Synthesis, Characterisation and Preliminary Antimicrobial Evaluation of Chitosan-4-Anisaldehyde Conjugates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Chitosan-4-Anisaldehyde Conjugate (ChT-AA)
2.3. Preparation of Gels Containing ChT-AA Conjugates and Chitosan
2.4. Physicochemical Characterisation
2.5. Rheological Measurements
2.6. Antimicrobial Activity
3. Results
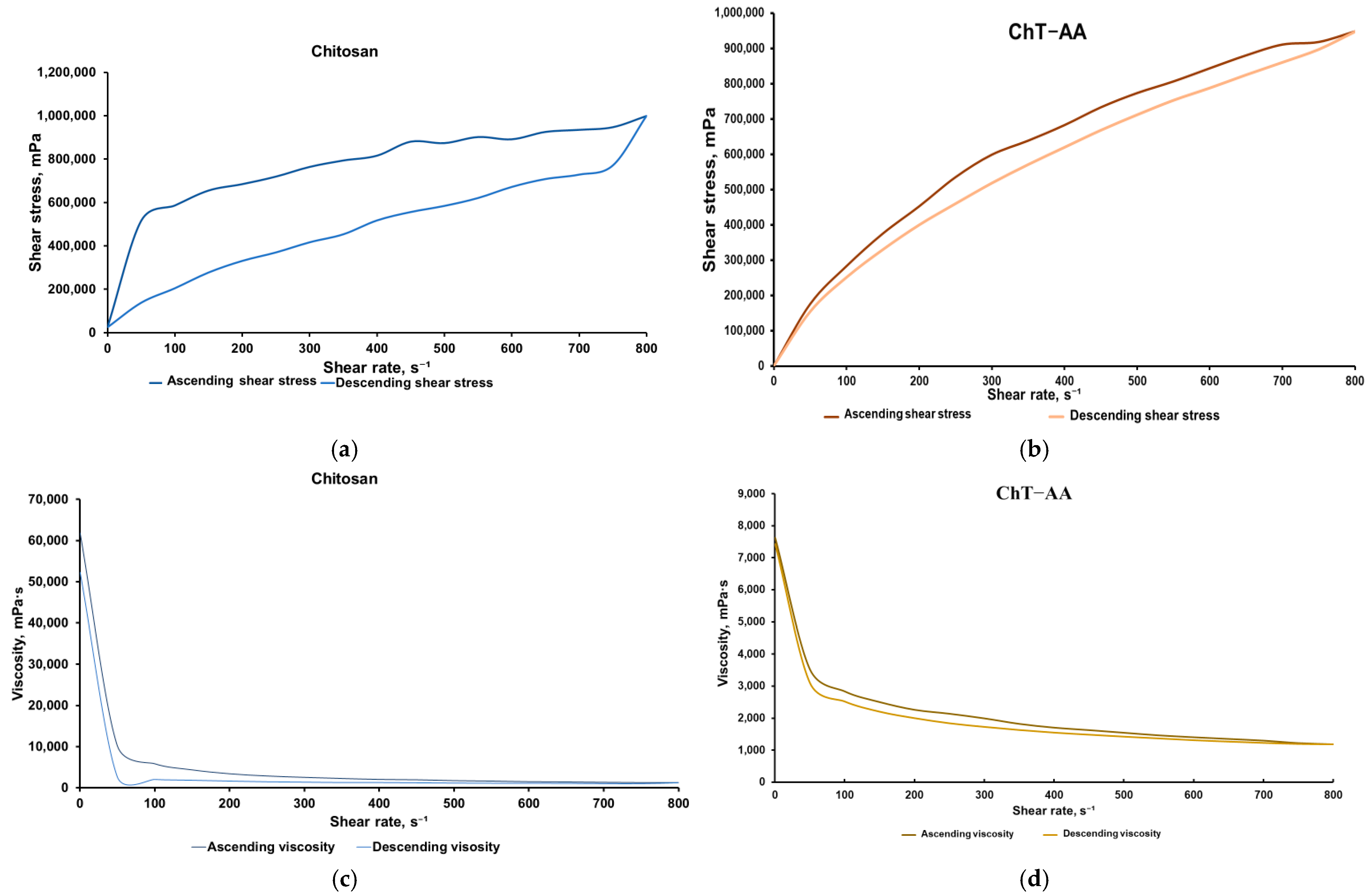
3.1. Rheological Analysis
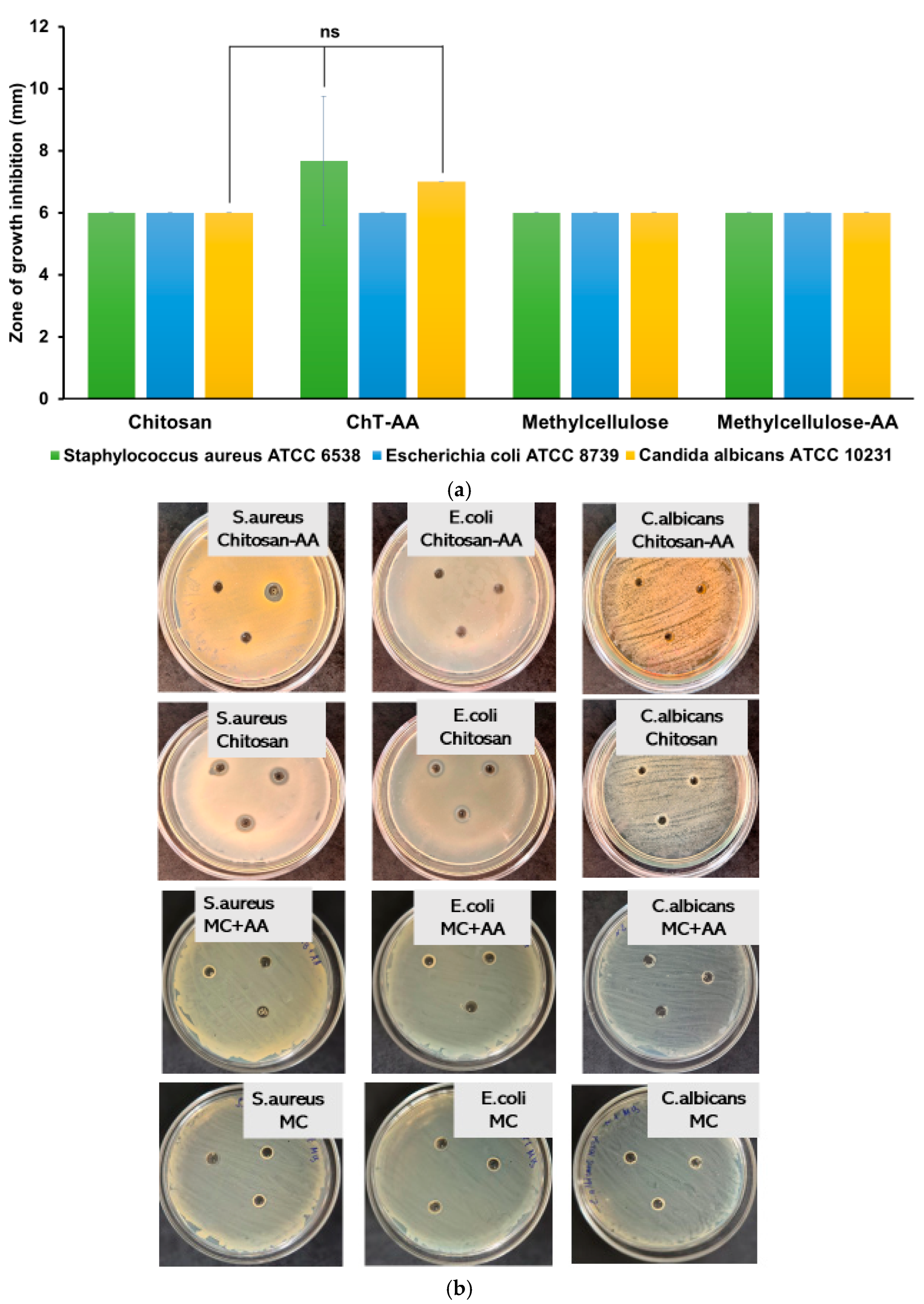
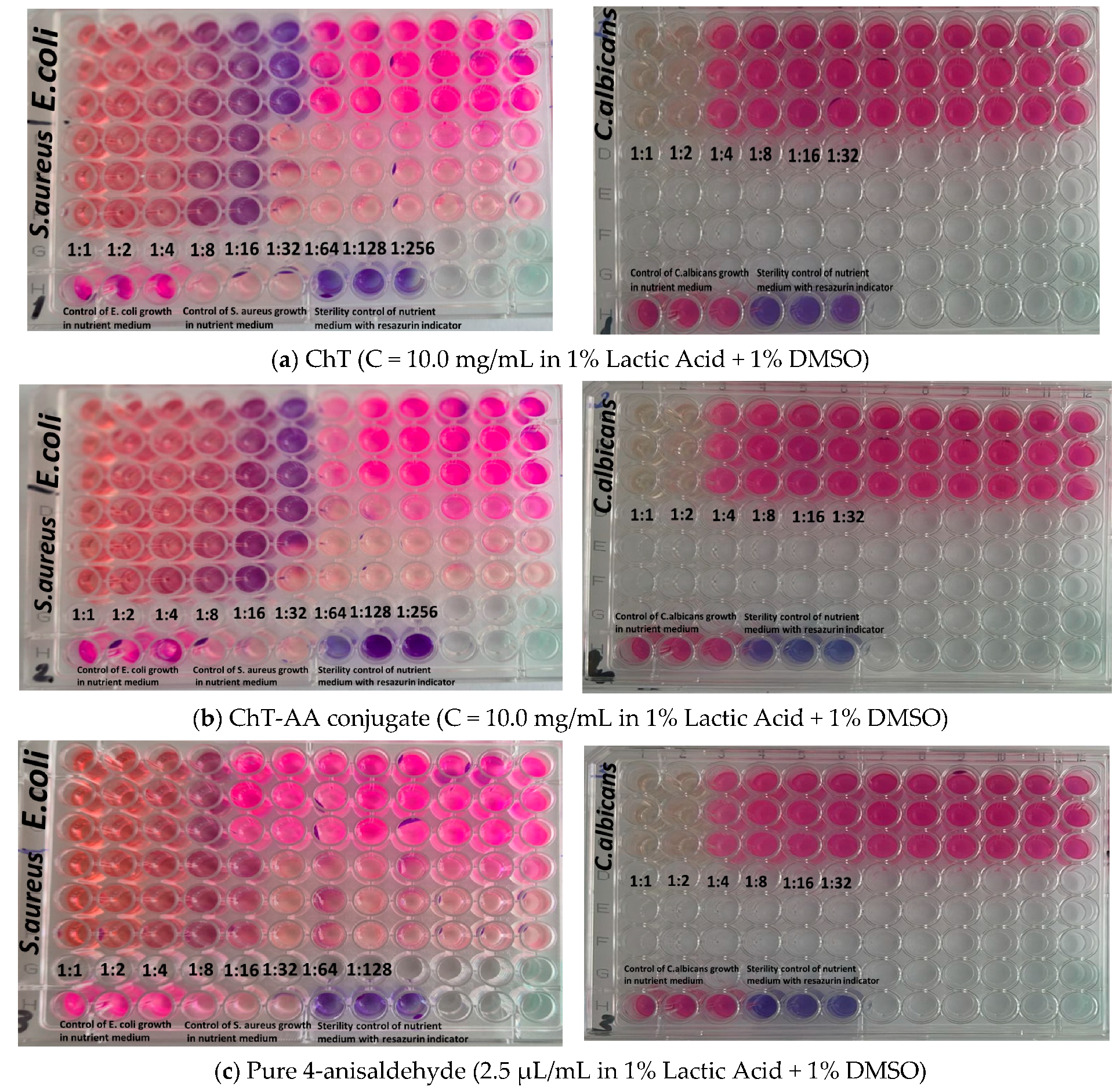
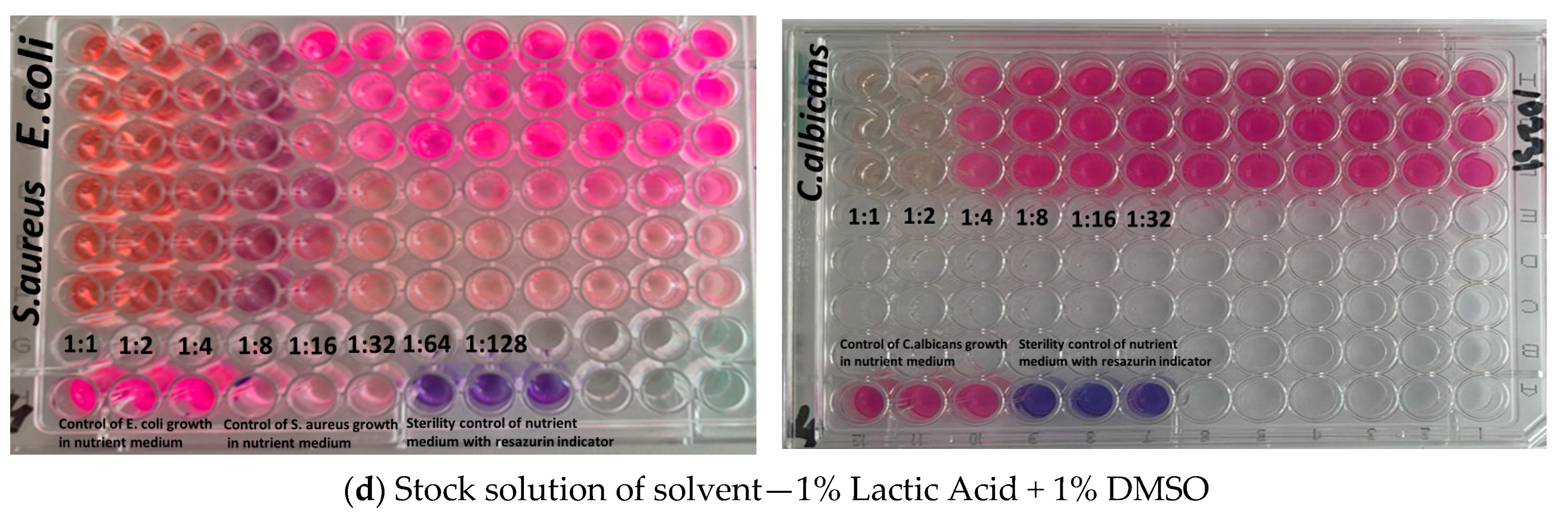
3.2. Antimicrobial Properties of Chitosan, ChT-AA, and Corresponding Gels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ChT | chitosan |
| AA | anisaldehyde |
| ChT-AA | chitosan-anisaldehyde conjugate |
| FTIR | Fourier-transform infrared spectroscopy |
| 1H NMR | proton nuclear magnetic resonance |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| NaFl | sodium fluorescein |
| Tg | glass transition temperature |
References
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Stevanovic, M.M.; Vukomanovic, M.; Milenkovic, M.; Boccaccini, A.R. Editorial: Antimicrobial Nanostructured Polymeric Materials and Nanocomposites. Front. Mater. 2021, 8, 748813. [Google Scholar] [CrossRef]
- Boateng, J. (Ed.) Therapeutic Dressings and Wound Healing Applications; Wiley: Hoboken, NJ, USA, 2020; ISBN 9781119433262. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Aljawish, A.; Chevalot, I.; Piffaut, B.; Rondeau-Mouro, C.; Girardin, M.; Jasniewski, J.; Scher, J.; Muniglia, L. Functionalization of Chitosan by Laccase-Catalyzed Oxidation of Ferulic Acid and Ethyl Ferulate under Heterogeneous Reaction Conditions. Carbohydr. Polym. 2012, 87, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitosan Composites with Inorganics, Morphogenetic Proteins and Stem Cells, for Bone Regeneration. Carbohydr. Polym. 2011, 83, 1433–1445. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Lu, W.; Hu, Y. Review on Chitosan-Based Antibacterial Hydrogels: Preparation, Mechanisms, and Applications. Int. J. Biol. Macromol. 2024, 255, 128080. [Google Scholar] [CrossRef]
- Haj, N.Q.; Mohammed, M.O.; Mohammood, L.E. Synthesis and Biological Evaluation of Three New Chitosan Schiff Base Derivatives. ACS Omega 2020, 5, 13948–13954. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, T.; Chen, X.; Tian, D.; Wang, L.; Gao, R. Preparation and Characterization of Vanillin-Conjugated Chitosan-Stabilized Emulsions via a Schiff-Base Reaction. Food Sci. Biotechnol. 2023, 32, 1489–1499. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils from Organically Cultivated Fennel Cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Meng, R.; Zhao, Z.; Liu, Z.; Zhao, X.; Shi, C.; Guo, N. Efficacy of a Combination of Nisin and P-Anisaldehyde against Listeria Monocytogenes. Food Control 2016, 66, 100–106. [Google Scholar] [CrossRef]
- Lin, J.; Meng, H.; Guo, X.; Tang, Z.; Yu, S. Natural Aldehyde-Chitosan Schiff Base: Fabrication, PH-Responsive Properties, and Vegetable Preservation. Foods 2023, 12, 2921. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Abdel-Rahman, R.M.; Hašová, M.; Šmejkalová, D.; Kolář, M.; Pekar, M.; Aly, A.S. Antibacterial Activity and Cell Viability of Hyaluronan Fiber with Silver Nanoparticles. Carbohydr. Polym. 2013, 92, 1177–1187. [Google Scholar] [CrossRef]
- Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and Chitosan Derivatives in Drug Delivery and Tissue Engineering. In Chitosan for Biomaterials II; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–44. [Google Scholar]
- Tamer, T.M.; Hassan, M.A.; Omer, A.M.; Baset, W.M.A.; Hassan, M.E.; El-Shafeey, M.E.A.; Eldin, M.S.M. Synthesis, Characterization and Antimicrobial Evaluation of Two Aromatic Chitosan Schiff Base Derivatives. Process Biochem. 2016, 51, 1721–1730. [Google Scholar] [CrossRef]
- Lokarev, A.V.; Ogai, M.A.; Slivkin, A.I.; Belenova, A.S. Development and Rheological Studies of Ointments with a Complex Extract from Medicinal Plant Raw Materials. Proc. Voronezh State Univ. Ser. Chem. Biol. Pharm. 2019, 2, 95–101. [Google Scholar]
- Lamy Rheology Instruments. User Manual RM200 Plus, Version UK03/2023; Lamy Rheology Instruments: Cham-pagne-au-Mont-d’Or, France, 2023. [Google Scholar]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2002, 49, 1049. [Google Scholar] [CrossRef]
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 9781562388393. [Google Scholar]
- Cockerill, F. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 1562389882. [Google Scholar]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Filippov, S.K.; Maji, S.; Glassner, M.; Cegłowski, M.; Hoogenboom, R.; King, S.; Lau, W.M.; Khutoryanskiy, V.V. Mucus-Penetrating Nanoparticles Based on Chitosan Grafted with Various Non-Ionic Polymers: Synthesis, Structural Characterisation and Diffusion Studies. J. Colloid Interface Sci. 2022, 626, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Dhawade, P.P.; Jagtap, R.N. Characterization of the Glass Transition Temperature of Chitosan and Its Oligomers by Temperature Modulated Differential Scanning Calorimetry. Adv. Appl. Sci. Res. 2012, 3, 1372. [Google Scholar]
- Alamri, A.A.; Borik, R.M.A.; El-Wahab, A.H.F.A.; Mohamed, H.M.; Ismail, K.S.; El-Aassar, M.R.; Al-Dies, A.-A.M.; El-Agrody, A.M. Synthesis of Schiff Bases Based on Chitosan, Thermal Stability and Evaluation of Antimicrobial and Antitumor Activities. Sci. Rep. 2025, 15, 892. [Google Scholar] [CrossRef]
- Kaldybekov, D.B.; Shatabayeva, E.O.; Polatkhan, A.A.; Tuleyeva, R.N.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Development and Investigation of Mucoadhesive Polymers Based on Chitosan for Intravesical Therapy. Eurasian J. Chem. 2024, 29, 13–21. [Google Scholar] [CrossRef]
- Wei, L.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. The Antioxidant and Antifungal Activity of Chitosan Derivatives Bearing Schiff Bases and Quaternary Ammonium Salts. Carbohydr. Polym. 2019, 226, 115256. [Google Scholar] [CrossRef]
- Dhlamini, K.S.; Selepe, C.T.; Ramalapa, B.; Tshweu, L.; Ray, S.S. Reimagining Chitosan-Based Antimicrobial Biomaterials to Mitigate Antibiotic Resistance and Alleviate Antibiotic Overuse: A Review. Macromol. Mater. Eng. 2024, 309, 2400018. [Google Scholar] [CrossRef]
- Available online: https://Hfpappexternal.Fda.Gov/Scripts/Fdcc/Index.Cfm?Set=FoodSubstances&id=METHOXYBENZALDEHYDEp&sort=Sortterm_ID&order=ASC&startrow=1&type=basic&search=anisole (accessed on 1 October 2025).
- Xin, Y.; Peng, S.; Wei, S.; Lei, Y.; Zhang, S.; Hu, Y.; Lv, Y. Antimicrobial and Biofilm Inhibition Effects of P-Anisaldehyde against Vibrio Parahaemolyticus. Food Control 2023, 154, 110021. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Costa, E.; Silva, S.; Tavaria, F.; Pintado, M. Antimicrobial and Antibiofilm Activity of Chitosan on the Oral Pathogen Candida albicans. Pathogens 2014, 3, 908–919. [Google Scholar] [CrossRef]
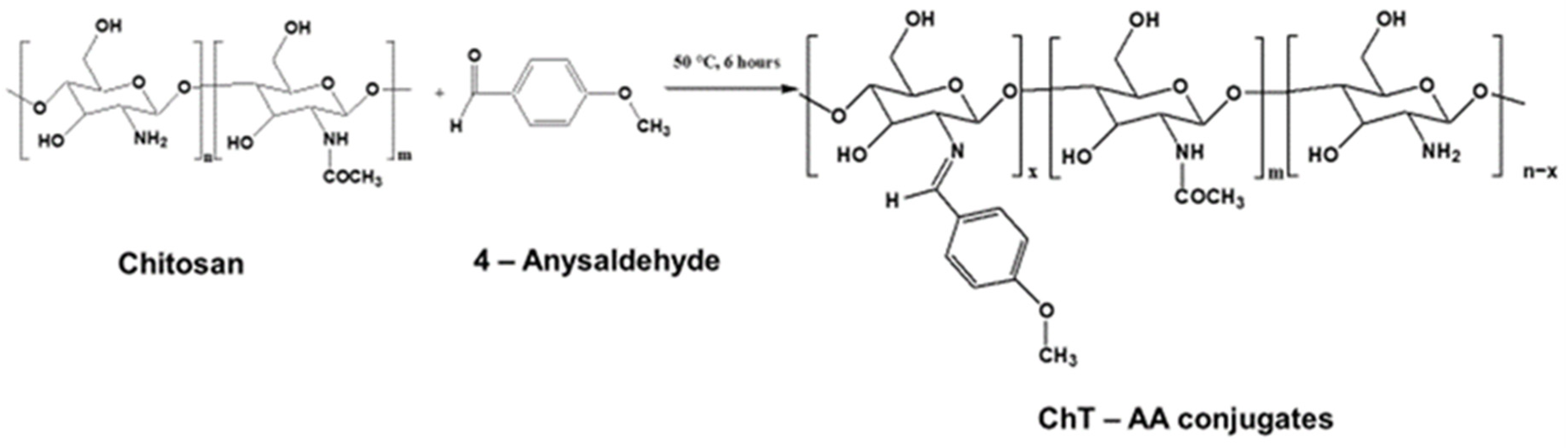
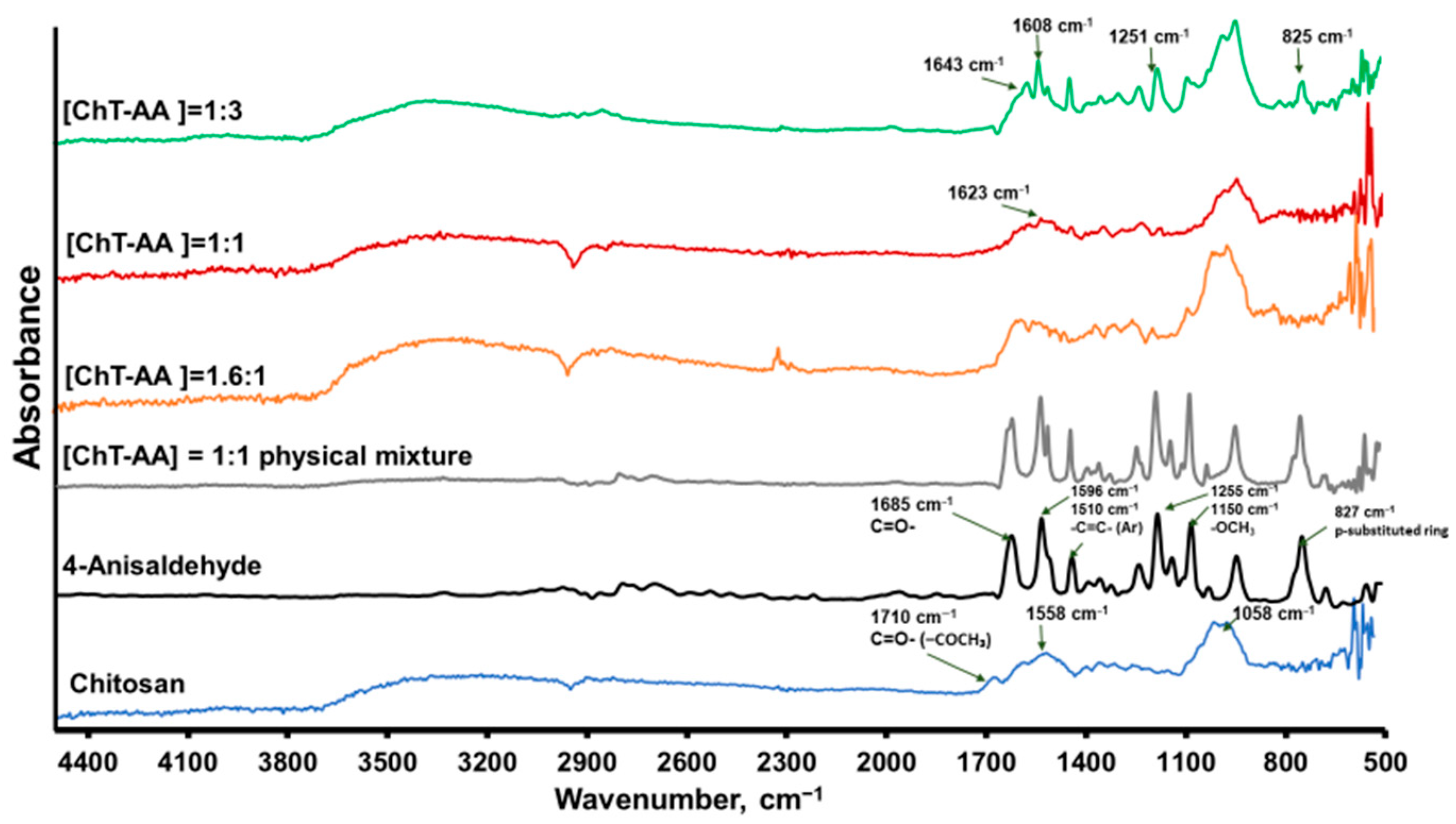

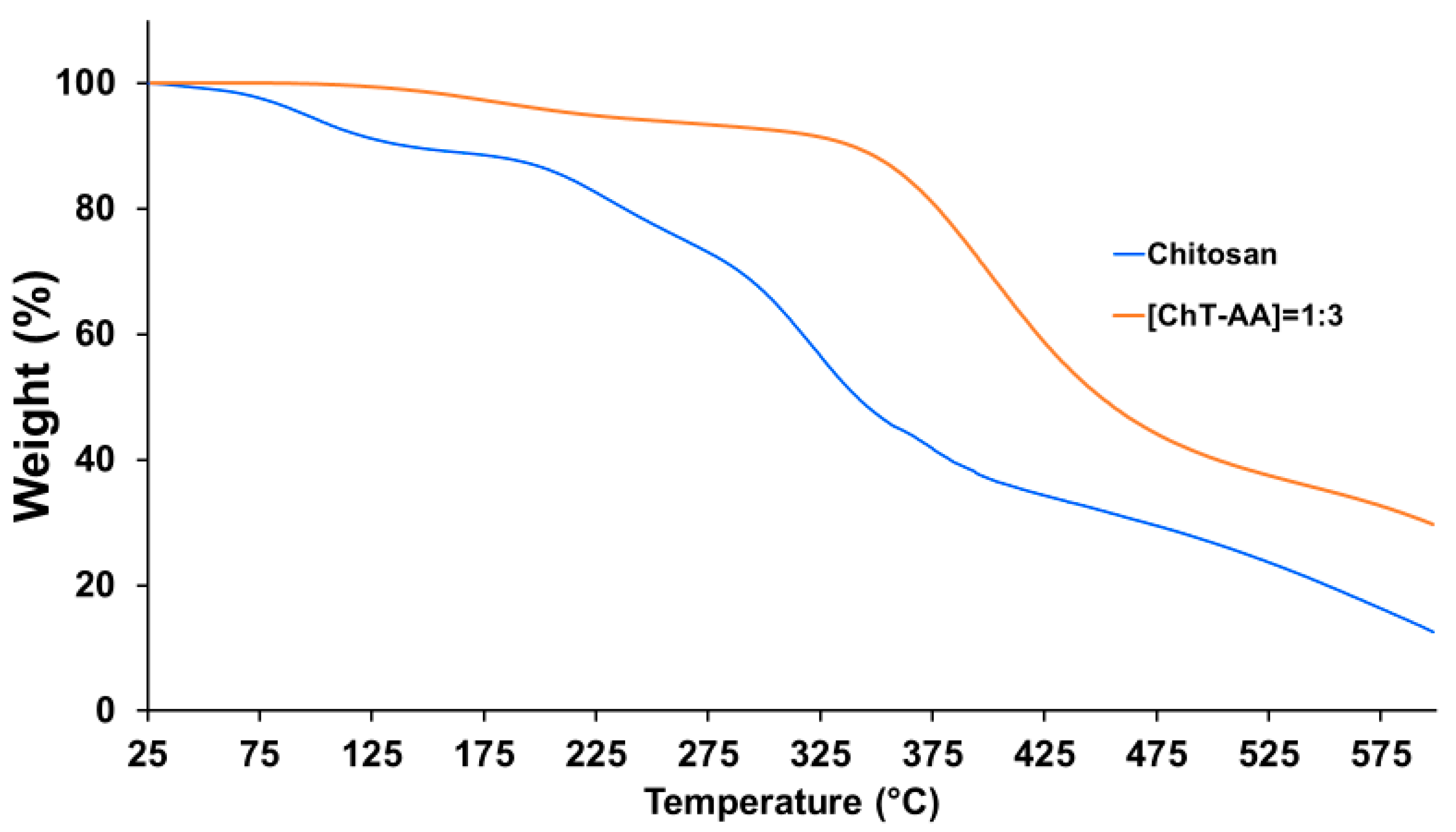
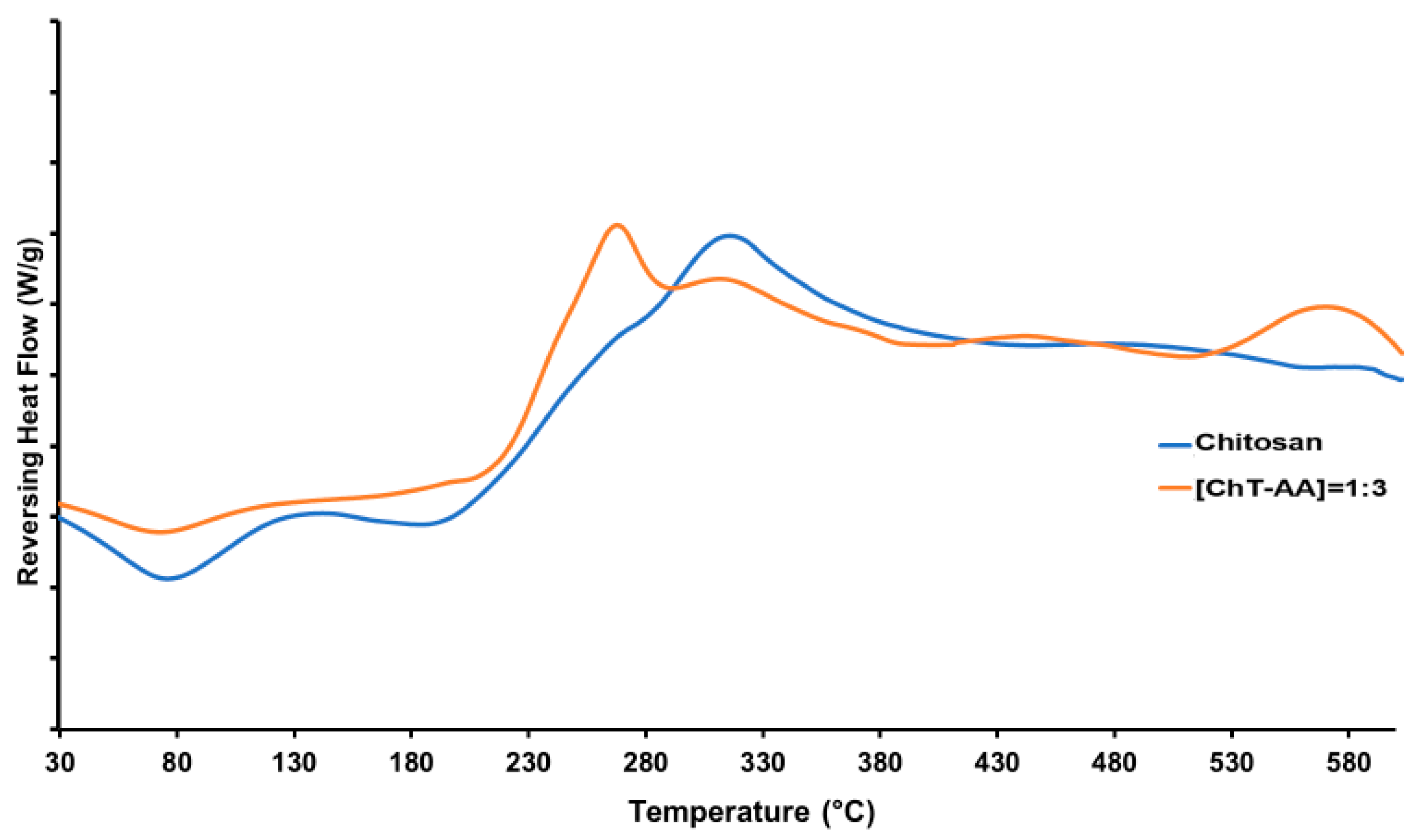
| Molar Ratio of [Chitosan]:[AA] | Weight of Chitosan, g | Weight of AA, g |
|---|---|---|
| 1.6:1 | 1.00 | 0.25 |
| 1:3 | 1.00 | 1.26 |
| 1:1 | 1.00 | 0.42 |
| Sample | Water Content in the Samples, % | T (Onset of Degradation), °C 1 | Tmax, °C 1 | Tg, °C 2 | Temperature of Exothermic Event, °C 2 | Temperature of Endothermic Event, °C 2 | Residual Weight at 600 °C, % |
|---|---|---|---|---|---|---|---|
| Chitosan | 10 | 200 | 350 | 117 | 314 | 76 | 13 |
| ChT-AA | 0.5 | 230 | 400 | 122.5 | 309 | 74 | 29.7 |
| Spindle Rotation Speed, rpm | Shear Rate, γ, s−1 | Gel with ChT-AA | Gel with Chitosan | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (γ = K·RPM) | Ascending | Descending | Ascending | Descending | |||||
| Shear Stress, τ, mPa | Viscosity, η, mPa·s | Shear Stress, τ, mPa | Viscosity, η, mPa·s | Shear Stress, τ, mPa | Viscosity, η, mPa·s | Shear Stress, τ, mPa | Viscosity, η, mPa·s | ||
| (τ = η·γ) | (τ = η·γ) | (τ = η·γ) | (τ = η·γ) | ||||||
| 0.5 | 0.5 | 3815 | 7630 ± 1145 | 3715 | 7430 ± 1115 | 30,916.5 | 61,833 ± 9275 | 26,137.5 | 52,275 ± 7841 |
| 50 | 50 | 176,900 | 3538 ± 531 | 155,600 | 3112 ± 467 | 516,100 | 10,322 ± 1548 | 138,200 | 2764 ± 415 |
| 100 | 100 | 284,000 | 2840 ± 426 | 252,300 | 2523 ± 378 | 586,300 | 5863 ± 879 | 205,000 | 2050 ± 308 |
| 150 | 150 | 376,050 | 2507 ± 376 | 330,750 | 2205 ± 331 | 655,200 | 4368 ± 655 | 277,500 | 1850 ± 278 |
| 200 | 200 | 453,000 | 2265 ± 340 | 400,600 | 2003 ± 300 | 684,600 | 3423 ± 513 | 331,000 | 1655 ± 248 |
| 250 | 250 | 535,500 | 2142 ± 321 | 460,750 | 1843 ± 276 | 719,500 | 2878 ± 432 | 370,000 | 1480 ± 222 |
| 300 | 300 | 599,100 | 1997 ± 300 | 519,000 | 1730 ± 260 | 764,100 | 2547 ± 382 | 416,700 | 1389 ± 208 |
| 350 | 350 | 638,750 | 1825 ± 274 | 571,550 | 1633 ± 245 | 793,800 | 2268 ± 340 | 453,600 | 1296 ± 194 |
| 400 | 400 | 682,800 | 1707 ± 256 | 620,400 | 1551 ± 233 | 816,000 | 2040 ± 306 | 517,600 | 1294 ± 194 |
| 450 | 450 | 733,050 | 1629 ± 244 | 668,700 | 1486 ± 223 | 880,650 | 1957 ± 294 | 556,200 | 1236 ± 185 |
| 500 | 500 | 773,500 | 1547 ± 232 | 712,500 | 1425 ± 214 | 874,000 | 1748 ± 262 | 584,500 | 1169 ± 175 |
| 550 | 550 | 806,300 | 1466 ± 220 | 753,500 | 1370 ± 206 | 902,000 | 1640 ± 246 | 620,950 | 1129 ± 169 |
| 600 | 600 | 843,600 | 1406 ± 211 | 788,400 | 1314 ± 197 | 891,600 | 1486 ± 223 | 672,000 | 1120 ± 168 |
| 650 | 650 | 880,100 | 1354 ± 203 | 825,500 | 1270 ± 191 | 925,600 | 1424 ± 214 | 708,500 | 1090 ± 164 |
| 700 | 700 | 910,700 | 1301 ± 195 | 861,000 | 1230 ± 185 | 935,200 | 1336 ± 200 | 728,700 | 1041 ± 156 |
| 750 | 750 | 918,000 | 1224 ± 184 | 897,750 | 1197 ± 180 | 947,250 | 1263 ± 189 | 771,000 | 1028 ± 154 |
| 800 | 800 | 948,000 | 1185 ± 178 | 948,000 | 1185 ± 178 | 999,200 | 1249 ± 187 | 999,200 | 1249 ± 187 |
| Sample | Minimum Inhibitory Concentration (MIC), mg/mL | ||
|---|---|---|---|
| E. coli ATCC 8739 | S. aureus ATCC 6538 | C. albicans ATCC 10231 | |
| 1. ChT (C = 10 mg/mL in 1% Lactic Acid + 1% DMSO) | 0.313 | 0.625 | 1.250 |
| 2. ChT-AA (C = 10 mg/mL in 1% Lactic Acid + 1% DMSO) | 0.313 | 0.313 | 1.250 |
| 3. Pure 4-anisaldehyde (2.5 μL/mL in 1% Lactic Acid + 1% DMSO) | 0.351 | 0.175 | 0.351 |
| 4. Stock solution of solvent—1% Lactic Acid + 1% DMSO (11 mg/mL of DMSO in 1 % Lactic acid + H2O) | 1.375 | 0.6875−1.375 | 1.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhayeva, D.N.; Mukhamediya, D.D.; Tairova, S.R.; Jumagaziyeva, A.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Synthesis, Characterisation and Preliminary Antimicrobial Evaluation of Chitosan-4-Anisaldehyde Conjugates. Polymers 2025, 17, 3017. https://doi.org/10.3390/polym17223017
Makhayeva DN, Mukhamediya DD, Tairova SR, Jumagaziyeva A, Irmukhametova GS, Khutoryanskiy VV. Synthesis, Characterisation and Preliminary Antimicrobial Evaluation of Chitosan-4-Anisaldehyde Conjugates. Polymers. 2025; 17(22):3017. https://doi.org/10.3390/polym17223017
Chicago/Turabian StyleMakhayeva, Danelya N., Dayana D. Mukhamediya, Saiyara R. Tairova, Ardak Jumagaziyeva, Galiya S. Irmukhametova, and Vitaliy V. Khutoryanskiy. 2025. "Synthesis, Characterisation and Preliminary Antimicrobial Evaluation of Chitosan-4-Anisaldehyde Conjugates" Polymers 17, no. 22: 3017. https://doi.org/10.3390/polym17223017
APA StyleMakhayeva, D. N., Mukhamediya, D. D., Tairova, S. R., Jumagaziyeva, A., Irmukhametova, G. S., & Khutoryanskiy, V. V. (2025). Synthesis, Characterisation and Preliminary Antimicrobial Evaluation of Chitosan-4-Anisaldehyde Conjugates. Polymers, 17(22), 3017. https://doi.org/10.3390/polym17223017







