Preparation and Paclitaxel-Loading of Regenerated Chitin Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitin Solution
2.3. Preparation of ChNps
2.4. Preparation of ChNps–PTX
2.5. Determination of DA for Chitin and ChNps
2.6. Determination of Drug Loading (DL)
2.7. Characterization
3. Results and Discussion
3.1. DA Analysis of Chitin and ChNps
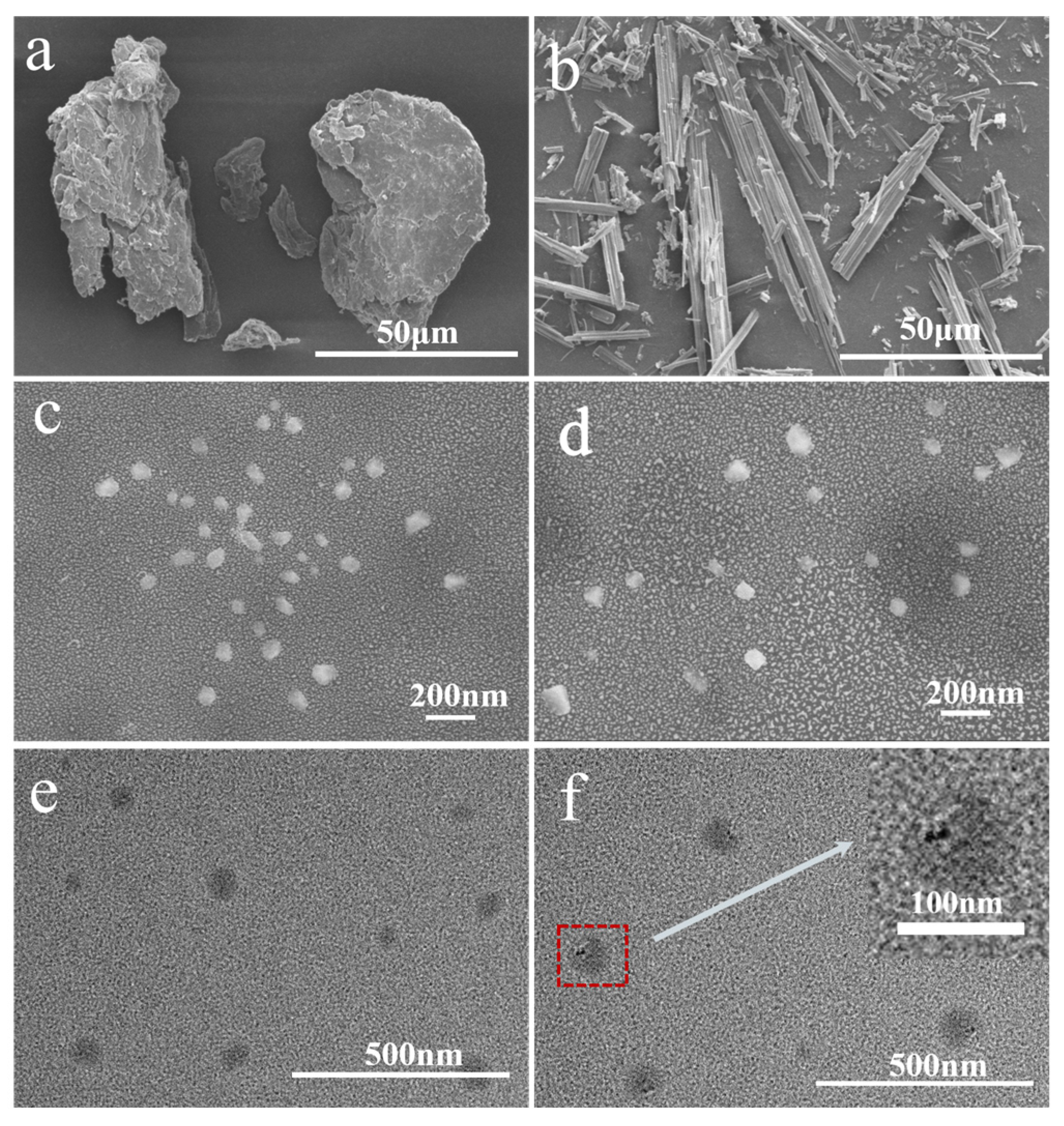
3.2. Morphological Analysis
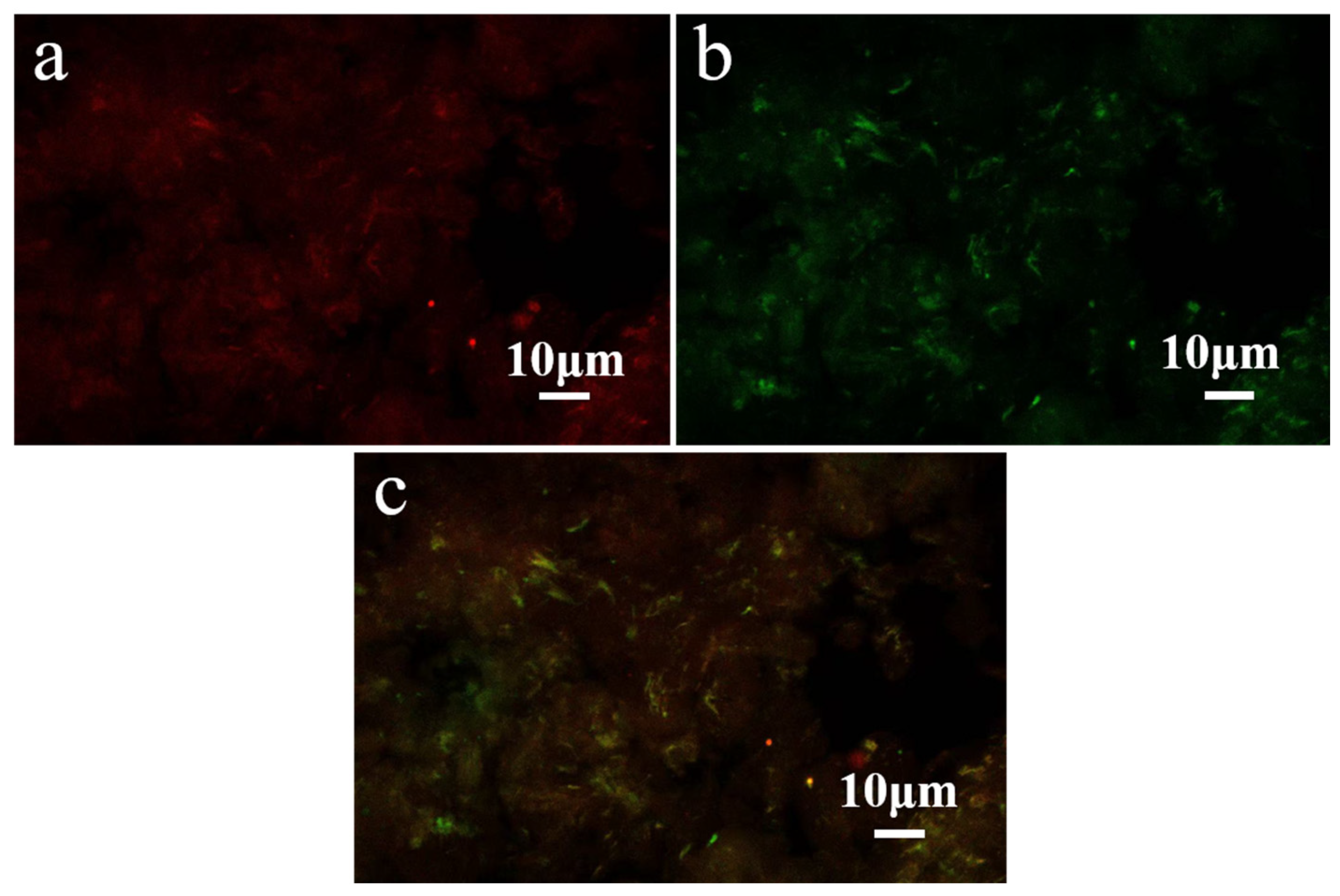
3.3. CLSM Analysis
3.4. Structural Characterization
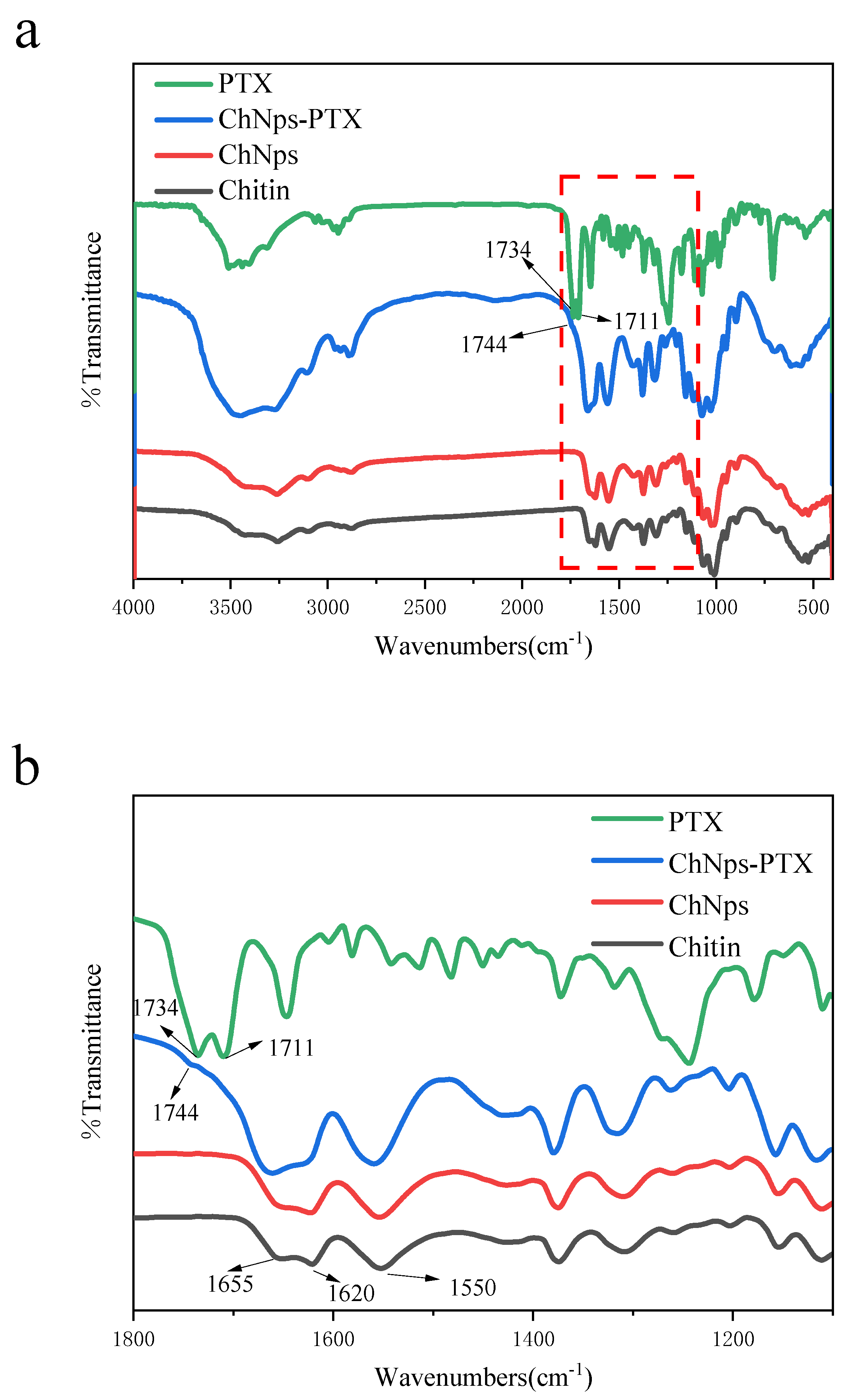
3.4.1. FT-IR Analysis
3.4.2. XRD Analysis
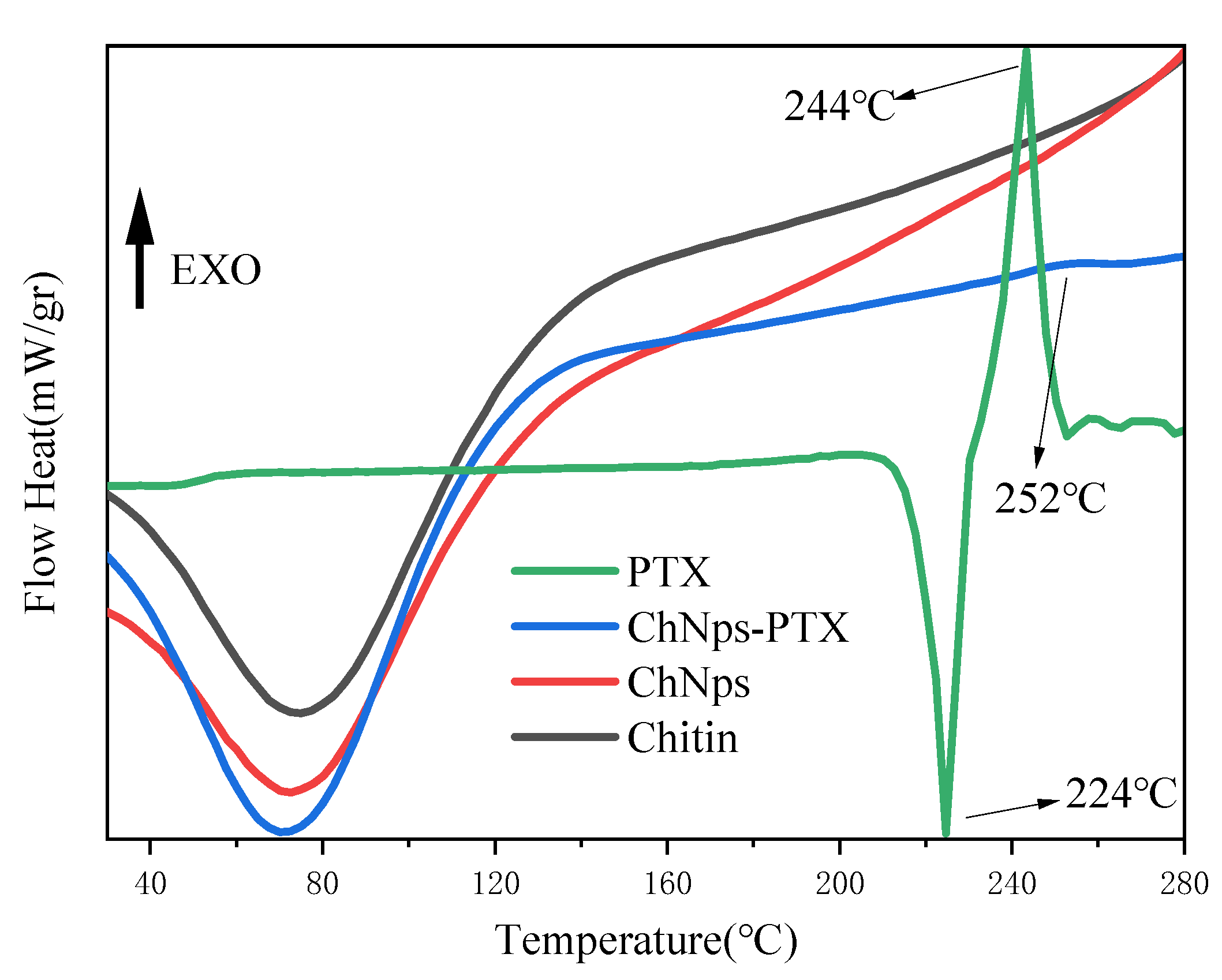
3.4.3. DSC Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ChNps | Chitin nanoparticles |
| PTX | Paclitaxel |
| ChNps–PTX | Chitin nanoparticles–paclitaxel |
| DA | degree of acetylation |
| DL | drug loading |
| FT-IR | Fourier Transform Infrared |
| DSC | Differential Scanning Calorimetry |
| XRD | X-ray Diffraction |
| CLSM | Confocal Laser Scanning Microscopy |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| 1H-NMR | Proton Nuclear Magnetic Resonance Spectroscopy |
| HPLC | high-performance liquid chromatography |
References
- Olugbojo, J.A.; Akinyemi, A.A.; Obasa, S.O.; Dare, E.O. Extraction and Utilization of Chitin and Chitosan from Waste Yields of Economically Important Crustaceans and Molluscs for Improved Fish Production. Asian J. Fish. Aquat. Res. 2024, 26, 95–114. [Google Scholar] [CrossRef]
- Ngasotter, S.; Xavier, K.M.; Meitei, M.M.; Waikhom, D.; Pathak, J.; Singh, S.K. Crustacean shell waste derived chitin and chitin nanomaterials for application in agriculture, food, and health–A review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100349. [Google Scholar] [CrossRef]
- Joseph, B.; Sam, R.M.; Balakrishnan, P.; Maria, H.J.; Gopi, S.; Volova, T.; Fernandes, S.C.M.; Thomas, S. Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances. Polymers 2020, 12, 1664. [Google Scholar] [CrossRef]
- Dutta, A.K.; Yamada, K.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of Chitin Nanofibers from Dry Chitin Powder by Star Burst System: Dependence on Number of Passes. J. Chitin Chitosan Sci. 2013, 1, 59–64. [Google Scholar] [CrossRef]
- Ben Cheikh, F.; Ben Mabrouk, A.; Magnin, A.; Putaux, J.L.; Boufi, S. Chitin nanocrystals as Pickering stabilizer for O/W emulsions: Effect of the oil chemical structure on the emulsion properties. Colloids Surf. B Biointerfaces 2021, 200, 111604. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, W.; Liang, J.; Li, L.; Zheng, L.; Shi, X.; Wang, C.; Dong, Y.; Li, C.; Zhu, X. In Situ Synthesis of Gold Nanoparticles from Chitin Nanogels and Their Drug Release Response to Stimulation. Polymers 2024, 16, 390. [Google Scholar] [CrossRef]
- Rigual, V.; Ovejero-Perez, A.; Martinez-Mangas, A.; Garcia-Sánchez, B.; Dominguez, J.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Comparison of bottom-up and top-down precipitation strategies for lignin nanoparticle obtention from organosolv and ionosolv Eucalyptus globulus liquors. Faraday Discuss. 2025. [Google Scholar] [CrossRef]
- Zewude, D.A.; Noguchi, T.; Sato, K.; Izawa, H.; Ifuku, S. Production of chitin nanoparticles by bottom-up approach from alkaline chitin solution. Int. J. Biol. Macromol. 2022, 210, 123–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Bian, W.; Feng, L.; Wu, Z.; Wang, P.; Zeng, X.; Wu, T. Stabilizing oil-in-water emulsions with regenerated chitin nanofibers. Food Chem. 2015, 183, 115–121. [Google Scholar] [CrossRef]
- Kadokawa, J.I. A Mini-Review: Fabrication of Polysaccharide Composite Materials Based on Self-Assembled Chitin Nanofibers. Materials 2024, 17, 1898. [Google Scholar] [CrossRef]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cai, J.; Zhang, L. A Review of Chitin Solvents and Their Dissolution Mechanisms. Chin. J. Polym. Sci. 2020, 38, 1047–1060. [Google Scholar] [CrossRef]
- Kätelhön, E.; Sokolov, S.V.; Bartlett, T.R.; Compton, R.G. The Role of Entropy in Nanoparticle Agglomeration. ChemPhysChem 2017, 18, 51–54. [Google Scholar] [CrossRef]
- Pyttlik, A.; Kuttich, B.; Kraus, T. Microgravity Removes Reaction Limits from Nonpolar Nanoparticle Agglomeration. Small 2022, 18, 2204621. [Google Scholar] [CrossRef] [PubMed]
- Al-Gebory, L.; Mengüç, M.P. The effect of pH on particle agglomeration and optical properties of nanoparticle suspensions. J. Quant. Spectrosc. Radiat. Transf. 2018, 219, 46–60. [Google Scholar] [CrossRef]
- Diephuis, W.R.; Molloy, A.L.; Boltz, L.L.; Porter, T.B.; Orozco, A.A.; Duron, R.; Crespo, D.; George, L.J.; Reiffer, A.D.; Escalera, G.; et al. The Effect of Agglomeration on Arsenic Adsorption Using Iron Oxide Nanoparticles. Nanomaterials 2022, 12, 1598. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Rameshthangam, P.; Venkatesan, S.; Singh, S.K.; Vijayan, S.R. In Vitro and In Silico Studies of Chitin and Chitosan Based Nanocarriers for Curcumin and Insulin Delivery. J. Polym. Environ. 2018, 26, 4095–4113. [Google Scholar] [CrossRef]
- Kumar, M.; Goswami, P.; Jha, A.; Manjit, M.; Satpute, A.P.; Koch, B.; Mishra, B. Formulation and evaluation of cetuximab functionalized phospholipid modified nanocrystals of paclitaxel for non-small cell lung cancer therapy. Sci. Rep. 2024, 14, 29114. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Liang, Q.; Wang, S.; Zhao, B.; Wang, Y.; Cai, Y.; Li, G. Bioavailability Enhancement of Paclitaxel via a Novel Oral Drug Delivery System: Paclitaxel-Loaded Glycyrrhizic Acid Micelles. Molecules 2015, 20, 4337–4356. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Cui, Y.; Zhao, Q.; Musetti, S.; Kinghorn, K.; Wang, S. Overcoming multiple gastrointestinal barriers by bilayer modified hollow mesoporous silica nanocarriers. Acta Biomater. 2018, 65, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, Y.; Chen, L. Intestinal Uptake of Barley Protein Based Nanoparticles for β-Carotene Delivery. Acta Pharm. Sin. B 2019, 9, 87–96. [Google Scholar] [CrossRef]
- Ravindran, S.; Suthar, J.K.; Rokade, R.; Deshpande, P.; Singh, P.; Pratinidhi, A.; Khambadkhar, R.; Utekar, S. Pharmacokinetics, Metabolism, Distribution and Permeability of Nanomedicine. Curr. Drug Metab. 2018, 19, 327–334. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, F.; Ling, J.; Ouyang, X.; Wang, G. Delivery of curcumin using a zein-xanthan gum nanocomplex: Fabrication, characterization, and in vitro release properties. Colloids Surf. B Biointerfaces 2021, 204, 111827. [Google Scholar] [CrossRef]
- Nsereko, S.; Amiji, M. Localized delivery of paclitaxel in solid tumors from biodegradable chitin microparticle formulations. Biomaterials 2002, 23, 2723–2731. [Google Scholar] [CrossRef]
- Smitha, K.T.; Anitha, A.; Furuike, T.; Tamura, H.; Nair, S.V.; Jayakumar, R. In vitro evaluation of paclitaxel loaded amorphous chitin nanoparticles for colon cancer drug delivery. Colloids Surf. B Biointerfaces 2013, 104, 245–253. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Wu, Y.; Lin, Y. Glycol chitin/PAA hydrogel composite incorporated bio-functionalized PLGA microspheres intended for sustained release of anticancer drug through intratumoral injection. Biotech Week 2018, 29, 1839–1858. [Google Scholar] [CrossRef]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of chitin hydrogel under mild conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Heux, L.; Brugnerotto, J.; Desbrières, J.; Versal, M.F.; Rinaudo, M. Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules 2000, 1, 746–751. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Zhuang, X.; Pang, M.; Qiu, D.; Chen, Y.; Mao, L.; Zhang, Z.; Xian, S.; Tang, X. Preparation, characterization and evaluation of loading paclitaxel into debranched corn starch. Ind. Crops Prod. 2025, 229, 120969. [Google Scholar] [CrossRef]
- Chu, L.; Niu, W.; Wu, S.; Ma, W.; Tang, B.; Zhang, S.; Li, H.; Liu, Z. A two-step approach for size controlled preparation of monodisperse polysaccharide-based nanospheres. Mater. Res. Express 2019, 6, 055013. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, S.; Li, H. Multiple Ethanolic-Precipitation: An Approach to the Separation of Dextrin Fractions with Narrow Molecular Weight Distributions from Acid-Hydrolyzed Waxy Corn Starch. Starch-Starke 2020, 72, 11–12. [Google Scholar] [CrossRef]
- Kasaai, M.R. Determination of the degree of N -acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2009, 79, 801–810. [Google Scholar] [CrossRef]
- Wu, T.; Wang, G.; Gao, C.; Chen, Z.; Feng, L.; Wang, P.; Zeng, X.; Wu, Z. Phosphoric acid-based preparing of chitin nanofibers and nanospheres. Cellulose 2016, 23, 477–491. [Google Scholar] [CrossRef]
- Zuo, R.; Kong, X.; Wang, Y.; Deng, S.G.; Zhuang, X.; Qiu, D. Isolation and characterization of natural nano starch from amaranth starch. Int. J. Biol. Macromol. 2024, 260 Pt 1, 129525. [Google Scholar] [CrossRef]
- Gayathri, N.K.; Aparna, V.; Maya, S.; Biswas, R.; Jayakumar, R.; Mohan, C.G. Preparation, characterization, drug release and computational modelling studies of antibiotics loaded amorphous chitin nanoparticles. Carbohydr. Polym. 2017, 177, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Falgayrac, A.; Pellerin, V.; Terrol, C.; Fernandes, S.C.M. Turning black soldier fly rearing by-products into valuable materials: Valorisation through chitin and chitin nanocrystals production. Carbohydr. Polym. 2024, 344, 122545. [Google Scholar] [CrossRef]
- Li, J.; Hao, Z.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. High-tensile chitin films regenerated from cryogenic aqueous phosphoric acid. Carbohydr. Polym. 2023, 312, 120826. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Chukhchin, D.G.; Gofman, I.V.; Pavlova, E.N.; Ushakov, V.A.; Vlasova, E.N.; Poshina, D.N.; Skorik, Y.A. Chitin Cryogels Prepared by Regeneration from Phosphoric Acid Solutions. Materials 2021, 14, 5191. [Google Scholar] [CrossRef]
- Ding, B.; Zhao, D.; Song, J.; Gao, H.; Xu, D.; Xu, M.; Cao, X.; Zhang, L.; Cai, J. Light weight, mechanically strong and biocompatible α-chitin aerogels from different aqueous alkali hydroxide/urea solutions. Sci. China Chem. 2016, 59, 1405–1414. [Google Scholar] [CrossRef]
- Kato, Y.; Kaminaga, J.; Matsuo, R.; Isogai, A. TEMPO-mediated oxidation of chitin, regenerated chitin and N -acetylated chitosan. Carbohydr. Polym. 2004, 58, 421–426. [Google Scholar] [CrossRef]
- Yu, P.; He, H.; Luo, Y.; Jia, D.; Dufresne, A. Elastomer Reinforced with Regenerated Chitin from Alkaline/Urea Aqueous System. ACS Appl. Mater. Interfaces 2017, 9, 26460–26467. [Google Scholar] [CrossRef] [PubMed]
- Ngasotter, S.; Xavier, K.A.M.; Porayil, L.; Balange, A.; Nayak, B.B.; Eapen, S.; Adarsh, K.J.; Sreekala, M.S.; Sharma, R.; Ninan, G. Optimized high-yield synthesis of chitin nanocrystals from shrimp shell chitin by steam explosion. Carbohydr. Polym. 2023, 16, 121040. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, H.N.; Zia, K.M.; Rehman, S.; Ilyas, R.; Sultana, S. Utilization of Shellfish Industrial Waste for Isolation, Purification, and Characterizations of Chitin from Crustacean’s Sources in Pakistan. J. Polym. Environ. 2021, 29, 2337–2348. [Google Scholar] [CrossRef]
- Farokhi, N.M.; Milani, J.M.; Amiri, Z.R. Production and comparison of structural, thermal and physical characteristics of chitin nanoparticles obtained by different methods. Sci. Rep. 2024, 14, 14594. [Google Scholar] [CrossRef]
- Zhang, C.; Ping, Q.; Zhang, H. Self-assembly and characterization of paclitaxel-loaded N-octyl-O-sulfate chitosan micellar system. Colloids Surf. B Biointerfaces 2004, 39, 69–75. [Google Scholar] [CrossRef]
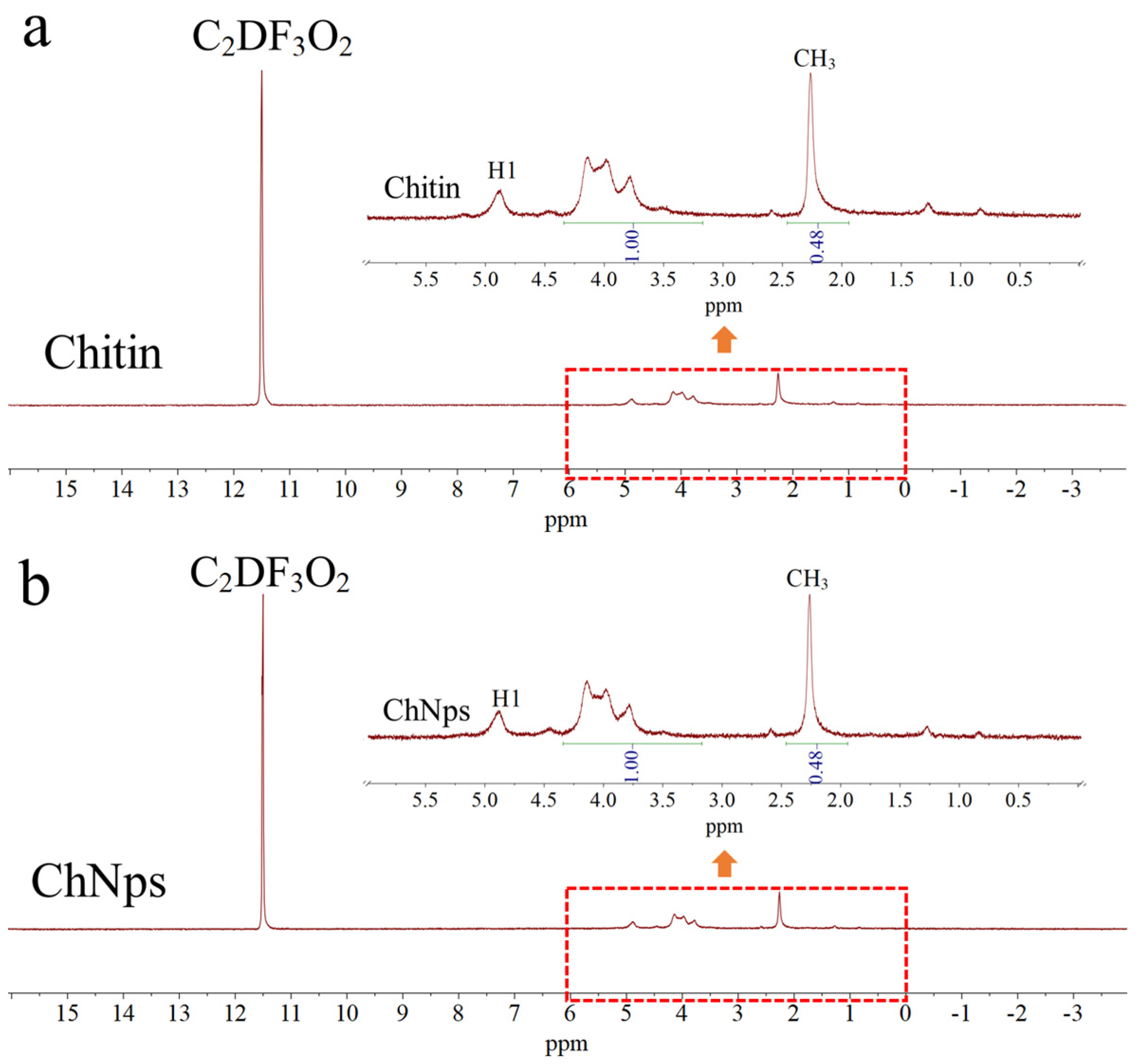


| Sample | Integral Area of -CH3 (1.94–2.64 ppm) | Integral Area of H2–H6 (3.17–4.34 ppm) | DA, % |
|---|---|---|---|
| Chitin | 0.48 | 1.00 | 96.03 |
| ChNps | 0.48 | 1.00 | 96.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qiu, D.; Zhuang, X.; Pei, Z.; Li, T.; Deng, S. Preparation and Paclitaxel-Loading of Regenerated Chitin Nanoparticles. Polymers 2025, 17, 3036. https://doi.org/10.3390/polym17223036
Li Y, Qiu D, Zhuang X, Pei Z, Li T, Deng S. Preparation and Paclitaxel-Loading of Regenerated Chitin Nanoparticles. Polymers. 2025; 17(22):3036. https://doi.org/10.3390/polym17223036
Chicago/Turabian StyleLi, Yanping, Dan Qiu, Xuechen Zhuang, Zhiduo Pei, Tao Li, and Shanggui Deng. 2025. "Preparation and Paclitaxel-Loading of Regenerated Chitin Nanoparticles" Polymers 17, no. 22: 3036. https://doi.org/10.3390/polym17223036
APA StyleLi, Y., Qiu, D., Zhuang, X., Pei, Z., Li, T., & Deng, S. (2025). Preparation and Paclitaxel-Loading of Regenerated Chitin Nanoparticles. Polymers, 17(22), 3036. https://doi.org/10.3390/polym17223036







