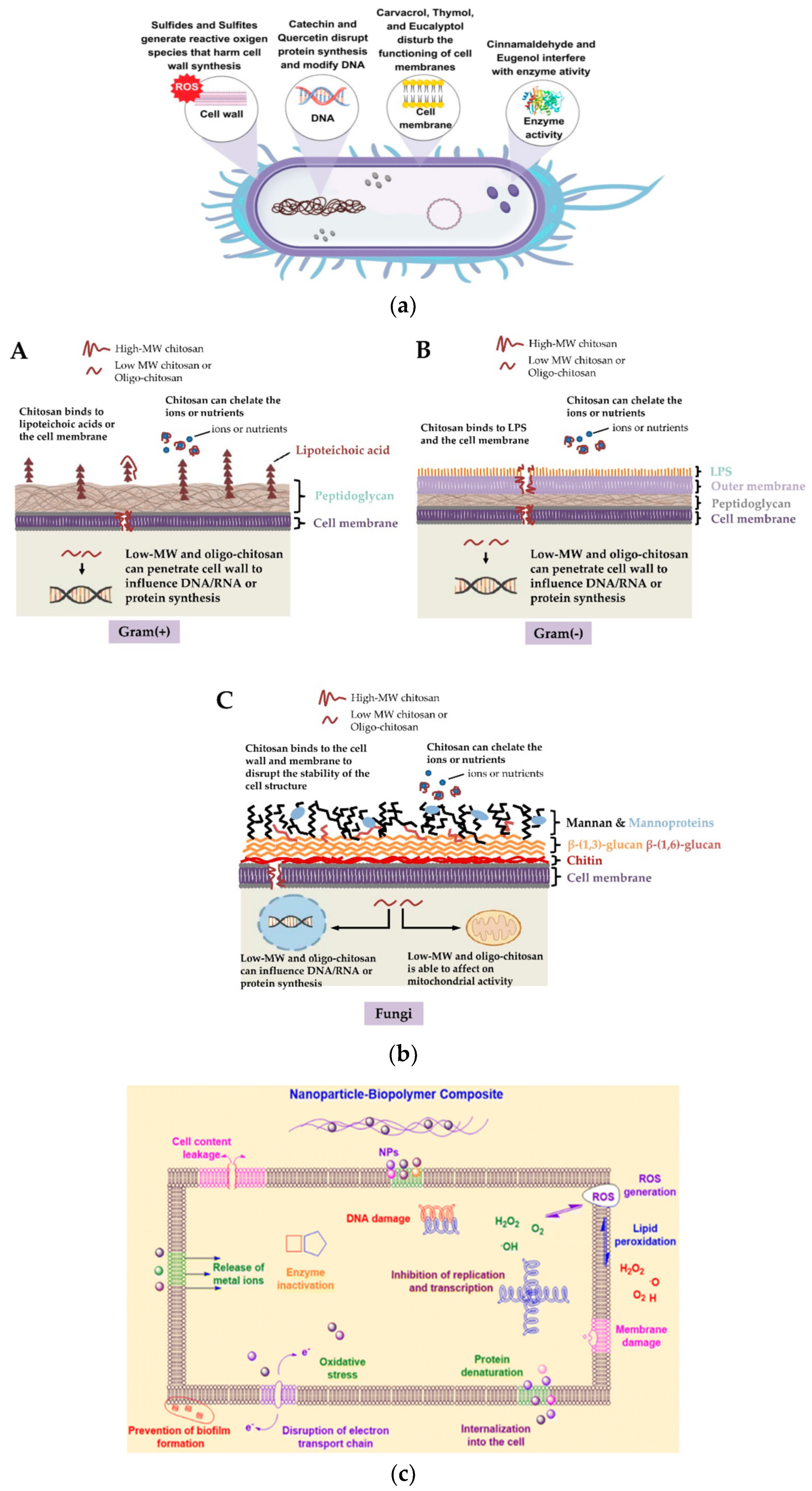
7.2. Mechanism of the Antimicrobial Activity of NPs
8. Some Considerations on the Mechanism of Antioxidant Activity
9. Toxicity Issues
10. Concluding Remarks and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Wu, H.; Wang, H.; Sun, A.; Kan, Z. Creation of fully degradable poly(lactic acid) composite by using biosourced poly(4-hydroxybutyrate) as bioderived toughening additives. Express Polym. Lett. 2022, 16, 996–1010. [Google Scholar] [CrossRef]
- Vallejos, S.; Trigo-López, M.; Arnaiz, A.; Garcia, J.M. From Classical to Advanced Use of Polymers in Food and Beverage Applications. Polymers 2022, 14, 4954. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; de Souza, F.M.; Dawsey, T.; Gupta, R.K. Biodegradable polymers and composites: Recent development and challenges. Polym. Compos. 2024, 45, 2896–2918. [Google Scholar] [CrossRef]
- Kumar, B.P.; Vijayakumar, S.; Thomas, J. Effect of polystyrene nanoplastics on its toxicity and reproduction in Philodina roseola. Sci. Rep. 2025, 15, 14206. [Google Scholar] [CrossRef] [PubMed]
- Alves, B. Plastic Waste Worldwide—Statistics & Facts. Energy & Environment. Waste Management. Worldhwide. Available online: https://www.statista.com/topics/5401/global-plastic-waste/ (accessed on 1 May 2025).
- Krawczak, P. Towards a circular, low-carbon emission plastics industry. Express Polym. Lett. 2022, 16, 901. [Google Scholar] [CrossRef]
- Talib, A.; Samad, A.; Hossain, M.J.; Muazzam, A.; Anwar, B.; Atique, R.; Hwang, Y.-H.; Joo, S.-T. Modern Trends and Techniques for Food Preservation. Food Life 2024, 1, 19–32. [Google Scholar] [CrossRef]
- Barone, A.S.; Matheus, J.R.V.; de Souza, T.S.P.; Moreira, R.F.A.; Fai, A.E.C. Green-based active packaging: Opportunities beyond COVID-19, food applications, and perspectives in circular economy—A brief review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4881–4905. [Google Scholar] [CrossRef]
- Koval, V.; Olczak, P.; Vdovenko, N.; Boiko, O.; Matuszewska, D.; Mikhno, I. Ecosystem of environmentally sustainable municipal infrastructure in the Ukraine. Sustainability 2021, 13, 10223. [Google Scholar] [CrossRef]
- Oliver-Cuenca, V.; Salaris, V.; Muñoz-Gimena, P.F.; Agüero, Á.; Peltzer, M.A.; Montero, V.A.; Arrieta, M.P.; Sempere-Torregrosa, J.; Pavon, C.; Samper, M.D.; et al. Bio-Based and Biodegradable Polymeric Materials for a Circular Economy. Polymers 2024, 16, 3015. [Google Scholar] [CrossRef]
- Wiley Science Solutions. Available online: https://sciencesolutions.wiley.com/request-quote/ (accessed on 9 October 2025).
- Ayllón-Gutiérrez, R.; Díaz-Rubio, L.; Montaño-Soto, M.; Haro-Vázquez, M.d.P.; Córdova-Guerrero, I. Applications of Plant Essential Oils in Pest Control and Their Encapsulation for Controlled Release: A Review. Agriculture 2024, 14, 1766. [Google Scholar] [CrossRef]
- Rath, D. A critical review on food adulteration and its risk on health. Int. J. Novel Res. Dev. 2022, 7, 353–356. [Google Scholar]
- Gigante, V.; Aliotta, L.; Ascrizzi, R.; Pistelli, L.; Zinnai, A.; Batoni, G.; Coltelli, M.-B.; Lazzeri, A. Innovative Biobased and Sustainable Polymer Packaging Solutions for Extending Bread Shelf Life: A Review. Polymers 2023, 15, 4700. [Google Scholar] [CrossRef]
- Demircan, B.; Velioglu, Y.S. Revolutionizing single-use food packaging: A comprehensive review of heat-sealable, water-soluble, and edible pouches, sachets, bags, or packets. Crit. Rev. Food Sci. Nutr. 2025, 65, 1497–1517. [Google Scholar] [CrossRef]
- Tăbărașu, A.-M.; Anghelache, D.-N.; Găgeanu, I.; Biriș, S.-Ș.; Vlăduț, N.-V. Considerations on the Use of Active Compounds Obtained from Lavender. Sustainability 2023, 15, 8879. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, J.; McClements, D.J.; Zhang, Z.; Zhang, R.; Jin, Z.; Chen, L. Stability enhancement methods for natural pigments in intelligent packaging: A review. Crit. Rev. Food Sci. Nutr. 2024, 65, 6233–6248. [Google Scholar] [CrossRef] [PubMed]
- Stoica, M.; Bichescu, C.I.; Crețu, C.-M.; Dragomir, M.; Ivan, A.S.; Podaru, G.M.; Stoica, D.; Stuparu-Crețu, M. Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods 2024, 13, 3027. [Google Scholar] [CrossRef] [PubMed]
- Dirpan, A.; Deliana, Y.; Ainani, A.F.; Irwan; Bahmid, N.A. Exploring the Potential of Pectin as a Source of Biopolymers for Active and Intelligent Packaging: A Review. Polymers 2024, 16, 2783. [Google Scholar] [CrossRef]
- Li, J.; Sun, H.; Weng, Y. Natural Extracts and Their Applications in Polymer-Based Active Packaging: A Review. Polymers 2024, 16, 625. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Khan, S.; Mehdizadeh, M.; Bahmid, N.A.; Adli, D.N.; Walkeri, T.R.; Perestrelo, R.; Camara, J.S. Phytochemicals and bioactive constituents in food packaging—A systematic review. Heliyon 2023, 9, E21196. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Alao, A.E.; Ayandele, A.A.; Adebayo, E.A.; Adewoyin, A.G.; Amao, J.A. Utilization potential of Pleurotus pulmonarius LAU09 (JF736658) on Crude oil contaminated Substrate. Trop. J. Nat. Prod. Res. 2025, 9, 1464–1470. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Y.; Zhang, Q.; Li, H.; Chen, L. Activity and safety evaluation of natural preservatives Review. Food Res. Int. 2024, 190, 114548. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tebar, N.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A Review. Polymers 2023, 15, 396. [Google Scholar] [CrossRef]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Chen, W.; Ma, S.; Wang, Q.; McClements, D.J.; Liu, X.; Ngai, T.; Liu, F. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. Nutr. 2022, 62, 5029–5055. [Google Scholar] [CrossRef] [PubMed]
- Alshangiti, D.M.; El-Damhougy, T.K.; Zaher, A.; Madani, M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef] [PubMed]
- Pawase, P.A.; Goswami, C.; Shams, R.; Pandey, V.K.; Tripathi, A.; Rustagi, S.; Darshan, G. A conceptual review on classification, extraction, bioactive potential and role of phytochemicals in human health. Future Foods 2024, 9, 100313. [Google Scholar] [CrossRef]
- Buriti, B.M.; Figueiredo, P.L.; Passos, M.F.; da Silva, J.K. Polymer-Based Wound Dressings Loaded with Essential Oil for the Treatment of Wounds: A Review. Pharmaceuticals 2024, 17, 897. [Google Scholar] [CrossRef]
- Orefice, N.S.; Di Raimo, R.; Mizzoni, D.; Logozzi, M.; Fais, S. Purposing Plant-Derived Exosomes-like Nanovesicles for Drug Delivery: Patents and Literature Review. Expert Opin. Ther. Pat. 2023, 33, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Iraji, A.; Esmaealzadeh, N.; Salehi, M.; Hashempur, M.H. Peppermint and menthol: A review on their biochemistry, pharmacological activities, clinical applications, and safety considerations. Crit. Rev. Food Sci. Nutr. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mhaske, A.; Shukla, R. Nanocarriers for the Delivery of Cosmeceuticals. In Nanotechnology Based Delivery of Phytoconstituents and Cosmeceuticals; Pooja, D., Kulhari, H., Eds.; Springer Nature: Singapore, 2024; pp. 305–328. ISBN 978-981-9953-14-1. [Google Scholar]
- Sarboland, S.S.; Mehrkhou, F.; Fattahi, M.; Forouzan, M. Biocontrol potential of aqueous and methanolic extracts of Russian knapweed, Acroptilon repens, L. (Asteraceae) against Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Sci. Rep. 2025, 15, 14928. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Sivertsvik, M.; Miteluţ, A.C.; Brebu, M.A.; Stoleru, E.; Rosnes, J.T.; Tănase, E.E.; Khan, W.; Pamfil, D.; Cornea, C.P.; et al. Comparative analysis of the composition and active property evaluation of certain essential oils to assess their potential applications in active food packaging. Materials 2017, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.M.; Costa, I.H.d.; Antunes, B.d.; Schirmann, G.d.S.; Oliveira, F.M.; Jansen-Alves, C.; Oliveira, E.G.d.; Zambiazi, R.C. Chemical Composition, Bioactive Compounds, Biological Activity, and Applications of Ora-Pro-Nóbis (Pereskia spp.): A Review. Food Res. Int. 2025, 221 Pt 1, 117239. [Google Scholar] [CrossRef]
- Kilictalip, M.; Sahan, S. Biochemical Properties and Nutritional Importance of some Medicinal and Aromatic Plants. In Bioactive Components and Biochemical Properties in Foods; İzol, E., Haspolat, Y.K., Eds.; Publisher Orient Enderun Publicity Service: Ankara, Turkey, 2025; pp. 66–85. [Google Scholar]
- Sithol, A.; Singh, S. Safety and Associated Legislation of Selected Food Contact Bio-Based Packaging. In Biobased Packaging Materials; Ahmed, S., Ed.; Springer: Singapore, 2024; pp. 247–277. [Google Scholar] [CrossRef]
- Food Additives and GRAS Ingredients—Information for Consumers. Available online: https://www.fda.gov/food/food-ingredients-packaging/food-additives-and-gras-ingredients-information-consumers (accessed on 21 August 2025).
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of Polysaccharides, Lipids and Proteins in Biodegradable Food Packaging Applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, K.B.; Mandemaker, L.D.; Ganjkhanlou, Y.; Vollmer, I.; Weckhuysen, B.M. Green Additives in Chitosan-based Bioplastic Films: Long-term Stability Assessment and Aging Effects. ChemSusChem 2024, 17, e202301426. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Prakash, B. Bioinformatics approaches: Elucidation of novel sites of action, toxicity prediction tool, and perception of bioactive compounds. In Green Products in Food Safety; Prakash, B., Freitas Brilhante de São José, J., Eds.; Academic Press: Amsterdam, The Netherlands, 2023; Chapter 11; pp. 309–327. [Google Scholar] [CrossRef]
- Liang, Z.; Veronica, V.; Huang, J.; Zhang, P.; Fang, Z. Combined effects of plant food processing by-products and high oxygen modified atmosphere packaging on the storage stability of beef patties. Food Control 2022, 133, 108586. [Google Scholar] [CrossRef]
- Muhammad, A.; Ahmad, Q.S.; Muhammad, B.; Hafiz, M.N.I. Biobased active food packaging materials: Sustainable alternative to conventional petrochemical based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Wongphan, P.; Promhuad, K.; Srisa, A.; Laorenza, Y.; Oushapjalaunchai, C.; Harnkarnsujarit, N. Unveiling the Future of Meat Packaging: Functional Biodegradable Packaging Preserving Meat Quality and Safety. Polymers 2024, 16, 1232. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A. Polymeric Nanoparticles for Biomedical Applications. Polymers 2024, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.W. Antioxidant pectin/pullulan edible coating incorporated with Vitis vinifera grape seed extract for extending the shelf life of peanuts. Postharvest Biol. Technol. 2022, 183, 111740. [Google Scholar] [CrossRef]
- Hu, X.; Lu, C.; Tang, H.; Pouri, H. Active Food Packaging Made of Biopolymer-Based Composites. Materials 2022, 16, 279. [Google Scholar] [CrossRef]
- Upadhyay, A.; Agbesi, P.; Arafat, K.M.; Urdaneta, F.; Dey, M.; Basak, M.; Hong, S.; Umeileka, C.; Argyropoulos, D. Bio-based smart packaging: Fundamentals and functions in sustainable food systems. Trends Food Sci. Technol. 2024, 145, 104369. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Fasihnia, S.H.; Peighambardoust, S.J.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M. Active polypropylene-based films incorporating combined antioxidants and antimicrobials: Preparation and characterization. Foods 2021, 10, 722. [Google Scholar] [CrossRef]
- Sharma, H.; Rajput, R. The science of food preservation: A comprehensive review of synthetic preservatives. J. Curr. Res. Food Sci. 2023, 4, 25–29. [Google Scholar]
- Mummaleti, G.; Udo, T.; Mohan, A.; Kong, F. Synthesis, characterization and application of microbial pigments in foods as natural colors. Crit. Rev. Food Sci. Nutr. 2024, 65, 5602–5631. [Google Scholar] [CrossRef]
- Li, H.; Gao, K.; Guo, H.; Li, R.; Li, G. Advancements in Gellan Gum-Based Films and Coatings for Active and Intelligent Packaging. Polymers 2024, 16, 2402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, J.; Liao, J. Physically fabricated chitin nanofibers for food applications. Cellulose 2024, 31, 10143–10163. [Google Scholar] [CrossRef]
- Van Hai, L.; Pham, D.H.; Roy, S.; Kim, J. Reinforcement effects of different types of chitin nanofibers on cellulose/chitin nanocomposite filaments. Cellulose 2024, 31, 10327–10339. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2021, 99, 323–336. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Chowdhury, N.R.; Dandapat, P.; Gagaoua, M.; Chauhan, P.; Pateiro, M.; Lorenzo, J.M. Application of pomegranate by-products in muscle foods: Oxidative indices, colour stability, shelf life and health benefits. Molecules 2021, 26, 467. [Google Scholar] [CrossRef] [PubMed]
- European Union. Register of Feed Additives, 226th ed.; European Commission: Luxembourg, 2016; ISBN 9789276150695.
- Erol, O. Use of Natural Antioxidants in Edible Films and Coatings. Eurasian J. Food Sci. Technol. 2024, 8, 114–121. [Google Scholar]
- Arrigoni, R.; Ballini, A.; Jirillo, E.; Santacroce, L. Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects. Antibiotics 2024, 13, 603. [Google Scholar] [CrossRef]
- Kumar, U.; Kumar, I.; Singh, P.K.; Yadav, J.S.; Dwivedi, A.; Singh, P.; Mishra, S.; Sharma, R.K. Total phenolic content and antioxidant activities in methanol extracts of medicinal herbs from Indo-Gangetic plains of India. J. Appl. Biol. Biotech. 2024, 12, 89–99. [Google Scholar] [CrossRef]
- Gopathy, S.; Seshadri, S.; Amudha, P.; Vidya, R.; Jayalakshmi, M.; Kulanthaivel, L.; Raju, M.; Subbarajet, K.G. Phytochemicals and Natural Extracts, Secondary Metabolites of Plants and Improvement of Brain Function. In Neuroprotective Effects of Phytochemicals in Brain Ageing; Pathak, S., Banerjee, A., Eds.; Springer: Singapore, 2024; pp. 199–219. [Google Scholar] [CrossRef]
- Caballero, B. (Ed.) Encyclopedia of Human Nutrition, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2023; pp. 513–522. ISBN 9780128218488. eBook ISBN: 9780323908160. [Google Scholar]
- Mtewa, A.G.; Egbuna, C.; Beressa, T.B.; Ngwira, K.J.; Lampiao, F. Phytopharmaceuticals: Efficacy, safety, and regulation. In Preparation of Phytopharmaceuticals for the Management of Disorders, The Development of Nutraceuticals and Traditional Medicine; Mishra, A., Egbuna, C., Goyal, M., Eds.; Academic Press: Amsterdam, The Netherlands, 2021; pp. 25–38. ISBN 9780128202852. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.-S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.-M.; Craciunescu, O.; Iosageanu, A.; Artem, V.; Ranca, A.; Motelica, L.; et al. Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. [Google Scholar] [CrossRef]
- Charles, A.P.R.; Chen, B.; Rao, J. Cannabidiol (CBD) as an emerging nutraceutical ingredient from industrial hemp: Regulation, production, extraction, nutraceutical properties, and functionality. Crit. Rev. Food Sci. Nutr. 2024, 65, 6030–6052. [Google Scholar] [CrossRef]
- Dwivedi, S.; Ahmad, I.Z. Role of Nanotechnology in the Development of Photoprotective Formulations. In Photoprotective Green Pharmacology: Challenges, Sources and Future Applications; Kannaujiya, V.K., Sinha, R.P., Rahman, M.A., Sundaram, S., Eds.; Springer Nature: Singapore, 2023; pp. 201–222. ISBN 978-981-9907-49-6. [Google Scholar]
- Al-Ouqaili, M.T.S.; Saleh, R.O.; Amin, H.I.M.; Jawhar, Z.H.; Akbarizadeh, M.R.; Naderifar, M.; Issa, K.D.; Gavilán, J.C.O.; Nobre, M.A.L.; Jalil, A.T.; et al. Synthesize of Pluronic-Based Nanovesicular Formulation Loaded with Pistacia atlantica Extract for Improved Antimicrobial Efficiency. Arab. J. Chem. 2023, 16, 104704. [Google Scholar] [CrossRef]
- Han, H.S.; Song, K.B. Noni (Morinda citrifolia) fruit polysaccharide films containing blueberry (Vaccinium corymbosum) leaf extract as an antioxidant packaging material. Food Hydrocoll. 2021, 112, 106372. [Google Scholar] [CrossRef]
- Han, H.S.; Song, K.B. Antioxidant properties of watermelon (Citrullus lanatus) rind pectin films containing kiwifruit (Actinidia chinensis) peel extract and their application as chicken thigh packaging. Food Packag. Shelf Life 2021, 28, 100636. [Google Scholar] [CrossRef]
- Pechangou, S.N.; Enang, B.E.; Ngohoba, V.S.; Njoya, E.M.; Njayou, F.N.; Moundipa, P.F. Crude Extracts of Codiaeum Variegatum Stem Exhibit Potent Antioxidant and Anti-inflammatory Activities in Vitro. J. Explor. Res. Pharmacol. 2023, 8, 25–35. [Google Scholar] [CrossRef]
- Report: Global Plant Extracts Market Report 2022: Growing Demand for Natural and Plant-Based Ingredients Driving Sector—ResearchAndMarkets.com, 13 October 2022. Dublin--(Business Wire)--The “Plant Extracts Market by Product Type (Oleoresins, Essential Oils, Flavonoids, Alkaloids, Carotenoids), Application (Food & Beverages, Cosmetics, Pharmaceuticals, Dietary Supplements), Form, Source and Region—Global Forecast to 2027”. Available online: https://www.businesswire.com/news/home/20221013005751/en/Global-Plant-Extracts-Market-Report-2022-Growing-Demand-for-Natural-and-Plant-Based-Ingredients-Driving-Sector---ResearchAndMarkets.com (accessed on 16 January 2025).
- Giannakas, A. Plant Extracts-Based Food Packaging Films. In Natural Materials for Food Packaging Application Book; Parameswaranpillai, J., Jayakumar, A., Radhakrishnan, E.K., Siengchin, S., Radoor, S., Eds.; Wiley-VCH GmbH: Weinheim, Germany, 2023; Chapter 2; pp. 23–49. [Google Scholar] [CrossRef]
- Ding, L.; He, L.; Wang, Y.; Zhao, X.; Ma, H.; Luo, Y.; Guan, F.; Xiong, Y. Research progress and challenges of composite wound dressings containing plant extracts. Cellulose 2023, 30, 11297–11322. [Google Scholar] [CrossRef]
- Kirk, M.D.; Angulo, F.J.; Havelaar, A.H.; Black, R.E. Diarrhoeal disease in children due to contaminated food. Bull. World Health Organ. 2017, 95, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.A.; Rahman, H.; Niaz, Z.; Qasim, M.; Khan, J.; Tayyaba; Rehman, B. Antibacterial activity of some medicinal plants against selected human pathogenic bacteria. Eur. J. Microbiol. Immunol. 2013, 3, 272–274. [Google Scholar] [CrossRef]
- Kursa, W.; Jamiołkowska, A.; Wyrostek, J.; Kowalski, R. Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium spp.—An In Vitro Study. Agronomy 2022, 12, 3204. [Google Scholar] [CrossRef]
- Behiry, S.I.; Hamad, N.A.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Kenawy, A.M.; Elsamra, I.A.; Youssef, N.H.; Elsharkawy, M.M.; Abdelkhalek, A.; et al. Antifungal and Antiaflatoxigenic Activities of Different Plant Extracts against Aspergillus flavus. Sustainability 2022, 14, 12908. [Google Scholar] [CrossRef]
- Kathait, P.; Omre, P.K.; Kumar, P.; Gaikwadet, K.K. Development of a PVA-starch Antioxidant Film Incorporating Beetroot Stem Waste Extract for Active Food Packaging. J. Polym. Environ. 2023, 31, 4160–4169. [Google Scholar] [CrossRef]
- Hosny, R.; Taha, T.; Elnouby, M.; El Desouky, E.A.; Alhudhaibiet, A.M.; Elsherif, M.A.; Moustafa, M.; Yahia, M.; Abu-Saied, M.A. Environmentally green film blends: Polyvinyl alcohol (PVA)/cellulose acetate (CA)/potato peel starch as an alternative to petroleum plastics. Cellulose 2024, 31, 6155–6172. [Google Scholar] [CrossRef]
- Lu, W.-C.; Chiu, C.-S.; Mulio, A.T.; Chan, Y.-J.; Li, P.-H. New perspectives on different Sacha inchi seed oil extractions and its applications in the food and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2023, 65, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Loan, L.T.K.; Vinh, B.T.; Tai, N.V. Elucidation of antioxidant compounds recovery capacity from “Cam” purple rice bran by different sustainable extraction techniques. J. Appl. Biol. Biotech. 2024, 12, 112–116. [Google Scholar] [CrossRef]
- Farhadi, S.; Javanmard, M. Development of fibrous casings based on sugarcane bagasse with natural antioxidant using rosemary and thyme extract in dried sausages. Food Meas. 2023, 17, 2475–2487. [Google Scholar] [CrossRef]
- Ansarifar, E.; Hedayati, S.; Zeinali, T.; Ebadi, F.A.; Asghar, Z.; Krystian, M.; Khaneghah, M.; Amin, K.M. Encapsulation of Jujube Extract in Electrospun Nanofiber: Release Profile, Functional Effectiveness, and Application for Active Packaging. Food Bioprocess Technol. 2022, 15, 2009–2019. [Google Scholar] [CrossRef]
- De Cristofaro, G.A.; Paolucci, M.; Pappalardo, D.; Pagliarulo, C.; Sessini, V.; Lo Re, G. Interface Interactions Driven Antioxidant Properties in Olive Leaf Extract/Cellulose Nanocrystals/Poly(Butylene Adipate-Co-Terephthalate) Biomaterials. Int. J. Biol. Macromol. 2024, 272, 132509. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Wangchuk, P. Chapter 11—Essential oils and their bioactive molecules in healthcare. In Herbal Biomolecules in Healthcare Applications; Mandal, S.C., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 215–237. [Google Scholar]
- Fokou, J.B.H.; Jazet Dongmo, P.M.; Boyom, F.F. Essential Oil’s Chemical Composition and Pharmacological Properties. In Essential Oils-Oils of Nature; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2020; Chapter 2. [Google Scholar] [CrossRef]
- Bezerra Girão, D.K.F.; Cardoso, C.C.; Silva, F.R.O. Effect of essential oils on pain management: What do we know and where do we go? Braz. J. Health Aromather. Essent. Oil Sect. 2024, 1. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Vasile, C. Encapsulation of Natural Bioactive Compounds by Electrospinning—Applications in Food Storage and Safety. Polymers 2021, 13, 3771. [Google Scholar] [CrossRef]
- Ismaili, D.; Parın, F.N.; Sıcak, Y.; Öztürk, M.; Terzioğlu, P. Electrospun lavender essential oil-loaded polylactic acid nanofibrous mats for antioxidant applications. Polym. Bull. 2024, 81, 13975–13992. [Google Scholar] [CrossRef]
- Tavares, L.; Noreña, C.P.Z.; Barros, H.L.; Smaoui, S.; Lima, P.L.; de Oliveira, M.M. Rheological and structural trends on encapsulation of bioactive compounds of essential oils: A global systematic review of recent research. Food Hydrocoll. 2022, 129, 107628. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Armanu, E.-G.; Secula, M.S.; Tofanica, B.-M.; Volf, I. The Impact of Biomass Composition Variability on the Char Features and Yields Resulted through Thermochemical Processes. Polymers 2024, 16, 2334. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.; Freire, L.; Carnielli-Queiroz, L.; Bragotto, A.P.A.; Silva, N.C.C.; Rocha, L.O. Essential oils: An eco-friendly alternative for controlling toxigenic fungi in cereal grains. Compr. Rev. Food Sci. Food Saf. 2023, 23, e13251. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. Sec. Agro-Food Saf. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Vishwakarma, A.; Wrona, M.; Bertella, A.; Rudawska, A.; Gierszewska, M.; Schmidt, B. Comparative Study of Crucial Properties of Packaging Based on Polylactide and Selected Essential Oils. Foods 2025, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Sulistiawan, S.S.; Sadeghi, K.; Kumar, R.; Kim, D.; Seo, J. In Situ Reactive Extrusion of LDPE Films with Methacrylated Pyrogallol for Antimicrobial and Antioxidant Active Packaging. Polymers 2025, 17, 325. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Qin, D.; Li, H.; Zhang, T.; Han, Y.; Huang, Y.Q.; He, D.; Wu, K.; Chai, X.; Chen, C. Study of antimicrobial activity and mechanism of vapor-phase cinnamaldehyde for killing Escherichia coli based on fumigation method. Front. Nutr. 2022, 9, 1040152. [Google Scholar] [CrossRef]
- Arneth, B.; Abdelmonem, R.; El-Nabarawi, M.A.; Teaima, M.H.; Rashwan, K.O.; Soliman, M.A.; Al-Samadi, I.E.I. Optimized Hesperidin-Loaded Lipid Nanoparticles with Tea Tree Oil for Enhanced Wound Healing: Formulation, Characterization, and Evaluation. Pharmaceuticals 2025, 18, 290. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.C.; Poitevin, C.G.; Bischoff, A.M.; Beger, M.; Siqueira da Luz, T.; Mazarotto, E.J.; Benatto, A.; Martins, C.E.N.; Sales Maia, B.H.L.N.; Sari, R.; et al. Insecticidal and antifungal activities of Melaleuca rhaphiophylla essential oil against insects and seed-borne pathogens in stored products. Ind. Crops Prod. 2022, 182, 114871. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Poitevin, C.G.; da Luz, T.S.; Mazarotto, E.J.; Furuie, J.L.; Martins, C.E.N.; do Amaral, W.; Cipriano, R.R.; da Rosa, J.M.; Pimentel, I.C.; et al. Antifungal activity of essential oils and their combinations against storage fungi. Environ. Sci. Pollut. Res. Int. 2023, 30, 48559–48570. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, I.; Paré, A.; Kaboré, D.; Montet, D.; Durand, N.; Bouajila, J.; Zida, E.P.; Sawadogo-Lingani, H.; Nikiéma, P.A.; Nebié, R.H.C.; et al. Antifungal and Antiaflatoxinogenic Effects of Cymbopogon citratus, Cymbopogon nardus, and Cymbopogon schoenanthus Essential Oils Alone and in Combination. J. Fungi 2022, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Al-Suwaytee, S.H.M.; Ben Hadj Ayed, O.; Chaâbane-Banaoues, R.; Kosksi, T.; Shleghm, M.R.; Chekir-Ghedira, L.; Babba, H.; Sfar, S.; Lassoued, M.A. Exploring the Antifungal Effectiveness of a Topical Innovative Formulation Containing Voriconazole Combined with Pinus sylvestris L. Essential Oil for Onychomycosis. Colloids Interfaces 2024, 8, 56. [Google Scholar] [CrossRef]
- Moradialvand, M.; Saniee, P.; McClements, D.J.; Rafati, H. Enhanced antimicrobial activity of Cinnamomum zeylanicum essential oil nanoemulsions against Helicobacter pylori: A microfluidic-based assessment. J. Nanopart. Res. 2025, 27, 27. [Google Scholar] [CrossRef]
- Kalmykova, A.D.; Yakupova, E.N.; Bekmuratova, F.A.; Fitsev, I.M.; Ziyatdinova, G.K. Evaluation of the antioxidant properties and GC-MSD analysis of commercial essential oils from plants of the Lamiaceae family. Uchenye Zap. Kazan. Univ. Seriya Estestv. Nauk. 2023, 165, 94–117. [Google Scholar] [CrossRef]
- Miri, Y.B.; Benabdallah, A.; Taoudiat, A.; Mahdid, M.; Djenane, D.; Tacer-Caba, Z.; Topkaya, C.; Simal-Gandara, J. Potential of essential oils for protection of Couscous against Aspergillus flavus and aflatoxin B1 contamination. Food Control 2023, 145, 109474. [Google Scholar] [CrossRef]
- Sevik, R.; Akarca, G.; Kilinc, M.; Ascioglu, Ç. Chemical Composition of Tea Tree (Melaleuca alternifolia) (Maiden & Betche) Cheel Essential Oil and Its Antifungal Effect on Foodborne Molds Isolated from Meat Products. J. Essent. Oil-Bear. Plants 2021, 24, 561–570. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of Novel Active Packaging Films Based on Whey Protein Isolate Incorporated with Chitosan Nanofiber and Nano-Formulated Cinnamon Oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Vasile, C.; Stoleru, E.; Darie-Niţa, R.N.; Dumitriu, R.P.; Pamfil, D.; Tarţau, L. Biocompatible Materials Based on Plasticized Poly(lactic acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(lactic acid) Materials. Polymers 2019, 11, 941. [Google Scholar] [CrossRef]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and Antioxidant Properties of Hydroxypropyl Methylcellulose-Based Active Composite Films Incorporating Oregano Essential Oil Nanoemulsions. LWT 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; He, Q. Effect of Orange (Citrus sinensis L.) Peel Essential Oil on Characteristics of Blend Films Based on Chitosan and Fish Skin Gelatin. Food Biosci. 2021, 41, 100927. [Google Scholar] [CrossRef]
- Sharma, P.; Ahuja, A.; Dilsad Izrayeel, A.M.; Samyn, P.; Rastogi, V.K. Physicochemical and Thermal Characterization of Poly (3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Films Incorporating Thyme Essential Oil for Active Packaging of White Bread. Food Control 2022, 133, 108688. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; de Vasconcelos, G.; Rosseto, M.; Dal Castel Krein, D.; Oliveira, F.; Freitas, C.P.; Antunes do Nascimento, C.; dos Santos, L.R.; Loss, R.A.; Dettmer, A.; et al. Composite Films from Steam-exploded Gelatin and Thyme Essential Oil: Production, Characterization and Application as Coatings. J. Polym. Environ. 2024, 32, 2616–2628. [Google Scholar] [CrossRef]
- Hedayati, S.; Ansarifar, E.; Moradinezhad, F. Assessment of Zataria Multiflora Essential Oil—Incorporated Electrospun Polyvinyl Alcohol Fiber Mat as Active Packaging. Polymers 2023, 15, 1048. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Dumka, A.; Masek, A. Eugenol and cloves as plant-origin stabilizers in epoxidized natural rubber compositions. Express Polym. Lett. 2024, 18, 1039–1050. [Google Scholar] [CrossRef]
- Masood, F.; Makhdoom, M.A.; Channa, I.A.; Gilani, S.J.; Khan, A.; Hussain, R.; Batool, S.A.; Konain, K.; Rahman, S.U.; Wadood, A.; et al. Development and Characterization of Chitosan and Chondroitin Sulfate Based Hydrogels Enriched with Garlic Extract for Potential Wound Healing/Skin Regeneration Applications. Gels 2022, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Furdak, P.; Bartosz, G.; Stefaniuk, I.; Cieniek, B.; Bieszczad-Bedrejczuk, E.; Soszyński, M.; Sadowska-Bartosz, I. Effect of Garlic Extract on the Erythrocyte as a Simple Model Cell. Int. J. Mol. Sci. 2024, 25, 5115. [Google Scholar] [CrossRef]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Iwiński, H.; Lech, K.; Carbonell-Barrachina, Á.A.; Szumny, A. Coriandrum sativum L.—Effect of Multiple Drying Techniques on Volatile and Sensory Profile. Foods 2021, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Arzani, A.; Arzani, V.; Roberts, T.H. Phenolic Compounds and Antimicrobial Properties of Mint and Thyme. J. Herb. Med. 2022, 36, 100604. [Google Scholar] [CrossRef]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. Int. J. Mol. Sci. 2023, 24, 6936. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Antibacterial and Antibiofilm Efficacy of Thyme (Thymus vulgaris L.) Essential Oil against Foodborne Illness Pathogens, Salmonella enterica subsp. enterica Serovar Typhimurium and Bacillus cereus. Antibiotics 2023, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Di Rosario, M.; Continisio, L.; Mantova, G.; Carraturo, F.; Scaglione, E.; Sateriale, D.; Forgione, G.; Pagliuca, C.; Pagliarulo, C.; Colicchio, R.; et al. Thyme Essential Oil as a Potential Tool Against Common and Re-Emerging Foodborne Pathogens: Biocidal Effect on Bacterial Membrane Permeability. Microorganisms 2025, 13, 37. [Google Scholar] [CrossRef]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Continisio, L.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Eco-Friendly Sanitization of Indoor Environments: Effectiveness of Thyme Essential Oil in Controlling Bioaerosol Levels and Disinfecting Surfaces. BioTech 2024, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Özen, İ.; Demir, A.; Bahtiyari, M.İ.; Wang, X.; Nilghaz, A.; Wu, P.; Shirvanimoghaddam, K.; Naebe, M. Multifaceted applications of thymol/carvacrol-containing polymeric fibrous structures. Adv. Ind. Eng. Polym. Res. 2024, 7, 182–200. [Google Scholar] [CrossRef]
- Giotopoulou, I.; Stamatis, H.; Barkoula, N.-M. Encapsulation of Thymol in Ethyl Cellulose-Based Microspheres and Evaluation of Its Sustained Release for Food Applications. Polymers 2024, 16, 3396. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agarwal, R.K.; Gurunathan, K. Bio-preservative effect of blends of essential oils: Natural anti-oxidant and anti-microbial agents for the shelf life enhancement of emulsion based chicken sausages. J. Food Sci. Technol. 2020, 57, 3040–3050. [Google Scholar] [CrossRef]
- Liang, L.; Su, Q.; Ma, Y.; Zhao, S.; Zhang, H.; Gao, X. Research progress on the polysaccharide extraction and antibacterial activity. Ann. Microbiol. 2024, 74, 17. [Google Scholar] [CrossRef]
- Chiappini, V.; Conti, C.; Astolfi, M.L.; Girelli, A.M. Characteristic study of Candida rugosa lipase immobilized on lignocellulosic wastes: Effect of support material. Bioprocess Biosyst. Eng. 2025, 48, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Sun, Z. Aromatic Biobased Polymeric Materials Using Plant Polyphenols as Sustainable Alternative Raw Materials: A Review. Polymers 2024, 16, 2752. [Google Scholar] [CrossRef]
- Zhan, K.; Ji, X.; Luo, L. Recent progress in research on Momordica charantia polysaccharides: Extraction, purification, structural characteristics and bioactivities. Chem. Biol. Technol. Agric. 2023, 10, 58. [Google Scholar] [CrossRef]
- Zheng, J.; Shang, M.; Dai, G.; Dong, J.; Wang, Y.; Duan, B. Bioactive polysaccharides from Momordica charantia as functional ingredients: A review of their extraction, bioactivities, structural-activity relationships, and application prospects. Crit. Rev. Food Sci. Nutr. 2023, 64, 12103–12126. [Google Scholar] [CrossRef]
- Liu, J.; Lei, Y.; Guo, M.; Wang, L. Research Progress on the Hypoglycemic Effects and Mechanisms of Action of Momordica charantia polysaccharide. J. Food Biochem. 2023, 8867155. [Google Scholar] [CrossRef]
- Nagarajan, V.; Kizhaeral, S.S.; Subramanian, M.; Rajendran, S.; Ranjan, J. Encapsulation of a volatile biomolecule (hexanal) in cyclodextrin metal-organic frameworks for slow release and its effect on preservation of mangoes. ACS Food Sci. Technol. 2021, 1, 1936–1944. [Google Scholar] [CrossRef]
- Li, R.; Chen, T.; Pan, X. Metal–organic-framework-based materials for antimicrobial applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Sun, X.; Zhou, L.; Du, H.; Zhu, Z.; Wen, Y. Electrospun pullulan/PVA nanofibers integrated with thymol-loaded porphyrin metal−organic framework for antibacterial food packaging. Carbohydr. Polym. 2021, 270, 118391. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Z.; He, Q.; Deng, Y.; Wei, F.; Xu, C.; Fu, L.; Lin, B. Enhanced aqueous stability and long-acting antibacterial of silver-based MOFs via chitosan-crosslinked for fruit fresh-keeping. Appl. Surf. Sci. 2022, 571, 151351. [Google Scholar] [CrossRef]
- Sultana, A.; Kathuria, A.; Gaikwad, K.K. Metal–organic frameworks for active food packaging. A review. Environ. Chem. Lett. 2022, 20, 1479–1495. [Google Scholar] [CrossRef]
- Peng, Y.; Tan, Q.; Huang, H.; Zhu, Q.; Kang, X.; Zhong, C.; Han, B. Customization of functional MOFs by a modular design strategy for target applications. Chem. Synth. 2022, 2, 15. [Google Scholar] [CrossRef]
- Khan, M.J.; Hafeez, F.; Islam, M.R.; Zhu, C.; Xianyu, Y. Advanced Antibacterial Packaging for Food Preservation Through Multifunctional Metal–Organic Framework Nanocomposite. Nano Micro Small 2025, 21, 2501111. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Fan, H.; Tsopmo, A.; Regenstein, J.M.; Ashaolu, T.J. Plant-based antioxidant peptides: Impact on oxidative stress and gut microbiota. Criti. Rev. Food Sci. Nutr. 2025, 65, 8006–8029. [Google Scholar] [CrossRef] [PubMed]
- Merenkova, S.; Zinina, O. Effect of Bioactive Packaging Materials Based on Sodium Alginate and Protein Hydrolysates on the Quality and Safety of Refrigerated Chicken Meat. Polymers 2024, 16, 3430. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Moon, J.-Y.; Lee, Y.-C. Insights into Bioactive Peptides in Cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Perea-Flores, M.d.J.; Peña-Barrientos, A.; Barrios-Francisco, R.; Rojas-Candelas, L.E.; Calderón-Domínguez, G. Encapsulation Efficiency of Electrosprayed Glucose Oxidase Capsules: Effect of the Drying Technique. Polymers 2025, 17, 488. [Google Scholar] [CrossRef]
- Zhang, X.; Tao, L.; Wei, G.; Yang, M.; Wang, Z.; Shi, C.; Shi, Y.; Huang, A. Plant-derived rennet: Research progress, novel strategies for their isolation, identification, mechanism, bioactive peptide generation, and application in cheese manufacturing. Crit. Rev. Food Sci. Nutr. 2025, 65, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, S.; Zhang, J.; Thakur, K.; Battino, M.; Cao, H.; Farag, M.A.; Xiao, J.; Wei, Z. Asparagus saponins: Effective natural beneficial ingredient in functional foods, from preparation to applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 12284–12302. [Google Scholar] [CrossRef] [PubMed]
- Gulin-Sarfraz, T.; Kalantzopoulos, G.N.; Haugen, J.-E.; Axelsson, L.; Raanaas Kolstad, H.; Sarfraz, J. Controlled Release of Volatile Antimicrobial Compounds from Mesoporous Silica Nanocarriers for Active Food Packaging Applications. Int. J. Mol. Sci. 2022, 23, 7032. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pradhan, S.; Vivek, S. Polyphenols in different plant parts of Inula grandiflora collected from two habitats of Uttarakhand Himalayas. J. Herbs Spices Med. Plants 2023, 29, 199–212. [Google Scholar] [CrossRef]
- Zejli, H.; EL Amrani, B.; Metouekel, A.; Bousseraf, F.Z.; Fitat, A.; Taleb, M.; Abdellaoui, A. Comparative assessment of total phenolics content and in vitro antioxidant capacity variations of leaf extracts of Origanum grossii and Thymus pallidus. Moroc. J. Chem. 2024, 12, 361–375. [Google Scholar]
- Mohammed, B.S.; Sanadelaslam, E.; Salwa, I.A.E.; Ahmed, S.J. HPLC-PDA-MS Identification of Phenolic Profile and In Vitro Antioxidant Activity of Adansonia digitata L. Leaves from Sudan. Moroc. J. Chem. 2024, 12, 221–232. [Google Scholar]
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in essential oils: Chemistry, classification, and potential impact on human health and industry. Phytomed. Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, H.I.; González-Torres, M.; Escutia-Guadarrama, L.; Sergio, A.; Bernal-Chávez, D.M.; Giraldo-Gomez, D.M.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. Sec. Neuropharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef] [PubMed]
- Mołdoch, J.; Agacka-Mołdoch, M.; Jóźwiak, G.; Wojtunik-Kulesza, K. Biological Activity of Monoterpene-Based Scaffolds: A Natural Toolbox for Drug Discovery. Molecules 2025, 30, 1480. [Google Scholar] [CrossRef]
- Shlosman, K.; Rein, D.M.; Shemesh, R.; Cohen, Y. Lyophilized Emulsions of Thymol and Eugenol Essential Oils Encapsulated in Cellulose. Polymers 2024, 16, 1422. [Google Scholar] [CrossRef] [PubMed]
- Fayyazbakhsh, A.; Hajinajaf, N.; Bakhtiari, H.; Feuchter, M.; Improta, I.; Salehi, E.; Shahsavar, S.K.; Julinova, M.; Ghasemi, A.; Ghasemi, B.; et al. Eco-friendly additives for biodegradable polyesters: Recent progress in performance optimization and environmental impact reduction. Sustain. Mater. Technol. 2025, 44, e01395. [Google Scholar] [CrossRef]
- de Souza Vieira, J.; Sales de Oliveira, V.; Junqueira Carneiro, M.; Labre da Silva, T.; Augusta, I.M.; de Carvalho, M.G.; Frankland Sawaya, A.C.H.; Saldanha, T. Phenolic composition and insights into the use of pink pepper (Schinus terebentifolius Raddi) fruit against lipid oxidation in food systems. Food Biosci. 2023, 53, 102556. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef] [PubMed]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Mihaylova, D.; Dimitrova-Dimova, M.; Popova, A. Dietary Phenolic Compounds-Wellbeing and Perspective Applications. Int. J. Mol. Sci. 2024, 25, 4769. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Jiménez, S.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Pereira-Caro, G. 9—Bioaccesibility and bioavailability of marine polyphenols. In Marine Phenolic Compounds. Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 265–298. [Google Scholar] [CrossRef]
- de Oliveira, I.; Santos-Buelga, C.; Aquino, Y.; Barros, L.; Heleno, S.A. New frontiers in the exploration of phenolic com-pounds and other bioactives as natural preservatives. Food Biosci. 2025, 68, 106571. [Google Scholar] [CrossRef]
- Yu, L.; Shi, H. Effect of two mulberry (Morus alba L.) leaf polyphenols on improving the quality of fresh-cut cantaloupe during storage. Food Control 2021, 121, 107624. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, L.; Cai, J.; Dong, Q.; Din, Z.U.; Hu, Z.Z.; Wang, G.Z.; Ding, W.P.; He, J.R.; Cheng, S.-Y. Starch/tea polyphenols nanofibrous films for food packaging application: From facile construction to enhance mechanical, antioxidant and hydrophobic properties. Food Chem. 2021, 360, 129922. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Cao, X.; Liu, Q.; Wang, H.; Kong, B. Preparation and functional properties of poly(vinyl alcohol)/ethylcellulose/tea polyphenol electrospun nanofibrous films for active packaging material. Food Control 2021, 130, 108331. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Zeng, F. Biological Functions and Health Benefits of Flavonoids in Fruits and Vegetables: A Contemporary Review. Foods 2025, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Chandran, G.U.; Kumar, A.A.; Menon, S.K.; Sambhudevan, S.; Shankar, B. The potential role of flavonoids in cellulose-based biopolymeric food packaging materials for UV radiation protection. Cellulose 2024, 31, 4733–4773. [Google Scholar] [CrossRef]
- Sharma, R.; Thakur, A. Health Promoting Phytochemicals in Vegetables: A Mini Review Health Promoting Phytochemicals in Vegetables: A Mini Review. Int. J. Food Ferment. Technol. 2018, 8, 107–117. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, E.S.; Park, B.J.; Jung, Y.W.; Kim, D.H.; Lee, S.J. Polycaprolactone/Anthocyanin-Based Electrospun Volatile Amines Gas Indicator with Improved Visibility by Varying Bi-Solvent Ratio: A Case of Intelligent Packaging of Mackerel. Foods 2023, 12, 3850. [Google Scholar] [CrossRef] [PubMed]
- Remedio, L.N.; Parada Quinayá, C. Intelligent Packaging Systems with Anthocyanin: Influence of Different Polymers and Storage Conditions. Polymers 2024, 16, 2886. [Google Scholar] [CrossRef] [PubMed]
- Oladzadabbasabadi, N.; Nafchia, A.M.; Ghasemlou, M.; Ariffin, F.; Singh, Z.; Al-Hassan, A. Natural anthocyanins: Sources, extraction, characterization, and suitability for smart packaging. Food Packag. Shelf Life 2022, 33, 100872. [Google Scholar] [CrossRef]
- Gençdağ, E.; Özdemir, E.E.; Demirci, K.; Görgüç, A.; Yılmaz, F.M. Copigmentation and stabilization of anthocyanins using organic molecules and encapsulation techniques. Curr. Plant Biol. 2022, 29, 100238. [Google Scholar] [CrossRef]
- Pirayesh, H.; Park, B.D.; Khanjanzadeh, H.; Park, H.-J.; Cho, Y.-J. Cellulosic material-based colorimetric films and hydrogels as food freshness indicators. Cellulose 2023, 30, 2791–2825. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, L.; Liu, Y.; Du, P.; Hu, P.; Cao, J.; Wang, W. Chitosan-Enhanced pH-Sensitive Anthocyanin Indicator Film for the Accurate Monitoring of Mutton Freshness. Polymers 2024, 16, 849. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Pons-Fuster Lopez, E.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxidative Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Fabbrizi, G.; Moya, A.J. Lignin from Plant-Based Agro-Industrial Biowastes: From Extraction to Sustainable Applications. Polymers 2025, 17, 952. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Stoleru, E.; Mitchell, G.R.; Vasile, C.; Brebu, M. Bioactive Electrospun Fibers of Poly(epsilon-Caprolactone) Incorporating alpha-Tocopherol for Food Packaging Applications. Molecules 2021, 26, 5498. [Google Scholar] [CrossRef] [PubMed]
- Janani, N.; Zare, E.N.; Salimi, F.; Makvandi, P. Antibacterial tragacanth gum-based nanocomposite films carrying ascorbic acid antioxidant for bioactive food packaging. Carbohydr. Polym. 2020, 247, 116678. [Google Scholar] [CrossRef]
- Hapsari, A.R.; Roto, R.; Siswanta, D. Release of alpha-tocopherol from chitosan/pectin polyelectrolyte complex film into fatty food simulant for the design of antioxidant active food package. J. Teknol. 2020, 82, 43–49. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefinery 2024, 14, 4419–4440. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits. A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Bruni, G.P.; de Oliveira, J.P.; Gómez-Mascaraque, L.G.; Fabra, M.J.; Martins, V.G.; Zavareze, E.R.; López-Rubio, A. Electrospun β-carotene–loaded SPI:PVA fiber mats produced by emulsion-electrospinning as bioactive coatings for food packaging. Food Packag. Shelf Life 2020, 23, 100426. [Google Scholar] [CrossRef]
- Pavón-Pérez, J.; Vallejos-Almirall, A.; Agurto-Muñoz, C.; Galarce-Bustos, O. Sustainable approaches for the study of alkaloids from plants using supercritical fluid-based processes. Green Chem. 2022, 24, 9450–9474. [Google Scholar] [CrossRef]
- Muñoz, I.J.; Schilman, P.E.; Barrozo, R.B. Impact of alkaloids in food consumption, metabolism and survival in a blood-sucking insects. Sci. Rep. 2020, 10, 9443. [Google Scholar] [CrossRef] [PubMed]
- Nabi-Afjadi, M.; Heydari, M.; Zalpoor, H.; Arman, I.; Sadoughi, A.; Sahami, P.; Aghazadeh, S. Lectins and lectibodies: Potential promising antiviral agents. Cell. Mol. Biol. Lett. 2022, 27, 37. [Google Scholar] [CrossRef]
- Mercer, D.K.; Torres, M.D.; Duay, S.S.; Lovie, E.; Simpson, L.; von Köckritz-Blickwede, M.; De la Fuente-Nunez, C.; O’Neil, D.A.; Angeles-Boza, A.M. Antimicrobial susceptibility testing of antimicrobial peptides to better predict efficacy. Front. Cell. Infect. Microbiol. 2020, 10, 326. [Google Scholar] [CrossRef]
- Banasaz, S.; Ferraro, V. Keratin from animal by-products: Structure, characterization, extraction and application—A Review. Polymers 2024, 16, 1999. [Google Scholar] [CrossRef]
- Yaman, M.; Balta, M.F.; Karakaya, O.; Kaya, T.; Necas, T.; Yildiz, E.; Dirim, E. Assessment of Fatty Acid Composition, Bioactive Compounds, and Mineral Composition in Hazelnut Genetic Resources: Implications for Nutritional Value and Breeding Programs. Horticulturae 2023, 9, 1008. [Google Scholar] [CrossRef]
- Lužaić, T.; Kravić, S.; Stojanović, Z.; Grahovac, N.; Jocić, S.; Cvejić, S.; Pezo, L.; Romanić, R. Investigation of oxidative characteristics, fatty acid composition and bioactive compounds content in cold pressed oils of sunflower grown in Serbia and Argentina. Heliyon 2023, 9, e18201. [Google Scholar] [CrossRef] [PubMed]
- Edayadulla, N.; Divakaran, D.; Sundari, C.S.; Malinee, S.; Indran, S.; Sanjay, M.R.; Suchartet, S. Suitability study of novel bio-plasticizer from Agave sisalana leaf for biofilm applications: A biomass to biomaterial approach. Biomass Convers. Biorefin. 2024, 14, 19675–19691. [Google Scholar] [CrossRef]
- Guo, Z.; Li, X. eBook: A Practical Guide to Nanoparticle Characterization by Light Scattering Techniques; Bettersize Instruments Ltd.: Dandong, China, Last Update 10/2025; Available online: https://www.bettersizeinstruments.com (accessed on 20 August 2025).
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/metal oxide nanocomposites for bactericidal effect: A review. Chemosphere 2021, 272, 128607. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.Y.; Hopkins, M.; Jaiswal, A.K.; Jaiswal, S. Nanoclays-containing bio-based packaging materials: Properties, applications, safety, and regulatory issues. J. Nanostruct. Chem. 2024, 14, 71–93. [Google Scholar] [CrossRef]
- Mamudu, U.; Kabyshev, A.; Bekmyrza, K.; Kuterbekov, K.A.; Baratova, A.; Omeiza, L.A.; Lim, R.C. Extraction, Preparation and Characterization of Nanocrystalline Cellulose from Lignocellulosic Simpor Leaf Residue. Molecules 2025, 30, 1622. [Google Scholar] [CrossRef]
- Oh, C.Y.; Henderson, E.R. In vitro transcription of self-assembling DNA nanoparticles. Sci. Rep. 2023, 13, 12961. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Tarmizi, A.S.; Othman, M.B.H.; Abdullah Sani, M.S.; Rafiae, N.N.; Rostan, N.R.; Mohamad Ibrahim, M.N. Conventional Blending Chitosan Lignin Nanocomposites Hydrogel (CsLNPs) for food coating application. Halalsphere 2025, 5, 15–23. [Google Scholar] [CrossRef]
- Sharma, N.; Yaqoob, M.; Singh, P.; Kaur, G.; Aggarwal, P. Nanoencapsulation of Bioactive Compounds. Chapter 9. In Nanotechnology Horizons in Food Process Engineering, Volume 3, Trends, Nanomaterials, and Food Delivery; Goyal, M.R., Mishra, S.K., Kumar, S., Eds.; AAP, CRC Press (Taylor & Francis Group): Palm Bay, FL, USA, 2023; pp. 285–462. ISBN 9781774910986. [Google Scholar]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-derived nanostructures: Types and applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Jantan, I.; Norahmad, N.A.; Yuandani, M.A.H.; Mohamed-Hussein, Z.A.; Mohd Abd Razak, M.R.; Mohamed, A.F.S.; Lam, K.W.; Ibrahim, S. Inhibitory effect of food-functioned phytochemicals on dysregulated inflammatory pathways triggered by SARS-CoV-2: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2024, 65, 2405–2430. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Conte-Junior, C.A. Recent Advances on Nanomaterials to COVID-19 Management: A Systematic Review on Antiviral/Virucidal Agents and Mechanisms of SARS-CoV-2 Inhibition/Inactivation. Glob. Health Ongoing Chall. 2021, 5, 2000115. [Google Scholar] [CrossRef]
- Greenwood, M. Trends in Nanobiomaterials. Available online: https://www.azonano.com/article.aspx?ArticleID=6385&utm_source=azonetwork_newsletter&utm_medium=email&utm_campaign=nanoparticles_and_colloids_newsletter_4_april_2023 (accessed on 21 August 2025).
- Malik, S.; Khan, A.; Ali, N.; Rahdar, A.; Yasin, G.; Hussain, S.; Bilal, M. Natural polymer-based nanostructures and their applications. In Smart Polymer Nanocomposites: Design, Synthesis, Functionalization, Properties, and Applications. Part III: Applications of Polymer Nanostructures and Polymer Nanocomposites; Ali, N., Khan, A., Gupta, R.K., Bilal, M., Nguyen, T.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2023; Chapter 24; pp. 529–541. [Google Scholar]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef]
- Terea, H.; Rebiai, A.; Selloum, D.; Tedjani, M.L. Cellulose/ZnO nanoparticles (CNC/ZnO NPs): Synthesis, characterization, and evaluation of their antibacterial and antifungal activities. Cellulose 2024, 31, 5027–5042. [Google Scholar] [CrossRef]
- Zulkarnain, N.N.; Abd Rahman, N.; Othman, A.R.; Md Saleh, N.; Mohd Ali, J.; Shukor, H.; Mohd Said, M.; Wan Zaki, W.R. Characterization and antibacterial activity of bacterial cellulose impregnated with Moringa oleifera leaf extract and silver nanoparticles. Cellulose 2024, 31, 5213–5227. [Google Scholar] [CrossRef]
- Parker, R.M.; Parton, T.G.; Chan, C.L.C.; Bay, M.M.; Frka-Petesic, B.; Vignolini, S. Bioinspired Photonic Materials from Cellulose: Fabrication, Optical Analysis, and Applications. Acc. Mater. Res. 2023, 4, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Maskur, M.; Prihanto, A.A.; Firdaus, M.; Kobun, R.; Nurdiani, R. Review of the potential of bioactive compounds in seaweed to reduce histamine formation in fish and fish products. Ital. J. Food Saf. 2025, 14, 12994. Available online: https://www.pagepressjournals.org/ijfs/article/view/12994 (accessed on 21 August 2025). [CrossRef]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of Agri-Food By-Products in the Food Industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Yadav, S.; Malik, K.; Moore, J.M.; Kamboj, B.R.; Malik, S.; Malik, V.K.; Arya, S.; Singh, K.; Mahanta, S.; Bishnoi, D.K. Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules 2024, 29, 2055. [Google Scholar] [CrossRef] [PubMed]
- Duguma, H.T.; Khule, P.; McArdle, A.; Fennell, K.; Almenar, E. Turning agricultural waste into packages for food: A literature review from origin to end-of-life. Food Packag. Shelf Life 2023, 40, 101166. [Google Scholar] [CrossRef]
- Vadivel, D.; Ferraro, F.; Dondi, D. Harnessing Biomass for a Sustainable Future: The Role of Starch and Lignin. Catalysts 2024, 14, 747. [Google Scholar] [CrossRef]
- Culqui-Arce, C.; Mori-Mestanza, D.; Fernández-Jeri, A.B.; Cruzalegui, R.J.; Mori Zabarburú, R.C.; Vergara, A.J.; Cayo-Colca, I.S.; da Silva, J.G.; Araujo, N.M.P.; Castro-Alayo, E.M.; et al. Polymers Derived from Agro-Industrial Waste in the Development of Bioactive Films in Food. Polymers 2025, 17, 408. [Google Scholar] [CrossRef]
- Forgione, G.; De Cristofaro, G.A.; Sateriale, D.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Pomegranate Peel and Olive Leaf Extracts to Optimize the Preservation of Fresh Meat: Natural Food Additives to Extend Shelf-Life. Microorganisms 2024, 12, 1303. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Bautista, M.; Espinosa-Juárez, J.V.; Moreno-Rocha, L.A.; Betanzos-Cabrera, G.; Salanță, L.C.; De la O Arciniega, M.; Olvera-Hernández, E.G.; Jaramillo-Morales, O.A. Differential Antinociceptive Efficacy of Peel Extracts and Lyophilized Juices of Three Varieties of Mexican Pomegranate (Punica granatum L.) in the Formalin Test. Plants 2023, 12, 131. [Google Scholar] [CrossRef]
- Wijaya, C.H.; Nuraida, L.; Nuramalia, D.R.; Hardanti, S.; Świąder, K. Oncom: A Nutritive Functional Fermented Food Made from Food Process Solid Residue. Appl. Sci. 2024, 14, 10702. [Google Scholar] [CrossRef]
- Basta, A.H.; Lotfy, V.F. The synergistic route for enhancing rice by-product derived nanoparticles in sustained release of bioactive compound. Cellulose 2023, 30, 11473–11491. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Clean. Prod. 2023, 434, 139989. [Google Scholar] [CrossRef]
- Zainul, R.; Albadn, Y.M.; Yahya, E.B.; Manoharadas, S.; Saharudin, N.I.; Abdul Khalil, H.P.S.; Khan, M.R.; Jaber, M. Eco-friendly approach for nanocellulose isolation from agricultural wastes and the fabrication of bioaerogel scaffolds. Express Polym. Lett. 2024, 18, 359–370. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Kossalbayev, B.D.; Belkozhayev, A.M.; Abaildayev, A.; Kadirshe, D.K.; Tastambek, K.T.; Kurmanbek, A.; Toleutay, G. Biodegradable Packaging from Agricultural Wastes: A Comprehensive Review of Processing Techniques, Material Properties, and Future Prospects. Polymers 2025, 17, 2224. [Google Scholar] [CrossRef] [PubMed]
- Carnaval, L.S.C.; Jaiswal, A.K.; Jaiswal, S. Agro-Food Waste Valorization for Sustainable Bio-Based Packaging. J. Compos. Sci. 2024, 8, 41. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Z.; Shi, Y.; Reynolds, A.G.; Duan, C.; Lan, Y. Volatile phenols in wine: Overview of origin, formation, analysis, and sensory expression. Crit. Rev. Food Sci. Nutr. 2024, 65, 3001–3026. [Google Scholar] [CrossRef]
- Chia, M.R.; Phang, S.-W.; Razali, N.S.M.; Ahmad, I. Approach towards sustainable circular economy: Waste biorefinery for the production of cellulose nanocrystals. Cellulose 2024, 31, 3377–3420. [Google Scholar] [CrossRef]
- Puglia, D.; Luzi, F.; Tolisano, C.; Rallini, M.; Priolo, D.; Brienza, M.; Costantino, F.; Torre, L.; Del Buono, D. Cellulose Nanocrystals and Lignin Nanoparticles Extraction from Lemna minor L.: Acid Hydrolysis of Bleached and Ionic Liquid-Treated Biomass. Polymers 2024, 16, 1395. [Google Scholar] [CrossRef]
- Machado, B.; Costa, S.M.; Costa, I.; Fangueiro, R.; Ferreira, D.P. The potential of algae as a source of cellulose and its derivatives for biomedical applications. Cellulose 2024, 31, 3353–3376. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-Food Wastes for Bioplastics: European Prospective on Possible Applications in Their Second Life for a Circular Economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef]
- Oliveira, T.C.G.; Caleja, C.; Oliveira, M.B.P.P.; Pereira, E.; Barros, L. Reuse of fruits and vegetables biowaste for sustainable development of natural ingredients. Food Biosci. 2023, 53, 102711. [Google Scholar] [CrossRef]
- Zuffi, V.; Puliga, F.; Mercatante, D.; Rodriguez-Estrada, M.T.; Sanchez-Cortes, S.; Zambonelli, A.; Francioso, O. Conversion of grape pomace into fungal biomass: A study of Pleurotus cultivation for a sustainable agro-residue management. Chem. Biol. Technol. Agric. 2025, 12, 22. [Google Scholar] [CrossRef]
- Sateriale, D.; Forgione, G.; Di Rosario, M.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Vine-Winery Byproducts as Precious Resource of Natural Antimicrobials: In Vitro Antibacterial and Antibiofilm Activity of Grape Pomace Extracts against Foodborne Pathogens. Microorganisms 2024, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Dapkevicius, M.L.E.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking by-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Nobile, M.A.D. Sustainable use of fruit and vegetable by-products to enhance food packaging performance. Food 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Haris, S.; Zubair Alam, M.; Galiwango, E.; Galiwango, E.; Mohamed, M.M.; Kamal-Eldin, A.; Al-Marzouqi, A.H. Characterization analysis of date fruit pomace: An underutilized waste bioresource rich in dietary fiber and phenolic antioxidants. Waste Manag. 2023, 163, 34–42. [Google Scholar] [CrossRef]
- Serratos, I.N.; García Torres, J.A.; Mendoza Téllez, J.L.; Silva Roy, D.; Soto Estrada, A.M.; Leyva López, N.E.; Rodríguez González, H.; Le Borgne, S.; Sánchez-Sánchez, K.L.; Sosa Fonseca, R. Banana Peel Based Cellulose Material for Agriculture and Aquiculture: Toward Circular Economy. Polymers 2025, 17, 1230. [Google Scholar] [CrossRef] [PubMed]
- Boutaleb, Y.; Zerdoum, R.; Bensid, N.; Abumousa, R.A.; Hattab, Z.; Bououdina, M. Adsorption of Cr(VI) by Mesoporous Pomegranate Peel Biowaste from Synthetic Wastewater under Dynamic Mode. Water 2022, 14, 3885. [Google Scholar] [CrossRef]
- Somasundaram, R.; Isaac, R.; Divakaran, D.; Suyambulingam, I.; Siengchin, S.; Manavalan, M. Agro-waste of Senna alata (L.) Roxb. stem: A sustainable biofiber material for lightweight composites and diverse applications. Cellulose 2025, 32, 383–412. [Google Scholar] [CrossRef]
- Vitiello, G.; Venezia, V.; Verrillo, M.; Nuzzo, A.; Houston, J.; Cimino, S.; D’Errico, G.; Aronne, A.; Paduano, L.; Piccolo, A.; et al. Hybrid humic acid/titanium dioxide nanomaterials as highly effective antimicrobial agents against gram (−) pathogens and antibiotic contaminants in wastewater. Environ. Res. 2021, 193, 110562. [Google Scholar] [CrossRef]
- Henry, S.; Dhital, S.; Sumer, H.; Butardo, V., Jr. Solid-State Fermentation of Cereal Waste Improves the Bioavailability and Yield of Bacterial Cellulose Production by a Novacetimonas sp. Isolate. Foods 2024, 13, 3052. [Google Scholar] [CrossRef]
- Pramana, A.; Kurnia, D.; Firmanda, A.; Rossi, E.; Hasnah, N.A.R.; Putri, V.J. Using palm oil residue for food nutrition and quality: From palm fatty acid distillate to vitamin E toward sustainability. J. Sci. Food Agric. 2025, 105, 4728–4740. [Google Scholar] [CrossRef]
- Santana, J.C.; Gardim, R.B.; Almeida, P.F.; Borini, G.B.; Quispe, A.P.; Llanos, S.A.; Heredia, J.A.; Zamuner, S.; Gamarra, F.M.C.; Farias, T.M.B.; et al. Valorization of chicken feet by-product of the poultry industry: High qualities of gelatin and biofilm from extraction of collagen. Polymers 2020, 12, 529. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Chandran, A.J.; Rangappa, S.M.; Suyambulingam, I.; Manik, G.; Siengchin, S. Marine waste as a resource: Developing bio-epoxy composites for a sustainable future. Mater. Today Sustain. 2024, 27, 100908. [Google Scholar] [CrossRef]
- Zaghbib, I.; Abdullah, J.A.A.; Romero, A. Development of a Multifunctional Chitosan-Based Composite Film from Crab Shell (Portunus segnis) and Algae (Ulva lactuca) with Enhanced Antioxidant and Antimicrobial Properties for Active Food Packaging. Foods 2025, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Yap, X.Y.; Gew, L.T.; Khalid, M.; Yow, Y.-Y. Algae-Based Bioplastic for Packaging: A Decade of Development and Challenges (2010–2020). J. Polym. Environ. 2023, 31, 833–851. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Erasmus, M. Green Synthesis of Bioplastics from Microalgae: A State-of-the-Art Review. Polymers 2024, 16, 1322. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.C.; Strauss, S.; Shpigel, M.; Guttman, L.; Stengel, D.B.; Rebours, C.; Gjorgovska, N.; Turan, G.; Balina, K.; Zammit, G.; et al. The green seaweed Ulva: Tomorrow’s “wheat of the sea” in foods, feeds, nutrition, and biomaterials. Crit. Revi. Food Sci. Nutr. 2024, 65, 3728–3763. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Lloret, V.; Martínez-Abad, A.; López-Rubio, A.; Martínez-Sanz, M. Sustainable Bio-Based Materials from Minimally Processed Red Seaweeds: Effect of Composition and Cell Wall Structure. J. Polym. Environ. 2023, 31, 886–899. [Google Scholar] [CrossRef]
- Sarker, N.K.; Kaparaju, P. Advances in Algae-Based Bioplastics: From Strain Engineering and Fermentation to Commercialization and Sustainability. Fermentation 2025, 11, 574. [Google Scholar] [CrossRef]
- Belkacem, M.; Bousemat, H.; Asmaa, B.; Mustapha, H.B.; Haddou, B. Biological Characteristics of Chitin and Chitosan. In Chitin and Chitosan; Physical and Chemical Proprities; Ahmed, S., Zeghoud, S., Amor, I.B., Hemmami, H., Eds.; Jenny Stanford Publishing: Singapore, 2025; Chapter 6. [Google Scholar] [CrossRef]
- Rigogliuso, S.; Campora, S.; Notarbartolo, M.; Ghersi, G. Recovery of Bioactive Compounds from Marine Organisms: Focus on the Future Perspectives for Pharmacological, Biomedical and Regenerative Medicine Applications of Marine Collagen. Molecules 2023, 28, 1152. [Google Scholar] [CrossRef]
- Pawłowicz, T.; Gabrysiak, K.A.; Wilamowski, K. Investigating the Potential of Polypore Fungi as Eco-Friendly Materials in Food Industry Applications. Forests 2024, 15, 1230. [Google Scholar] [CrossRef]
- Boufetacha, M.; Gharibi, E.; Benali, M. Optimising Supercritical Carbon Dioxide Extraction of Rosmarinic Acid from Rosmarinus officinalis L. and Enhancing Yield Through Soxhlet Coupling. Processes 2025, 13, 655. [Google Scholar] [CrossRef]
- BhadangeJitendra, Y.A.; Carpenter, J.; Saharan, V.K. A Comprehensive Review on Advanced Extraction Techniques for Retrieving Bioactive Components from Natural Sources. ACS Omega 2024, 9, 31274–31297. [Google Scholar] [CrossRef]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and modern techniques for bioactive compounds recovery from plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Abdulkareem, M.H. Insights on Extracting Bio-Active Compounds from Plants that Act Against Bacteria (Article Review). Cent. Asian J. Med. Nat. Sci. 2025, 6, 1–6. [Google Scholar]
- Younes, A.; Karboune, S.; Liu, L.; Andreani, E.S.; Dahman, S. Extraction and Characterization of Cocoa Bean Shell Cell Wall Polysaccharides. Polymers 2023, 15, 745. [Google Scholar] [CrossRef]
- Mora, M.; Fàbregas, E.; Céspedes, F.; Puy, N. Pine bio-oil based biorefinery procedure for the production of antioxidants, acids, and sugars. Biofuels Bioprod. Biorefin. 2025, 19, 1030–1043. [Google Scholar] [CrossRef]
- Rojas, K.; Verdugo-Molinares, M.G.; Vallejo, A.A. Use of encapsulating polymers of active compounds in the pharmaceutical and food industry. Food Chem. Adv. 2024, 4, 100619. [Google Scholar] [CrossRef]
- Coelho, S.C.; Tița, O.; Constantinescu, M.A.; Rusu, L.; Tița, M.A. Natural Polymers as Carriers for Encapsulation of Volatile Oils: Applications and Perspectives in Food Products. Polymers 2024, 16, 1026. [Google Scholar] [CrossRef]
- Kłosowska, A.; Wawrzyńczak, A.; Feliczak-Guzik, A. Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances. Cosmetics 2023, 10, 26. [Google Scholar] [CrossRef]
- Šola, I.; Gmižić, D.; Pinterić, M.; Tot, A.; Ludwig-Müller, J. Adjustments of the Phytochemical Profile of Broccoli to Low and High Growing Temperatures: Implications for the Bioactivity of Its Extracts. Int. J. Mol. Sci. 2024, 25, 3677. [Google Scholar] [CrossRef] [PubMed]
- Boostani, S.; Sarabandi, K.; Tarhan, O.; Rezaei, A.; Assadpour, E.; Rostamabadi, H.; Falsafi, S.R.; Tan, C.; Zhang, F.; Jafari, S.M. Multiple Pickering emulsions stabilized by food-grade particles as innovative delivery systems for bioactive compounds. Historical Perspective. Adv. Coll. Interf. Sci. 2024, 328, 103174. [Google Scholar] [CrossRef]
- El Kader, A.A.; Hashish, H.A. Encapsulation techniques of food bioproduct. Egypt. J. Chem. 2020, 63, 1881–1909. [Google Scholar] [CrossRef]
- de Almeida, N.T.; Pereira, A.L.S.; de Oliveira Barros, M.; Mattos, A.L.A.; Rosa, M.d.F. Enhancing Starch Film Properties Using Bacterial Nanocellulose-Stabilized Pickering Emulsions. Polymers 2024, 16, 3346. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Bazana, M.T.; Franco Codevilla, C.; Ragagnin de Menezes, C. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Ali Aboudzadeh, M.; Hamzehlou, S. Low-Energy Emulsification Methods for Encapsulation of Antioxidants. In Emulsion-Based Encapsulation of Antioxidants Design and Performance; Part of the Book Series: Food Bioactive Ingredients, (FBC); Aboudzadeh, A., Ed.; Springer: Berlin, Germany, 2020; Chapter 3; pp. 109–147. [Google Scholar]
- Pimentel-Moral, S.; Verardo, V.; Robert, P.; Segura-Carretero, A.; Martínez-Férez, A. Nanoencapsulation strategies applied to maximize target delivery of intact polyphenols. In Encapsulations and Nanotechnology in the Agri-Food Industry Volume 2; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 13; pp. 559–595. [Google Scholar]
- McClements, D.J.; Das, A.K.; Dhar, P.; Nanda, P.K.; Chatterjee, N. Nanoemulsion-Based Technologies for Delivering Natural Plant-Based Antimicrobials in Foods. Front. Sustain. Food Syst. Sec. Agro-Food Saf. 2021, 5, 643208. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Sharma, R.; Agarwal, A.; Haleem, D.R. Nanoemulsions based edible coatings with potential food applications. Int. J. Biobased Plast. 2021, 3, 112–125. [Google Scholar] [CrossRef]
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in sustainable food packaging: From eco-friendly materials to innovative technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- Csuti, A.; Zheng, B.; Zhou, H. Post pH-driven encapsulation of polyphenols in next-generation foods: Principles, formation and applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 12892–12906. [Google Scholar] [CrossRef] [PubMed]
- Günal-Köroğlu, D.; Karabulut, G.; Catalkaya, G.; Capanoglu, E. The Effect of Polyphenol-Loaded Electrospun Fibers in Food Systems. Food Bioprocess Technol. 2025, 18, 5094–5116. [Google Scholar] [CrossRef]
- Duong, T.H.; Dinh, K.B.; Luu, T.; Chapman, J.; Baji, A.; Truong, V.K. Nanoengineered sustainable antimicrobial packaging: Integrating essential oils into polymer matrices to combat food waste. Int. J. Food Sci. Technol. 2024, 59, 5887–5901. [Google Scholar] [CrossRef]
- Stanley, J.; John, A.; Pušnik Črešnar, K.; Fras Zemljič, L.; Lambropoulou, D.A.; Bikiaris, D.N. Active Agents Incorporated in Polymeric Substrates to Enhance Antibacterial and Antioxidant Properties in Food Packaging Applications. Macromol 2023, 3, 1–27. [Google Scholar] [CrossRef]
- John, A.; Črešnar, K.P.; Bikiaris, D.N.; Zemljič, L.F. Colloidal Solutions as Advanced Coatings for Active Packaging Development: Focus on PLA Systems. Polymers 2023, 15, 273. [Google Scholar] [CrossRef] [PubMed]
- Chelaghema, A.; Durand, N.; Servent, A.; Mamouni, M.; Poucheret, P.; Schorr-Galindo, S.; Fontana, A.; Strub, C. Antifungal and antimycotoxic activities of 3 essential oils against 3 mycotoxinogenic fungi. Arch. Microbiol. 2022, 204, 504. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Karaça, A.C.; Rashidinejad, A.; Jafari, S.M. Recent advancements in curcumin extraction, chemical/bio-synthesis, purification, and food applications. Crit. Rev. Food Sci. Nutr. 2025, 65, 2714–2730. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Modarres-Gheisari, S.M.M.; Gavagsaz-Ghoachani, R.; Malaki, M.; Safarpour, P.; Zandi, M. Ultrasonic nano-emulsification—A review. Ultrason. Sonochem. 2019, 52, 88–105. [Google Scholar] [CrossRef]
- Espitia, P.J.; Fuenmayor, C.A.; Otoni, C.G. Nanoemulsions: Synthesis, characterization, and application in bio-based active food packaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 264–285. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Yan, J.; Zhang, S.; Shi, C.; McClements, D.J.; Liu, X.; Liu, F. Development of antibacterial nanoemulsions incorporating thyme oil: Layer-by-layer self-assembly of whey protein isolate and chitosan hydrochloride. Food Chem. 2021, 339, 128016. [Google Scholar] [CrossRef]
- Cai, J.; Guo, Y.; Liang, Y.; He, Y. A curcumin sustained-release hydrogel prepared from Pickering emulsions stabilized by modified amorphous calcium phosphate nanoparticles. Colloid. Polym. Sci. 2024, 302, 781–790. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Figueiredo, M.P.; Magri, V.R.; Eulálio, D.; Cunha, V.R.R.; Alcântara, A.C.S.; Perotti, G.F. Biomaterials Based on Organic Polymers and Layered Double Hydroxides Nanocomposites: Drug Delivery and Tissue Engineering. Pharmaceutics 2023, 15, 413. [Google Scholar] [CrossRef] [PubMed]
- Marcello, E.; Chiono, V. Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting. Int. J. Mol. Sci. 2023, 24, 3390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- Mishra, M.K. (Ed.) Handbook of Encapsulation and Controlled Release; Taylor and Francis Group, CRC Press: Boca Raton, FL, USA, 2016; 1455p. [Google Scholar]
- Kawee-ai, A. Advancing Gel Systems with Natural Extracts: Antioxidant, Antimicrobial Applications, and Sustainable Innovations. Gels 2025, 11, 125. [Google Scholar] [CrossRef]
- Bazzaz, S.; Abbasi, A.; Ghotbabad, A.G.; Pourjafar, H.; Hosseini, H. Novel Encapsulation Approaches in the Functional Food Industry: With a Focus on Probiotic Cells and Bioactive Compounds. Probiotics Antimicrob. Proteins 2025, 17, 1132–1170. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze Drying and Spray-Drying as Encapsulation Techniques. Molecules 2022, 27, 5069. [Google Scholar] [CrossRef]
- Barbosa-Nuñez, J.A.; Espinosa-Andrews, H.; Vallejo Cardona, A.A.; Haro-González, J.N. Polymer-based encapsulation in food products: A comprehensive review of applications and advancements. J. Future Foods 2025, 5, 36–49. [Google Scholar] [CrossRef]
- Soto-Madrid, D.; Arrau, F.; Zúñiga, R.N.; Gutiérrez-Cutiño, M.; Matiacevich, S. Development and Characterization of a Natural Antioxidant Additive in Powder Based on Polyphenols Extracted from Agro-Industrial Wastes (Walnut Green Husk): Effect of Chickpea Protein Concentration as an Encapsulating Agent during Storage. Polymers 2024, 16, 777. [Google Scholar] [CrossRef]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials 2021, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Suja Rani, S.; Latha, C. Nanoencapsulation for Controlled Release of Active Components. In Handbook of Nutraceuticals. Science, Technology and Engineering; Rajakumari, R., Thomas, S., Eds.; Springer: Cham, Switzerland, 2026. [Google Scholar]
- Parente, J.F.; Sousa, V.I.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Biodegradable Polymers for Microencapsulation Systems. Adv. Polym. Technol. 2022, 2022, 4640379. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, G.; Choi, E.; Ryu, J.-H. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater. Sci. 2022, 6, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation strategies to enhance the antibacterial properties of essential oils in food system. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Xue, J.; Luo, Y. Protein-polysaccharide nanocomplexes as nanocarriers for delivery of curcumin: A comprehensive review on preparation methods and encapsulation mechanisms. J. Future Foods 2023, 3, 99–114. [Google Scholar] [CrossRef]
- Singh, A.; Goswami, L.; Panda, S.K. Enhancing Encapsulation Efficiency of Bioactive Compounds Through Electrospraying: A Case Study on Encapsulating Epigallocatechin Gallate. In Basic Protocols in Encapsulation of Food Ingredients. Methods and Protocols in Food Science; Gomez-Zavaglia, A., Ed.; Humana: New York, NY, USA, 2025. [Google Scholar] [CrossRef]
- Mouro, C.U.; Gouveia, I.C. Engineering electrospun fibers for encapsulation of essential oils. Chapter 6. In Electrospinning and Electrospraying Encapsulation of Food Bioactive Compounds; Rostamabadi, H., Falsafi, S.R., Luo, Y., Eds.; Academic Press: Amsterdam, The Netherlands, 2025; Chapter 6; pp. 111–128. [Google Scholar] [CrossRef]
- Khalafi, N.; Gharachorloo, M.; Ganjloo, A.; Yousefi, S. Release kinetics, color stability and antioxidant activity of red cabbage anthocyanins encapsulated in zein electrospun nanoribbons. J. Food Measur. Charact. Food Meas. 2024, 18, 1363–1371. [Google Scholar] [CrossRef]
- Stoleru, E.; Brebu, M. Stabilization Techniques of Essential Oils by Incorporation into Biodegradable Polymeric Materials for Food Packaging. Molecules 2021, 26, 6307. [Google Scholar] [CrossRef] [PubMed]
- Loesch-Zhang, A.; Bellmann, M.; Lachmann, K.; Biesalski, M.; Geissler, A. Plasma Polymerization of Vegetable Oils onto Paper Substrates of Varying Porosity for Improved Hydrophobicity. Adv. Mater. Interfaces 2024, 11, 2400507. [Google Scholar] [CrossRef]
- Stoleru, E.; Vasile, C.; Irimia, A.; Brebu, M. Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils. Molecules 2021, 26, 4500. [Google Scholar] [CrossRef]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P.S. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification (Review Article). Sustain. Food Technol. 2023, 1, 500–510. [Google Scholar] [CrossRef]
- Dang, Z.; Ma, X.; Yang, Z.; Wen, X.; Zhao, P. Electrospun Nanofiber Scaffolds Loaded with Metal-Based Nanoparticles for Wound Healing. Polymers 2024, 16, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, M.; Yu, Y.; Wen, B.; Cheng, L. A Novel Polyaniline-Coated Bagasse Fiber Composite with Core–Shell Heterostructure Provides Effective Electromagnetic Shielding Performance. ACS Appl. Mater. Interfaces 2017, 9, 809–818. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef] [PubMed]
- Akdaşçi, E.; Eker, F.; Duman, H.; Bechelany, M.; Karav, S. Microbial-Based Green Synthesis of Silver Nanoparticles: A Comparative Review of Bacteria- and Fungi-Mediated Approaches. Int. J. Mol. Sci. 2025, 26, 10163. [Google Scholar] [CrossRef] [PubMed]
- Rami, M.R.; Forouzandehdel, S.; Aalizadeh, F. Enhancing Biodegradable Smart Food Packaging: Fungal-Synthesized Nanoparticles for Stabilizing Biopolymers. Heliyon 2024, 10, e37692. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Ganeshamoorthy, N.; Shobana, M.K. Influence of phytochemicals with iron oxide nanoparticles for biomedical applications: A review. Polym. Bull. 2023, 80, 11715–11758. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Januskevice, V.; Saunoriute, S.; Raubyte, U.; Viskelis, J.; Memvanga, P.B.; Viskelis, P. Antimicrobial Antioxidant Polymer Films with Green Silver Nanoparticles from Symphyti radix. Polymers 2024, 16, 317. [Google Scholar] [CrossRef]
- Yadav, J.; Chauhan, P. Green synthesis of silver nanoparticles using Citrus X sinensis (Orange) fruit extract and assessment of their catalytic reduction. Mater. Today Proc. 2022, 62, 6177–6181. [Google Scholar] [CrossRef]
- Michalec, S.; Nieckarz, W.; Klimek, W.; Lange, A.; Matuszewski, A.; Piotrowska, K.; Hotowy, A.; Kunowska-Slósarz, M.; Sosnowska, M. Green Synthesis of Silver Nanoparticles from Chlorella vulgaris Aqueous Extract and Their Effect on Salmonella enterica and Chicken Embryo Growth. Molecules 2025, 30, 1521. [Google Scholar] [CrossRef]
- Liaqat, N.; Jahan, N.; Khalil-ur-Rahman; Anwar, T.; Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef] [PubMed]
- EL Megdar, S.; Fayzi, L.; Elkheloui, R.; Laktib, A.; Bourouache, M.; EL Boulani, A.; Oualid, H.A.; Cherifi, K.; Msanda, F.; Hassi, M.; et al. Biological Synthesis of Silver Nanoparticles from Lavandula mairei Humbert: Antibacterial and Antioxidant Activities. Curr. Microbiol. 2024, 81, 151. [Google Scholar] [CrossRef]
- Asadi, Z.; Saki, M.; Khosravi, R.; Amin, M.; Ghaemi, A.; Akrami, S. Green Synthesis of Silver Nanoparticles Using Extracts of Cocculus Pendulus: Morphology and Antibacterial Efficacy Against Common Nosocomial Pathogens. Indian J. Microbiol. 2024, 64, 1598–1607. [Google Scholar] [CrossRef]
- Tran, M.Q.; Vu, H.T.; Hoang, V.; Luu, T.X.T. Ultrasound accelerated the green and mild preparation of bioactive, coated, and biosynthesized silver nanoparticles by different leaf extracts. Inorg. Nano-Met. Chem. 2025, 1–9. [Google Scholar] [CrossRef]
- Kurian, S.; Gande, V.V.; Subramaniam, P.; Bauri, R. Synthesis and separation of silver nanoparticles using an aqueous two-phase system with citrate as reducing agent. J. Nanopart. Res. 2024, 26, 51. [Google Scholar] [CrossRef]
- Dzhagan, V.; Mazur, N.; Smirnov, O.; Yeshchenko, O.; Isaieva, O.; Kovalenko, M.; Vuichyk, M.; Skoryk, M.; Pirko, Y.; Yemets, A.; et al. SERS application of Ag nanoparticles synthesized with aqueous fungi extract. J. Nanopart. Res. 2023, 25, 37. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Song, S.; Liu, M.; Zhang, P.; Zhong, D.; Wang, Y.; Niu, Y.; Xu, Y. Controllable Silver Release for Efficient Treatment of Drug-Resistant Bacterial-Infected Wounds. ChemBioChem 2024, 25, e202400406. [Google Scholar] [CrossRef] [PubMed]
- Sibuyi, N.R.S.; Thipe, V.C.; Panjtan-Amiri, K.; Meyer, M.; Katti, K.V. Green synthesis of gold nanoparticles using Acai berry and Elderberry extracts and investigation of their effect on prostate and pancreatic cancer cells. Nanobiomedicine 2021, 8, 1849543521995310. [Google Scholar] [CrossRef]
- Fan, Y.; Li, N.; Wang, J.; Liao, L.; Wei, J. Green Synthesis of Biocompatible Chiral Gold Nanoparticles. Polymers 2024, 16, 3333. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Mou, W.; Li, X.; Li, Y.; Liu, Y. Study on the antibacterial and antifungal properties of a highly stable zinc oxide nanofluid. J. Nanopart. Res. 2024, 26, 175. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: Characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef]
- Sachin; Jaishree; Singh, N.; Singh, R.; Shah, K.; Pramanik, B.K. Green synthesis of zinc oxide nanoparticles using lychee peel and its application in anti-bacterial properties and CR dye removal from wastewater. Chemosphere 2023, 327, 138497. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, T.; Ji, B.; Chou, Y.; Du, X. Green Synthesis of Zinc Oxide Nanoparticles Using Aloe vera Leaf Extract and Evaluation of the Antimicrobial and Antioxidant Properties of the ZnO/Regenerated Cellulose Film. Cellulose 2024, 31, 4849–4864. [Google Scholar] [CrossRef]
- Hamouda, R.; Alharbi, A.A.; Al-Tuwaijri, M.M.; Makharita, R.R. The Antibacterial Activities and Characterizations of Biosynthesized Zinc Oxide Nanoparticles, and Their Coated with Alginate Derived from Fucus vesiculosus. Polymers 2023, 15, 2335. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.; Baruah, C.; Babu, A.; Kalita, P. Biological and Non-conventional Synthesis of Zinc Oxide Nanoparticles (ZnONPs): Their Potential Applications. J. Nanotechnol. Nanomater. 2022, 3, 79–89. [Google Scholar]
- Afonso, I.S.; Cardoso, B.; Nobrega, G.; Minas, G.; Ribeiro, J.E.; Lima, R.A. Green synthesis of nanoparticles from olive oil waste for environmental and health applications: A review. J. Environ. Chem. Eng. 2024, 12, 114022. [Google Scholar] [CrossRef]
- Sani, A.; Hassan, D.; Chanihoon, G.Q.; Melo Máximo, D.V.; Sánchez-Rodríguez, E.P. Green Chemically Synthesized Iron Oxide Nanoparticles–Chitosan Coatings for Enhancing Strawberry Shelf-Life. Polymers 2024, 16, 3239. [Google Scholar] [CrossRef]
- Anjum, V.; Dixit, V.; Jha, P.; Potoroko, I. Food products regulations in the European Union. In Global Regulations of Medicinal, Pharmaceutical, and Food Products; Anjum, V., Dixit, V., Jha, P., Potoroko, I., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 255–287. [Google Scholar]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef]
- Dáger-López, D.; Chenché, Ó.; Ricaurte-Párraga, R.; Núñez-Rodríguez, P.; Bajaña, J.M.; Fiallos-Cárdenas, M. Advances in the Production of Sustainable Bacterial Nanocellulose from Banana Leaves. Polymers 2024, 16, 1157. [Google Scholar] [CrossRef]
- Heacock, J.A.; Sun, Y.; Li, Y.V. Mechanical and morphological properties of cellulose nanocrystals extracted from industrial hemp agro-waste. Cellulose 2024, 31, 10861–10877. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Franco, T.S.; Viana, L.C.; Bonfatti Júnior, E.A.; de Muñiz, G.I.B. Micro and nanoengineered structures and compounds: Nanocellulose. Cellulose 2023, 30, 10595–10632. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Electrospun Nanomaterials Based on Cellulose and Its Derivatives for Cell Cultures: Recent Developments and Challenges. Polymers 2023, 15, 1174. [Google Scholar] [CrossRef] [PubMed]
- Muthoka, R.M.; Panicker, P.S.; Agumba, D.O.; Kim, J. Polydopamine nanocoating on cellulose nanofiber film and its multifunctional behaviors. Cellulose 2024, 31, 887–906. [Google Scholar] [CrossRef]
- Hozman-Manrique, A.S.; Garcia-Brand, A.J.; Hernandez-Carrion, M.; Porras, A. Isolation and Characterization of Cellulose Microfibers from Colombian Cocoa Pod Husk via Chemical Treatment with Pressure Effects. Polymers 2023, 15, 664. [Google Scholar] [CrossRef]
- Sreejit, V.; Preetha, R.; Mubeena, S.A.; Dhananjay, S. Green synthesized nano starch: Preparation and emerging avenues in food industry. J. Nanopart. Res. 2025, 27, 105. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Shiha, A.M.; Mahrous, H.; Abeer Mohammed, A.B. Green synthesis of chitosan nanoparticles, optimization, characterization and antibacterial efficacy against multi drug resistant biofilm-forming Acinetobacter baumannii. Sci. Rep. 2022, 12, 19869. [Google Scholar] [CrossRef] [PubMed]
- Amrish Varshan, G.S.; Karthick Raja Namasivayam, S. A Green Chemistry Principle for the Biotransformation of Fungal Biomass Derived Chitosan into Versatile Nano Scale Materials with High Biocompatibility and Potential Biological Activities—A Review. BioNanoScience 2024, 14, 4145–4166. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P.; Sukatta, U.; Beaumont, M.; Rosenau, T. Efficient extraction of lignin-rich nanofibers from rambutan peel using a two-step delignification process. Cellulose 2025, 32, 3685–3700. [Google Scholar] [CrossRef]
- Hadi, S.T.; Mohamed, A.M. Green synthesis optimization and characterization of selenium nanoparticles using aqueous extract of peel bitter orange (Citrus aurantium). Funct. Food Sci. 2025, 5, 238–248. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Zhao, S.; Liu, P.; Xie, F.; Zhang, B. Biofunctional chitosan–biopolymer composites for biomedical applications. Mater. Sci. Eng. R Rep. 2024, 159, 100775. [Google Scholar] [CrossRef]
- Bejenaru, C.; Radu, A.; Segneanu, A.-E.; Biţă, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bradu, I.A.; Vlase, T.; Vlase, G.; Bejenaru, L.E. Pharmaceutical Applications of Biomass Polymers: Review of Current Research and Perspectives. Polymers 2024, 16, 1182. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Mahna, A. A review of biomedical applications of low-frequency magnetic fields and nanoparticles (LFMFs/NPs). J. Nanopart. Res. 2025, 27, 40. [Google Scholar] [CrossRef]
- Malekjani, N.; Jafari, S.M. Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3–47. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Ma, S.; Wang, F.; Wang, F.; Wang, L. A comprehensive review of intelligent controlled release antimicrobial packaging in food preservation. Food Sci. Biotechnol. 2023, 32, 1459–1478. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Drusch, S.; Acevedo, F.; Castro-Alvarez, A.; Benie, A.; Poncelet, D.; Dragosavac, M.M.; Defain Tesoriero, M.V.; Löwenstei, P.; Yonaha, V.; et al. Structure, controlled release mechanisms and health benefits of pectins as an encapsulation material for bioactive food components. Food Funct. 2022, 13, 10870–10881. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Controll. Release 1987, 5, 23–36, 37–42. [Google Scholar] [CrossRef]
- Cid, A.G.; Sonvico, F.; Bettini, R.; Colombo, P.; Gonzo, E.; Jimenez-Kairuz, A.F.; Bermúdez, J.M. Evaluation of the Drug Release Kinetics in Assembled Modular Systems Based on the Dome Matrix Technology. J. Pharm. Sci. 2020, 109, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Somaratne, G.; Ye, A.; Nau, F.; Ferrua, M.J.; Dupont, D.; Paul Singh, R.; Singh, J. Role of biochemical and mechanical disintegration on β-carotene release from steamed and fried sweet potatoes during in vitro gastric digestion. Food Res. Int. 2020, 136, 109481. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Mehany, T.; Shyu, D.J.H.; Assadpour, E.; Malekjani, N.; Castro-Muñoz, R.; Jafari, S.M. Release of Encapsulated Bioactive Compounds from Active Packaging/Coating Materials and its Modeling: A Systematic Review. Colloids Interfaces 2023, 7, 25. [Google Scholar] [CrossRef]
- Djuris, J.; Cvijic, S.; Djekic, L. Model-Informed Drug Development: In Silico Assessment of Drug Bioperformance following Oral and Percutaneous Administration. Pharmaceuticals 2024, 17, 177. [Google Scholar] [CrossRef]
- Li, Q.; Huang, K.X.; Pan, S.; Su, C.; Bi, J.; Lu, X. Thymol Disrupts Cell Homeostasis and Inhibits the Growth of Staphylococcus aureus. Contrast Media Mol. Imaging 2022, 22, 8743096. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R. Exploring the Molecular Mechanisms and Therapeutic Potentials of Essential Oils: A Systems Biology Approach. Futur. Integr. Med. 2024, 3, 116–131. [Google Scholar] [CrossRef]
- Roy, S.; Sarkhel, S.; Bisht, D.; Hanumantharao, S.N.; Rao, S.; Jaiswal, A. Antimicrobial mechanisms of biomaterials: From macro to nano. Biomater. Sci. 2022, 10, 4392–4423. [Google Scholar] [CrossRef]
- Yu, L.; Shi, H. Recent advances in anti-adhesion mechanism of natural antimicrobial agents on fresh produce. Curr. Opin. Food Sci. 2021, 42, 8–14. [Google Scholar] [CrossRef]
- Upadhyay, P.; Zubair, M.; Roopesh, M.S.; Ullah, A. An Overview of Advanced Antimicrobial Food Packaging: Emphasizing Antimicrobial Agents and Polymer-Based Films. Polymers 2024, 16, 2007. [Google Scholar] [CrossRef] [PubMed]
- Ovung, A.; Bhattacharyya, J. Viral, Parasitic, Bacterial, and Fungal Infections. Antimicrobial, Host Defense, and Therapeutic Strategies. In Bacterial Infections: Antimicrobial Mechanism of Action and Bacterial Resistance; Bagchi, D., Das, A., Downs, B.W., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 40; pp. 479–487. [Google Scholar]
- Eshboev, F.; Mamadalieva, N.; Nazarov, P.A.; Hussain, H.; Katanaev, V.; Egamberdieva, D.; Azimova, S. Antimicrobial Action Mechanisms of Natural Compounds Isolated from Endophytic Microorganisms. Antibiotics 2024, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, Antifungal, Antiviral Activity, and Mechanisms of Action of Plant Polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Pizzo, J.S.; Visentainer, J.V.; da Silva, A.L.B.R.; Rodrigues, C. Application of essential oils as sanitizer alternatives on the postharvest washing of fresh produce. Food Chem. 2023, 407, 135101. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Pirouz, A.; Papakonstantinou, I.; Michalska, M. Antimicrobial mechanisms of nanopatterned surfaces—A developing story. Front. Chem. Sec. Nanosci. 2024, 12, 1354755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, L.; Li, Z.; Chu, X. Metal-based nanoparticles in antibacterial application in biomedical field: Current development and potential mechanisms. Biomed Microdevices 2024, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Krishnan, G.P.; Sethuraman, S.; Abraham, S.V.P.I.; Krishnan, S.T.; Venkateswar, A.; Arunkumar, J.; Shi, C.; Mubarak, A.D. Exploring Possible Ways to Enhance the Potential and Use of Natural Products through Nanotechnology in the Battle against Biofilms of Foodborne Bacterial Pathogens. Pathogens 2023, 12, 270. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Applicationin Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Figueira, F.; Barbosa, J.S.; Mendes, R.F.; Braga, S.S.; Paz, F.A. Virus meet metal-organic frameworks: A nanoporous solution to a world-sized problem? Mater Today 2021, 43, 84–98. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Kongolo Kalemba, M.R.; Makhuvele, R.; Njobeh, P.B. Phytochemical screening, antioxidant activity of selected methanolic plant extracts and their detoxification capabilities against AFB1 toxicity. Heliyon 2024, 10, e24435. [Google Scholar] [CrossRef]
- Nailwal, N.; Bhatia, N.; Ali, A.; Ansari, A.; Raheja, R.; Godad, A.; Doshi, G. Antioxidants obtained from marine sources. In Marine Antioxidants Preparations, Syntheses, and Applications; Kim, S.-K., Shin, K.-H., Venkatesan, J., Eds.; Academic Press: Amsterdam, The Netherlands, 2023; Chapter 4; pp. 45–56. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. Sec. Food Chem. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Semenya, J.; Anamoah, C.; Chapman, K.N.; Barone, V. Computational study of ortho-substituent effects on antioxidant activities of phenolic dendritic antioxidants. Antioxidants 2020, 9, 189. [Google Scholar] [CrossRef]
- Gordon, M.H. The Mechanism of Antioxidant Action in Vitro. In Food Antioxidants; Hudson, B.J.F., Ed.; Elsevier Applied Food Science Series; Springer: Dordrecht, The Netherlands, 1990; Chapter 1. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Kashtiban, A.E.; Okpala, C.O.R.; Karimidastjerd, A.; Zahedinia, S. Recent advances in nano-related natural antioxidants, their extraction methods and contributions to the food industry. Explor. Foods Foodomics 2024, 2, 125–154. [Google Scholar] [CrossRef]
- Pavlovskyi, D.; Vorobyova, V. A Comprehensive Overview of Chemical Additives in Single-Use Polymeric Products: Functionality, Environmental Impact and the Analytical Greenness Assessment. Water Air Soil Pollut. 2025, 236, 186. [Google Scholar] [CrossRef]
- Fuentes, C.; Fuentes, A.; Barat, J.M.; Ruiz, M.J. Relevant Essential Oil Components: A Minireview on Increasing Applications and Potential Toxicity. Toxicol. Mech. Methods 2021, 31, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanopart. Res. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Faraz, A.; Saleem, F.; Arslan, M.A.; Ishaq, H.M.; Hussain, S.M.; Iqbal, Z.M.; Nazir, S.; Qureshi, T.M.; Akram, A.; Ameen, M.; et al. Safety and Regulatory Issues of Organic Nanomaterial. In Organic-Based Nanomaterials in Food Packaging; Younis, K., Yousuf, O., Ul Islam, S., Eds.; Springer: Cham, Switzerland, 2024; Chapter 11; pp. 209–225. [Google Scholar] [CrossRef]
- Acharya, D.; Satapathy, S.; Yadav, K.K.; Somu, P.; Mishra, G. Systemic evaluation of mechanism of cytotoxicity in human colon cancer HCT-116 cells of silver nanoparticles synthesized using marine algae Ulva lactuca extract. J. Inorg. Organomet. Polym. Mater. 2022, 32, 596–605. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Kolesnyk, L.A.; Kryvtsova, M.; Salamon, I.; Kolesnyk, O.; Csabai, J. Development of in vitro cultivation technology, and study of biochemical and antimicrobial properties of the essential oil of promising chemotypes of Salvia rosmarinus. Kertgazdaság 2025, 57, 68–83. [Google Scholar]
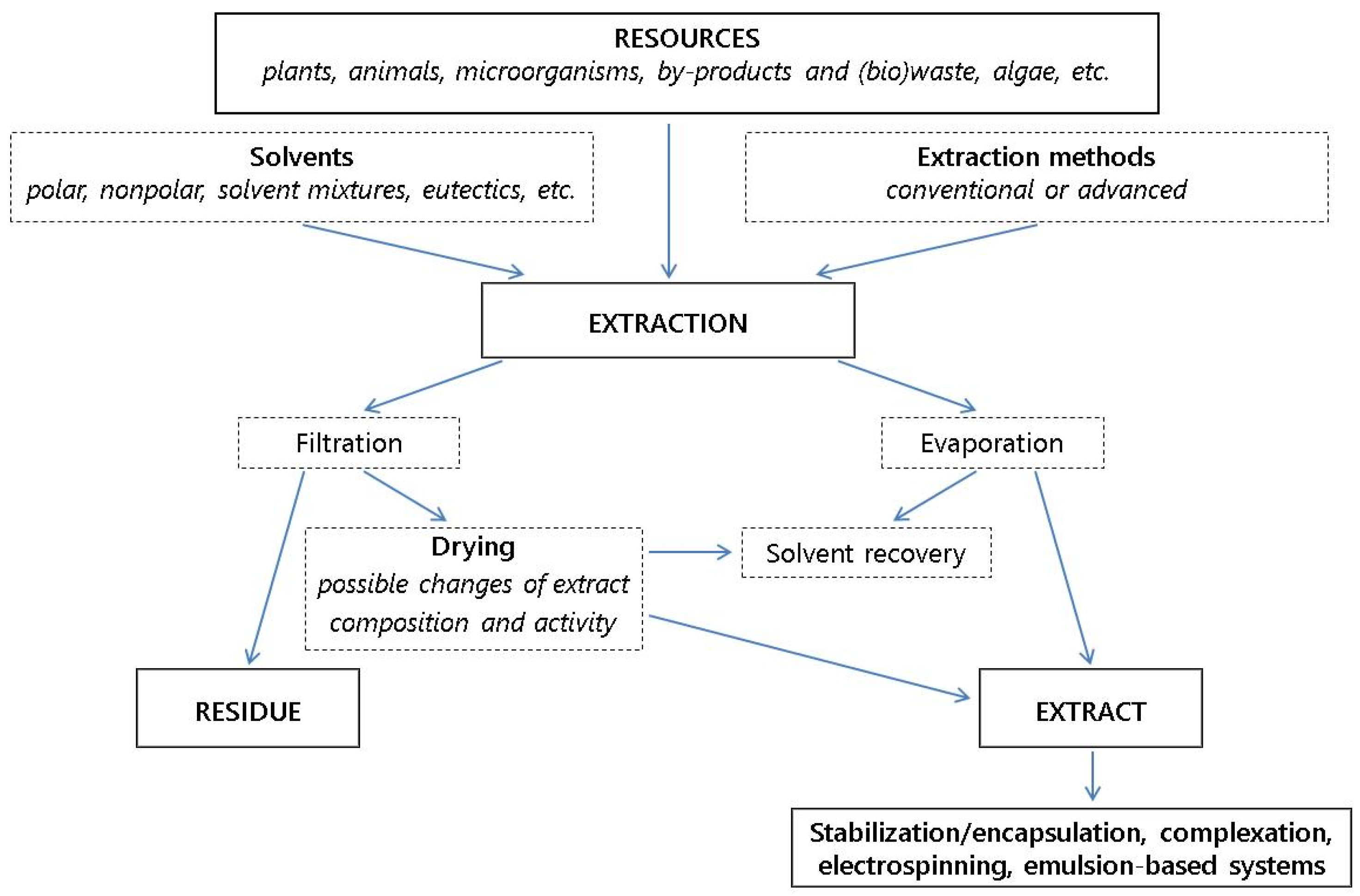
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, C.; Tantaru, G.; Creteanu, A. Recent Insights into the Research of (Bio)Active Additives for Advanced Polymer Materials. Polymers 2025, 17, 3139. https://doi.org/10.3390/polym17233139
Vasile C, Tantaru G, Creteanu A. Recent Insights into the Research of (Bio)Active Additives for Advanced Polymer Materials. Polymers. 2025; 17(23):3139. https://doi.org/10.3390/polym17233139
Chicago/Turabian StyleVasile, Cornelia, Gladiola Tantaru, and Andreea Creteanu. 2025. "Recent Insights into the Research of (Bio)Active Additives for Advanced Polymer Materials" Polymers 17, no. 23: 3139. https://doi.org/10.3390/polym17233139
APA StyleVasile, C., Tantaru, G., & Creteanu, A. (2025). Recent Insights into the Research of (Bio)Active Additives for Advanced Polymer Materials. Polymers, 17(23), 3139. https://doi.org/10.3390/polym17233139






