Polyhedral Oligomeric Silsesquioxanes (POSS) for Transparent Coatings: Material Properties and Applications
Abstract
1. Introduction
2. The Basic Characteristics of POSS
2.1. Definition and Core Structure
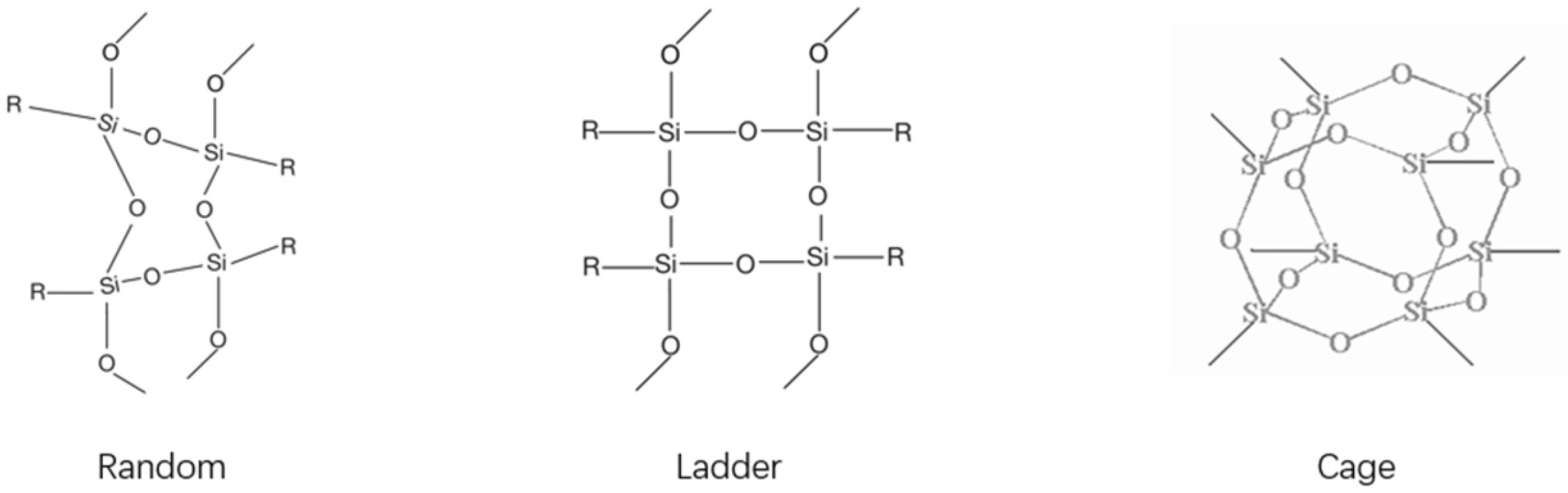
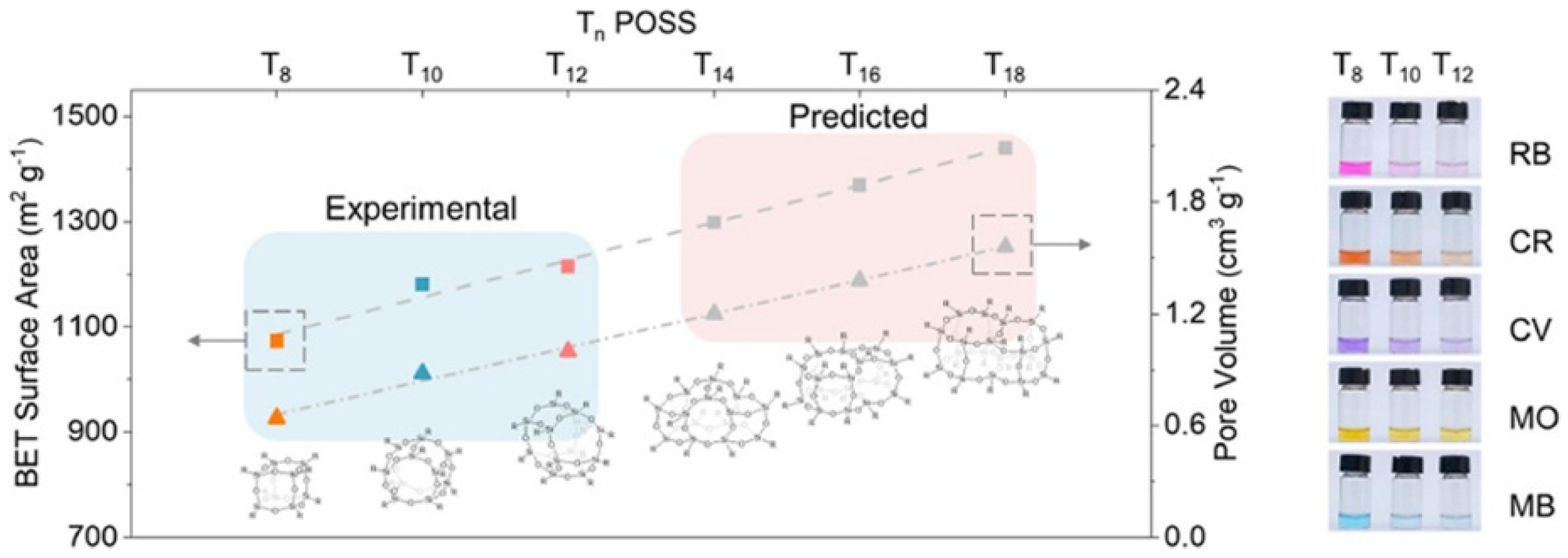
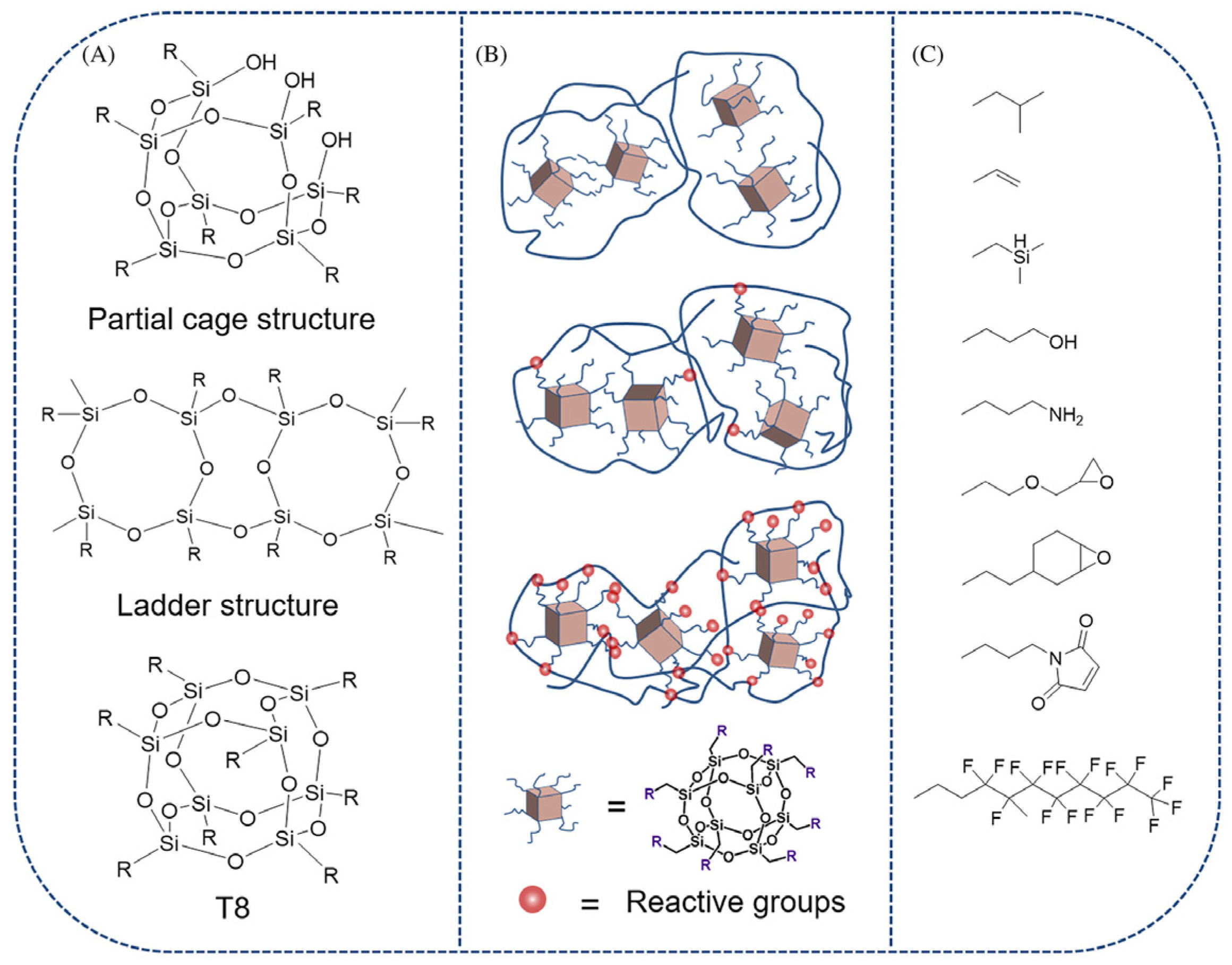
2.2. Classification and Structural Divergences
3. POSS Cage-like Structure: Advantages and Synthesis Methods
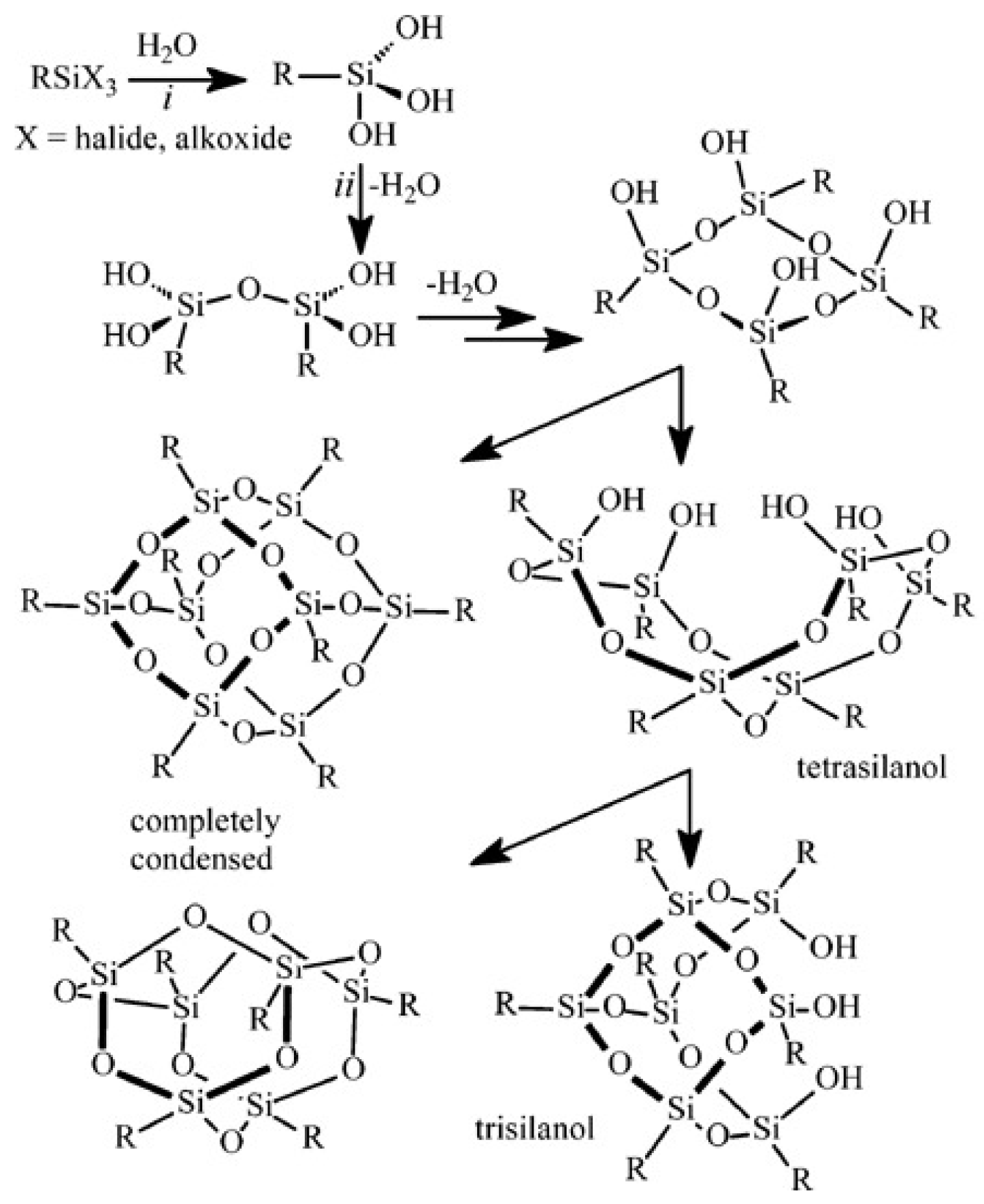
3.1. Sol–Gel Method
3.2. Corner Sealing Method
3.3. Rearrangement Method
3.4. Characterization Method
4. Functionalization Strategies of POSS
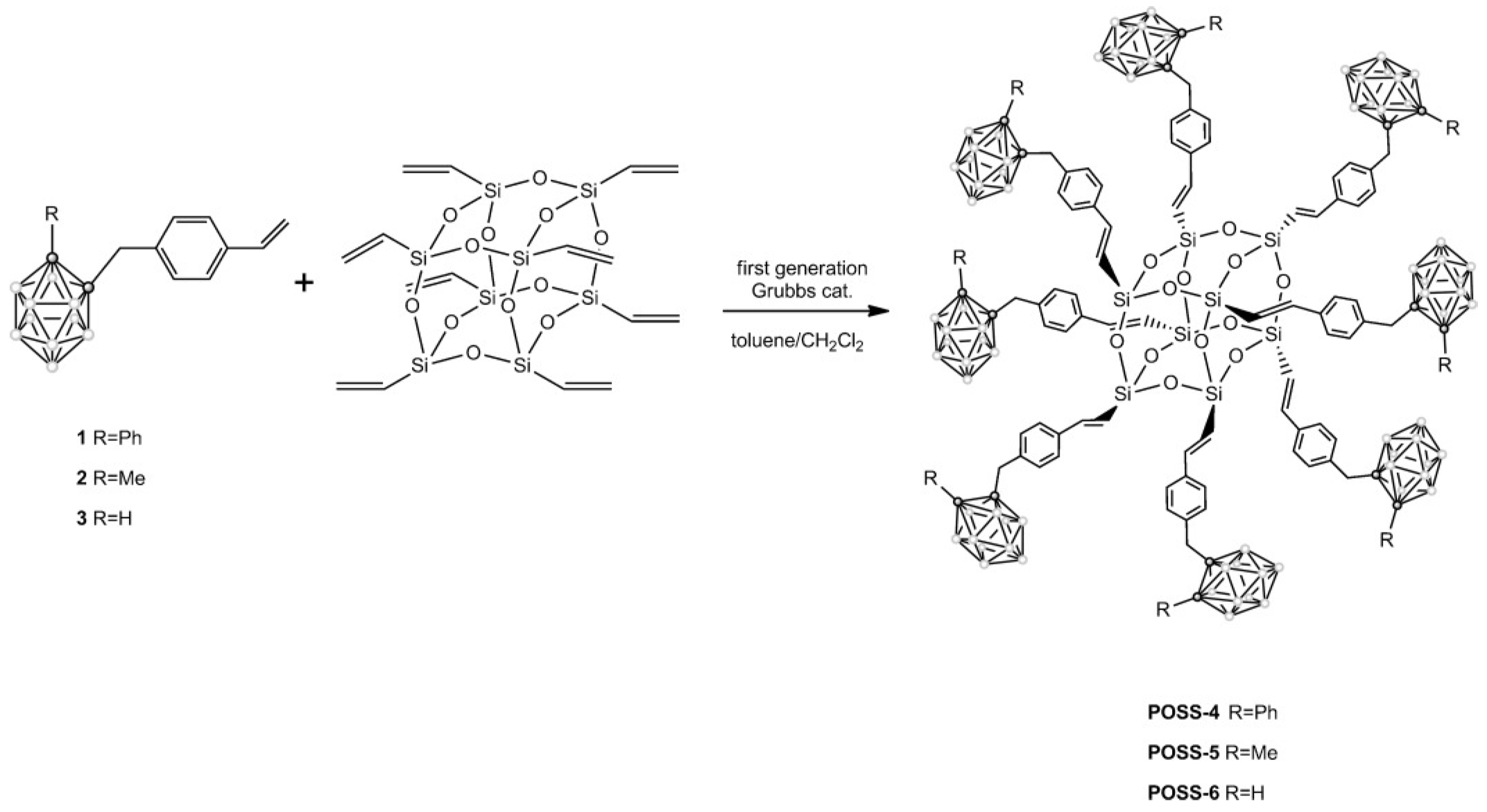
4.1. The Introduction Method of Functional Groups
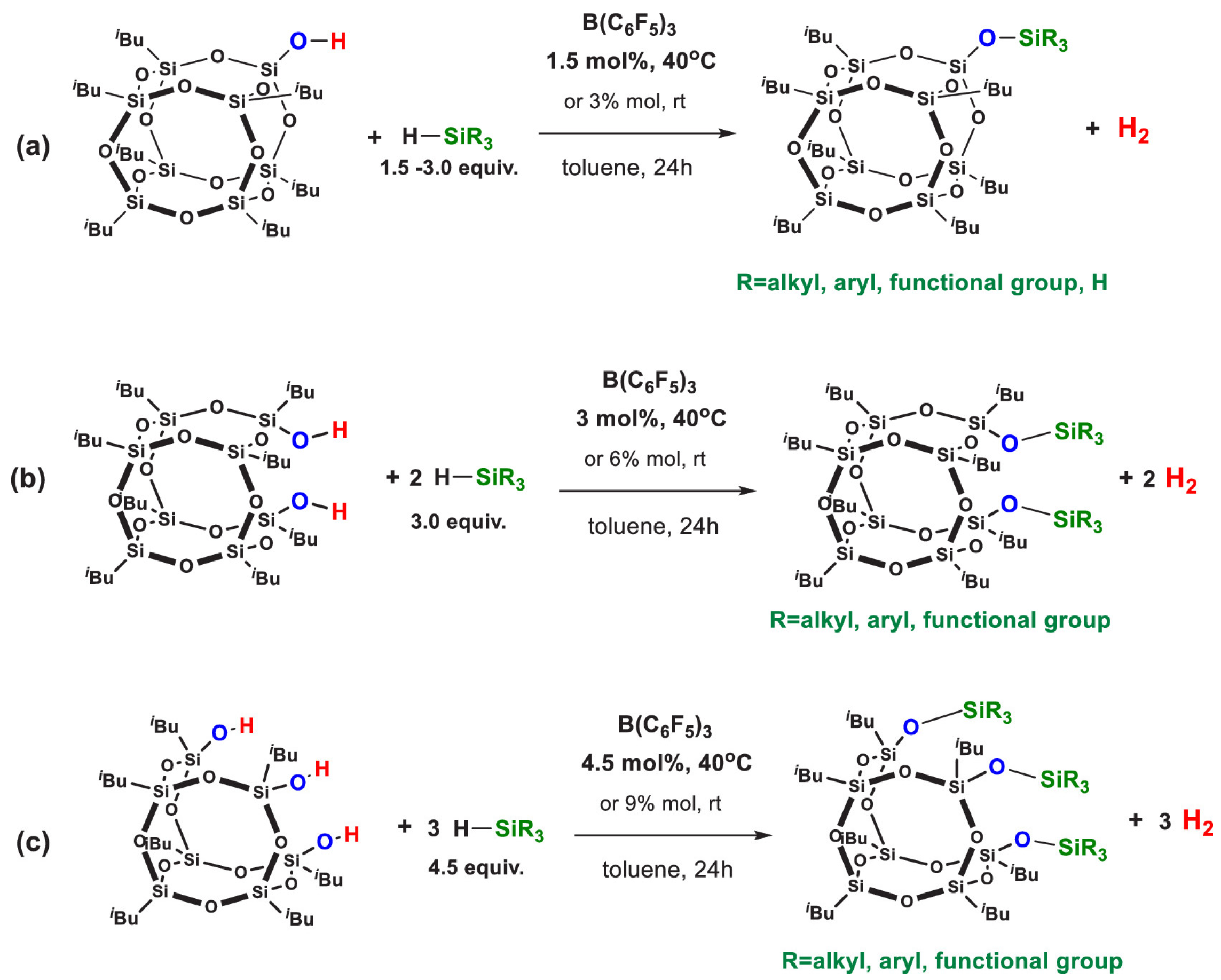
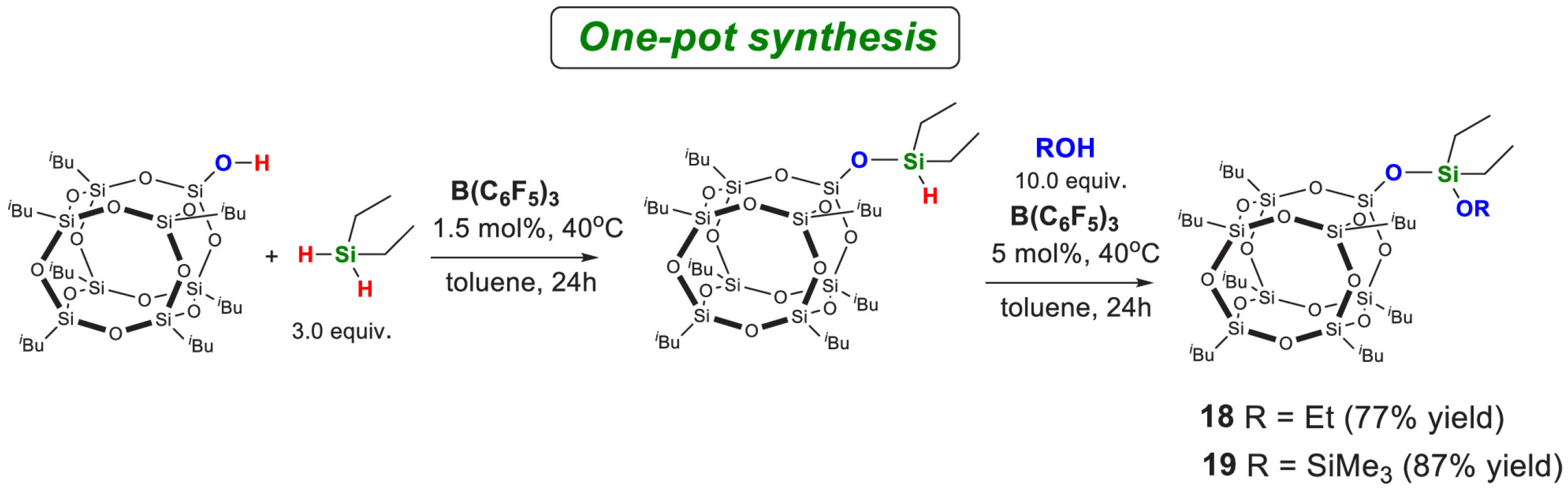
4.1.1. Metal Catalysis
4.1.2. Metal-Free Catalysis
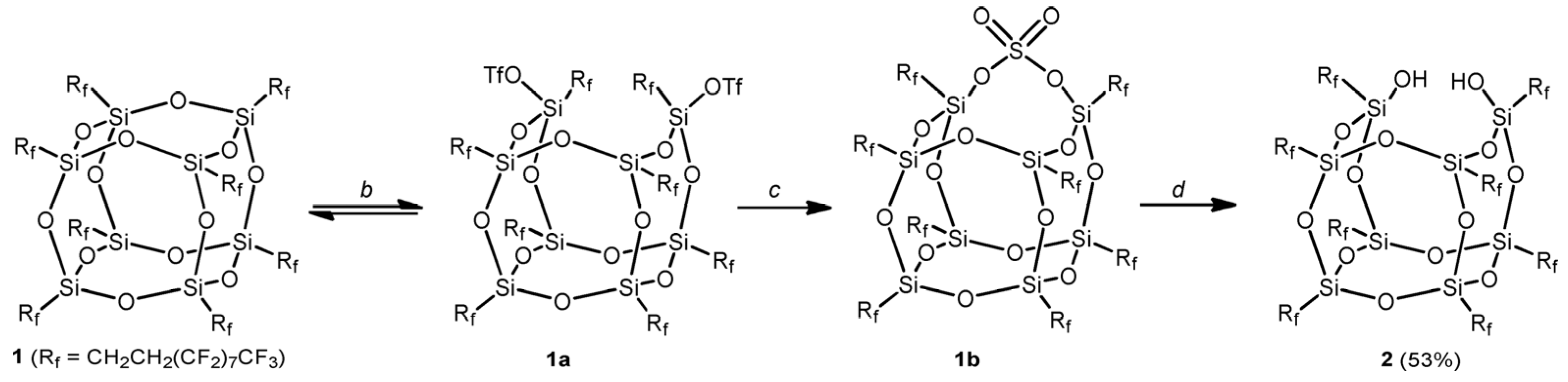
4.1.3. Si-O-Si Bond Ring-Opening
4.2. Property Modulation of POSS-Based Materials via Functional Group and Applications in Transparent Cover Plate Coatings
4.2.1. Solubility
4.2.2. Thermal Stability
4.2.3. Electronic Property
5. Integration Techniques for POSS Transparent Cover Coatings
5.1. Conventional Integration Methods
5.1.1. Physical Blending
5.1.2. Covalent Grafting
5.1.3. Chemical Crosslinking
5.2. Advanced Integration Techniques
5.2.1. UV–Thermal Dual Curing
5.2.2. In Situ Polymerization
6. The Performance of POSS Transparent Cover Plate Coatings
6.1. Light Transmittance
6.2. Mechanical Properties
6.3. Electronic Properties
6.4. Thermal Stability
6.5. Hydrophobicity
7. Application of POSS in Transparent Coatings
7.1. Space Environment Applications
7.2. Architectural and Agricultural Applications
7.3. Display Technology Application
7.4. New Energy Applications
7.5. Biological Applications
8. Discussion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Wu, Y.; Huang, Y.; Liu, L. Strong, Transparent and Flexible Aramid Nanofiber/POSS Hybrid Organic/Inorganic Nanocomposite Membranes. Compos. Sci. Technol. 2018, 156, 269–275. [Google Scholar] [CrossRef]
- Kausar, A. State-of-the-Art Overview on Polymer/POSS Nanocomposite. Polym.-Plast. Technol. Eng. 2017, 56, 1401–1420. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Mayer, P.; Lubecki, M.; Stosiak, M.; Robakowska, M. Effects of Surface Preparation on the Adhesion of UV-Aged Polyurethane Coatings. Int. J. Adhes. Adhes. 2022, 117, 103183. [Google Scholar] [CrossRef]
- Jalarvo, N.; Gourdon, O.; Ehlers, G.; Tyagi, M.; Kumar, S.K.; Dobbs, K.D.; Smalley, R.J.; Guise, W.E.; Ramirez-Cuesta, A.; Wildgruber, C.; et al. Structure and Dynamics of Octamethyl-POSS Nanoparticles. J. Phys. Chem. C 2014, 118, 5579–5592. [Google Scholar] [CrossRef]
- Lin, X.; Deng, Y.-Y.; Zhang, Q.; Han, D.; Fu, Q. Effect of POSS Size on the Porosity and Adsorption Performance of Hybrid Porous Polymers. Macromolecules 2023, 56, 1243–1252. [Google Scholar] [CrossRef]
- Han, S.; Wang, X.; Shao, Y.; Guo, Q.; Li, Y.; Zhang, W. Janus POSS Based on Mixed [2:6] Octakis-Adduct Regioisomers. Chem. A Eur. J. 2016, 22, 6397–6403. [Google Scholar] [CrossRef]
- Harrison, P.G.; Hall, C. Preparation and Characterization of Octasilsequioxane Cage Monomers. Main Group Met. Chem. 1997, 20, 515–529. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, J. Rational Design of Clear POSS Coatings with High Hardness, Excellent Flexibility, and Multifunctionality for Foldable Displays. ACS Appl. Polym. Mater. 2024, 6, 7621–7630. [Google Scholar] [CrossRef]
- Köytepe, S.; Demirel, M.H.; Gültek, A.; Seçkin, T. Metallo-Supramolecular Materials Based on Terpyridine-Functionalized polyhedral Silsesquioxane. Polym. Int. 2013, 63, 778–787. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Lewandowski, D.; Hreczycho, G. B(C6F5)3-Catalyzed Dehydrocoupling of POSS Silanols with Hydrosilanes: A Metal-Free Strategy for Effecting Functionalization of Silsesquioxanes. Inorg. Chem. 2020, 59, 9206–9214. [Google Scholar] [CrossRef]
- Ramirez, S.M.; Diaz, Y.J.; Campos, R.; Stone, R.L.; Haddad, T.S.; Mabry, J.M. Incompletely Condensed Fluoroalkyl Silsesquioxanes and Derivatives: Precursors for Low Surface Energy Materials. J. Am. Chem. Soc. 2011, 133, 20084–20087. [Google Scholar] [CrossRef]
- Xu, Y.; Long, J.; Zhang, R.; Du, Y.; Guan, S.; Wang, Y.; Huang, L.; Wei, H.; Liu, L.; Huang, Y. Greatly Improving Thermal Stability of Silicone Resins by Modification with POSS. Polym. Degrad. Stab. 2020, 174, 109082. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; Juárez-Pérez, E.J.; Teixidor, F.; Viñas, C.; Núñez, R. Synthesis, Characterization, and Thermal Behavior of Carboranyl–Styrene Decorated Octasilsesquioxanes: Influence of the Carborane Clusters o n Photoluminescence. Chem. A Eur. J. 2013, 19, 17021–17030. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.-G.; Becker, U.; Kieffer, J. Tuning Electronic Properties of Functionalized Polyhedral Oligomeric Silsesquioxanes: A DFT and TDDFT Study. J. Phys. Chem. A 2009, 113, 9707–9714. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Adachi, S.; Chujo, Y. Structure–Property Relationship of Octa-substituted POSS in Thermal and Mechanical Reinforcements of Conventional Polymers. J. Polym. Sci. A Polym. Chem. 2009, 47, 5690–5697. [Google Scholar] [CrossRef]
- Ma, Y.; He, L.; Zhao, L.; Pan, A. POSS-Based Glycidyl Methacrylate Copolymer for Transparent and Permeable Coatings. Soft Mater. 2016, 14, 253–263. [Google Scholar] [CrossRef]
- Monticelli, O.; Fina, A.; Cozza, E.S.; Prato, M.; Bruzzo, V. POSS Vapor Phase Grafting: A Novel Method to Modify Polymer Films. J. Mater. Chem. 2011, 21, 18049. [Google Scholar] [CrossRef]
- Jae, H.; Park, J.; Byun, J.; Chi, W.S.; Kim, J.H.; Jung, H.W.; Roh, D. Dual-Cured Organic–Inorganic Hard Coating with High Flexibility and Transparency Based on Polysilsesquioxane and a Functional Polymer. Prog. Org. Coat. 2024, 191, 108416. [Google Scholar] [CrossRef]
- Di Luca, C.; Soulé, E.R.; Zucchi, I.A.; Hoppe, C.E.; Fasce, L.A.; Williams, R.J.J. In-Situ Generation of a Dispersion of POSS Crystalline Platelets in an Epoxy Matrix Induced by Polymerization. Macromolecules 2010, 43, 9014–9021. [Google Scholar] [CrossRef]
- Yuasa, S.; Sato, Y.; Imoto, H.; Naka, K. Fabrication of Composite Films with Poly(Methyl Methacrylate) and Incompletely Condensed Cage-silsesquioxane Fillers. J Appl. Polym. Sci 2017, 135, 46033. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, K.; Chujo, Y. Synthesis of POSS Derivatives Having Dual Types of Alkyl Substituents and Their Application as a Molecular Filler for Low-Refractive and Highly Durable Materials. Bull. Chem. Soc. Jpn. 2017, 90, 205–209. [Google Scholar] [CrossRef]
- Hwang, S.O.; Lee, J.Y.; Lee, J.-H. Effect of the Silsesquioxane Structure on the Mechanical Properties of the Silsesquioxane-Reinforced Polymer Composite Films. Prog. Org. Coat. 2019, 137, 105316. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral Oligosilsesquioxane-Modified Boron Nitride Nanotube Based E Poxy Nanocomposites: An Ideal Dielectric Material with High Thermal Conductivity. Adv. Funct. Mater. 2012, 23, 1824–1831. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Cheng, F.; Jiao, X.; Fan, Y.; Lai, G.; Hua, X.; Yang, X. UV Cured Transparent Coatings with Good Thermal Stability Prepared from a Liquid Polyhedral Oligomeric Silsesquioxane with Allyl Sulphur-containing Carbosilane Substitutes. Prog. Org. Coat. 2020, 148, 105886. [Google Scholar] [CrossRef]
- Kozuka, H.; Tanaka, K.; Chujo, Y. Enhancement of Thermal Stability of Structural Color by the Substituent Effect in Polyhedral Oligomeric Silsesquioxane in Block Copolymers. Eur. Polym. J. 2022, 175, 111360. [Google Scholar] [CrossRef]
- Marcinkowska, A.; Prządka, D.; Andrzejewska, E. POSS Functionalized with Mixed Fluoroalkyl and Methacryloxy Substituents as Modifiers for UV-Curable Coatings. J. Coat. Technol. Res. 2018, 16, 167–178. [Google Scholar] [CrossRef]
- Park, J.; Lim, T.; Yang, K.-H.; Ju, S.; Jeong, S.-M. Dipping-Press Coating Method for Retaining Transparency and Imparting Hydrophobicity Regardless of Plastic Substrate Type. Polymers 2021, 13, 403. [Google Scholar] [CrossRef]
- Fina, A.; Monticelli, O.; Camino, G. POSS-Based Hybrids by Melt/Reactive Blending. J. Mater. Chem. 2010, 20, 9297. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic–Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. (Ed.) Applications of Polyhedral Oligomeric Silsesquioxanes; Advances in Silicon Science; Springer: Dordrecht, The Netherlands, 2011; Volume 3, ISBN 978-90-481-3786-2. [Google Scholar]
- Wang, J.; Zhou, D.-L.; Lin, X.; Li, J.-H.; Han, D.; Bai, H.; Fu, Q. Preparation of Low-k Poly(Dicyclopentadiene) Nanocomposites with Excel Lent Comprehensive Properties by Adding Larger POSS. Chem. Eng. J. 2022, 439, 135737. [Google Scholar] [CrossRef]
- Zhou, D.; Li, J.; Guo, Q.; Lin, X.; Zhang, Q.; Chen, F.; Han, D.; Fu, Q. Polyhedral Oligomeric Silsesquioxanes Based Ultralow-k Material s: The Effect of Cage Size. Adv. Funct. Mater. 2021, 31, 2102074. [Google Scholar] [CrossRef]
- Heeley, E.L.; Hughes, D.J.; El Aziz, Y.; Williamson, I.; Taylor, P.G.; Bassindale, A.R. Properties and Self-Assembled Packing Morphology of Long Alkyl-Chained Substituted Polyhedral Oligomeric Silsesquioxanes (POSS) Cages. Phys. Chem. Chem. Phys. 2013, 15, 5518. [Google Scholar] [CrossRef]
- Imai, K.; Kaneko, Y. Preparation of Ammonium-Functionalized Polyhedral Oligomeric Silsesquioxanes with High Proportions of Cagelike Decamer and Their Facile Separation. Inorg. Chem. 2017, 56, 4133–4140. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, P.; Wei, S.Y.; Hu, L.J. Influence of Molecular Structure of POSS on Gas-Molecule Diffusion Coefficients Using Molecular Dynamic Simulation. Mater. Sci. Forum 2011, 689, 114–121. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, P.; Wei, S.Y.; Hu, L.J. Influence of Molecular Structure on the Optical Property of POSS: A DFT Calculation Based Quantum Chemistry Calculation. Mater. Sci. Forum 2011, 689, 58–63. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; He, C. Some Recent Developments of Polyhedral Oligomeric Silsesquioxane (POSS)-Based Polymeric Materials. J. Mater. Chem. 2011, 21, 2775–2782. [Google Scholar] [CrossRef]
- Liu, J.; Kaneko, Y. Preparation of Polyhedral Oligomeric Silsesquioxanes Containing Carboxyl Side-Chain Groups and Isolation of a Cage-Like Octamer Using Clay Mineral. Bull. Chem. Soc. Jpn. 2018, 91, 1120–1127. [Google Scholar] [CrossRef]
- Janeta, M.; John, Ł.; Ejfler, J.; Szafert, S. High-Yield Synthesis of Amido-Functionalized Polyoctahedral Oligomeric Silsesquioxanes by Using Acyl Chlorides. Chem. A Eur. J. 2014, 20, 15966–15974. [Google Scholar] [CrossRef]
- Laird, M.; Yokoyama, J.; Carcel, C.; Unno, M.; Bartlett, J.R.; Wong Chi Man, M. Sol–Gel Processing of Polyhedral Oligomeric Silsesquioxanes: Nanohybrid Materials Incorporating T8 and T10 Cages. J. Sol.-Gel. Sci. Technol. 2020, 95, 760–770. [Google Scholar] [CrossRef]
- Perry, K.; Sui, B.; Li, Y. Mercapto-Functionalized Polyhedral Oligomeric Silsesquioxane as a Soluble Support for the Synthesis of Peptide Thioesters. Tetrahedron Lett. 2021, 85, 153483. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, R.W.J.M.; van Santen, R.A.; Abbenhuis, H.C.L. The Dynamic Status Quo of Polyhedral Silsesquioxane Coordination Chemistry. Eur. J. Inorg. Chem. 2004, 2004, 675–683. [Google Scholar] [CrossRef]
- Wang, H.; Nie, M.-X.; Lin, X.; Li, X.-Q.; Liu, H.; Guo, Q.-Y.; Han, D.; Fu, Q. Cage-Rearranged and Cage-Intact Syntheses of Azido-Functionalized Larger T10 and T12 POSSs. Dalton Trans. 2024, 53, 9467–9472. [Google Scholar] [CrossRef]
- Furgal, J.C.; Goodson Iii, T.; Laine, R.M. D5h [PhSiO1.5 ]10 Synthesis via F− Catalyzed Rearrangement of [PhSiO1.5 ]n. An Experimental/Computational Analysis of Likely Reaction Pathways. Dalton Trans. 2016, 45, 1025–1039. [Google Scholar] [CrossRef]
- Yandek, G.R.; Moore, B.M.; Ramirez, S.M.; Mabry, J.M. Effects of Peripheral Architecture on the Properties of Aryl Polyhedral Oligomeric Silsesquioxanes. J. Phys. Chem. C 2012, 116, 16755–16765. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Bottino, P. Influence of Symmetry/Asymmetry of the Nanoparticles Structure on the Thermal Stability of Polyhedral Oligomeric Silsesquioxane/Polystyrene Nanocomposites. Polym. Compos. 2012, 33, 1903–1910. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, X.; Yin, G.-Z.; Han, S.-Y.; Han, D.; Fu, Q.; Yang, S.; Zhang, W.-B. Symmetry-Dictated Mesophase Formation and Phase Diagram of Perfluorina Ted Polyhedral Oligomeric Silsesquioxanes. Macromolecules 2019, 52, 2361–2370. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.-B.; Hsieh, I.-F.; Zhang, G.; Cao, Y.; Li, X.; Wesdemiotis, C.; Lotz, B.; Xiong, H.; Cheng, S.Z.D. Breaking Symmetry toward Nonspherical Janus Particles Based on Polyhedal Oligomeric Silsesquioxanes: Molecular Design, “Click” Synthesis, a Nd Hierarchical Structure. J. Am. Chem. Soc. 2011, 133, 10712–10715. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Q.; Han, S.; Wang, J.; Han, D.; Fu, Q.; Zhang, W. Stochastic/Controlled Symmetry Breaking of the T8-POSS Cage s toward Multifunctional Regioisomeric Nanobuilding Blocks. Chem. A Eur. J. 2015, 21, 15246–15255. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Liu, H.; Sun, D.; Li, X. Synthesis and Aggregation Properties of a Series of Dumbbell Polyhedral Oligosilsesquioxane-Perylene Diimide Triads. CrystEngComm 2015, 17, 1453–1463. [Google Scholar] [CrossRef]
- Leong, F.Y.; Low, L.E.; Chew, I.M.L. Theoretical Analysis of Substituent- and Cage-Dependent Electronic pro Perties of POSS. AIP Adv. 2023, 13, 065216. [Google Scholar] [CrossRef]
- Matějka, L.; Dukh, O.; Hlavatá, D.; Meissner, B.; Brus, J. Cyclization and Self-Organization in Polymerization of Trialkoxysilane s. Macromolecules 2001, 34, 6904–6914. [Google Scholar] [CrossRef]
- Schwab, J.J.; Lichtenhan, J.D. Polyhedral Oligomeric Silsesquioxane(POSS)-Based Polymers. Appl. Organometal. Chem. 1998, 12, 707–713. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U. Polyhedral Oligomeric Silsesquioxane (POSS) Polymers and Copolymers: A Review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Jagannathan, J.R.; Targos, K.; Franz, A.K. Synthesis of Functionalized Silsesquioxane Nanomaterials by Rhodium- tCatalyzed Carbene Insertion into Si−H Bonds. Angew. Chem. Int. Ed. 2021, 61, e2021104170. [Google Scholar] [CrossRef]
- Walczak, M.; Januszewski, R.; Franczyk, A.; Marciniec, B. Synthesis of Monofunctionalized POSS through Hydrosilylation. J. Organomet. Chem. 2018, 872, 73–78. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Kuciński, K.; Lewandowski, D.; Hreczycho, G. Ru-Catalyzed Dehydrogenative Silylation of POSS-Silanols with Hydrosilanes: Its Introduction to One-Pot Synthesis. Inorg. Chem. 2019, 58, 1201–1207. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Z.; Zhang, M.; Yang, F.; Zhang, W.; Wu, X.; Yang, R. Facile Synthesis of Alkali Metal Polyhedral Oligomeric Silsesquioxane Salt and Its Application in Flame-Retardant Epoxy Resins. ACS Appl. Polym. Mater. 2023, 5, 3848–3857. [Google Scholar] [CrossRef]
- Ramirez, S.M.; Diaz, Y.J.; Campos, R.; Haddad, T.S.; Mabry, J.M. Functionalization of Fluoroalkyl Polyhedral Oligomeric Silsesquioxanes (F-POSS). In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; pp. 95–109. ISBN 978-0-8412-2792-7. [Google Scholar]
- Chhatre, S.S.; Guardado, J.O.; Moore, B.M.; Haddad, T.S.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Fluoroalkylated Silicon-Containing Surfaces-Estimation of Solid-Surface Energy. ACS Appl. Mater. Interfaces 2010, 2, 3544–3554. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Zhao, X.; Jiang, P.; Yu, H. Flame Retardancy and Thermal Stability of Polyhedral Oligomeric Silsesquioxane Nanocomposites. Fire Mater. 2011, 37, 1–16. [Google Scholar] [CrossRef]
- Lin; He, C.; Xiao, Y. Theoretical Studies of Monosubstituted and Higher Phenyl-Substituted O Ctahydrosilsesquioxanes. J. Phys. Chem. B 2003, 107, 13788–13792. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, S.; Zhang, Q.; Zhu, H.; Zhu, S. Polyhedral Oligomeric Silsesquioxane-based Functional Coatings: A Review. Can. J. Chem. Eng. 2023, 101, 4979–4991. [Google Scholar] [CrossRef]
- Mituła, K.; Duszczak, J.; Rzonsowska, M.; Żak, P.; Dudziec, B. Polysiloxanes Grafted with Mono(Alkenyl)Silsesquioxanes—Particular Con Cept for Their Connection. Materials 2020, 13, 4784. [Google Scholar] [CrossRef]
- Kim, D.; Jung, B.; Kim, K.; Cho, K.Y.; Jeong, Y. Highly Robust and Transparent Flexible Cover Window Films Based on UV- Curable Polysilsesquioxane Nano Sol. J. Appl. Polym. Sci. 2020, 137, 49012. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, Y.; He, J.; Dong, X. Transparent Superhydrophobic Coatings with Mechanical and Chemical Sta Bility Prepared by Modified Polyhedral Oligosilsesquioxanes via UV-Cur Able Method. Coatings 2023, 13, 498. [Google Scholar] [CrossRef]
- Loh, T.C.; Ng, C.M.; Kumar, R.N.; Ismail, H.; Ahmad, Z. Improvement of Thermal Ageing and Transparency of Methacrylate Based Poly(Siloxane–Silsesquioxane) for Optoelectronic Application. J. Appl. Polym. Sci. 2017, 134, 45285. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Yeh, C.-C.; Li, K.-F.; Yan, C.-J.; Chen, C.-C.; Hong, F.C.-N. Synthesis and Applications of Functionalized Polysiloxane Nanoparticles Uniformly Dispersed in UV-Cured Resin. In Proceedings of the 2015 10th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 21–23 October 2015; pp. 362–364. [Google Scholar]
- Ngoi, K.H.; Wong, J.C.; Chia, C.H.; Jin, K.S.; Kim, H.; Kim, H.-C.; Kim, H.-J.; Ree, M. Inorganic-Organic Nanocomposite Networks: Structure, Curing Reaction, Properties, and Hard Coating Performance. Compos. Sci. Technol. 2022, 218, 109112. [Google Scholar] [CrossRef]
- Xu, H.; Cao, X.; Shi, Y.; Cong, T.; Liu, H.; Gao, Y. In Situ Formation of POSS Layer on the Surface of Polyimide Film and Anti-Atomic Oxygen of SiO2/POSS Coatings. Prog. Org. Coat. 2023, 182, 107703. [Google Scholar] [CrossRef]
- Xu, C.; Gao, Z.; Guo, Y.; Shu, M.; Gao, Y. Study on In-Situ Growth of Polyhedral Oligomeric Silsesquioxane (POSS) Layer on Kapton Surface and the Properties of SiO2/POSS Coatings. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124720. [Google Scholar] [CrossRef]
- Chen, P.; Liu, T.; Wang, D.; Zhang, Q.; Hu, L. Modified Vinyl–POSS Materials as Sun Protection Factor and as Films for Greenhouse Cover. Ferroelectrics 2010, 411, 36–43. [Google Scholar] [CrossRef]
- Chen, P.; Liu, T.; Wang, D.; Liu, Y.; Hu, L.J. Silsesquioxane Materials as Sun Protection Factor Ingredients and as Films for Greenhouse Covers. Mater. Sci. Forum 2009, 610–613, 104–108. [Google Scholar] [CrossRef]
- Wang, D.; Wei, S.Y.; Hu, L.J. POSS/TiO2 Nanohybrids as Sun Protection Ingredients for Greenhouse Covers. Adv. Mater. Res. 2010, 113–116, 2077–2080. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, S.; Liu, H.; Zheng, Y.; Zhao, K.; Huang, J.; Lai, Y. High-Performance Anti-Fouling Coating: Achieving High Transparency, Durability, and Flexibility via Epoxy-Amine Curing with Dense Cross-Linking Design. J. Mater. Sci. Technol. 2026, 242, 255–263. [Google Scholar] [CrossRef]
- Mahfuz, H.; Powell, F.; Granata, R.; Hosur, M.; Khan, M. Coating of Carbon Fiber with Polyhedral Oligomeric Silsesquioxane (POSS) to Enhance Mechanical Properties and Durability of Carbon/Vinyl Ester Composites. Materials 2011, 4, 1619–1631. [Google Scholar] [CrossRef]
- Nezakati, T.; Tan, A.; Seifalian, A.M. Enhancing the Electrical Conductivity of a Hybrid POSS–PCL/Graphene Nanocomposite Polymer. J. Colloid Interface Sci. 2014, 435, 145–155. [Google Scholar] [CrossRef]
- Huang, H.; Lin, H.; Kershaw, S.V.; Susha, A.S.; Choy, W.C.H.; Rogach, A.L. Polyhedral Oligomeric Silsesquioxane Enhances the Brightness of Perovskite Nanocrystal-Based Green Light-Emitting Devices. J. Phys. Chem. Lett. 2016, 7, 4398–4404. [Google Scholar] [CrossRef]
- Paajanen, M.; Karttunen, M.; Kortet, S.; Härkki, O.; Orko, I. Thermally More Durable Electromechanical Films by POSS Nanomodification. Key Eng. Mater. 2013, 538, 65–68. [Google Scholar] [CrossRef]
- Zhu, W.; He, J.; Dong, X. Study of Polyhedral Oligomeric Silsesquioxane-Modified Superwetting Transparent Coating for Anti-Fogging, Stain Resistance, Self-Cleaning and Anti-Biological Application. Coatings 2025, 15, 936. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Nair, A.S.; Raut, H.K.; Yuan Tan, T.T.; He, C.; Ramakrishna, S.; Xu, J. Superhydrophobic Fluorinated POSS–PVDF-HFP Nanocomposite Coating on Glass by Electrospinning. J. Mater. Chem. 2012, 22, 18479. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Zhao, Z.; Zhou, X.; Wu, L.; Weng, Z. High-Transparency, Long-Life Fluorinated POSS-Based Liquid-like Coating for Anti-Icing Glass Applications. Coatings 2025, 15, 745. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, C.; He, J.; Dong, X. Study of Polyhedral Oligomeric Silsesquioxane-Modified Superhydrophilic Transparent Coating in Antifogging, Antifrost and Self-Cleaning. Polymers 2025, 17, 599. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Shoiriki, M.; Mizumo, T.; Kaneko, Y. Preparation of Low-Crystalline POSS Containing Two Types of Alkylammonium Groups and Its Optically Transparent Film. J. Mater. Chem. C 2014, 2, 2496–2501. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Yuan, S.; Ren, X.; Yang, C.; Han, S.; Qi, Y.; Li, D.; Liu, J. Preparation and Characterization of Atomic Oxygen-Resistant, Optically Transparent and Dimensionally Stable Copolyimide Films from Fluorinated Monomers and POSS-Substituted Diamine. Polymers 2024, 16, 2845. [Google Scholar] [CrossRef]
- Lei, X.-F.; Qiao, M.-T.; Tian, L.-D.; Yao, P.; Ma, Y.; Zhang, H.-P.; Zhang, Q.-Y. Improved Space Survivability of Polyhedral Oligomeric Silsesquioxane (POSS) Polyimides Fabricated via Novel POSS-Diamine. Corros. Sci. 2015, 90, 223–238. [Google Scholar] [CrossRef]
- Atar, N.; Grossman, E.; Gouzman, I.; Bolker, A.; Murray, V.J.; Marshall, B.C.; Qian, M.; Minton, T.K.; Hanein, Y. Atomic-Oxygen-Durable and Electrically-Conductive CNT-POSS-Polyimide Flexible Films for Space Applications. ACS Appl. Mater. Interfaces 2015, 7, 12047–12056. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Qian, Y.; Qi, H.; Li, J.; Sun, J. Mechanically Robust Atomic Oxygen-Resistant Coatings Capable of Autonomously Healing Damage in Low Earth Orbit Space Environment. Adv. Mater. 2018, 30, 1803854. [Google Scholar] [CrossRef]
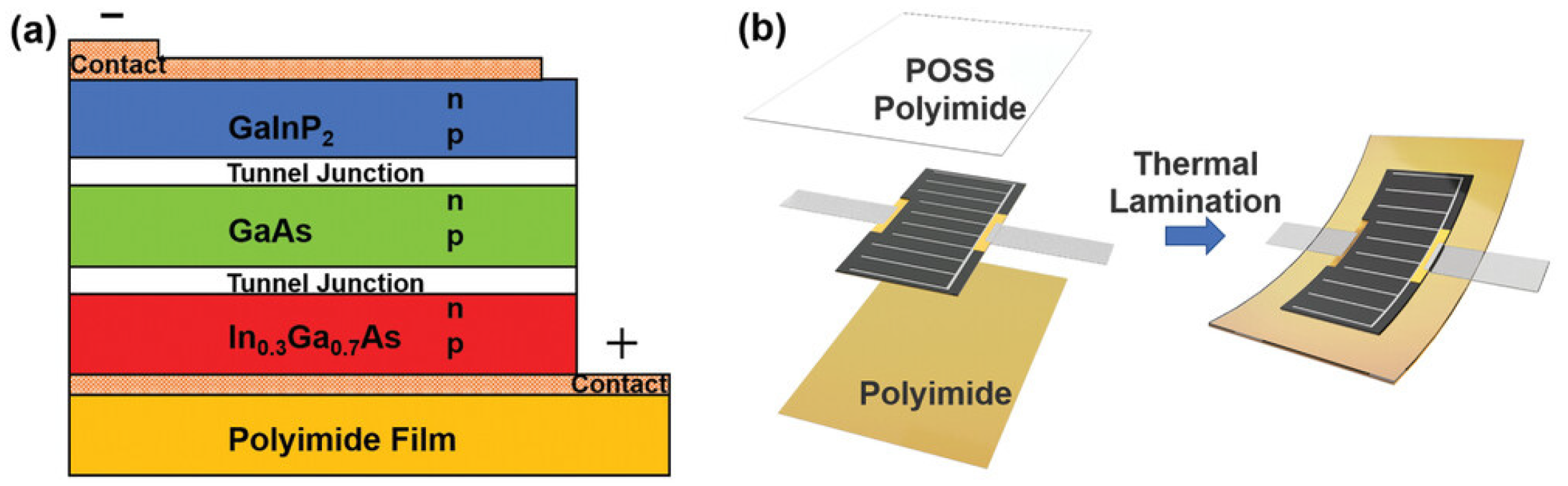
- Qian, M.; Mao, X.; Wu, M.; Cao, Z.; Liu, Q.; Sun, L.; Gao, Y.; Xuan, X.; Pan, Y.; Niu, Y.; et al. POSS Polyimide Sealed Flexible Triple-Junction GaAs Thin-Film Solar Cells for Space Applications. Adv Mater. Technol. 2021, 6, 2100603. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Wu, X.; Yi, W.; Xiao, Q. Bio-Based Polyurethane Nanocomposite Thin Coatings from Two Comparable POSS with Eight Same Vertex Groups for Controlled Release Urea. Sci. Rep. 2021, 11, 9917. [Google Scholar] [CrossRef]
- Lyu, B.; Lu, X.; Gao, D.; Wu, H.; Ma, J. Construction and Evaluation of Environment-Friendly POSS Multi-Crosslinked Mulch Film Based on Bone Gelatin. Int. J. Biol. Macromol. 2023, 247, 125829. [Google Scholar] [CrossRef]
- Li, L.; Dong, S.; Li, H.; Wang, M.; Liang, L.; Pang, M. Introducing Hydroxyl-Containing POSS to Liquefaction of Biomass: An Alternative Route to Bio-Based Polyurethane Nanocomposites for Coated Fertilizers. Eur. Polym. J. 2023, 192, 112082. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, J.; Ahmed, N.; Shi, J.; Wen, M.; Yan, N. Construction of Biomass-Based Flame-Retardant, Antimicrobial and Hydrophobic Wood Coatings with POSS Organic-Inorganic Nanoparticles. Eur. Polym. J. 2024, 219, 113370. [Google Scholar] [CrossRef]
- Li, Z.; Kong, J.; Wang, F.; He, C. Polyhedral Oligomeric Silsesquioxanes (POSSs): An Important Building Block for Organic Optoelectronic Materials. J. Mater. Chem. C 2017, 5, 5283–5298. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Yan, Z.; Chen, K.; Zhang, M.; Zhang, H.; Liu, X.; Xing, R.; Li, J. P-211: Double Capping Layers Technology for Transparent OLED Display. SID Symp. Dig. Tech. Pap. 2024, 55, 2184–2187. [Google Scholar] [CrossRef]
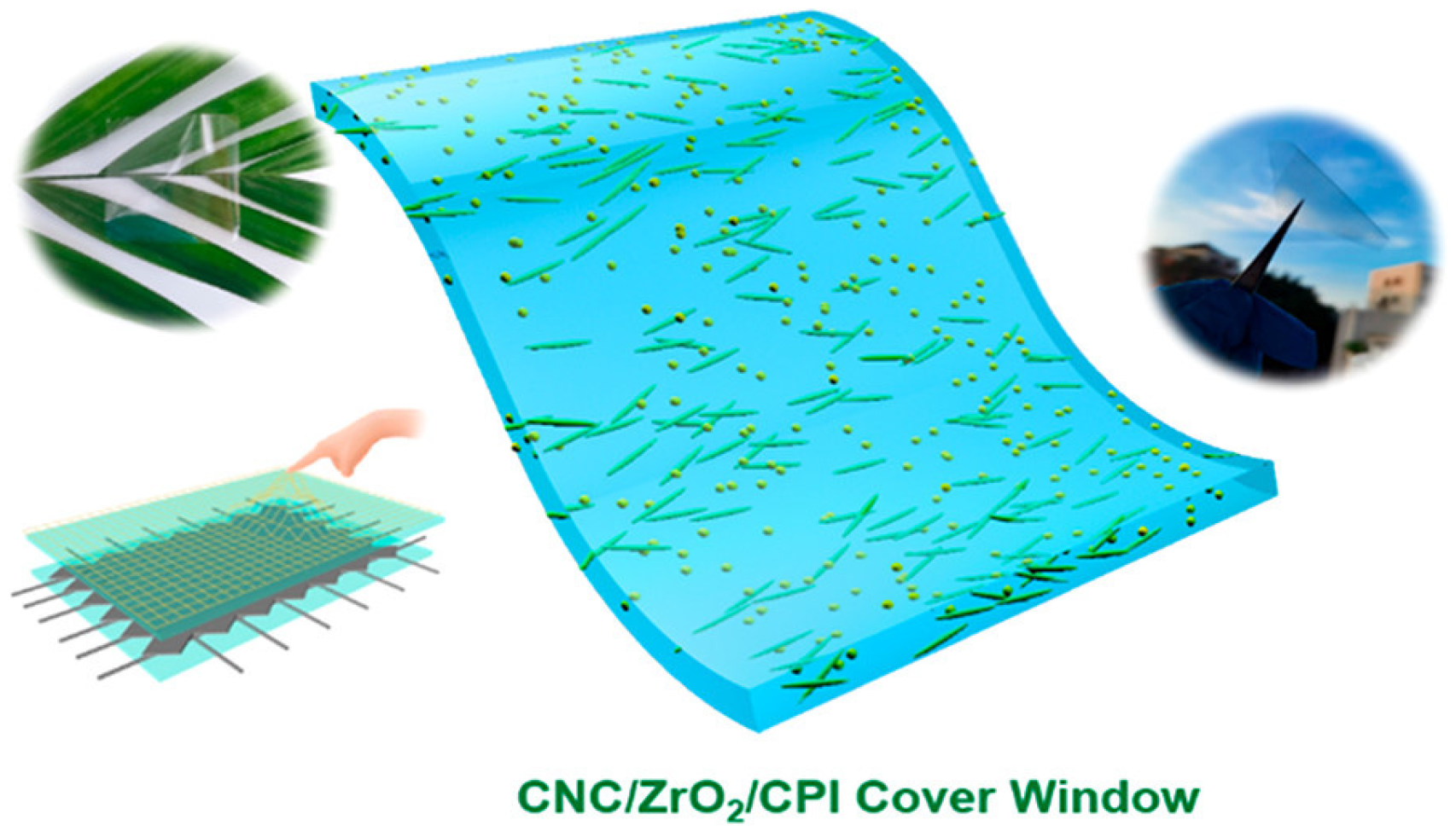
- Xie, J.; Jia, D.; Dirican, M.; Xia, Y.; Li, C.; Liu, Y.; Cui, M.; Yan, C.; Wan, J.; Liu, H.; et al. Highly Foldable, Super-Sensitive, and Transparent Nanocellulose/Ceramic/Polymer Cover Windows for Flexible OLED Displays. ACS Appl. Mater. Interfaces 2022, 14, 16658–16668. [Google Scholar] [CrossRef]
- Ghani, K.; Kiomarsipour, N.; Ranjbar, M. New High-Efficiency Protective Coating Containing Glycidyl-POSS Nanocage for Improvement of Solar Cell Electrical Parameters. J. Nanostruct. 2019, 9, 103–111. [Google Scholar] [CrossRef]
- He, Z.; Yu, P.; Gao, J.; Ma, C.; Xu, J.; Duan, W.; Zhao, Y.; Miao, Z. An Energy-Efficient and Low-Driving-Voltage Flexible Smart Window Enhanced by POSS and CsxWO3. Sol. Energy Mater. Sol. Cells 2023, 250, 112096. [Google Scholar] [CrossRef]
- Ozimek, J.; Pielichowski, K. Recent Advances in Polyurethane/POSS Hybrids for Biomedical Applications. Molecules 2021, 27, 40. [Google Scholar] [CrossRef]
- Feghhi, M.; Rezaie, J.; Akbari, A.; Jabbari, N.; Jafari, H.; Seidi, F.; Szafert, S. Effect of Multi-Functional Polyhydroxylated Polyhedral Oligomeric Silsesquioxane (POSS) Nanoparticles on the Angiogenesis and Exosome Biogenesis in Human Umbilical Vein Endothelial Cells (HUVECs). Mater. Des. 2021, 197, 109227. [Google Scholar] [CrossRef]
- Ge, X.; Dong, L.; Sun, L.; Song, Z.; Wei, R.; Shi, L.; Chen, H. New Nanoplatforms Based on UCNPs Linking with Polyhedral Oligomeric Silsesquioxane (POSS) for Multimodal Bioimaging. Nanoscale 2015, 7, 7206–7215. [Google Scholar] [CrossRef]
- Wei, G.; Yao, Y.; Zhang, H.; Cui, W.; Lu, Y. Cage-Shaped Silsesquioxane with Multiactive Sites for Impeding Aggregation of Small Insoluble Organic Molecule. Chem. Eng. J. 2023, 470, 144330. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A. Self-Healing Silsesquioxane-Based Materials. Polymers 2022, 14, 1869. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, S.W.; Lee, G.; Yoon, J.; Kim, S.; Hong, J.-H.; Jo, S.-C.; Jeong, U. Fabrication of Practical Deformable Displays: Advances and Challenges. Light Sci. Appl. 2023, 12, 61. [Google Scholar] [CrossRef]


| Performance Property | Method/Strategy | Key Mechanism/Effect |
|---|---|---|
| Thermal Stability | Introduction of carborane cages into POSS framework | Mass loss below 10% after combustion at 1000 °C [14] |
| Use of phenyl-functionalized POSS or longer/unsaturated side chains | Improved thermal stability and elasticity of polymer matrix [16] | |
| Cyclopentyl-substituted POSS (vs. isobutyl) | Thermally reinforced POSS domains enhance structural color stability [26] | |
| Electronic Properties | Functionalization of POSS-T8 with electron-withdrawing/donating groups | Modulates HOMO/LUMO levels, bandgap, charge transport, and exciton binding energy [15] |
| Insertion of molecules (e.g., N2) inside POSS cage | Lowers LUMO energy level [15] | |
| POSS as hole-blocking layer in perovskite LEDs | Deep HOMO level suppresses hole leakage, improves EQE [80] | |
| POSS-functionalized BNNTs in epoxy nanocomposites | Reduces dielectric constant and loss, enhances thermal conductivity [24] | |
| Light Transmittance | Use of partially condensed POSS (vs. fully condensed) | Better dispersion in polymer matrix, higher transparency [21] |
| Incorporation of TTB-modified POSS | UV-blocking while maintaining high visible-light transmittance [76] | |
| Low-crystallinity POSS with asymmetric substituents | Suppresses crystallization, forms optically transparent films [86] | |
| Dual curing (UV-thermal) with cycloaliphatic epoxy-POSS and acrylic resin A multi-point, highly cross-linked network was constructed through the efficient curing of a polyamine curing agent, tetraethylene-pentamine, with EP-POSS. | Highly transparent films with superior thermal stability [19] High transparency, low surface roughness, and efficient repellency against contaminants [77] | |
| Mechanical Properties | In situ sol–gel synthesis of modified POSS with alkylsilanes | Reduces refractive index, improves thermal and mechanical properties of PMMA [22] |
| POSS coating on carbon fibers via combined chemical-mechanical processing | Enhances interlaminar shear and impact resistance by 17–38% [78] | |
| Incorporation of diverse silsesquioxanes (SQ) into polyurethane acrylate (PUA) | Increases hardness and scratch resistance, reduces elastic modulus [23] | |
| Covalent grafting or chemical crosslinking of POSS | Prevents phase separation, enhances toughness, thermal stability, and network density [18] | |
| Hydrophobicity | Copolymerization with 4M4F-POSS (four methacryloxy and four fluoroalkyl groups) | Enhances scratch resistance and hydrophobicity [27] |
| Electrospinning of fluoroPOSS-PVDF-HFP nanocomposites | Forms transparent superhydrophobic coatings [83] | |
| Two-step dip-coating and hot-pressing with PDMS-ODA and fluorinated alumina nanoparticles | Retains transparency, imparts hydrophobicity on plastic substrates [28] | |
| Surface modification with fluorinated monomer (TFOA) | Imparts oil and water repellency without compromising other properties [9] | |
| Developing a fluorinated GPOSS-based coating with an inorganic/organic composite structure. Incorporation of poly(ethylene glycol) methyl ether methacry-late-functionalized POSS. | Significantly reduces the minimum de-icing pressure of the fluorinated modified coating [84]. Developing a highly hydrophilic coating, exhibiting a water contact angle of less than 10° [85]. |
| Performance Metric | Conventional Organic Coating (Reference Case) | Conventional Inorganic Coating (Reference Case) | POSS-Based Coating (Specific Example from This Review) |
|---|---|---|---|
| Optical Transmittance | Good: ~92% (Typical for clear polymers) | Excellent: >95% (e.g., Fused silica) | >95% at 550 nm achieved by using partially condensed POSS for superior dispersion in PMMA matrix, avoiding light scattering. |
| Hardness | Poor to Fair: 2B–3H (e.g., Soft PU/Acrylics) | Excellent but Brittle: 6H–9H (e.g., sol–gel SiO2) | 9H Pencil Hardness achieved via a crosslinked network of MA-POSS and MP-POSS, providing inorganic-like rigidity. |
| Flexibility | Excellent: Can be folded | Very Poor: Cracks easily under strain | Withstands > 1000 bending cycles (r ~0.8 mm) due to the flexible thiol-ene network built from the same MA-POSS/MP-POSS system. |
| Thermal Stability | Poor: Ta or Td < 200 °C | Excellent: Td > 400 °C | Significantly increased decomposition temperature of silicone resin by incorporating aminopropyl-POSS forming reinforcing hydrogen bonds. |
| Environmental Resistance | Poor: Degrades under UV/AO | Excellent: Inert to UV/AO | AO erosion rate reduced to 3.6% of pristine Kapton using an in situ-grown aminopropyl-POSS adhesion layer under SiO2. |
| Surface Hydrophobicity | Moderate: ~95° (e.g., standard coatings) | Hydrophilic: <90° | Superhydrophobic (Contact Angle > 110°) achieved by copolymerizing with 4M4F-POSS in a UV-curable acrylate resin. |
| Key Limitation | Low durability, soft, poor thermal resistance | Brittle, poor adhesion, difficult to process | Higher raw material cost; More complex synthesis and integration required. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Bian, Z.; Wei, Y.; He, X.; Lu, X.; Lu, Q. Polyhedral Oligomeric Silsesquioxanes (POSS) for Transparent Coatings: Material Properties and Applications. Polymers 2025, 17, 3050. https://doi.org/10.3390/polym17223050
Chen Y, Bian Z, Wei Y, He X, Lu X, Lu Q. Polyhedral Oligomeric Silsesquioxanes (POSS) for Transparent Coatings: Material Properties and Applications. Polymers. 2025; 17(22):3050. https://doi.org/10.3390/polym17223050
Chicago/Turabian StyleChen, Yujia, Zhiwei Bian, Yunhao Wei, Xiaojie He, Xuemin Lu, and Qinghua Lu. 2025. "Polyhedral Oligomeric Silsesquioxanes (POSS) for Transparent Coatings: Material Properties and Applications" Polymers 17, no. 22: 3050. https://doi.org/10.3390/polym17223050
APA StyleChen, Y., Bian, Z., Wei, Y., He, X., Lu, X., & Lu, Q. (2025). Polyhedral Oligomeric Silsesquioxanes (POSS) for Transparent Coatings: Material Properties and Applications. Polymers, 17(22), 3050. https://doi.org/10.3390/polym17223050





