Analysis of Tyre Pyrolysis Oil as Potential Diesel Fuel Blend with Focus on Swelling Behaviour of Nitrile-Butadiene Rubber
Abstract
1. Introduction
2. Materials
3. Experiment
3.1. Analysis of Fuels
| Parameter | Norm |
|---|---|
| Viscosity | ASTM D7042-21a [37] |
| Density | |
| Polyaromatic content | DIN EN 12916 [38] |
| Flash point | DIN EN ISO 2719 [39] |
| HFRR 1-test | DIN EN ISO 12156-1 [40] |
| CFPP 2 | DIN EN 116 [41] |
| Cupper corrosion | DIN EN ISO 2160 [42] |
| Oxidation stability | DIN EN 16091 [43] |
| Water content | DIN 51777-2 [44] |
| Carbon residue | DIN EN ISO 10370 [45] |
| Ash | DIN EN ISO 6245 [46] |
| Sulphur content | DIN EN ISO 20884 [47] |
| Distillation process | DIN EN ISO 3405 [48] |
3.2. Sorption Experiments
4. Results
4.1. Comparison of the Chemical Composition of DF and TPO
4.1.1. GC/MS Analysis
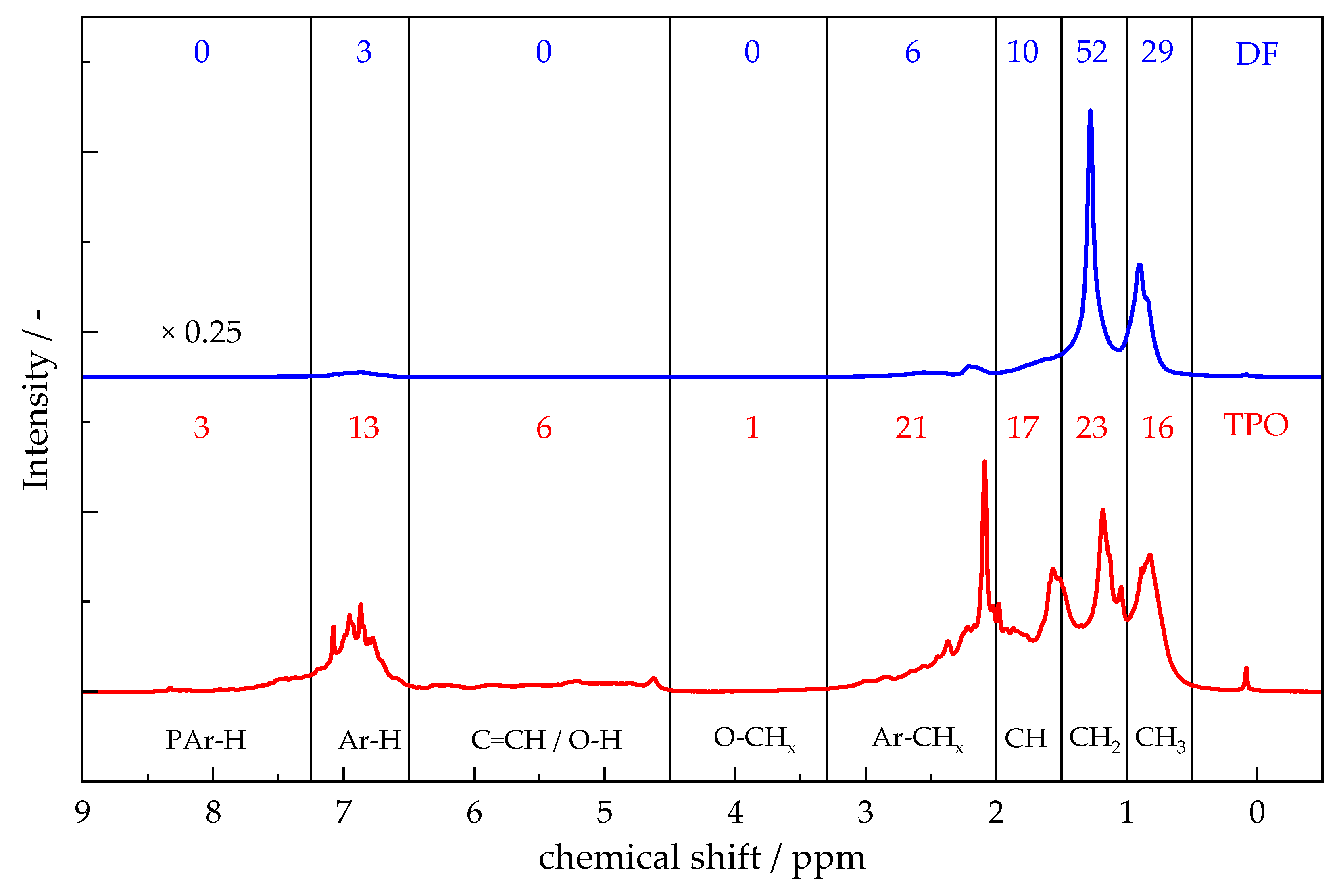
4.1.2. 1H-NMR-Spetroscopy
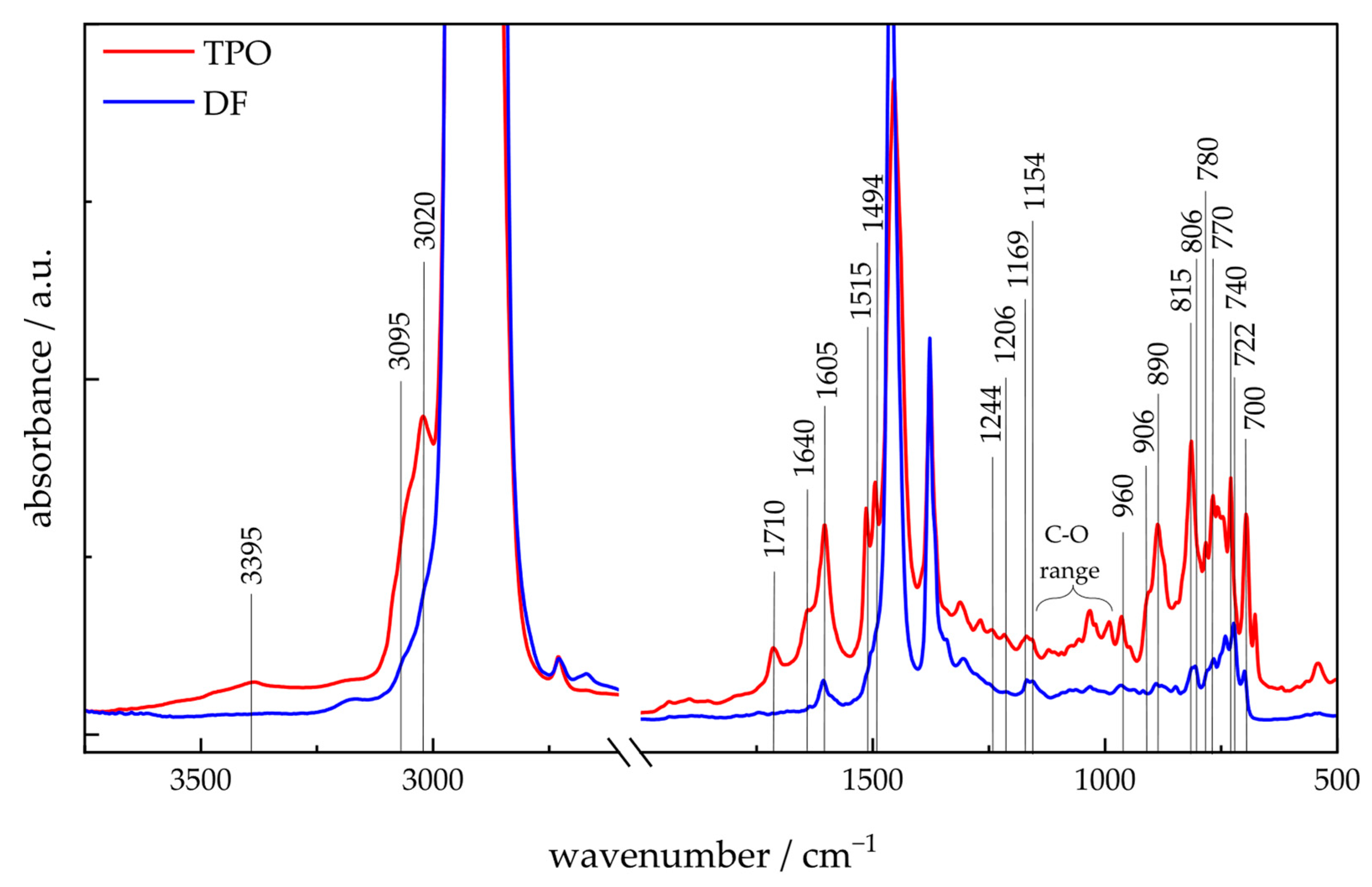
4.1.3. ATR-FTIR-Spectroscopy
4.1.4. Further Chemical Analysis
4.2. Characteristics of TPO and DF-TPO-Blends
4.3. Swelling Behaviour of NBR and Its Influence on Mechanical Properties
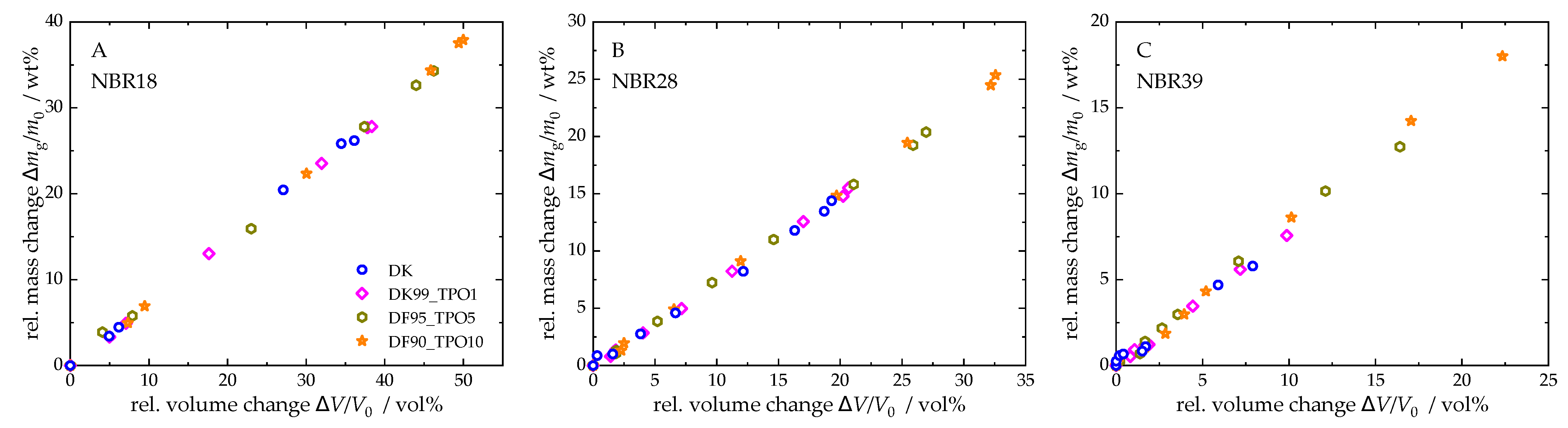
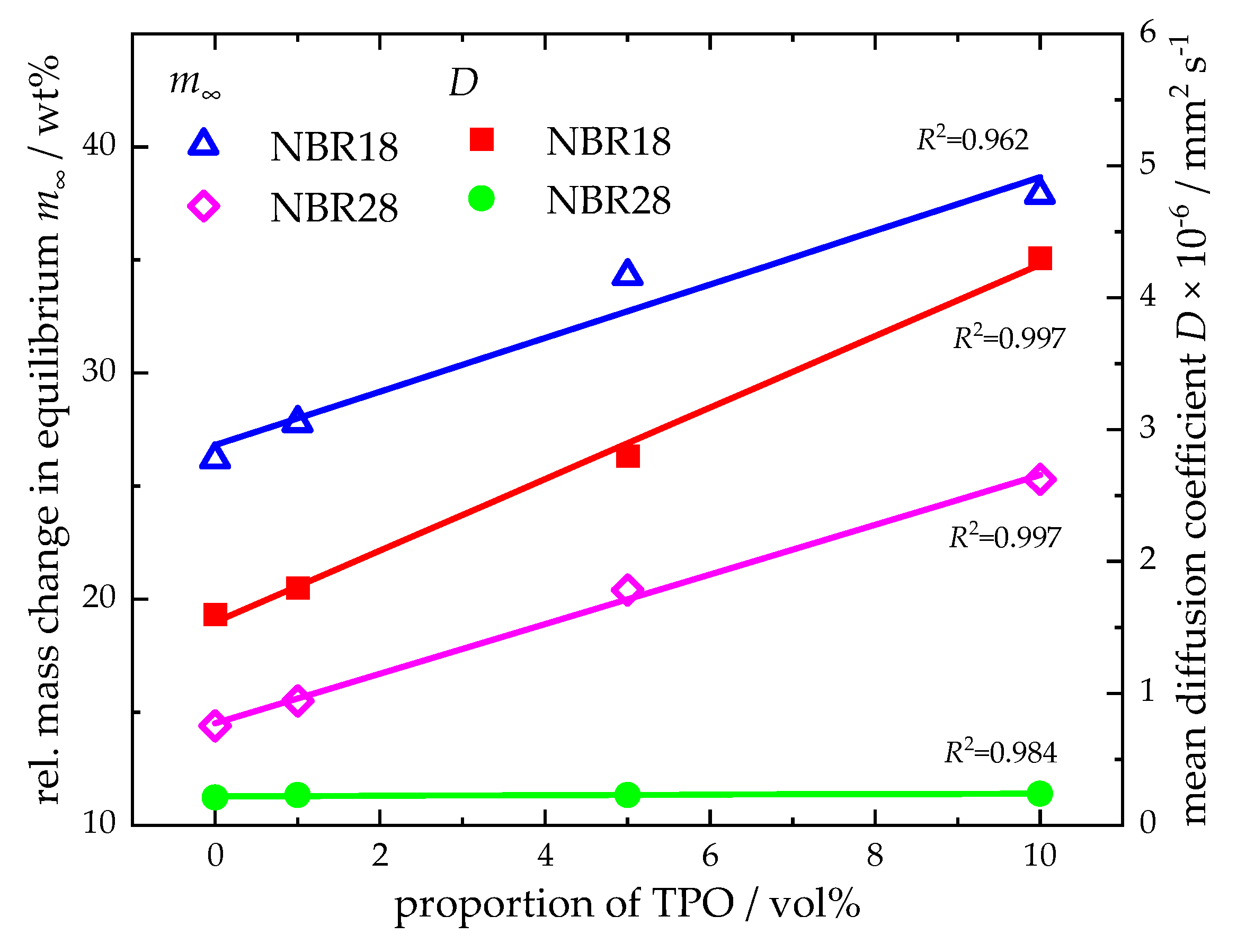
4.3.1. Mass and Volume Change in NBR After the Sorption
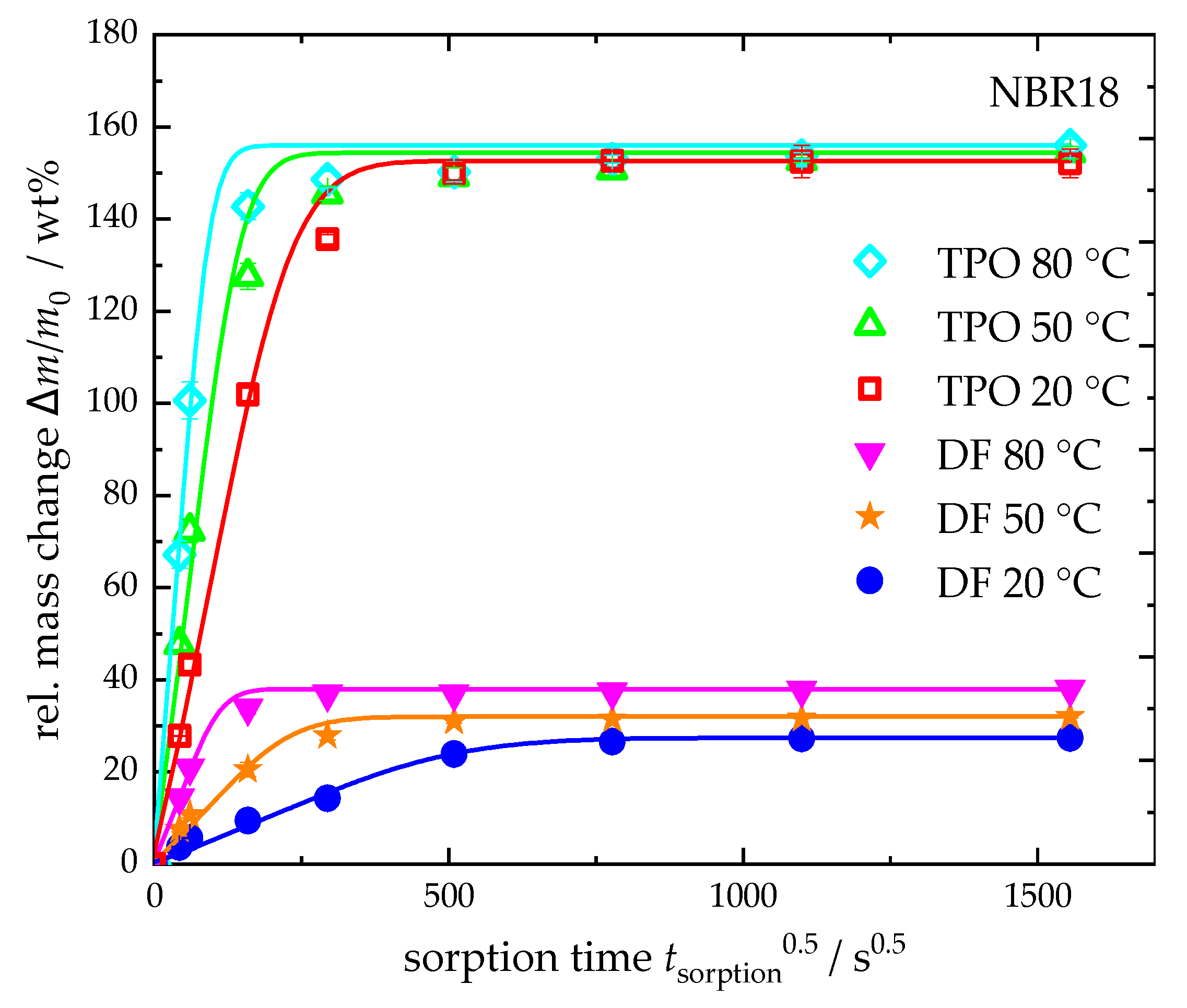
4.3.2. Temperature Dependence of the Diffusion Process of DF and TPO
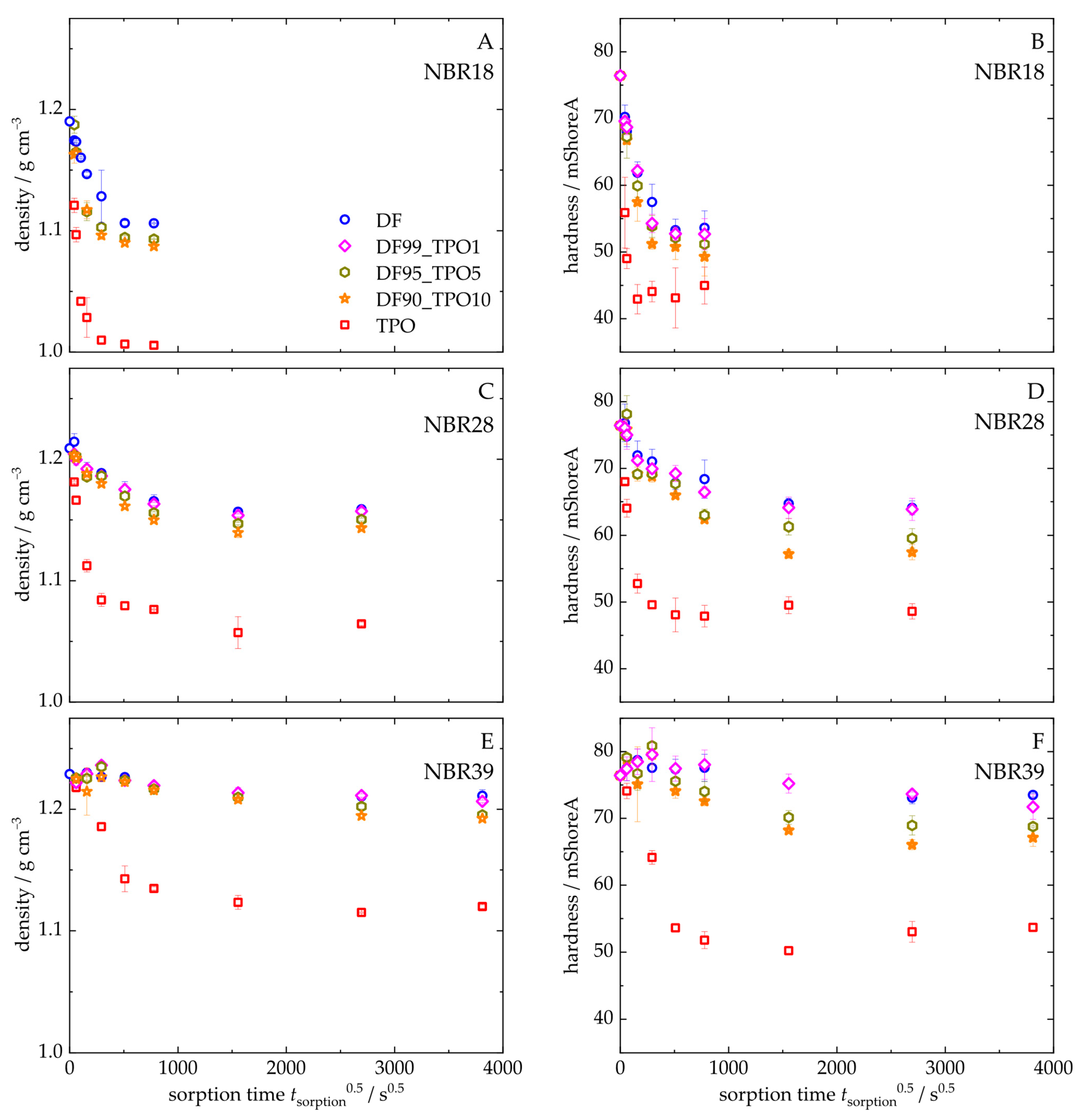
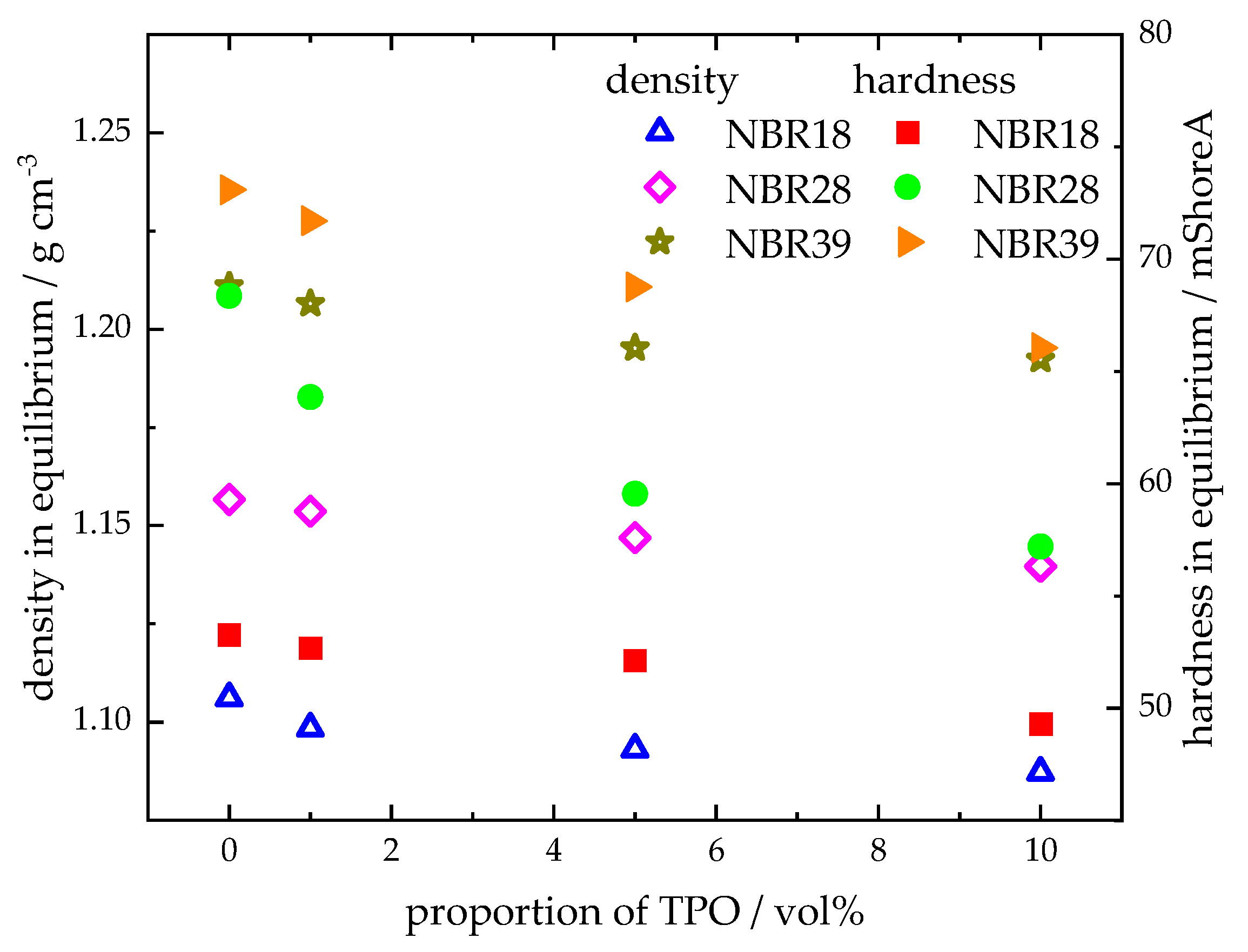
4.3.3. Change in the Mechanical and Physical Properties of the NBR
5. Conclusions
- Higher monoaromatic and polyaromatic contents. This property makes TPO a potential blending component for aromatic-free synthetic fuels.
- Higher sulphur content. Desulfurization is required to reduce the sulphur concentration to within the target range. Alternatively, due to its high sulphur content and correspondingly low value in the high frequency reciprocating rig test, TPO could be considered as a blend component for lubricating oils.
- Higher proportions of olefinic compounds. Hydrogenation is necessary to saturate olefins and improve oxidation stability, thereby expanding the possible applications of both neat TPO and its blends with other fuels and oils.
- Larger amounts of low- and high-boiling components. Distillation of TPO to separate these fractions could produce a cut similar to DF, potentially lowering the polyaromatic content, as these are typically found in the high-boiling fraction, and raising the flash point by removing low-boiling compounds.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DF | Disel fuel |
| TPO | Tyre pyrolysis oil |
| NBR | Nitrile-butadiene rubber |
| NR | natural rubber |
| SBR | Styrene-butadiene rubber |
| BR | Butadiene rubber |
| phr | Parts-per-hundred rubber |
| DPHP | Di(2-propylheptyl)phthalate |
| 6-PPD | N-(4-Methylpentan-2-yl)-N-phenylbenzene-1,4-diamine |
| TAN | Total acid number |
| HFRR | High frequency reciprocating rig |
| CFPP | cold filter plugging point |
| 2D-GC/MS | Two-dimensional gas chromatography coupled to mass spectrometry |
| SPE | Solid phase extraction |
| GC/MS | Gas chromatography coupled to mass spectrometry |
| 1H-NMR | 1H-Nuclear magnetic resonance |
| FTIR | Fourier transform infrared |
| HPLC | High-Performance Liquid Chromatography |
| PAHs | Polyaromatic hydrocarbons |
| KOH | Potassium hydroxide |
References
- Continental Reifen Deutschland GmbH. Reifengrundlagen. Available online: https://www.continental-reifen.de/tire-knowledge/tire-mixture/ (accessed on 19 August 2025).
- Conesa, J.A.; Martín-Gullón, I.; Font, R.; Jauhiainen, J. Complete Study of the Pyrolysis and Gasification of Scrap Tires in a Pilot Plant Reactor. Environ. Sci. Technol. 2004, 38, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Chaala, A.; Darmstadt, H. The Vacuum Pyrolysis of Used Tires End-Uses for Oil and Carbon Black Products. J. Anal. Appl. Pyrolysis 1999, 51, 201–221. [Google Scholar] [CrossRef]
- Dai, X.; Yin, X.; Wu, C.; Zhang, W.; Chen, Y. Pyrolysis of Waste Tires in a Circulating Fluidized-Bed Reactor. Energy 2001, 26, 385–399. [Google Scholar] [CrossRef]
- Uçar, S.; Karagöz, S.; Yanik, J.; Saglam, M.; Yuksel, M. Copyrolysis of Scrap Tires with Waste Lubricant Oil. Fuel Process. Technol. 2005, 87, 53–58. [Google Scholar] [CrossRef]
- González, J.F.; Encinar, J.M.; Canito, J.L.; Rodríguez, J.J. Pyrolysis of Automobile Tyre Waste. Influence of Operating Variables and Kinetics Study. J. Anal. Appl. Pyrolysis 2001, 58–59, 667–683. [Google Scholar] [CrossRef]
- De, I.; Rodriguez, M.; Laresgoiti, M.F.; Cabrero, M.A.; Torres, A.; Chomon, M.J.; Caballeró, B. Pyrolysis of Scrap Tyres. Fuel Process. Technol. 2001, 72, 9–22. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of Waste Tyres: A Review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Okoye, C.O.; Jones, I.; Zhu, M.; Zhang, Z.; Zhang, D. Manufacturing of Carbon Black from Spent Tyre Pyrolysis Oil–A Literature Review. J. Clean. Prod. 2021, 279, 123336. [Google Scholar] [CrossRef]
- Groves, S.A.; Lehrle, R.S.; Blazso, M.; Szckely, T. Natural Rubber Pyrolysis: Study of Temperature and Thickness-Dependence Indicates Dimer Formation Mechanism. J. Anal. Appl. Pyrolysis 1991, 19, 301–309. [Google Scholar] [CrossRef]
- Chen, F.; Qian, J. Studies on the Thermal Degradation of Polybutadiene. Fuel Process. Technol. 2000, 67, 53–60. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Meng, H.; Tong, H.; Cai, X.; Liu, J. Studies on Pyrolysis Mechanisms of Syndiotactic Polystyrene Using DFT Method. Chem. Phys. Lett. 2020, 747, 137334. [Google Scholar] [CrossRef]
- Gardner, P.; Lehrle, R. Polystyrene Pyrolysis Mechanisms-as Deduced from the Dependence of Product Yields in Film Thickness. Eur. Polym. J. 1993, 29, 425–435. [Google Scholar] [CrossRef]
- Liqiu, F.; Jin, J.; Zang, Z.; Zhang, Q.; Sun, X.; Deng, L.; Zhang, J. Mechanism of Remarkable Interference of Styrene-Butadiene to Gasoline Identification for Fire Debris Analysis Based on TG-IR-GC/MS. J. Anal. Appl. Pyrolysis 2025, 186, 106958. [Google Scholar] [CrossRef]
- Chen, F.; Qian, J. Studies on the Thermal Degradation of Cis-1,4-Polyisoprene. Fuel 2002, 81, 2071–2077. [Google Scholar] [CrossRef]
- Williams, P.; Taylor, D. Aromatization of Tyre Pyrolysis Oil to Yield Polycyclic Aromatic Hydrocarbons. Fuel 1993, 72, 1469–1474. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. Pyrolysis-Thermogravimetric Analysis of Tyres and Tyre Components. Fuel 1995, 14, 1277–1283. [Google Scholar] [CrossRef]
- Sainz-Diaz, C.I.; Kelly, D.R.; Avenell, C.S.; Griffiths, A.G. Pyrolysis of Furniture and Tire Wastes in a Flaming Pyrolyzer Minimizes Discharges to the Environment. Energy Fuels 1997, 11, 1061–1072. [Google Scholar] [CrossRef]
- Williams, P.T.; Brindle, A.J. Aromatic Chemicals from the Catalytic Pyrolysis of Scrap Tyres. J. Anal. Appl. Pyrolysis 2003, 67, 143–164. [Google Scholar] [CrossRef]
- Fairburn, J.A.; Behie, L.A.; Svrcek, W.Y. Ultrapyrolysis of N-Hexadecane in a Novel Micro-Reactor. Fuel 1990, 69, 1537–1545. [Google Scholar] [CrossRef]
- Brazier, D.W.; Schwartz, N. The Effect of Heating Rate on the Thermal Degradation of Polybutadiene. J. Appl. Polym. Sci. 1978, 22, 113–124. [Google Scholar] [CrossRef]
- Frigo, S.; Seggiani, M.; Puccini, M.; Vitolo, S. Liquid Fuel Production from Waste Tyre Pyrolysis and Its Utilisation in a Diesel Engine. Fuel 2014, 116, 399–408. [Google Scholar] [CrossRef]
- Arya, S.; Sharma, A.; Rawat, M.; Agrawal, A. Tyre Pyrolysis Oil as an Alternative Fuel: A Review. Mater. Today Proc. 2020, 28, 2481–2484. [Google Scholar] [CrossRef]
- Goyal, A.; Kumar, N. Some Experimental Studies on the Use of Tyre Pyrolysis Oil (TPO) in an Agricultural Diesel Engine. SAE Tech. Pap. 2019, 1, 796. [Google Scholar] [CrossRef]
- Graham, J.L.; Striebich, R.C.; Myers, K.J.; Minus, D.K.; Harrison, W.E. Swelling of Nitrile Rubber by Selected Aromatics Blended in a Synthetic Jet Fuel. Energy Fuels 2006, 20, 759–765. [Google Scholar] [CrossRef]
- Blivernitz, A. Untersuchung Der Verträglichkeit von Elastomeren. Ph.D. Thesis, Universität der Bundeswehr, Neubibierg, Germany, 2019. [Google Scholar]
- Musil, B.; Demmel, B.; Lion, A.; Johlitz, M. A Contribution to the Chemomechanics of Elastomers Surrounded by Liquid Media: Continuum Mechanical Approach for Parameter Identification Using the Example of Sorption Experiments. J. Rubber Res. 2021, 24, 271–279. [Google Scholar] [CrossRef]
- Gormley, R.J.; Link, D.D.; Baltrus, J.P.; Zandhuis, P.H. Interactions of Jet Fuels with Nitrile O-Rings: Petroleum-Derived versus Synthetic Fuels. Energy Fuels 2009, 23, 857–861. [Google Scholar] [CrossRef]
- Lou, W.; Zhang, W.; Wang, H.; Jin, T.; Liu, X. Influence of Hydraulic Oil on Degradation Behavior of Nitrile Rubber O-Rings at Elevated Temperature. Eng. Fail. Anal. 2018, 92, 1–11. [Google Scholar] [CrossRef]
- Röthemeyer, F. Kautschuk Technologie, 3rd ed.; Hanser: München, Germany, 2013; Volume 3. [Google Scholar]
- Starmer, P. Swelling of Nitrile Rubber Vulcanizates-Part 3: Factors Affecting Maximum Swelling. Elastomers Plast. 1993, 25, 188–215. [Google Scholar] [CrossRef]
- Lachat, V. Understanding Oil Resistance of Nitrile Rubber Group Interactions at Interfaces. Ph.D. Thesis, University of Akron, Akron, OH, USA, 2008. [Google Scholar]
- DIN EN 590; Kraftstoffe–Dieselkraftstoff–Anforderungen Und Prüfverfahren. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2022.
- DIN 53504:2017-03; Testing of Rubber-Determination of Tensile Strength at Break, Tensile Stress at Yield, Elongation at Break and Stress Values in a Tensile Test. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2017.
- D7042-21a; ASTM International Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). ASTM International: West Conshohocken, PA, USA, 2022.
- DIN EN 12916; Mineralölerzeugnisse–Bestimmung von Aromatischen Kohlenwasserstoffgruppen in Mitteldestillaten–Hochleistungsflüssigkeitschromatographie-Verfahren Mit-Detektion. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2022.
- DIN EN ISO 2719; Bestimmung Des Flammpunktes–Verfahren Nach Pensky-Martens Mit Geschlossenem Tiegel. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2021.
- DIN EN ISO 12156-1; Dieselkraftstoff –Bestimmung Der Schmierfähigkeit Unter Verwendung Eines-Prüfgerätes (HFRR). DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2019.
- DIN EN 116; Dieselkraftstoffe Und Haushaltsheizöle–Bestimmung Des Temperaturgrenzwertes Der Filtrierbarkeit–Verfahren Mit Einem Stufenweise Arbeitenden Kühlbad. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2018.
- DIN EN ISO 2160; Mineralölerzeugnisse Auf Kupfer-Kupferstreifenprüfung. DIN Deutsches Institut für Normung e.V: Berlin, Germany, 1999.
- DIN EN 16091; Bestimmung Der Oxidationsstabilität Mit Beschleunigtem Und Kleiner Probenmenge. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2022.
- DIN 51777; Mineralölerzeugnisse–Bestimmung Des Wassergehaltes Durch Titration Nach Karl Fischer. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2020.
- DIN EN ISO 10370; Mineralölerzeugnisse–Bestimmung Des Koksrückstandes–Mikroverfahren. DIN Deutsches Institut für Normung e.V: Berlin, Germany, 2015.
- DIN EN ISO 6245; Mineralölerzeugnisse Bestimmung Der Asche. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2003.
- DIN EN ISO 20884; Mineralölerzeugnisse–Bestimmung Des Schwefelgehaltes in Kraftstoffen–Wellenlängendispersive Röntgenfluoreszenz-Spektrometrie. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2022.
- DIN EN ISO 3405; Mineralölerzeugnisse Und Verwandte Produkte Mit Natürlichem Oder Ursprung–Bestimmung Des Destillationsverlaufes Bei Atmosphärendruck. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2019.
- D664−24; ASTM International Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2024.
- DIN EN ISO 3961; Tierische Und Pflanzliche Fette Und Öle–Bestimmung Der Iodzahl. DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2025.
- John, C. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975; ISBN 0198533446. [Google Scholar]
- DIN ISO 48-4; Elastomere Oder Thermoplastische Elastomere-Bestimmung Der Härte-Teil 4: Eindringhärte Durch Durometer-Verfahren (Shore-Härte). DIN Deutsches Institut für Normung e. V: Berlin, Germany, 2021.
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire Wear Particles in the Aquatic Environment-A Review on Generation, Analysis, Occurrence, Fate and Effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef]
- Di Salvi, W.; de Santo, C.E.; Matsumoto, A.; Calhabeu, A.M.; Lopes, É.S.N.; Gabriel, L.P. Characterization of Thermal-Oxidative Aging Mechanism of Commercial Tires. Eng. Fail. Anal. 2023, 154, 107631. [Google Scholar] [CrossRef]
- Uebe, J.; Kryzevicius, Z.; Majauskiene, R.; Dulevicius, M.; Kosychova, L.; Zukauskaite, A. Use of Polypropylene Pyrolysis Oil in Alternative Fuel Production. Waste Manag. Res. 2022, 40, 1220–1230. [Google Scholar] [CrossRef]
- Islam, M.R.; Tushar, M.S.H.K.; Haniu, H. Production of Liquid Fuels and Chemicals from Pyrolysis of Bangladeshi Bicycle/Rickshaw Tire Wastes. J. Anal. Appl. Pyrolysis 2008, 82, 96–109. [Google Scholar] [CrossRef]
- Gupta, P.L.; Dogra, P.V.; Kuchhal, R.K.; Kumar, P. Estimation of Average Structural Parameters of Petroleum Crudes and Coal-Derived Liquids by 13C and 1H n.m.r. Fuel 1985, 65, 515–519. [Google Scholar] [CrossRef]
- Banar, M.; Akyildiz, V.; Özkan, A.; Çokaygil, Z.; Onay, Ö. Characterization of Pyrolytic Oil Obtained from Pyrolysis of TDF (Tire Derived Fuel). Energy Convers. Manag. 2012, 62, 22–30. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Gani, A.; Hourani, N.; Emwas, A.H.; Sarathy, S.M.; Roberts, W.L. TG/DTG, FT-ICR Mass Spectrometry, and NMR Spectroscopy Study of Heavy Fuel Oil. Energy Fuels 2015, 29, 7825–7835. [Google Scholar] [CrossRef]
- Rodriguez, J.; Tierney, J.W.; Wender, I. Evaluation of a Delayed Coking Process by 1H and 13C n.m.r. Spectroscopy: 1. Material Balances. Fuel 1994, 73, 1863–1869. [Google Scholar] [CrossRef]
- Rodriguez, J.; Tierney, J.W.; Wender, I. Evaluation of a Delayed Coking Process by 1H and 13C n.m.r. Spectroscopy: 2. Detailed Interpretation of Liquid n.m.r. Spectra. Fuel 1994, 73, 1870–1875. [Google Scholar] [CrossRef]
- Hummel, O.; Scholl, F. Atlas of Polymer and Plastics Analysis; Carl Hanser: Munich, Germany, 1988; Volume 2. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 2nd ed.; John Wiley & Sons: Chichester, UK, 1994. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fatley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Caracteristic Frequencies of Organic Molecules; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Scheuermann, S.S.; Forster, S.; Eibl, S. In-Depth Interpretation of Mid-Infrared Spectra of Various Synthetic Fuels for the Chemometric Prediction of Aviation Fuel Blend Properties. Energy Fuels 2017, 31, 2934–2943. [Google Scholar] [CrossRef]
- Smith, B. The C=O Bond, Part III Carboxylic Acids. Spectroscopy 2018, 33, 14–20. [Google Scholar]
- Cunliffe, A.M.; Williams, P.T. Composition of Oils Derived from the Batch Pyrolysis of Tyres. J. Anal. Appl. Pyrolysis 1998, 44, 131–152. [Google Scholar] [CrossRef]
- Mastral, A.M.; Murillo, R.; Callén, M.S.; García, T.; Snape, C.E. Influence of Process Variables on Oils from Tire Pyrolysis and Hydropyrolysis in a Swept Fixed Bed Reactor. Energy Fuels 2000, 14, 739–744. [Google Scholar] [CrossRef]
- Day, M.; Cooney, J.D.; Shen, Z. Pyrolysis of Automobile Shredder Residue: An Analysis of the Products of a Commercial Screw Kiln Process. J. Anal. Appl. Pyrolysis 1996, 37, 49–61. [Google Scholar] [CrossRef][Green Version]
- Totton, T.S.; Misquitta, A.J.; Kraft, M. A Quantitative Study of the Clustering of Polycyclic Aromatic Hydrocarbons at High Temperatures. Phys. Chem. Chem. Phys. 2012, 14, 4081–4094. [Google Scholar] [CrossRef]
- Elvati, P.; Violi, A. Thermodynamics of Poly-Aromatic Hydrocarbon Clustering and the Effects of Substituted Aliphatic Chains. Proc. Combust. Inst. 2013, 34, 1837–1843. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An Overview of Biodiesel Oxidation Stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Lopez, G.; Alvarez, J.; Amutio, M.; Mkhize, N.M.; Danon, B.; van der Gryp, P.; Görgens, J.F.; Bilbao, J.; Olazar, M. Waste Truck-Tyre Processing by Flash Pyrolysis in a Conical Spouted Bed Reactor. Energy Convers. Manag. 2017, 142, 523–532. [Google Scholar] [CrossRef]
- Stockwell, W.R.; Calvert, J.G. The Mechanism of the HO-SO2 Reaction. Atmos. Environ. 1983, 17, 2231–2235. [Google Scholar] [CrossRef]
- Serefentse, R.; Ruwona, W.; Danha, G.; Muzenda, E. A Review of the Desulphurization Methods Used for Pyrolysis Oil. Procedia Manuf. 2019, 35, 762–768. [Google Scholar] [CrossRef]
- Williams, P.T.; Bottrill, R.P. Sulfur-Polycyclic Aromatic Hydrocarbons in Tyre Pyrolysis Oil. Fuel 1995, 74, 736–742. [Google Scholar] [CrossRef]
- Blivernitz, A.; Förster, T.; Eibl, S. Simultaneous and Time Resolved Investigation of Diffusion Processes of Individual Model Fuel Components in Acrylonitrile-Butadiene-Rubber in the Light of Swelling Phenomena. Polym. Test. 2018, 70, 47–56. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Masjuki, H.H.; Siang, C.T.; Fazal, M.A. Compatibility of Elastomers in Palm Biodiesel. Renew. Energy 2010, 35, 2356–2361. [Google Scholar] [CrossRef]
- Kass, M.; Theiss, T.; Janke, C.; Pawel, S.; Technology, J.C.-S. Compatibility of Elastomers with Test Fuels of Gasoline Blended with Ethanol. Seal. Technol. 2012, 2012, 7–12. [Google Scholar] [CrossRef]
- Zhu, L.; Cheung, C.S.; Zhang, W.G.; Huang, Z. Compatibility of Different Biodiesel Composition with Acrylonitrile Butadiene Rubber (NBR). Fuel 2015, 158, 288–292. [Google Scholar] [CrossRef]
- Lachat, V.; Varshney, V.; Dhinojwala, A.; Yeganeh, M.S. Molecular Origin of Solvent Resistance of Polyacrylonitrile. Macromolecules 2009, 42, 7103–7107. [Google Scholar] [CrossRef]
- George, S.; Varughese, K.T.; Thomas, S. Molecular Transport of Aromatic Solvents in Isotactic Polypropylene/ Acrylonitrile-Co-Butadiene Rubber Blends. Polymer 2000, 41, 579–594. [Google Scholar] [CrossRef]
- Chai, A.B.; Andriyana, A.; Verron, E.; Johan, M.R. Mechanical Characteristics of Swollen Elastomers under Cyclic Loading. Mater. Des. 2013, 44, 566–572. [Google Scholar] [CrossRef]




| Ingredient | Concentration/phr | ||
|---|---|---|---|
| Acrylonitrile content/wt% | 18 | 28 | 39 |
| Perbunan 1846 | 100 | ||
| Perbunan 2846 | 100 | ||
| Perbunan 3946 | 100 | ||
| Carbon black (Typ N550) | 60 | ||
| Di(2-propylheptyl) phthalate (DPHP) | 20 | ||
| N-(4-Methylpentan-2-yl)-N-phenylbenzene-1,4-diamine (6-PPD) | 2 | ||
| Zinc oxide | 5 | ||
| Stearic acid | 1 | ||
| Sulphur | 2 | ||
| N-Cyclohexyl-2-benzothiazolylsulfenamide (CBS) | 1.5 | ||
| Tetramethyl thiuram monosulfide (TMTM-80) | 0.5 | ||
| Range | Substance Class | Intensity Percentage Area% | |
|---|---|---|---|
| DF | TPO | ||
| 1 | Alkanes/Alkenes | 74 | 8 |
| 2 | Cycloalkanes | 9 | 7 |
| 3 | Aromatics | 11 | 40 |
| 4 | Indenes | 4 | 12 |
| 5 | Diaromatics | 2 | 22 |
| 6 | Polyaromatics | - | 5 |
| Others * | ≤1 | 6 | |
| Characteristic | Units | DF | DF99TPO1 | DF95TPO5 | DF90TPO10 | TPO |
|---|---|---|---|---|---|---|
| Total acid number | mg g−1 | 0 | 0.05 | 0.14 | 0.23 | 2.08 |
| Iodine value | g 100 g−1 | 2.9 | 3.1 | 3.7 | 14 | 93 |
| Aromatic content | wt% | 24.1 | 24.4 | 26.0 | 27.7 | ~60 * |
| Characteristic | Unit | Target | DF | DF99TPO1 | DF95TPO5 | DF90TPO10 | TPO |
|---|---|---|---|---|---|---|---|
| Density | kg m−3 | 822.0–845.0 | 833.3 | 834.2 | 838.1 | 843.1 | 917.1 |
| Viscosity | mm2 s−1 | 2.000–4.500 | 2.840 | 2.836 | 2.830 | 2.826 | 1.985 |
| Polyaromatic content | wt% | ≤8.0 | 2.9 | 3.0 | 3.8 | 4.7 | 21.8 * |
| Flash point | °C | >55 | 65.5 | 64.0 | 58.0 | 53.1 | - |
| HFRR 1-Test | µm | ≤460 | 420.5 | 359.5 | 222.5 | 209.5 | 175.5 |
| CFPP 2 | °C | ≤−20 | −25 | −25 | −13 | −14 | - |
| Cu corrosion | extent | 1 | 1a | 1a | 1a | 1a | 1a |
| Oxidation stability | min | ≥60 | 85 | 196 | 183 | 93 | 22 |
| Water content | mg kg−1 | ≤200 | 37 | 30 | 60 | 40 | 2054 |
| Coke residue | wt% | ≤0.30 | 0.02 | 0.14 | 0.75 | 1.39 | 2.53 |
| Ash | wt% | ≤0.010 | <0.001 | 0.001 | 0.001 | 0.001 | - |
| Sulphur content | mg kg−1 | ≤10.0 | 7.3 | 134 | 648 | 1292 | 11,600 (1.16%) |
| Distillation curve | |||||||
| Start | vol% | 171.3 | 177.9 | 165.3 | 157.0 | 36.0 | |
| 10% v/v | 210.7 | 208.0 | 203.8 | 200.4 | 124.0 | ||
| 50% v/v | 273.2 | 275.1 | 274.3 | 274.8 | 226.0 | ||
| 90% v/v | ≤360 | 334.5 | 336.2 | 338.1 | 342.2 | 387.0 | |
| 95% v/v | ≤360 | 349.4 | 349.9 | 353.1 | 358.8 | - | |
| End | 358.6 | 356.9 | 357.9 | 363.4 | 390.1 |
| Fuel | NBR18 | NBR28 | NBR39 | |||
|---|---|---|---|---|---|---|
| m∞ | D × 10−6 | m∞ | D × 10−6 | m∞ | D × 10−6 | |
| wt% | mm2 s−1 | wt% | mm2 s−1 | wt% | mm2 s−1 | |
| DF | 26 | 1.6 | 14 | 0.21 | - | - |
| DF99TPO1 | 28 | 1.8 | 16 | 0.23 | - | - |
| DF95TPO5 | 34 | 2.8 | 20 | 0.23 | - | - |
| DF90TPO10 | 38 | 4.3 | 25 | 0.24 | - | - |
| TPO | 153 | 11.8 | 106 | 1.2 | 74 | 0.21 |
| Sorption Temperature °C | DF | TPO | ||
|---|---|---|---|---|
| m∞ | D × 10−6 | m∞ | D × 10−6 | |
| wt% | mm2 s−1 | wt% | mm2 s−1 | |
| 20 | 27 | 0.73 | 153 | 3.43 |
| 50 | 32 | 3.36 | 154 | 8.83 |
| 80 | 38 | 15.3 | 156 | 21.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitz, S.; Förster, T.; Eibl, S. Analysis of Tyre Pyrolysis Oil as Potential Diesel Fuel Blend with Focus on Swelling Behaviour of Nitrile-Butadiene Rubber. Polymers 2025, 17, 3016. https://doi.org/10.3390/polym17223016
Seitz S, Förster T, Eibl S. Analysis of Tyre Pyrolysis Oil as Potential Diesel Fuel Blend with Focus on Swelling Behaviour of Nitrile-Butadiene Rubber. Polymers. 2025; 17(22):3016. https://doi.org/10.3390/polym17223016
Chicago/Turabian StyleSeitz, Steffen, Tobias Förster, and Sebastian Eibl. 2025. "Analysis of Tyre Pyrolysis Oil as Potential Diesel Fuel Blend with Focus on Swelling Behaviour of Nitrile-Butadiene Rubber" Polymers 17, no. 22: 3016. https://doi.org/10.3390/polym17223016
APA StyleSeitz, S., Förster, T., & Eibl, S. (2025). Analysis of Tyre Pyrolysis Oil as Potential Diesel Fuel Blend with Focus on Swelling Behaviour of Nitrile-Butadiene Rubber. Polymers, 17(22), 3016. https://doi.org/10.3390/polym17223016







