Electrospun PVA-CTS-HA Wound Dressings with Ag-ZnO Nanoparticles for Diabetic Foot Ulcers Treatment: Physicochemical Properties, Hemocompatibility, and Cell Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
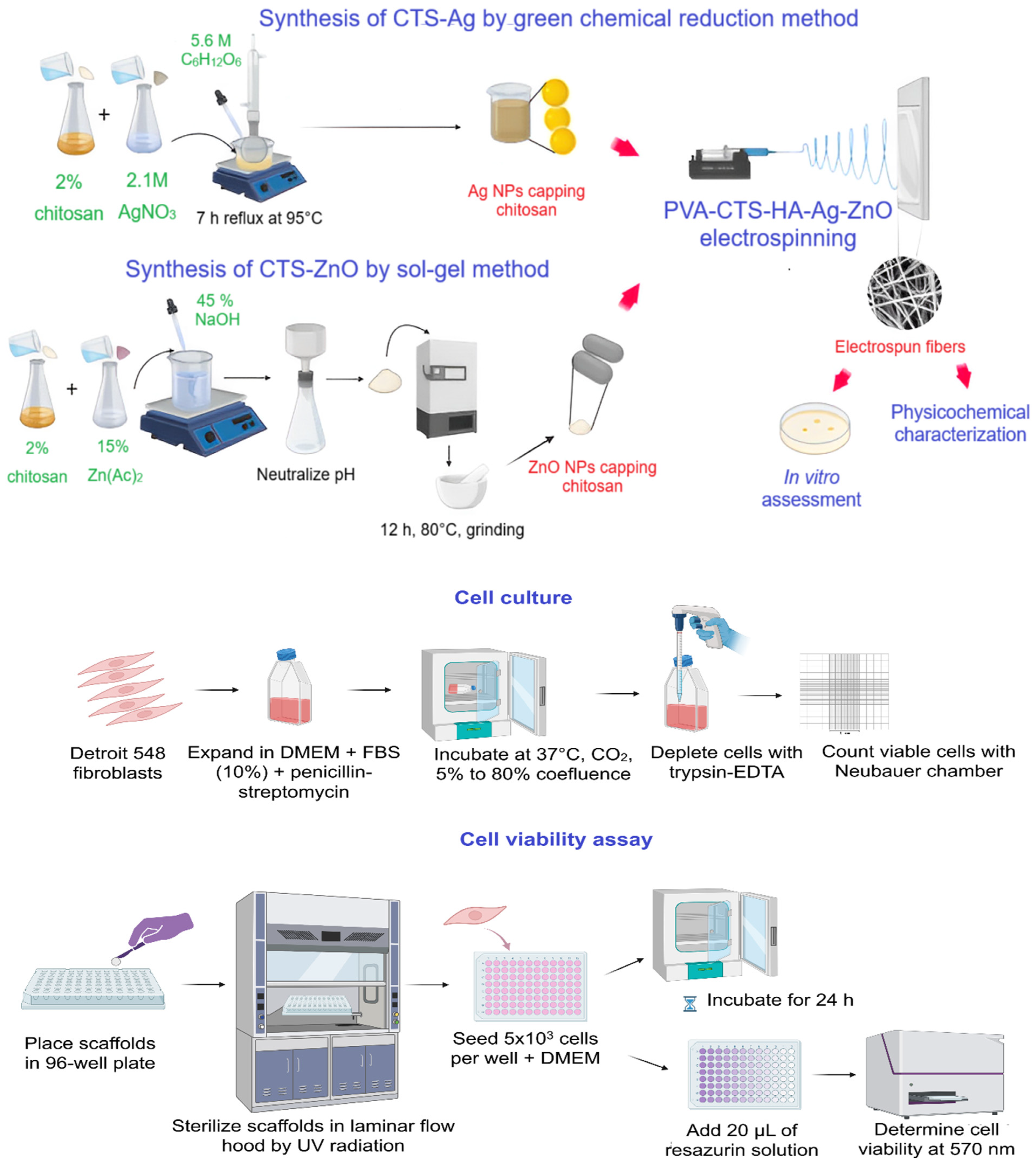
2.2. Methodology
2.2.1. Physicochemical Characterization of the Electrospun Scaffolds
2.2.2. Biological Assessment
3. Results and Discussion
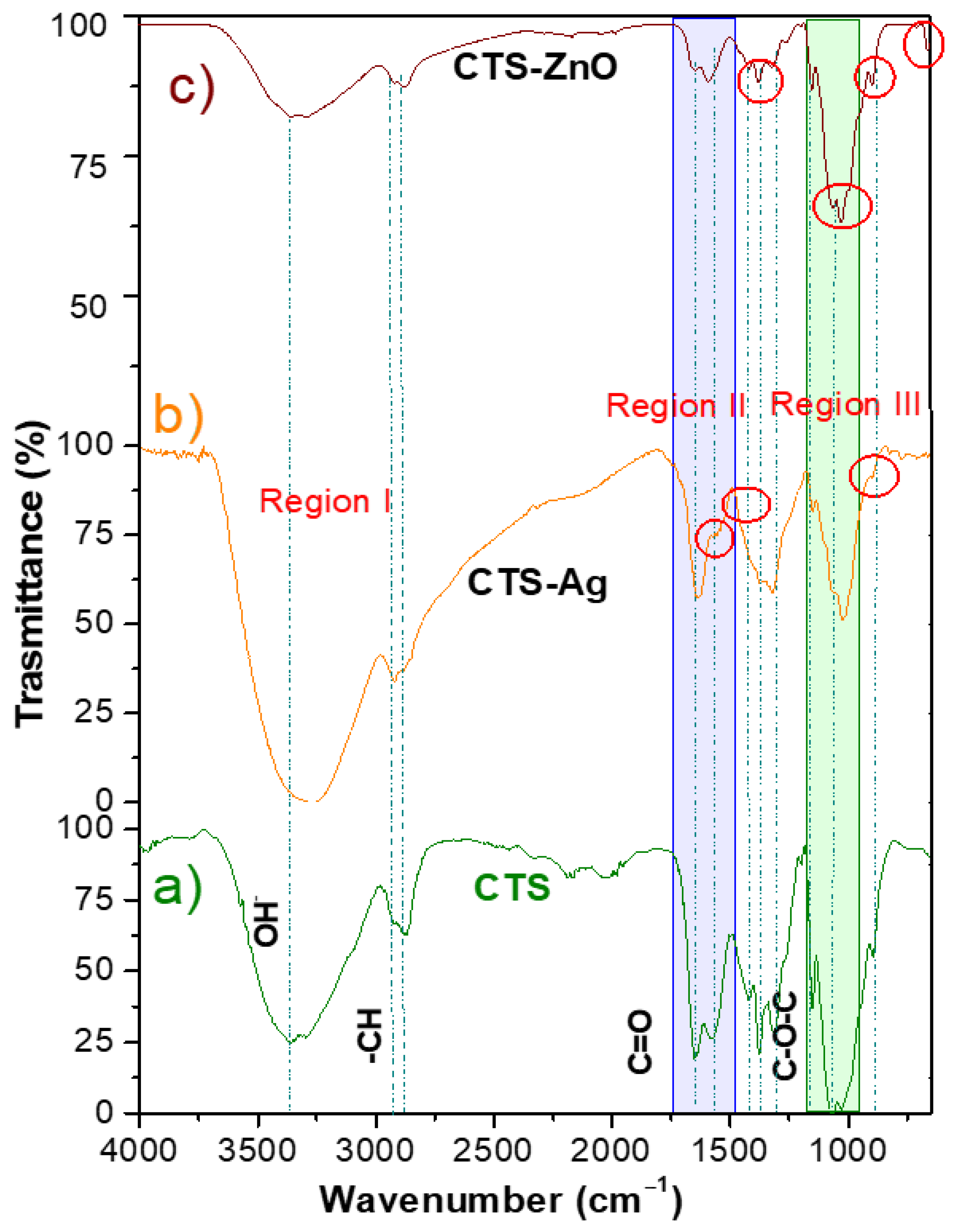
3.1. Chemical Characterization of Binary CTS-Ag and CTS-ZnO Compounds
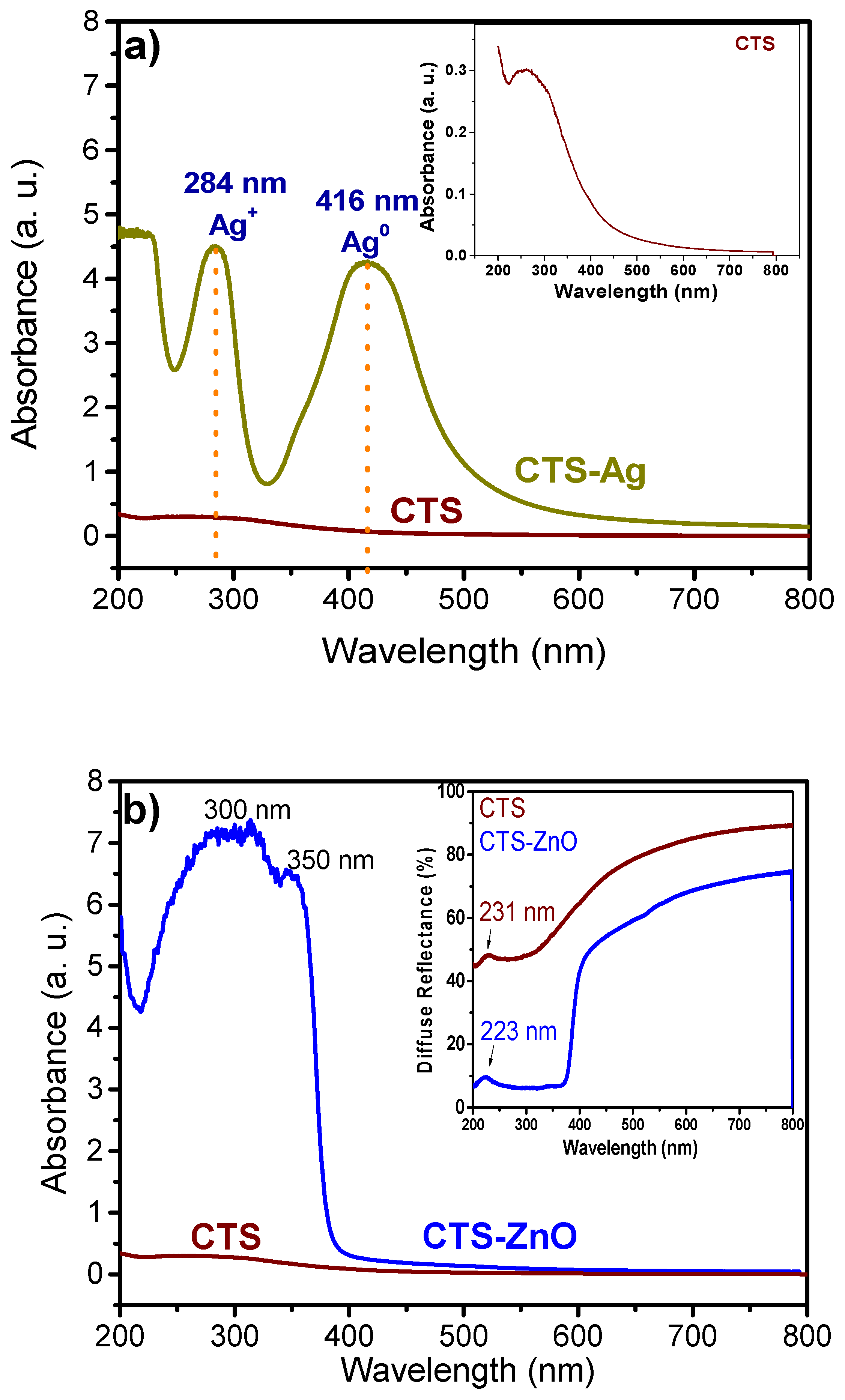
3.2. UV-Vis Spectroscopy of CTS-Ag and CTS ZnO Compounds
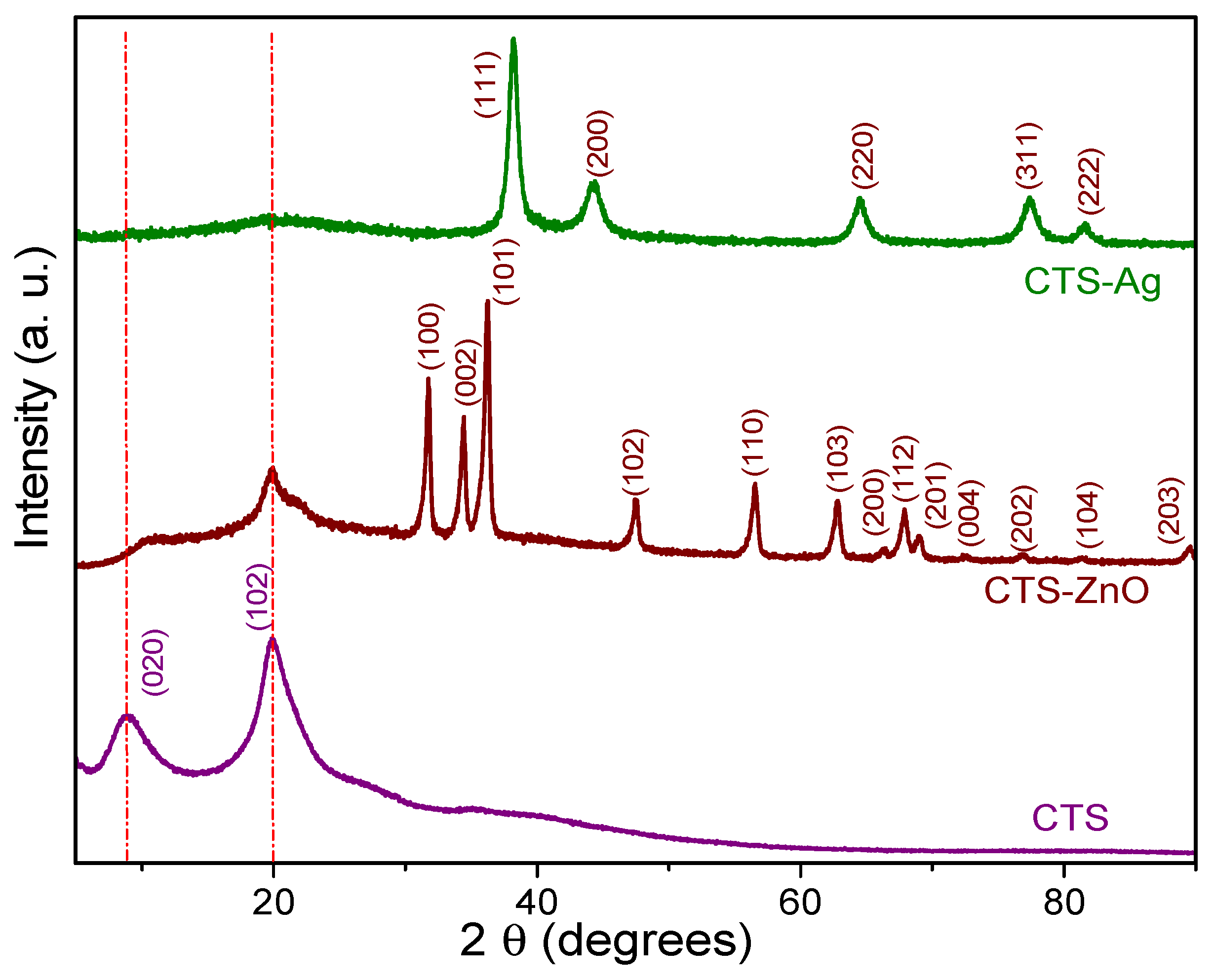
3.3. XRD of CTS-Ag and CTS-ZnO Compounds
3.4. Morphological and Microstructural Observations
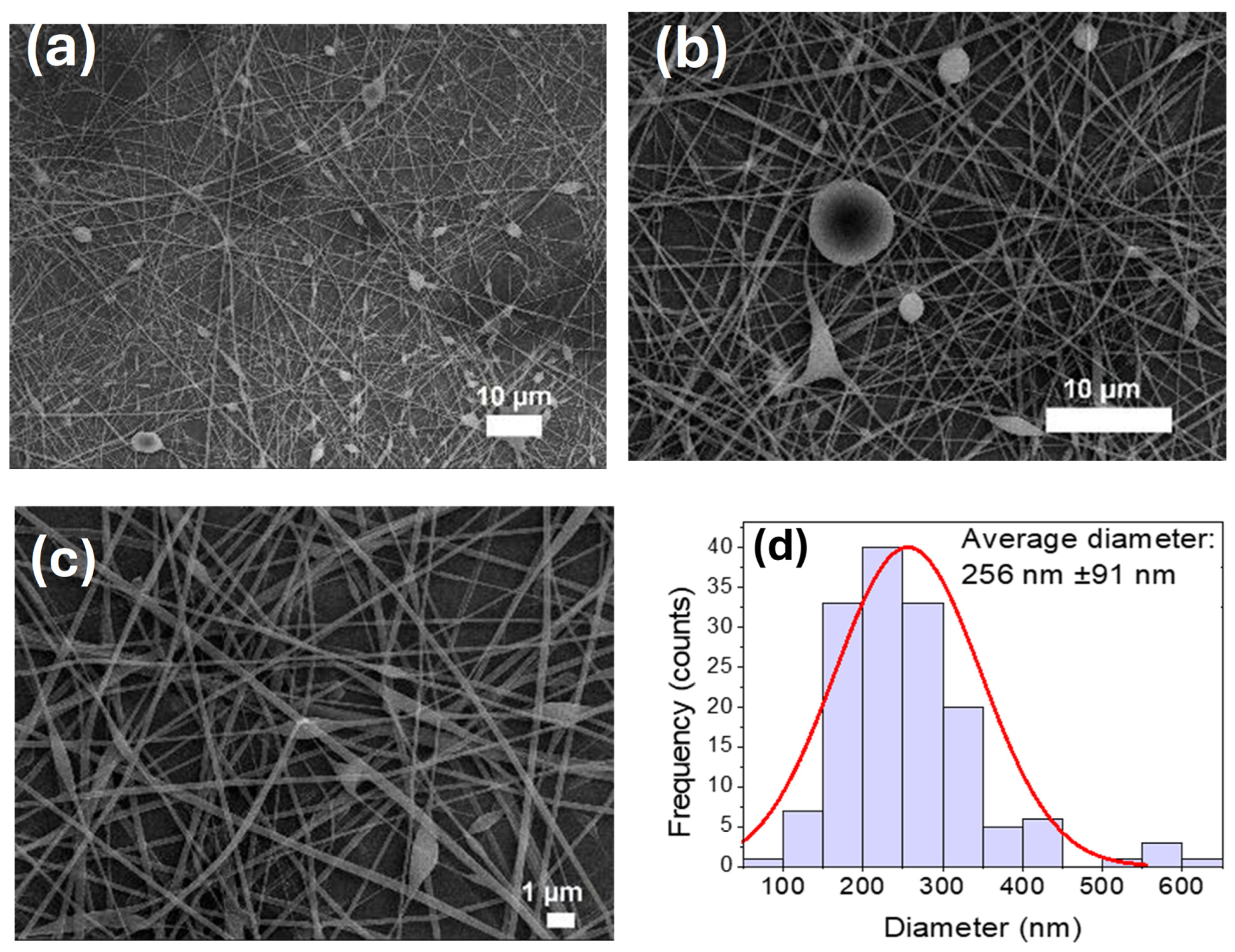
3.5. Fabrication of PVA-CTS Electrospun Fibers
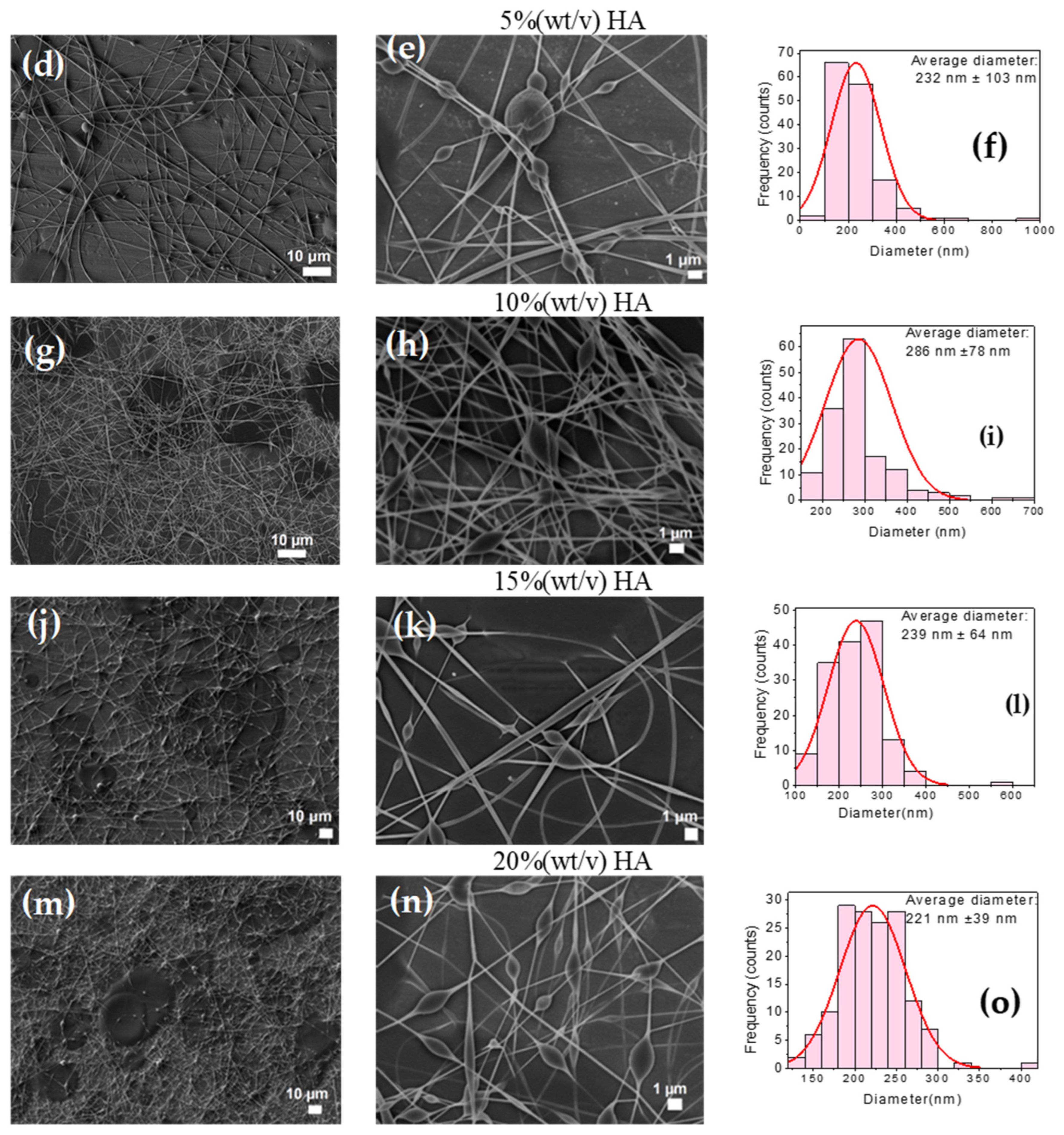
3.6. Fabrication of PVA-CTS-HA Electrospun Fibers
3.7. Thickness and Chemical Characterization of Electrospun Fibers
3.8. In Vitro Analysis of Electrospun Fiber Scaffolds: Determination of Hemolysis Percentage and Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhat, S.Z.; Al-Hajri, N.; Kanbour, S.; Ahmadzada, M.; Borovoy, A.; Abusamaan, M.S.; Canner, J.K.; Nass, C.; Sherman, R.L.; Hines, K.F.; et al. Glycemic management in diabetic foot ulcers: A comparative analysis of wound and wound-free periods in adults with type 1 and type 2 diabetes. Can. J. Diabetes 2024, 48, 517–523.e2. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, J.; Bakker, K.; van Houtum, W.H.; Schaper, N.C. Practical guidelines on the management and prevention of the diabetic foot: Based upon the international consensus on the diabetic foot (2007) prepared by the international working group on the diabetic foot. Diabetes Metab. Res. Rev. 2008, 24 (Suppl. 1), S181–S187. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jiang, Y.-b.; Liu, Z.-b.; Li, M.-h.; Niu, W.-j.; Lei, Z.-c.; Wang, B.-w.; Lu, D.-y.; Zhu, Y.-w. Benefits of liquid dressings in post-operative wound dressing of diabetic foot ulcer. Curr. Probl. Surg. 2025, 65, 101730. [Google Scholar] [CrossRef]
- Brouwer, R.J.; van Reijen, N.S.; Dijkgraaf, M.G.; Hoencamp, R.; Koelemay, M.J.; van Hulst, R.A.; Ubbink, D.T. Economic analysis of hyperbaric oxygen therapy for the treatment of ischaemic diabetic foot ulcers. Diving Hyperb. Med. 2024, 54, 265–274. [Google Scholar] [CrossRef]
- Castellani, L.; Arruda, S. Hooked on healing—Fish skin grafts for diabetic foot ulcers. NEJM Evid. 2024, 3, EVIDe2400373. [Google Scholar] [CrossRef]
- Hinchliffe, R.J.; Andros, G.; Apelqvist, J.; Bakker, K.; Friederichs, S.; Lammer, J.; Lepantalo, M.; Mills, J.L.; Reekers, J.; Shearman, C.P.; et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab. Res. Rev. 2012, 28 (Suppl. 1), 179–217. [Google Scholar] [CrossRef]
- Clark, R.A.F. Cutaneous tissue repair: Basic biologic considerations. I. J. Am. Acad. Dermatol. 1985, 13, 701–725. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Biondo, M.; Tomasello, L.; Giordano, C.; Arnaldi, G.; Pizzolanti, G. The promising approach of 3D bioprinting for diabetic foot ulcer treatment: A concise review of recent developments. Heliyon 2024, 10, e36707. [Google Scholar] [CrossRef]
- Paiva, M.D.O.; Rojas, S.D.N. Pie diabético: ¿podemos prevenirlo? Rev. Méd. Clín. Condes 2016, 27, 227–234. [Google Scholar] [CrossRef][Green Version]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wu, J.; Bian, Y.; Cai, L.; Hu, G.; Zhou, X. A comprehensive review of the pathophysiology and management of amniotic fluid embolism. Clin. Exp. Obstet. Gynecol. 2025, 52, 39277. [Google Scholar] [CrossRef]
- Raja, J.; Maturana, M.; Kayali, S.; Khouzam, A.; Efeovbokhan, N. Diabetic foot ulcer: A comprehensive review of pathophysiology and management modalities. World J. Clin. Cases 2023, 11, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.; Creager, M.; Libby, P. Diabetes and atherosclerosis. JAMA J. Am. Med. Assoc. 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Building vs. Rebuilding epidermis: Comparison embryonic development and adult wound repair. Front. Cell Dev. Biol. 2022, 9, 796080. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.; Herzig, S.; Nawroth, P. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Dawi, J.; Tumanyan, K.; Tomas, K.; Misakyan, Y.; Gargaloyan, A.; Gonzalez, E.; Hammi, M.; Tomas, S.; Venketaraman, V. Diabetic foot ulcers: Pathophysiology, immune dysregulation, and emerging therapeutic strategies. Biomedicines 2025, 13, 1076. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Sheikholeslami, S.A.; Esmaeili, J.; Jalise, S.Z.; Barati, A. A response surface methodology study on the development of ph-sensitive wound dressings using rhodamine b-loaded chitosan nanoparticles and sodium alginate-based films. Heliyon 2024, 10, e40670. [Google Scholar] [CrossRef] [PubMed]
- Koupai, A.A.; Varshosaz, J.; Tavakoli, M.; Mirhaj, M.; Salehi, S.; Dobakhti, F. Multifunctional tri-layer wound dressing containing zno nanoparticles and igf-1 as an efficient biomaterial for healing of full thickness skin injuries. Asian J. Pharm. Sci. 2025, 20, 101039. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lu, D.; Sun, S.; Cai, M.; Lin, L.; Zhu, M. Material design, fabrication strategies, and the development of multifunctional hydrogel composites dressings for skin wound management. Biomacromolecules 2025, 26, 1419–1460. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, Y.; Zhang, Y.; Zhuang, P.; Wang, J. Breathable functional aerogel dressings facilitate the healing of diabetic wounds. Biomed. Technol. 2025, 9, 100071. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Dang, H.; Niu, B.; Yan, H.; Guo, R.; Wang, H.; Zhou, P. An adhesive, antibacterial hydrogel wound dressing fabricated by dopamine-grafted oxidized sodium alginate and methacrylated carboxymethyl chitosan incorporated with Cu(II) complex. Biomater. Adv. 2025, 170, 214217. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.u.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Shakil, U.A.; Abu Hassan, S.B.; Yahya, M.Y.; Rejab, M.R.M. A focused review of short electrospun nanofiber preparation techniques for composite reinforcement. Nanotechnol. Rev. 2022, 11, 1991–2014. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Li, W.; He, J.; Chen, Q.; Bao, F.; Huo, Y.; Deng, J.; Lin, Q.; Luo, F. Enhancement of oryzanol application by constructing modified β-cd inclusion complex and polycaprolactone-chitosan electrospun fiber membranes: Perspectives on wound dressings and grape preservation. Food Chem. 2025, 473, 143025. [Google Scholar] [CrossRef]
- Madani, M.; Cruz, C.D.; Gounani, Z.; Baniasadi, H.; Tammela, P.; Laaksonen, T.; Niskanen, J.; Seppälä, J. Functionalized cellulose nanocrystals reinforced pla-gelatin electrospun fibers for potential antibacterial wound dressing and coating applications. Int. J. Biol. Macromol. 2025, 287, 138389. [Google Scholar] [CrossRef]
- Breitenbach, G.L.; Caldas, B.S.; Pellá, M.C.G.; Muniz, E.C.; Dragunski, D.C. Graphene oxide (go)-reinforced curcumin (cur)-loaded zein-based electrospun nanofibers for potential wound dressing purposes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134872. [Google Scholar] [CrossRef]
- Pirzadeh, K.; Torkian, L.; Asli, M.D. Coaxial electrospun wound dressing integrated with ag-doped hydroxyapatite for wound healing: Tetracycline delivery. Mater. Today Commun. 2024, 41, 110439. [Google Scholar] [CrossRef]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Antibacterial and bioactive multilayer electrospun wound dressings based on hyaluronic acid and lactose-modified chitosan. Biomater. Adv. 2023, 154, 213613. [Google Scholar] [CrossRef]
- Marjani, M.E.; Hmtshirazi, R.; Mohammadi, T. CDI crosslinked chitosan/poly (vinyl alcohol) electrospun nanofibers loaded with achillea millefolium and viola extract: A promising wound dressing. Carbohydr. Polym. 2024, 336, 122117. [Google Scholar] [CrossRef]
- Zou, P.; Lee, W.-H.; Gao, Z.; Qin, D.; Wang, Y.; Liu, J.; Sun, T.; Gao, Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with oh-cath30 nanoparticles. Carbohydr. Polym. 2020, 232, 115786. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Nassiri, M.; Farahmandian, N.; Farshi, P.; Aliakbar Ahovan, Z.; Majidi, J.; Hashemi, A.; Shafikhani, S.H.; Moroni, L.; Gholipourmalekabadi, M. Engineering of a bilayer antibacterial wound dressing from bovine pericardium and electrospun chitosan/PVA/antibiotics for infectious skin wounds management: An in vitro and in vivo study. Int. J. Biol. Macromol. 2024, 282, 137055. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Cui, H.; Wang, Y.; Wang, C.; Li, Y.; Wang, C. Biocompatible AIE material from natural resources: Chitosan and its multifunctional applications. Carbohydr. Polym. 2020, 227, 115338. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Wang, M.; Roy, A.K.; Webster, T.J. Development of chitosan/poly(vinyl alcohol) electrospun nanofibers for infection related wound healing. Front. Physiol. 2017, 7, 683. [Google Scholar] [CrossRef]
- Zhang, W.; Khan, A.; Ezati, P.; Priyadarshi, R.; Sani, M.A.; Rathod, N.B.; Goksen, G.; Rhim, J.-W. Advances in sustainable food packaging applications of chitosan/polyvinyl alcohol blend films. Food Chem. 2024, 443, 138506. [Google Scholar] [CrossRef]
- Chen, Y.; Etxabide, A.; Seyfoddin, A.; Ramezani, M. Fabrication and characterisation of poly(vinyl alcohol)/chitosan scaffolds for tissue engineering applications. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J. Biol. Eng. 2020, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Ghale, R.A.; Asl, R.M.; Tabandeh, F. Advancements in characterization and preclinical applications of hyaluronic acid-based biomaterials for wound healing: A review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100706. [Google Scholar] [CrossRef]
- Séon-Lutz, M.; Couffin, A.-C.; Vignoud, S.; Schlatter, G.; Hébraud, A. Electrospinning in water and in situ crosslinking of hyaluronic acid/cyclodextrin nanofibers: Towards wound dressing with controlled drug release. Carbohydr. Polym. 2019, 207, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Geng, X.; Zhang, Y.; Shi, Y.; Zhao, L. Microenvironmental pH modulating oxygen self-boosting microalgal prodrug carboxymethyl chitosan/hyaluronic acid/puerarin hydrogel for accelerating wound healing in diabetic rats. Int. J. Biol. Macromol. 2024, 282, 136669. [Google Scholar] [CrossRef]
- Blinov, A.; Rekhman, Z.; Yasnaya, M.; Gvozdenko, A.; Golik, A.; Kravtsov, A.; Shevchenko, I.; Askerova, A.; Prasolova, A.; Pirogov, M.; et al. Enhancement of stability and activity of zinc carbonate nanoparticles using chitosan, hydroxyethyl cellulose, methyl cellulose and hyaluronic acid for multifaceted applications in medicine. Int. J. Biol. Macromol. 2025, 298, 139768. [Google Scholar] [CrossRef]
- Sayyar, Z.; Hosseini, Z.; Beheshtizadeh, N. Developing curcumin loaded-magnetic montmorillonite nanoparticles/polyvinyl alcohol/hyaluronic acid/chitosan nanofiber mats as a wound dressing. J. Drug Deliv. Sci. Technol. 2024, 93, 105408. [Google Scholar] [CrossRef]
- Bozdag, M.; Urek, F.; Cesur, S.; Sahin, A.; Gunduz, O. Bovine serum albumin (bsa)-loaded polyvinyl alcohol (pva)/chitosan (ch)/hydroxyapatite (ha) electrospun nanofibers for bone tissue regeneration. J. Drug Deliv. Sci. Technol. 2025, 106, 106712. [Google Scholar] [CrossRef]
- Salim, S.A.; Taha, A.A.; Khozemy, E.E.; El-Moslamy, S.H.; Kamoun, E.A. Electrospun zinc-based metal organic framework loaded-pva/chitosan/hyaluronic acid interfaces in antimicrobial composite nanofibers scaffold for bone regeneration applications. J. Drug Deliv. Sci. Technol. 2022, 76, 103823. [Google Scholar] [CrossRef]
- Alkabli, J. Recent advances in the development of chitosan/hyaluronic acid-based hybrid materials for skin protection, regeneration, and healing: A review. Int. J. Biol. Macromol. 2024, 279, 135357. [Google Scholar] [CrossRef]
- Hosseini, H.; Shahraky, M.K.; Amani, A.; Landi, F.S. Electrospinning of polyvinyl alcohol/chitosan/hyaluronic acid nanofiber containing growth hormone and its release investigations. Polym. Adv. Technol. 2021, 32, 574–581. [Google Scholar] [CrossRef]
- Mohamady Hussein, M.A.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Sezgin Mansuroglu, D.; Sahin, Y.M.; Gunduz, O.; Muhammed, M.; et al. Dual-drug delivery of ag-chitosan nanoparticles and phenytoin via core-shell pva/pcl electrospun nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef] [PubMed]
- Rehan Ansari, M.; Agrohi, P.; Rao Peta, K. Effect of citric acid on structural, optical, morphological properties of ZnO and the bactericidal applications against human pathogenic bacteria E. coli DH5α. Mater. Today Proc. 2024; in press. [Google Scholar] [CrossRef]
- Cao, S.; Li, Q.; Zhang, S.; Liu, Z.; Lv, X.; Chen, J. Preparation of biodegradable carboxymethyl cellulose/dopamine/Ag nps cryogel for rapid hemostasis and bacteria-infected wound repair. Int. J. Biol. Macromol. 2022, 222, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-j.; Nisar, S.S.; Choe, H.-C. Enhanced surface of collagen-Zn-Ag-Ha coated ti-6al-4v alloy for biocompatibility. Surf. Interfaces 2025, 59, 105920. [Google Scholar] [CrossRef]
- Li, X.; Pang, L.; Duan, J.; Huang, N.; Chen, X.; Huang, W.; Liu, Y.; Fu, C.; Zhang, C.; Tu, H.; et al. Eco-friendly antibacterial electrospinning nanofibrous film containing nano-silver green-synthesized by natural glycoprotein for infected wound healing. J. Colloid Interface Sci. 2025, 683, 256–268. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-nps/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef]
- Mujeeb Rahman, P.; Muraleedaran, K.; Mujeeb, V.M.A. Applications of chitosan powder with in situ synthesized nano ZnO particles as an antimicrobial agent. Int. J. Biol. Macromol. 2015, 77, 266–272. [Google Scholar] [CrossRef]
- Santiago-Castillo, K.; Del Angel-López, D.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Willcock, H.; Brachetti-Sibaja, S.B. Effect on the processability, structure and mechanical properties of highly dispersed in situ Zno:Cs nanoparticles into pva electrospun fibers. J. Mater. Res. Technol. 2021, 11, 929–945. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Yang, J.-M.; Chang, Y.-H. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Zhang, Y.; Yu, Z.; Qi, C. Preparation of a novel chitosan based biopolymer dye and application in wood dyeing. Polymers 2016, 8, 338. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H.; Su, W.-W. Modification of different molecular weights of chitosan by p-coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A ftir-atr study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Berthomieu, C.; Hienerwadel, R. Fourier transform infrared (ftir) spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef]
- Nie, B.; Stutzman, J.; Xie, A. A vibrational spectral maker for probing the hydrogen-bonding status of protonated asp and glu residues. Biophys. J. 2005, 88, 2833–2847. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Ansari, M.A.; Ali, K.; Farooqui, Z.; Al-Dossary, H.A.; Zubair, M.; Musarrat, J. Nanotechnology and diabetic foot ulcer: Future prospects. In Diabetic Foot Ulcer: An Update; Springer: Singapore, 2021; pp. 331–357. [Google Scholar]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from uv-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.; Rita, A.; Sahaya Jude Dhas, S.; Martin Britto Dhas, S.A. Tuning of surface plasmon resonance of silver nano particles by shock waves for plasmonic device applications. Opt. Laser Technol. 2020, 128, 106235. [Google Scholar] [CrossRef]
- Huang, Q.; Jiao, Z.; Li, M.; Qiu, D.; Liu, K.; Shi, H. Preparation, characterization, antifungal activity, and mechanism of chitosan/TiO2 hybrid film against bipolaris maydis. J. Appl. Polym. Sci. 2013, 128, 2623–2629. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Ocakoglu, K.; Mansour, S.A.; Yildirimcan, S.; Al-Ghamdi, A.A.; El-Tantawy, F.; Yakuphanoglu, F. Microwave-assisted hydrothermal synthesis and characterization of ZnO nanorods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 148, 362–368. [Google Scholar] [CrossRef]
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of crystalline structural differences between α- and β-chitosan on their nanoparticle formation via ionic gelation and superoxide radical scavenging activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Banica, R.; Ursu, D.; Svera, P.; Sarvas, C.; Rus, S.F.; Novaconi, S.; Kellenberger, A.; Racu, A.V.; Nyari, T.; Vaszilcsin, N. Electrical properties optimization of silver nanowires supported on polyethylene terephthalate. Part Sci. Technol. 2016, 34, 217–222. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Asgari-Targhi, G.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Hatami Tooski, A. Synthesis and characterization of chitosan encapsulated zinc oxide (ZnO) nanocomposite and its biological assessment in pepper (Capsicum annuum) as an elicitor for in vitro tissue culture applications. Int. J. Biol. Macromol. 2021, 189, 170–182. [Google Scholar] [CrossRef]
- Ahmad Yusof, N.A.; Mat Zain, N.; Pauzi, N. Synthesis of chitosan/zinc oxide nanoparticles stabilized by chitosan via microwave heating. Bull. Chem. React. Eng. Catal. 2019, 14, 9. [Google Scholar] [CrossRef]
- Mata, G.C.; Morais, M.S.; Oliveira, W.P.; Aguiar, M.L. Composition effects on the morphology of pva/chitosan electrospun nanofibers. Polymers 2022, 14, 4856. [Google Scholar] [CrossRef]
- Langwald, S.; Ehrmann, A.; Sabantina, L. Measuring physical properties of electrospun nanofiber mats for different biomedical applications. Membranes 2023, 13, 488. [Google Scholar] [CrossRef]
- Kausar, A. Polymeric nanocomposite via electrospinning: Assessment of morphology, physical properties and applications. J. Plast. Film Sheeting 2020, 37, 70–92. [Google Scholar] [CrossRef]
- Telemeco, T.A.; Ayres, C.; Bowlin, G.L.; Wnek, G.E.; Boland, E.D.; Cohen, N.; Baumgarten, C.M.; Mathews, J.; Simpson, D.G. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005, 1, 377–385. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Ma, P.X. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv. Drug Deliv. Rev. 2012, 64, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Cooper-White, J. A simple method for fabricating 3-D multilayered composite scaffolds. Acta Biomater. 2013, 9, 4599–4608. [Google Scholar] [CrossRef] [PubMed]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing. Int. Wound J. 2014, 11, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Water-induced shape memory behavior of poly (vinyl alcohol) and p-coumaric acid-modified water-soluble chitosan blended membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, N.; Gupta, A.; Tiwari, S.; Tiwari, S.K.; Dhakate, S.R. Electrospun chitosan–polyvinyl alcohol composite nanofibers loaded with cerium for efficient removal of arsenic from contaminated water. J. Mater. Chem. A 2014, 2, 16669–16677. [Google Scholar] [CrossRef]





| Parameter | Range | CTS + NPs 2 wt % | PVA Solution 8 wt % | HA Solution 2 wt % | Ag-ZnO Nanoparticles in Situ CTS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag NPs Solution 2 wt % | ZnO NPs Solution 2 wt % | ||||||||||
| % | g | % | g | % | g | % | g | % | g | ||
| Needle gauge | 20–22 G | 39.00 | 1.95 | 60.00 | 3.00 | 1.00 | 0.05 | 30 | 0.585 | 70 | 1.365 |
| Voltage | 15, 20, 25 and 30 kV | 50 | 0.975 | 50 | 0.975 | ||||||
| Flow rate | 1, 2, 3 and 4 µL/min | 70 | 1.365 | 30 | 0.585 | ||||||
| Distance | 15, 20, 25 and 30 cm | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Castillo, K.; Torres-Huerta, A.M.; Cervantes-Uc, J.M.; Rodríguez-Salazar, A.E.; Brachetti-Sibaja, S.B.; Dorantes-Rosales, H.J.; Márquez-Rocha, F.J.; Domínguez-Crespo, M.A. Electrospun PVA-CTS-HA Wound Dressings with Ag-ZnO Nanoparticles for Diabetic Foot Ulcers Treatment: Physicochemical Properties, Hemocompatibility, and Cell Viability. Polymers 2025, 17, 3001. https://doi.org/10.3390/polym17223001
Santiago-Castillo K, Torres-Huerta AM, Cervantes-Uc JM, Rodríguez-Salazar AE, Brachetti-Sibaja SB, Dorantes-Rosales HJ, Márquez-Rocha FJ, Domínguez-Crespo MA. Electrospun PVA-CTS-HA Wound Dressings with Ag-ZnO Nanoparticles for Diabetic Foot Ulcers Treatment: Physicochemical Properties, Hemocompatibility, and Cell Viability. Polymers. 2025; 17(22):3001. https://doi.org/10.3390/polym17223001
Chicago/Turabian StyleSantiago-Castillo, Karina, Aidé Minerva Torres-Huerta, José. Manuel Cervantes-Uc, Adela Eugenia Rodríguez-Salazar, Silvia Beatriz Brachetti-Sibaja, Héctor Javier Dorantes-Rosales, Facundo Joaquín Márquez-Rocha, and Miguel Antonio Domínguez-Crespo. 2025. "Electrospun PVA-CTS-HA Wound Dressings with Ag-ZnO Nanoparticles for Diabetic Foot Ulcers Treatment: Physicochemical Properties, Hemocompatibility, and Cell Viability" Polymers 17, no. 22: 3001. https://doi.org/10.3390/polym17223001
APA StyleSantiago-Castillo, K., Torres-Huerta, A. M., Cervantes-Uc, J. M., Rodríguez-Salazar, A. E., Brachetti-Sibaja, S. B., Dorantes-Rosales, H. J., Márquez-Rocha, F. J., & Domínguez-Crespo, M. A. (2025). Electrospun PVA-CTS-HA Wound Dressings with Ag-ZnO Nanoparticles for Diabetic Foot Ulcers Treatment: Physicochemical Properties, Hemocompatibility, and Cell Viability. Polymers, 17(22), 3001. https://doi.org/10.3390/polym17223001








