Design and Fabrication of Low-Temperature 3D-Printed Bioactive Polyurethane/MnO2 Scaffolds for Bone Repair
Abstract
1. Introduction
2. Materials and Methods
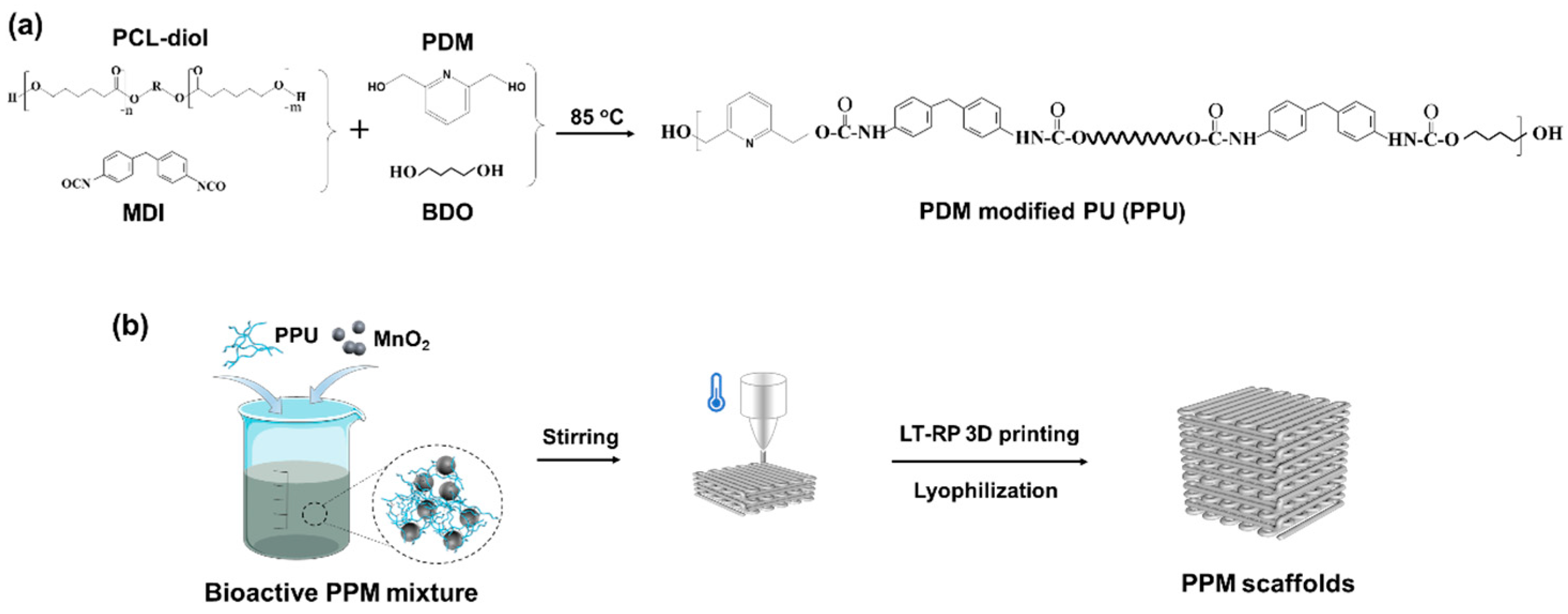
2.1. Synthesis of the PPU
2.2. Fabrication of the Low-Temperature 3D-Printed Scaffolds
2.3. Characterization of the 3D-Printed Scaffolds
2.4. In Vitro Bioactivity of the 3D-Printed Scaffolds
2.5. Evaluation of Degradation of the PPM Scaffolds
2.6. Statistical Analysis
3. Results and Discussion
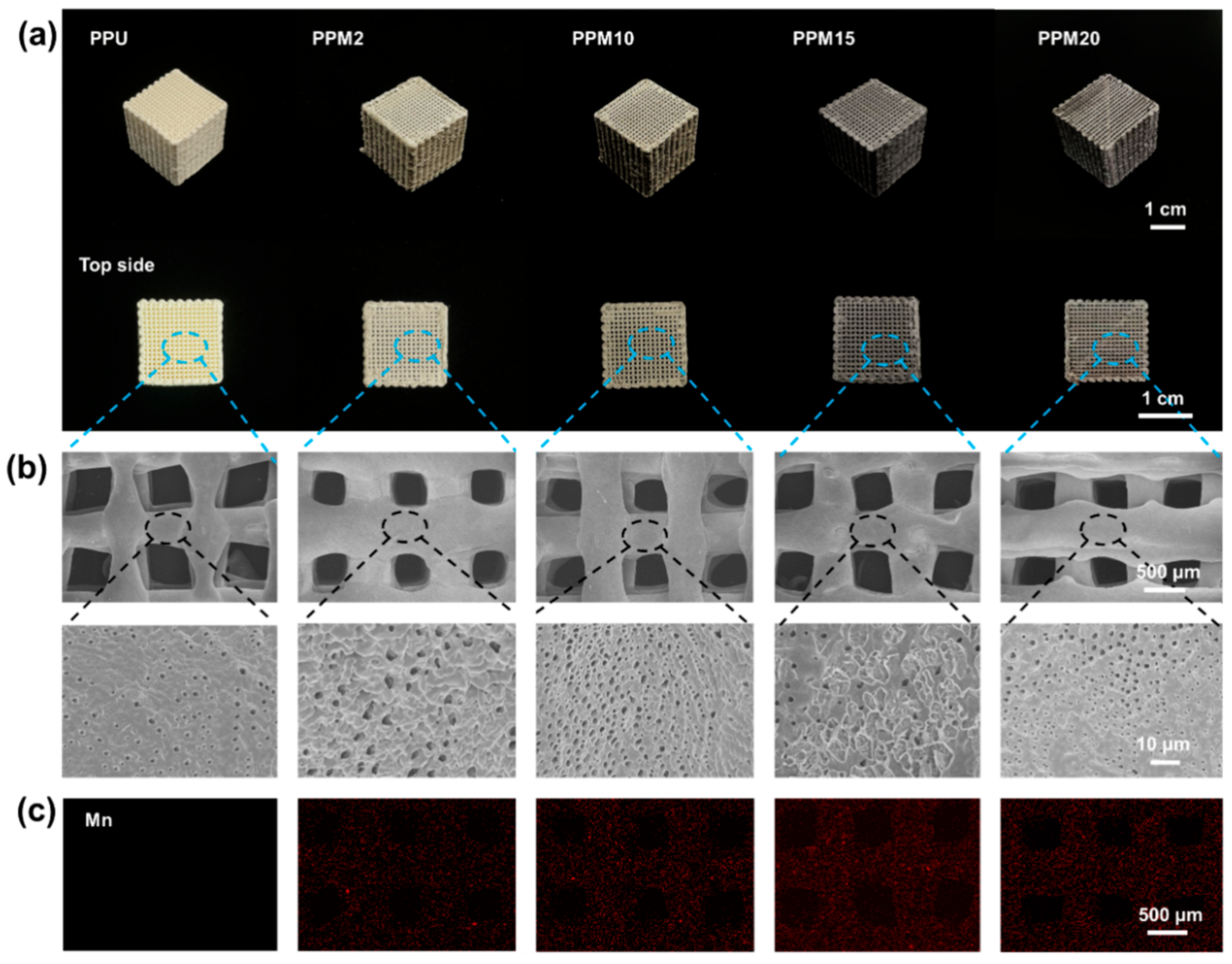
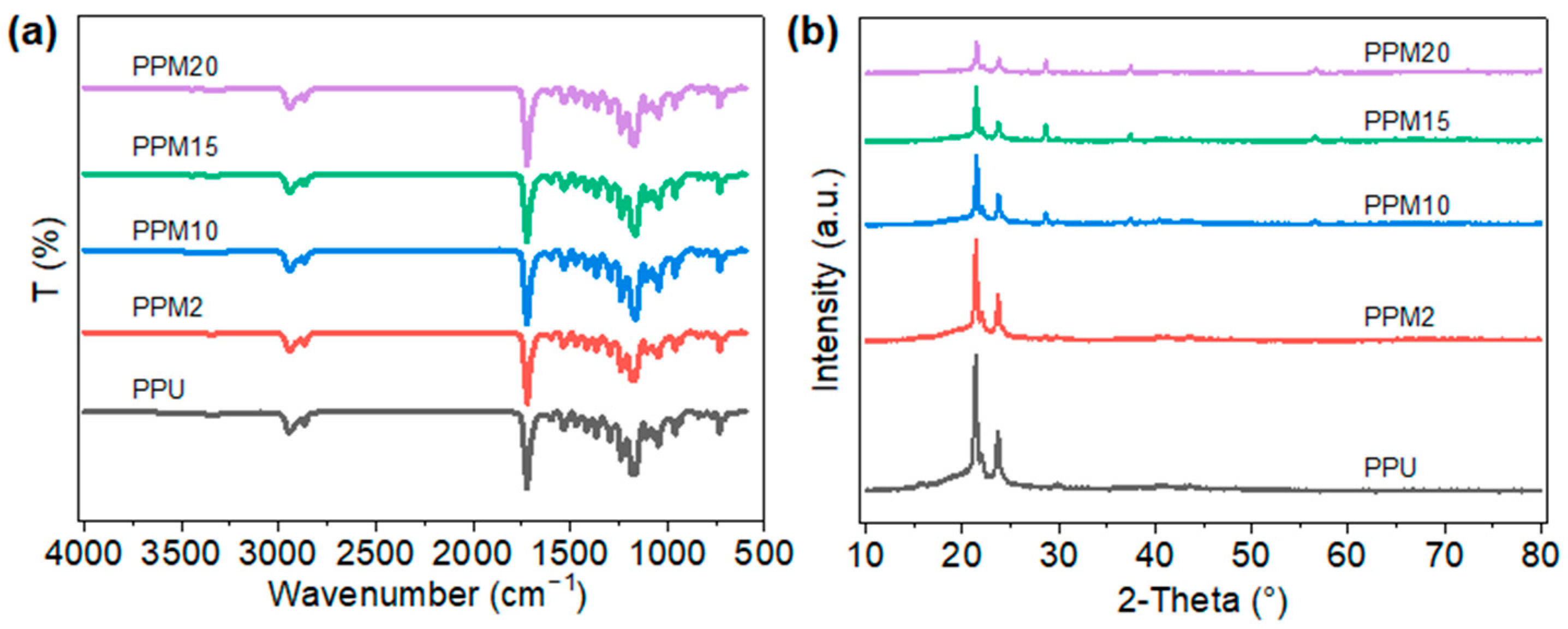
3.1. Characterization of the Low-Temperature 3D-Printed Bioactive Scaffolds
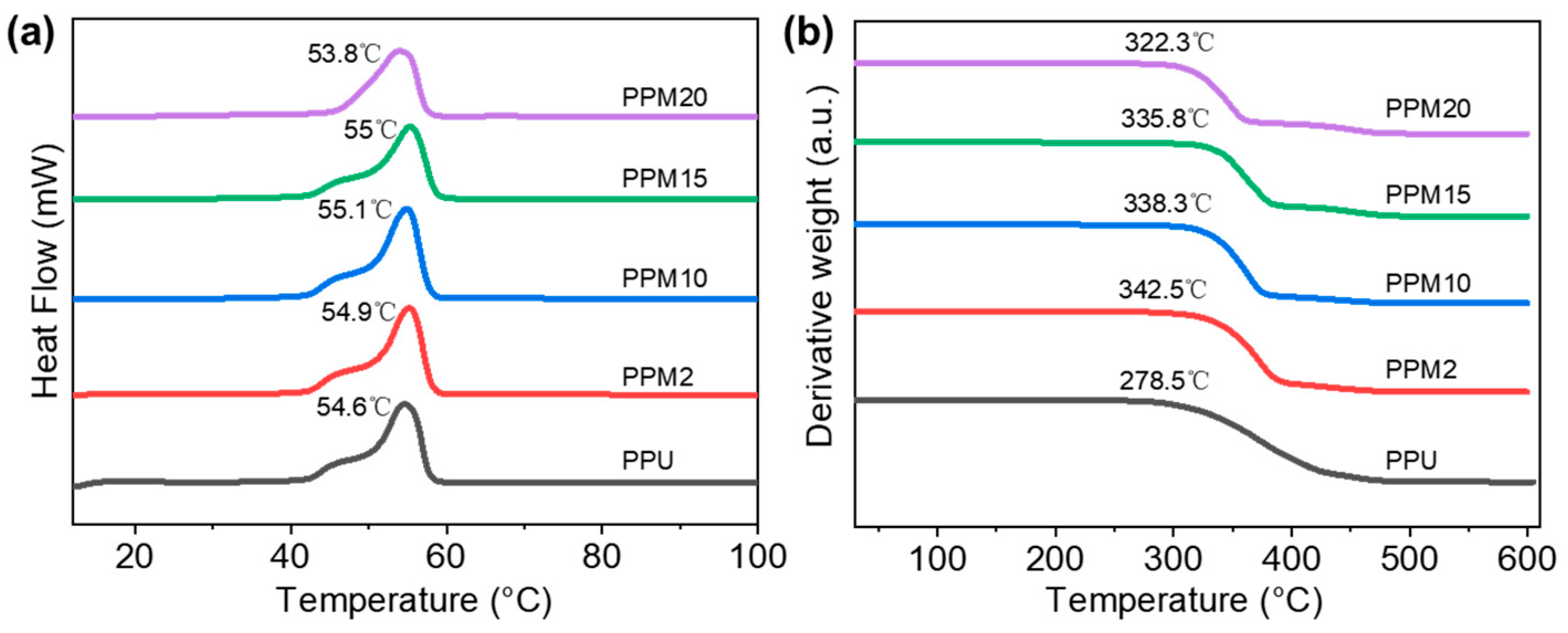
3.2. Thermal Properties of the Low-Temperature 3D-Printed Bioactive Scaffolds
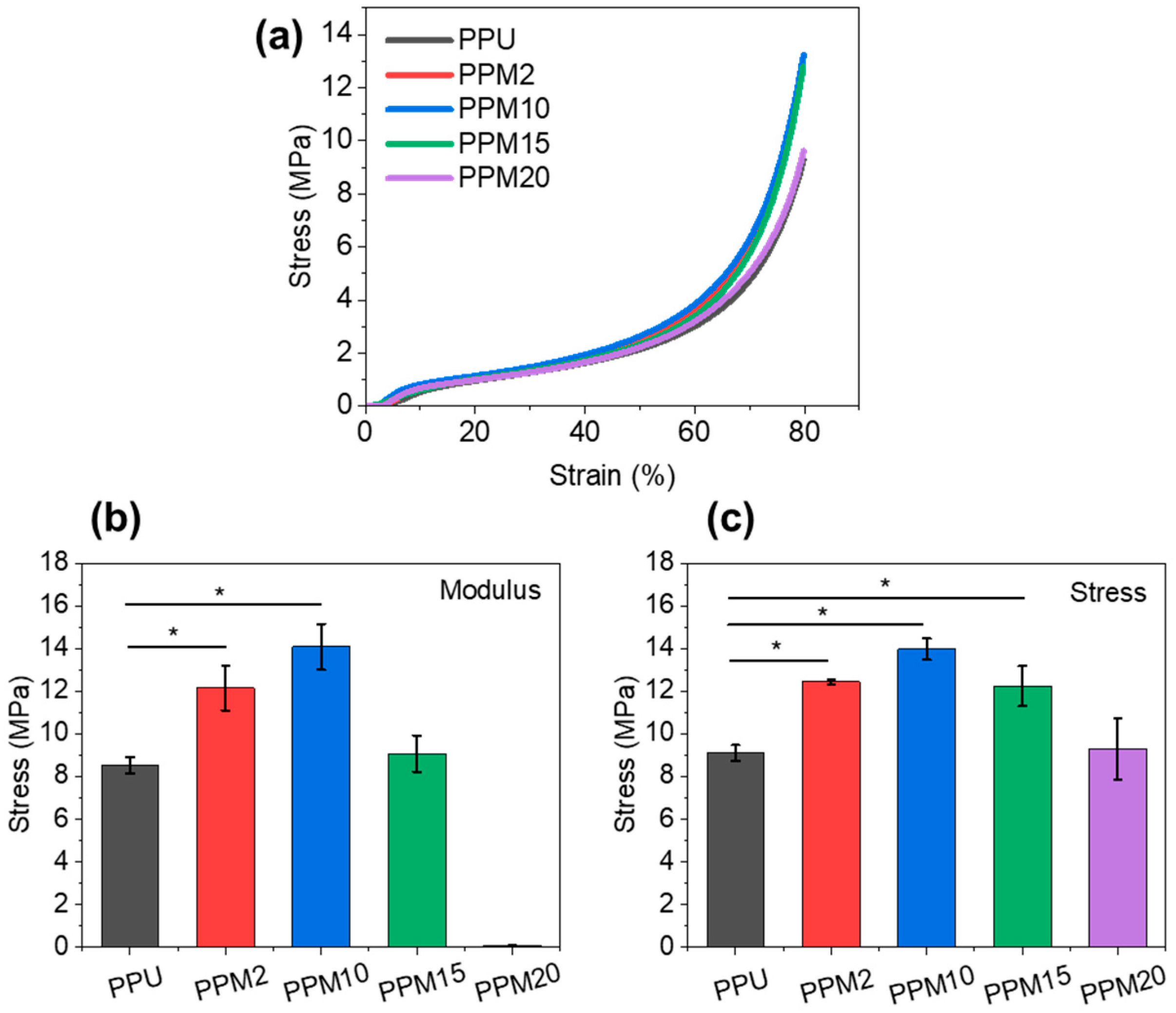
3.3. Mechanical Properties of the Low-Temperature 3D-Printed Bioactive Scaffolds
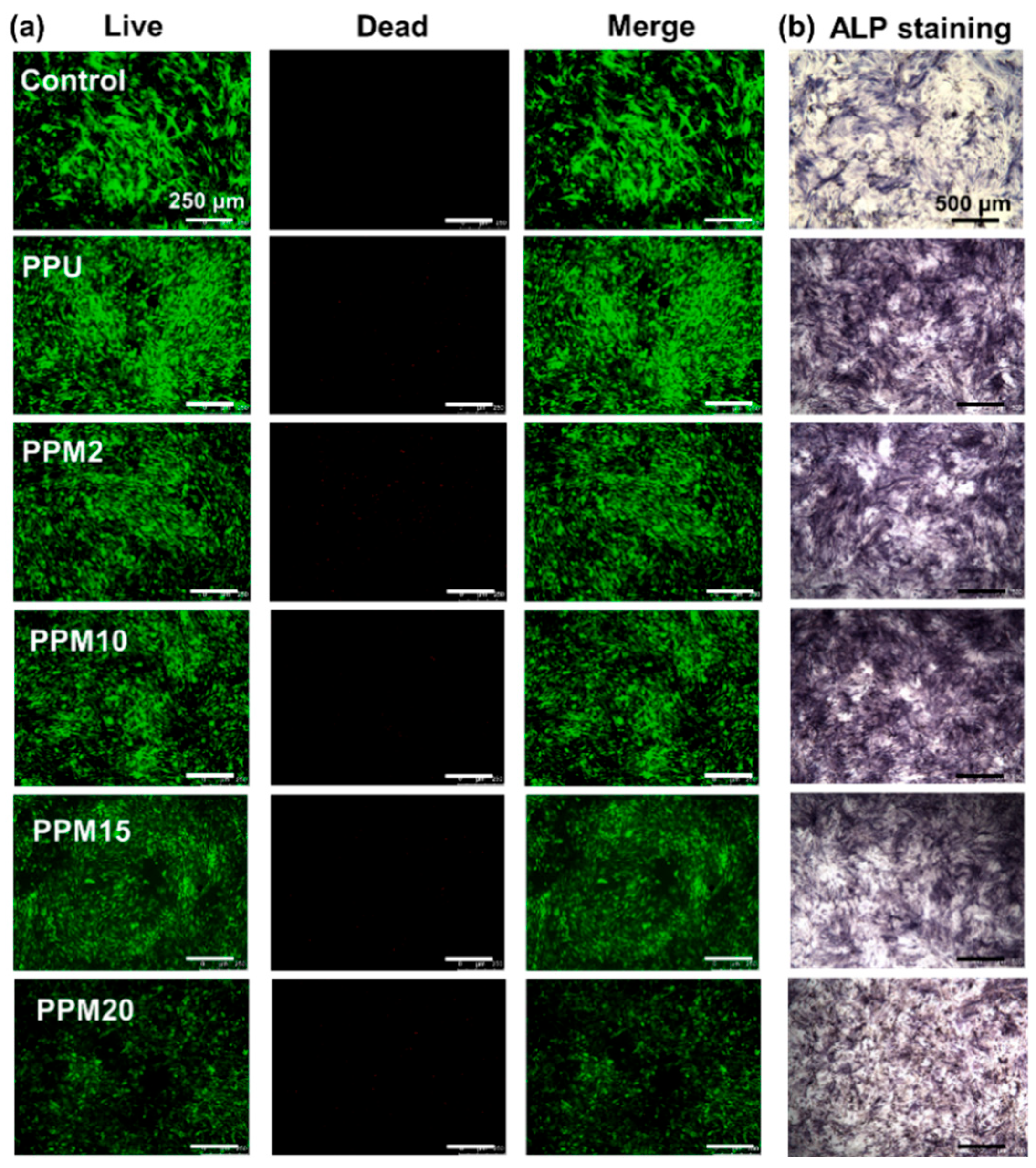
3.4. In Vitro Cell Studies
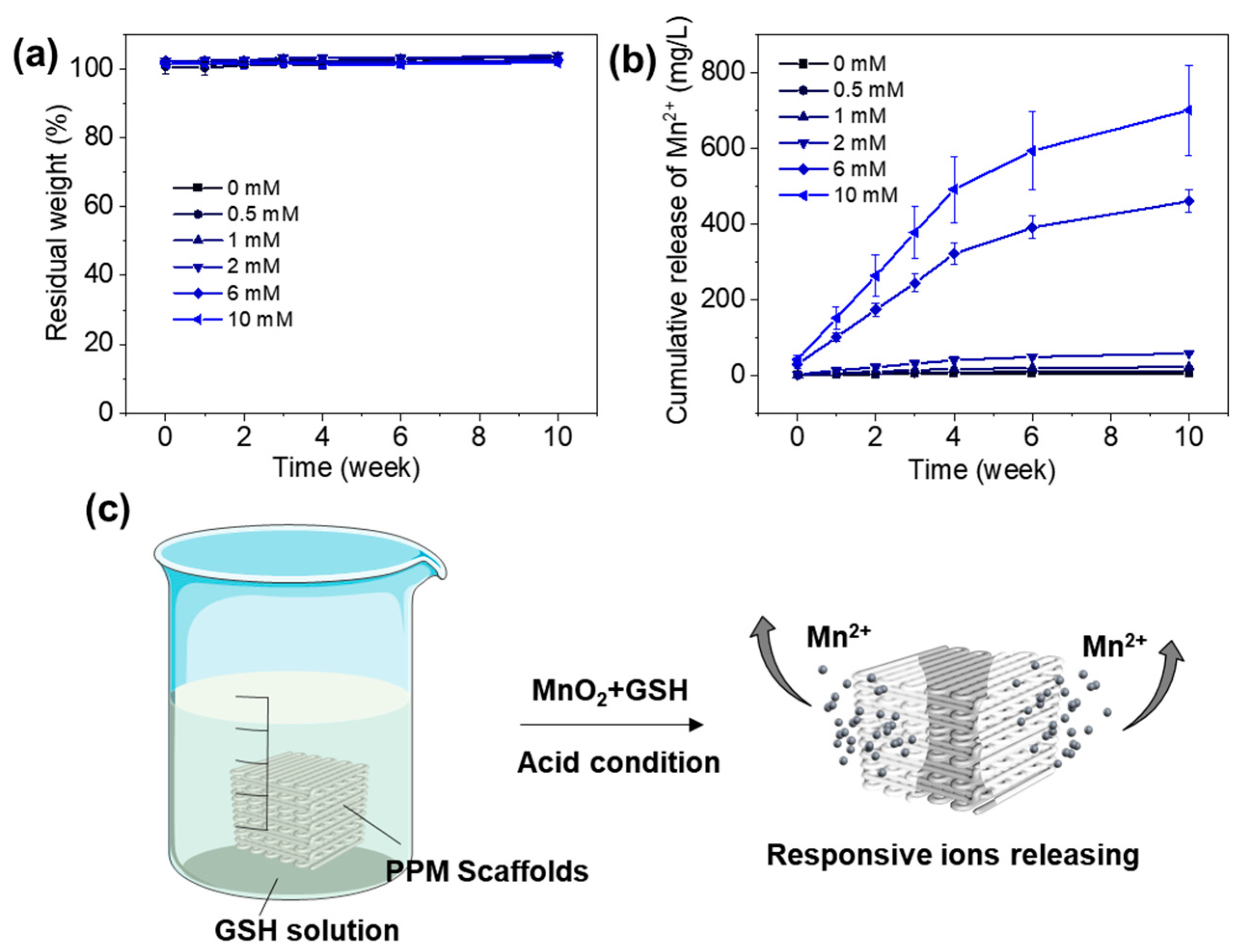
3.5. Degradation and Mn2+ Releasing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LT-RP | Low-temperature rapid prototyping |
| PU | Polyurethane |
| MnO2 | Manganese dioxide |
| PDM | 2, 6-pyridinedimethanol |
| PPMx | The scaffolds with x wt% MnO2 |
| PCL | Polycaprolactone |
| MDI | 4,4′-methylenebis (phenyl isocyanate) |
| BDO | 1, 4-butanediol |
| Mn2+ | Mn ions |
| PPU | PDM modified PU |
| SEM | Scanning electron microscope |
| EDS | Energy-dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared |
| XRD | Wide-angle X-ray diffraction |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| rBMSC | Rat bone marrow mesenchymal stem cells |
| α-MEM | α-minimum essential medium |
| FBS | Fetal bovine serum |
| ALP | Alkaline phosphatase |
| GSH | Glutathione |
| PBS | Phosphate-buffered saline |
| FDM | Fused deposition modeling |
| DIW | Direct ink writing |
References
- Zhang, Y.; Hu, J.; Xie, R.; Yang, Y.; Cao, J.; Tu, Y.; Zhang, Y.; Qin, T.; Zhao, X. A programmable, fast-fixing, osteo-regenerative, biomechanically robust bone screw. Acta Biomater. 2020, 103, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, Z.; Lu, M.; Ma, H.; Wu, C.; Lu, H. Hierarchically structured biomaterials for tissue regeneration. Microstructures 2024, 4, 2024014. [Google Scholar] [CrossRef]
- Pyla, K.R.; Wang, H.; Escobedo-Diaz, J.P. Biomechanical Performance of Additively Manufactured Bone-Mimicking Scaffolds with Graded Architectures. Adv. Mater. Technol. 2025, 10, e00504. [Google Scholar] [CrossRef]
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24, S36–S40. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Montufar, E.; Casas-Luna, M.; Horynová, M.; Tkachenko, S.; Fohlerová, Z.; Diaz-De-La-Torre, S.; Dvořák, K.; Čelko, L.; Kaiser, J. High strength, biodegradable and cytocompatible alpha tricalcium phosphate-iron composites for temporal reduction of bone fractures. Acta Biomater. 2018, 70, 293–303. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Lv, X.; Zhang, M.; Huang, X.; Shi, Y.; Lu, C.; Song, J.; Yang, H. Bioinspired mineral–organic bone adhesives for stable fracture fixation and accelerated bone regeneration. Adv. Funct. Mater. 2020, 30, 1908381. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhang, W.; Deng, J.; Nie, Y.; Du, X.; Qin, L.; Lai, Y. 3D-printed NIR-responsive shape memory polyurethane/magnesium scaffolds with tight-contact for robust bone regeneration. Bioact. Mater. 2022, 16, 218–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J. Isocyanate Modified GO Shape-Memory Polyurethane Composite. Polymers 2020, 12, 118. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, X.; Li, X.; Yu, Y.; Kaplan, D.L.; Cai, Q. Biodegradable and electroactive cryogel microspheres for neurovascularized bone regeneration. Matter 2025, 8, 102366. [Google Scholar] [CrossRef]
- Zili, X.; Junjie, D.; Deju, G.; Yuhan, D.; Yuanchi, Z.; Yuxiao, L. Stimuli-responsive biomedical polymeric films for tissue regeneration. Microstructures 2025, 5, 2025055. [Google Scholar] [CrossRef]
- Zhang, P.; Ruan, H.; Li, Q.; He, Y.; Yang, C. Resolving hyperelasticity-adhesiveness conflict in polymer networks by in situ constructing mechanical heterogeneities. Nat. Commun. 2025, 16, 6094. [Google Scholar] [CrossRef]
- Du, R.; Zhao, B.; Luo, K.; Wang, M.-X.; Yuan, Q.; Yu, L.-X.; Yang, K.-K.; Wang, Y.-Z. Shape memory polyester scaffold promotes bone defect repair through enhanced osteogenic ability and mechanical stability. ACS Appl. Mater. Interfaces 2023, 15, 42930–42941. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; Mahmood, T.; De Groot, K.; Van Blitterswijk, C. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Dong, Z.; Wang, Y.; Han, C.; Liao, Z.; Li, Y.; Huang, H.; Mou, J.; Mi, J.; et al. Additive manufacturing of personalized, semipermeable and biodegradable polymer/metal composite membrane for guided bone regeneration. Mater. Today 2025, 83, 181–197. [Google Scholar] [CrossRef]
- Salehabadi, M.; Mirzadeh, H. 3D Printing of Polyester Scaffolds for Bone Tissue Engineering: Advancements and Challenges. Adv. Mater. Technol. 2025, 10, 2401522. [Google Scholar] [CrossRef]
- Momeni, F.; Liu, X.; Ni, J. A review of 4D printing. Mater. Des. 2017, 122, 42–79. [Google Scholar] [CrossRef]
- Saadi, M.A.S.R.; Maguire, A.; Pottackal, N.T.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct Ink Writing: A 3D Printing Technology for Diverse Materials. Adv. Mater. 2022, 34, 2108855. [Google Scholar] [CrossRef]
- Nguyen, K.Q.; Vuillaume, P.Y.; Hu, L.; Vachon, A.; Diouf-Lewis, A.; Marcoux, P.-L.; Robert, M.; Elkoun, S. Effect of in situ thermal treatment on interlayer adhesion of 3D printed polyetherimide (PEI) parts produced by fused deposition modeling (FDM). Mater. Today Commun. 2024, 39, 108588. [Google Scholar] [CrossRef]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Nie, Y.; Du, X.; Huang, C.; Li, L.; Long, J.; Wang, X.; Tong, W.; Qin, L.; et al. Time-Sequential and Multi-Functional 3D Printed MgO2/PLGA Scaffold Developed as a Novel Biodegradable and Bioactive Bone Substitute for Challenging Postsurgical Osteosarcoma Treatment. Adv. Mater. 2024, 36, 2308875. [Google Scholar] [CrossRef]
- Long, J.; Yao, Z.; Zhang, W.; Liu, B.; Chen, K.; Li, L.; Teng, B.; Du, X.-F.; Li, C.; Yu, X.-F.; et al. Regulation of Osteoimmune Microenvironment and Osteogenesis by 3D-Printed PLAG/black Phosphorus Scaffolds for Bone Regeneration. Adv. Sci. 2023, 10, 2302539. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhao, X.; Xie, R.; Qin, T.; Ji, F. Mechanically Robust Shape Memory Polyurethane Nanocomposites for Minimally Invasive Bone Repair. ACS Appl. Bio Mater. 2019, 2, 1056–1065. [Google Scholar] [CrossRef]
- Yu, R.; Wang, Q.; Wang, W.; Xiao, Y.; Wang, Z.; Zhou, X.; Zhang, X.; Zhu, X.; Fang, C. Polyurethane/graphene oxide nanocomposite and its modified asphalt binder: Preparation, properties and molecular dynamics simulation. Mater. Des. 2021, 209, 109994. [Google Scholar] [CrossRef]
- Kim, S.-R.; Jeon, J.; Kim, Y.-C.; Park, J.-W. Transparent and Skin-Attachable Silver Nanowire Electrodes Embedded on Dissolvable Polyurethane for Highly Conformable Wearable Electronics. Adv. Mater. Technol. 2023, 8, 2200968. [Google Scholar] [CrossRef]
- Zeng, X.; Miao, J.; Xia, R.; Qian, J.; Zhu, S.; Chen, P.; Tai, Y. Design and fabrication of shape memory polyurethane network with rapid recoverable plastic deformation. Mater. Today Commun. 2023, 35, 105777. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape–memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Qiu, H.; Wang, C.; Dong, X.; Du, J.; Li, X.; Yang, X.; Fang, H.; Ding, Y. A robust bio-based polyurethane employed as surgical suture with help to promote skin wound healing. Biomater. Adv. 2025, 166, 214048. [Google Scholar] [CrossRef]
- Dang, G.-p.; Gu, J.-t.; Song, J.-h.; Li, Z.-t.; Hao, J.-x.; Wang, Y.-z.; Wang, C.-y.; Ye, T.; Zhao, F.; Zhang, Y.-F.; et al. Multifunctional polyurethane materials in regenerative medicine and tissue engineering. Cell Rep. Phys. Sci. 2024, 5, 102053. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhu, S.; Qin, T.; Ji, F. A “trampoline” nanocomposite: Tuning the interlayer spacing in graphene oxide/polyurethane to achieve coalesced mechanical and memory properties. Compos. Sci. Technol. 2019, 180, 14–22. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, P.; Ma, P.a.; Lin, J. Manganese oxide nanomaterials: Synthesis, properties, and theranostic applications. Adv. Mater. 2020, 32, 1905823. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 2018, 48, 675–687.e7. [Google Scholar] [CrossRef] [PubMed]
- Lüthen, F.; Bulnheim, U.; Müller, P.D.; Rychly, J.; Jesswein, H.; Nebe, J.B. Influence of manganese ions on cellular behavior of human osteoblasts in vitro. Biomol. Eng. 2007, 24, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Miola, M.; Brovarone, C.V.; Maina, G.; Rossi, F.; Bergandi, L.; Ghigo, D.; Saracino, S.; Maggiora, M.; Canuto, R.A.; Muzio, G. In vitro study of manganese-doped bioactive glasses for bone regeneration. Mater. Sci. Eng. C 2014, 38, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, Z.; Sun, Y.; Nie, Y.; Zhang, Y.; Li, Z.; Luo, Z.; Zhang, W.; Wang, X.; Du, Y.; et al. 3D-printed manganese dioxide incorporated scaffold promotes osteogenic-angiogenic coupling for refractory bone defect by remodeling osteo-regenerative microenvironment. Bioact. Mater. 2025, 44, 354–370. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Guan, Y.; Wei, X.; Sha, M.; Yi, M.; Jing, M.; Lv, M.; Guo, W.; Xu, J. Manganese salts function as potent adjuvants. Cell. Mol. Immunol. 2021, 18, 1222–1234. [Google Scholar] [CrossRef]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.-D.; Mauceri, H.; Beckett, M.; Darga, T. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, S.; Lu, Z.; Li, J.; Yang, X.; Qiao, Y.; Men, Y.; Sun, J. Healable, Recyclable, and Mechanically Tough Polyurethane Elastomers with Exceptional Damage Tolerance. Adv. Mater. 2020, 32, 2005759. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Liu, Y.; Gong, T.; Wang, L.; Zhou, S. Highly pH-sensitive polyurethane exhibiting shape memory and drug release. Polym. Chem. 2014, 5, 5168–5174. [Google Scholar] [CrossRef]
- Ma, B.; Nishina, Y.; Bianco, A. A glutathione responsive nanoplatform made of reduced graphene oxide and MnO2 nanoparticles for photothermal and chemodynamic combined therapy. Carbon 2021, 178, 783–791. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Wang, J.; Pu, X.; Zhang, G.; Wang, S.; Wang, N.; Li, X. Engineering p-Band Center of Oxygen Boosting H+ Intercalation in δ-MnO2 for Aqueous Zinc Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202215654. [Google Scholar] [CrossRef]
- Long, J.; Zhang, W.; Chen, Y.; Teng, B.; Liu, B.; Li, H.; Yao, Z.; Wang, D.; Li, L.; Yu, X.-F.; et al. Multifunctional magnesium incorporated scaffolds by 3D-Printing for comprehensive postsurgical management of osteosarcoma. Biomaterials 2021, 275, 120950. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, G.; Zhang, J.; Sang, Z.; Chen, Z.; Xu, Q.; Wang, S.; Zhang, X. Synthesis and properties of temperature-responsive shape memory polyurethane with secondary crosslinked network structure based on LPO. Polymer 2024, 311, 127559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Guo, A.; Nie, Y.; Xu, Z.; Deng, J.; Zhang, Y.; Yao, Z.; Zhang, W.; Lai, Y.; Zhang, Y. Design and Fabrication of Low-Temperature 3D-Printed Bioactive Polyurethane/MnO2 Scaffolds for Bone Repair. Polymers 2025, 17, 3101. https://doi.org/10.3390/polym17233101
Li L, Guo A, Nie Y, Xu Z, Deng J, Zhang Y, Yao Z, Zhang W, Lai Y, Zhang Y. Design and Fabrication of Low-Temperature 3D-Printed Bioactive Polyurethane/MnO2 Scaffolds for Bone Repair. Polymers. 2025; 17(23):3101. https://doi.org/10.3390/polym17233101
Chicago/Turabian StyleLi, Long, Along Guo, Yangyi Nie, Zili Xu, Junjie Deng, Yuyang Zhang, Zhenyu Yao, Wei Zhang, Yuxiao Lai, and Yuanchi Zhang. 2025. "Design and Fabrication of Low-Temperature 3D-Printed Bioactive Polyurethane/MnO2 Scaffolds for Bone Repair" Polymers 17, no. 23: 3101. https://doi.org/10.3390/polym17233101
APA StyleLi, L., Guo, A., Nie, Y., Xu, Z., Deng, J., Zhang, Y., Yao, Z., Zhang, W., Lai, Y., & Zhang, Y. (2025). Design and Fabrication of Low-Temperature 3D-Printed Bioactive Polyurethane/MnO2 Scaffolds for Bone Repair. Polymers, 17(23), 3101. https://doi.org/10.3390/polym17233101







