The Role of Time in the Structural Ordering of Poly-3-Hexylthiophene
Abstract
1. Introduction
2. Materials and Methods
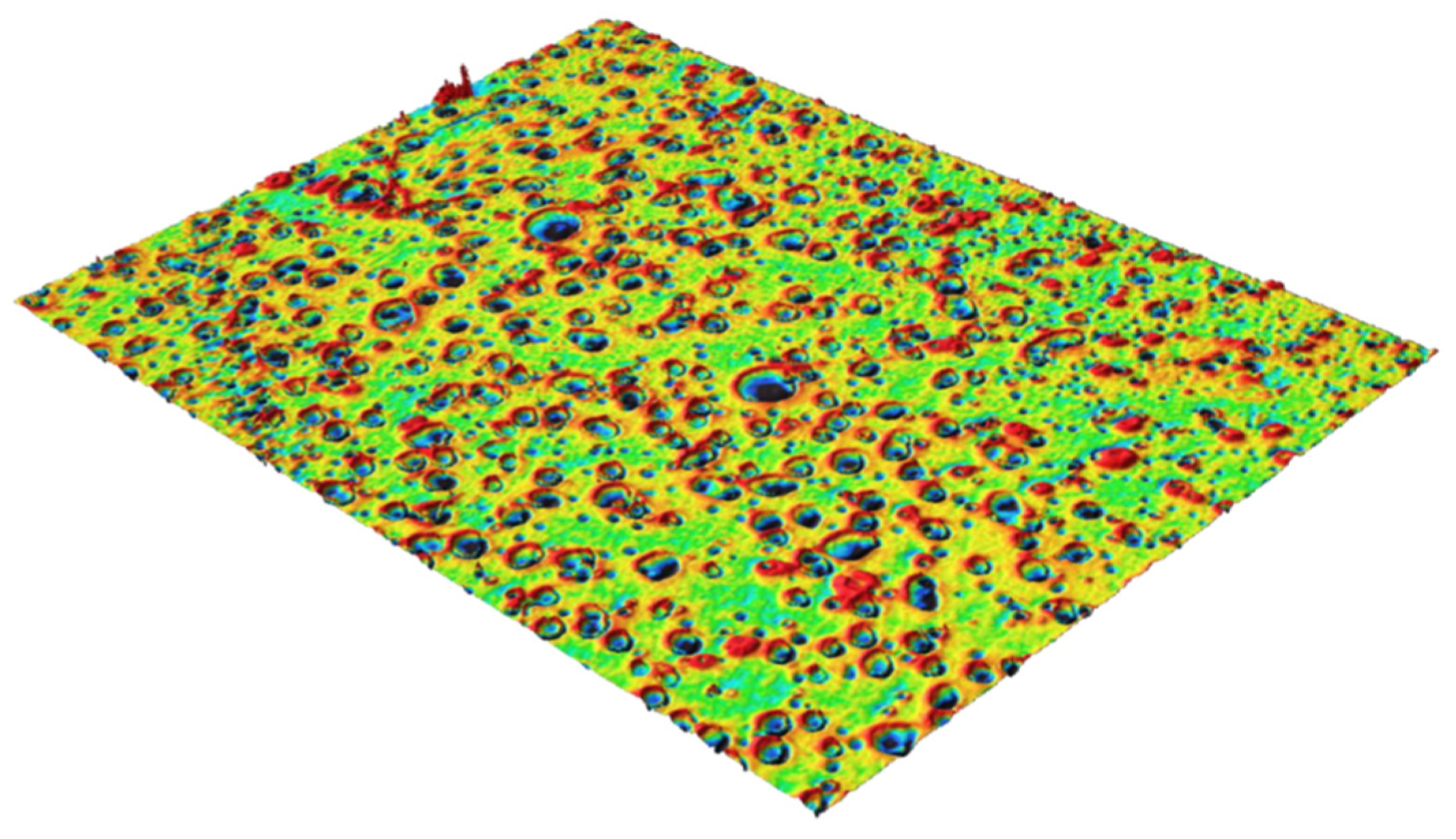
3. Results and Discussion
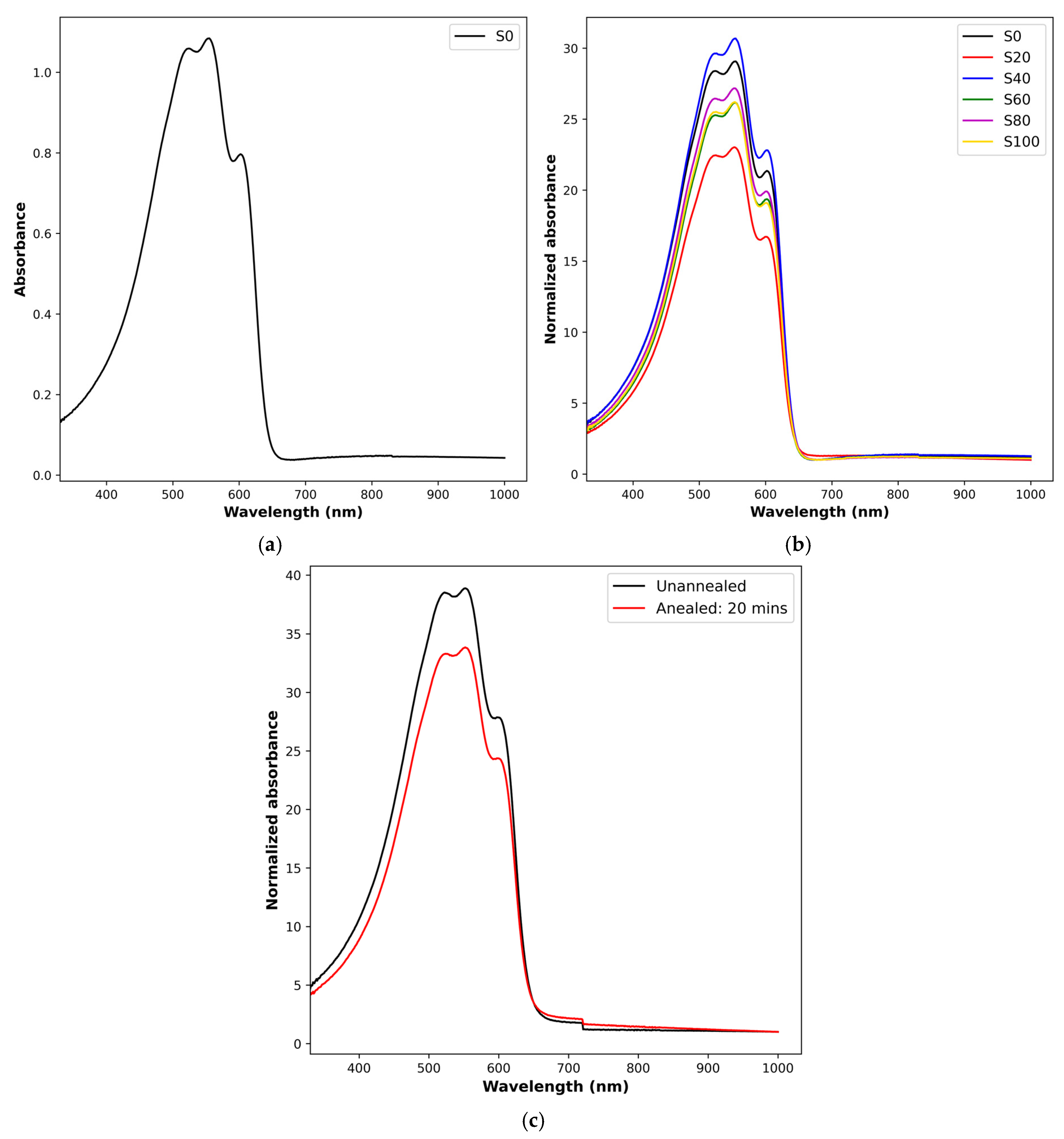
3.1. Annealing and Absorbance
3.2. Optoelectronic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nava-Sanchez, R.; Casados-Cruz, G.; Morales-Acevedo, A. Effect of the P3HT Concentration in the Precursor Solution on the Crystallinity of Annealed P3HT Thin Films Prepared by Spin-Coating. In Proceedings of the 19th International Conference on Electrical Engineering, Computing Science and Automatic Control, Mexico City, Mexico, 9–11 November 2022. [Google Scholar] [CrossRef]
- Kline, R.J.; McGehee, M.D.; Kadnikova, E.N.; Liu, J.; Frechet, J.M.J. Controlling the Field-Effect Mobility of regioregular Polythiophene by Changing the Molecular Weight. Adv. Mater. 2003, 15, 1519–1522. [Google Scholar] [CrossRef]
- Zen, A.; Pflaum, J.; Hirschamann, S.; Zhuang, W.; Jaiser, F.; Asawapirom, U.; Rabe, J.P.; Scherf, U.; Neher, D. Effect of Molecular Weight and Annealing of Poly(3-hexylthiophene)s on the Performance of Organic Field-Effect Transistors. Adv. Funct. Mater. 2004, 14, 757–764. [Google Scholar] [CrossRef]
- Urien, M.; Bailly, L.; Vignau, L.; Cloutet, E.; Cuendias, A.; Wantz, G.; Cramail, H.; Hirsch, L.; Parneix, J.P. Effect of the regioregularity of poly(3-hexylthiophene) on the performances of organic photovoltaic devices. Polym. Int. 2008, 57, 764–769. [Google Scholar] [CrossRef]
- Peng, R.; Zhu, J.; Pang, W.; Cui, Q.; Wu, F.; Liu, K.; Wang, M.; Pan, G. Thermal Annealing Effects on the Absorption and Structural Properties of Regioregular Poly(3-Hexylthiophene) Films. J. Macromol. Sci. B 2011, 50, 624–636. [Google Scholar] [CrossRef]
- Yang, H.; Shin, T.J.; Yang, L.; Cho, K.; Ryu, C.Y.; Bao, Z. Effect of mesoscale crystalline structureon the field-effect mobility of rigioregular poly(3-hexyl thiophene) in thin-film transistors. Adv. Funct. Mater. 2005, 15, 671–676. [Google Scholar] [CrossRef]
- Kobashi, M.; Takeuchi, H. Inhomogeneity of spin-coated and cast non-regioregular poly(3-hexylthiophene) films structures and electrical and photophysical properties. Macromolecules 1998, 31, 7273–7278. [Google Scholar] [CrossRef]
- Al-Ibrahim, M.; Ambacher, O.; Sensfuss, S.; Gobsch, G. Effects of solvent and annealing on the improved performance of solar cells based on poly. 3-hexylthiophene: Fullerene. Appl. Phys. Lett. 2005, 86, 201120. [Google Scholar] [CrossRef]
- Grigorian, S.; Joshi, S.; Pietsch, U. Temperature-dependent structural properties of P3HT films. In IOP Conference Series: Materials Science and Engineering, Proceedings of the Synchrotron Radiation in Polymer Science (SRPS 4), Kerkrade, Netherlands, 8–11 September 2009; IOP Publishing: Bristol, UK, 2010; Volume 14, p. 012007. [Google Scholar] [CrossRef]
- Cho, S.; Lee, K.; Yuen, J.; Wang, G.; Moses, D.; Heeger, A.J.; Surin, M.; Lazzaroni, R. Thermal annealing-induced enhancement of the field-effect mobility of regioregular poly(3-hexylthiophene) films. J. Appl. Phys. 2006, 100, 114503. [Google Scholar] [CrossRef]
- Chirvase, D.; Parisi, J.; Hummelen, J.C.; Dyakonov, V. Influence of nanomorphology on the photovoltaic action of polymer: Fullerene composites. Nanotechnology 2004, 15, 1317–1323. [Google Scholar] [CrossRef]
- Gurau, M.C.; Delongchamp, D.M.; Vogel, B.M.; Lin, E.K.; Fischer, D.A.; Sambasivan, S.; Richter, L.J. Measuring molecular order in poly(3-alkylthiophene) thin films with polarizing spectroscopies. Langmuir 2007, 23, 834–842. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, F.S. Synergistic Effect of Processing Additives and Thermal Annealing on Nanomorphology and Hole Mobility of Poly(3-hexylthiophene) Thin Films. Polymers 2019, 11, 112. [Google Scholar] [CrossRef]
- Prasad, S.S.; Divya, G.; Kumar, K.S. P3HT Thin Films and Their Optical Characterization. In Proceedings of the IEEE 2021 2nd International Conference on Advances in Computing, Communication, Embedded and Secure Systems (ACCESS), Ernakulam, India, 2–4 September 2021. [Google Scholar] [CrossRef]
- Benhaliliba, M.; Ben Ahmed, A.; Kaleli, M.; Meftah, S.E. Structural, optical, nonlinear optical, HUMO-LUMO properties and electrical characterization of Poly(3-hexylthiophene) (P3HT). Opt. Mater. 2022, 132, 112782. [Google Scholar] [CrossRef]
- Kesornsit, S.; Direksilp, C.; Phasuksom, K.; Thummarungsan, N.; Sakunpongpitiporn, P.; Rotjanasuworapong, K.; Sirivat, A.; Niamlang, S. Synthesis of Highly Conductive Poly(3-hexylthiophene) by Chemical Oxidative Polymerization Using Surfactant Templates. Polymers 2022, 14, 3860. [Google Scholar] [CrossRef] [PubMed]
- Delongchamp, D.M.; Kline, R.J.; Jung, Y.; Lin, E.K.; Fischer, D.A.; Gundlach, D.J.; Cotts, S.K.; Moad, A.J.; Richter, L.J.; Toney, M.F.; et al. Molecular basis of mesophase ordering in a thiophene-based copolymer. Macromolecules 2008, 41, 5709–5715. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.H.; Wang, S.C.; Zhang, L.; Liu, X.-Y.; Zhang, Q.; Han, Y.-C. Control Aggregation of P3HT in Solution for High Efficiency Doping: Ensuring Structural Order and the Distribution of Dopants. Chin. J. Polym. Sci. 2023, 41, 811–823. [Google Scholar] [CrossRef]
- Kim, J.; Guo, J.; Sini, G.; Sørensen, M.K.; Andreasen, J.W.; Woon, K.L.; Coropceanu, V.; Paleti, S.H.K.; Wei, H.; Peralta, S.; et al. Remarkable conductivity enhancement in P-doped polythiophenes via rational engineering of polymer-dopant interactions. Mater. Today Adv. 2023, 18, 100360. [Google Scholar] [CrossRef]
- Morfa, A.J.; Barnes, T.M.; Ferguson, A.J.; Levi, D.H.; Rumbles, G.; Rowlen, K.L.; van de Lagemaat, J. Optical characterization of pristine poly(3-hexyl thiophene) films. J. Polym. Sci. B Polym. Phys. 2011, 49, 186–194. [Google Scholar] [CrossRef]
- Chang, J.F.; Clark, J.; Zhao, N.; Sirringhaus, H.; Breiby, D.W.; Andreasen, J.W.; Nielsen, M.M.; Giles, M.; Heeney, M.; McCulloch, I. Molecular-weight dependence of interchain polaron delocalization and exciton bandwidth in high-mobility conjugated polymers. Phys. Rev. B 2006, 74, 115318. [Google Scholar] [CrossRef]
- Trznadel, M.; Pron, A.; Zagorska, M.; Chrzaszcz, R.; Pielichowski, J. Effect of molecular weight on spectroscopic and spectroelectrochemical properties of regioregular poly(3-hexylthiophene). Macromolecules 1998, 31, 5051–5058. [Google Scholar] [CrossRef]
- Benchaabane, A.; Hajlaoui, M.E.; Hnainia, N.; Al-Tabbakh, A.; Zeinert, A.; Bouchriha, H. Optical properties enhancement of hybrid nanocomposites thin films based on P3HT matrix and ZnO@SiO2 core-shell nanoparticles. Opt. Mater. 2020, 102, 109829. [Google Scholar] [CrossRef]
- Wemple, S.H.; DiDomenico, M. Behavior of the electronic dielectric constant in covalent and ionic materials. Phys. Rev. B 1971, 3, 1338. [Google Scholar] [CrossRef]
- Shakra, A.M.; Mohamed, R.A.; El-Bakry, M.Y.; Ali, R.M.; Habashy, D.M. A Combined Experimental and Theoretical Study of the Optical Behavior of Se-Ge-Ga-Sb Chalcogenide Thin Films. Egypt J. Solids 2024, 46, 33–76. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Ahmad, A.A.; Alsaad, A.M.; Telfah, A.D. Optical characterizations of PMMA/metal oxide nanoparticles thin films: Bandgap engineering using a novel derived model. Heliyon 2021, 7, e05952. [Google Scholar] [CrossRef] [PubMed]
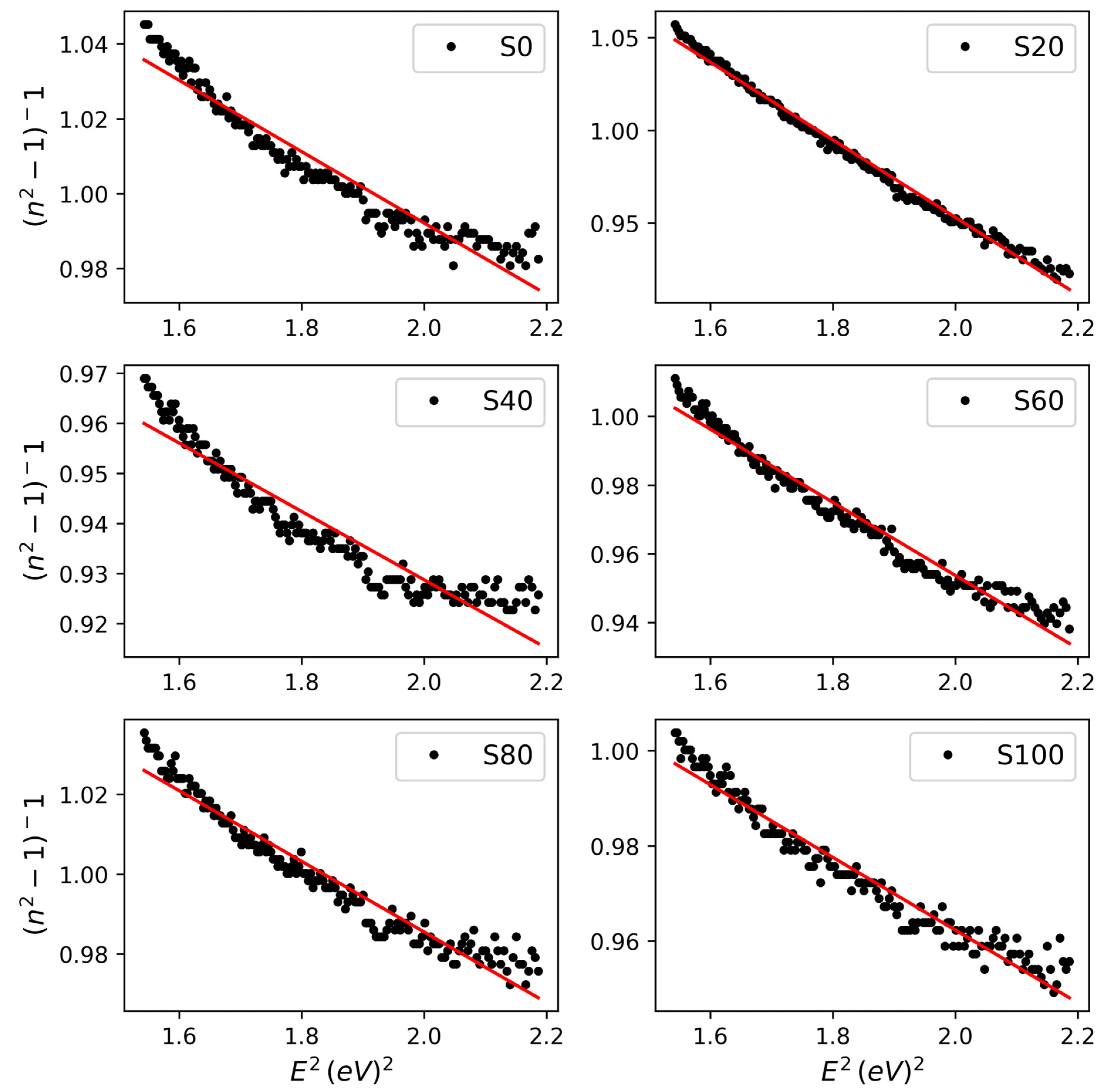
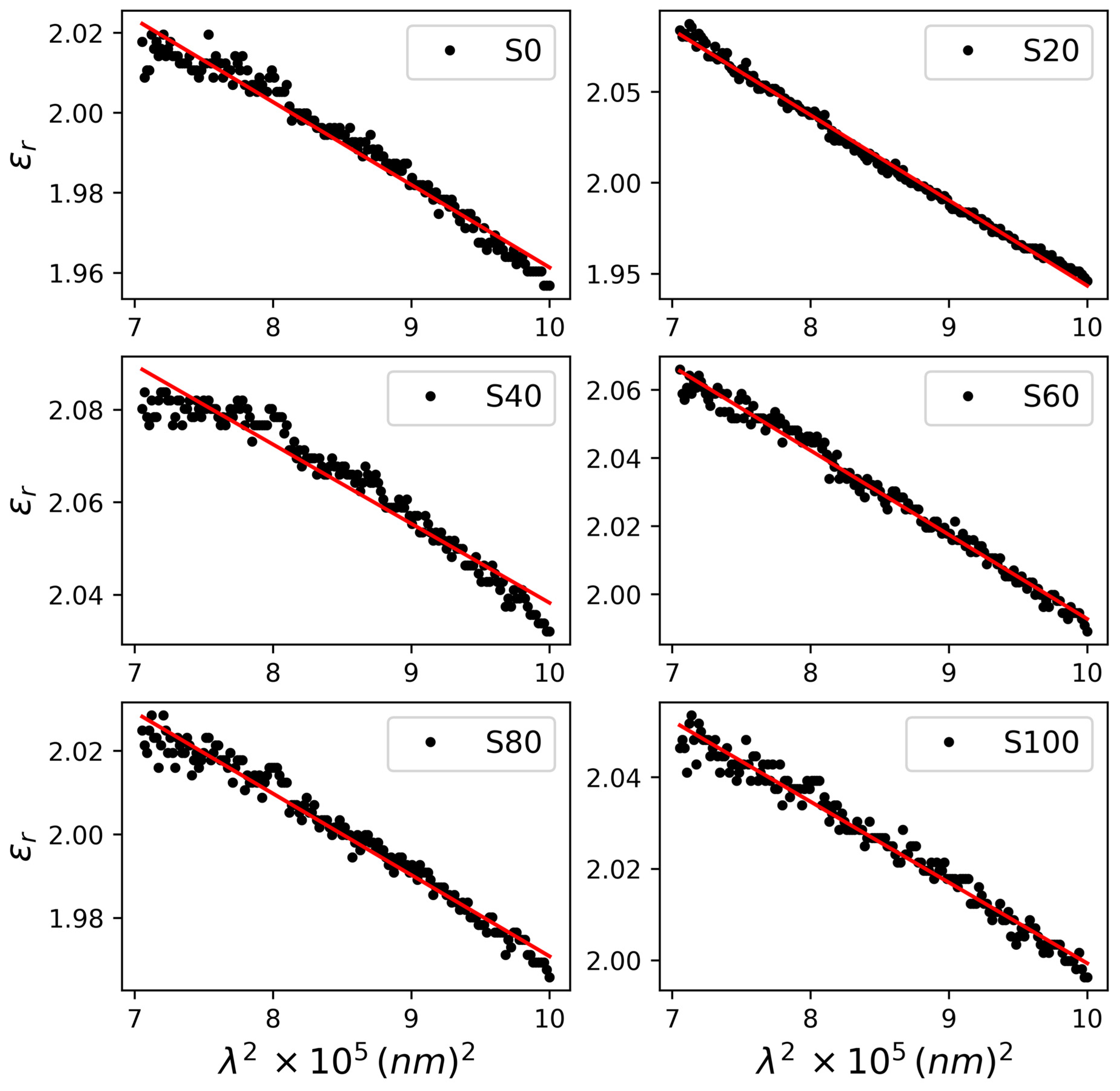

| Model | WDD Model | SF Model | ||||
|---|---|---|---|---|---|---|
| Parameter | Ed (eV) | Eo (eV) | ++ εo | ε∞ | N/m∗ (m−3kg−1) | ** N × 108 (m−3) |
| S0 | 9.33 ± 0.13 | 11.01 ± 0.17 | 1.48 ± 0.06 | 2.37 ± 0.09 | 3.01 ± 0.15 | 2.74 ± 0.14 |
| S20 | 3.81 ± 0.10 | 5.16 ± 0.16 | 1.49 ± 0.07 | 2.62 ± 0.08 | 6.08 ± 0.16 | 5.54 ± 0.14 |
| S40 | 14.10 ± 0.15 | 14.86 ± 0.10 | 1.58 ± 0.08 | 2.27 ± 0.03 | 2.32 ± 0.086 | 2.11 ± 0.08 |
| S60 | 8.30 ± 0.07 | 9.73 ± 0.14 | 1.51 ± 0.05 | 2.40 ± 0.06 | 3.48 ± 0.16 | 3.17 ± 0.15 |
| S80 | 9.98 ± 0.09 | 11.76 ± 0.16 | 1.49 ± 0.08 | 2.38 ± 0.06 | 2.65 ± 0.12 | 2.42 ± 0.11 |
| S100 | 12.10 ± 0.12 | 13.44 ± 0.10 | 1.52 ± 0.06 | 2.28 ± 0.04 | 2.50 ± 0.14 | 2.27 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uba, I.; Jagoi, W.; Forrest, B.; Hamidu, A.-M.; Granderson, K.; Baskerville, E.; Outsey, L.; Birdow, R.; Mann, K.; Ash, J. The Role of Time in the Structural Ordering of Poly-3-Hexylthiophene. Polymers 2025, 17, 3077. https://doi.org/10.3390/polym17223077
Uba I, Jagoi W, Forrest B, Hamidu A-M, Granderson K, Baskerville E, Outsey L, Birdow R, Mann K, Ash J. The Role of Time in the Structural Ordering of Poly-3-Hexylthiophene. Polymers. 2025; 17(22):3077. https://doi.org/10.3390/polym17223077
Chicago/Turabian StyleUba, Ikemefuna, Wisdom Jagoi, Brenden Forrest, Abdul-Majeed Hamidu, Kenneth Granderson, Emmanuel Baskerville, Lailah Outsey, Robert Birdow, Kamar Mann, and Justice Ash. 2025. "The Role of Time in the Structural Ordering of Poly-3-Hexylthiophene" Polymers 17, no. 22: 3077. https://doi.org/10.3390/polym17223077
APA StyleUba, I., Jagoi, W., Forrest, B., Hamidu, A.-M., Granderson, K., Baskerville, E., Outsey, L., Birdow, R., Mann, K., & Ash, J. (2025). The Role of Time in the Structural Ordering of Poly-3-Hexylthiophene. Polymers, 17(22), 3077. https://doi.org/10.3390/polym17223077





