Degradation Mechanism of Perfluoroelastomer (FFKM) in the Acidic SC2 Solution of Semiconductor Radio Corporation of America (RCA) Cleaning
Abstract
1. Introduction
2. Materials and Methods
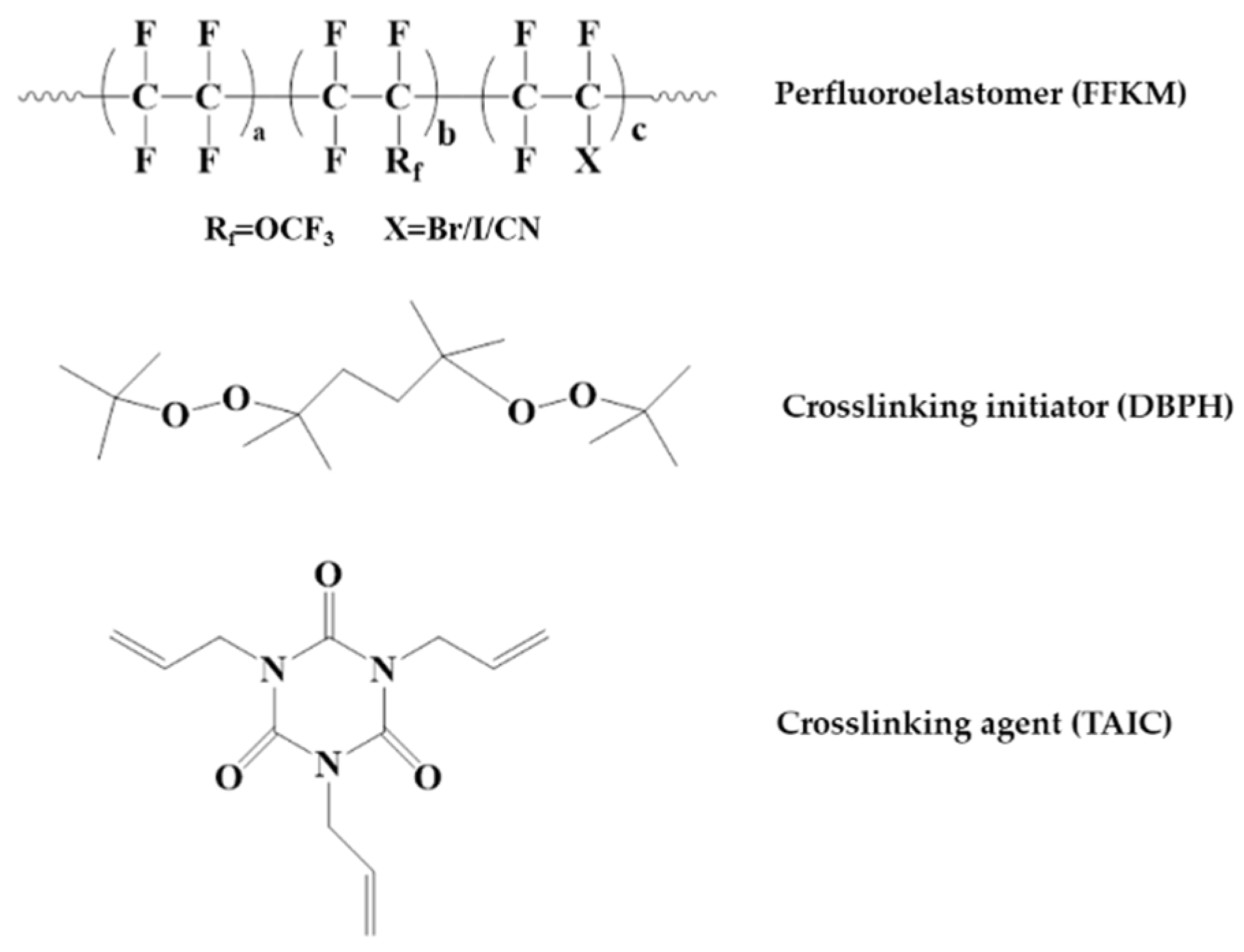
2.1. Sample Preparation
2.2. Degradation Test
2.3. Physical and Chemical Characterization
2.3.1. Mass
2.3.2. Micro-Structure
2.3.3. ATR-FTIR
2.3.4. XPS
2.3.5. TGA
2.3.6. TGA-FTIR
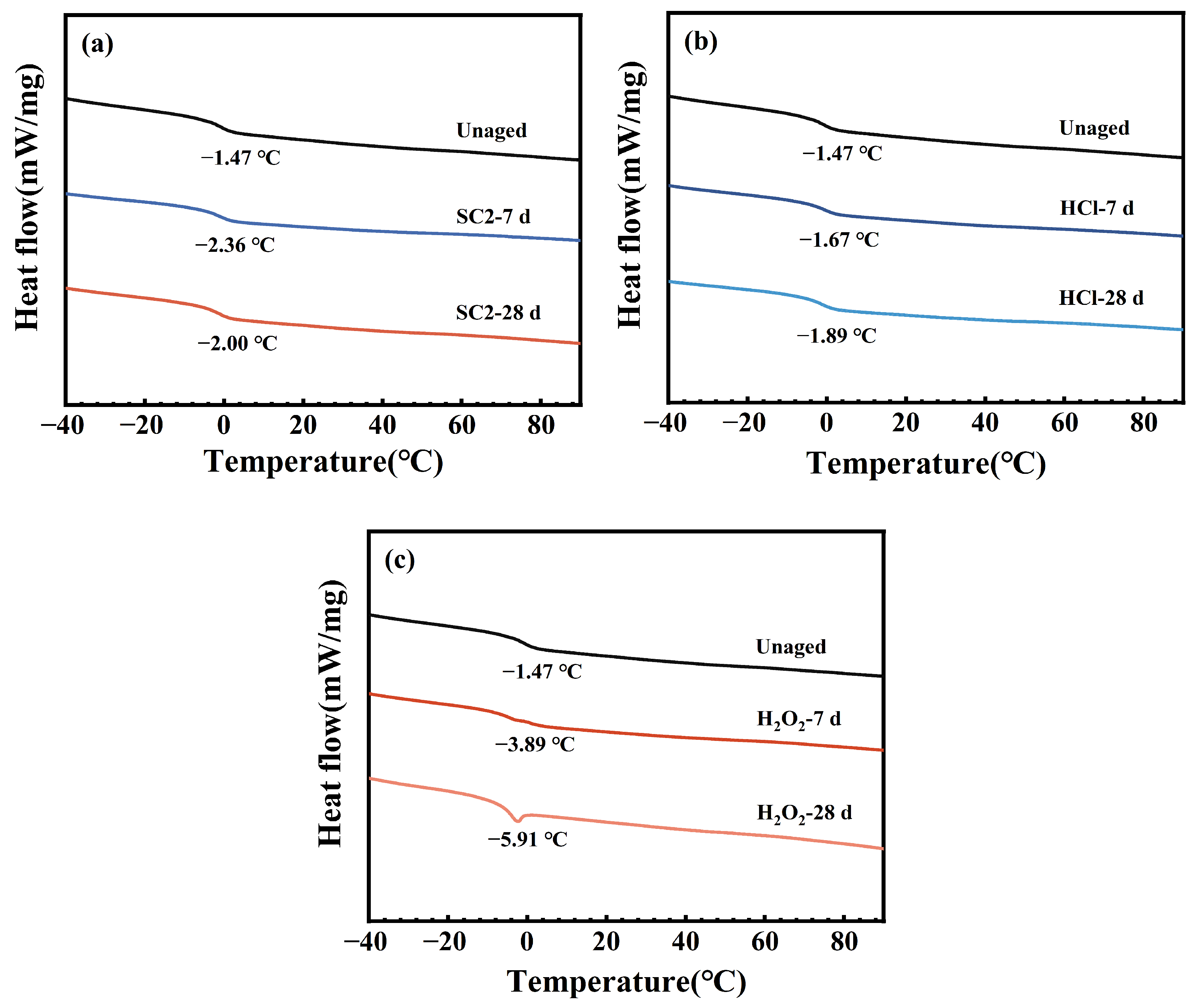
2.3.7. DSC
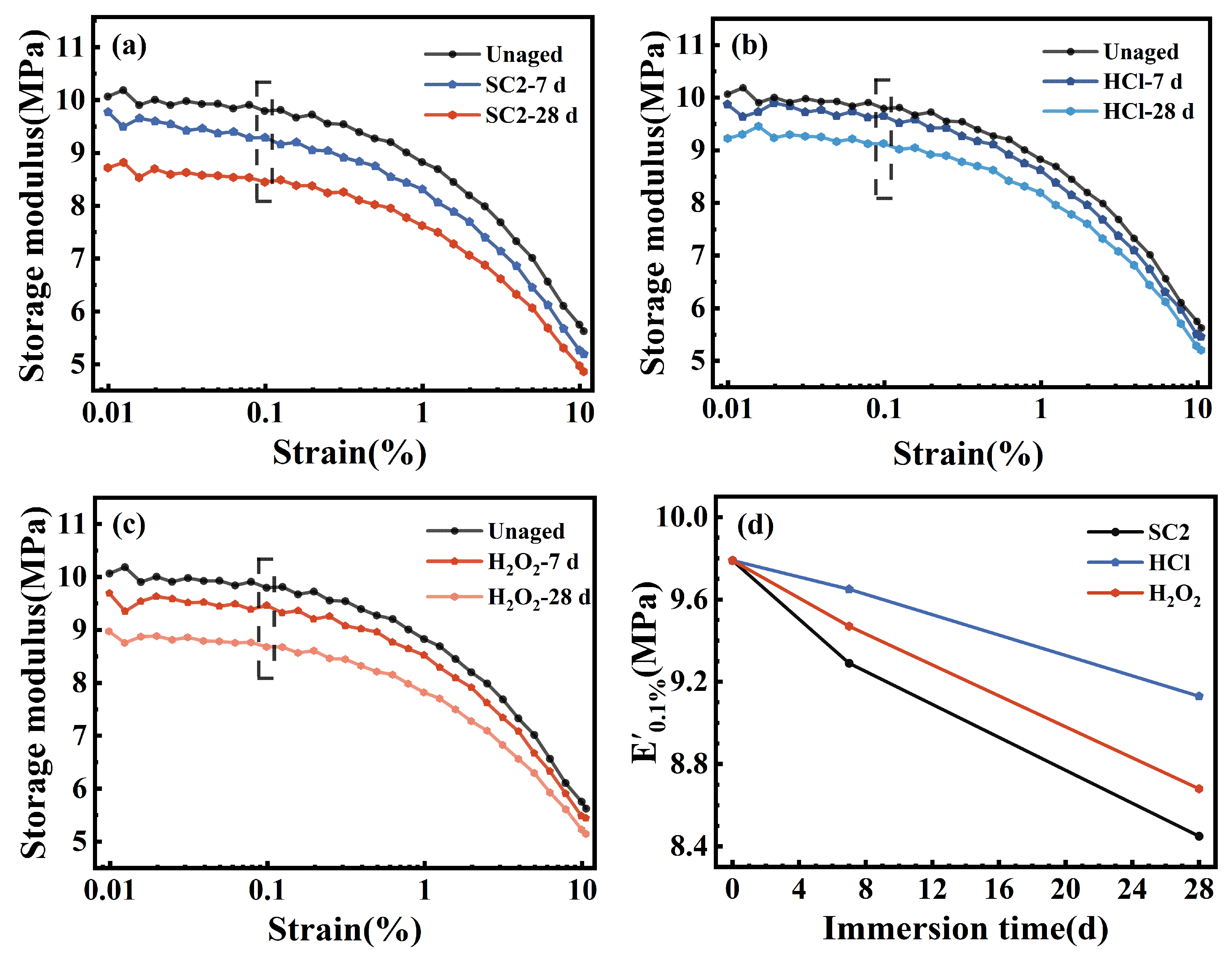
2.3.8. DMA
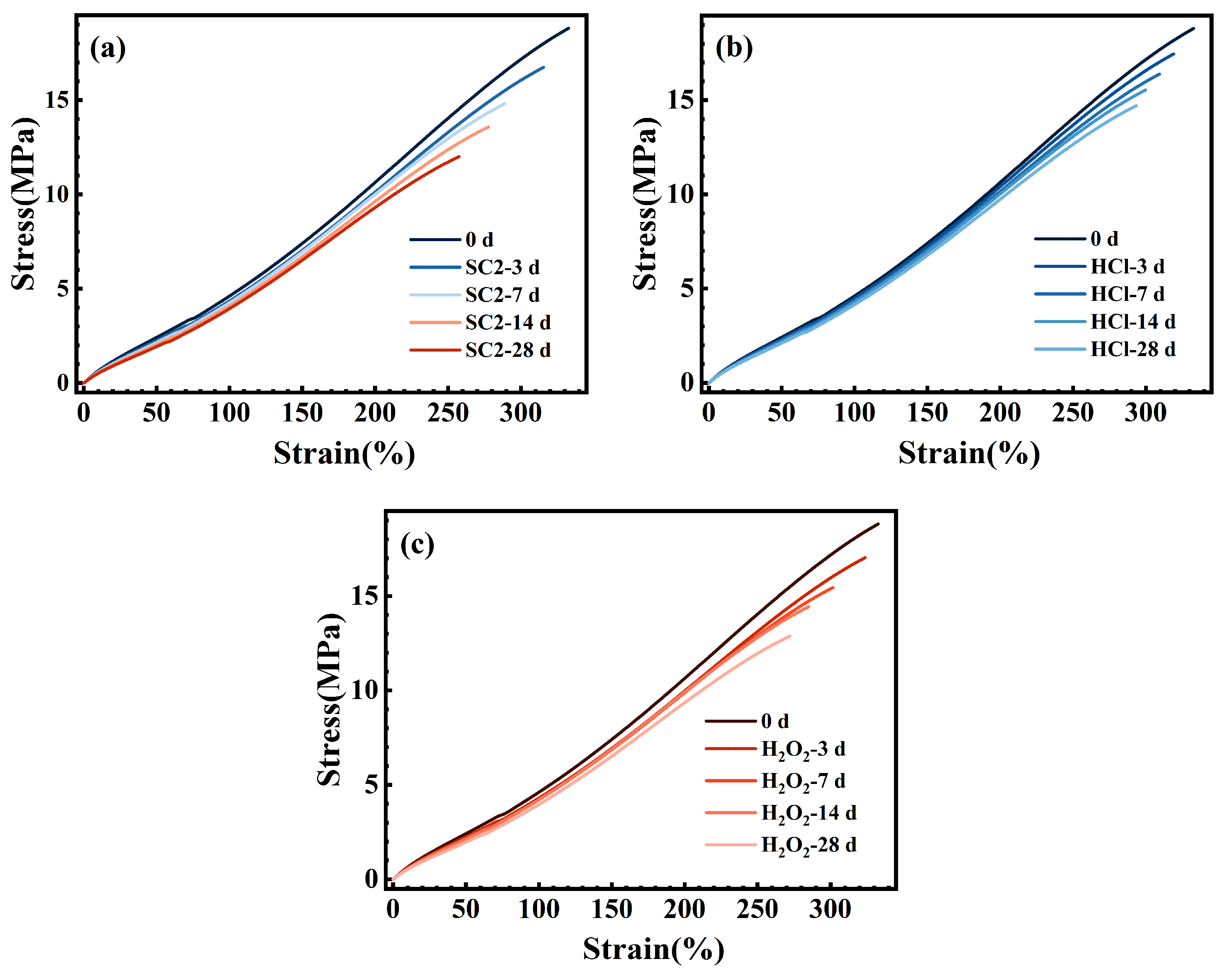
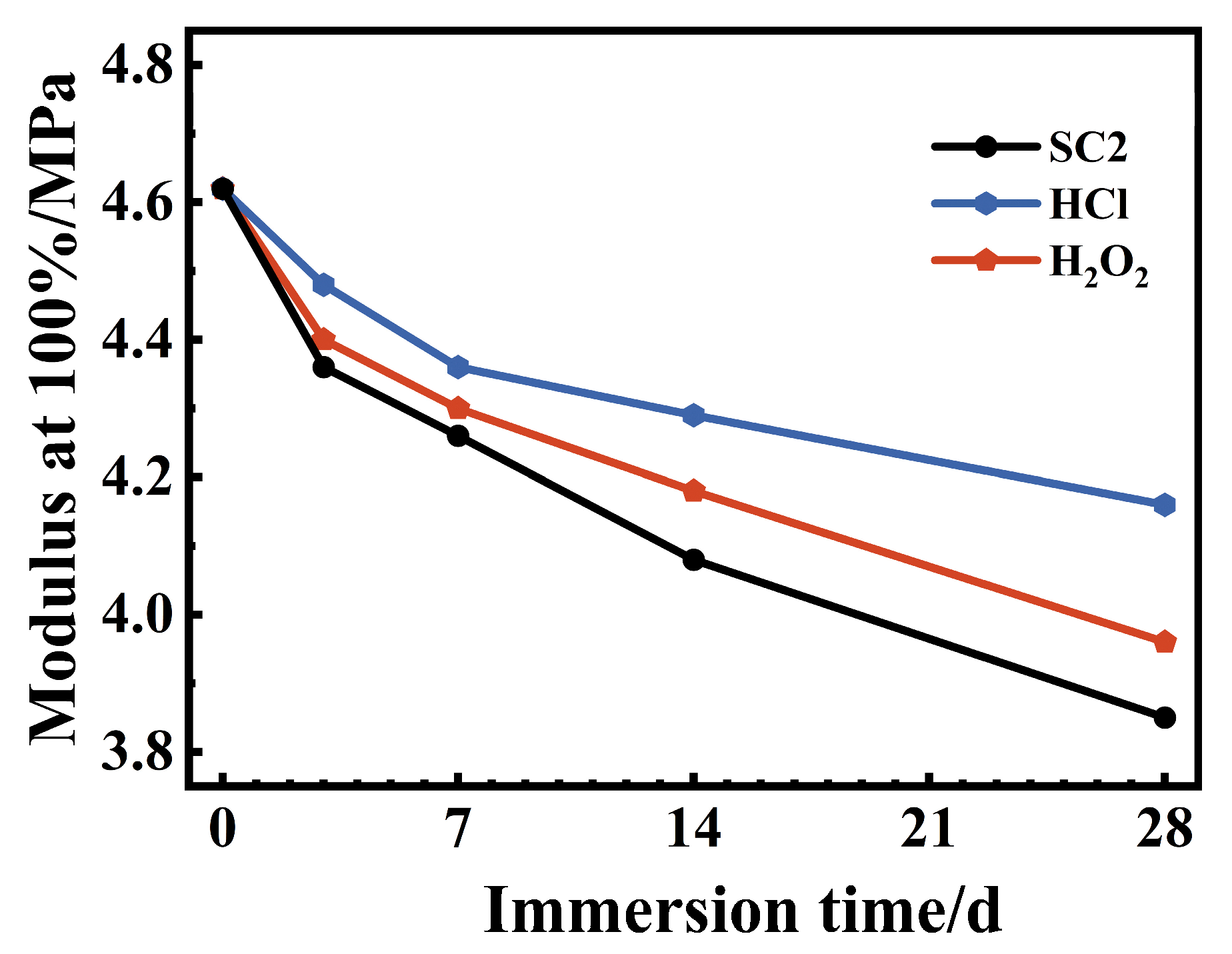
2.3.9. Tensile Test
3. Results
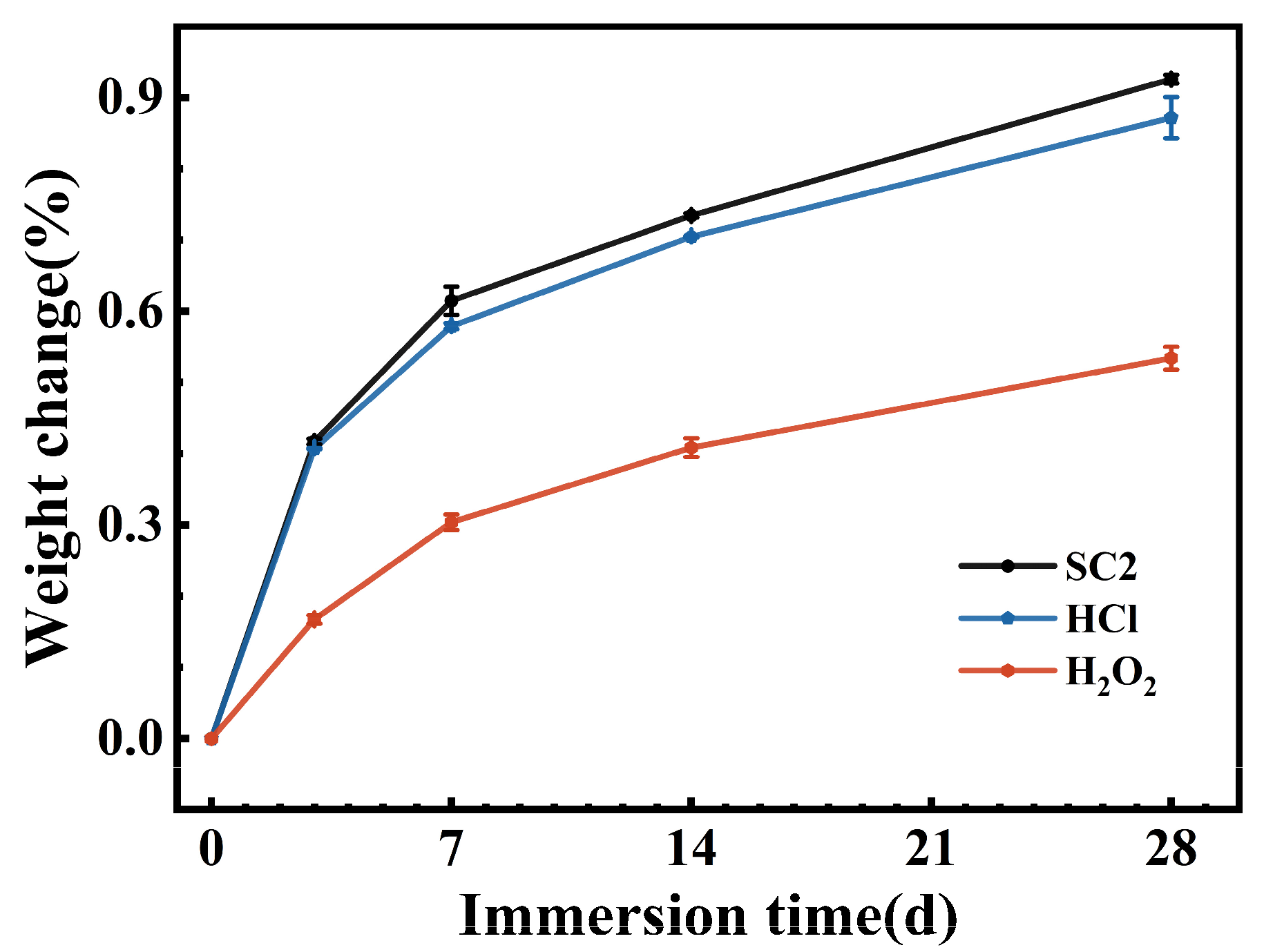
3.1. Mass Test
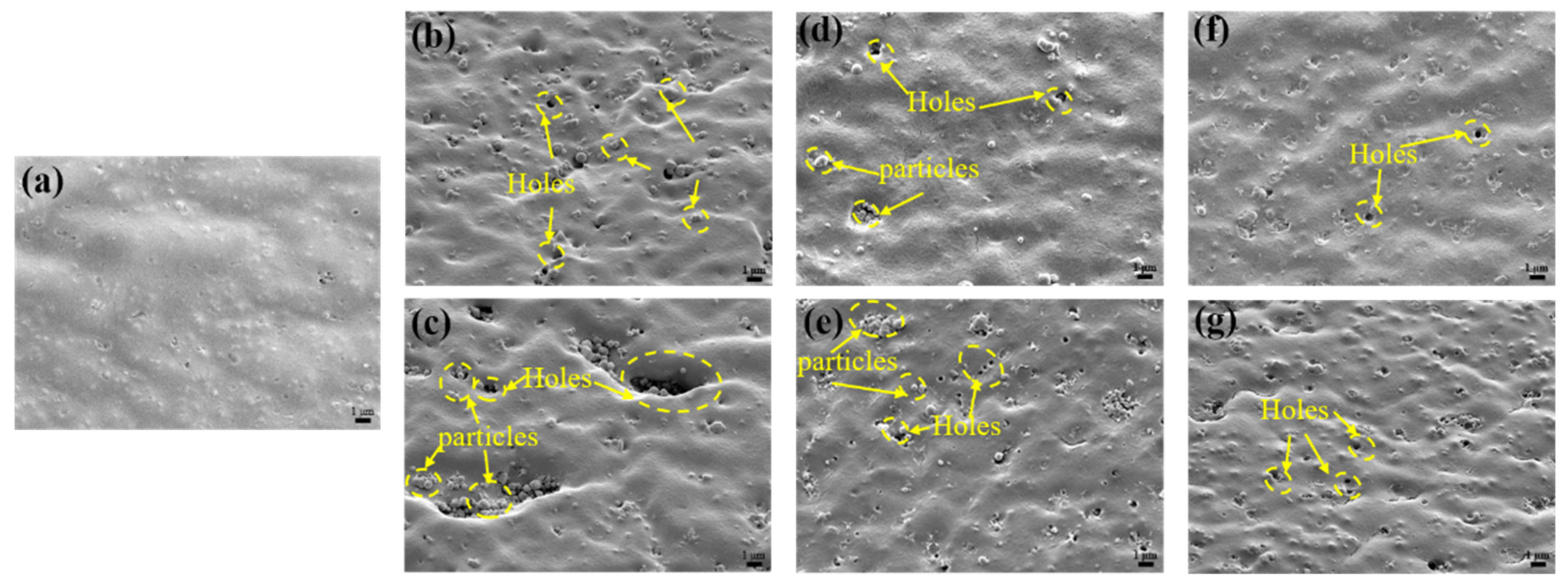
3.2. Microscopic Morphology
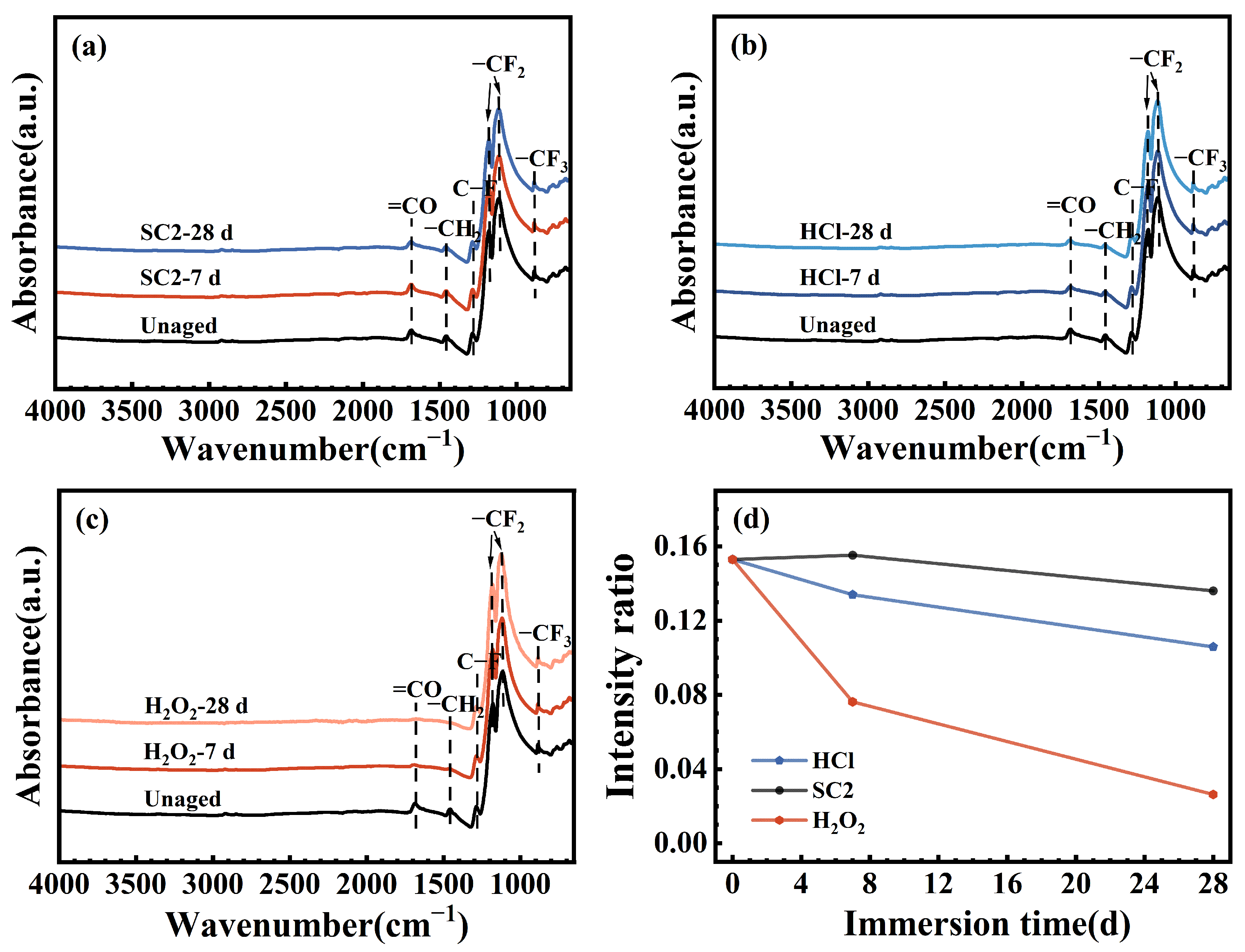
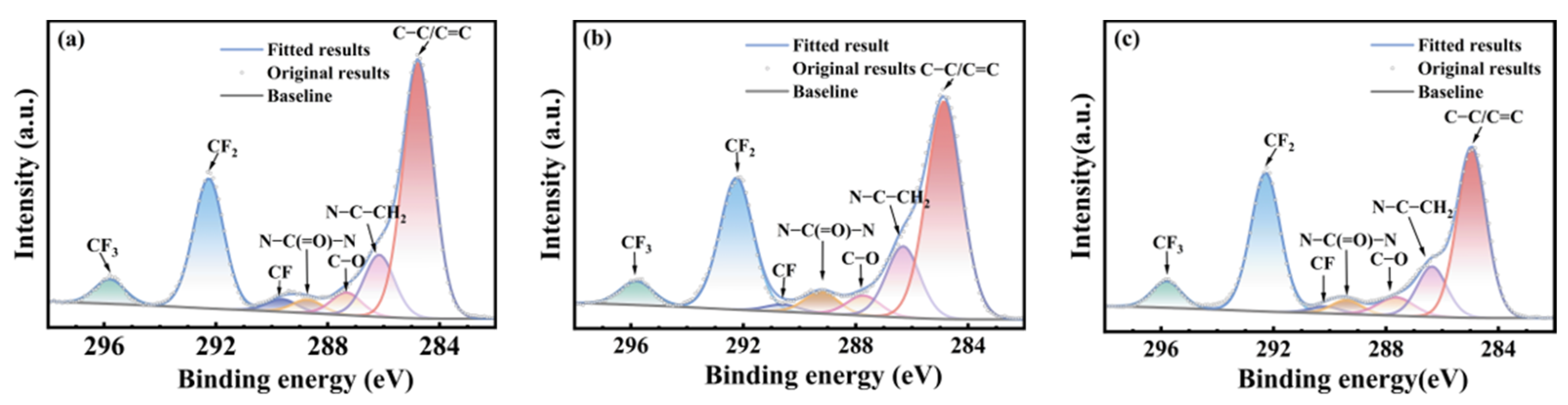
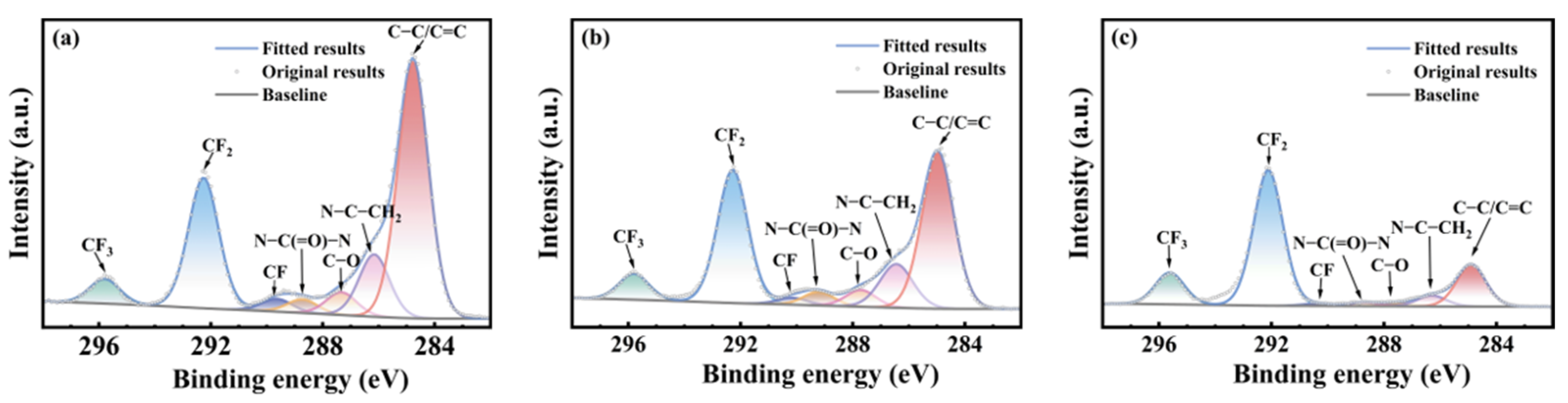
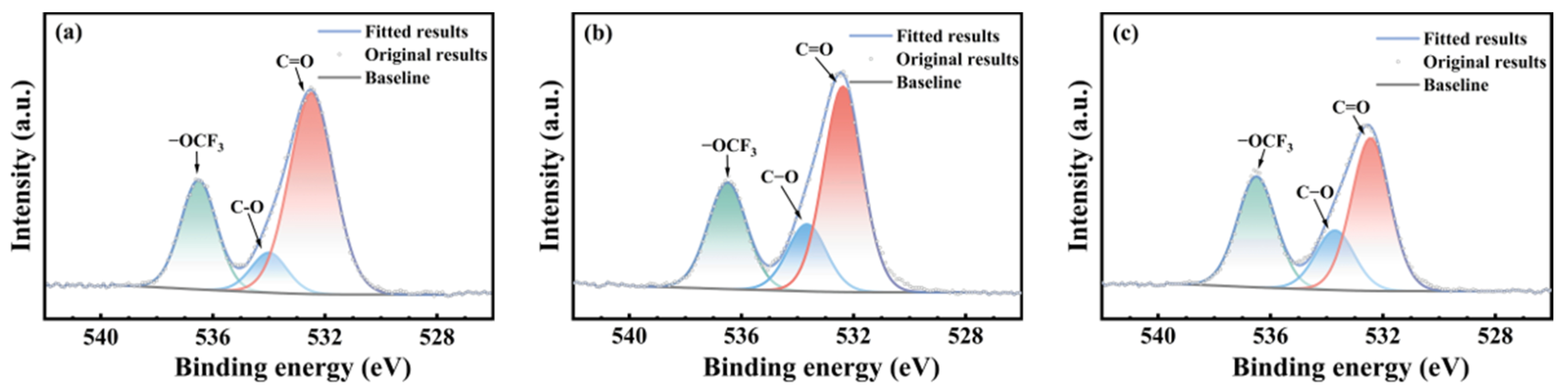
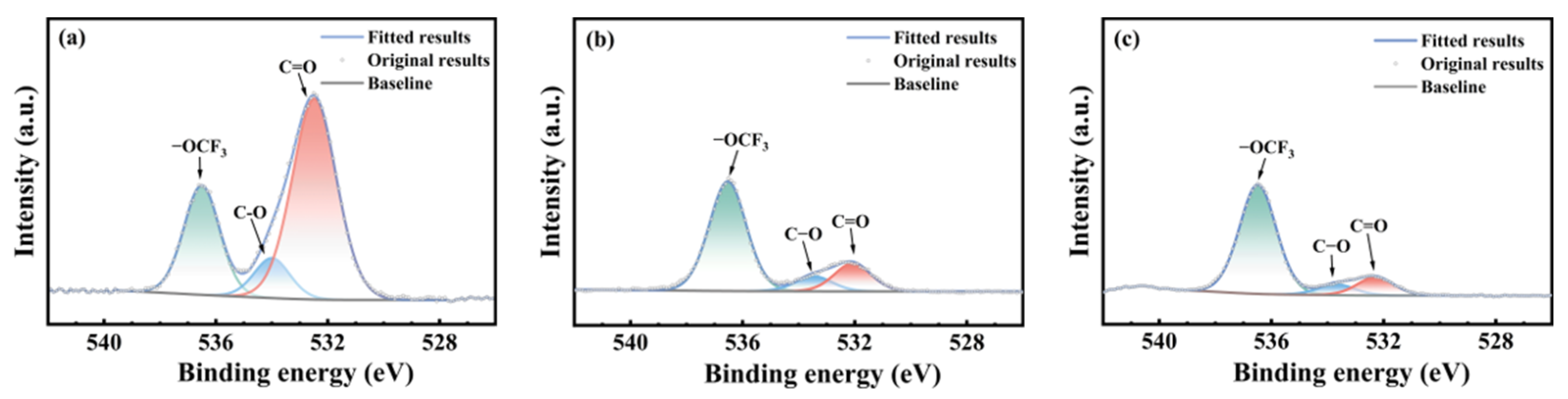
3.3. Chemical Structural Evolution
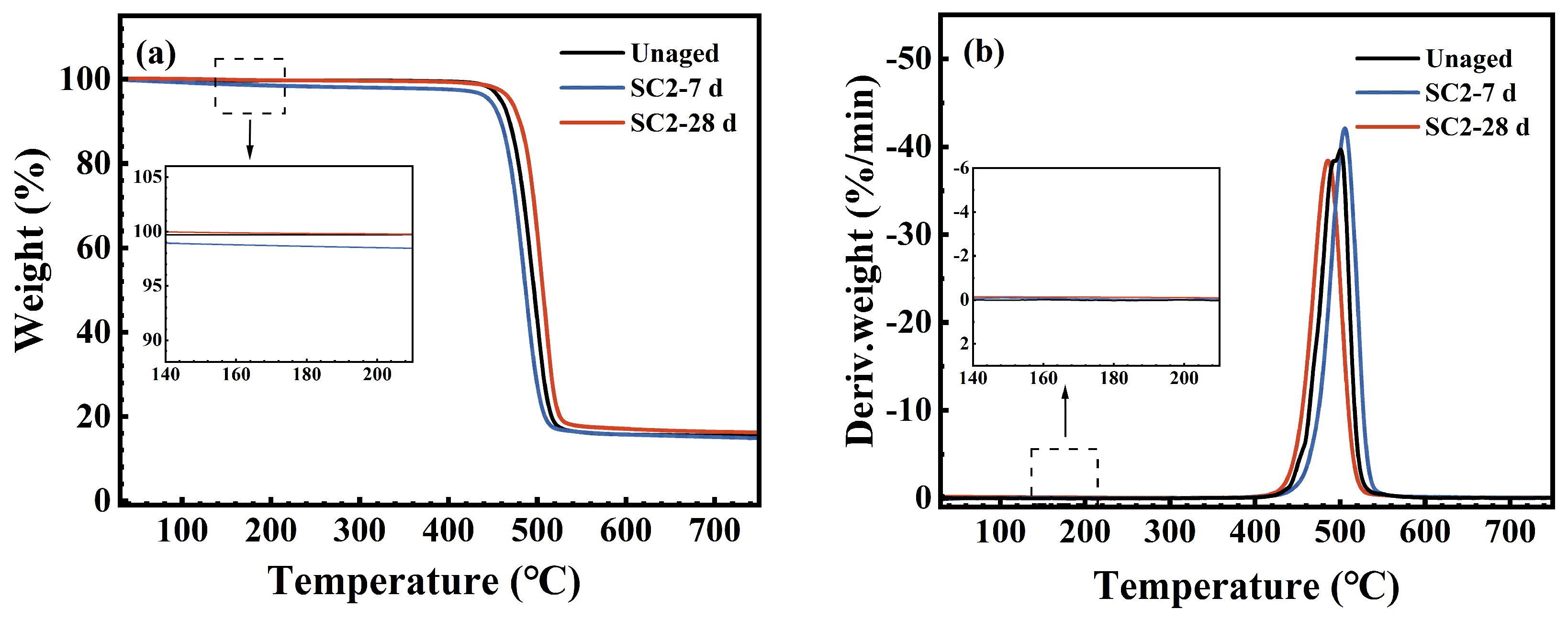
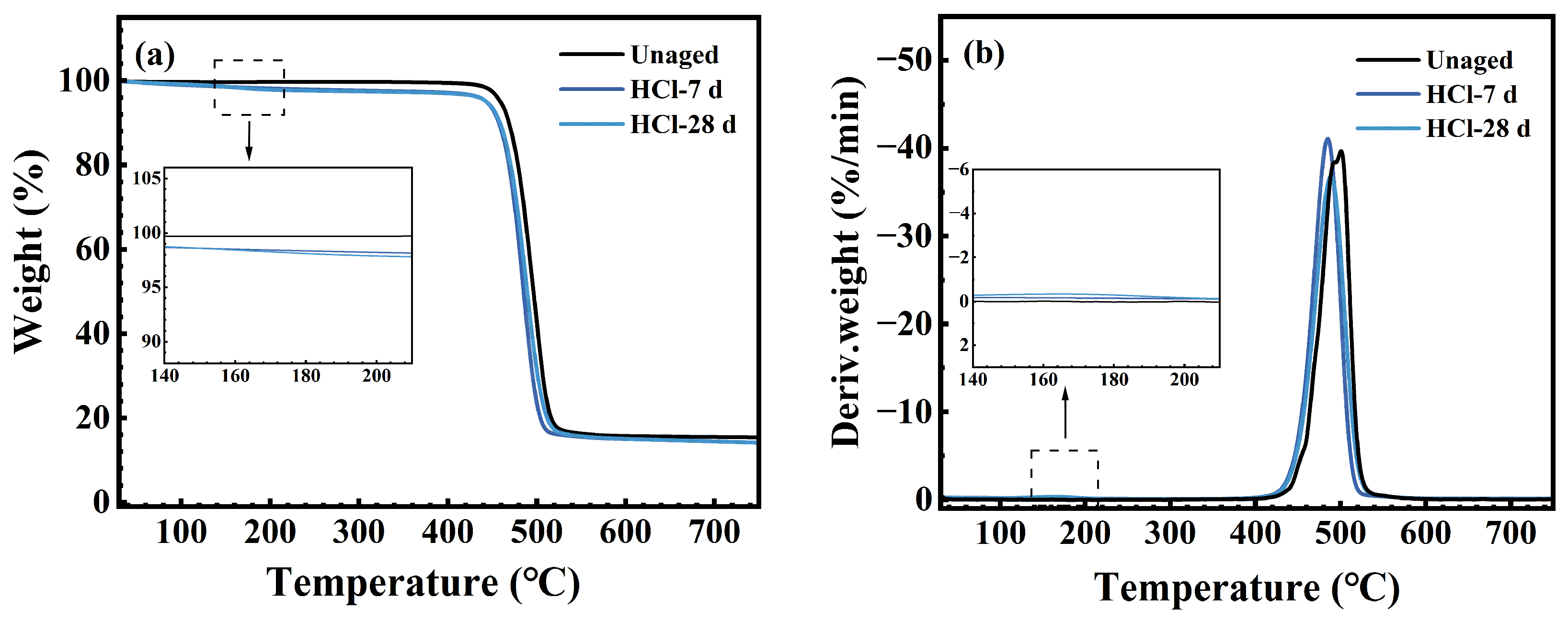
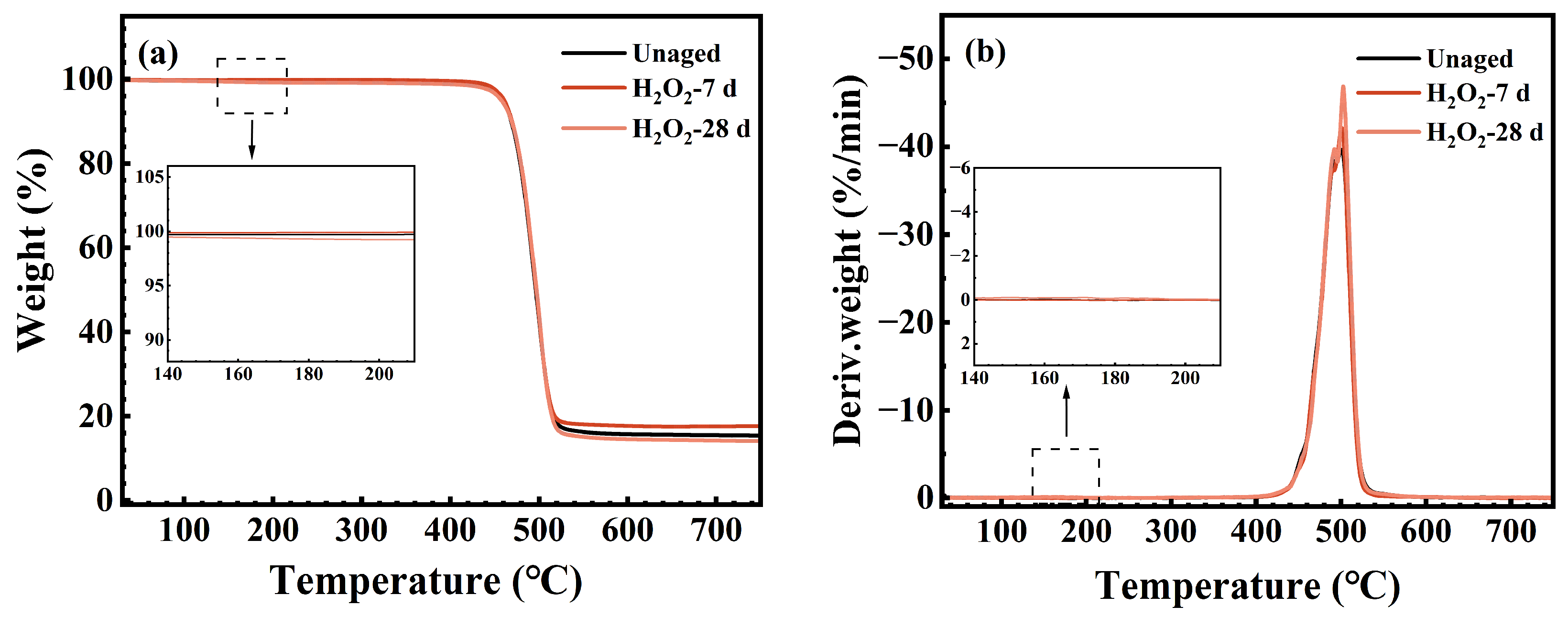
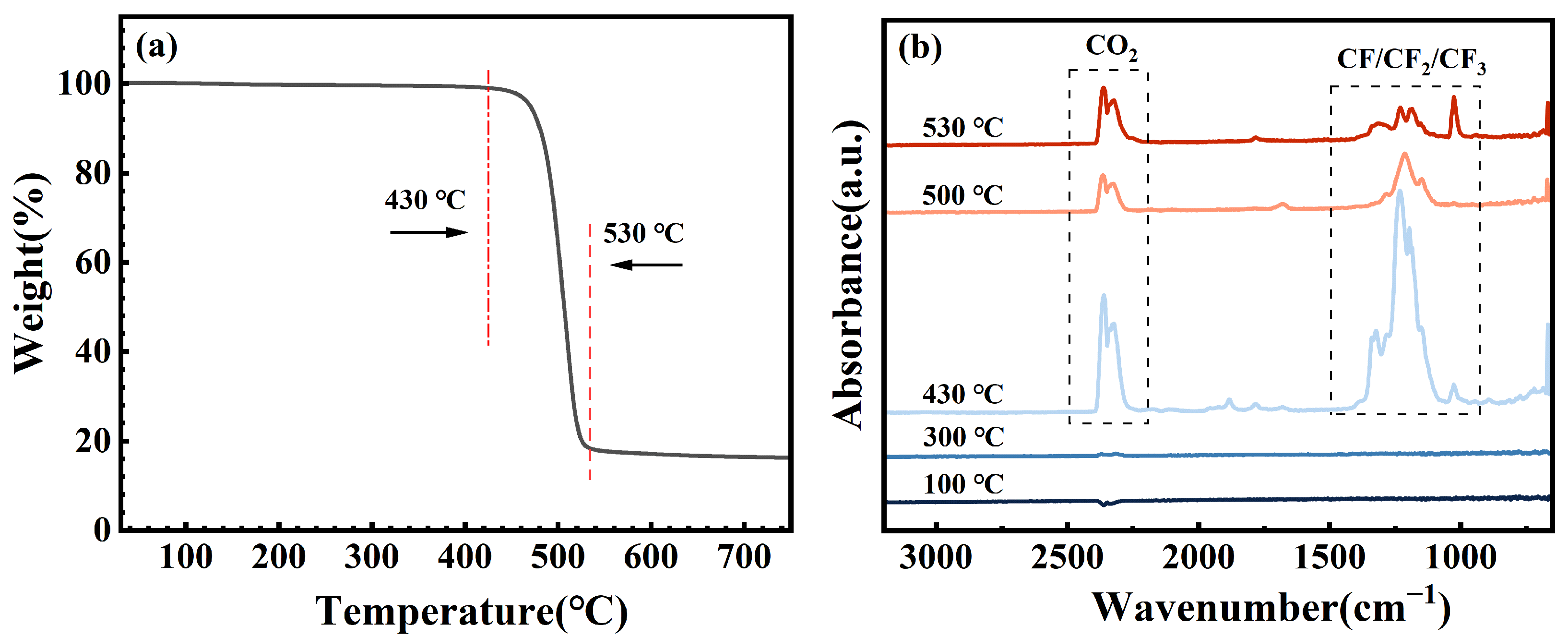
3.4. Thermal Stability
3.5. Cross-Linked Network
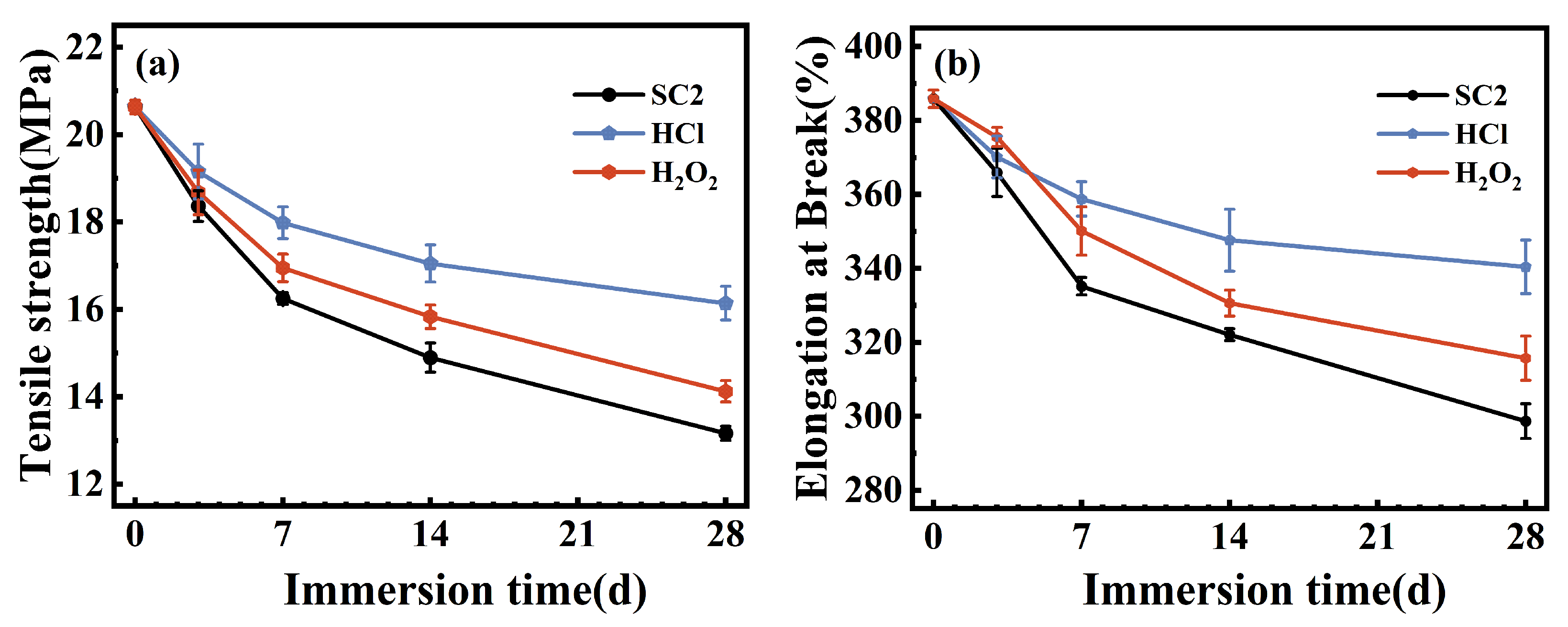
3.6. Mechanical Properties
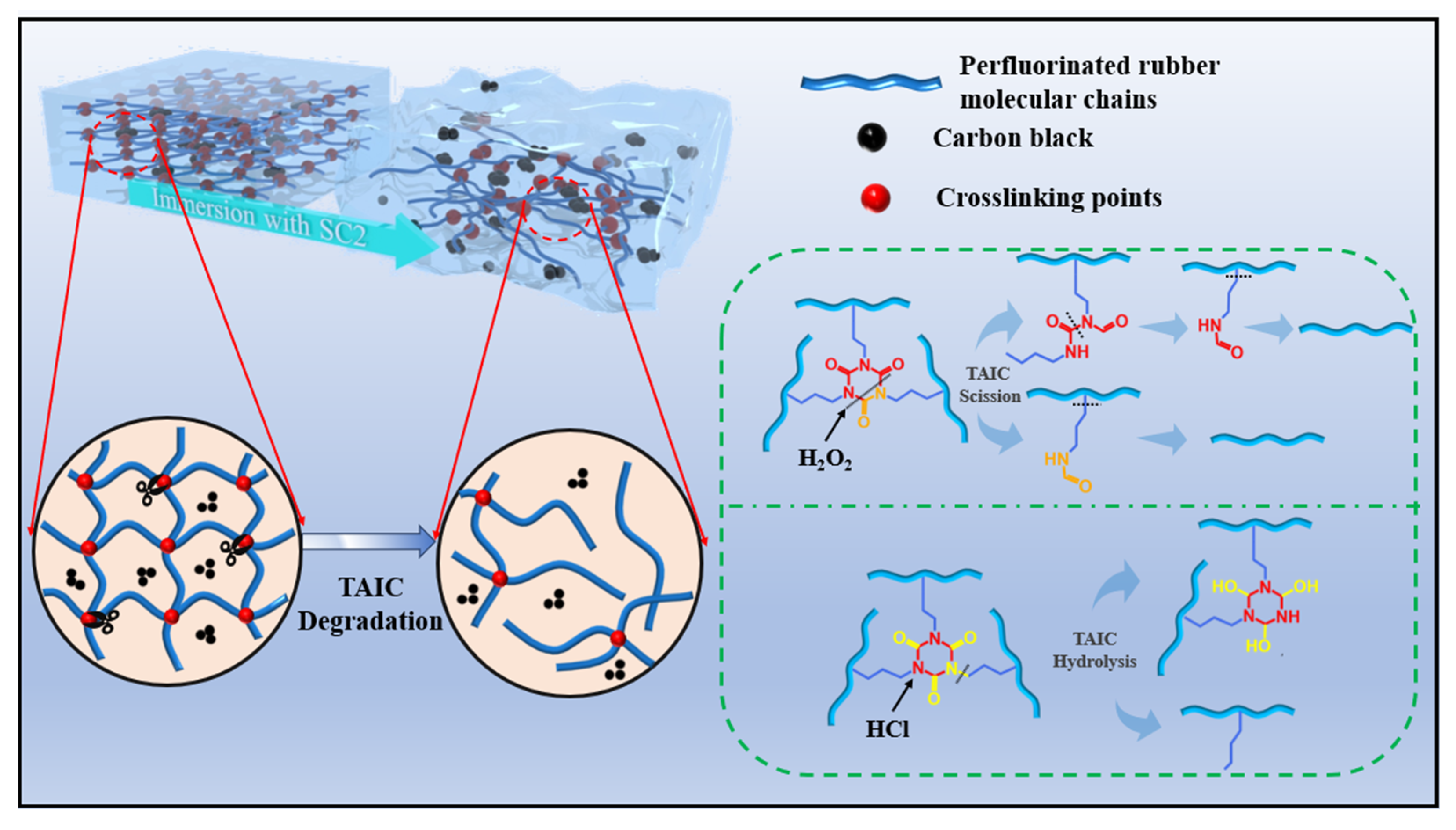
3.7. Degradation Mechanisms of FFKM in Acidic SC2 Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Periyasamy, M.; Quartapella, C.J.; Piacente, N.P.; Reichl, G.; Lynn, B. Smart Quantum Tunneling Composite Sensors to Monitor FKM and FFKM Seals. Sensors 2023, 23, 1342. [Google Scholar] [CrossRef] [PubMed]
- Paillard, J.; Dunn, T.; Durn, F.; Astbury, A.; Gulcur, M. Predicting Seal Longevity: Advanced Simulations for Plasma and Rotary Applications in Semiconductor Processes: EO: Equipment Optimization. In Proceedings of the 2025 36th Annual SEMI Advanced Semiconductor Manufacturing Conference (ASMC), Albany, NY, USA, 5–8 May 2025; pp. 1–5. [Google Scholar]
- Wang, S.; Legare, J.M. Perfluoroelastomer and fluoroelastomer seals for semiconductor wafer processing equipment. J. Fluor. Chem. 2003, 122, 113–119. [Google Scholar] [CrossRef]
- Atkinson, S. Essential high-purity sealing materials for semiconductor manufacturing. Seal. Technol. 2018, 2018, 5–7. [Google Scholar] [CrossRef]
- Améduri, B.; Boutevin, B.; Kostov, G. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Dai, C.Q.; Chen, P.Q.; Qi, S.C.; Hu, Y.B.; Song, Z.T.; Dai, M.Z. Ultrathin flexible InGaZnO transistor for implementing multiple functions with a very small circuit footprint. Nano Res. 2021, 14, 2469. [Google Scholar] [CrossRef]
- Stern, C.; Schwab, C.; Kindelmann, M.; Stamminger, M.; Weirich, T.E.; Park, I.; Hausen, F.; Finsterbusch, M.; Bram, M.; Guillon, O. Correlative characterization of plasma etching resistance of various aluminum garnets. J. Am. Ceram. Soc. 2024, 107, 7105–7118. [Google Scholar] [CrossRef]
- Osburn, C.; Berger, H.; Donovan, R.; Jones, G. The Effects of Contamination on Semiconductor Manufacturing Yield. J. IEST 2006, 31, 45–57. [Google Scholar] [CrossRef]
- Connolly, N.; Hysick, M.; Barlaz, D.E.; Garza, R.; Lunardi, G.; Ruzic, D.N. Characterization of elastomer degradation in O2/Ar plasma via mass and surface morphology changes. J. Vac. Sci. Technol. A 2024, 42, 023004. [Google Scholar] [CrossRef]
- Legare, J.M.; Wang, S.; Vigliotti, M.; Sogo, S. Contamination Considerations for Perfluoroelastomer Seals Used in Deposition Processes. In Proceedings of the 2008 IEEE/SEMI Advanced Semiconductor Manufacturing Conference, Cambridge, MA, USA, 5–7 May 2008; pp. 297–300. [Google Scholar]
- DuPont Performance, P. Outgassing Characterization of Elastomeric Seals Used in Semiconductor Wafer Processing. In Proceedings of the AVS Symposium, Wilmington, DE, USA, 9–13 November 2009. [Google Scholar]
- The Impact of a Potential PFAS Restriction on the Semiconductor Sector; RINA Tech UK Ltd.: London, UK, 2023.
- Puga, E.V.; Wachtendorf, V.; Kmmling, A.; Brendelberger, S.; Jaunich, M.; Sattler, C. Lifetime prediction and degradation assessment of FKM and FFKM O-rings under high temperature thermo-oxidative ageing. Polym. Test. 2025, 147, 108820. [Google Scholar] [CrossRef]
- Zhuo, W.-Y.; Wang, Q.-L.; Li, G.; Yang, G.; Zhang, H.; Xu, W.; Niu, Y.-H.; Li, G.-X. Detection of the Destruction Mechanism of Perfluorinated Elastomer (FFKM) Network under Thermo-oxidative Aging Conditions. Chin. J. Polym. Sci. 2022, 40, 504–514. [Google Scholar] [CrossRef]
- Goto, T.; Obara, S.; Shimizu, T.; Inagaki, T.; Shirai, Y.; Sugawa, S. Study on CF4/O2 plasma resistance of O-ring elastomer materials. J. Vac. Sci. Technol. A Vac. Surf. Film. 2020, 38, 013002. [Google Scholar] [CrossRef]
- Sugama, T.; Toshifumi, S.; Tatiana, P.; Tatiana, P.; Erica Marie, R.; Erica Marie, R.; James, R.M.; James, R.M.; Douglas, A.B.; Douglas, A.B. Degradation of different elastomeric polymers in simulated geothermal environments at 300 °C. Polym. Degrad. Stab. 2015, 120, 328–339. [Google Scholar] [CrossRef]
- Heller, M.; Legare, J.; Wang, S.; Fukuhara, S. Thermal stability and sealing performance of perfluoroelastomer seals as a function of crosslinking chemistry. J. Vac. Sci. Technol. A Vac. Surf. Film. 1999, 17, 2119–2124. [Google Scholar] [CrossRef]
- DuPont. DuPont™ Kalrez® Perfluoroelastomer Parts in Semiconductor Industry—Thermal Processes: Semicon Product Selector Guide (KZE-A40112-00-A0422, Final Rev.); DuPont: Wilmington, DE, USA, 2022. [Google Scholar]
- DuPont. DuPont™ Kalrez® Perfluoroelastomer Parts in Semiconductor Industry—Plasma Processes: Semicon Product Selector Guide (KZE-A40111-00-A0422, Final Rev.); DuPont: Wilmington, DE, USA, 2022. [Google Scholar]
- Kern, W.; Puotinen, D.A. Cleaning Solutions Based on Hydrogen Peroxide for Use in Silicon Semiconductor Technology. RCA Rev. 1970, 31, 187–206. [Google Scholar]
- Celler, G.K.; Barr, D.L.; Rosamilia, J.M. Etching of silicon by the RCA Standard Clean 1. Electrochem. Solid State Lett. 2000, 3, 47–49. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, F.; Zhang, T.; Liu, L. Aging behavior of perfluorinated elastomer (FFKM) in the SC1 solution used in the semiconductor wet etching process. Polym. Degrad. Stab. 2025, 240, 111454. [Google Scholar] [CrossRef]
- Han, W.; Du, H.; Li, S.; Kang, H.; Fang, Q. Mechanical properties and creep behavior of fluoroelastomer under hydrochloric acid environments. Polym. Bull. 2020, 77, 5967–5983. [Google Scholar] [CrossRef]
- Bayerstadler, A. Reinigung und Gasphasenepitaxie in einem Ultrahochvakuum-Mehrkammersystem für Zukünftige CMOS-Technologien; Cuvillier Verlag: Göttingen, Germany, 2006; pp. 25–26. [Google Scholar]
- ASTM D412-16(2021); Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension. ASTM International: West Conshohocken, PA, USA, 2021.
- Saville, B.; Watson, A.A. Structural Characterization of Sulfur-Vulcanized Rubber Networks. Rubber Chem. Technol. 1967, 40, 100–148. [Google Scholar] [CrossRef]
- Hagen, R.; Salmén, L.; Stenberg, B. Effects of the type of crosslink on viscoelastic properties of natural rubber. J. Polym. Sci. Part B Polym. Phys. 2015, 34, 1997–2006. [Google Scholar] [CrossRef]
- Guth, E. Theory of Filler Reinforcement. J. Appl. Phys. 1945, 16, 20–25. [Google Scholar] [CrossRef]
- Douglas, J.F.; Horkay, F. Influence of swelling on the elasticity of polymer networks cross-linked in the melt state: Test of the localization model of rubber elasticity. J. Chem. Phys. 2024, 160, 12. [Google Scholar] [CrossRef]
- Surve, K.; Cai, M.; Yun, J.; Zolfaghari, A.; Krishnamoorti, R.; Bhowmick, A.K. Effect of extreme environments on aging of fluoroelastomers. Polym. Degrad. Stab. 2024, 227, 16. [Google Scholar] [CrossRef]
- Piwowarczyk, J.; Jdrzejewski, R.; Moszyński, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR Studies of Polytetrafluoroethylene Thin Films Obtained by Physical Methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Thermo Fisher, S. XPS Reference Table of Elements: Carbon (C1s) and Oxygen (O1s). Available online: https://www.thermofisher.com/ch/en/home/materials-science/learning-center/periodic-table.html (accessed on 15 May 2025).
- Fengyi, H.; Fengyi, H.; Yihu, S.; Yihu, S.; Qiang, Z.; Qiang, Z. Payne effect of thermo-oxidatively aged isoprene rubber vulcanizates. Polymer 2020, 195, 122432. [Google Scholar] [CrossRef]
- Parker Hannifin, C. ORD 5700: O-Ring Handbook; Parker Hannifin Corporation, O-Ring Division: Cleveland, OH, USA, 2012. [Google Scholar]
- Parker Hannifin, C. O-Ring Guide; Parker Hannifin Corporation, O-Ring Division: Cleveland, OH, USA, 2021. [Google Scholar]
- DuPont. Seal Design Considerations Using Kalrez® Perfluoroelastomer Parts; DuPont Electronics & Industrial: Wilmington, DE, USA, 2021. [Google Scholar]
- Simon, A.; Pepin, J.; Berthier, D.; Méo, S. Degradation mechanism of FKM during thermo-oxidative aging from mechanical and network structure correlations. Polym. Degrad. Stab. 2023, 208, 110271. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, Y.; Su, Y.; Hu, S.; Shi, Y.; Shi, X. Thermal oxidative aging behavior and lifetime prediction of fluoroether rubber. Polym. Degrad. Stab. 2025, 232, 111106. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Wen, X.; Chen, Q.; Zhang, T.; Liu, L. Degradation Mechanism of Perfluoroelastomer (FFKM) in the Acidic SC2 Solution of Semiconductor Radio Corporation of America (RCA) Cleaning. Polymers 2025, 17, 3081. https://doi.org/10.3390/polym17223081
Meng F, Wen X, Chen Q, Zhang T, Liu L. Degradation Mechanism of Perfluoroelastomer (FFKM) in the Acidic SC2 Solution of Semiconductor Radio Corporation of America (RCA) Cleaning. Polymers. 2025; 17(22):3081. https://doi.org/10.3390/polym17223081
Chicago/Turabian StyleMeng, Fandi, Xiaolong Wen, Qishan Chen, Tao Zhang, and Li Liu. 2025. "Degradation Mechanism of Perfluoroelastomer (FFKM) in the Acidic SC2 Solution of Semiconductor Radio Corporation of America (RCA) Cleaning" Polymers 17, no. 22: 3081. https://doi.org/10.3390/polym17223081
APA StyleMeng, F., Wen, X., Chen, Q., Zhang, T., & Liu, L. (2025). Degradation Mechanism of Perfluoroelastomer (FFKM) in the Acidic SC2 Solution of Semiconductor Radio Corporation of America (RCA) Cleaning. Polymers, 17(22), 3081. https://doi.org/10.3390/polym17223081






