Biomechanical Analysis of Silk as a Tendon or Ligament Replacement
Abstract
1. Introduction
2. Anatomy of Tendons and Ligaments
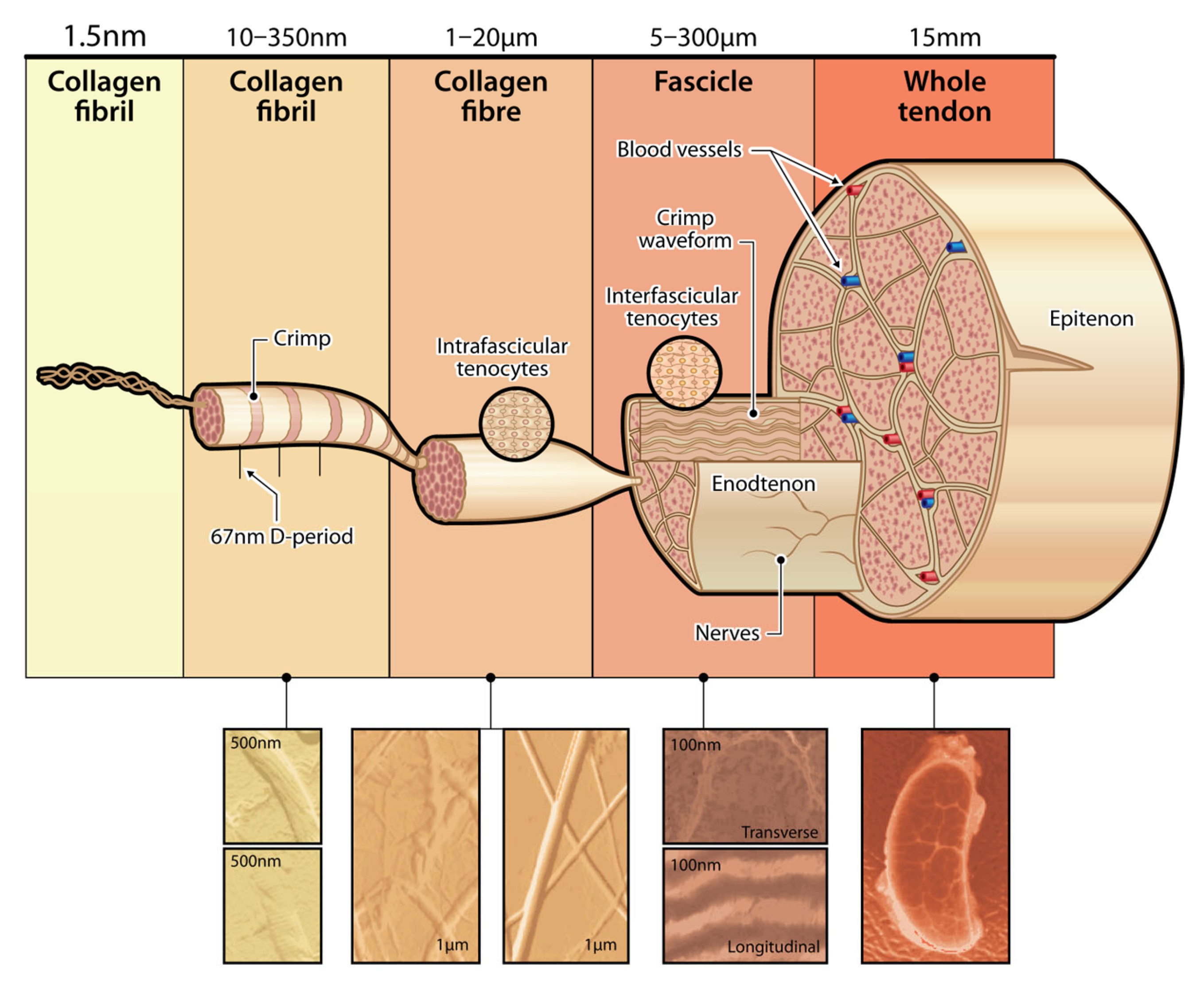
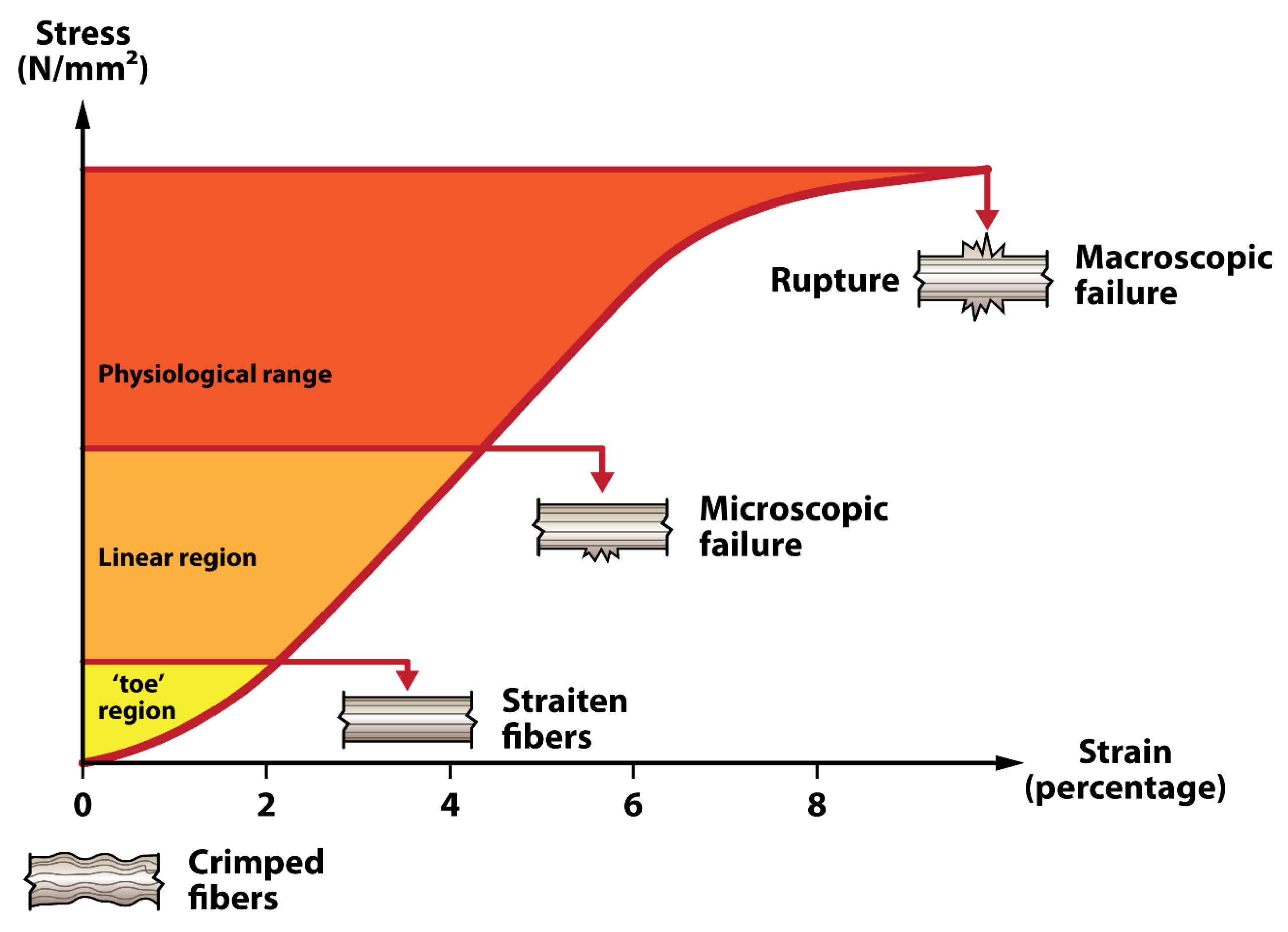
2.1. Anatomy of Tendons
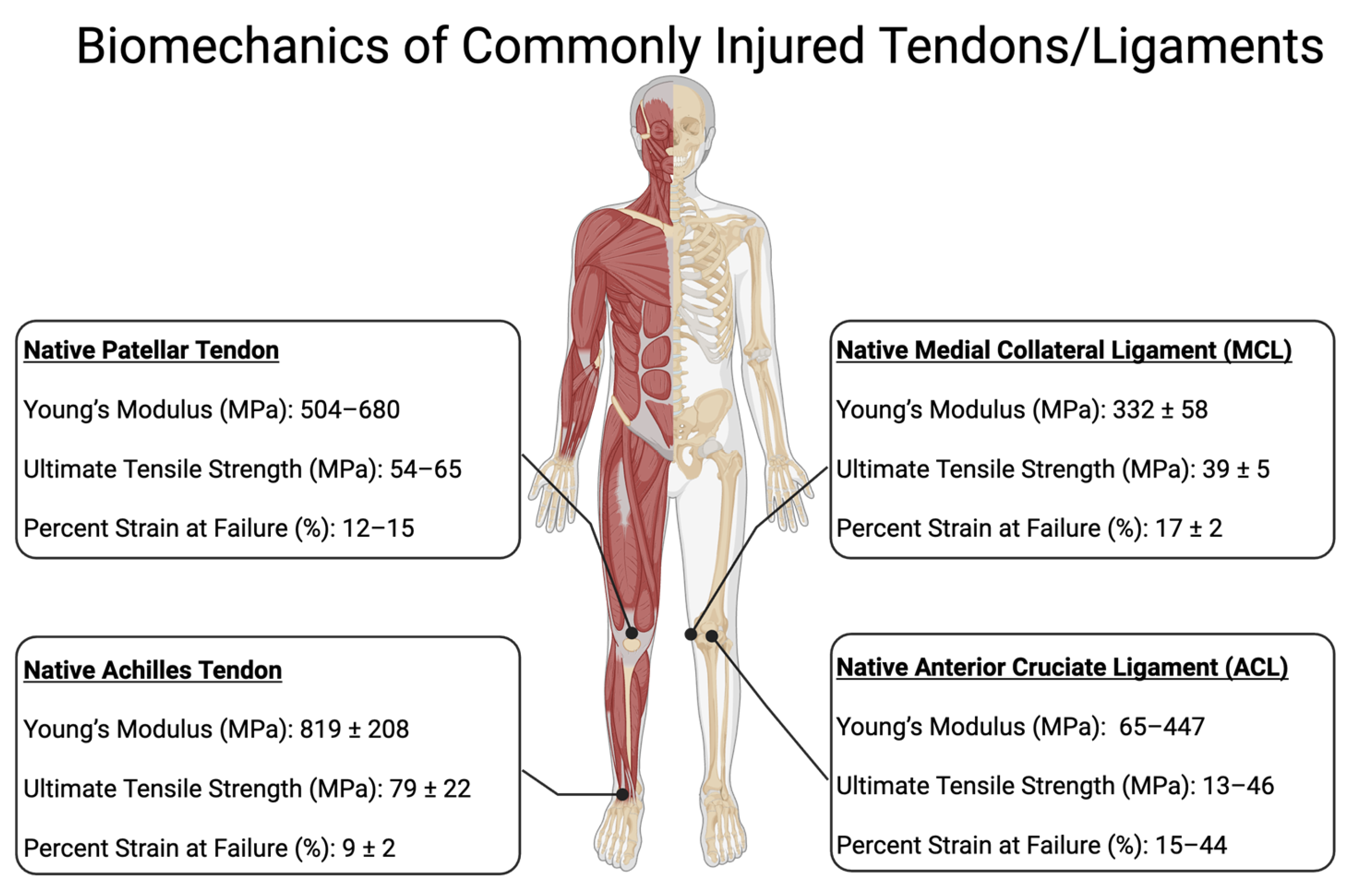
2.2. Tendon Injury
2.3. Anatomy of Ligaments
2.4. Ligament Injury
3. T/L Graft Options
3.1. Artificial
3.2. Conventional Grafts
3.3. Silk Grafts
4. Factors Affecting Regeneration
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACL | Anterior Cruciate Ligament |
| ATFL | Anterior Talofibular Ligament |
| BPB | Bone-Patellar-Bone |
| ECM | Extracellular Matrix |
| FHL | Flexor Hallucis Longus |
| Kennedy LAD | Kennedy ligament augmentation device |
| LARS | Ligament Advanced Reinforcement System |
| LCL | Lateral Collateral Ligament |
| MCL | Medial Collateral Ligament |
| MPa | Megapascal |
| P.B. | Peroneus Brevis |
| PCL | Posterior Cruciate Ligament |
| PDGF | Platelet-Derived Growth Factor |
| PET | Polyethylene Terephthalate |
| RGD | Arginyl-Glycyl-Aspartic acid |
| T/L | Tendon and Ligament |
| TGF-β | Transforming Growth Factor-Beta |
| UCL | Ulnar Collateral Ligament |
| UTS | Ultimate Tensile Strength |
| VEGF | Vascular Endothelial Growth Factor |
References
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef]
- Stone, K.R.; Walgenbach, A.; Galili, U. Induced Remodeling of Porcine Tendons to Human Anterior Cruciate Ligaments by α-GAL Epitope Removal and Partial Cross-Linking. Tissue Eng. Part B Rev. 2017, 23, 412–419. [Google Scholar] [CrossRef]
- Colatruglio, M.; Flanigan, D.C.; Long, J.; DiBartola, A.C.; Magnussen, R.A. Outcomes of 1- Versus 2-Stage Revision Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2021, 49, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef] [PubMed]
- Seyler, T.M.; Bracey, D.N.; Plate, J.F.; Lively, M.O.; Mannava, S.; Smith, T.L.; Saul, J.M.; Poehling, G.G.; Van Dyke, M.E.; Whitlock, P.W. The Development of a Xenograft-Derived Scaffold for Tendon and Ligament Reconstruction Using a Decellularization and Oxidation Protocol. Arthroscopy 2017, 33, 374–386. [Google Scholar] [CrossRef]
- Ratcliffe, A.; Butler, D.L.; Dyment, N.A.; Cagle, P.J.; Proctor, C.S.; Ratcliffe, S.S.; Flatow, E.L. Scaffolds for Tendon and Ligament Repair and Regeneration. Ann. Biomed. Eng. 2015, 43, 819–831. [Google Scholar] [CrossRef]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef]
- Rees, J.D.; Wilson, A.M.; Wolman, R.L. Current concepts in the management of tendon disorders. Rheumatology 2006, 45, 508–521. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef]
- Adam, N.C.; Smith, C.R.; Herzog, W.; Amis, A.A.; Arampatzis, A.; Taylor, W.R. In Vivo Strain Patterns in the Achilles Tendon During Dynamic Activities: A Comprehensive Survey of the Literature. Sports Med. Open 2023, 9, 60. [Google Scholar] [CrossRef]
- Dt, B.; Jd, H.; Pe, C.; Ke, V. Acute Tibialis Posterior Tendon Rupture with Pronation-Type Ankle Fractures. Orthopedics 2016, 39, e970–e975. [Google Scholar] [CrossRef]
- Kim, H.M.; Dahiya, N.; Teefey, S.A.; Middleton, W.D.; Stobbs, G.; Steger-May, K.; Yamaguchi, K.; Keener, J.D. Location and initiation of degenerative rotator cuff tears: An analysis of three hundred and sixty shoulders. J. Bone Jt. Surg. Am. 2010, 92, 1088–1096. [Google Scholar] [CrossRef]
- Patellar Tendon Rupture—Knee & Sports—Orthobullets. Available online: https://www.orthobullets.com/knee-and-sports/3024/patellar-tendon-rupture (accessed on 10 September 2025).
- Physiopedia. Ligament. Available online: https://www.physio-pedia.com/Ligament (accessed on 10 September 2025).
- Hsu, S.-L.; Liang, R.; Woo, S.L. Functional tissue engineering of ligament healing. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010, 2, 12. [Google Scholar] [CrossRef]
- Smith, K.D.; Vaughan-Thomas, A.; Spiller, D.G.; Innes, J.F.; Clegg, P.D.; Comerford, E.J. The organisation of elastin and fibrillins 1 and 2 in the cruciate ligament complex. J. Anat. 2011, 218, 600–607. [Google Scholar] [PubMed]
- Henninger, H.B.; Valdez, W.R.; Scott, S.A.; Weiss, J.A. Elastin governs the mechanical response of medial collateral ligament under shear and transverse tensile loading. Acta Biomater. 2015, 25, 304–312. [Google Scholar] [CrossRef]
- Physiopedia. Proteoglycans. Available online: https://www.physio-pedia.com/Proteoglycans (accessed on 10 September 2025).
- Clinical Gate. Connective Tissue. 2015. Available online: https://clinicalgate.com/connective-tissue/ (accessed on 10 September 2025).
- Azar, F.M.; Canale, S.T.; Beaty, J.H. Campbell’s Operative Orthopaedics, 14th ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 4, ISBN 9780443117633. Available online: https://evolve.elsevier.com/cs/product/9780323672177?role=student (accessed on 10 September 2025).
- Benjamin, M.; Ralphs, J.R. Fibrocartilage in tendons and ligaments—An adaptation to compressive load. J. Anat. 1998, 193 Pt 4, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Susanne, H.; Klaus, S. Epidemiology of athletic knee injuries: A 10-year study. Knee 2006, 13, 184–188. [Google Scholar] [CrossRef]
- Fong, D.T.P.; Hong, Y.; Chan, L.K.; Yung, P.S.H.; Chan, K.M. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007, 37, 73–94. [Google Scholar] [CrossRef]
- Swanson, B.; Hewitt, M.A.; Buckley, S.E.; Collins, C.; Hunt, K.J. Epidemiology of Ankle Ligament Injuries in US High School Athletes. Foot Ankle Orthop. 2022, 7, 2473011421S00961. [Google Scholar] [CrossRef]
- Rao, R.; Bhattacharyya, R.; Andrews, B.; Varma, R.; Chen, A. The management of combined ACL and MCL injuries: A systematic review. J. Orthop. 2022, 34, 21–30. [Google Scholar] [CrossRef]
- Woo, S.L.Y.; Abramowitch, S.D.; Kilger, R.; Liang, R. Biomechanics of knee ligaments: Injury, healing, and repair. J. Biomech. 2006, 39, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Longo, U.G.; Denaro, V. Anterior cruciate ligament tear. N. Engl. J. Med. 2009, 360, 1463. [Google Scholar]
- Murray, M.M.; Fleming, B.C. Biology of anterior cruciate ligament injury and repair: Kappa delta ann doner vaughn award paper 2013. J. Orthop. Res. 2013, 31, 1501–1506. [Google Scholar] [CrossRef]
- Musahl, V.; Karlsson, J. Anterior Cruciate Ligament Tear. N. Engl. J. Med. 2019, 380, 2341–2348. [Google Scholar] [CrossRef]
- Phisitkul, P.; James, S.L.; Wolf, B.R.; Amendola, A. MCL Injuries of the Knee: Current Concepts Review. Iowa Orthop. J. 2006, 26, 77–90. [Google Scholar] [PubMed]
- van der List, J.P.; Mintz, D.N.; DiFelice, G.S. The Location of Anterior Cruciate Ligament Tears: A Prevalence Study Using Magnetic Resonance Imaging. Orthop. J. Sports Med. 2017, 5, 2325967117709966. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Nam, S.W. Rupture of Posterior Cruciate Ligament: Diagnosis and Treatment Principles. Knee Surg. Relat. Res. 2011, 23, 135–141. [Google Scholar] [CrossRef]
- Yaras, R.J.; O’Neill, N.; Mabrouk, A.; Yaish, A.M. Lateral Collateral Ligament Knee Injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK560847/ (accessed on 10 September 2025).
- Bergman, R.; Shuman, V.L. Acute Ankle Sprain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK459212/ (accessed on 10 September 2025).
- Maffulli, N.; Ziello, S.; Maisto, G.; Migliorini, F.; Oliva, F. Local Tendon Transfers for Chronic Ruptures of the Achilles Tendon: A Systematic Review. J. Clin. Med. 2023, 12, 707. [Google Scholar] [CrossRef]
- Feng, S.M.; Maffulli, N.; Oliva, F.; Saxena, A.; Hao, Y.F.; Hua, Y.H.; Xu, H.-L.; Tao, X.; Xu, W.; Migliorini, F.; et al. Surgical management of chronic Achilles tendon rupture: Evidence-based guidelines. J. Orthop. Surg. 2024, 19, 132. [Google Scholar] [CrossRef]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 3, 7–12. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Tranovich, M.J.; Kropf, E.J.; Fu, F.H.; Harner, C.D. Bone-patellar tendon-bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: A retrospective matched analysis with 2–10 year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1520–1527. [Google Scholar] [CrossRef]
- Buerba, R.A.; Boden, S.A.; Lesniak, B. Graft Selection in Contemporary Anterior Cruciate Ligament Reconstruction. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e21. [Google Scholar] [CrossRef]
- Kunze, K.N.; Moran, J.; Polce, E.M.; Pareek, A.; Strickland, S.M.; Williams, R.J. Lower donor site morbidity with hamstring and quadriceps tendon autograft compared with bone-patellar tendon-bone autograft after anterior cruciate ligament reconstruction: A systematic review and network meta-analysis of randomized controlled trials. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tang, Q.; Ernst, S.; Linde, M.A.; Smolinski, P.; Wu, S.; Fu, F. Peroneus longus tendon autograft has functional outcomes comparable to hamstring tendon autograft for anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Runer, A.; Keeling, L.; Wagala, N.; Nugraha, H.; Özbek, E.A.; Hughes, J.D.; Musahl, V. Current trends in graft choice for anterior cruciate ligament reconstruction—Part I: Anatomy, biomechanics, graft incorporation and fixation. J. Exp. Orthop. 2023, 10, 37. [Google Scholar] [CrossRef]
- Tian, P.; Hu, W.-Q.; Li, Z.-J.; Sun, X.-L.; Ma, X.-L. Comparison of autograft and allograft tendons in posterior cruciate ligament reconstruction: A meta-analysis. Medicine 2017, 96, e7434. [Google Scholar] [CrossRef]
- Lansdown, D.A.; Riff, A.J.; Meadows, M.; Yanke, A.B.; Bach, B.R. What Factors Influence the Biomechanical Properties of Allograft Tissue for ACL Reconstruction? A Systematic Review. Clin. Orthop. 2017, 475, 2412–2426. [Google Scholar] [CrossRef]
- Hoburg, A.; Keshlaf, S.; Schmidt, T.; Smith, M.; Gohs, U.; Perka, C.; Pruss, A.; Scheffler, S. High-dose electron beam sterilization of soft-tissue grafts maintains significantly improved biomechanical properties compared to standard gamma treatment. Cell Tissue Bank. 2015, 16, 219–226. [Google Scholar] [CrossRef]
- Giannini, S.; Buda, R.; Di Caprio, F.; Agati, P.; Bigi, A.; De Pasquale, V.; Ruggeri, A. Effects of freezing on the biomechanical and structural properties of human posterior tibial tendons. Int. Orthop. 2008, 32, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Gut, G.; Marowska, J.; Jastrzebska, A.; Olender, E.; Kamiński, A. Structural mechanical properties of radiation-sterilized human Bone-Tendon-Bone grafts preserved by different methods. Cell Tissue Bank. 2016, 17, 277–287. [Google Scholar] [CrossRef]
- Colaço, H.B.; Shah, Z.; Back, D.; Davies, A.; Ajuied, A. (iv) Xenograft in orthopaedics. Orthop. Trauma 2015, 29, 253–260. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Lin, H.; Zhu, T.; Cai, J.; Su, W.; Chen, J.; Xu, J.; Li, Y.; Wang, J.; et al. Advances in Regenerative Sports Medicine Research. Front. Bioeng. Biotechnol. 2022, 10, 908751. [Google Scholar] [CrossRef] [PubMed]
- Dahlstedt, L.J.; Netz, P.; Dalén, N. Poor results of bovine xenograft for knee cruciate ligament repair. Acta Orthop. Scand. 1989, 60, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Stone, K.R. In Situ “Humanization” of Porcine Bioprostheses: Demonstration of Tendon Bioprostheses Conversion into Human ACL and Possible Implications for Heart Valve Bioprostheses. Bioengineering 2021, 8, 10. [Google Scholar] [CrossRef]
- Mall, N.A.; Chalmers, P.N.; Moric, M.; Tanaka, M.J.; Cole, B.J.; Bach, B.R.; Paletta, G.A. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am. J. Sports Med. 2014, 42, 2363–2370. [Google Scholar] [CrossRef]
- Ardern, C.L.; Webster, K.E.; Taylor, N.F.; Feller, J.A. Return to sport following anterior cruciate ligament reconstruction surgery: A systematic review and meta-analysis of the state of play. Br. J. Sports Med. 2011, 45, 596–606. [Google Scholar] [CrossRef]
- Paterno, M.V.; Rauh, M.J.; Schmitt, L.C.; Ford, K.R.; Hewett, T.E. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin. J. Sport Med. 2012, 22, 116–121. [Google Scholar] [CrossRef]
- Vermeijden, H.D.; Yang, X.A.; Mintz, D.N.; Rademakers, M.V.; van der List, J.P.; Kerkhoffs, G.M.M.J.; DiFelice, G.S. Age and Bone Bruise Patterns Predict Tear Location in the Anterior Cruciate Ligament. Arthrosc. Sports Med. Rehabil. 2022, 5, e41–e50. [Google Scholar] [CrossRef]
- Sonin, A.H.; Fitzgerald, S.W.; Hoff, F.L.; Friedman, H.; Bresler, M.E. MR imaging of the posterior cruciate ligament: Normal, abnormal, and associated injury patterns. Radiographics 1995, 15, 551–561. [Google Scholar] [CrossRef]
- Wijdicks, C.A.; Griffith, C.J.; Johansen, S.; Engebretsen, L.; LaPrade, R.F. Injuries to the medial collateral ligament and associated medial structures of the knee. J. Bone Jt. Surg. Am. 2010, 92, 1266–1280. [Google Scholar]
- Alaia, E.F.; Rosenberg, Z.S.; Alaia, M.J. Stener-Like Lesions of the Superficial Medial Collateral Ligament of the Knee: MRI Features. AJR Am. J. Roentgenol. 2019, 213, W272–W276. [Google Scholar]
- Kahan, J.B.; Li, D.; Schneble, C.A.; Huang, P.; Bullock, J.; Porrino, J.; Medvecky, M.J. The Pathoanatomy of Posterolateral Corner Ligamentous Disruption in Multiligament Knee Injuries Is Predictive of Peroneal Nerve Injury. Am. J. Sports Med. 2020, 48, 3541–3548. [Google Scholar] [CrossRef]
- LCL Injury of the Knee—Knee & Sports—Orthobullets. Available online: https://www.orthobullets.com/knee-and-sports/3011/lcl-injury-of-the-knee (accessed on 3 March 2025).
- Jeong, M.S.; Choi, Y.S.; Kim, Y.J.; Kim, J.S.; Young, K.W.; Jung, Y.Y. Deltoid ligament in acute ankle injury: MR imaging analysis. Skeletal Radiol. 2014, 43, 655–663. [Google Scholar] [CrossRef]
- Kaminski, T.W.; Hertel, J.; Amendola, N.; Docherty, C.L.; Dolan, M.G.; Hopkins, J.T.; Nussbaum, E.; Poppy, W.; Richie, D. National Athletic Trainers’ Association position statement: Conservative management and prevention of ankle sprains in athletes. J. Athl. Train. 2013, 48, 528–545. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Liu, T.; Xiang, F.; Deng, Y.; Li, Z.; Wei, D. Long-term outcome of flexor hallucis longus tendon transfer for chronic Achilles tendon rupture with large defect: A retrospective series. Medicine 2023, 102, e35302. [Google Scholar] [CrossRef]
- Friederichsen, P.; Zwicky, S.; Stephan, A.; Stadelmann, V.A.; Rippstein, P. Transosseous Flexor Hallucis Longus Tendon Transfer for Large Achilles Tendon Defects: Surgery Technique and Outcome. Foot Ankle Int. 2025, 46, 159–167. [Google Scholar] [CrossRef]
- Huang, L.; Chen, L.; Chen, H.; Wang, M.; Jin, L.; Zhou, S.; Gao, L.; Li, R.; Li, Q.; Wang, H.; et al. Biomimetic Scaffolds for Tendon Tissue Regeneration. Biomimetics 2023, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Deptuch, T.; Dams-Kozlowska, H. Silk Materials Functionalized via Genetic Engineering for Biomedical Applications. Materials 2017, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Teulé, F.; Cooper, A.R.; Furin, W.A.; Bittencourt, D.; Rech, E.L.; Brooks, A.; Lewis, R.V. A protocol for the production of recombinant spider silk-like proteins for artificial fiber spinning. Nat. Protoc. 2009, 4, 341–355. [Google Scholar] [CrossRef]
- Hahn, J.; Gögele, C.; Schulze-Tanzil, G. Could an Anterior Cruciate Ligament Be Tissue-Engineered from Silk? Cells 2023, 12, 2350. [Google Scholar] [CrossRef]
- Brooks, A.E.; Brothers, T.J.; Creager, M.S.; Lewis, R.V. A novel methodology to explore the viscoelasticity of spider major ampullate silk. J. Appl. Biomater. Biomech. 2007, 5, 158–165. [Google Scholar]
- Mechanical Properties of Ligament and Tendon Musculoskeletal Key. Available online: https://musculoskeletalkey.com/mechanical-properties-of-ligament-and-tendon/ (accessed on 3 March 2025).
- Applications of Silk-Based Biomaterials in Biomedicine and Biotechnology. Available online: https://www.researchgate.net/publication/378143482_Applications_of_silk-based_biomaterials_in_biomedicine_and_biotechnology (accessed on 3 March 2025).
- Kardestuncer, T.; McCarthy, M.B.; Karageorgiou, V.; Kaplan, D.; Gronowicz, G. RGD-tethered silk substrate stimulates the differentiation of human tendon cells. Clin. Orthop. Relat. Res. 2006, 448, 234–239. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Meinel, L.; Fajardo, R.; Hofmann, S.; Langer, R.; Chen, J.; Snyder, B.; Vunjak-Novakovic, G.; Kaplan, D. Silk implants for the healing of critical size bone defects. Bone 2005, 37, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Han, J.; Yu, Y.; Chen, F.; Yao, J. Boost Tendon/Ligament Repair with Biomimetic and Smart Cellular Constructs. Front. Bioeng. Biotechnol. 2021, 9, 726041. [Google Scholar] [CrossRef]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burns Trauma 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Biomaterials as Tendon and Ligament Substitutes: Current Developments. Available online: http://ouci.dntb.gov.ua/en/works/40rqxoW9/ (accessed on 15 September 2025).
- Liu, X.; Zhu, B.; Li, Y.; Liu, X.; Guo, S.; Wang, C.; Li, S.; Wang, D. The Role of Vascular Endothelial Growth Factor in Tendon Healing. Front. Physiol. 2021, 12, 766080. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Fan, B.; Wang, X.; Huang, X.; Guan, J.; Sun, Z.; Xu, B.; Yang, M.; Chen, Z.; Jiang, D.; et al. A Systematic Review of Tissue Engineering Scaffold in Tendon Bone Healing in vivo. Front. Bioeng. Biotechnol. 2021, 9, 621483. [Google Scholar] [CrossRef]
- Skutek, M.; van Griensven, M.; Zeichen, J.; Brauer, N.; Bosch, U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur. J. Appl. Physiol. 2001, 86, 48–52. [Google Scholar] [CrossRef]
- Beyene, R.T.; Derryberry, S.L.; Barbul, A. The Effect of Comorbidities on Wound Healing. Surg. Clin. N. Am. 2020, 100, 695–705. [Google Scholar] [CrossRef]
- Fadini, G.P.; Spinetti, G.; Santopaolo, M.; Madeddu, P. Impaired Regeneration Contributes to Poor Outcomes in Diabetic Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 34–44. [Google Scholar] [CrossRef] [PubMed]

| Ligament/Tendon Injury | Mechanism of Injury | Common Causes |
|---|---|---|
| Anterior Cruciate Ligament (ACL) Tear | Non-contact, multi-planar movement involving sudden deceleration, an aggressive pivot or cut, and/or awkward landing from a jump | Common in sports like soccer, football, and basketball |
| Medial Collateral Ligament (MCL)/Lateral Collateral Ligament (LCL) Tear | Direct Impact: Direct blow to the Outside (MCL) or Inside (LCL) of the knee Non-contact force: Sudden, forceful movement that twists the knee | Direct Impact: contact sports such as hockey or football Non-contact: skier’s foot getting caught or a soccer player cutting and pivoting suddenly |
| Posterior Cruciate Ligament (PCL) Tear | Direct Impact: Direct blow to the front of the knee Non-contact force: Hyperflexion of the knee, or Sudden forceful movement that twists the knee | Direct Impact: Dashboard injury or falling on a bent knee Non-contact: a misstep causing knee hyperflexion, soccer player cutting and pivoting suddenly |
| Ulnar Collateral Ligament (UCL) Injury | Repetitive stress from throwing or overhead activities, leading to gradual wear and tear, or a sudden traumatic event like a fall on an outstretched arm | Most common in Baseball Pitchers |
| Anterior Talofibular Ligament (ATFL) (ankle) | Sudden twisting or inversion of the ankle commonly from a misstep or quick pivot motion | Common in sports like basketball, football, soccer |
| Achilles Tendon Tear | Sudden, forceful plantarflexion (e.g., pushing off the foot while the knee is extended) or Sudden, unexpected dorsiflexion (e.g., landing from a jump or fall) | Common in sports like gymnastics, basketball, and soccer |
| Patellar Tendon Tear | Landing from a jump in sports (e.g., basketball, volleyball): The tendon may tear as the quadriceps contract to control landing with the knee flexed. | Common in sports like, basketball, and volleyball |
| Biceps Tendon Tear | Sudden, forceful resistance against biceps contraction | Lifting heavy weight with arms Dog suddenly and forcefully pulling on leash with outstretched arm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, C.; McCloskey, C.; Williams, K.; Teixeira, K.; Brooks, B.D.; Brooks, A.E. Biomechanical Analysis of Silk as a Tendon or Ligament Replacement. Polymers 2025, 17, 3052. https://doi.org/10.3390/polym17223052
Wagner C, McCloskey C, Williams K, Teixeira K, Brooks BD, Brooks AE. Biomechanical Analysis of Silk as a Tendon or Ligament Replacement. Polymers. 2025; 17(22):3052. https://doi.org/10.3390/polym17223052
Chicago/Turabian StyleWagner, Caleb, Colin McCloskey, Kaitlin Williams, Katherine Teixeira, Benjamin D. Brooks, and Amanda E. Brooks. 2025. "Biomechanical Analysis of Silk as a Tendon or Ligament Replacement" Polymers 17, no. 22: 3052. https://doi.org/10.3390/polym17223052
APA StyleWagner, C., McCloskey, C., Williams, K., Teixeira, K., Brooks, B. D., & Brooks, A. E. (2025). Biomechanical Analysis of Silk as a Tendon or Ligament Replacement. Polymers, 17(22), 3052. https://doi.org/10.3390/polym17223052








