Mechanical Enhancement of Polychloroprene Adhesives via Reinforcement with Aluminum Oxide Nanofibers
Abstract
1. Introduction
2. Materials and Methods
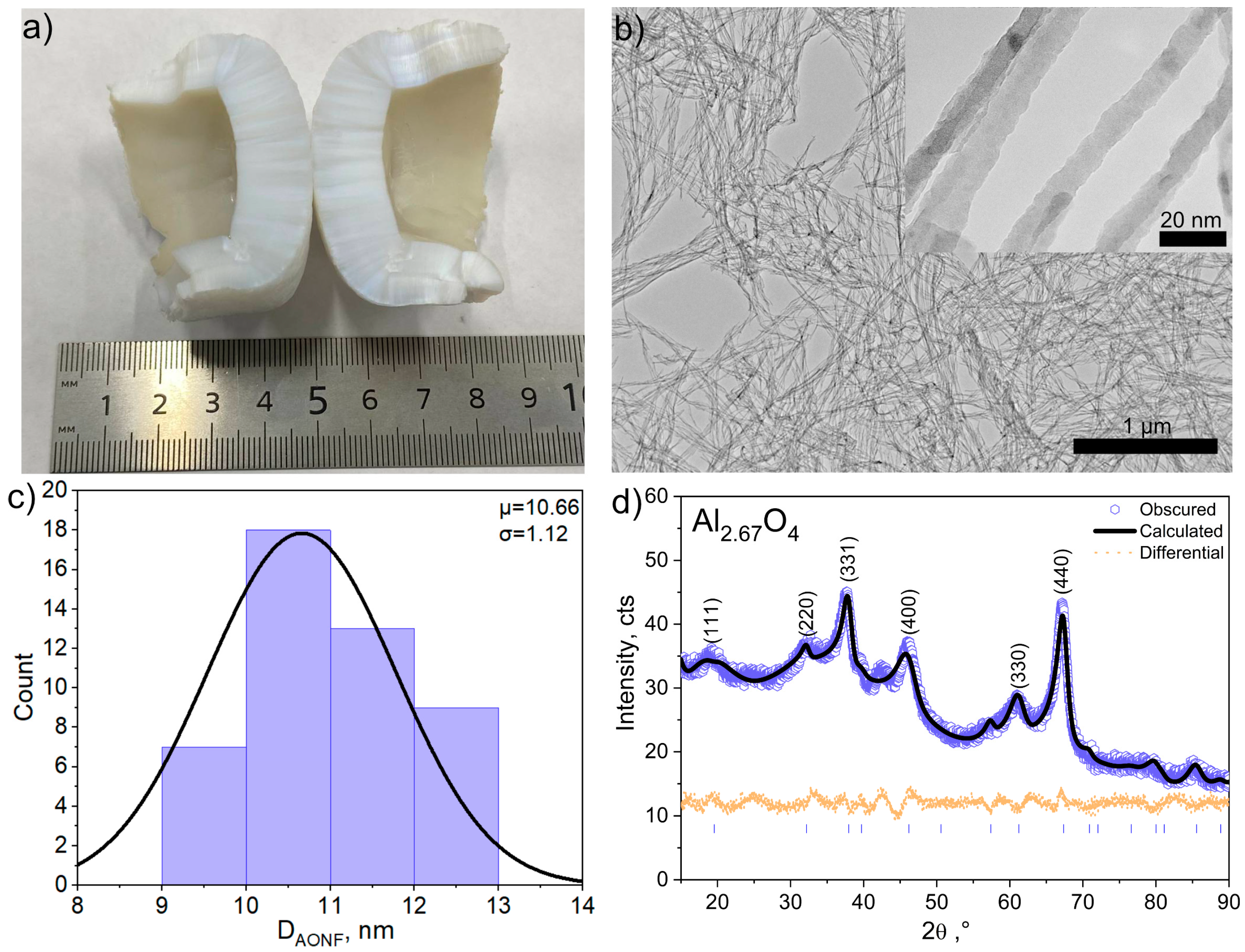
2.1. Alumina Nanofiber (AONF) Synthesis
2.2. AONF Characterization
2.2.1. Microscopy
2.2.2. XRD
2.3. AONF Characterization
2.3.1. CR Composition Synthesis
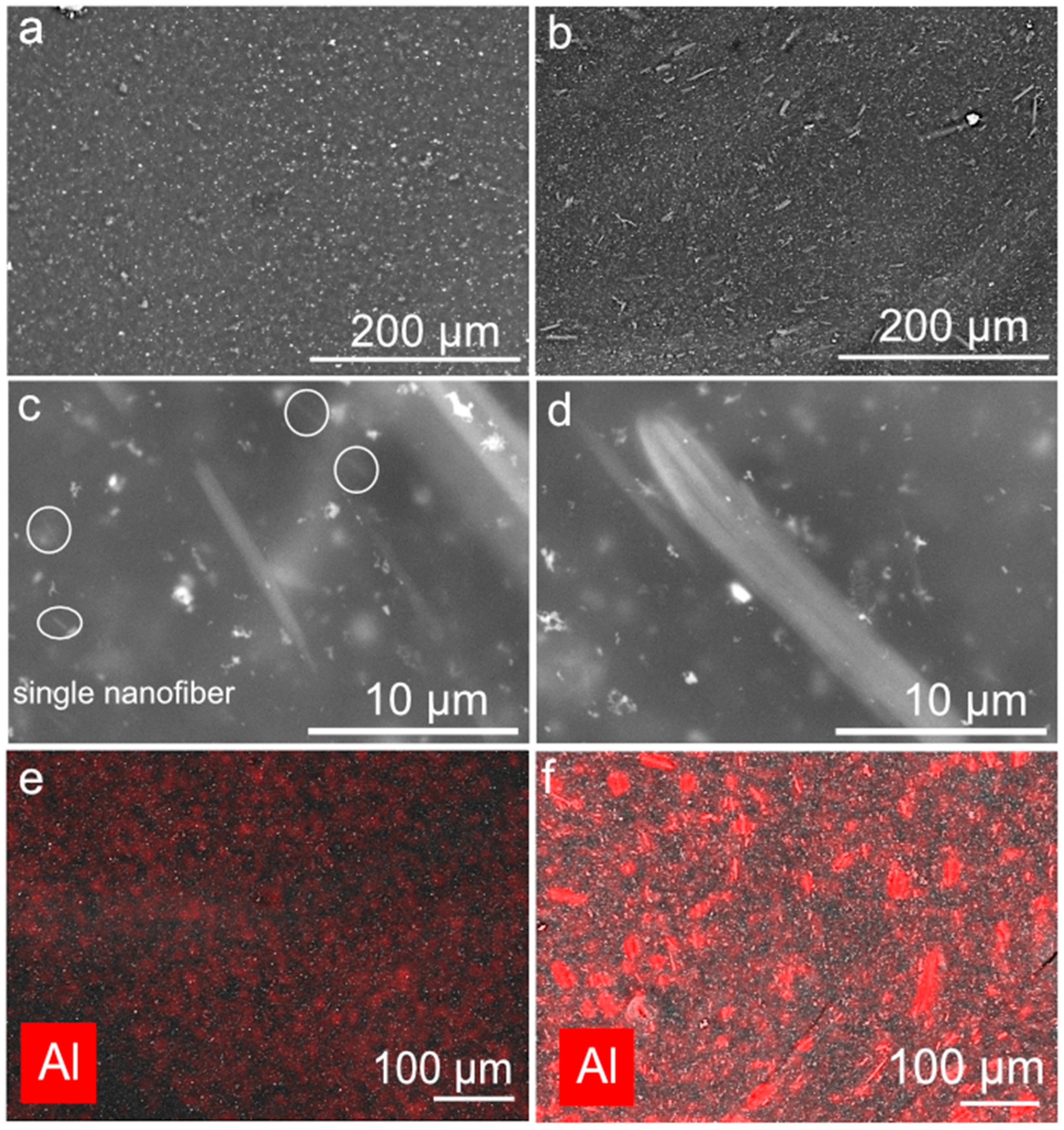
2.3.2. Microscopy
2.4. Thermomechanical Test
2.4.1. Shear Strength Tests
2.4.2. Tensile Tests
2.4.3. TGA, DTGA, DSC Analysis
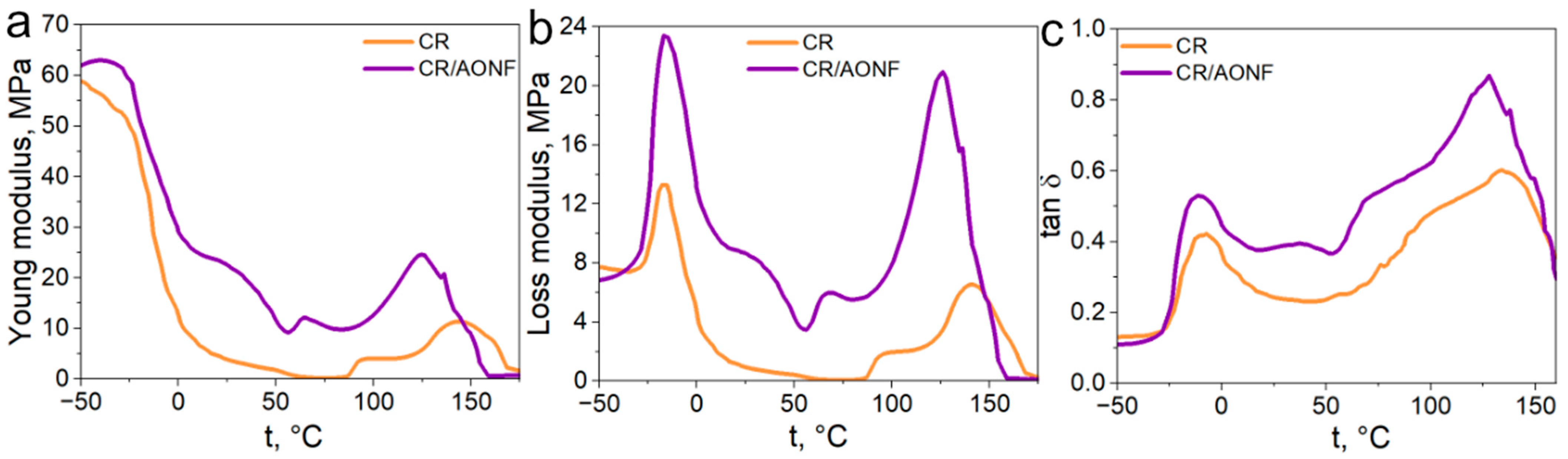
2.4.4. DMA Analysis
3. Results and Discussion
3.1. Structure of Aluminum Oxide Nanofibers
3.2. Microscopy Results
4. Mechanical Properties of CR/AONF
5. Thermic Analysis and DMA
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CR | Chloroprene rubber |
| AONF | Aluminum oxide nanofiber |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
References
- Ismail, H.; Leong, H.C. Curing Characteristics and Mechanical Properties of Natural Rubber/chloroprene Rubber and Epoxidized Natural Rubber/chloroprene Rubber Blends. Polym. Test. 2001, 20, 509–516. [Google Scholar] [CrossRef]
- Suresh, K.; Megavarnan, R.; Vengatachalam, P.; Krishnamurthy, S.; Balakrishnan, P. A Review on the Effect of Various Nano Fillers on the Mechanical Properties and Thermal Stability of Chloroprene Rubber Composites. Mater. Today Proc. 2022, 68, 2560–2568. [Google Scholar] [CrossRef]
- Treger, Y.A.; Morozov, K.A.; Dasaeva, G.S.; Frolkova, A.K. Chloroprene Rubber: Application and Production. Fine Chem. Technol. 2018, 13, 26–38. [Google Scholar] [CrossRef]
- Wu, W.L.; Li, J.K. Study on Carbon Fiber Reinforced Chloroprene Rubber Composites. Adv. Mater. Res. 2014, 1052, 254–257. [Google Scholar] [CrossRef]
- Olejnik, A.; Smejda-Krzewicka, A.; Strzelec, K.; Szynkowska, M.I. Curing and Properties of Chloroprene and Butadiene Rubber (CR/BR) Blends Cross-Linked with copper(I) Oxide or copper(II) Oxide. Int. J. Polym. Anal. Charact. 2018, 24, 18–31. [Google Scholar] [CrossRef]
- Graves, D.F. Rubber. In Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; pp. 689–718. [Google Scholar]
- Fukahori, Y. The Mechanics and Mechanism of the Carbon Black Reinforcement of Elastomers. Rubber Chem. Technol. 2003, 76, 548–566. [Google Scholar] [CrossRef]
- De Sarkar, M.; Sunada, T.; Tomizawa, S.; Kondo, A. Effect of Carbon Black on Properties of Acrylonitrile-Chloroprene Rubber. J. Appl. Polym. Sci. 2022, 139, 51588. [Google Scholar] [CrossRef]
- Lawandy, S.N.; Abd-El-Nour, K.N. Dielectric Properties and Stress–strain Measurements of Chloroprene Rubber Based on Different Carbon Black Fillers. J. Appl. Polym. Sci. 1986, 31, 841–848. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Salleh, S.Z.; Ismail, H. Using Chloroprene Rubber Waste in Rubber Blends: Optimizing Performance by Adding Fillers. Green Mater. 2019, 7, 156–167. [Google Scholar] [CrossRef]
- Kapgate, B.P.; Das, C.; Das, A.; Basu, D.; Wiessner, S.; Reuter, U.; Heinrich, G. Reinforced Chloroprene Rubber by in Situ Generated Silica Particles: Evidence of Bound Rubber on the Silica Surface. J. Appl. Polym. Sci. 2016, 133, 43717. [Google Scholar] [CrossRef]
- Smejda-Krzewicka, A.; Rybiński, P.; Bradło, D.; Żukowski, W. The Morphology, Mechanical and Dynamic Properties, Fire Hazard and Toxicity of Chloroprene and Butadiene Rubber Composites Cross-Linked with Zinc. Materials 2023, 16, 1240. [Google Scholar] [CrossRef]
- Keibal, N.A.; Kablov, V.F.; Kochetkov, V.G.; Kryukova, D.A.; Urzhumov, D.A.; Gunko, Y.A. The Use of Pipe-Production Waste as Polyfunctional Modifiers of Polychloroprene Adhesives. Polym. Sci. Ser. D 2025, 18, 277–282. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Wu, S.; Wang, W.; Zhang, L. Novel Percolation Phenomena and Mechanism of Strengthening Elastomers by Nanofillers. Phys. Chem. Chem. Phys. 2010, 12, 3014–3030. [Google Scholar] [CrossRef]
- Philip, T.; Jayadas, N.H. Enhancing Tribological and Dynamic Mechanical Properties of Carbon Black-Filled Polychloroprene Rubber Composites Through the Incorporation of Modified Nanoclay. Arab. J. Sci. Eng. 2025, 1–12. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, H.; Zhang, Y. Nonlinear Viscoelasticity and Stress-Softening Behavior of Chloroprene Rubber Reinforced by Multiwalled Carbon Nanotubes. Polym. Compos. 2014, 35, 2194–2202. [Google Scholar] [CrossRef]
- Li, W.; Liang, J.; Zhu, Y.; Li, C.; Gong, Q.; Liang, C. Study on the Influence of Carbon Nanotube Modification on the Mechanical and Magnetic Properties of Chloroprene Rubber-Based Magnetorheological Elastomers. Polym. Compos. 2024, 45, 14177–14190. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, L.; Wen, S.; Liu, L. Graphene Oxide-Supported Zinc Oxide Nanoparticles for Chloroprene Rubber with Improved Crosslinking Network and Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105492. [Google Scholar] [CrossRef]
- Maya, M.G.; George, S.C.; Jose, T.; Kailas, L.; Thomas, S. Development of a Flexible and Conductive Elastomeric Composite Based on Chloroprene Rubber. Polym. Test. 2018, 65, 256–263. [Google Scholar] [CrossRef]
- Kopylov, V.M.; Kostyleva, E.I.; Kostylev, I.M.; Koviazin, A.V. Silica Fillers for Silicone Rubber. Int. Polym. Sci. Technol. 2011, 38, 35–47. [Google Scholar] [CrossRef]
- Rattanasom, N.; Kueseng, P.; Deeprasertkul, C. Improvement of the Mechanical and Thermal Properties of Silica-Filled Polychloroprene Vulcanizates Prepared from Latex System. J. Appl. Polym. Sci. 2012, 124, 2657–2668. [Google Scholar] [CrossRef]
- Roy, K.; Alam, M.N.; Mandal, S.K.; Debnath, S.C. Silica-Coated Nano Calcium Carbonate Reinforced Polychloroprene Rubber Nanocomposites: Influence of Silica Coating on Cure, Mechanical and Thermal Properties. J. Nanostruct. Chem. 2015, 6, 15–24. [Google Scholar] [CrossRef]
- Whelan, A.; Lee, K.S. (Eds.) Developments in Rubber Technology—2; Springer: Dordrecht, The Netherlands, 1981; ISBN 9789400981089. [Google Scholar]
- Xiao, C.B.; Zhang, Z.P.; Liu, J.H.; Li, R.; Zhang, Z.P. Preparation and Properties of Chloroprene Rubber/Montmorillonite Nanocomposites. Key Eng. Mater. 2007, 334–335, 865–868. [Google Scholar] [CrossRef]
- Yeh, M.-H.; Hwang, W.-S.; Chang, Y.-C. Preparation and Mechanical Properties of Polychloroprene–Montmorillonite Composites. Jpn. J. Appl. Phys. 2005, 44, 6847. [Google Scholar] [CrossRef]
- Simunin, M.M.; Voronin, A.S.; Fadeev, Y.V.; Dobrosmyslov, S.S.; Kuular, A.A.; Shalygina, T.A.; Shabanova, K.A.; Chirkov, D.Y.; Voronina, S.Y.; Khartov, S.V. Influence of the Addition of Alumina Nanofibers on the Strength of Epoxy Resins. Materials 2023, 16, 1343. [Google Scholar] [CrossRef]
- Teoh, G.L.; Liew, K.Y.; Mahmood, W.A.K. Synthesis and Characterization of Sol–gel Alumina Nanofibers. J. Sol-Gel Sci. Technol. 2007, 44, 177–186. [Google Scholar] [CrossRef]
- Noordin, M.R.; Liew, K.Y. Synthesis of Alumina Nanofibers and Composites. In Nanofibers; IntechOpen: London, UK, 2010; ISBN 9789537619862. [Google Scholar]
- Sudhakaran, G.; Avirah, S.A. Studies on Reinforcing Effect of Industrial Nano Silica in Chloroprene Vulcanizates Containing Carboxy-Terminated Liquid Natural Rubber. J. Elastomers Plast. 2022, 54, 509–517. [Google Scholar] [CrossRef]
- Kutuzov, M. Method and System for Alumina Nanofibers Synthesis from Molten Aluminum. U.S. Patent 13/756,366, 1 August 2013. [Google Scholar]
- Minakov, A.V.; Pryazhnikov, M.I.; Simunin, M.M.; Dobrosmyslov, S.S.; Kuular, A.A.; Molokeev, M.S.; Volochaev, M.N.; Khartov, S.V.; Voronin, A.S. Rheological Properties of Colloidal Suspensions of Alumina Nanofibers. J. Mol. Liq. 2022, 367, 120385. [Google Scholar] [CrossRef]
- Kuular, A.A.; Simunin, M.M.; Bermeshev, T.V.; Voronin, A.S.; Dobrosmyslov, S.S.; Fadeev, Y.V.; Molokeev, M.S.; Volochaev, M.N.; Khartov, S.V. The Influence of Alumina Nanofibers on the Physical and Mechanical Properties of Mineral-Filled Polyethylene: An Experimental Study. Tech. Phys. Lett. 2020, 46, 1215–1218. [Google Scholar] [CrossRef]
- Sukhanova, A.; Boyandin, A.; Ertiletskaya, N.; Simunin, M.; Shalygina, T.; Voronin, A.; Vasiliev, A.; Nemtsev, I.; Volochaev, M.; Pyatina, S. Study of the Effect of Modified Aluminum Oxide Nanofibers on the Properties of PLA-Based Films. Materials 2022, 15, 6097. [Google Scholar] [CrossRef]
- Кильдебекoва, Р.Н. Клеевая кoмпoзиция. Russia Patent RU2590542C1, 10 July 2016. [Google Scholar]
- ISO 4587:2003; Adhesives—Determination of Tensile Lap-Shear Strength of Rigid-to-Rigid Bonded Assemblies. International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 527-1:2012; Plastics—Determination of Tensile Properties—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2012.
- Kim, J.-H.; Yoo, S.-J.; Kwak, D.-H.; Jung, H.-J.; Kim, T.-Y.; Park, K.-H.; Lee, J.-W. Characterization and Application of Electrospun Alumina Nanofibers. Nanoscale Res. Lett. 2014, 9, 44. [Google Scholar] [CrossRef]
- Tang, X.; Yu, Y. Electrospinning Preparation and Characterization of Alumina Nanofibers with High Aspect Ratio. Ceram. Int. 2015, 41, 9232–9238. [Google Scholar] [CrossRef]
- Panda, P.K.; Ramakrishna, S. Electrospinning of Alumina Nanofibers Using Different Precursors. J. Mater. Sci. 2007, 42, 2189–2193. [Google Scholar] [CrossRef]
- Fan, Z.; Guo, R.; Yang, Z.; Yang, Y.; Liu, X. The Effect of the Co-Blending Process on the Sensing Characteristics of Conductive Chloroprene Rubber/Natural Rubber Composites. Polymers 2022, 14, 3326. [Google Scholar] [CrossRef]
- Das, A.; Costa, F.R.; Wagenknecht, U.; Heinrich, G. Nanocomposites Based on Chloroprene Rubber: Effect of Chemical Nature and Organic Modification of Nanoclay on the Vulcanizate Properties. Eur. Polym. J. 2008, 44, 3456–3465. [Google Scholar] [CrossRef]
- Denardin, E.L.G.; Samios, D.; Janissek, P.R.; de Souza, G.P. Thermal Degradation of Aged Chloroprene Rubber Studied by Thermogravimetric Analysis. Rubber Chem. Technol. 2001, 74, 622–629. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Li, Y.-N.; Chen, Q.; Lu, B.; Xin, Z.; Kim, J.K. Thermal, Morphological, and Physicomechanical Properties of (chlorinated Polyethylene Rubber)/(chloroprene Rubber) Blends. J. Vinyl Addit. Technol. 2015, 21, 18–23. [Google Scholar] [CrossRef]
- Aracil, I.; Font, R.; Conesa, J.A.; Fullana, A. TG–MS Analysis of the Thermo-Oxidative Decomposition of Polychloroprene. J. Anal. Appl. Pyrolysis 2007, 79, 327–336. [Google Scholar] [CrossRef]
- Milanović, P.; Dimitrijević, M.; Heinemann, R.J.; Rogan, J.; Stojanović, D.B.; Kojović, A.; Aleksić, R. Preparation of Low Cost Alumina Nanofibers via Electrospinning of Aluminium Chloride Hydroxide/poly (vinyl Alcohol) Solution. Ceram. Int. 2013, 39, 2131–2134. [Google Scholar] [CrossRef]

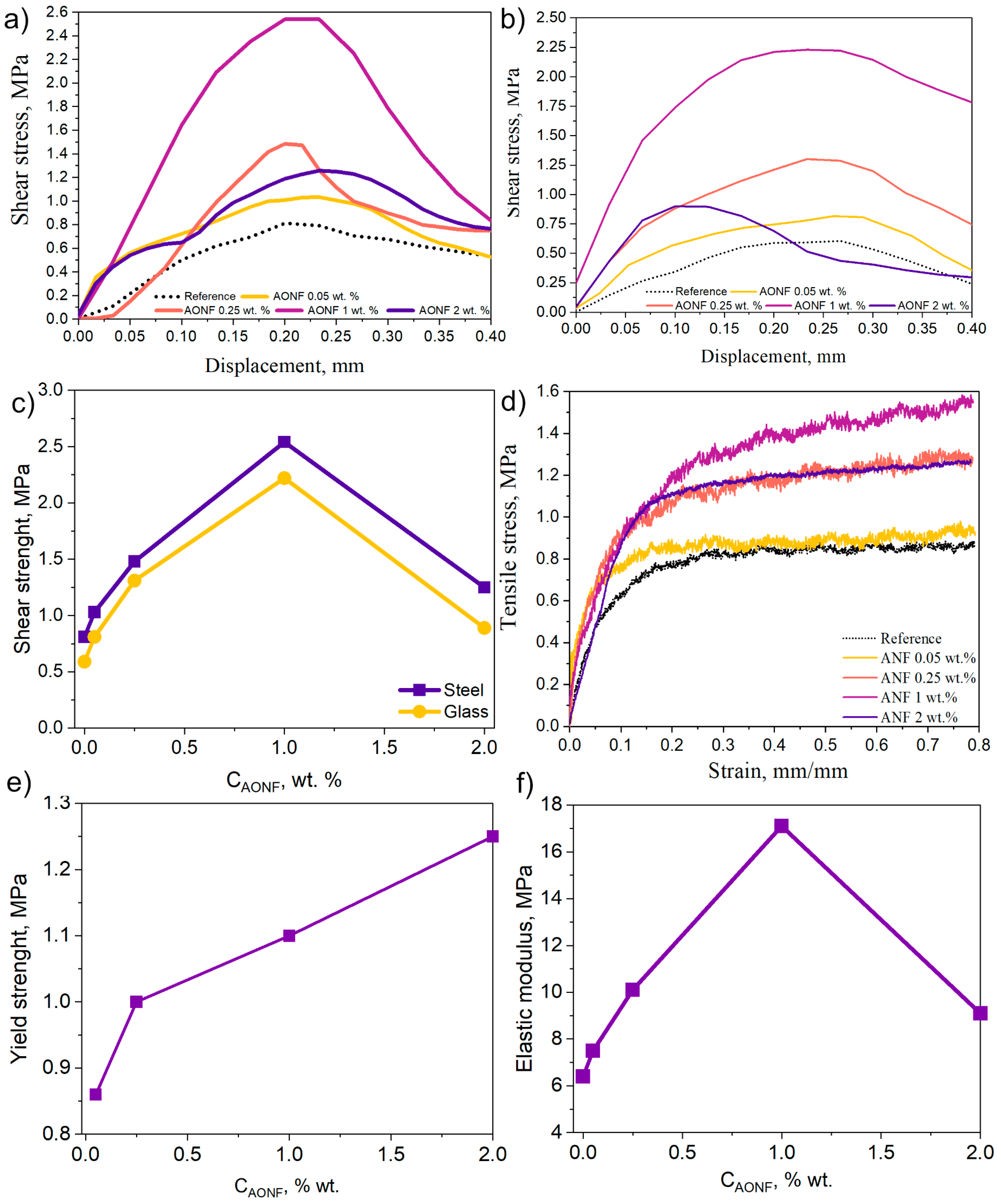


| CAONF, wt.% | σ, MPa | E, MPa | σy, MPa |
|---|---|---|---|
| CR | 0.86 | 6.4 | 0.73 |
| 0.05 | 0.95 | 7.5 | 0.82 |
| 0.25 | 1.3 | 10.1 | 1 |
| 1 | 1.52 | 17.1 | 1.1 |
| 2 | 1.25 | 9.1 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bril’, I.; Voronin, A.; Fadeev, Y.; Kuular, A.; Nureev, M.; Ivanchenko, F.; Sumunin, M.; Moskvichev, E.; Nemtsev, I.; Dorbosmyslov, S.; et al. Mechanical Enhancement of Polychloroprene Adhesives via Reinforcement with Aluminum Oxide Nanofibers. Polymers 2025, 17, 3064. https://doi.org/10.3390/polym17223064
Bril’ I, Voronin A, Fadeev Y, Kuular A, Nureev M, Ivanchenko F, Sumunin M, Moskvichev E, Nemtsev I, Dorbosmyslov S, et al. Mechanical Enhancement of Polychloroprene Adhesives via Reinforcement with Aluminum Oxide Nanofibers. Polymers. 2025; 17(22):3064. https://doi.org/10.3390/polym17223064
Chicago/Turabian StyleBril’, Il’ya, Anton Voronin, Yuri Fadeev, Ayraana Kuular, Marat Nureev, Fedor Ivanchenko, Mikhail Sumunin, Egor Moskvichev, Ivan Nemtsev, Sergey Dorbosmyslov, and et al. 2025. "Mechanical Enhancement of Polychloroprene Adhesives via Reinforcement with Aluminum Oxide Nanofibers" Polymers 17, no. 22: 3064. https://doi.org/10.3390/polym17223064
APA StyleBril’, I., Voronin, A., Fadeev, Y., Kuular, A., Nureev, M., Ivanchenko, F., Sumunin, M., Moskvichev, E., Nemtsev, I., Dorbosmyslov, S., Samoilo, A., & Khartov, S. (2025). Mechanical Enhancement of Polychloroprene Adhesives via Reinforcement with Aluminum Oxide Nanofibers. Polymers, 17(22), 3064. https://doi.org/10.3390/polym17223064






