Innovative Carbon Black Replacement in Rubber Compound: Impact of Pyrolytic Carbon Black and Energy-Gypsum By-Products on Vulcanization and Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Rubber Compound Preparation
2.2. Vulcanization Parameters and Process
2.3. Payne Effect
2.4. Hardness Determination
2.5. Tensile Properties
2.6. Morphology Analysis
3. Results and Discussion
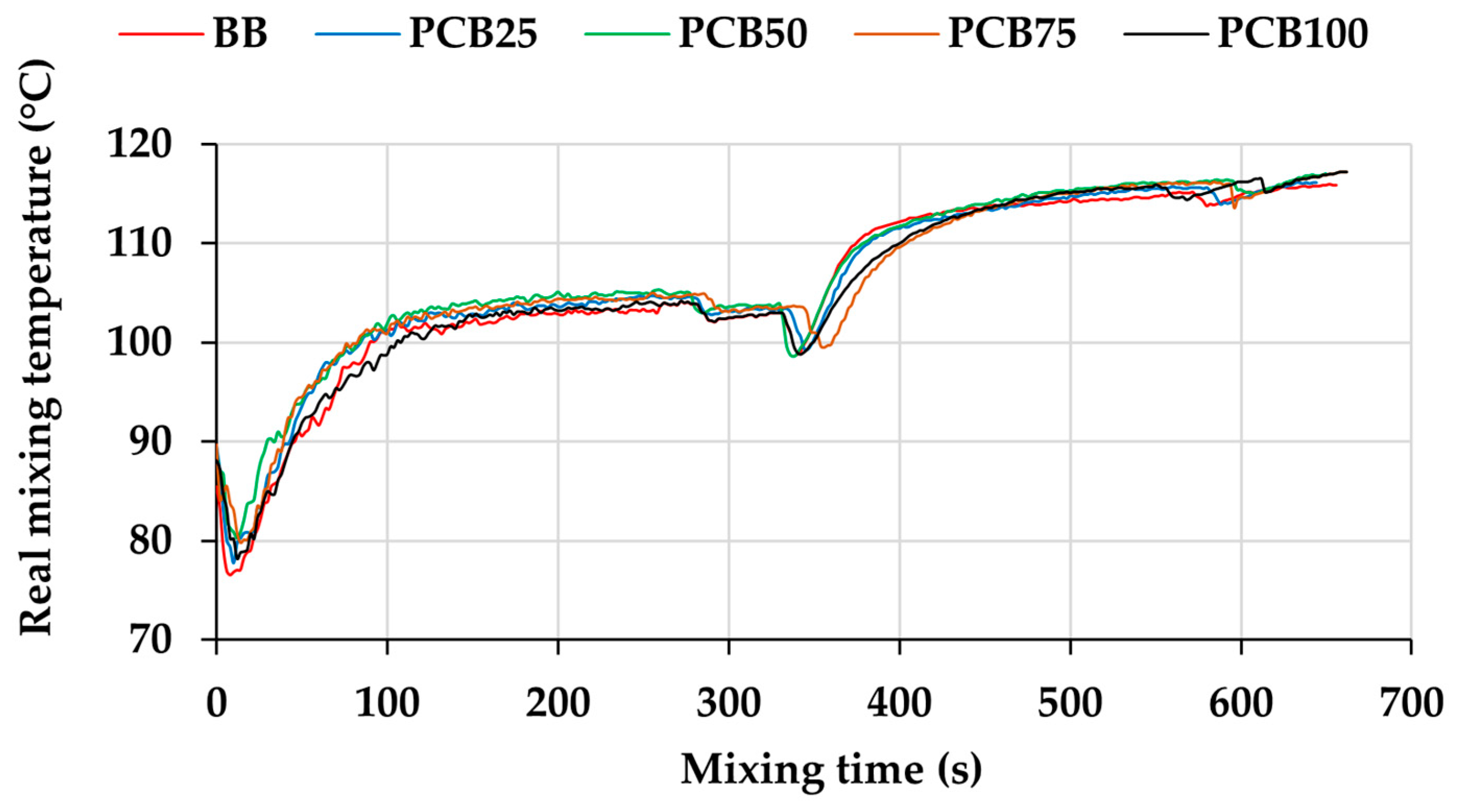
3.1. Mixing Process Parameters
3.2. Rheological Properties
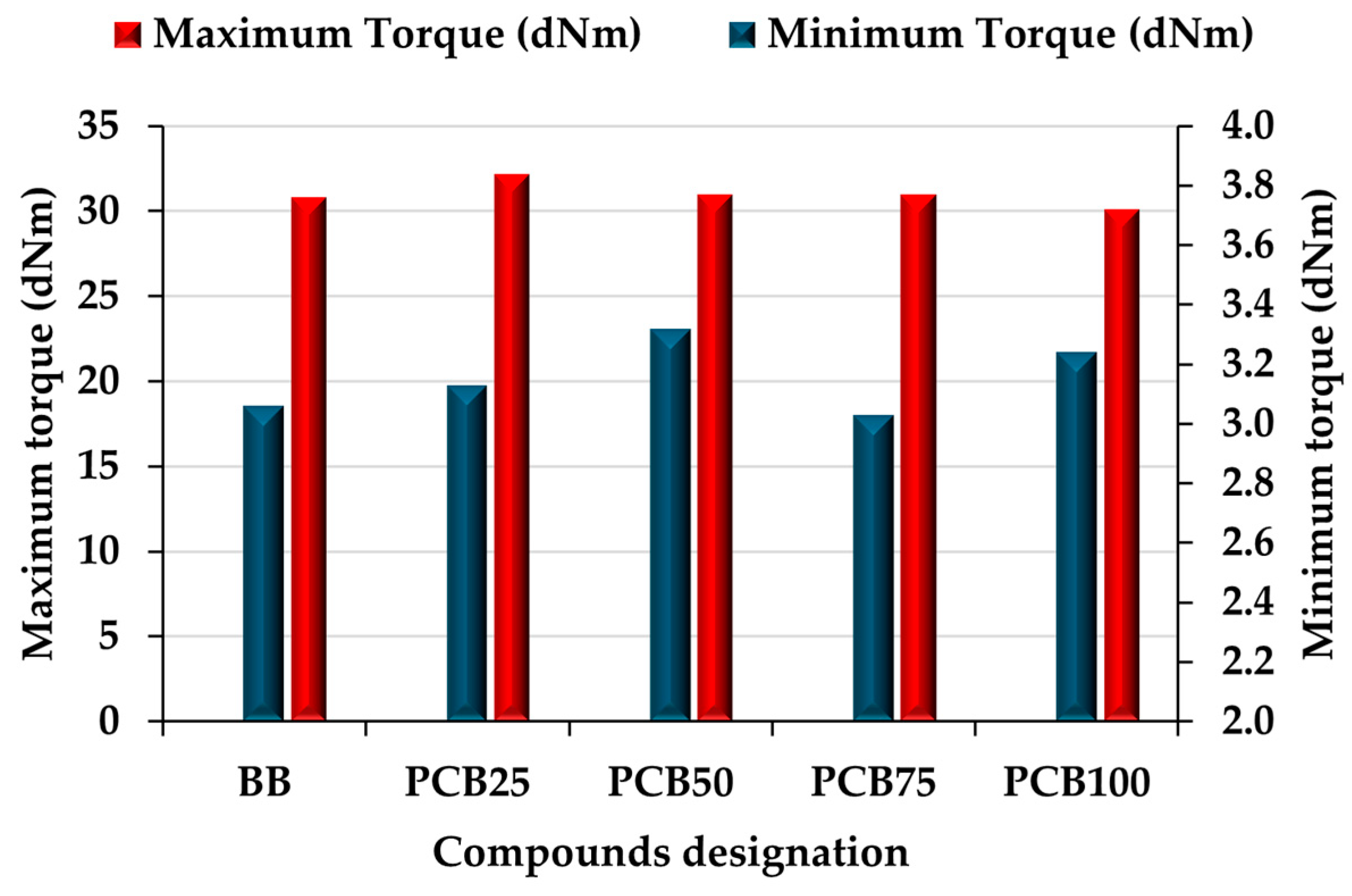
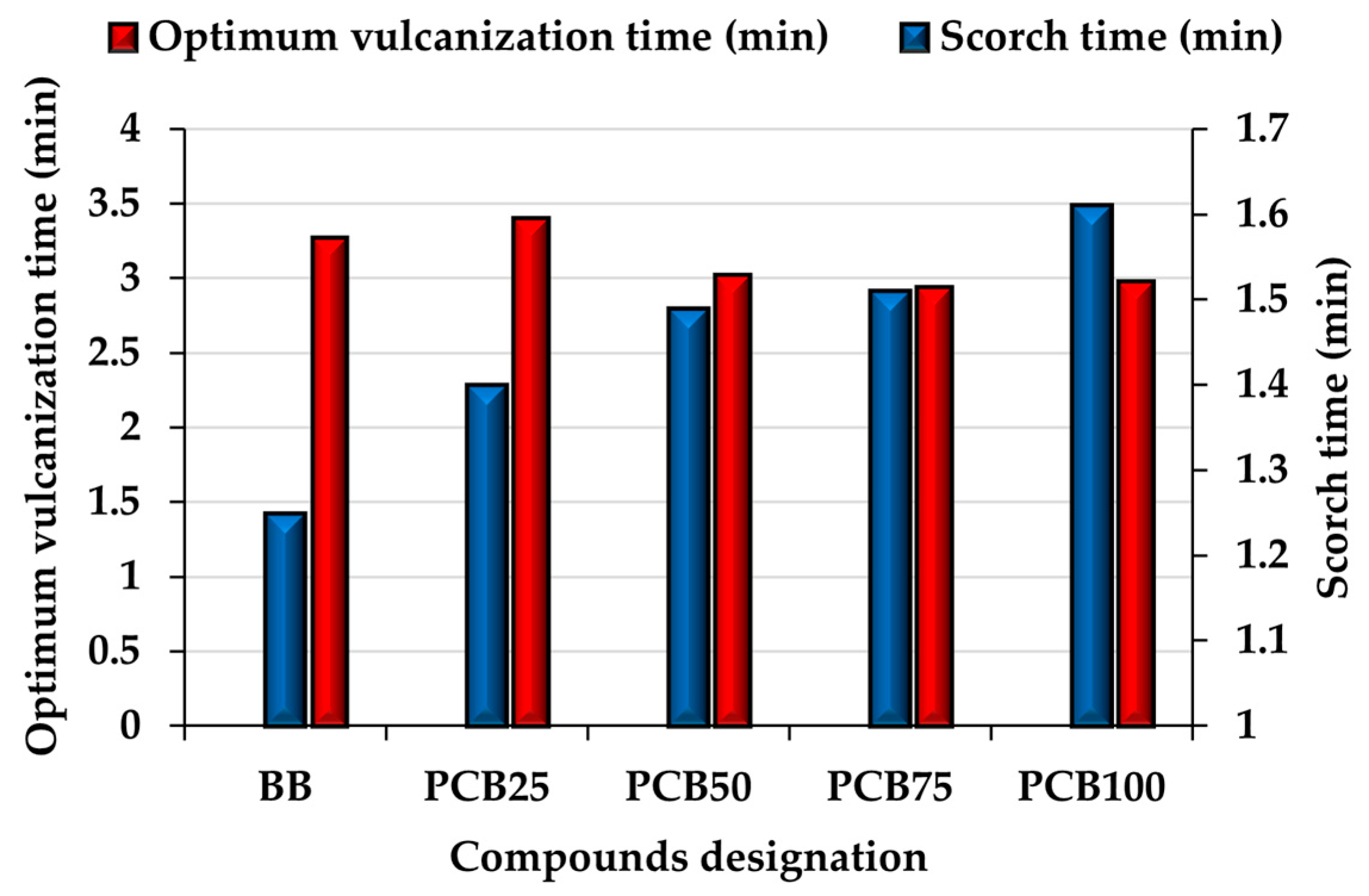
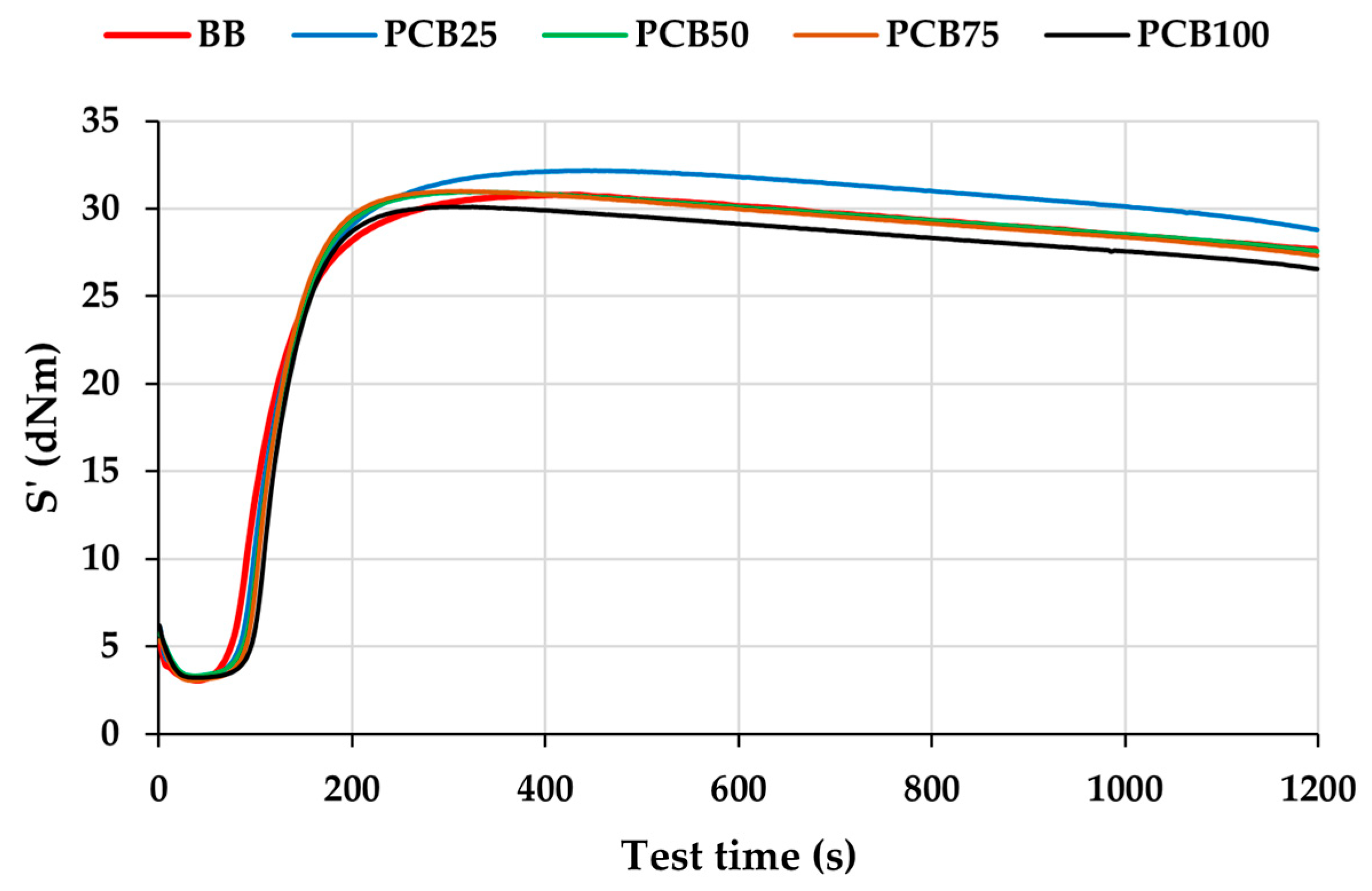
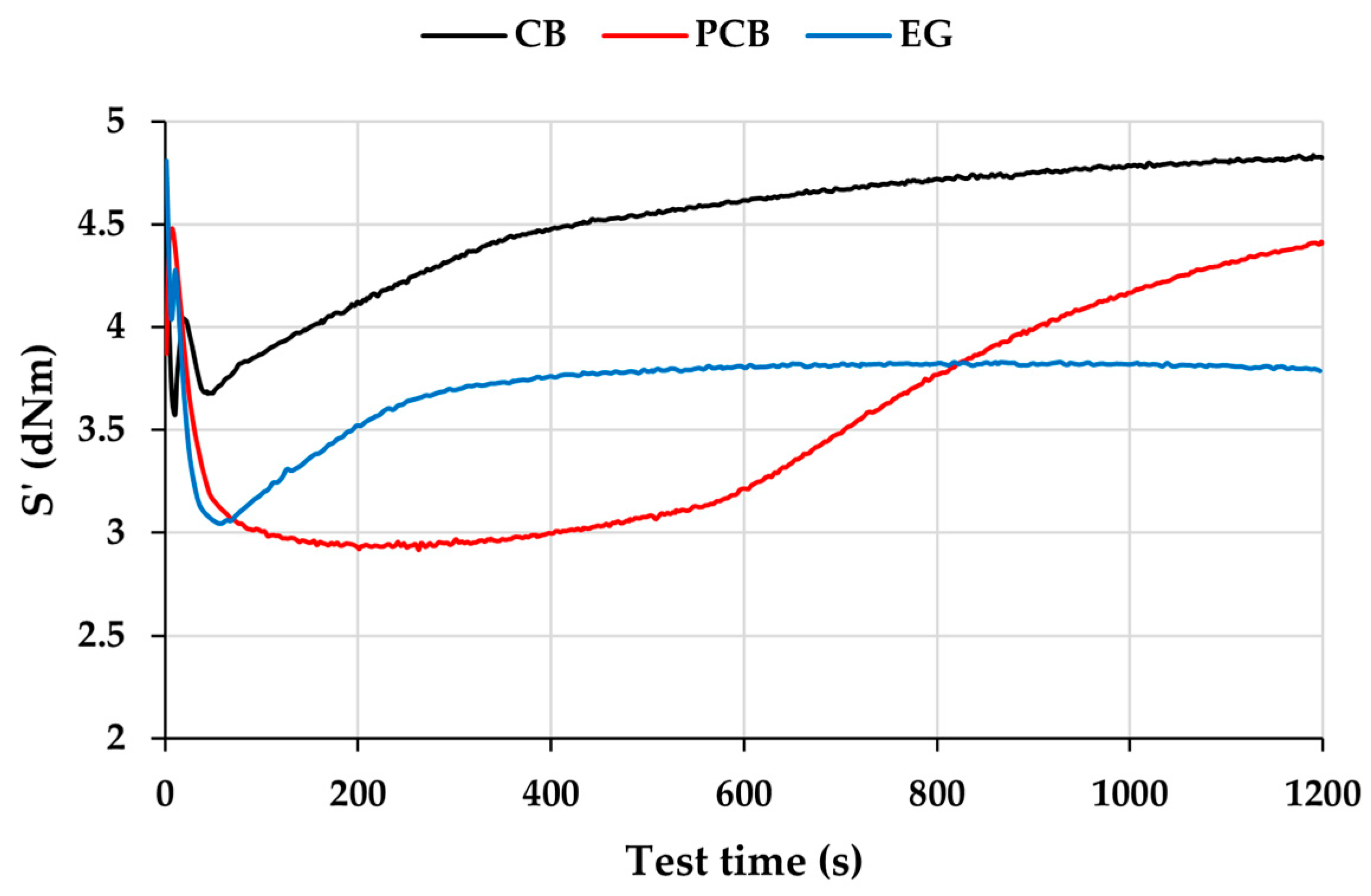
3.3. Vulcanization Characteristics
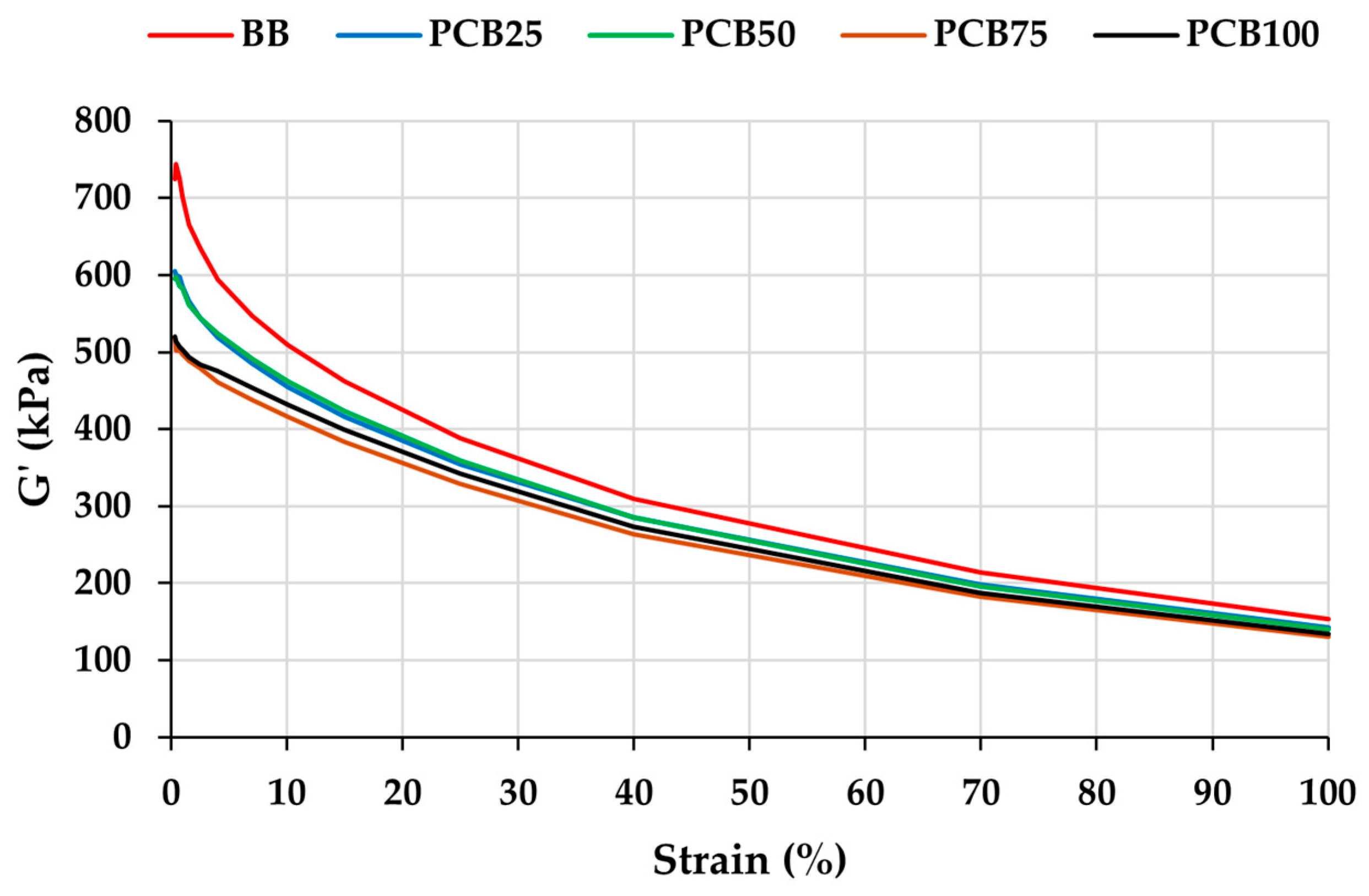
3.4. Payne Effect
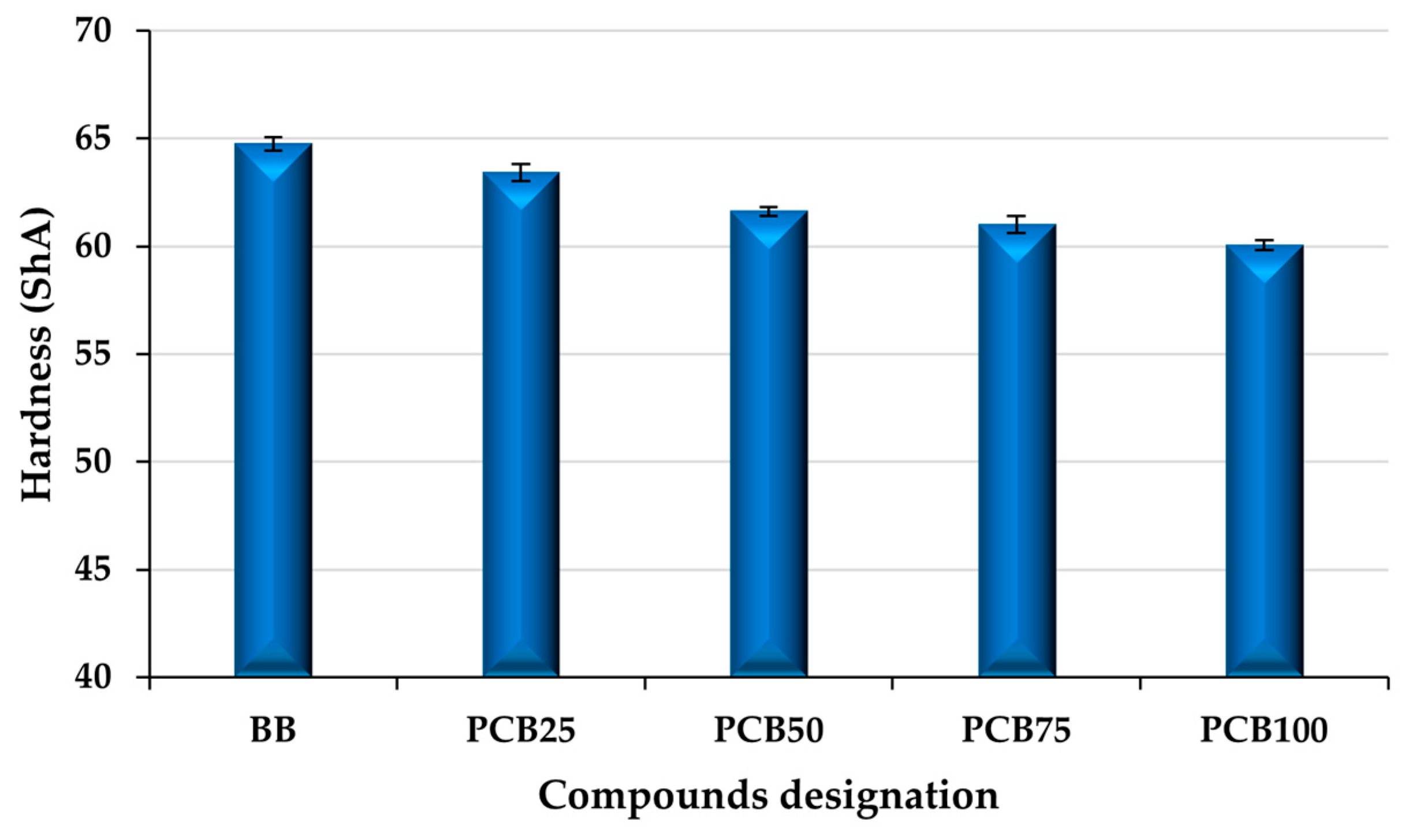
3.5. Hardness
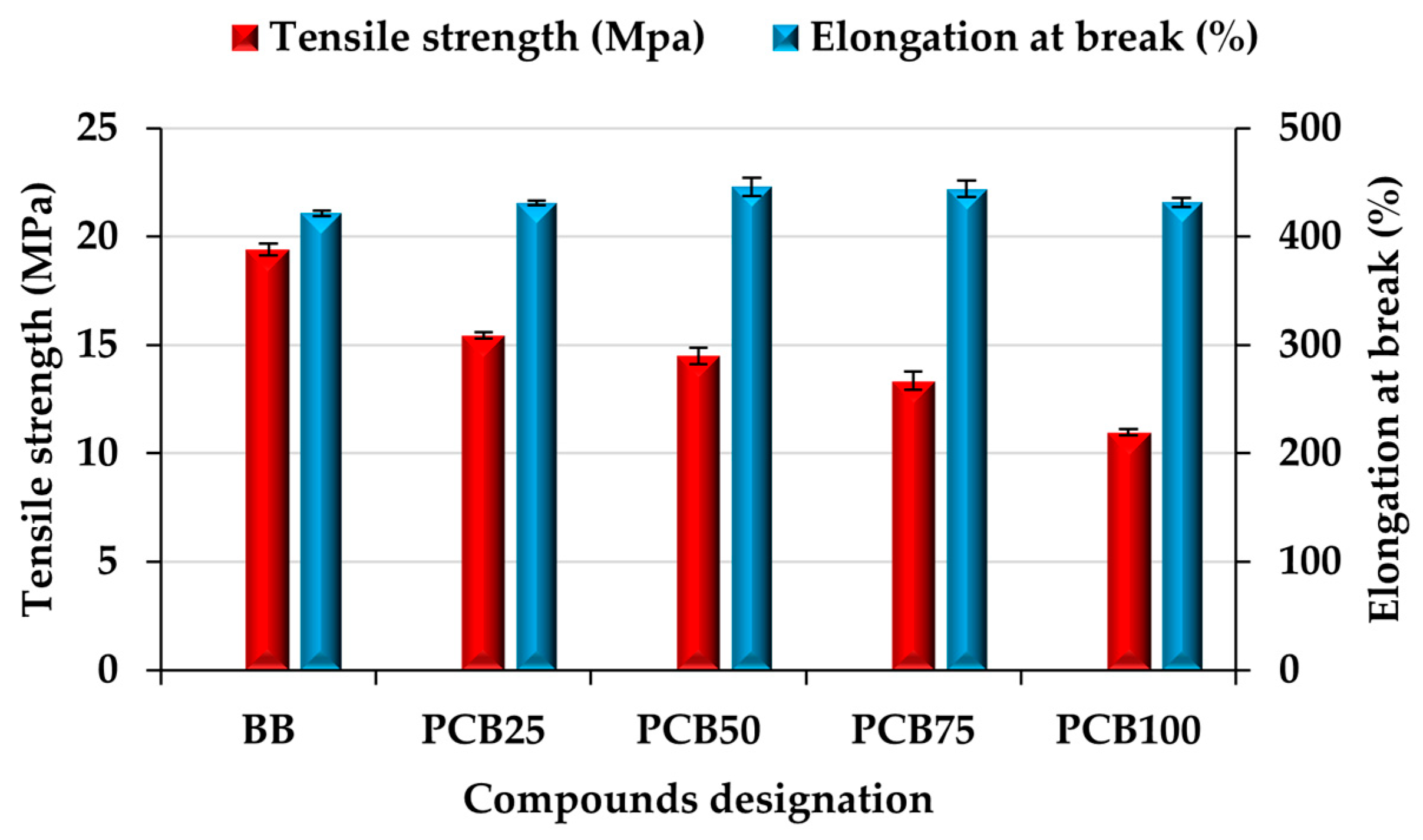
3.6. Tensile Properties
3.7. Morphology Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yangthong, H.; Buaksuntear, K.; Suethao, S.; Chworos, A.; Smitthipong, W. Waste material fly ash as an alternative filler for elastomers. Polym. Eng. Sci. 2023, 63, 2624–2644. [Google Scholar] [CrossRef]
- Ondrušová, D.; Labaj, I.; Vršková, J.; Pajtášová, M.; Mezencevová, V.Z. Application of alternative additives in the polymer composite systems used in automotive industry. IOP Conf. Ser. Mater. Sci. Eng. 2020, 776, 012101. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, G.-W.; Kim, H.-J.; Chung, K.; Jang, K.-S. Effects of Filler Functionalization on Filler-Embedded Natural Rubber/Ethylene-Propylene-Diene Monomer Composites. Polymers 2022, 14, 3502. [Google Scholar] [CrossRef]
- Masa, A.; Krem-Ae, A.; Ismail, H.; Hayeemasae, N. Possible Use of Sepiolite as Alternative Filler for Natural Rubber. Mater. Res. 2020, 23, e20200100. [Google Scholar] [CrossRef]
- Ren, X.; Barrera, C.S.; Tardiff, J.L.; Cornish, K. Sustainable Epoxidized Guayule Natural Rubber, Blends and Composites with Improved Oil Resistance and Greater Stiffness. Materials 2022, 15, 3946. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, G.; Zhang, J.; Qian, D. Prediction of the Strength of Rubberized Concrete by an Evolved Random Forest Model. Adv. Civ. Eng. 2019, 2019, 5198583. [Google Scholar] [CrossRef]
- Pajtášová, M.; Mičicová, Z.; Ondrušová, D.; Božeková, S.; Janík, R.; Pecušová, B.; Raník, L. Use of waste materials in rubber matrix. MATEC Web Conf. 2018, 157, 07009. [Google Scholar] [CrossRef]
- Nuzaimah, M.; Sapuan, S.M.; Nadlene, R.; Jawaid, M. Recycling of waste rubber as fillers: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012016. [Google Scholar] [CrossRef]
- Torres, G.B.; Hiranobe, C.T.; da Silva, E.A.; Cardim, G.P.; Cardim, H.P.; Cabrera, F.C.; Lozada, E.R.; Gutierrez-Aguilar, C.M.; Sánchez, J.C.; Carvalho, J.A.J.; et al. Eco-Friendly Natural Rubber–Jute Composites for the Footwear Industry. Polymers 2023, 15, 4183. [Google Scholar] [CrossRef] [PubMed]
- Rikmann, E.; Mäeorg, U.; Liiv, J. Recycling of Low-Quality Carbon Black Produced by Tire Pyrolysis. Appl. Sci. 2024, 14, 2192. [Google Scholar] [CrossRef]
- Kong, D.; Wang, S.; Shan, R.; Gu, J.; Yuan, H.; Chen, Y. Characteristics and chemical treatment of carbon black from waste tires pyrolysis. J. Anal. Appl. Pyrolysis 2024, 178, 106419. [Google Scholar] [CrossRef]
- Huang, J.; Sun, Y. Viscoelastic Analysis of the Damping Asphalt Mixtures (DAMs) Made with a High Content of Asphalt Rubber (AR). Adv. Civ. Eng. 2020, 2020, 8826926. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Natural Rubber Composites Filled with Crop Residues as an Alternative to Vulcanizates with Common Fillers. Polymers 2019, 11, 972. [Google Scholar] [CrossRef]
- Nia, S.B.; Shafei, B. Carbon footprint reduction through repurposing solid wastes into sustainable construction materials: A state-of-the-art review. Clean. Responsible Consum. 2025, 18, 100310. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; de Bomfim, A.S.C.; Benini, K.C.C.d.C.; Cioffi, M.O.H.; Voorwald, H.J.C.; Rodrigue, D. Waste Paper as a Valuable Resource: An Overview of Recent Trends in the Polymeric Composites Field. Polymers 2023, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Ayar, M.; Dalkiran, A.; Kale, U.; Nagy, A.; Karakoc, T.H. Investigation of the Substitutability of Rubber Compounds with Environmentally Friendly Materials. Sustainability 2021, 13, 5251. [Google Scholar] [CrossRef]
- Lotfy, V.F.; Basta, A.H.; Shafik, E.S. Assessment the performance of chemical constituents of agro wastes in production safety alternative carbon black filler in rubber composite purpose. Sci. Rep. 2025, 15, 11035. [Google Scholar] [CrossRef]
- Dadkhah, M.; Messori, M. A comprehensive overview of conventional and bio-based fillers for rubber formulations sus-tainability. Mater. Today Sustain. 2024, 27, 100886. [Google Scholar]
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef]
- Jiang, H.; Shao, J.; Hu, Q.; Zhu, Y.; Cheng, W.; Zhang, J.; Fan, T.; Yu, J.; Yang, H.; Zhang, X.; et al. Carbon black production characteristics and mechanisms from pyrolysis of rubbers. Fuel Process. Technol. 2023, 253, 108011. [Google Scholar] [CrossRef]
- Lai, S.-M.; Chu, Y.-L.; Chiu, Y.; Chang, M.-C.; Hsieh, T.-Y.; Hsieh, M.-H. Effect of pyrolysis carbon black from waste tires on the properties of styrene–butadiene rubber compounds. Polym. Polym. Compos. 2021, 29, 75–86. [Google Scholar] [CrossRef]
- Malinova, P. Effect of modified pyrolysis carbon on the properties of elastomer composites based on styrene-butadiene rubber. J. Chem. Technol. Metall. 2022, 57, 657–670. [Google Scholar]
- Sagar, M.; Nibedita, K.; Manohar, N.; Kumar, K.R.; Suchismita, S.; Pradnyesh, A.; Reddy, A.B.; Sadiku, E.R.; Gupta, U.; Lachit, P.; et al. A potential utilization of end-of-life tyres as recycled carbon black in EPDM rubber. Waste Manag. 2018, 74, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Moulin, L.; Da Silva, S.; Bounaceur, A.; Herblot, M.; Soudais, Y. Assessment of Recovered Carbon Black Obtained by Waste Tires Steam Water Thermolysis: An Industrial Application. Waste Biomass-Valoriz. 2017, 8, 2757–2770. [Google Scholar] [CrossRef]
- ISO 37:2024; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2024.
- ISO 48-2:2018; Rubber, Vulcanized or Thermoplastic—Determination of Hardness (Hardness Between 10 IRHD and 100 IRHD). ISO: Geneva, Switzerland, 2018.
- Das, S.; Chattopadhyay, S.; Dhanania, S.; Bhowmick, A.K. Improved dispersion and physico-mechanical properties of rubber/silica composites through new silane grafting. Polym. Eng. Sci. 2020, 60, 3115–3134. [Google Scholar] [CrossRef]
- Pan, Y.; Sui, Z.; Chen, Y.; Pan, Y.; Tang, S.; Wang, C.; Han, W. Effect of Interfacial Lubrication between Rubber and Metal on Reducing Mixer Chamber Wall Wear in Mixing Process. Polymers 2022, 14, 3473. [Google Scholar] [CrossRef]
- Robertson, C.G.; Hardman, N.J. Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Rochmadi; Sulistyo, H.; Honggokusumo, S. Rubber mixing process and its relationship with bound rubber and crosslink density. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 012048. [Google Scholar] [CrossRef]
- Kraibut, A.; Saiwari, S.; Kaewsakul, W.; Noordermeer, J.W.M.; Sahakaro, K.; Dierkes, W.K. Dynamic Response and Molecular Chain Modifications Associated with Degradation during Mixing of Silica-Reinforced Natural Rubber Compounds. Polymers 2022, 15, 160. [Google Scholar] [CrossRef]
- Akkenzheyeva, A.; Haritonovs, V.; Bussurmanova, A.; Merijs-Meri, R.; Imanbayev, Y.; Riekstins, A.; Serikbayeva, A.; Sydykov, S.; Aimova, M.; Mustapayeva, G. Study of the Viscoelastic and Rheological Properties of Rubber-Bitumen Binders Obtained from Rubber Waste. Polymers 2023, 16, 114. [Google Scholar] [CrossRef]
- Lin, Y.; Peng, Q. (Eds.) Stearic Acid: Synthesis, Properties and Applications; Nova Science Publishers Inc.: New York, NY, USA, 2014; pp. 25–58. [Google Scholar]
- Wang, J.; Rishi, K.; Narayanan, V.; Beaucage, G.; Yavitt, B.; McGlasson, A.; Chauby, M.; Tang, A.; Okoli, U.; Ilavsky, J. Impact of melt viscosity on filler dispersion in elastomeric nanocomposites. Polymer 2025, 326, 128237. [Google Scholar] [CrossRef]
- McGlasson, A.; Rishi, K.; Beaucage, G.; Narayanan, V.; Chauby, M.; Mulderig, A.; Kuppa, V.K.; Ilavsky, J.; Rackaitis, M. The effects of staged mixing on the dispersion of reinforcing fillers in elastomer compounds. Polymer 2019, 181, 121765. [Google Scholar] [CrossRef]
- Pornprasit, R.; Pornprasit, P.; Boonma, P.; Natwichai, J. Determination of the Mechanical Properties of Rubber by FT-NIR. J. Spectrosc. 2016, 2016, 4024783. [Google Scholar] [CrossRef]
- Bao, X.; Nian, G.; Kutsovsky, Y.; Kim, J.; Jiao, Q.; Suo, Z. Low-intensity mixing process of high molecular weight polymer chains leads to elastomers of long network strands and high fatigue threshold. Soft Matter 2023, 19, 5956–5966. [Google Scholar] [CrossRef]
- De Carvalho, A.P.; Dos Santos, H.F.; Ribeiro, G.D.; Hiranobe, C.T.; Goveia, D.; Gennaro, E.M.; Paim, L.L.; Dos Santos, R.J. Sustainable Composites: Analysis of Filler–Rubber Interaction in Natural Rubber–Styrene–Butadiene Rubber/Polyurethane Composites Using the Lorenz–Park Method and Scanning Electron Microscopy. Polymers 2024, 16, 471. [Google Scholar] [CrossRef]
- Abbasi Shahdehi, I.; Abbasi-Soureshjani, M.; Alimardani, M. Mechanistic study of filler dispersion enhancement in rubbers with environmentally safe processing oils. Results Eng. 2025, 27, 106758. [Google Scholar] [CrossRef]
- Alam, N.; Kumar, V.; Jeong, S.U.; Park, S.-S. Enhancing Rubber Vulcanization Cure Kinetics: Lowering Vulcanization Temperature by Addition of MgO as Co-Cure Activator in ZnO-Based Cure Activator Systems. Polymers 2024, 16, 876. [Google Scholar] [CrossRef]
- Al-Nesrawy, E.F.; Al-Nesrawy, S.H.H. Rheological Properties Of (NR/SBR/CMC/C.B) Nanocomposites. J. Phys. Conf. Ser. 2021, 1973, 012161. [Google Scholar] [CrossRef]
- Lubura, J.D.; Kojic, P.; Pavlicevic, J.; Ikonic, B.; Omorjan, R.; Bera, O. Prediction of rubber vulcanization using an artificial neural network. Chem. Ind. 2021, 75, 277–283. [Google Scholar] [CrossRef]
- Kopal, I.; Labaj, I.; Vršková, J.; Harničárová, M.; Valíček, J.; Ondrušová, D.; Krmela, J.; Palková, Z. A Generalized Regression Neural Network Model for Predicting the Curing Characteristics of Carbon Black-Filled Rubber Blends. Polymers 2022, 14, 653. [Google Scholar] [CrossRef]
- Aboelmagd, A.; Fathy, N.; Abadir, E.; Ismael, A.; Shafik, E.S. Effect of Different Accelerators on Some Mechanical Properties of Natural Rubber. Egypt. J. Chem. 2024, 68, 239–250. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, J.; Chen, S.J. Effects of carbon blacks with various structures on vulcanization and reinforcement of filled ethylene-propylene-diene rubber. Express Polym. Lett. 2008, 2, 695–704. [Google Scholar] [CrossRef]
- Dierkes, W.K.; Anjum, A.; Noordermeer, J.W.M.; Blume, A.; Ramani, B.; Bramer, E.; Brem, G. Influence of sulfur in pyrolytic carbon black on in-rubber properties. In Proceedings of the 200th Technical Meeting of the Rubber Division, ACS 2022, Knoxville, TN, USA, 10–13 October 2022. [Google Scholar]
- Pöschl, M.; Sathi, S.G.; Stoček, R. A chemical interpretation for the post-reversion upturn in the natural rubber/accelerated sulfur system based on the viscoelastic properties and cross-link density measurements. Polym. Bull. 2024, 81, 12521–12541. [Google Scholar] [CrossRef]
- Sathi, S.G.; Stocek, R.; Kratina, O. Reversion free high-temperature vulcanization of cis-polybutadiene rubber with the accelerated-sulfur system. Express Polym. Lett. 2020, 14, 823–837. [Google Scholar] [CrossRef]
- Ngamsurat, S.; Boonkerd, K.; Leela-Adisorn, U.; Potiyaraj, P. Curing Characteristics of Natural Rubber Filled with Gypsum. Energy Procedia 2011, 9, 452–458. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Z.; Wang, W.; Zheng, Q. Payne effect of carbon black filled natural rubber nanocomposites: Influences of extraction, crosslinking, and swelling. J. Rheol. 2021, 65, 807–820. [Google Scholar] [CrossRef]
- Jovanović, V.; Samaržija-Jovanović, S.; Budinski-Simendić, J.; Marković, G.; Marinović-Cincović, M. Composites based on carbon black reinforced NBR/EPDM rubber blends. Compos. Part B Eng. 2013, 45, 333–340. [Google Scholar] [CrossRef]
- Aggarwal, A.; Hackel, N.; Grunert, F.; Ilisch, S.; Beiner, M.; Blume, A. Investigation of Rheological, Mechanical, and Viscoelastic Properties of Silica-Filled SSBR and BR Model Compounds. Polymers 2024, 16, 3212. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, Y.; Wang, Z.; Han, S.; Han, W.; Bian, H. Effect of Adding Pyrolysis Carbon Black (CBp) on Soft Friction and Metal Wear during Mixing. Polymers 2022, 14, 1319. [Google Scholar] [CrossRef]
- Pöschl, M.; Vašina, M.; Zádrapa, P.; Měřínská, D.; Žaludek, M. Study of Carbon Black Types in SBR Rubber: Mechanical and Vibration Damping Properties. Materials 2020, 13, 2394. [Google Scholar] [CrossRef]
- Honorato, L.; Dias, M.L.; Azuma, C.; Nunes, R.C.R. Rheological properties and curing features of natural rubber compositions filled with fluoromica ME 100. Polim. E Tecnol. 2016, 26, 249–253. [Google Scholar] [CrossRef]
- Gumel, S.M.; Adam, J.L.; Ladan, M.; Habibu, S. Effects of treated wood flour on physico-mechanical properties of filled natural rubber. Chemsearch J. 2013, 4, 42–46. [Google Scholar]
- Virág, L.; Egedy, A.; Varga, C.; Erdős, G.; Berezvai, S.; Kovács, L.; Ulbert, Z. Determination of the most significant rubber components influencing the hardness of natural rubber (NR) using various statistical methods. Heliyon 2024, 10, e25170. [Google Scholar] [CrossRef]
- Kratina, O.; Pöschl, M.; Stoček, R. The effect of apparent density of sulfidic crosslink and their chemical nature on self-heat build-up in carbon black filled Natural Rubber under cyclic mechanical loading. Polym. Degrad. Stab. 2024, 227, 110871. [Google Scholar] [CrossRef]
- Dijkhuis, K.A.; Noordermeer, J.W.; Dierkes, W.K. The relationship between crosslink system, network structure and material properties of carbon black reinforced EPDM. Eur. Polym. J. 2009, 45, 3302–3312. [Google Scholar] [CrossRef]
- Mostoni, S.; Milana, P.; Marano, C.; Conzatti, L.; Mauri, M.; D’ARienzo, M.; Di Credico, B.; Simonutti, R.; Stagnaro, P.; Scotti, R. Localizing the cross-links distribution in elastomeric composites by tailoring the morphology of the curing activator. Compos. Sci. Technol. 2022, 230, 109780. [Google Scholar] [CrossRef]
- Bieliński, D.M.; Klajn, K.; Gozdek, T.; Kruszyński, R.; Świątkowski, M. Influence of n-ZnO Morphology on Sulfur Crosslinking and Properties of Styrene-Butadiene Rubber Vulcanizates. Polymers 2021, 13, 1040. [Google Scholar] [CrossRef] [PubMed]
- Hayeemasae, N.; Waesateh, K.; Soontaranon, S.; Masa, A. Effect of vulcanization systems and crosslink density on tensile properties and network structures of natural rubber. J. Teknol. 2022, 84, 181–187. [Google Scholar] [CrossRef]
- Labaj, I.; Ondrušová, D.; Skalková, P.; Vršková, J.; Pajtášová, M.; Dobrovská, J. The effect of alternative filler content on mixing and vulcanization process of elastomeric blends. AIP Conf. Proc. 2023, 2976, 080007. [Google Scholar] [CrossRef]
- Ekwueme, C.C.; Igwe, I.O. Cure Characteristics and Mechanical Properties of Pineapple Leaf Fibre Filled Natural Rubber. J. Miner. Mater. Charact. Eng. 2018, 06, 601–617. [Google Scholar] [CrossRef]



| Compounds Designation | |||||
|---|---|---|---|---|---|
| Ingredients | BB | PCB25 | PCB50 | PCB75 | PCB100 |
| Content (phr) | |||||
| SMR 10 | 100 | 100 | 100 | 100 | 100 |
| CB N339 | 30.00 | 22.50 | 15.00 | 7.50 | 0.00 |
| EG | 47 | 47 | 47 | 47 | 47 |
| PCB | 0.00 | 7.50 | 15.00 | 22.50 | 30.00 |
| Zinc oxide (ZnO) | 3 | 3 | 3 | 3 | 3 |
| Stearic acid (SA) | 2 | 2 | 2 | 2 | 2 |
| Sulfur Crystex OT33 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| TBBS | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Chemical Element | Filler Type | ||
|---|---|---|---|
| CB N399 | EG | PCB | |
| Composition (wt.%) | |||
| C | 99.32 | - | 94.53 |
| Zn | 0.01 | - | 0.84 |
| S | 0.53 | 29.55 | 1.66 |
| Si | 0.10 | 1.16 | 2.31 |
| Ca | 0.02 | 68.64 | 0.66 |
| Compounds Designation | |||
|---|---|---|---|
| Ingredients | CB | PCB | EG |
| Content (phr) | |||
| SMR 10 | 100 | 100 | 100 |
| CB N339 | 30 | 0 | 0 |
| EG | 0 | 0 | 47 |
| PCB | 0 | 30 | 0 |
| Zinc oxide (ZnO) | 3 | 3 | 3 |
| Stearic acid (SA) | 2 | 2 | 2 |
| Sulfur Crystex OT33 | 0 | 0 | 0 |
| TBBS | 1.5 | 1.5 | 1.5 |
| Compounds Designation | |||||
|---|---|---|---|---|---|
| Properties | BB | PCB25 | PCB50 | PCB75 | PCB100 |
| CRI (min−1) | 49.50 | 50.00 | 65.36 | 69.93 | 72.99 |
| ΔM (MH − MEnd) (dNm) | 3.09 | 3.38 | 3.39 | 3.69 | 3.55 |
| Compounds Designation | |||||
|---|---|---|---|---|---|
| BB | PCB25 | PCB50 | PCB75 | PCB100 | |
| G′ 0.28% (kPa) | 725.2 | 604.98 | 595.22 | 517.96 | 520.12 |
| G′ 100% (kPa) | 153.51 | 142.14 | 139.74 | 130.7 | 134.48 |
| ΔG′ (kPa) | 571.69 | 462.84 | 455.48 | 387.26 | 385.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labaj, I.; Vršková, J.; Kopal, I.; Dubec, A.; Ondrušová, D. Innovative Carbon Black Replacement in Rubber Compound: Impact of Pyrolytic Carbon Black and Energy-Gypsum By-Products on Vulcanization and Properties. Polymers 2025, 17, 3080. https://doi.org/10.3390/polym17223080
Labaj I, Vršková J, Kopal I, Dubec A, Ondrušová D. Innovative Carbon Black Replacement in Rubber Compound: Impact of Pyrolytic Carbon Black and Energy-Gypsum By-Products on Vulcanization and Properties. Polymers. 2025; 17(22):3080. https://doi.org/10.3390/polym17223080
Chicago/Turabian StyleLabaj, Ivan, Juliána Vršková, Ivan Kopal, Andrej Dubec, and Darina Ondrušová. 2025. "Innovative Carbon Black Replacement in Rubber Compound: Impact of Pyrolytic Carbon Black and Energy-Gypsum By-Products on Vulcanization and Properties" Polymers 17, no. 22: 3080. https://doi.org/10.3390/polym17223080
APA StyleLabaj, I., Vršková, J., Kopal, I., Dubec, A., & Ondrušová, D. (2025). Innovative Carbon Black Replacement in Rubber Compound: Impact of Pyrolytic Carbon Black and Energy-Gypsum By-Products on Vulcanization and Properties. Polymers, 17(22), 3080. https://doi.org/10.3390/polym17223080








