Hierarchically Porous Polyaniline Exhibiting Enhanced Pseudocapacitive Property from Copolymerization of Aniline and Tetrakis(4-aminophenyl)methane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
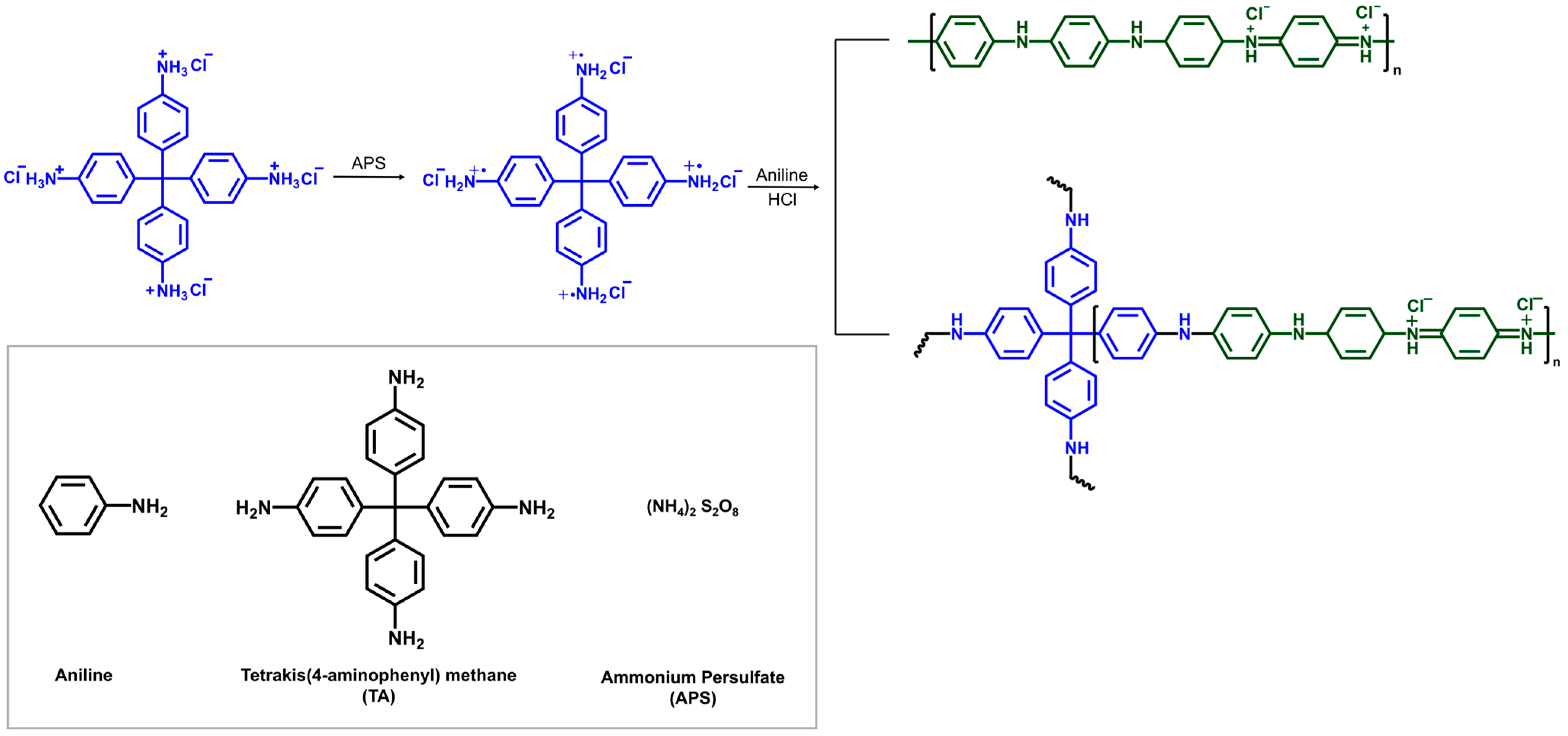
2.2. Synthesis of PANI
2.3. Synthesis of PANI (TA x%)
2.4. Electrode Ink Preparation
2.5. Electrode Preparation for Capacitance Measurement
2.6. Characterization
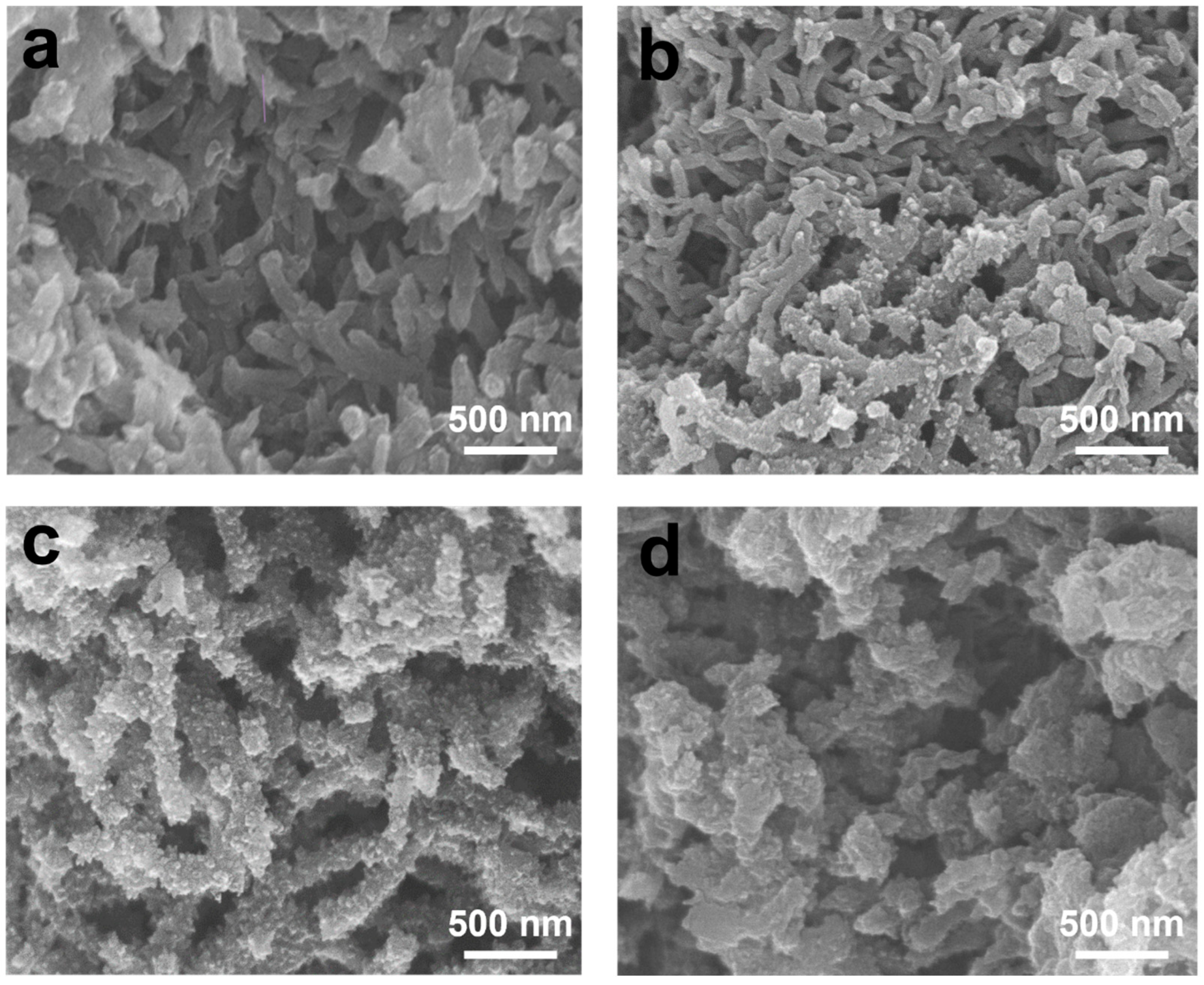
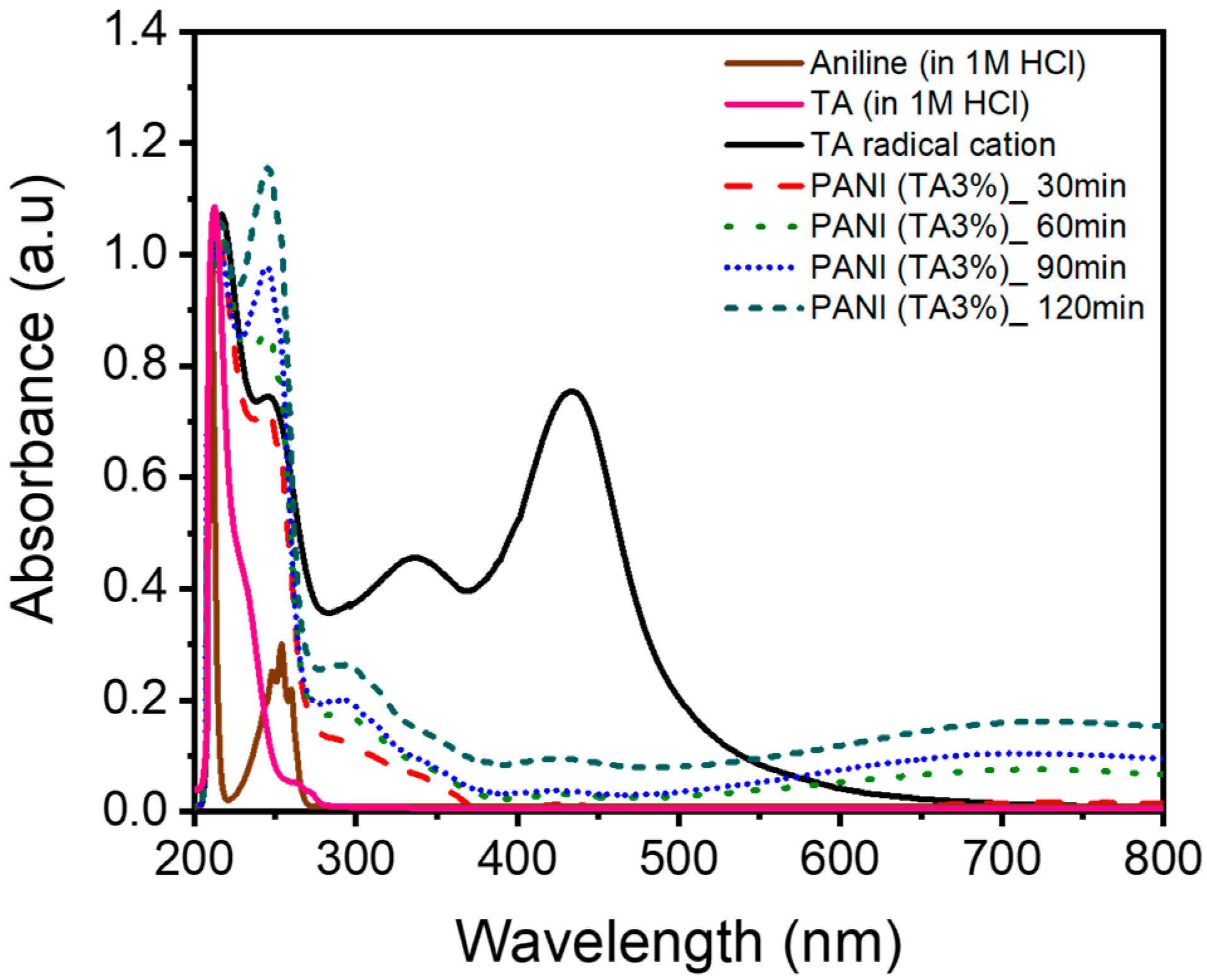
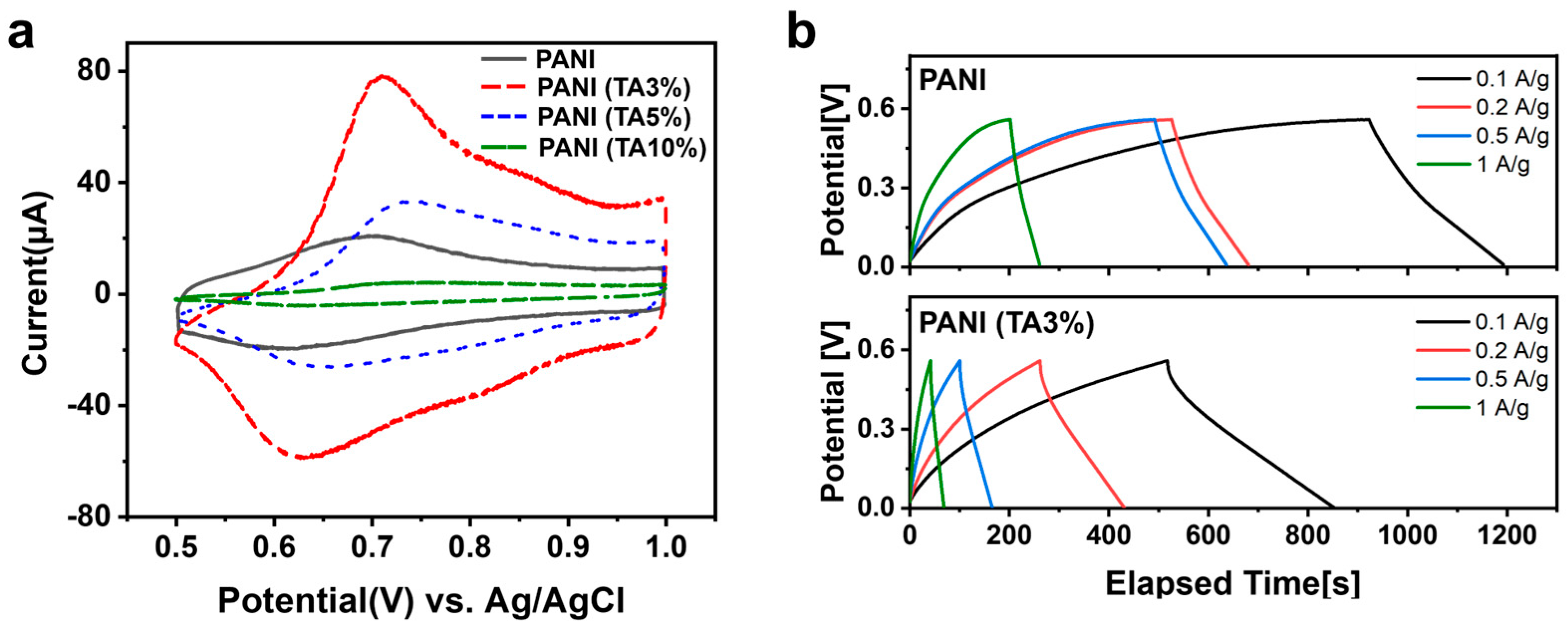
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TA | Tetrakis(4-aminophenyl)methane |
| APS | Ammonium Persulfate |
| PANI | Polyaniline |
| PANI(TA x%) | Polyaniline from copolymerization of aniline and TA (x mol% relative to aniline) |
References
- Naresh, N.; Zhu, Y.; Fan, Y.; Luo, J.; Wang, T.; Parkin, I.P.; Boruah, B.D. Advanced Porous Gold-PANI Micro-Electrodes for High-Performance On-Chip Micro-Supercapacitors. Nano Lett. 2024, 24, 11059–11066. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, Y.; Miao, R.; Liu, L.; Lu, Z.; Zhang, N. Redox Active Lignosulfonate Functionalized Polyaniline-Based Hydrogel Electrodes for Wide Temperature Tolerant Flexible Supercapacitors. ACS Sustain. Chem. Eng. 2024, 12, 13220–13230. [Google Scholar] [CrossRef]
- Deepak, N.; Kumar, A.; Sarkar, A.; Shukla, S.; Saxena, S. Synergistic Interaction of Siloxene and Polyaniline Nanocomposite for High-Performance Supercapacitor Electrodes. Energy Fuels 2024, 38, 13333–13343. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Q.; Chen, Q.; Li, C.M.; Chen, J. Synergistically boosting the electrochemical performance of polypyrrole-coated activated carbon derived from carbon dots for a high-performance supercapacitor. Chem. Commun. 2021, 57, 9264–9267. [Google Scholar] [CrossRef]
- Namsheer, K.; Kenz, M.K.; Lakshmy, S.; Sharma, C.S.; Jeong, S.M.; Rout, C.S. Carbon Nanotube Interconnected Polypyrrole@ E-MXene Organic-Inorganic Hybrids for Interdigitated In-Plane Supercapacitor Applications. Adv. Mater. Technol. 2025, 10, 2401838. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, J.; Yang, C.; Zang, L.; Zhang, G.; Sakai, E. High-Performance PVA/PEDOT:PSS Hydrogel Electrode for All-Gel-State Flexible Supercapacitors. Adv. Mater. Technol. 2021, 6, 2000919. [Google Scholar] [CrossRef]
- Su, Z.; Jin, Y.; Wang, H.; Li, Z.; Huang, L.; Wang, H. PEDOT:PSS and Its Composites for Flexible Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 11915–11932. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N.; Zhang, M.; Zhang, X.; Zhang, Z. Flexible Ti3C2Tx/PEDOT:PSS films with outstanding volumetric capacitance for asymmetric supercapacitors. Dalton Trans. 2019, 48, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.B.; Kiefer, R.; Vo, P.N.X.; Kazantseva, N.E.; Saha, P. Conducting polymers for pseudocapacitors. In Pseudocapacitors: Fundamentals to High Performance Energy Storage Devices; Springer: Cham, Switzerland, 2023; pp. 157–175. [Google Scholar]
- De, B.; Banerjee, S.; Pal, T.; Verma, K.D.; Tyagi, A.; Manna, P.K.; Kar, K.K. Transition Metal Oxide/Electronically Conducting Polymer Composites as Electrode Materials for Supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials II: Performance; Kar, K.K., Ed.; Springer International Publishing: Cham, Germany, 2020; pp. 353–385. [Google Scholar]
- Agarwal, A.; Tolani, R.; Sankapal, B.R. Conducting Polymers–Metal Chalcogenides Hybrid Composite: Current Trends and Future Prospects Toward Supercapacitor Applications. Energy Technol. 2024, 12, 2400133. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Yan, Z.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.-H. Three-Dimensional Tubular MoS2/PANI Hybrid Electrode for High Rate Performance Supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 28294–28302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Wei, B.; Kang, F. Facile synthesis of hierarchical conducting polypyrrole nanostructures via a reactive template of MnO2 and their application in supercapacitors. RSC Adv. 2014, 4, 199–202. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Huang, X. Improved electrochemical performance of hierarchical porous carbon/polyaniline composites. Electrochim. Acta 2012, 74, 98–104. [Google Scholar] [CrossRef]
- Itoi, H.; Hayashi, S.; Matsufusa, H.; Ohzawa, Y. Electrochemical synthesis of polyaniline in the micropores of activated carbon for high-performance electrochemical capacitors. Chem. Commun. 2017, 53, 3201–3204. [Google Scholar] [CrossRef]
- Leal, J.; Garcia, B. Effects of Substituents on UV Spectra of Aniline and Anilinium Ion Derivatives. Hammett Correlations. Z. Phys. Chem. 1988, 269, 26–32. [Google Scholar] [CrossRef]
- Kasemthaveechok, S.; Abella, L.; Crassous, J.; Autschbach, J.; Favereau, L. Organic radicals with inversion of SOMO and HOMO energies and potential applications in optoelectronics. Chem. Sci. 2022, 13, 9833–9847. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; MacDiarmid, A. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Kobaisi, M.A.; Bhosale, R.S.; El-Khouly, M.E.; La, D.D.; Padghan, S.D.; Bhosale, S.V.; Jones, L.A.; Antolasic, F.; Fukuzumi, S.; Bhosale, S.V. The sensitivity of donor–acceptor charge transfer to molecular geometry in DAN–NDI based supramolecular flower-like self-assemblies. Sci. Rep. 2017, 7, 16501. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Ramesh, C.; Elango, M.; Thamilselvan, M. Optical and magnetic studies on Cu2O/PANI nanocomposite prepared by chemical polymerization method. Int. Sch. Res. Not. 2014, 2014, 567927. [Google Scholar] [CrossRef]
- Bairi, V.G.; Bourdo, S.E.; Sacre, N.; Nair, D.; Berry, B.C.; Biris, A.S.; Viswanathan, T. Ammonia gas sensing behavior of tanninsulfonic acid doped polyaniline-TiO2 composite. Sensors 2015, 15, 26415–26429. [Google Scholar] [CrossRef]
- Grivin, A.V.; Kraynik, I.y.I.; Fedko, I.S.; Nechaeva, A.M.; Markova, G.D.; Baranov, O.V.; Suvorov, D.S.; Zakharova, V.A.; Raitman, O.A.; Polunin, S.V. The Kinetics of the Synthesis of Polyaniline Nanoparticles Stabilized by Branched Polyvinyl Alcohol. Macromol. Chem. Phys. 2025, e00283. [Google Scholar] [CrossRef]
- Tzou, K.; Gregory, R. Kinetic study of the chemical polymerization of aniline in aqueous solutions. Synth. Met. 1992, 47, 267–277. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Stejskal, J. The effect of pH on the oxidative polymerization of aniline and the morphology and properties of products. Russ. Chem. Rev. 2010, 79, 1123. [Google Scholar] [CrossRef]
- Mav, I.; Žigon, M. 1H NMR study of the kinetics of substituted aniline polymerization. I. Homopolymerization of 2-methoxyaniline. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2471–2481. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Shishov, M. Oxidative polymerization of aniline: Molecular synthesis of polyaniline and the formation of supramolecular structures. New Polym. Spec. Appl. 2012, 740, 272. [Google Scholar]
- Dhand, C.; Das, M.; Sumana, G.; Srivastava, A.K.; Pandey, M.K.; Kim, C.G.; Datta, M.; Malhotra, B.D. Preparation, characterization and application of polyaniline nanospheres to biosensing. Nanoscale 2010, 2, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-M.; Lin, Y.-W.; Liao, C.-S. Preparation and characterization of polyaniline/multi-walled carbon nanotube composites. Carbon 2005, 43, 734–740. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Zhao, X.; Yu, P.; Wu, L.; Chen, D. Enhanced electrochemical performance of polyaniline/sulfonated polyhedral oligosilsesquioxane nanocomposites with porous and ordered hierarchical nanostructure. J. Mater. Chem. 2012, 22, 1884–1892. [Google Scholar] [CrossRef]
- Tawde, S.; Mukesh, D.; Yakhmi, J. Redox behavior of polyaniline as influenced by aromatic sulphonate anions: Cyclic voltammetry and molecular modeling. Synth. Met. 2001, 125, 401–413. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Gawli, Y.; Banerjee, A.; Dhakras, D.; Deo, M.; Bulani, D.; Wadgaonkar, P.; Shelke, M.; Ogale, S. 3D polyaniline architecture by concurrent inorganic and organic acid doping for superior and robust high rate supercapacitor performance. Sci. Rep. 2016, 6, 21002. [Google Scholar] [CrossRef] [PubMed]
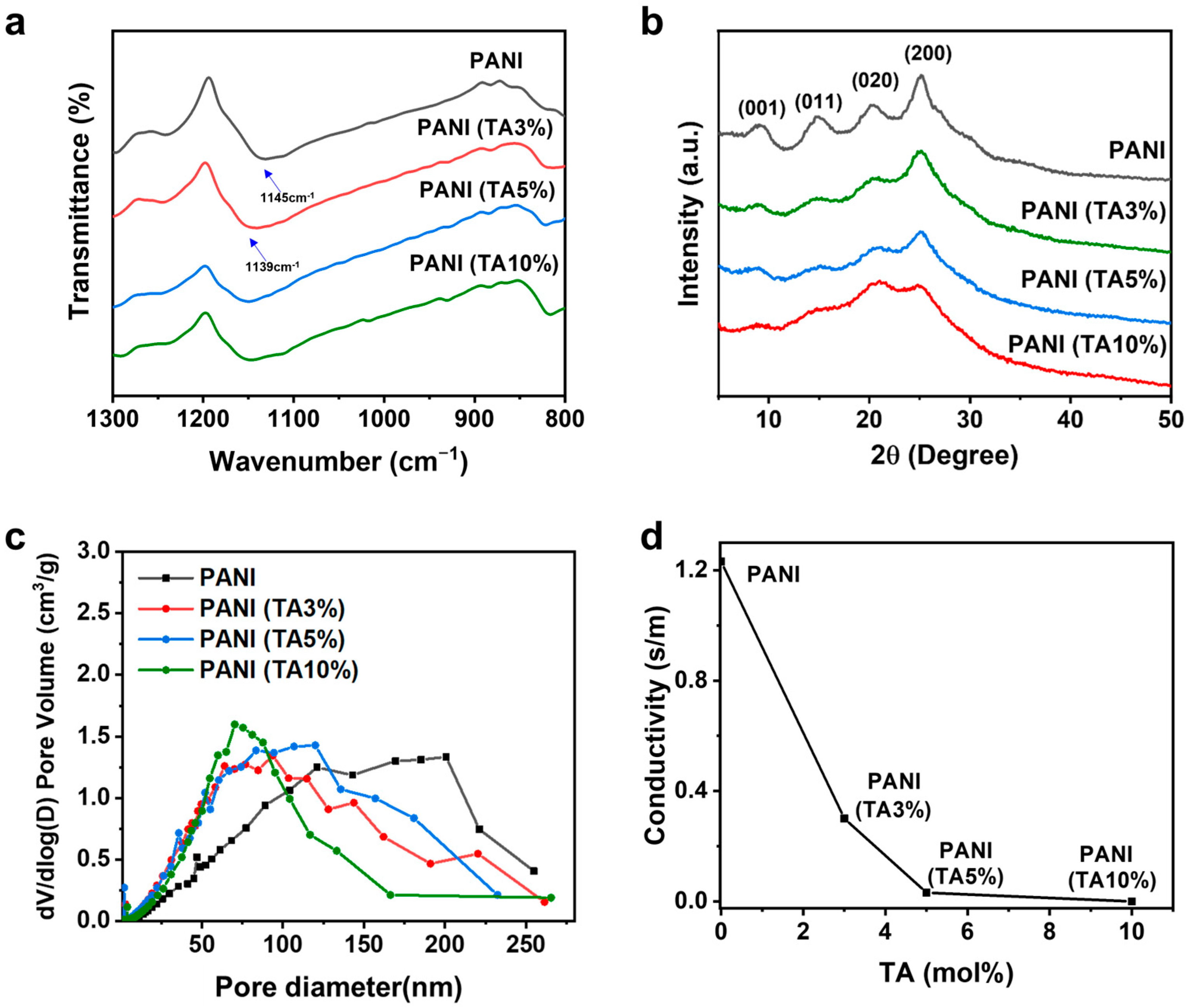
| Sample | BET Surface Area (m2/g) | Average Pore Diameter (Nm) | Conductivity (S/cm) | Specific Capacitance (F/g) |
|---|---|---|---|---|
| PANI | 45 | 73 | 1.23 | 398 |
| PANI (TA 3%) | 77 | 48 | 0.30 | 556 |
| PANI (TA 5%) | 95 | 45 | 0.032 | 530 |
| PANI (TA 10%) | 77 | 48 | 1.02 × 10−6 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Yeo, K.E.; Park, J.-W. Hierarchically Porous Polyaniline Exhibiting Enhanced Pseudocapacitive Property from Copolymerization of Aniline and Tetrakis(4-aminophenyl)methane. Polymers 2025, 17, 3062. https://doi.org/10.3390/polym17223062
Choi J, Yeo KE, Park J-W. Hierarchically Porous Polyaniline Exhibiting Enhanced Pseudocapacitive Property from Copolymerization of Aniline and Tetrakis(4-aminophenyl)methane. Polymers. 2025; 17(22):3062. https://doi.org/10.3390/polym17223062
Chicago/Turabian StyleChoi, Jinsoon, Kyeong Eun Yeo, and Ji-Woong Park. 2025. "Hierarchically Porous Polyaniline Exhibiting Enhanced Pseudocapacitive Property from Copolymerization of Aniline and Tetrakis(4-aminophenyl)methane" Polymers 17, no. 22: 3062. https://doi.org/10.3390/polym17223062
APA StyleChoi, J., Yeo, K. E., & Park, J.-W. (2025). Hierarchically Porous Polyaniline Exhibiting Enhanced Pseudocapacitive Property from Copolymerization of Aniline and Tetrakis(4-aminophenyl)methane. Polymers, 17(22), 3062. https://doi.org/10.3390/polym17223062





