An Anti-Freezing Ionic Conductive Hydrogel for Strain Sensing and Energy Harvesting Devices
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of AICH
2.3. Microstructures
2.4. Anti-Freezing Characteristics
2.5. Mechanical Property
2.6. Conductivity
2.7. Fabrication of AICH-Based Devices
3. Results and Discussion
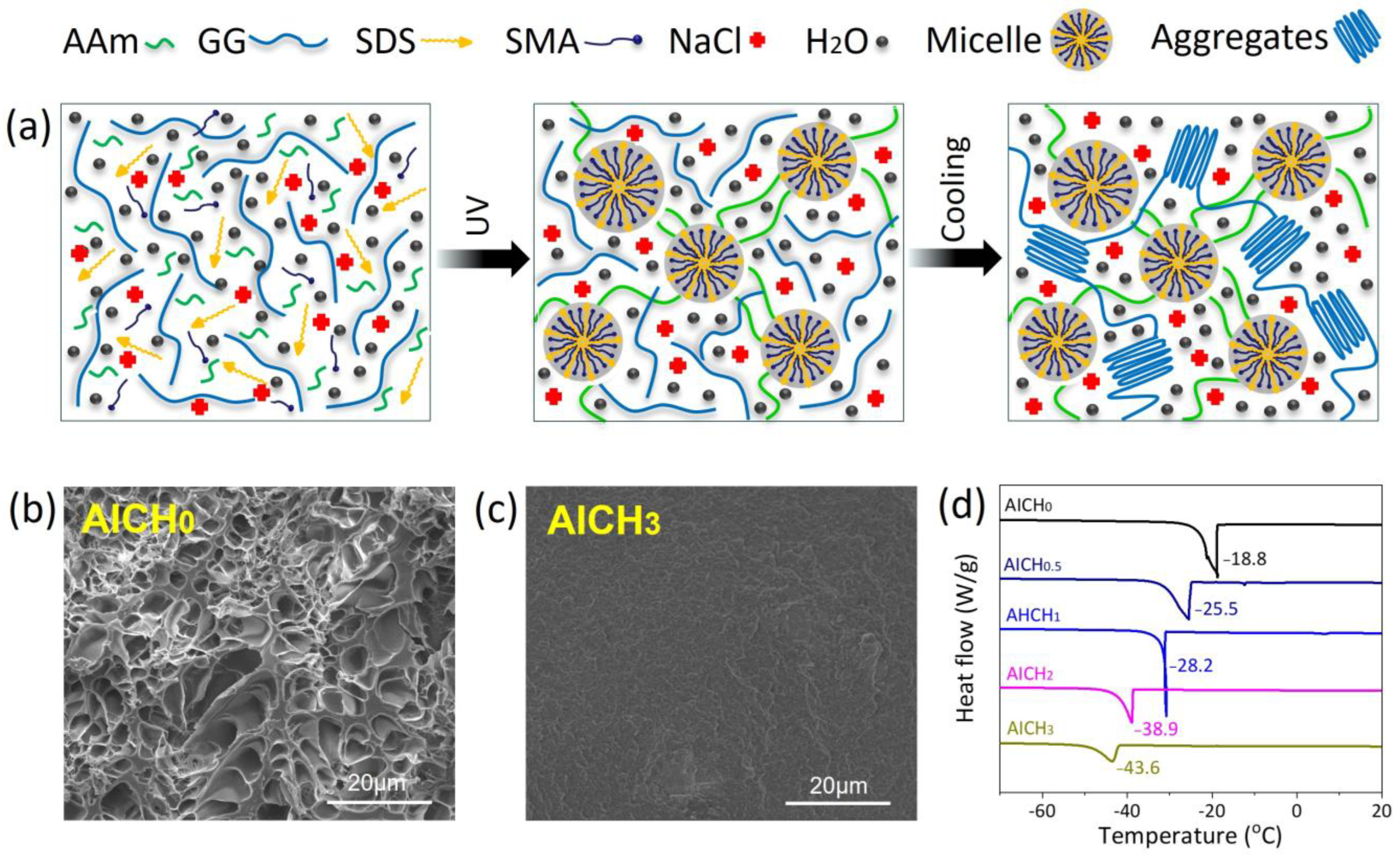
3.1. Preparation of AICH
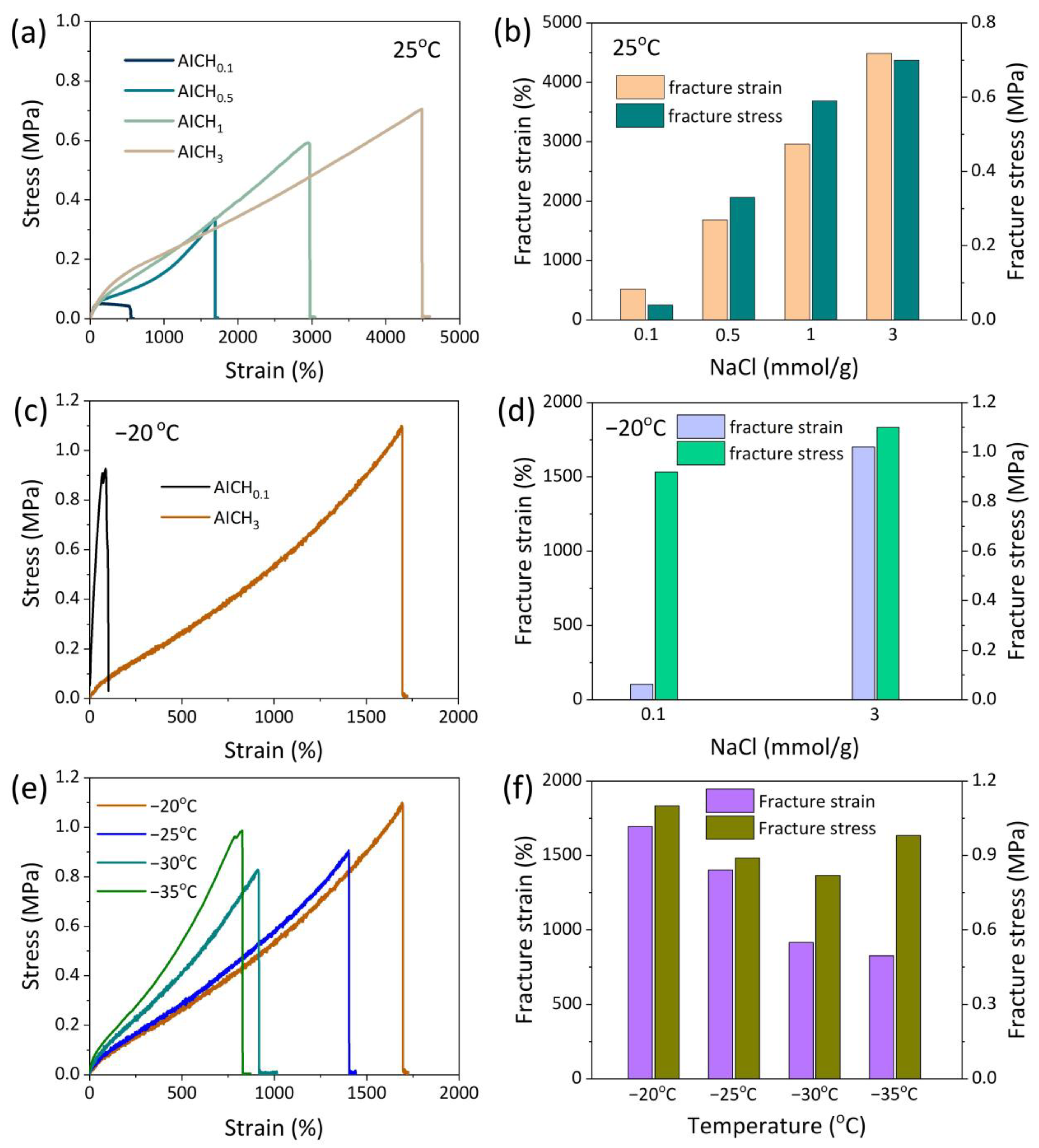
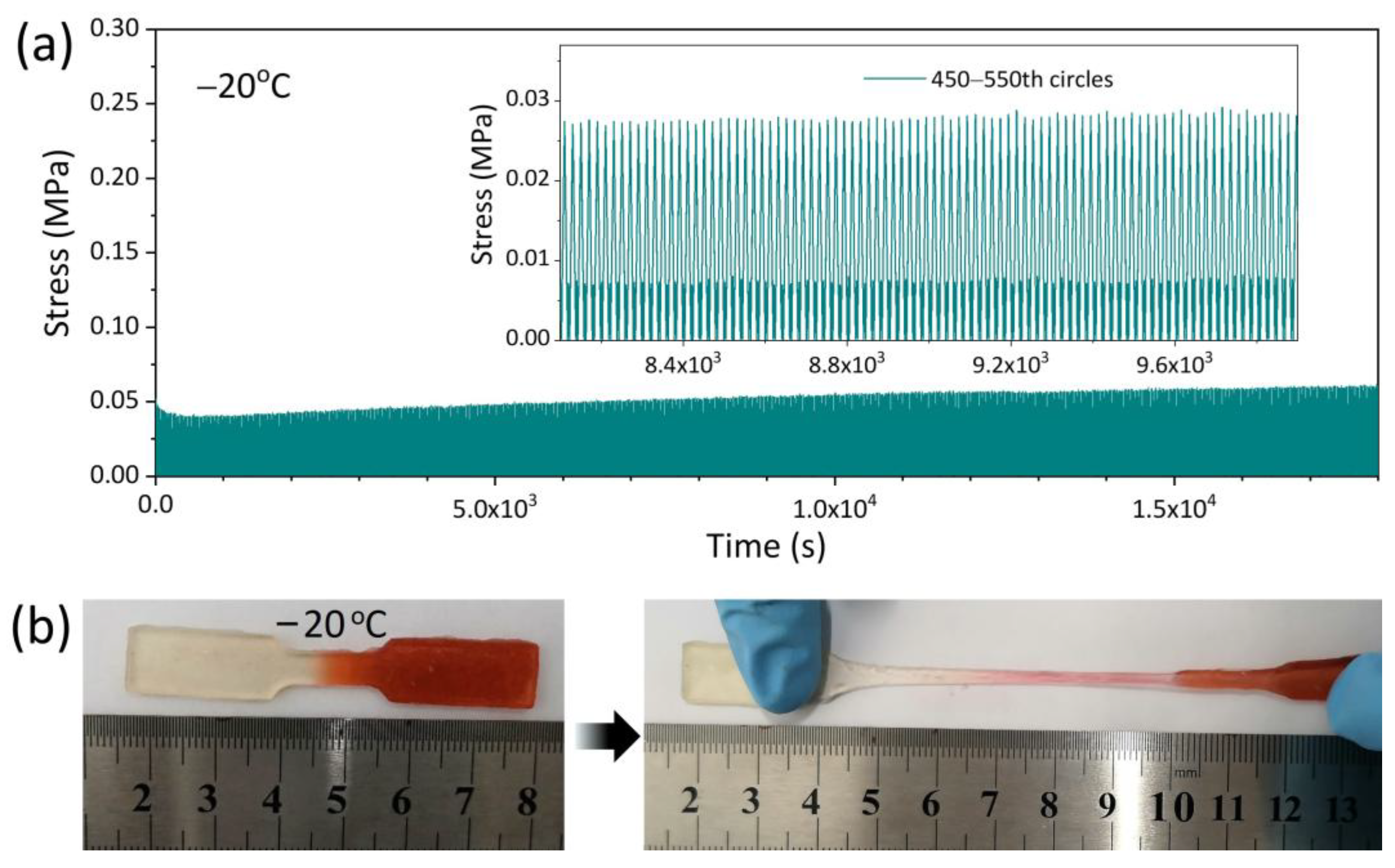
3.2. Mechanical Property of AICH
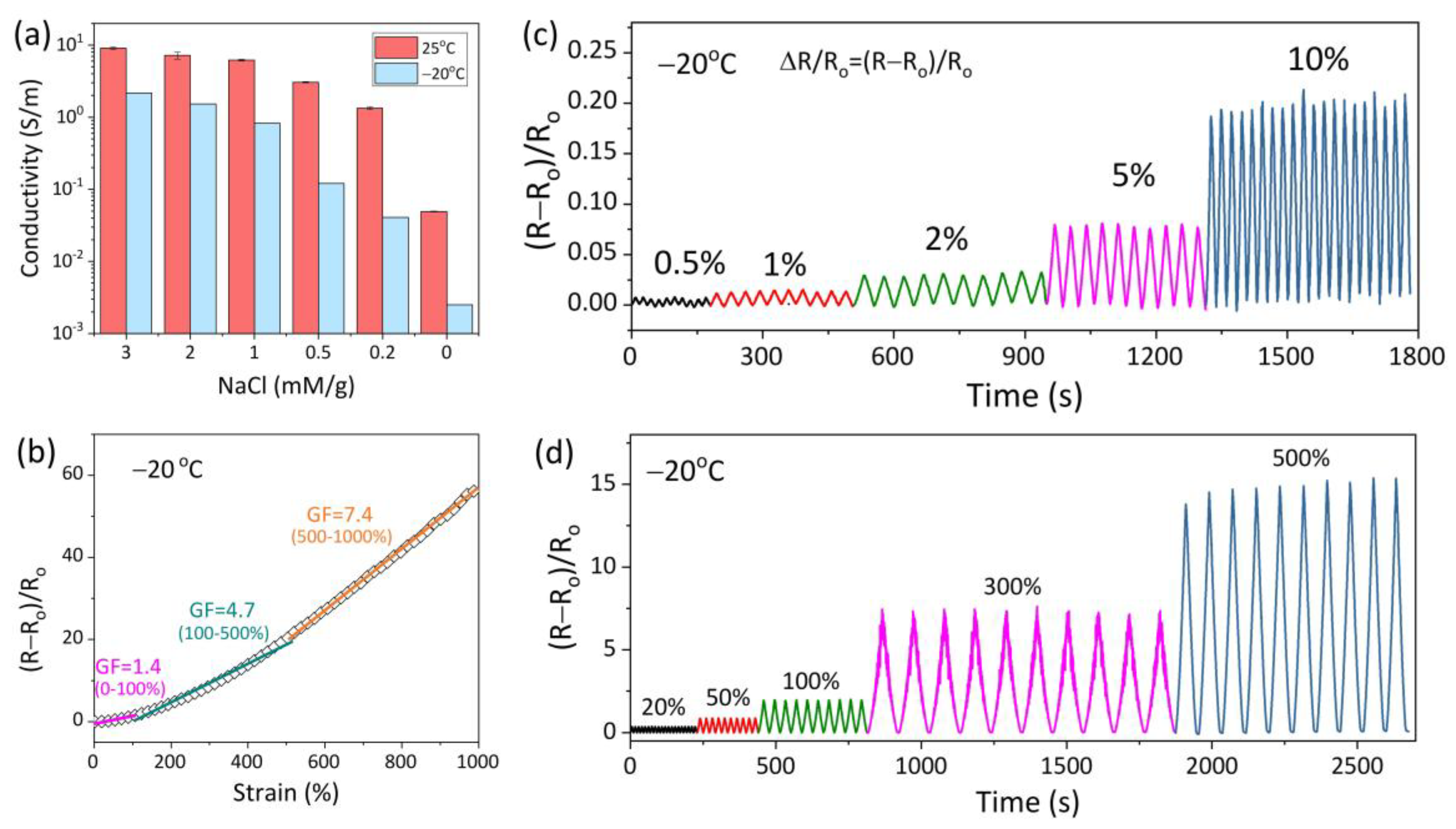
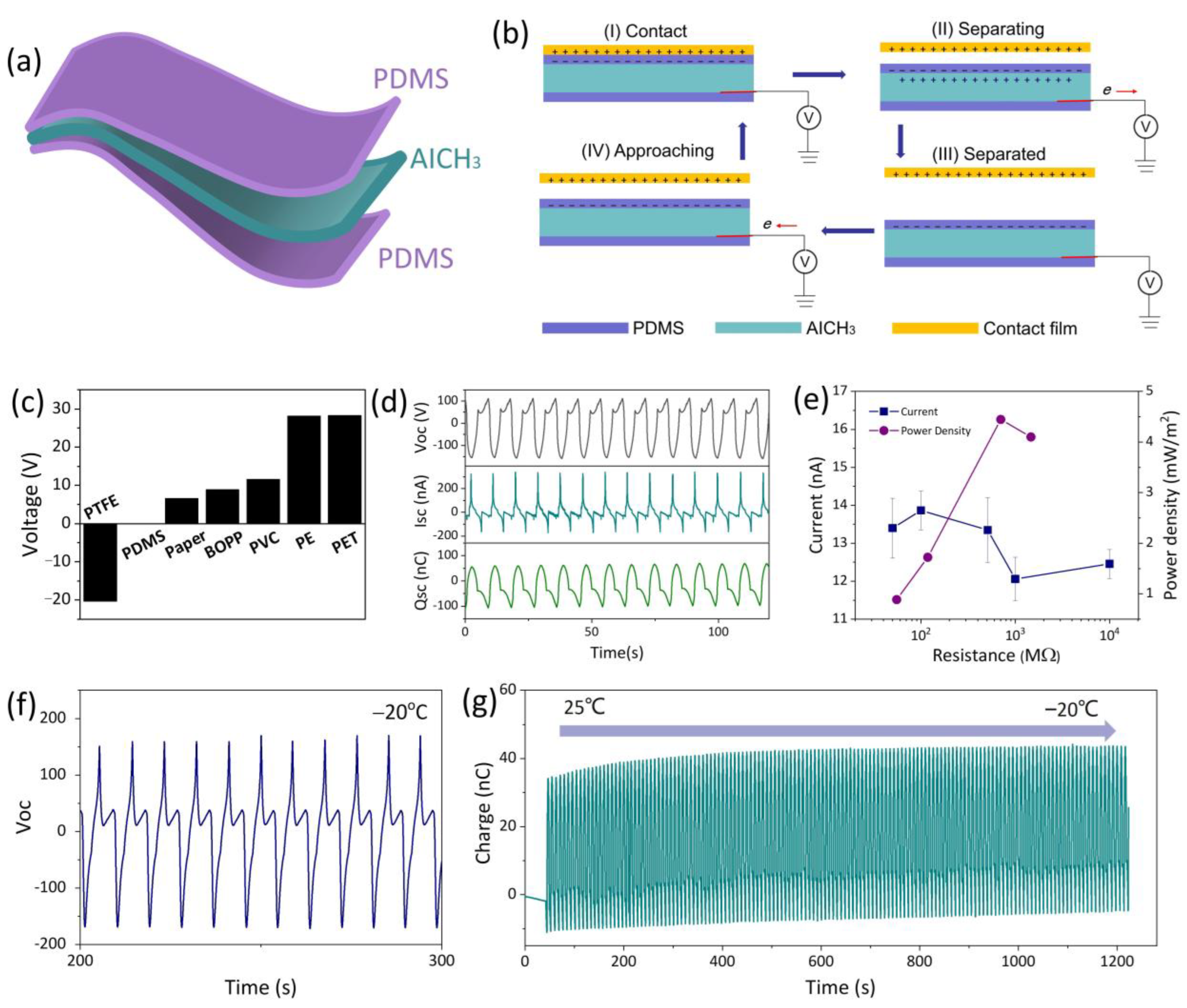
3.3. Conductivity and Strain Sensitivity of AICH
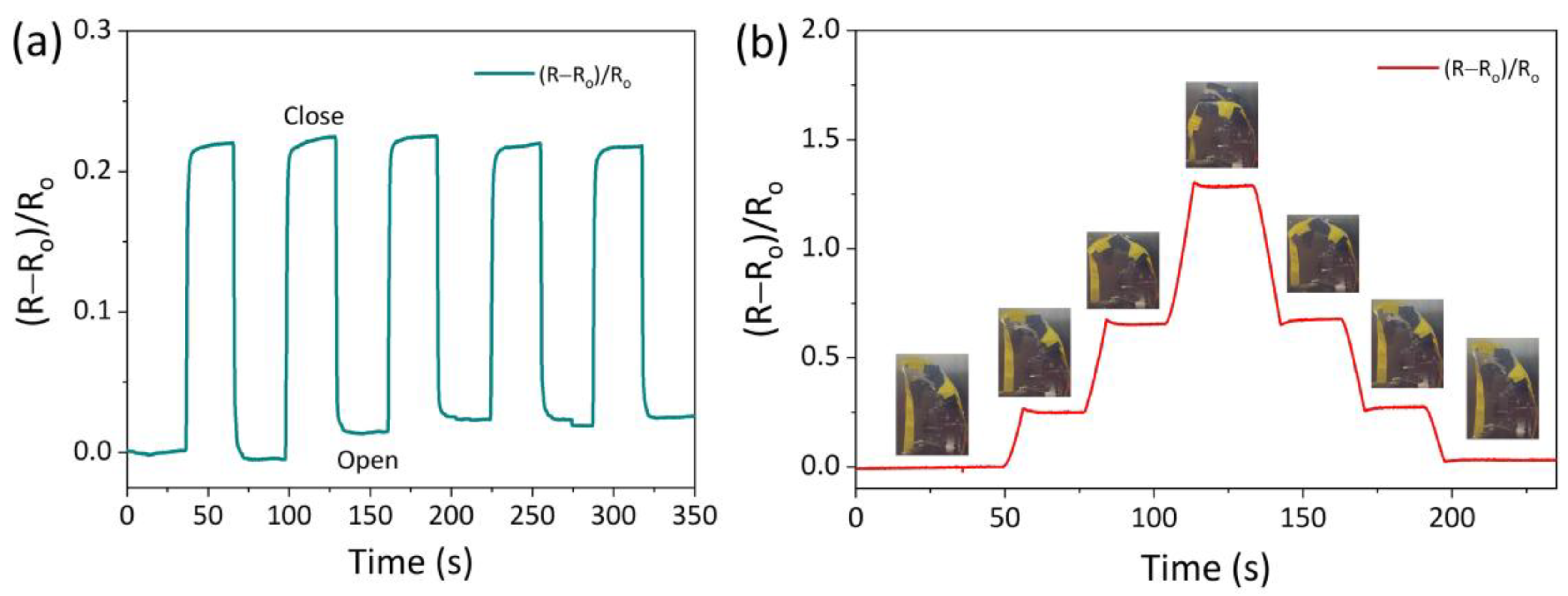
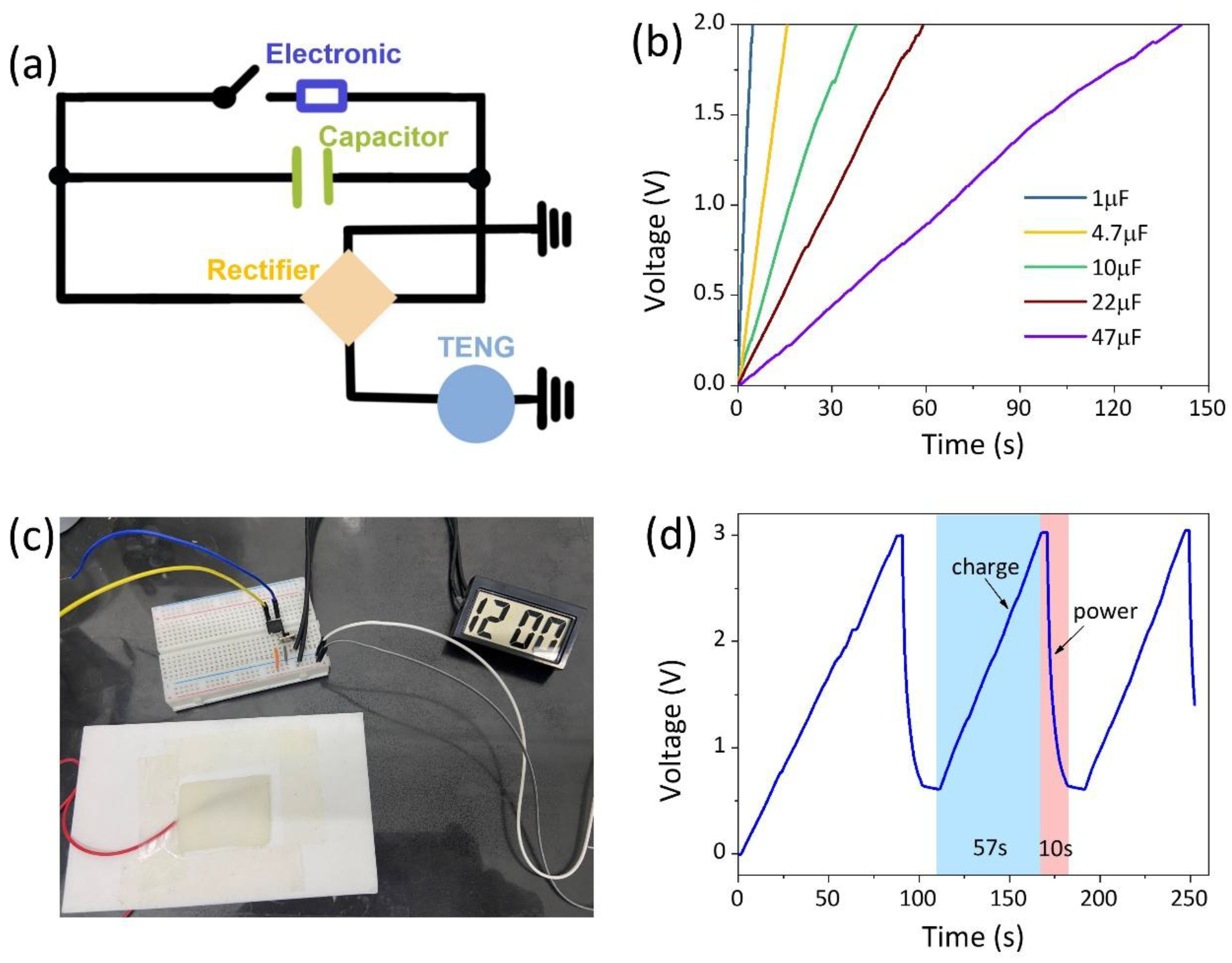
3.4. AICH3-Based Flexible Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Wang, G.Q.; Sun, G.F.; Bao, C.C.; Li, Y.; Meng, C.Z.; Yao, Z. A Flexible-Integrated Multimodal Hydrogel-Based Sensing Patch. Nano-Micro Lett. 2025, 17, 156. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhang, L.Y.; Wang, M.; Zang, D.F.; Long, H.Y.; Weng, L.; Guo, N.; Gao, J.G.; Liu, Y.H.; Xu, B.B. A wearable ionic hydrogel strain sensor with double cross-linked network for human-machine interface. Adv. Compos. Hybrid Mater. 2025, 8, 17. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, Y.R.; Liao, Y.T.; Zhang, H.Y. Bioinspired Super-Robust Conductive Hydrogels for Machine Learning-Assisted Tactile Perception System. Adv. Mater. 2025, 37, 2416275. [Google Scholar] [CrossRef]
- Guo, X.K.; Zhang, S.L.; Patel, S.; Sun, X.L.; Zhu, Y.L.; Wei, Z.C.; Wang, R.G.; He, X.D.; Wang, Z.K.; Yu, C.J.; et al. A skin-mimicking multifunctional hydrogel via hierarchical, reversible noncovalent interactions. Sci. Adv. 2025, 11, eadv8523. [Google Scholar] [CrossRef]
- Lin, W.J.; Zhang, J.Y.; Pan, G.Y.; Lin, Y.B.; Chen, D.Q.; Lu, J.H.; Li, J.X.; Huang, J.H.; Li, Z.H.; Lin, X.F.; et al. Multifunctional Wide-Temperature Threshold Conductive Eutectic Hydrogel Sensor for Shoulder Pain Monitoring and Photothermal Therapy. Adv. Funct. Mater. 2025, e09779. [Google Scholar] [CrossRef]
- Shi, H.R.; Kuang, F.; Huo, H.X.; Gao, Y.H.; Duan, X.; Shen, J.J.; Wan, J.Y.; Li, Y.M.; Du, G.B.; Yang, L. Temperature-Responsive Cellulose-Based Janus Hydrogel as Underwater Electronic Skin. Nano Lett. 2025, 25, 7835–7844. [Google Scholar] [CrossRef]
- Zhang, K.Q.; You, H.B.; Liu, Y.X.; Lin, F.L.; Li, Y.N.; Jia, L.N.; Bao, J.X.; Ge, X.; Pan, G.F.; Yu, H.T.; et al. Development of a multifunctional and biocompatible hydrogel for flexible implantable sensors. Chem. Eng. J. 2025, 522, 167403. [Google Scholar] [CrossRef]
- Choudhury, S.; Wang, Z.L.; Kim, S.W. Hydrogel-based piezoelectric materials and devices for implantable bioelectronics. Biomaterials 2026, 327, 123768. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Zhang, H.; Yao, J.; Cao, X.; Luo, Y.; Wang, Q.G.; Yu, Y.; Su, R.; Yao, Y.D.; Li, G.H.; et al. Biodegradable Anti-Stenosis Microsurgical Implantable Hydrogel Stent for Efficient Vas Deferens Recanalization. Adv. Funct. Mater. 2025, e15388. [Google Scholar] [CrossRef]
- Liu, H.; Deng, Y.; Li, J.H.; Liu, Q.H.; Mu, L.H.; Zhang, R.Z.; Sun, C.L.; He, J.M.; Qu, M.N. Stretchable, anti-freezing, self-healing, and degradable high-performance conductive hydrogel-based triboelectric nanogenerator for energy harvesting and human activity recognition. Mater. Today Energy 2025, 53, 102013. [Google Scholar] [CrossRef]
- Chen, H.; Zu, G.Q.; Ju, Q.Q.; Yang, X.J. Hydrogel-based sandwich-structured triboelectric nanogenerator energy harvesting storage system for multi-functional sensing and monitoring. Chem. Eng. J. 2025, 523, 168739. [Google Scholar] [CrossRef]
- Zhao, J.W.; Yang, J.H.; Chen, J.; Hou, C.L.; Wang, Y.Z.; Wang, Y.J. A stretchable triboelectric nanogenerator with an organohydrogel as electrode for biomechanical energy harvesting and self-powered sensing. Mater. Today Commun. 2025, 49, 113704. [Google Scholar] [CrossRef]
- Yang, B.H.; Li, Y.W.; Zheng, K.; Xiong, Y.; Jin, X.K.; Zhu, L.X.; Cai, R.; Hu, B. Bi-modal synergistic hydrogel sensors coupled with machine learning enable gesture parsing and identity recognition. Chem. Eng. J. 2025, 523, 168174. [Google Scholar] [CrossRef]
- Chen, J.Y.; Liu, F.F.; Abdiryim, T.; Liu, X. An overview of conductive composite hydrogels for flexible electronic devices. Adv. Compos. Hybrid Mater. 2024, 7, 35. [Google Scholar] [CrossRef]
- Yim, Y.J.; Yoon, Y.H.; Kim, S.H.; Lee, J.H.; Chung, D.C.; Kim, B.J. Carbon Nanotube/Polymer Composites for Functional Applications. Polymers 2025, 17, 119. [Google Scholar] [CrossRef]
- Wu, Z.J.; Deng, X.S.; Yu, X.; Gu, J.W.; El-Bahy, Z.M.; Mersal, G.A.M.; Zhang, J.; Alhadhrami, A.; Xu, H.Y.; Guo, N.; et al. Electrospun thermoplastic polyurethane membrane decorated with carbon nanotubes: A platform of flexible strain sensors for human motion monitoring. Polymer 2024, 303, 127120. [Google Scholar] [CrossRef]
- Ran, J.; Liu, Y.F.; Feng, H.X.; Shi, H.X.; Ma, Q. A review on graphene-based electrode materials for supercapacitor. J. Ind. Eng. Chem. 2024, 137, 106–121. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sun, Q.; Liang, X.; Gu, P.Z.; Hu, Z.Y.; Yang, X.; Liu, M.X.; Sun, Z.J.; Huang, J.; Wu, G.M.; et al. Stretchable and negative-Poisson-ratio porous metamaterials. Nat. Commun. 2024, 15, 44707. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, X.R.; Zhao, X.Y.; Chen, H.L.; Jia, P. Significantly Enhanced Acidic Oxygen Evolution Reaction Performance of RuO2 Nanoparticles by Introducing Oxygen Vacancy with Polytetrafluoroethylene. Polymers 2025, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.G.; Li, Y.X.; Feng, S.Y.; Wang, Z.Y.; Xiao, H.; Chen, S.X.; Wang, Y.Q.; He, L.C.; Mao, X. Innovations and Applications of Composite Hydrogels: From Polymer-Based Systems to Metal-Ion-Doped and Functional Nanomaterial-Enhanced Architectures. Small 2025, 21, 03147. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Lu, Z.; Jiang, Y.C.; Gao, Y.T.; Liu, W.; Hu, W.F.; Xin, B.J. Hierarchical MXene/PANI porous film electrodes with improved ion transport for supercapacitors. Int. J. Electrochem. Sci. 2025, 20, 101199. [Google Scholar] [CrossRef]
- Setiawan, K.M.; Humaidi, A.R.; Hidayat, F.; Elsoraya, N.; Hanifah, A.; Alfiah, M.; Steky, F.V.; Milana, P.; Fukutani, K.; Rizal, C.; et al. Synthesis of Polyaniline/Sulfonated Polyaniline (PANI:SPAN) Acid-Base Pair Mimicking PEDOT:PSS Intermolecular Structure as a Hole-Transporting Layer. ACS Appl. Electron. Mater. 2025, 7, c01316. [Google Scholar] [CrossRef]
- Khan, M.; Inamuddin. Electrochemical characterization of recycled polyethylene terephthalate-polyaniline bioanode for enzymatic biofuel cell applications. Electrochim. Acta 2025, 539, 146934. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Guo, C.J.; Ji, A.S.; Zhou, H.L.; Zhou, W.L.; Liu, W.Z.; Wu, T.; Qu, J.P. Economical approach to thermoelectric cooling: Development of conductive polyethylene/polypyrrole@constantan composite using extensional rheological technology. Sci. China Mater. 2025, 68, 4125–4134. [Google Scholar] [CrossRef]
- Hu, C.N.; He, J.; He, Y.T.; Peng, Y.J. Anti-Freezing Conductive Gelatin Hydrogel Reinforced with Polypyrrole-decorated Cellulose Nanofibers for Strain Sensors and Triboelectric Nanogenerators. Chin. J. Polym. Sci. 2025, 43, 2083–2093. [Google Scholar] [CrossRef]
- Tao, S.X.; Yang, Q.; Su, J.; Zhang, N.; Xu, L.H.; Pan, H.; Zhou, T.C.; Zhang, H.J.; Jin, K.L.; Wang, J.P. High-performance flexible conductive films based on bacterial cellulose/polypyrrole: Synergistic effects of polyvinyl alcohol and fluorinated graphene in surface, interface and functional optimization. Colloids Surf. A Physicochem. Eng. Asp. 2025, 724, 137447. [Google Scholar] [CrossRef]
- Hou, S.Y.; Fan, X.Y.; Jin, T.Y.; Li, H.X.; Han, Y.C. A Highly Conductive, Stretchable, and Healable Sensing Electrode Consisting of PEDOT Conductive Pathway and Semi-Interpenetrating Network. Adv. Funct. Mater. 2025, 19643. [Google Scholar] [CrossRef]
- Saghir, S.; Ramirez-Sarabia, J.; Imenes, K.; Schiavone, G. Electropolymerised PEDOT:Polydopamine enables high-performance bioelectrode coatings. Sci. Rep. 2025, 15, s41598. [Google Scholar] [CrossRef]
- Fan, Z.; Pan, X.Y.; Gu, Z.M.; Gao, Y.H.; Gao, X.Y.; Pan, L.J.; Gao, W.J.; Jiang, D.Y. Highly Conductive and Mechanically Robust PEDOT:PSS-Based Fibers: Fabrication Methods, Structure-Property Relations, and Potential Applications. Macromol. Rapid Commun. 2025, 00639. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Zhou, P.C.; Lin, S.H.; Zhong, J.W.; Wang, Y. A Review of Conductive Hydrogel-Based Wearable Temperature Sensors. Adv. Healthc. Mater. 2024, 13, 01503. [Google Scholar] [CrossRef]
- Imani, K.B.C.; Dodda, J.M.; Yoon, J.; Torres, F.G.; Bin Imran, A.; Deen, G.R.; Al-Ansari, R. Seamless Integration of Conducting Hydrogels in Daily Life: From Preparation to Wearable Application. Adv. Sci. 2024, 11, 06784. [Google Scholar] [CrossRef]
- Guo, W.Y.; Ma, M.G. Conductive nanocomposite hydrogels for flexible wearable sensors. J. Mater. Chem. A 2024, 12, 9371–9399. [Google Scholar] [CrossRef]
- He, Q.H.; Cheng, Y.; Deng, Y.J.; Wen, F.; Lai, Y.K.; Li, H.Q. Conductive Hydrogel for Flexible Bioelectronic Device: Current Progress and Future Perspective. Adv. Funct. Mater. 2024, 34, 08974. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.W.; Guo, Z.H.; Pan, C.X.; Pu, X. Multi-crosslinked strong, tough and anti-freezing organohydrogels for flexible sensors. Nanoscale 2025, 17, 1400–1410. [Google Scholar] [CrossRef]
- Guo, R.Y.; Bao, Y.; Zheng, X.; Chen, J.; Zhang, W.B.; Liu, C.; Ma, J.Z. Organohydrogel Based Electronic Skin Reinforced by Dual-Mode Conduction and Hierarchical Collagen Fibers Skeleton. Adv. Sci. 2025, 12, 12934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Lin, Y.; Shen, S.T.; Du, Z.H.; Lin, Z.Q.; Zhou, P.P.; Huang, H.L.; Lyu, X.L.; Zou, Z.G. Intrinsic Anti-Freezing, Tough, and Transparent Hydrogels for Smart Optical and Multi-Modal Sensing Applications. Adv. Mater. 2025, 37, 13856. [Google Scholar] [CrossRef]
- Yu, Y.F.; Wang, S.; Yu, H.T.; Liao, X.J.; Feng, W. Anti-freezing conductive hydrogels with exceptional mechanical properties and stable sensing performance at-30 °C. Mater. Horiz. 2025, 12, 2679–2688. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, H.; Zhang, Y.X.; Yang, J.T.; Chen, Q.; Wu, J.; Liu, Y.L.; Zhao, C.; Tang, Y.J.; Zheng, J. Antifreezing hydrogels: From mechanisms and strategies to applications. Chem. Soc. Rev. 2025, 54, 00718b. [Google Scholar] [CrossRef]
- Huang, J.; Morin, F.J.; Laaser, J.E. Charge-Density-Dominated Phase Behavior and Viscoelasticity of Polyelectrolyte Complex Coacervates. Macromolecules 2019, 52, 4957–4967. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, X.; Cao, Y.X.; Yang, Y.Y.; Yang, Y.T.; Gao, Y.F.; Ma, Z.S.; Wang, J.F.; Wang, W.J.; Wu, D.C. Freezing-Tolerant, Highly Sensitive Strain and Pressure Sensors Assembled from Ionic Conductive Hydrogels with Dynamic Cross-Links. ACS Appl. Mater. Interfaces 2020, 12, 25334–25344. [Google Scholar] [CrossRef]
- Hu, C.X.; Zhang, Y.L.; Wang, X.D.; Xing, L.; Shi, L.Y.; Ran, R. Stable, Strain-Sensitive Conductive Hydrogel with Antifreezing Capability, Remoldability, and Reusability. Acs Appl. Mater. Interfaces 2018, 10, 44000–44010. [Google Scholar] [CrossRef]
- Ye, Y.H.; Zhang, Y.F.; Chen, Y.; Han, X.S.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 03430. [Google Scholar] [CrossRef]
- Sui, X.J.; Guo, H.S.; Chen, P.G.; Zhu, Y.N.; Wen, C.Y.; Gao, Y.H.; Yang, J.; Zhang, X.Y.; Zhang, L. Zwitterionic Osmolyte-Based Hydrogels with Antifreezing Property, High Conductivity, and Stable Flexibility at Subzero Temperature. Adv. Funct. Mater. 2020, 30, 07986. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.X.; Lu, X.; Han, S.J.; Yang, B.R.; Gui, X.C.; Tao, K.; Miao, J.M.; Liu, C. Ultrastretchable and Stable Strain Sensors Based on Antifreezing and Self-Healing Ionic Organohydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 9405–9414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.Q.; Zhang, J.; Hou, L.X.; Ye, D.Z.; Jiang, X.C. Preparation of tough and anti-freezing hybrid double-network carboxymethyl chitosan/poly(acrylic amide) hydrogel and its application for flexible strain sensor. J. Mater. Sci. 2022, 57, 19666–19680. [Google Scholar] [CrossRef]
- Chen, D.W.; Bai, H.Y.; Zhu, H.Y.; Zhang, S.W.; Wang, W.; Dong, W.F. Anti-freezing, tough, and stretchable ionic conductive hydrogel with multi-crosslinked double-network for a flexible strain sensor. Chem. Eng. J. 2024, 480, 148192. [Google Scholar] [CrossRef]
| References | Temperature (°C) | Conductivity (S/m) | GF at Strain Range |
|---|---|---|---|
| This work | −20 | 2.2 | 1.4 at 0–100% |
| This work | −20 | 2.2 | 4.7 at 100–500% |
| This work | −20 | 2.2 | 7.4 at 500–1000% |
| Ref. [40] | −20 | 0.01 | 3.9 at 0–280% |
| Ref. [41] | −20 | 0.32 | - |
| Ref. [42] | −70 | 1.1 | 1.2 at 120% |
| Ref. [43] | −40 | 2.7 | - |
| Ref. [44] | −18 | 1.9 | 1.9 at 0–200% |
| Ref. [45] | −20 | 0.1 | 1.5 at 0–150% |
| Ref. [46] | −30 | 0.32 | 0.6 at 0–100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yu, W.; Liu, S. An Anti-Freezing Ionic Conductive Hydrogel for Strain Sensing and Energy Harvesting Devices. Polymers 2025, 17, 3102. https://doi.org/10.3390/polym17233102
Wang Y, Yu W, Liu S. An Anti-Freezing Ionic Conductive Hydrogel for Strain Sensing and Energy Harvesting Devices. Polymers. 2025; 17(23):3102. https://doi.org/10.3390/polym17233102
Chicago/Turabian StyleWang, Yanjie, Wei Yu, and Sijun Liu. 2025. "An Anti-Freezing Ionic Conductive Hydrogel for Strain Sensing and Energy Harvesting Devices" Polymers 17, no. 23: 3102. https://doi.org/10.3390/polym17233102
APA StyleWang, Y., Yu, W., & Liu, S. (2025). An Anti-Freezing Ionic Conductive Hydrogel for Strain Sensing and Energy Harvesting Devices. Polymers, 17(23), 3102. https://doi.org/10.3390/polym17233102








