Low-Cost Biomass Nanofibers from Chitosan and Phytic Acid for Efficient Uranium Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Fabrication of Pure Chitosan Nanofibers
2.3. Modification of Phytic Acid on Chitosan Nanofibers
2.4. Adsorption Experiments from Uranium Spiked Sotluion
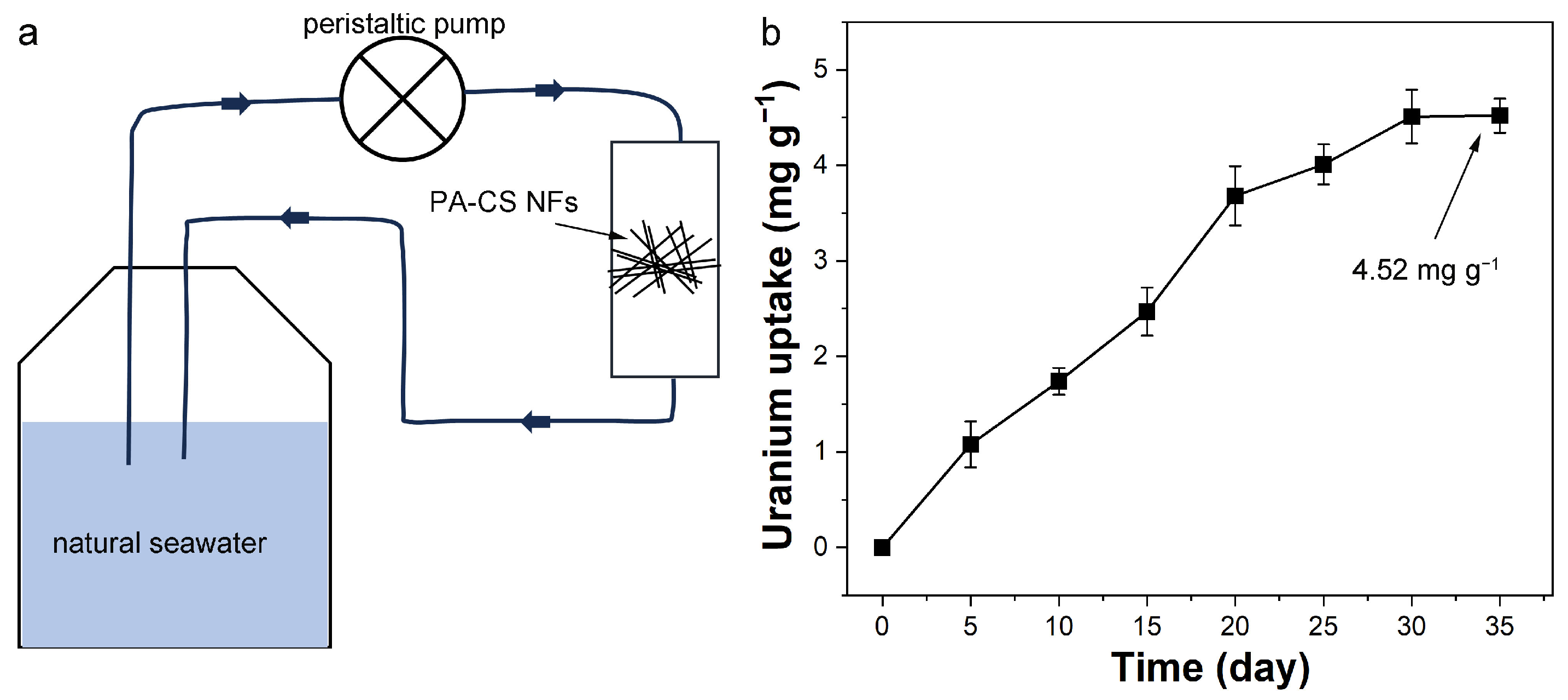
2.5. Uranium Extraction from Real Seawater
3. Results
3.1. Synthesis of Nanofiber Adsorbent
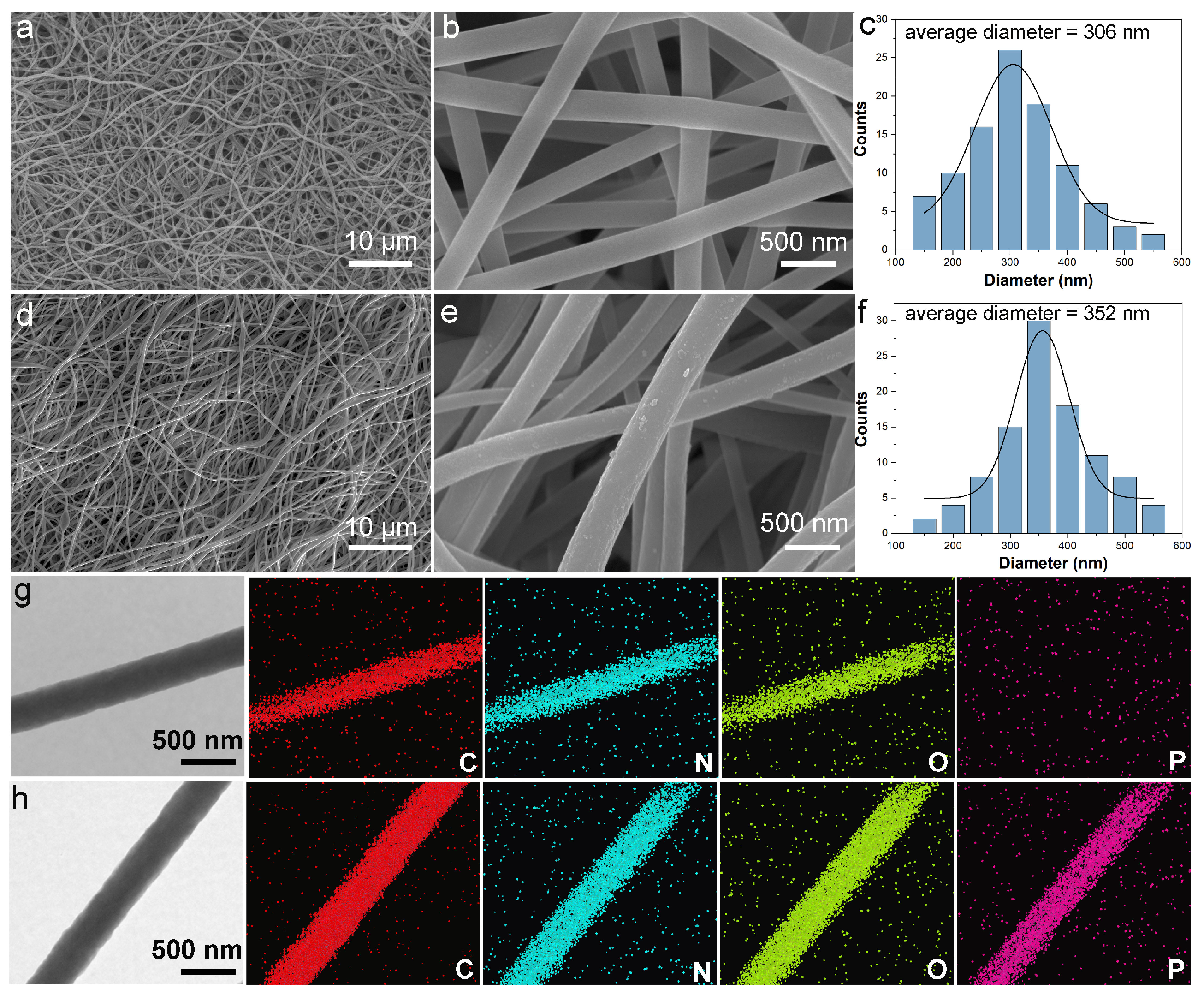
3.2. Morphological Analysis
3.3. Characterization of Chemical Structures
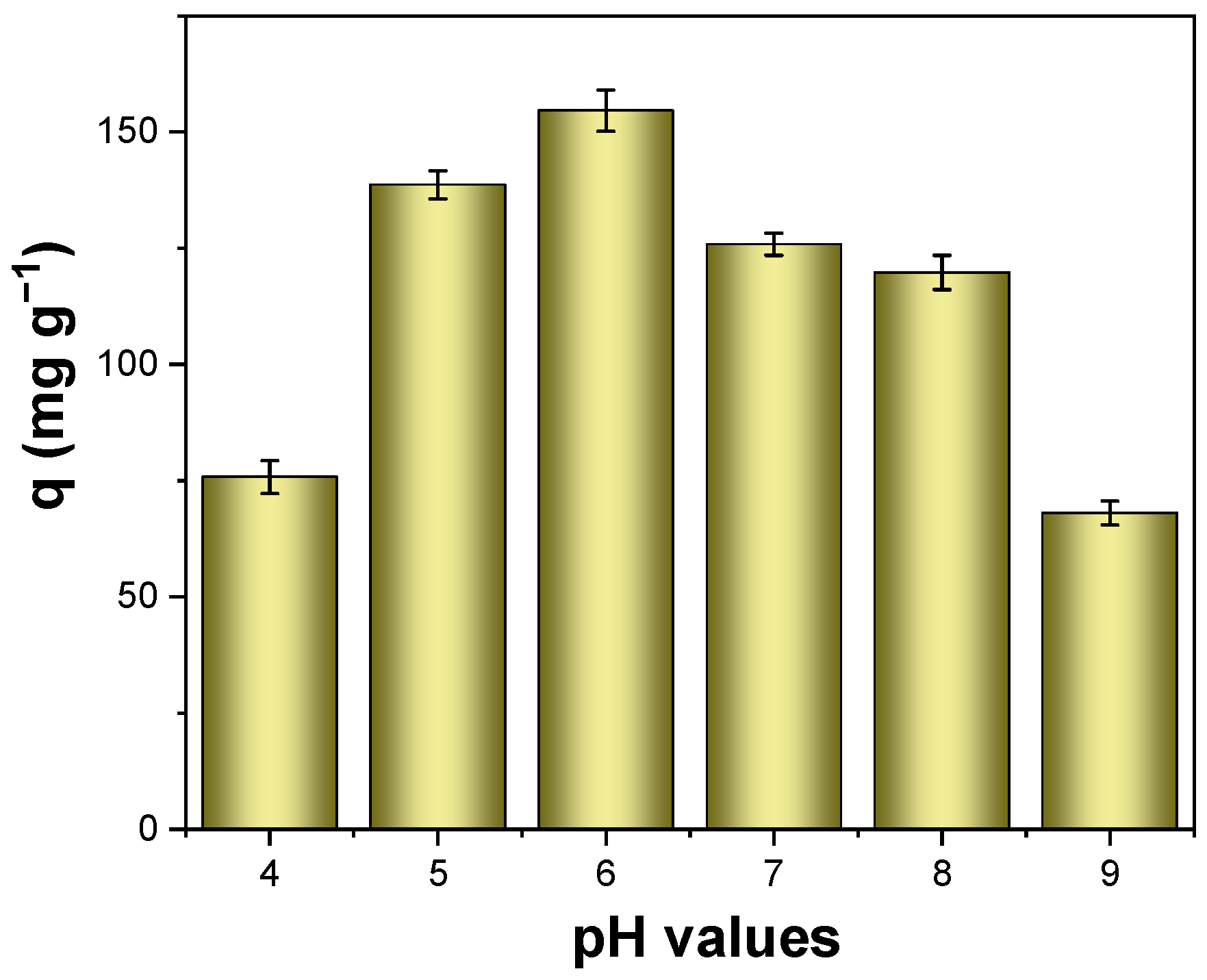
3.4. Effect of Solution pH on Uranium Adsorption
3.5. Effect of PA Modification
3.6. Adsorption Kinetics and Isotherm
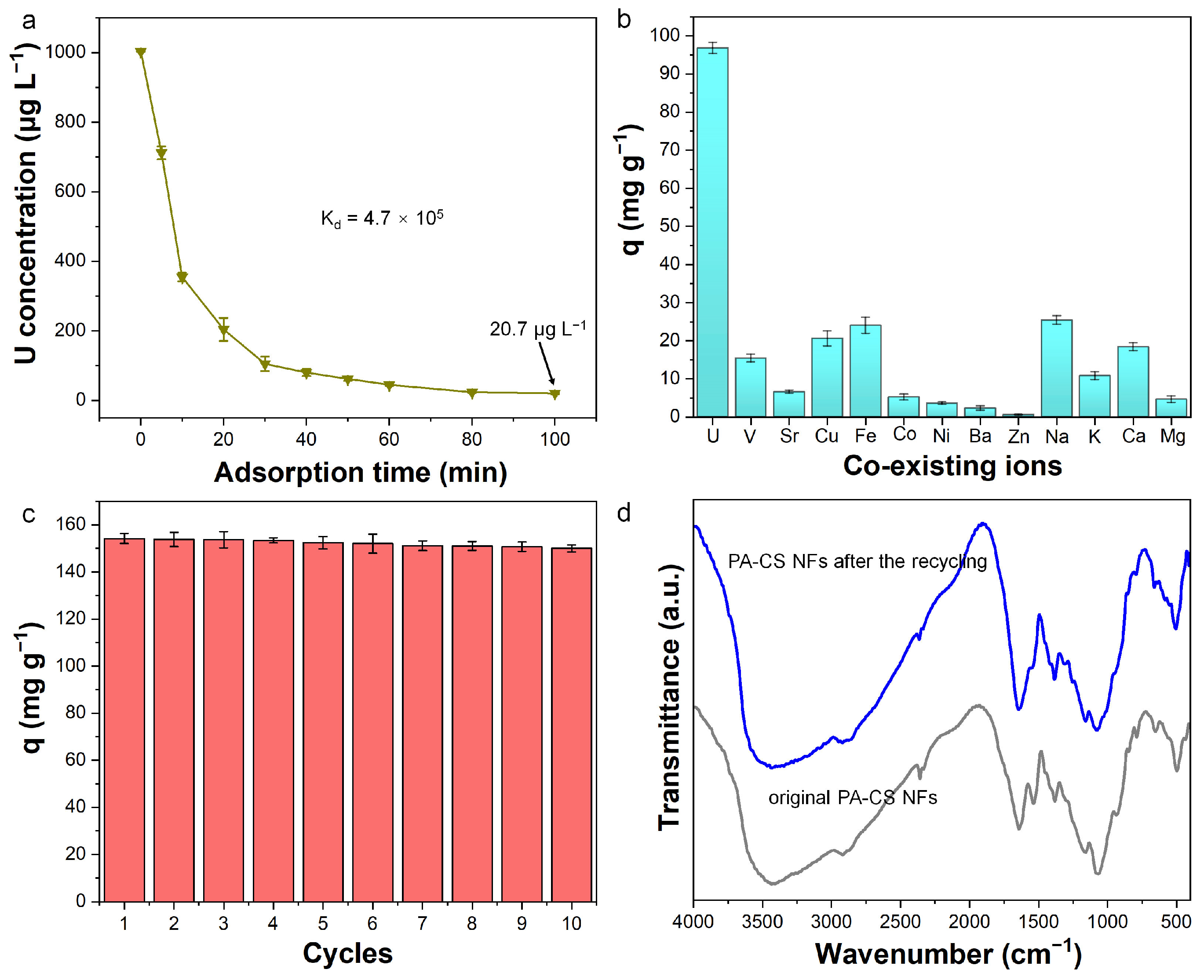
3.7. Deep Removal, Selectivity, and Recyclability
3.8. Uranium Extraction from Natural Seawater
3.9. Adsorption Mechanism Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS NFs | Chitosan nanofibers |
| EDS | Energy-dispersive X-ray spectroscopy |
| EDTA | Ethylenediaminetetraacetic acid |
| EPA | Environmental Protection Agency |
| ESP | Electrostatic potential |
| FT-IR | Fourier-transform infrared |
| ICP-MS | Inductively coupled plasma mass spectrometer |
| Kd | Distribution coefficient |
| PA | Phytic acid |
| PA-CS NFs | Biomass nanofiber adsorbents |
| PEO | Poly(ethylene oxide) |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| UES | Uranium extraction from seawater |
| UO22+ | Uranyl ion |
| U-PA-CS NFs | Biomass nanofibers after uranium adsorption |
| XPS | X-ray photoelectron spectroscopy |
References
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Chen, M.; Liu, D.; Liu, T.; Wei, T.; Qiao, Q.; Yuan, Y.; Wang, N. Constructing 2D Polyphenols-Based Crosslinked Networks for Ultrafast and Selective Uranium Extraction from Seawater. Small 2024, 20, 2401528. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, S.; Wang, L.; Yu, J.; Yuan, L.; Li, Z.; Wu, W.; Chai, Z.; Shi, W. Adhesive Tape-Inspired COF/Polymer Crosslinked Networks for Efficient Uranium Extraction from Seawater. Sep. Purif. Technol. 2025, 364, 132611. [Google Scholar] [CrossRef]
- Ma, X.; Meihaus, K.R.; Yang, Y.; Zheng, Y.; Cui, F.; Li, J.; Zhao, Y.; Jiang, B.; Yuan, Y.; Long, J.R.; et al. Photocatalytic Extraction of Uranium from Seawater Using Covalent Organic Framework Nanowires. J. Am. Chem. Soc. 2024, 146, 23566–23573. [Google Scholar] [CrossRef]
- Guo, H.; Hu, E.; Wang, Y.; Ou, Z.; Huang, B.; Lei, J.; Liu, H.; He, R.; Zhu, W. A Synergistic Coordination-Reduction Interface for Electrochemical Reductive Extraction of Uranium with Low Impurities from Seawater. Nat. Commun. 2025, 16, 2012. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Goto, A.; Zhao, Y.; Wang, R. Uranium and Lithium Extraction from Seawater: Challenges and Opportunities for A Sustainable Energy Future. J. Mater. Chem. A 2023, 11, 22551–22589. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Li, G.-Y.; Ding, D.-X.; Zhang, Z.-Y.; Chen, J.; Hu, N.; Li, L. Column Leaching of Uranium Ore with Fungal Metabolic Products and Uranium Recovery by Ion Exchange. J. Radioanal. Nucl. Chem. 2015, 304, 1139–1144. [Google Scholar] [CrossRef]
- Dong, W.; Xie, G.; Miller, T.R.; Franklin, M.P.; Oxenberg, T.P.; Bouwer, E.J.; Ball, W.P.; Halden, R.U. Sorption and Bioreduction of Hexavalent Uranium at a Military Facility by the Chesapeake Bay. Environ. Pollut. 2006, 142, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Kelly, S.D.; Kemner, K.M.; Watson, D.B.; Zhou, J.; Jardine, P.M.; Gu, B. Sequestering Uranium and Technetium through Co-Precipitation with Aluminum in a Contaminated Acidic Environment. Environ. Sci. Technol. 2009, 43, 7516–7522. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Ma, R.; Cao, J.; Ruan, X.; Cao, D.; Song, Y.; Chen, S.; Song, Y.; Wang, F.; et al. Overcoming Chemical Dissociation Processes: Electrochemical Modulation of High-Affinity Binding Sites for Rapid Uranium Extraction from Seawater. Adv. Funct. Mater. 2025, 35, 2412712. [Google Scholar] [CrossRef]
- Xin, Q.; Wang, H.; Hu, E.; Luo, K.; Lei, Z.; Hu, F.; Liu, X.; Hu, J.; Wang, Q. Investigation into Highly Selective Uranium Adsorption Using a Water-Stable Chitosan/Ellagic Acid/Cu-Metal–Organic Framework Material. Desalination 2025, 606, 118768. [Google Scholar] [CrossRef]
- Warner, W.C.L.; Mackie, K.E.; Warner, M.G.; Gill, G.A.; Addleman, R.S. Nanostructured Metal Oxide Sorbents for the Collection and Recovery of Uranium from Seawater. Ind. Eng. Chem. Res. 2016, 55, 4195–4207. [Google Scholar] [CrossRef]
- Liu, P.; Yu, Q.; Xue, Y.; Chen, J.; Ma, F. Adsorption Performance of U(VI) by Amidoxime-Based Activated Carbon. J. Radioanal. Nucl. Chem. 2020, 324, 813–822. [Google Scholar] [CrossRef]
- Ahmad, Z.; Li, Y.; Yang, J.; Geng, N.; Fan, Y.; Gou, X.; Sun, Q.; Chen, J. A Membrane-Supported Bifunctional Poly(amidoxime-ethyleneimine) Network for Enhanced Uranium Extraction from Seawater and Wastewater. J. Hazard. Mater. 2022, 425, 127995. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, Q.; Li, Y.; Cao, L.; Li, W. Amidoximated Cellulose Fiber Membrane for Uranium Extraction from Simulated Seawater. Carbohydr. Polym. 2020, 245, 116627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Aguila, B.; Earl, L.D.; Abney, C.W.; Wojtas, L.; Thallapally, P.K.; Ma, S. Covalent Organic Frameworks as a Decorating Platform for Utilization and Affinity Enhancement of Chelating Sites for Radionuclide Sequestration. Adv. Mater. 2018, 30, 1705479. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Yadav, A.; Yadav, S.; Panghal, P.; Singh, S.; Deep, A.; Kumar, S. Biomass-Based Adsorbents for Wastewater Remediation: A Systematic Review on Removal of Emerging Contaminants. Microchem. J. 2024, 207, 111880. [Google Scholar] [CrossRef]
- Chen, D.; Sun, M.; Zhao, X.; Shi, M.; Fu, X.; Hu, W.; Zhao, R. High-Efficiency and Economical Uranium Extraction from Seawater with Easily Prepared Supramolecular Complexes. J. Colloid Interface Sci. 2024, 668, 343–351. [Google Scholar] [CrossRef]
- Fu, X.; Shi, M.; Chen, D.; Ren, Z.; Bai, Q.; Zhao, R. Facile and Scalable Synthesis of Bio-Based Adsorbents from Tannin for Economical Uranium Extraction from Seawater. Desalination 2025, 601, 118553. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Teng, Y.; Jia, S.; Huang, H.; Li, Y.; Wang, C. Ce-MOF Composite Electrospinning as Antibacterial Adsorbent for the Removal of 2,4-Dichlorophenoxyacetic Acid. Chem. Eng. J. 2023, 462, 142195. [Google Scholar] [CrossRef]
- Fu, X.; Shi, M.; Chen, D.; Zhao, X.; Jiang, T.; Zhao, R. Theory-Driven Tailoring of the Microenvironment of Quaternary Ammonium Binding Sites on Electrospun Nanofibers for Efficient Bilirubin Removal in Hemoperfusion. Polymers 2024, 16, 1599. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Du, W.; Dong, C.; Xiong, M.; Yang, N.; Zhao, S.; He, H.; Nie, Z. An Ionic Liquid-Modified PVC Nanofiber Facilitates Gold Recovery from Wastewater by a Light-Enhancing Effect. Chem. Eng. J. 2025, 505, 159419. [Google Scholar] [CrossRef]
- Wang, J.; You, C.; Xu, Y.; Xie, T.; Wang, Y. Research Advances in Electrospun Nanofiber Membranes for Non-Invasive Medical Applications. Micromachines 2024, 15, 1226. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, D.; Zhang, N.; Shi, M.; Hu, W.; Yu, G.; Zhao, R. Biodegradable Chitosan-Zirconium Composite Adsorptive Membranes for Potential Arsenic (III/V) Capture Electrodialysis. Int. J. Biol. Macromol. 2023, 256, 128356. [Google Scholar] [CrossRef]
- Wang, H.; Jin, C.; Li, X.; Ma, J.; Ye, Y.; Tang, L.; Si, J.; Cui, B. A Green Biocatalyst Fabricated by Fungal Laccase Immobilized onto Fe3O4@Polyaniline-Chitosan Nanofibrous Composites for the Removal of Phenolic Compounds. Chem. Eng. J. 2025, 507, 160486. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, X.; Hu, J.; Li, N.; Sun, L.; Li, W.; Liu, H. Preparation of Salt-Tolerant Chitosan Hydrogels and Their Anti-Biofouling Behavior Study. Surf. Coat. Technol. 2023, 460, 129403. [Google Scholar] [CrossRef]
- Li, Y.; Dai, Y.; Tao, Q.; Xu, L. Synthesis and Characterization of Amino Acid-Functionalized Chitosan/Poly(Vinyl Alcohol) for Effective Adsorption of Uranium. J. Radioanal. Nucl. Chem. 2022, 331, 4753–4765. [Google Scholar] [CrossRef]
- Wang, C.; Guo, B.; Guo, L.; Chen, H.; Fan, J.; Wang, L.; Yang, G.; Zou, S.; Zeng, G. Rapid Capture of Uranium Over Acid-Tolerant Hybrid Nanofibrous Membrane. Sep. Purif. Technol. 2024, 340, 126717. [Google Scholar] [CrossRef]
- He, Y.; Du, W.; Fu, X.; Yuan, D.; Na, B.; Zhang, S.; Liu, H. Hypercross-Linked Porous Polymer Bearing Phosphonate Ligand for Efficient Removal of Uranium in Water: A Combined Experimental and Density Functional Theory Study. J. Radioanal. Nucl. Chem. 2025, 334, 2487–2499. [Google Scholar] [CrossRef]
- Tuo, K.; Li, J.; Li, Y.; Liang, C.; Shao, C.; Hou, W.; Fan, C.; Li, Z.; Pu, S.; Chen, Z.; et al. Phytic Acid Functionalized Hierarchical Porous Metal-Organic Framework Microspheres for Efficient Extraction of Uranium from Seawater. Small 2025, 21, 2407272. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xian, H.; He, H.; Bai, H.; Jiang, P.; Wang, G. Performance and Mechanism of Phytic Acid-Modified Mesoporous Tobermorite for U(VI) Adsorption in Water. Colloids Surf. A 2024, 699, 134691. [Google Scholar] [CrossRef]
- Ojha, S.S.; Stevens, D.R.; Hoffman, T.J.; Stano, K.; Klossner, R.; Scott, M.C.; Krause, W.; Clarke, L.I.; Gorga, R.E. Fabrication and Characterization of Electrospun Chitosan Nanofibers Formed via Templating with Polyethylene Oxide. Biomacromolecules 2008, 9, 2523–2529. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Xing, W.; Li, L.; Wu, H.; Wang, H.; Wang, Z.; Huang, L.; Tang, J. In Situ Growth of ZIF-8 Nanoparticles on Pure Chitosan Nanofibrous Membranes for Efficient Antimicrobial Wound Dressings. Int. J. Biol. Macromol. 2025, 297, 139921. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Jing, X.; Zhao, R.; Zhu, G.; Wang, C. Bio-Inspired Functionalization of Electrospun Nanofibers with Anti-Biofouling Property for Efficient Uranium Extraction from Seawater. Chem. Eng. J. 2023, 465, 142844. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Shi, M.; Fu, X.; Hu, W.; Shi, X.; Zhao, R.; Zhu, G. Enhanced and Selective Uranium Extraction onto Electrospun Nanofibers by Regulating the Functional Groups and Photothermal Conversion Performance. Chem. Eng. J. 2024, 480, 148108. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Sun, J. Degradable Poly(vinyl alcohol)-Based Supramolecular Plastics with High Mechanical Strength in a Watery Environment. Adv. Mater. 2021, 33, 2007371. [Google Scholar] [CrossRef]
- Lei, H.; Pan, N.; Zou, H.; Wang, X. Xianguo Tuo, Hollow Self-Assembled Hybrid Framework Based on Phytic Acid for U(VI) Capture from Highly Acidic Aqueous Media. Chem. Eng. J. 2023, 472, 144919. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.; Zhang, J.; Qin, Y.; Wang, D. Phytic Acid Intercalated Graphene Oxide for Anticorrosive Reinforcement of Waterborne Epoxy Resin Coating. Polymers 2019, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhong, X.; Lu, L.; Shi, H.; Liu, X.; Zhang, W.; Zhang, L. Incorporation Phytic Acid-Modified Chitosan/Sodium Alginate Melamine Sponge Composite for Highly Efficient Uranium Adsorption. Inter. J. Biol. Macromol. 2025, 318, 145207. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, R.; Bi, R.; Peng, Z.; Liu, X.; Shi, T.; Zhang, L.; Liang, R.; Qiu, J. Calcium Phytate Cross-Linked Polysaccharide Hydrogels for Selective Removal Of U(VI) from Tailings Wastewater. Water Res. 2025, 278, 123343. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.; Hosseini-Bandegharaei, A.; Chao, H. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Ye, T.; Wang, Y.; Zhou, L. Removal of Uranyl Ions from Aqueous Media by Tannic Acid-Chitosan Hydrothermal Carbon: Equilibria, Kinetics and Thermodynamics. J. Radioanal. Nucl. Chem. 2020, 326, 1843–1852. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Z.; Song, F.; Guo, Z.; Liu, B. Efficient Removal Of U(VI) Ions from Aqueous Solutions by Tannic Acid/Graphene Oxide Composites. Appl. Sci. 2020, 10, 8870. [Google Scholar] [CrossRef]
- Yang, H.; Ding, H.; Zhang, X.; Luo, X.; Zhang, Y. Immobilization of Dopamine on Aspergillus Niger Microspheres (AM/PDA) and its Effect on the U(VI) Adsorption Capacity in Aqueous Solutions. Colloids Surf. A 2019, 583, 123914. [Google Scholar] [CrossRef]
- Yang, A.; Yang, P.; Huang, C.P. Preparation of Graphene Oxide–Chitosan Composite and Adsorption Performance for Uranium. J. Radioanal. Nucl. Chem. 2017, 313, 371–378. [Google Scholar] [CrossRef]
- Rostamian, R.; Firouzzare, M.; Irandoust, M. Preparation and Neutralization of Forcespun Chitosan Nanofibers from Shrimp Shell Waste and Study on Its Uranium Adsorption in Aqueous Media. React. Funct. Polym. 2019, 143, 104335. [Google Scholar] [CrossRef]
- Huang, G.; Peng, W.; Yang, S. Synthesis of Magnetic Chitosan/Graphene Oxide Nanocomposites and Its Application for U(VI) Adsorption from Aqueous Solution. J. Radioanal. Nucl. Chem. 2018, 317, 337–344. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, M.; Chen, D. Production of Three-Dimensional Porous Polydopamine-Functionalized Attapulgite/Chitosan Aerogel for Uranium(VI) Adsorption. J. Radioanal. Nucl. Chem. 2018, 316, 635–647. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, D.; Chen, B.; Kong, L.; Su, M. Enhanced Uranium(VI) Adsorption by Chitosan Modified Phosphate Rock. Colloids Surf. A 2018, 547, 141–147. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Lin, M.; Wang, N.; Hu, S.; Wen, J. Photothermal Enhanced Separation and Extraction of Uranium: Zwitterion/Chitosan Functionalized Graphene Oxide Aerogel with Lamellar Structure and Microchannels. Sep. Purif. Technol. 2024, 348, 127695. [Google Scholar] [CrossRef]
- You, Y.; Liu, X.; Yang, W.; Zhang, X.; Qin, Z.; Yin, X. Thermosensitive Hydrogel from Amidoximated Bacterial Cellulose for Uranium Adsorption. Cellulose 2025, 32, 5539–5558. [Google Scholar] [CrossRef]
- Huang, Z.; Li, W.; Xu, S.; Xu, X.; Ou, M. A Novel Sponge-Like Composite Biosorbent Fabricated by Sodium Alginate and Polyethyleneimine for Uranium(VI) Extraction from Seawater. Int. J. Biol. Macromol. 2024, 279, 135004. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Chen, S.; Song, Y.; Liu, Q.; Yu, J.; Chen, R.; Zhu, J.; Li, R.; Wang, J. A Novel Anti-Biofouling Collagen Fiber Grafted with Hyperbranched Polyethyleneimine/Amidoxime for Efficient Uranium Extraction from Seawater. Desalination 2024, 586, 117894. [Google Scholar] [CrossRef]
- Mei, P.; Wu, R.; Shi, S.; Li, B.; Ma, C.; Hu, B.; Yuan, Y.; Wang, H.; Liu, T.; Wang, N. Conjugating Hyaluronic Acid with Porous Biomass to Construct Anti-Adhesive Sponges for Rapid Uranium Extraction from Seawater. Chem. Eng. J. 2021, 420, 130382. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, D.; Zhao, X.; Zhao, R. Tailoring the Coordination Microenvironment of Electrospun Nanofibers for The Separation of Thorium Ions from Ore Wastewater. J. Colloid Interface Sci. 2025, 697, 137957. [Google Scholar] [CrossRef] [PubMed]





| Raw Material | Price per Unit | Quantity (kg) | Total Price |
|---|---|---|---|
| chitosan | $14.3/kg | 0.60 kg | $8.58 |
| polyethylene oxide | $5.10/kg | 0.17 kg | $0.87 |
| phytic acid | $3.80/L | 0.80 L | $3.04 |
| acetic acid | $0.21/L | 13.50 L | $2.84 |
| other consumption | -- a | -- | $1.07 |
| (labor, electricity, etc.) | |||
| Total | $16.40 |
| Isotherm Model | PA-CS NFs |
|---|---|
| Langmuir isotherm | |
| qmax (mg g−1) | 457.8 |
| b (L mg−1) | 0.112 |
| R2 | 0.9897 |
| Freundlich isotherm | |
| KF | 53.2 |
| n | 2.26 |
| R2 | 0.8786 |
| Adsorbents | Equilibrium Time (min) a | C0 (mg L−1) b | m/V (g L−1) c | Adsorption Capacity (mg g−1) d | C0 (mg L−1) e | m/V (g L−1) | Ref., Year |
|---|---|---|---|---|---|---|---|
| tannic acid–chitosan hydrothermal carbon | 250 | 25 | 0.4 | 96.7 | 10~100 | 0.4 | [42], 2020 |
| tannic acid/graphene oxide | 120 | 167 | 0.6 | 266.6 | 10~170 | 0.6 | [43], 2020 |
| fungal mycelium microspheres | 300 | 40~160 | -- f | 250.7 | 25~175 | -- | [44], 2019 |
| graphene oxide–chitosan composite | 70 | 10 | 1.0 | 50.5 | 5~100 | 1.0 | [45], 2017 |
| forcespun chitosan nanofibers | 180 | 086 | 0.5 | 110.0 | 10~130 | 0.5 | [46], 2019 |
| magnetic chitosan/graphene oxide | 90 | 100 | 0.1 | 204.1 | 5~120 | 0.1 | [47], 2018 |
| functionalized attapulgite/chitosan aerogel | 120 | 50 | 0.4 | 175.1 | 25~80 | 0.4 | [48], 2018 |
| chitosan modified phosphate rock | 300 | 10 | 2.0 | 8.1 | 1~40 | 2.0 | [49], 2018 |
| phytic acid modified chitosan nanofibers | 60 | 50 | 0.3 | 457.8 | 5~250 | 0.1 | This work |
| Adsorbents | Feed Solution (U Concentration) | Extraction Days | Extraction Capacity (mg g−1) | Ref., Year |
|---|---|---|---|---|
| chitosan functionalized aerogel | natural seawater (3.3 μg L−1) | 28 | 3.59 | [50], 2024 |
| amidoximated bacteria cellulose | natural seawater (3.3 μg L−1) | 2 | 0.39 | [51], 2025 |
| tannin loaded melamine foam | natural seawater (3.2 μg L−1) | 16 | 4.20 | [19], 2025 |
| sodium alginate-based sponge | natural seawater (3.3 μg L−1) | 14 | 3.58 | [52], 2024 |
| collagen fiber | natural seawater (3.3 μg L−1) | 15 | 1.04 | [53], 2024 |
| hyaluronic acid conjugated biomass | natural seawater (3.3 μg L−1) | 30 | 2.12 | [54], 2021 |
| phytic acid modified chitosan nanofibers | natural seawater (3.3 μg L−1) | 35 | 4.52 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Geng, D.; Chen, D.; Shi, M.; Bai, Q.; Zhao, R. Low-Cost Biomass Nanofibers from Chitosan and Phytic Acid for Efficient Uranium Extraction. Polymers 2025, 17, 2725. https://doi.org/10.3390/polym17202725
Ren Z, Geng D, Chen D, Shi M, Bai Q, Zhao R. Low-Cost Biomass Nanofibers from Chitosan and Phytic Acid for Efficient Uranium Extraction. Polymers. 2025; 17(20):2725. https://doi.org/10.3390/polym17202725
Chicago/Turabian StyleRen, Zixu, Dongqi Geng, Dingyang Chen, Minsi Shi, Qing Bai, and Rui Zhao. 2025. "Low-Cost Biomass Nanofibers from Chitosan and Phytic Acid for Efficient Uranium Extraction" Polymers 17, no. 20: 2725. https://doi.org/10.3390/polym17202725
APA StyleRen, Z., Geng, D., Chen, D., Shi, M., Bai, Q., & Zhao, R. (2025). Low-Cost Biomass Nanofibers from Chitosan and Phytic Acid for Efficient Uranium Extraction. Polymers, 17(20), 2725. https://doi.org/10.3390/polym17202725







