Effect of Corn Starch as Stabilizer Particle in Combination with Egg White Proteins in Natural Rubber Latex Biofoams Produced by Microwave Foaming
Abstract
1. Introduction
2. Materials and Production Process
2.1. Materials
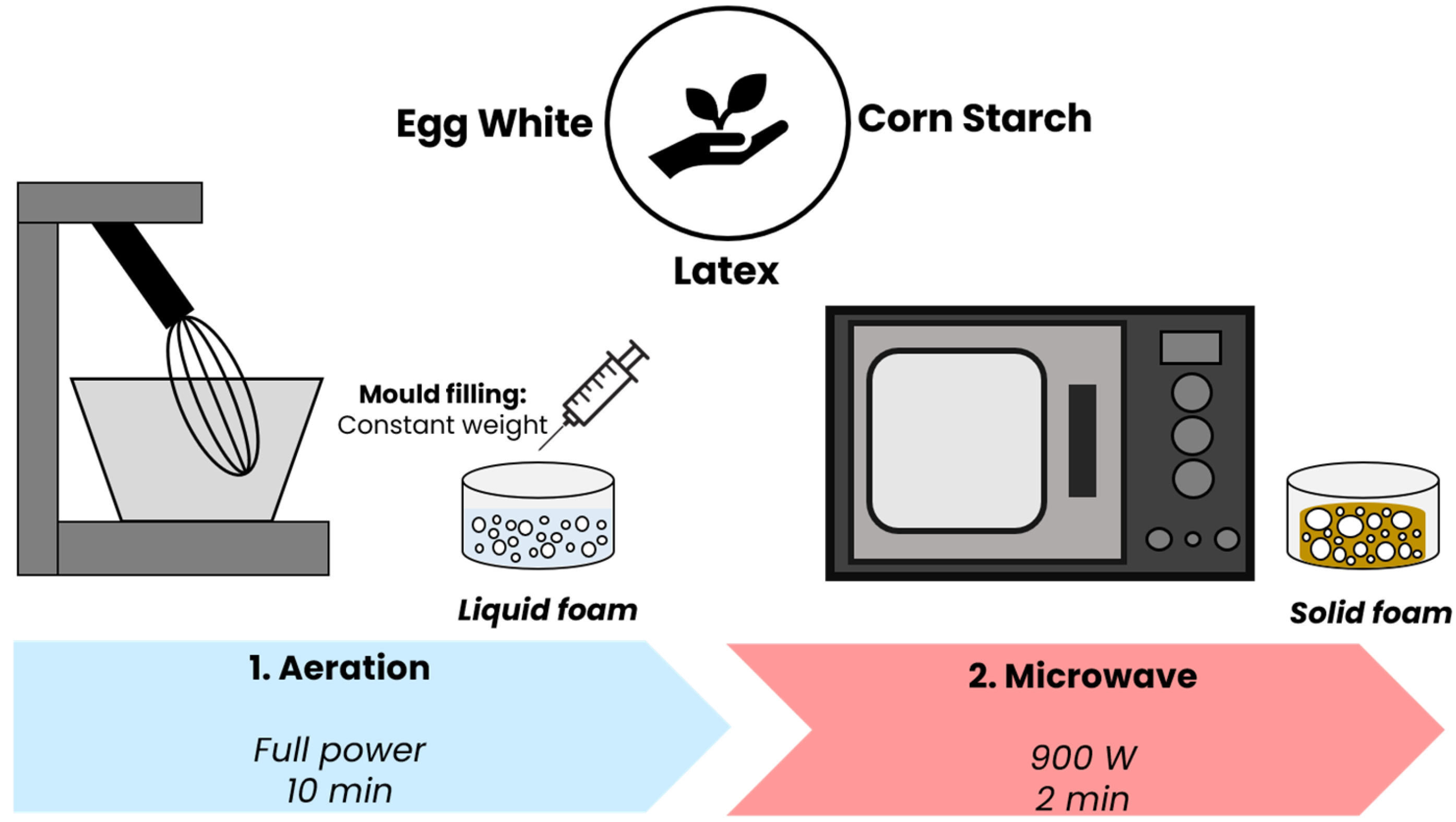
2.2. Production Process of Natural Rubber Latex Foams
3. Characterization Techniques
3.1. Density
3.2. Cellular Structure
3.3. Thermogravimetric Analysis (TGA)
3.4. Dehydration of Solid Samples
3.5. Mechanical Tests
4. Results and Discussion
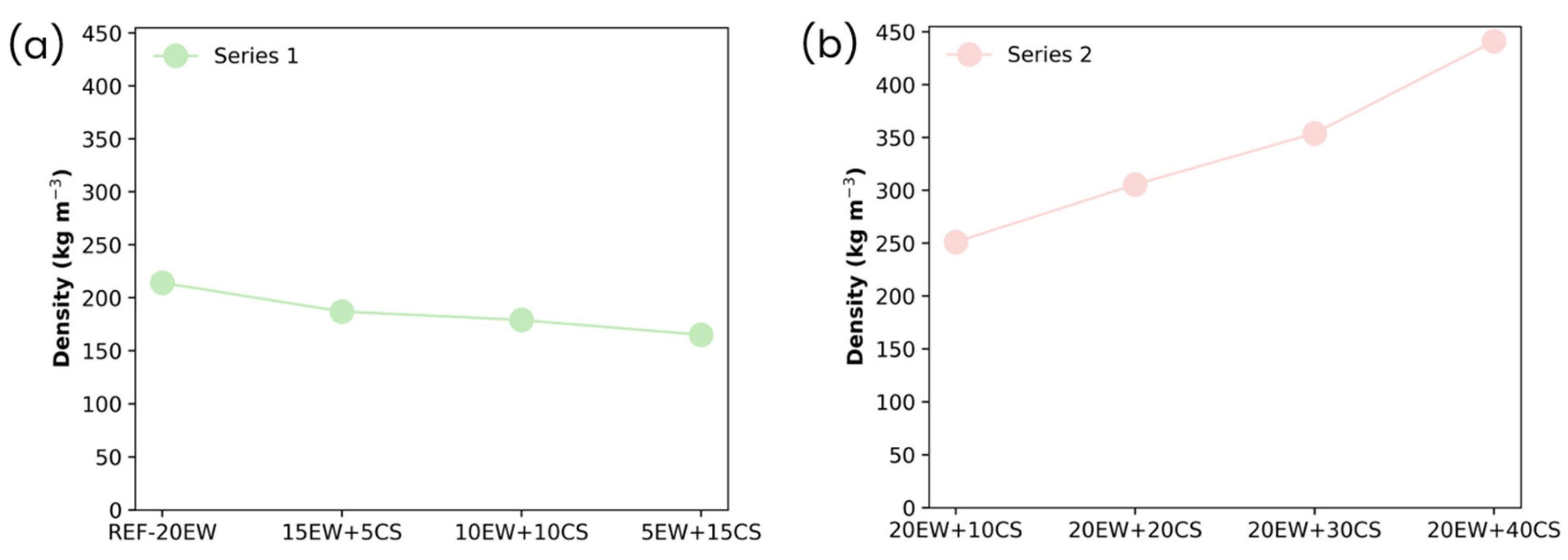
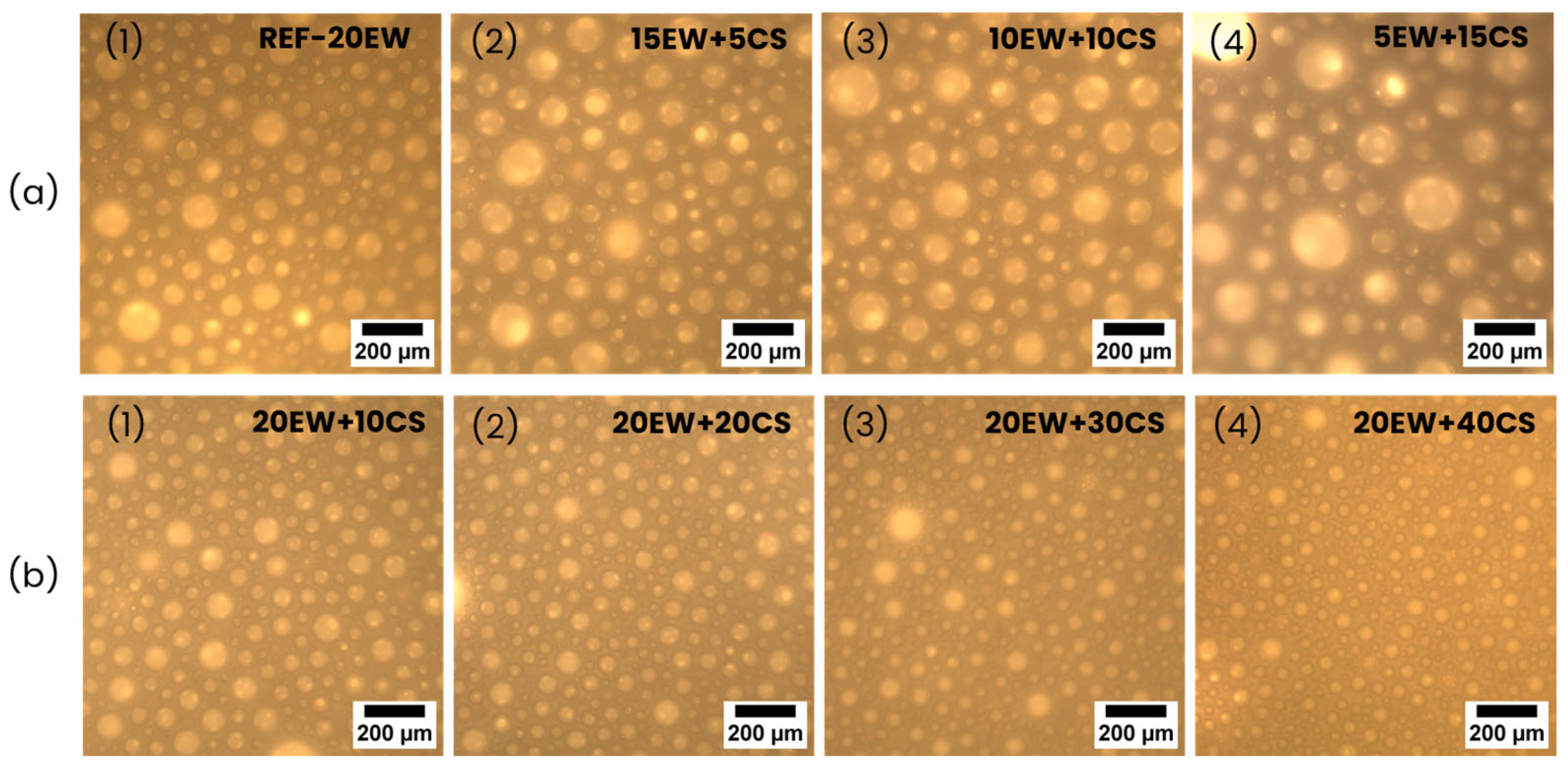
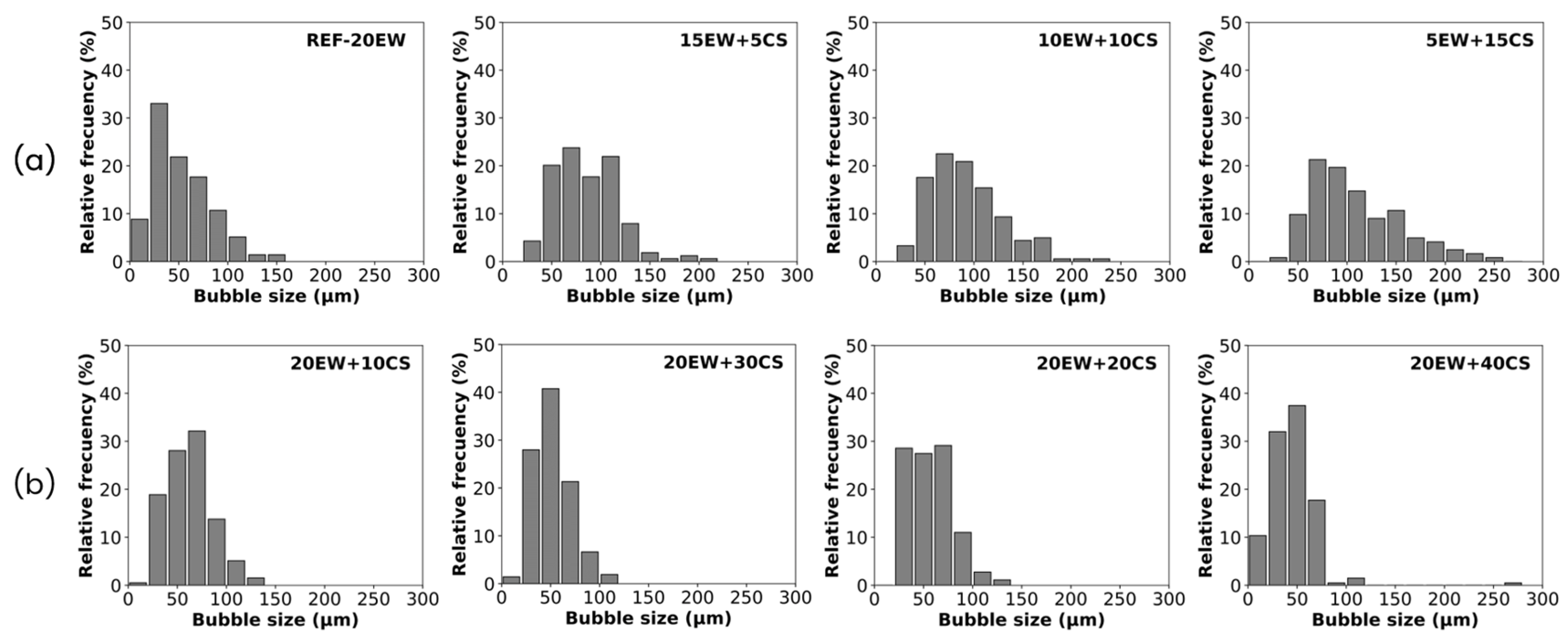
4.1. Liquid Foams
4.2. Solid Foams
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Gil, A.; Rodriguez-Perez, M.A.; De Saja, J.A.; Bellucci, F.S.; Ardanuy, M. Strategies to Improve the Mechanical Properties of Starch-Based Materials: Plasticization and Natural Fibers Reinforcement. Polímeros Ciência e Tecnol. 2014, 24, 36–42. [Google Scholar] [CrossRef]
- European Environment Agency. Available online: https://www.eea.europa.eu/en/topics/in-depth/plastics (accessed on 8 July 2025).
- Rio, E.; Drenckhan, W.; Salonen, A.; Langevin, D. Unusually stable liquid foams. Adv. Colloid Interface Sci. 2014, 205, 74–86. [Google Scholar] [CrossRef]
- van der Net, A.; Gryson, A.; Ranft, M.; Elias, F.; Stubenrauch, C.; Drenckhan, W. Highly structured porous solids from liquid foam templates. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 5–10. [Google Scholar] [CrossRef]
- Briceño-Ahumada, Z.; Mikhailovskaya, A.; Staton, J.A. The role of continuous phase rheology on the stabilization of edible foams: A review. Phys. Fluids 2022, 34, 031302. [Google Scholar] [CrossRef]
- Zhang, X.D.; Macosko, C.W.; Davis, H.T.; Nikolov, A.D.; Wasan, D.T. Role of silicone surfactant in flexible polyurethane foam. J. Colloid Interface Sci. 1999, 215, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, S.H.; Kim, B.K. Effects of silicon surfactant in rigid polyurethane foams. Express Polym. Lett. 2008, 2, 194–200. [Google Scholar] [CrossRef]
- Kudori, S.N.I.; Ismail, H.; Khimi, S.R. Tensile and morphological properties on kenaf core or bast filled natural rubber latex foam (NRLF). Mater. Today Proc. 2019, 17, 609–615. [Google Scholar] [CrossRef]
- Salmazo, L.O.; Lopez-Gil, A.; Ariff, Z.M.; Job, A.E.; Rodriguez-Perez, M.A. Influence of the irradiation dose in the cellular structure of natural rubber foams cross-linked by electron beam irradiation. Ind. Crops Prod. 2016, 89, 339–349. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J.; Shi, Z. Biodegradable PLA/PBS open-cell foam fabricated by supercritical CO2 foaming for selective oil-adsorption. Sep. Purif. Technol. 2021, 257, 117949. [Google Scholar] [CrossRef]
- Hu, D.; Xue, K.; Liu, Z.; Xu, Z.; Zhao, L. The essential role of PBS on PBAT foaming under supercritical CO2 toward green engineering. J. CO2 Util. 2022, 60, 101965. [Google Scholar] [CrossRef]
- Ivanič, F.; Kováčová, M.; Chodák, I. The effect of plasticizer selection on properties of blends poly(butylene adipate-co-terephthalate) with thermoplastic starch. Eur. Polym. J. 2019, 116, 99–105. [Google Scholar] [CrossRef]
- Averous, L.; Boquillon, N. Biocomposites based on plasticized starch: Thermal and mechanical behaviours. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Fourati, Y.; Tarrés, Q.; Mutjé, P.; Boufi, S. PBAT/thermoplastic starch blends: Effect of compatibilizers on the rheological, mechanical and morphological properties. Carbohydr. Polym. 2018, 199, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Quilez-Molina, A.I.; Oliveira-Salmazo, L.; Amezúa-Arranz, C.; López-Gil, A.; Rodríguez-Pérez, M.Á. Evaluation of the acid hydrolysis as pre-treatment to enhance the integration and functionality of starch composites filled with rich-in-pectin agri-food waste orange peel. Ind. Crops Prod. 2023, 205, 117407. [Google Scholar] [CrossRef]
- Lopez-Gil, A.; Silva-Bellucci, F.; Velasco, D.; Ardanuy, M.; Rodriguez-Perez, M.A. Cellular structure and mechanical properties of starch-based foamed blocks reinforced with natural fibers and produced by microwave heating. Ind. Crops Prod. 2015, 66, 194–205. [Google Scholar] [CrossRef]
- Quilez-Molina, A.I.; Oliveira-Salmazo, L.; López-Gil, A.; Rodríguez-Pérez, M.Á. Novel flexible and active expanded-starch films enriched with Agrifood waste via microwave irradiation. Future Foods 2024, 10, 100508. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palm oil-based bio-polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Amezúa-Arranz, C.; Santiago-Calvo, M.; Rodríguez-Pérez, M.Á. A new synthesis route to produce isocyanate-free polyurethane foams. Eur. Polym. J. 2023, 197, 112366. [Google Scholar] [CrossRef]
- Blattmann, H.; Lauth, M.; Mülhaupt, R. Flexible and Bio-Based Nonisocyanate Polyurethane (NIPU) Foams. Macromol. Mater. Eng. 2016, 301, 944–952. [Google Scholar] [CrossRef]
- Pinrat, S.; Dittanet, P.; Seubsai, A.; Prapainainar, P. Fabrication of Natural Rubber Latex Foam Composite Filled with Pineapple-leaf Cellulose Fibres. J. Phys. Conf. Ser. 2022, 2175, 012038. [Google Scholar] [CrossRef]
- Jitkokkruad, K.; Jarukumjorn, K.; Raksakulpiwat, C.; Chaiwong, S.; Rattanakaran, J.; Trongsatitkul, T. Effects of Bamboo Leaf Fiber Content on Cushion Performance and Biodegradability of Natural Rubber Latex Foam Composites. Polymers 2023, 15, 654. [Google Scholar] [CrossRef]
- Kudori, S.N.I.; Ismail, H. The effects of filler contents and particle sizes on properties of green kenaf-filled natural rubber latex foam. Cell. Polym. 2020, 39, 57–68. [Google Scholar] [CrossRef]
- Bashir, A.S.M.; Manusamy, Y.; Chew, T.L.; Ismail, H.; Ramasamy, S. Mechanical, thermal, and morphological properties of (eggshell powder)-filled natural rubber latex foam. J. Vinyl Addit. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef]
- Amezúa-Arranz, C.; Salmazo, L.O.; López-Gil, A.; Rodríguez-Pérez, M.Á. Effect of Egg White Protein and Water Content on the Stabilization Mechanisms of Natural Rubber Latex Foams Obtained from Microwave Radiation. Adv. Eng. Mater. 2024, 15, 2301922. [Google Scholar] [CrossRef]
- Ariff, Z.M.; Afolabi, L.O.; Salmazo, L.O.; Rodriguez-Perez, M.A. Effectiveness of microwave processing approach and green blowing agents usage in foaming natural rubber. J. Mater. Res. Technol. 2020, 9, 9929–9940. [Google Scholar] [CrossRef]
- Mine, Y. Recent advances in the understanding of egg white protein functionality. Trends Food Sci. Technol. 1995, 6, 225–232. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Geera, B.; Rybak, D.A. Effects of egg and egg replacers on yellow cake product quality. J. Food Process. Preserv. 2012, 36, 21–29. [Google Scholar] [CrossRef]
- Tan, T.C.; Kanyarat, K.; Azhar, M.E. Evaluation of functional properties of egg white obtained from pasteurized shell egg as ingredient in angel food cake. Int. Food Res. J. 2012, 19, 303–308. [Google Scholar]
- Li, X.; Li, J.; Chang, C.; Wang, C.; Zhang, M.; Su, Y.; Yang, Y. Foaming characterization of fresh egg white proteins as a function of different proportions of egg yolk fractions. Food Hydrocoll. 2019, 90, 118–125. [Google Scholar] [CrossRef]
- Guo, X.N.; Gao, F.; Zhu, K.X. Effect of fresh egg white addition on the quality characteristics and protein aggregation of oat noodles. Food Chem. 2020, 330, 127319. [Google Scholar] [CrossRef]
- Dickinson, E.; Izgi, E. Foam stabilization by protein-polysaccharide complexes. Colloids Surf. A Physicochem. Eng. Asp. 1996, 113, 191–201. [Google Scholar] [CrossRef]
- Gharbi, N.; Labbafi, M. Influence of treatment-induced modification of egg white proteins on foaming properties. Food Hydrocoll. 2019, 90, 72–81. [Google Scholar] [CrossRef]
- Lechevalier, V.; Jeantet, R.; Arhaliass, A.; Legrand, J.; Nau, F. Egg white drying: Influence of industrial processing steps on protein structure and functionalities. J. Food Eng. 2007, 83, 404–413. [Google Scholar] [CrossRef]
- Järviö, N.; Parviainen, T.; Maljanen, N.L.; Kobayashi, Y.; Kujanpää, L.; Ercili-Cura, D.; Landowski, C.P.; Ryynänen, T.; Nordlund, E.; Tuomisto, H.L. Ovalbumin production using Trichoderma reesei culture and low-carbon energy could mitigate the environmental impacts of chicken-egg-derived ovalbumin. Nat. Food 2021, 2, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xiao, N.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Wu, N.; Tu, Y. Effect of polysaccharides on the functional properties of egg white protein: A review. J. Food Sci. 2021, 86, 656–666. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, X.; Yang, W.; Liu, H.; Yu, L. Impact of citrus pectin on the foaming behavior, structures, and barrier properties of the starch foam blocks. Food Chem. 2025, 463, 141500. [Google Scholar] [CrossRef]
- Sjöqvist, M.; Gatenholm, P. The effect of starch composition on structure of foams prepared by microwave treatment. J. Polym. Environ. 2005, 13, 29–37. [Google Scholar] [CrossRef]
- Peng, L.; Zhongdong, L.; Kennedy, J.F. The study of starch nano-unit chains in the gelatinization process. Carbohydr. Polym. 2007, 68, 360–366. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; Xu, B. Morphological, physicochemical, and pasting properties of pre-gelatinized starch prepared by high-pressure homogenizer: A comparative study on starches from different resources. Food Res. Int. 2024, 197, 115294. [Google Scholar] [CrossRef] [PubMed]
- ASTM D1622-08; Standard Test Method for Apparent Density of Rigid Cellular Plastics. ASTM: West Conshohocken, PA, USA, 2008. Available online: https://store.astm.org/d1622-08.html (accessed on 12 November 2025).
- Pinto, J.; Solórzano, E.; Rodriguez-Perez, M.A.; De Saja, J.A. Characterization of the cellular structure based on user-interactive image analysis procedures. J. Cell. Plast. 2013, 49, 555–575. [Google Scholar] [CrossRef]
- ASTM D1621-16; Standard Test Method for Compressive Properties of Rigid Cellular Plastics. ASTM: West Conshohocken, PA, USA, 2023. [CrossRef]
- ISO 291:2008; Plastics—Standard Atmospheres for Conditioning and Testing. ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/50572.html (accessed on 12 November 2025).
- Seguchi, M.; Yamada, Y. Hydrophobic Character of Heat-Treated Wheat Starch. Cereal Chem. 1998, 65, 375–376. [Google Scholar]
- Zhang, Y.; Chang, Z.; Luo, W.; Gu, S.; Li, W.; An, J. Effect of starch particles on foam stability and dilational viscoelasticity of aqueous-foam. Chin. J. Chem. Eng. 2015, 23, 276–280. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.L.; Wang, L.; Jiang, J. Unlocking the Potential of Plant-Based Egg Analogs: Mechanisms, Challenges, and Strategies for Diverse Food Systems. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70287. [Google Scholar] [CrossRef]
- Patino, J.M.R.; Pilosof, A.M.R. Protein-polysaccharide interactions at fluid interfaces. Food Hydrocoll. 2011, 25, 1925–1937. [Google Scholar] [CrossRef]
- Murray, B.S.; Durga, K.; Yusoff, A.; Stoyanov, S.D. Stabilization of foams and emulsions by mixtures of surface active food-grade particles and proteins. Food Hydrocoll. 2011, 25, 627–638. [Google Scholar] [CrossRef]
- Asghari, A.K.; Norton, I.; Mills, T.; Sadd, P.; Spyropoulos, F. Interfacial and foaming characterisation of mixed protein-starch particle systems for food-foam applications. Food Hydrocoll. 2016, 53, 311–319. [Google Scholar] [CrossRef]
- Brownsey, G.J.; Orford, P.D.; Ridout, M.J.; Ring, S.G. A study of the mechanical behaviour and microstructure of a mixed starch—Egg-white protein gel. Top. Catal. 1989, 3, 7–17. [Google Scholar] [CrossRef]
- Sadahira, M.S.; Rodrigues, M.I.; Akhtar, M.; Murray, B.S.; Netto, F.M. Influence of pH on foaming and rheological properties of aerated high sugar system with egg white protein and hydroxypropylmethylcellulose. LWT—Food Sci. Technol. 2018, 89, 350–357. [Google Scholar] [CrossRef]
- Schmidt, I.; Novales, B.; Boué, F.; Axelos, M.A.V. Foaming properties of protein/pectin electrostatic complexes and foam structure at nanoscale. J. Colloid Interface Sci. 2010, 345, 316–324. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, L.; Karrar, E.; Qi, X.; Zhang, H.; Wu, G. Pickering foams stabilized by protein-based particles: A review of characterization, stabilization, and application. Trends Food Sci. Technol. 2023, 133, 148–159. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, L.; Zhang, H.; Liu, T.; Wu, G. Characteristic of the interaction mechanism between soy protein isolate and functional polysaccharide with different charge characteristics and exploration of the foaming properties. Food Hydrocoll. 2024, 150, 109615. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; Xu, Z.; Feng, X.; Kong, Q.; Ren, X. Efficient binding paradigm of protein and polysaccharide: Preparation of isolated soy protein-chitosan quaternary ammonium salt complex system and exploration of its emulsification potential. Food Chem. 2023, 407, 135111. [Google Scholar] [CrossRef]
- Sadahira, M.S.; Lopes, F.C.R.; Rodrigues, M.I.; Netto, F.M. Influence of protein-pectin electrostatic interaction on the foam stability mechanis. Carbohydr. Polym. 2014, 103, 55–61. [Google Scholar] [CrossRef]
- Jamilah, B.; Mohamed, A.; Abbas, K.A.; Rahman, R.A.; Karim, R.; Hashim, D.M. Protein-starch interaction and their effect on thermal and rheological characteristics of a food system: A review. J. Food Agric. Environ. 2009, 7, 169–174. [Google Scholar]
- Murray, B.S. Recent developments in food foams. Curr. Opin. Colloid Interface Sci. 2020, 50, 101394. [Google Scholar] [CrossRef]
- Asami, J.; Quevedo, B.V.; Santos, A.R.; Giorno, L.P.; Komatsu, D.; de Rezende Duek, E.A. The impact of non-deproteinization on physicochemical and biological properties of natural rubber latex for biomedical applications. Int. J. Biol. Macromol. 2023, 253, 126782. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, I.; Jridi, M.; Nasri, R.; Dammak, A.; Hajji, M.; Nasri, M.; Barkia, A. Characteristics and functional properties of gelatin from thornback ray skin obtained by pepsin-aided process in comparison with commercial halal bovine gelatin. Food Hydrocoll. 2014, 41, 309–318. [Google Scholar] [CrossRef]
- Luciano, G.; Vignali, A.; Vignolo, M.; Utzeri, R.; Bertini, F.; Iannace, S. Biocomposite Foams with Multimodal Cellular Structures Based on Cork Granulates and Microwave Processed Egg White Proteins. Materials 2023, 16, 3063. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Xie, F.; Li, M.; Chen, L.; Li, X. Kinetics and mechanism of thermal decomposition of cornstarches with different amylose/amylopectin ratios. Starch/Staerke 2010, 62, 139–146. [Google Scholar] [CrossRef]
- Pineda-Gõmez, P.; Angel-Gil, N.C.; Valencia-Muñoz, C.; Rosales-Rivera, A.; Rodríguez-García, M.E. Thermal degradation of starch sources: Green banana, potato, cassava, and corn—Kinetic study by non-isothermal procedures. Starch/Staerke 2014, 66, 691–699. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Pergamon Press: Oxford, UK, 1997. [Google Scholar]
- Schirmer, M.; Jekle, M.; Becker, T. Starch gelatinization and its complexity for analysis. Starch-Starke 2014, 67, 30–41. [Google Scholar] [CrossRef]
- Hu, D.; Yang, G.; Tian, Y.; Li, M.; Fan, L.; Li, R.; Wang, S. Effect of radio frequency heating on structure and physicochemical properties of protein and starch based on gelatinization degree of rice flour. Food Res. Int. 2025, 218, 116902. [Google Scholar] [CrossRef] [PubMed]
- Ignatzy, L.M.; Kern, K.; Muranyi, I.S.; Alpers, T.; Becker, T.; Gola, S.; Schweiggert-Weisz, U. Thermal, rheological, and microstructural characterization of composite gels from fava bean protein and pea starch. Food Hydrocoll. 2025, 172. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Min, G.; Qiao, D.; Zhang, B.; Niu, M.; Jia, C.; Xu, Y.; Lin, Q. Starch-protein interplay varies the multi-scale structures of starch undergoing thermal processing. Int. J. Biol. Macromol. 2021, 175, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Awika, J.M. Effect of protein–starch interactions on starch retrogradation and implications for food product quality. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2081–2111. [Google Scholar] [CrossRef]
- Waziiroh, E.; Bender, D.; Fuhrmann, P.L.; Schoenlechner, R. Effect of non-covalent interactions on gluten-free batter stability and bread properties. LWT 2024, 215. [Google Scholar] [CrossRef]
- Crockett, R.; Ie, P.; Vodovotz, Y. Effects of soy protein isolate and egg white solids on the physicochemical properties of gluten-free bread. Food Chem. 2011, 129, 84–91. [Google Scholar] [CrossRef]

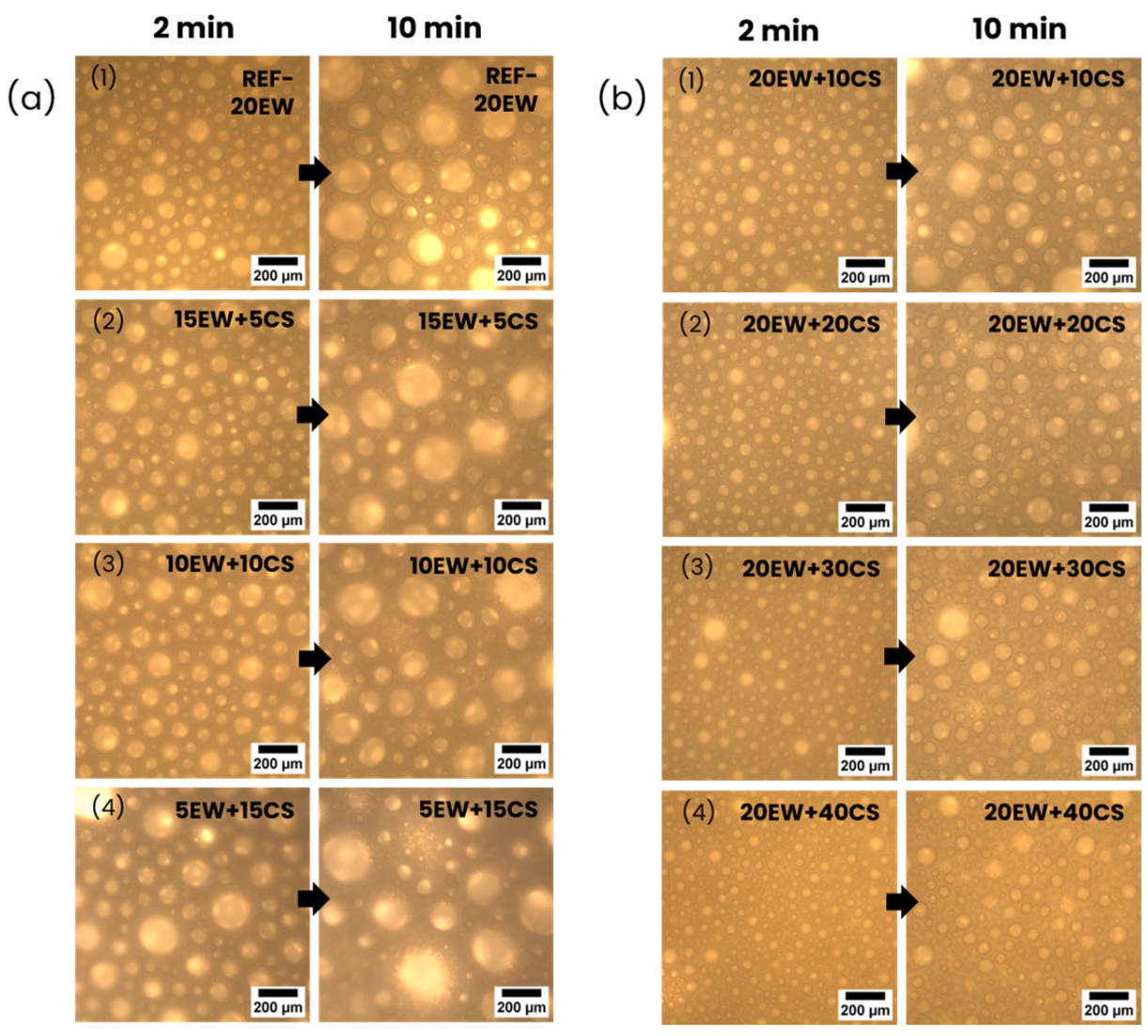
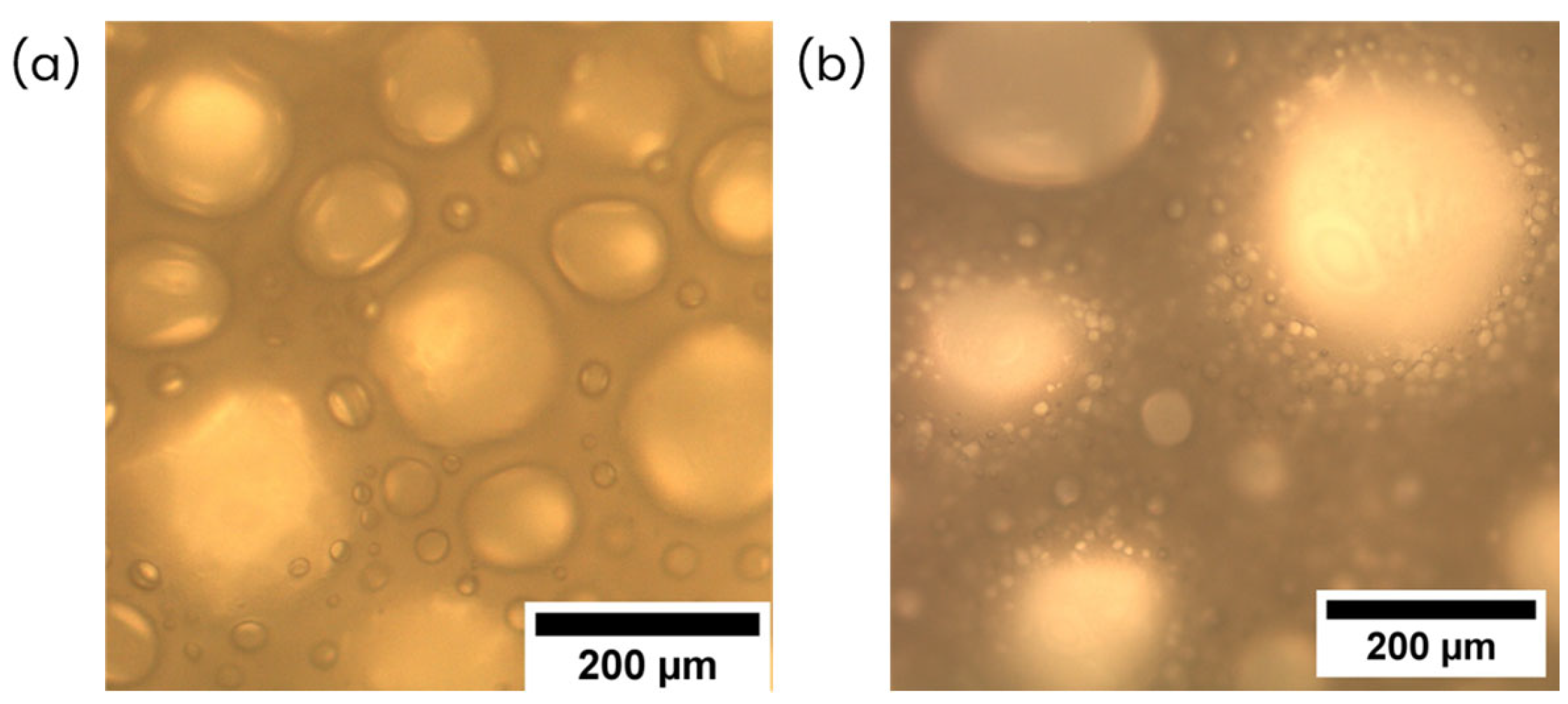
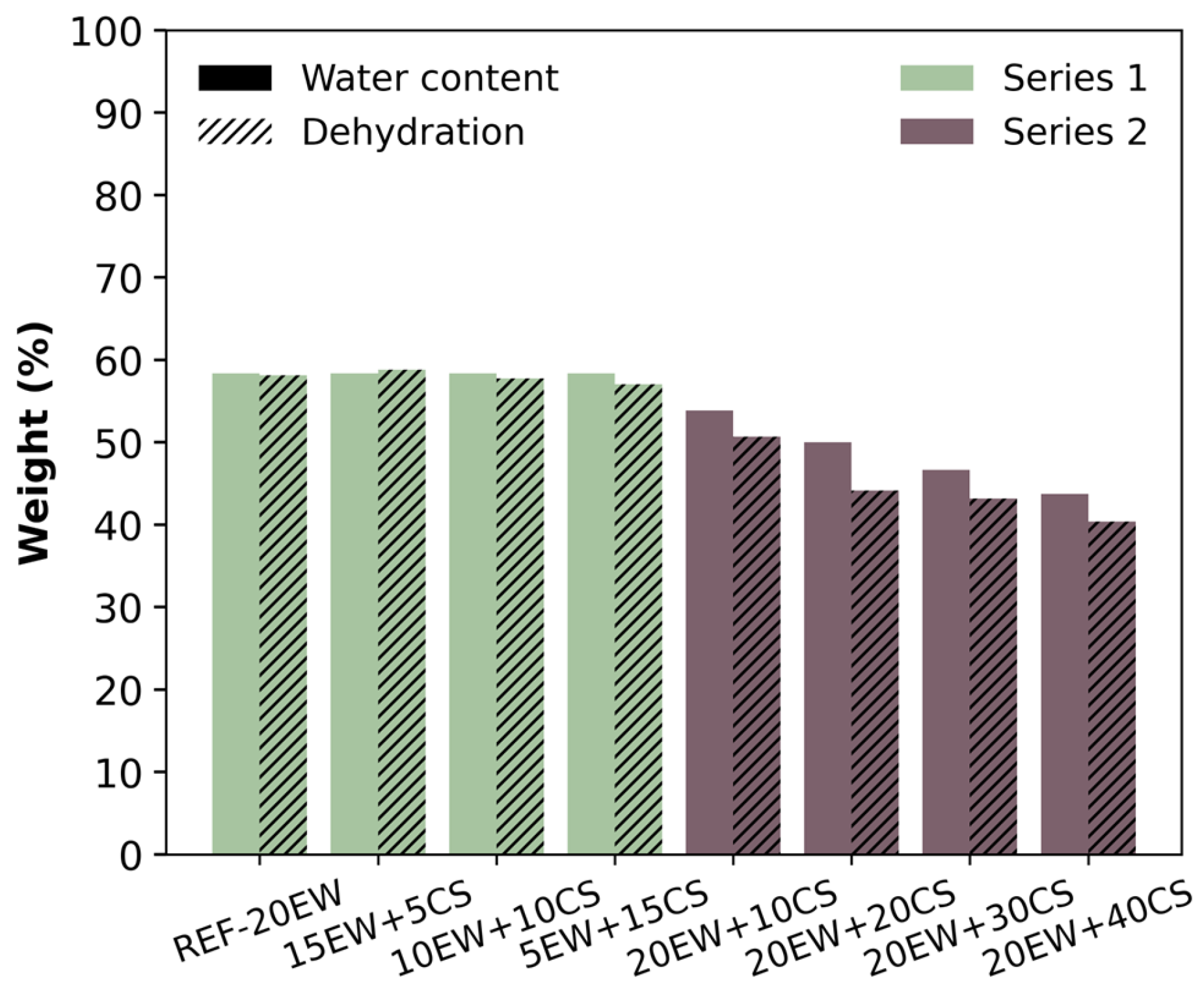

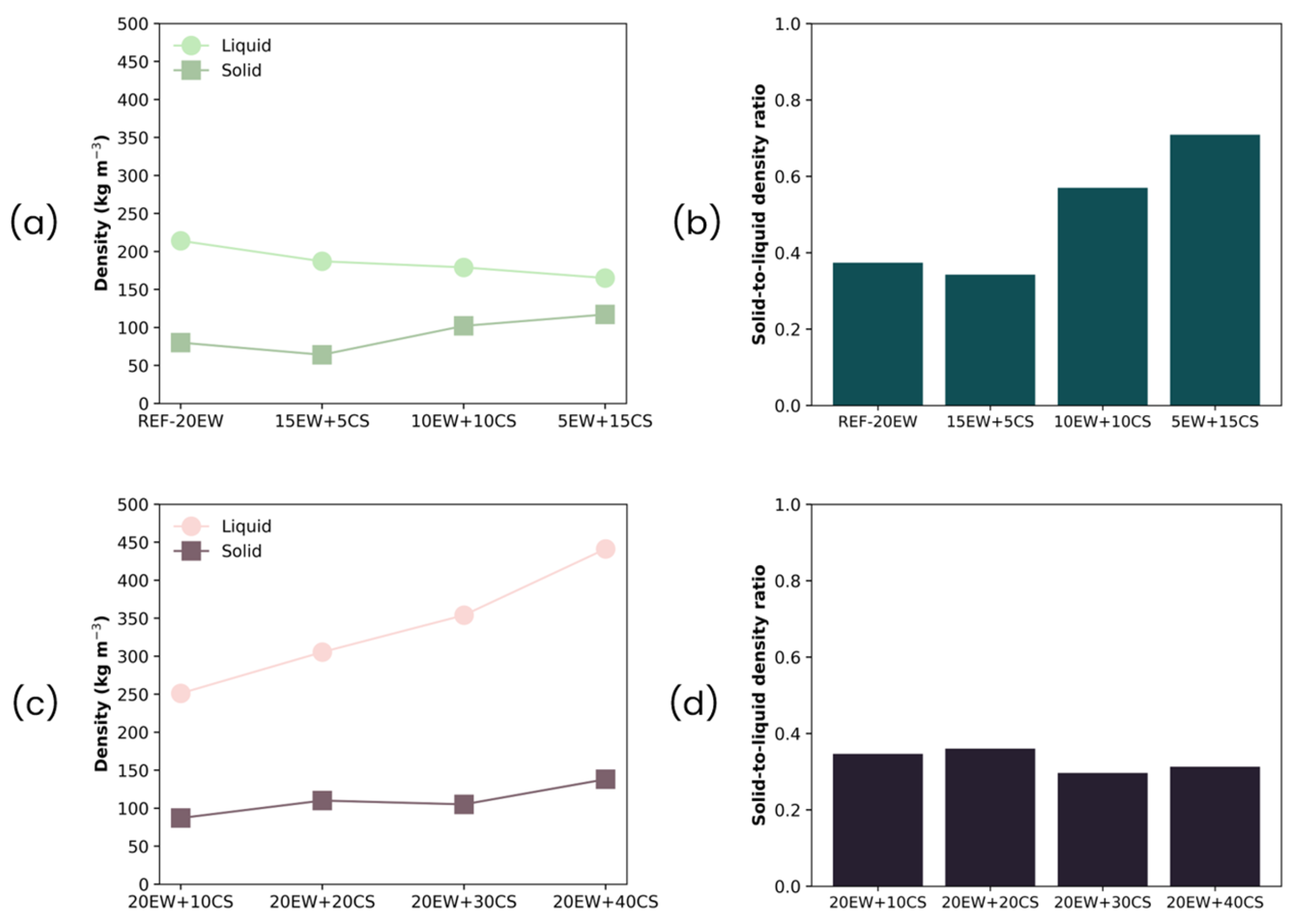
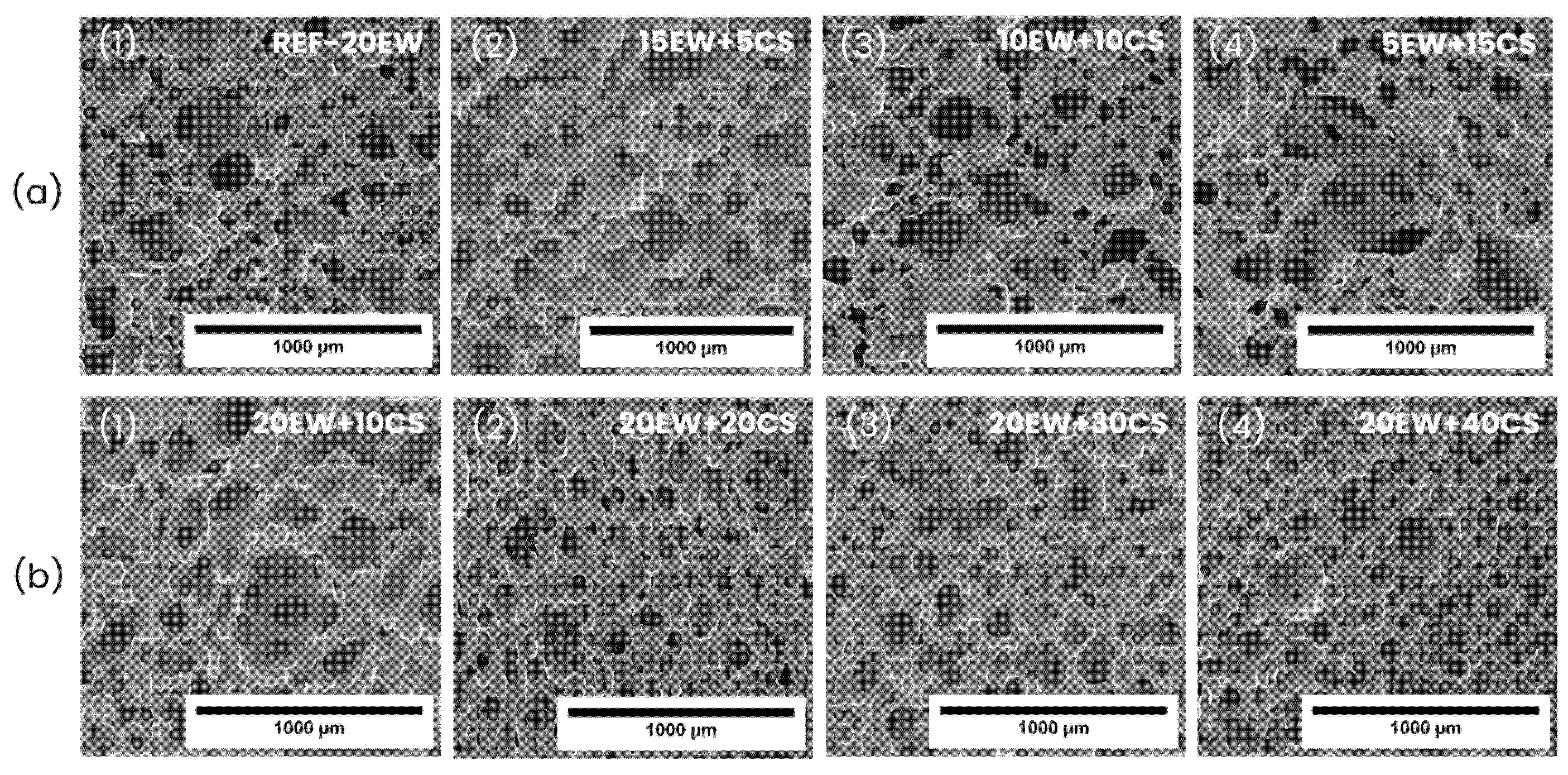
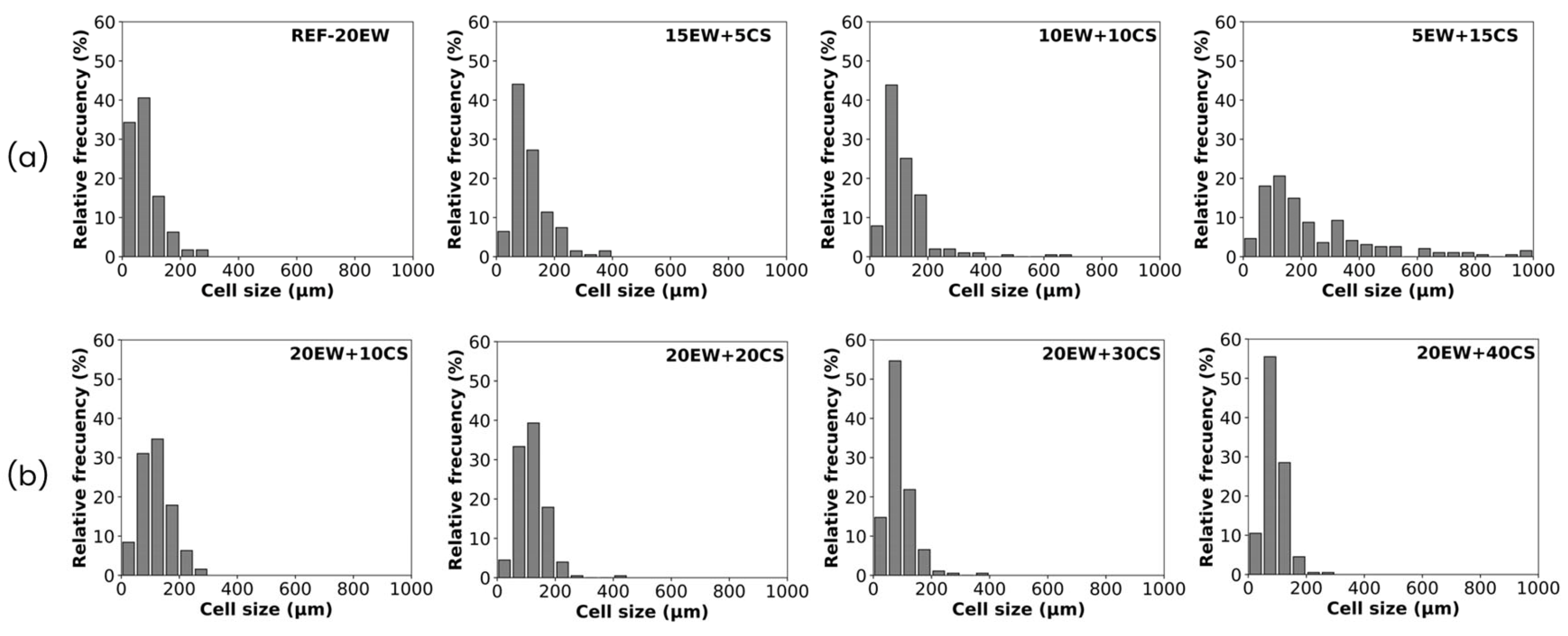

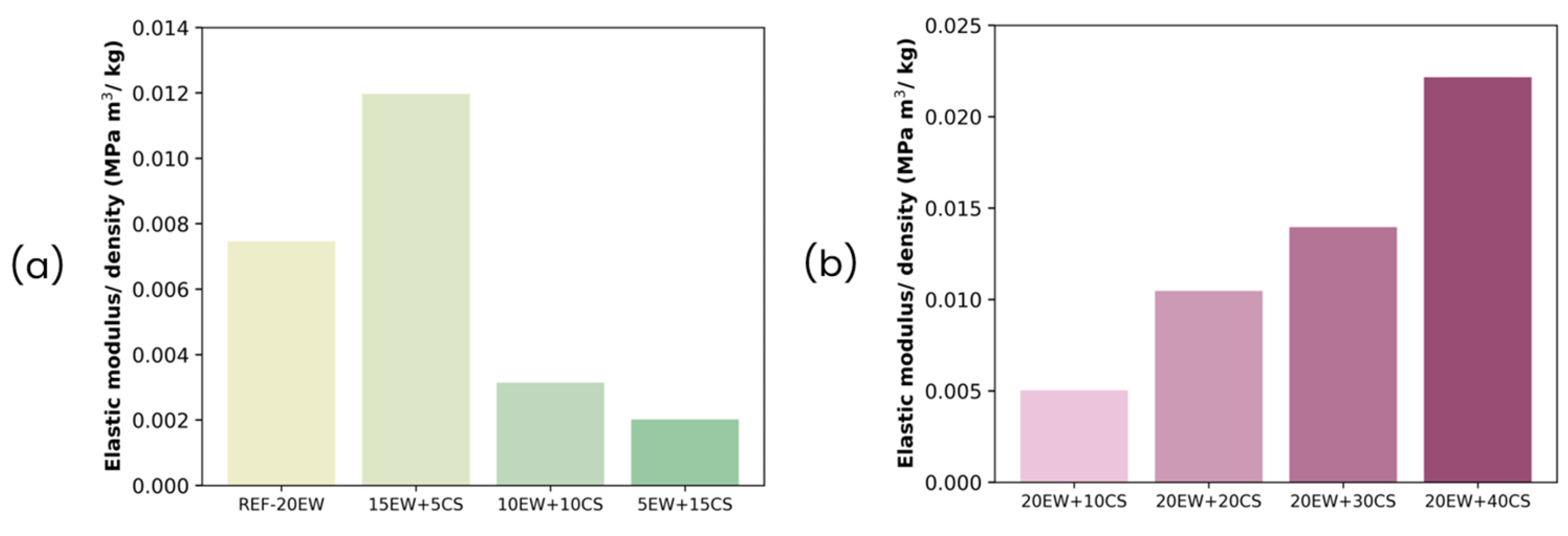
| Series | Sample | Egg White Protein (phr) a | Corn Starch (phr) a |
|---|---|---|---|
| Series 1 REPLACING EW BY CS: 20 phr stabilizer additives (sum of EW and CS) | REF-20 | 20 | 0 |
| 15EW + 5CS | 15 | 5 | |
| 10EW + 10CS | 10 | 10 | |
| 5EW + 15CS | 5 | 15 | |
| Series 2 ADDING CS TO EW: 20 phr EW + different contents of CS | 20EW + 10CS | 20 | 10 |
| 20EW + 20CS | 20 | 20 | |
| 20EW + 30CS | 20 | 30 | |
| 20EW + 40CS | 20 | 40 |
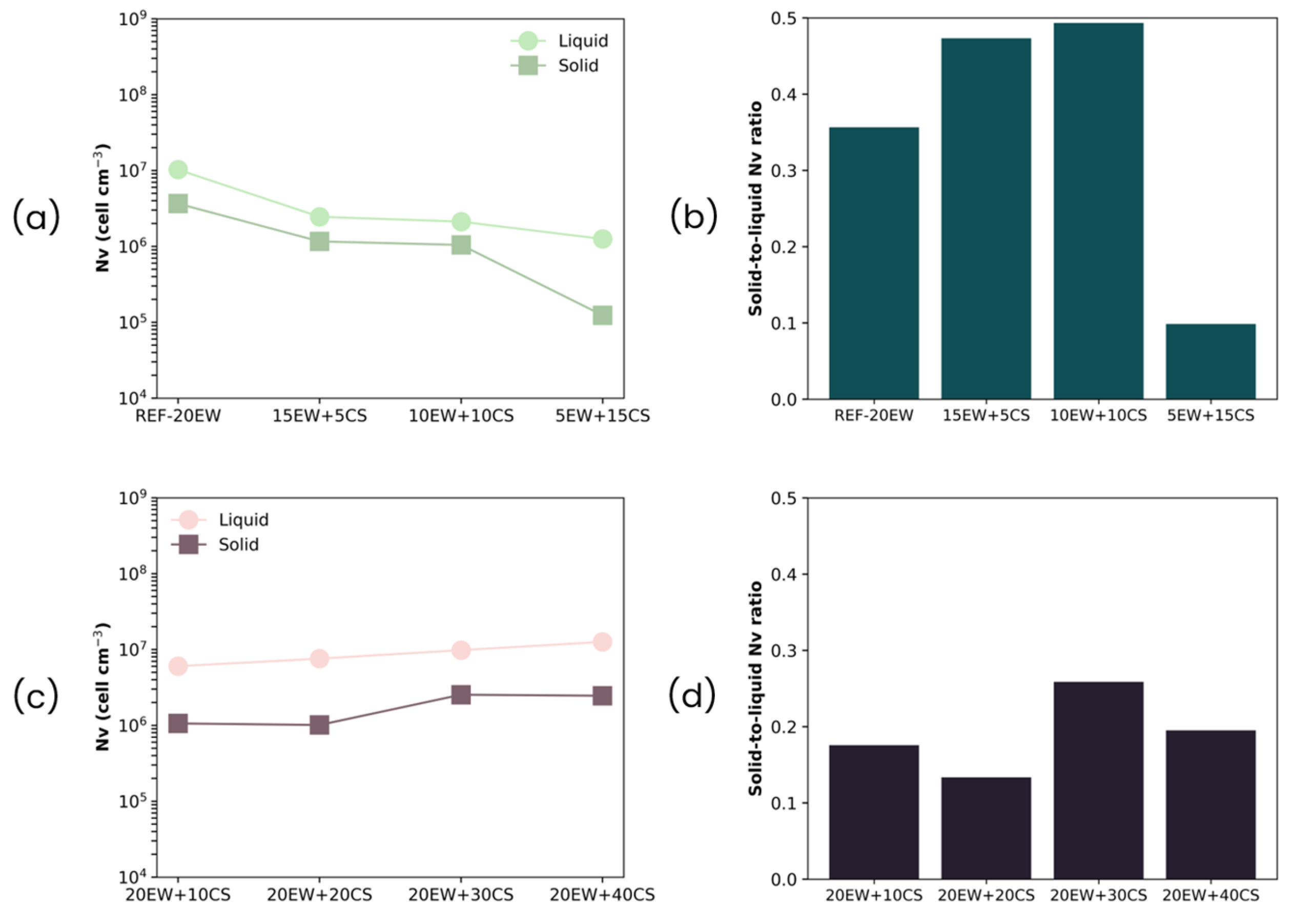
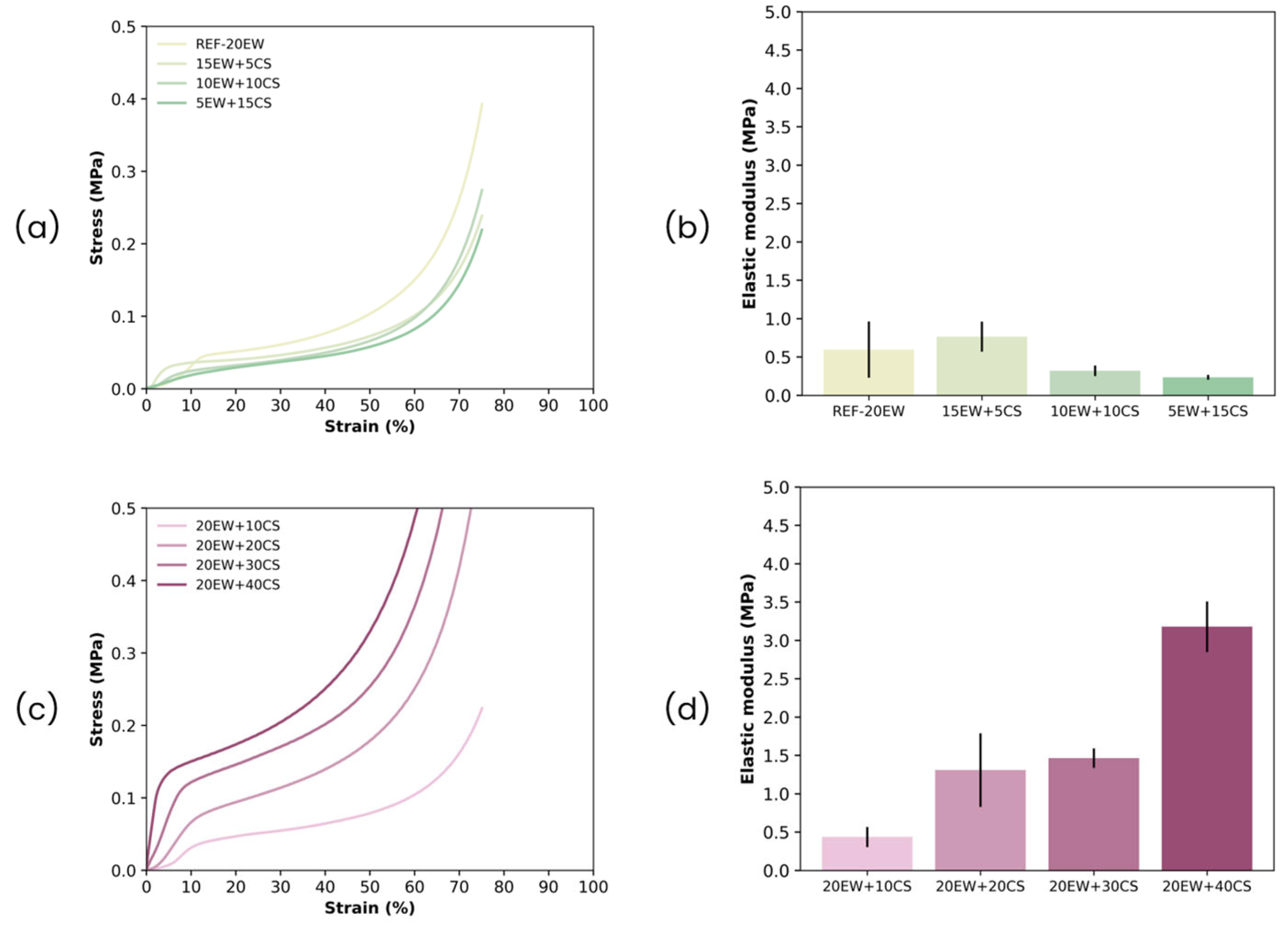
| Sample | ρ (kg/m3) | ρr | Φ3D (µm) | NSD | Ry/x | Nv × 106 (cells/cm3) |
|---|---|---|---|---|---|---|
| REF-20 | 214 ± 13 | 0.206 | 53 ± 29 | 0.56 | 1.02 | 10.26 |
| 15EW + 5CS | 187 ± 11 | 0.177 | 86 ± 33 | 0.38 | 0.98 | 2.45 |
| 10EW + 10CS | 179 ± 16 | 0.167 | 91 ± 36 | 0.40 | 1.00 | 2.12 |
| 5EW + 15CS | 165 ± 18 | 0.151 | 109 ± 47 | 0.43 | 0.98 | 1.25 |
| 20EW + 10CS | 251 ± 15 | 0.231 | 62 ± 24 | 0.38 | 0.98 | 6.04 |
| 20EW + 20CS | 305 ± 17 | 0.271 | 57 ± 23 | 0.40 | 1.03 | 7.57 |
| 20EW + 30CS | 354 ± 14 | 0.305 | 51 ± 19 | 0.37 | 0.97 | 9.80 |
| 20EW + 40CS | 441 ± 25 | 0.372 | 46 ± 24 | 0.53 | 0.99 | 12.60 |
| Sample | ρ (kg/m3) | ρr | Φ3D (µm) | NSD | Ry/x | Nv × 106 (cells/cm3) |
|---|---|---|---|---|---|---|
| REF-20 | 80 ± 5 | 0.075 | 78 ± 40 | 0.51 | 1.12 | 3.66 |
| 15EW + 5CS | 64 ± 2 | 0.058 | 116 ± 50 | 0.43 | 1.21 | 1.16 |
| 10EW + 10CS | 102 ± 11 | 0.089 | 119 ± 66 | 0.56 | 1.06 | 1.04 |
| 5EW + 15CS | 117 ± 20 | 0.099 | 241 ± 157 | 0.65 | 1.00 | 0.12 |
| 20EW + 10CS | 87 ± 6 | 0.075 | 119 ± 41 | 0.35 | 1.02 | 1.06 |
| 20EW + 20CS | 110 ± 3 | 0.090 | 120 ± 40 | 0.34 | 1.18 | 1.01 |
| 20EW + 30CS | 105 ± 5 | 0.083 | 88 ± 36 | 0.40 | 1.30 | 2.53 |
| 20EW + 40CS | 138 ± 18 | 0.106 | 89 ± 29 | 0.33 | 1.26 | 2.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amezúa-Arranz, C.; Oliveira Salmazo, L.; López-Gil, A.; Rodríguez-Pérez, M.-Á. Effect of Corn Starch as Stabilizer Particle in Combination with Egg White Proteins in Natural Rubber Latex Biofoams Produced by Microwave Foaming. Polymers 2025, 17, 3057. https://doi.org/10.3390/polym17223057
Amezúa-Arranz C, Oliveira Salmazo L, López-Gil A, Rodríguez-Pérez M-Á. Effect of Corn Starch as Stabilizer Particle in Combination with Egg White Proteins in Natural Rubber Latex Biofoams Produced by Microwave Foaming. Polymers. 2025; 17(22):3057. https://doi.org/10.3390/polym17223057
Chicago/Turabian StyleAmezúa-Arranz, Clara, Leandra Oliveira Salmazo, Alberto López-Gil, and Miguel-Ángel Rodríguez-Pérez. 2025. "Effect of Corn Starch as Stabilizer Particle in Combination with Egg White Proteins in Natural Rubber Latex Biofoams Produced by Microwave Foaming" Polymers 17, no. 22: 3057. https://doi.org/10.3390/polym17223057
APA StyleAmezúa-Arranz, C., Oliveira Salmazo, L., López-Gil, A., & Rodríguez-Pérez, M.-Á. (2025). Effect of Corn Starch as Stabilizer Particle in Combination with Egg White Proteins in Natural Rubber Latex Biofoams Produced by Microwave Foaming. Polymers, 17(22), 3057. https://doi.org/10.3390/polym17223057








