Performance and Durability of Biopolymer Blends Containing Modified Metal Oxide Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Metal Oxides
2.2.1. Modification of ZnO to f-ZnO
2.2.2. Sonication of TiO2 to s-TiO2
2.3. Melt Blending
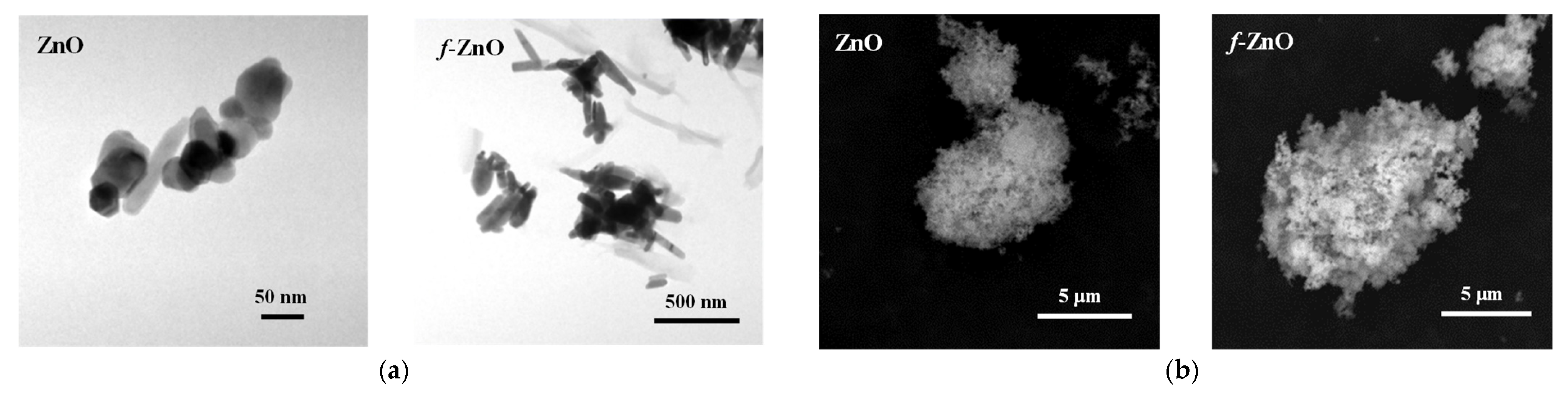
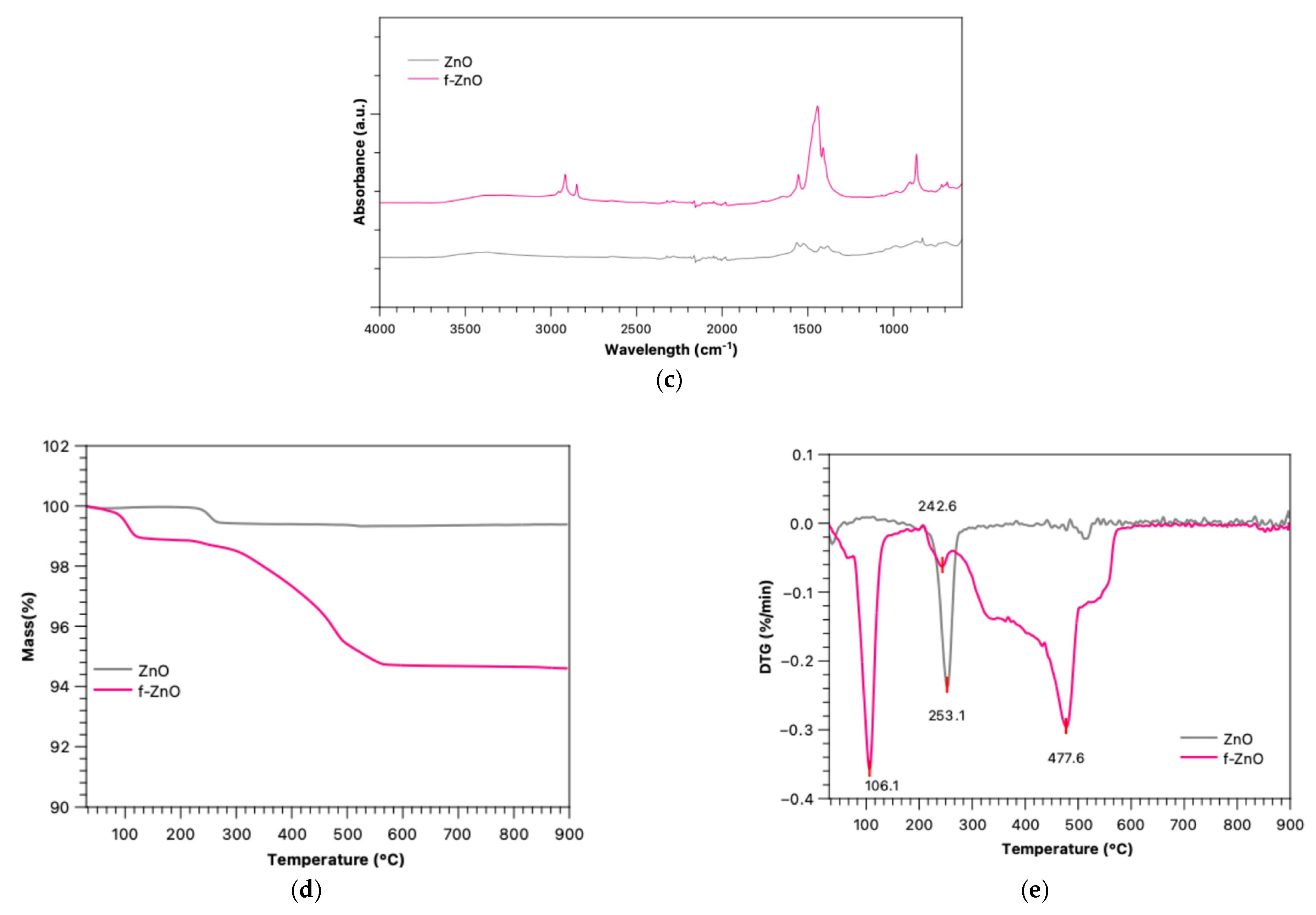
2.4. Characterisations of Particles
2.4.1. ATR-FTIR Spectroscopy
2.4.2. TGA/DTG Analysis
2.4.3. SEM and TEM Analysis
2.5. Characterisations of Composites
2.5.1. FTIR Spectroscopy
2.5.2. Water Contact Angle (WCA)
2.5.3. Tensile Tests
2.5.4. Rheological Analysis
2.5.5. Scanning Electron Microscopy (SEM)
2.5.6. Differential Scanning Calorimetry (DSC)
2.6. Photo-Oxidation Exposure
3. Results and Discussion
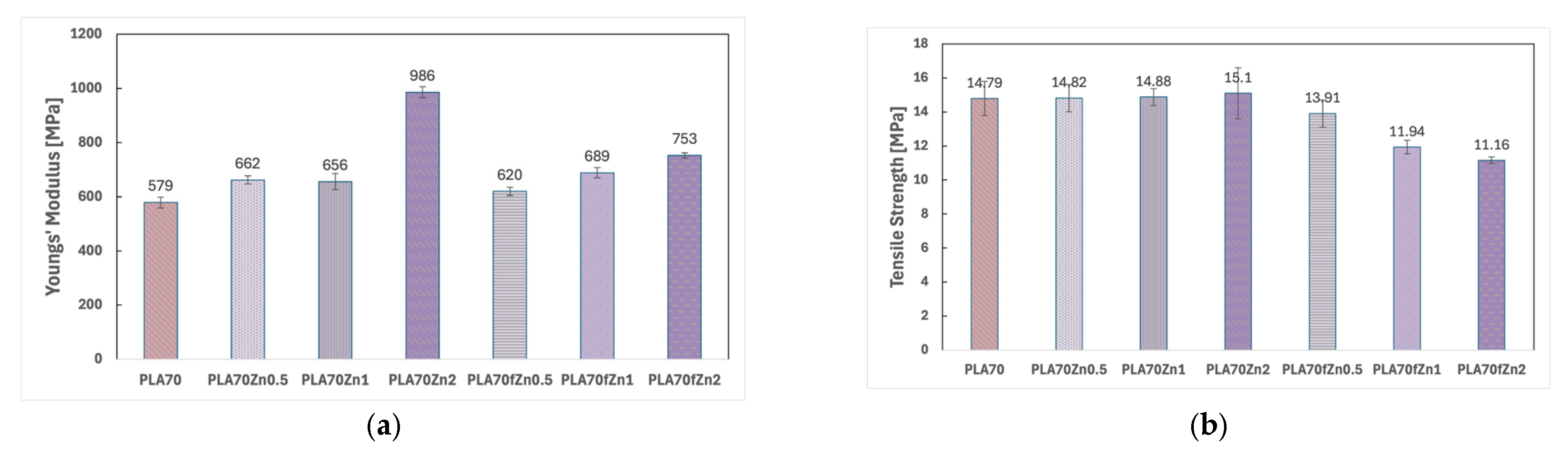
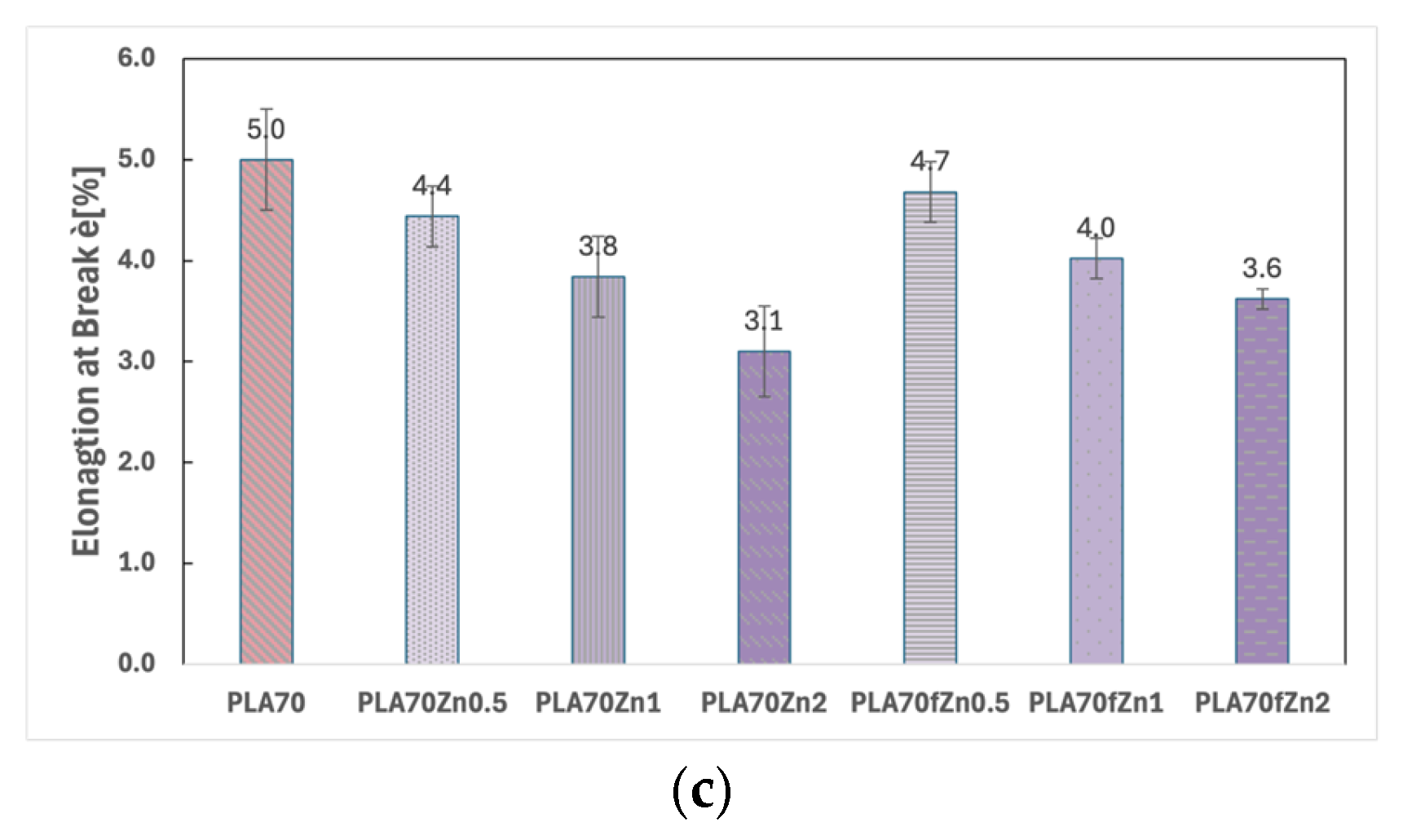
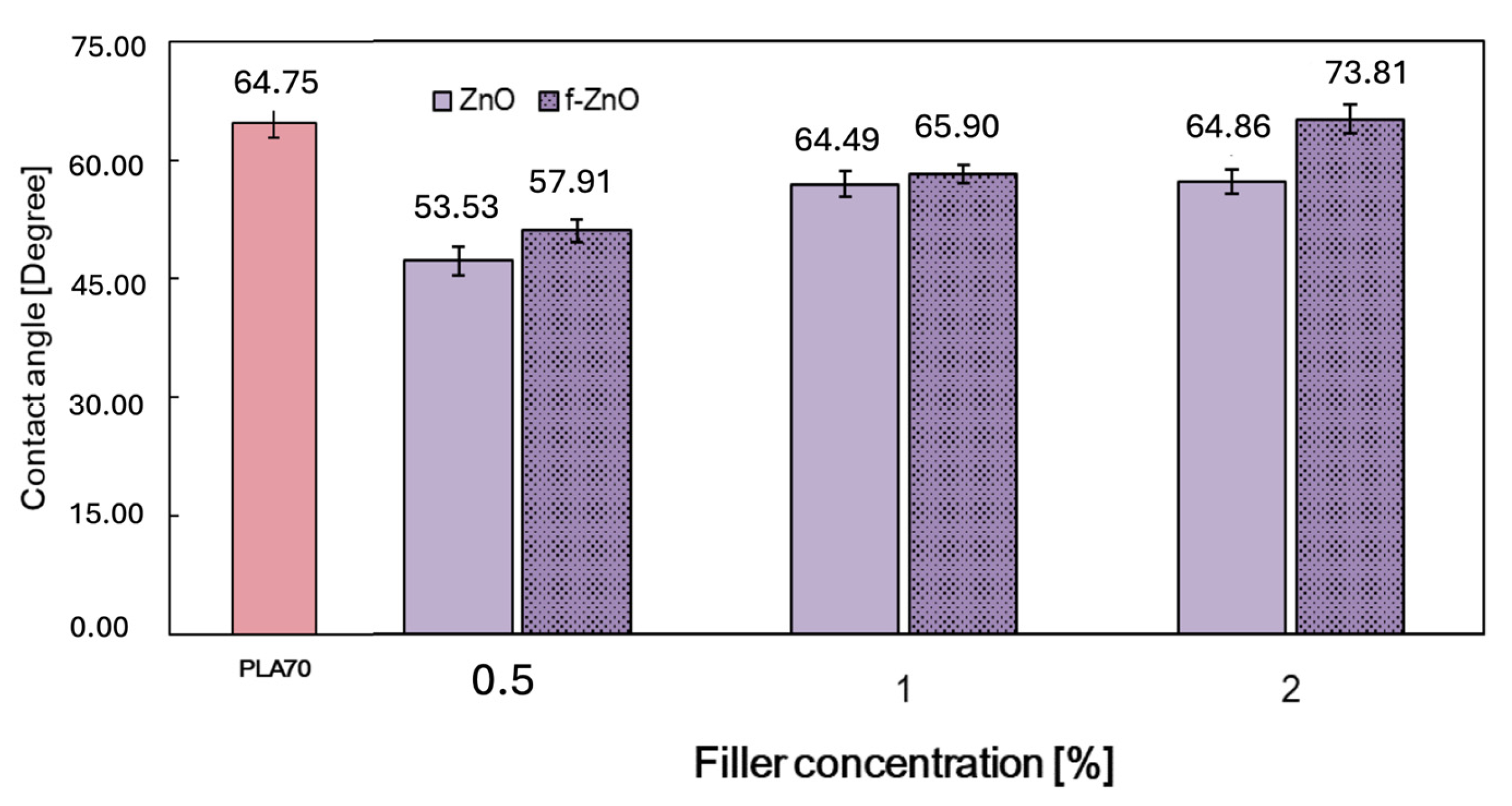
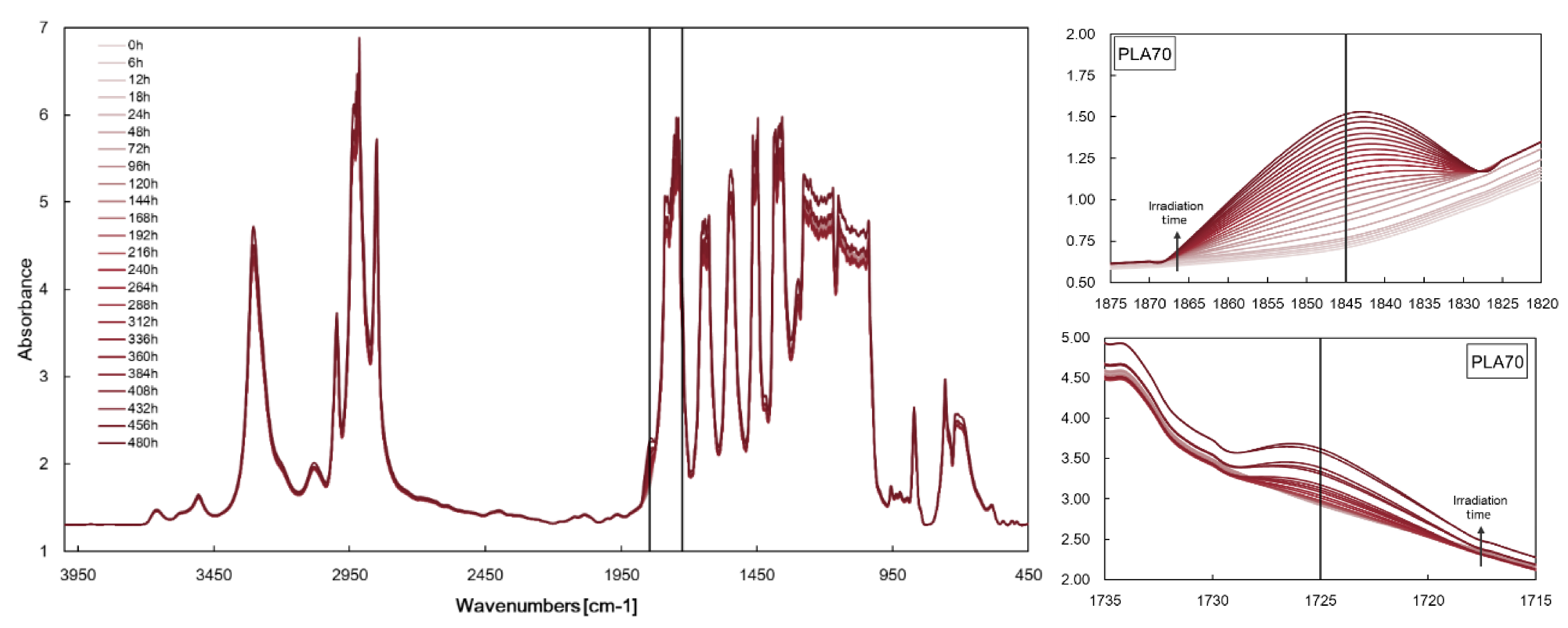
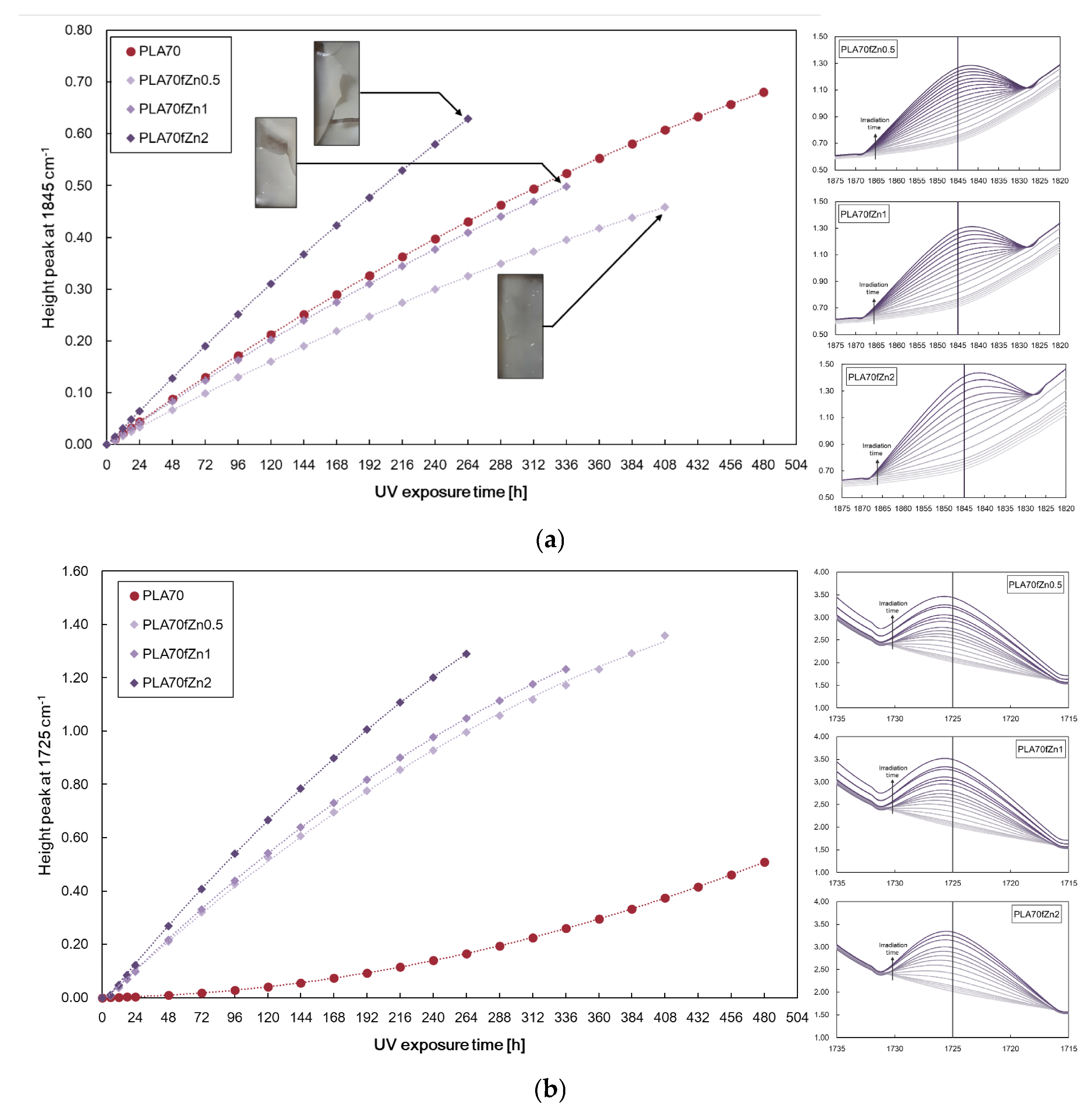
3.1. Characterisations of Biopolymer Blend Containing f-ZnO
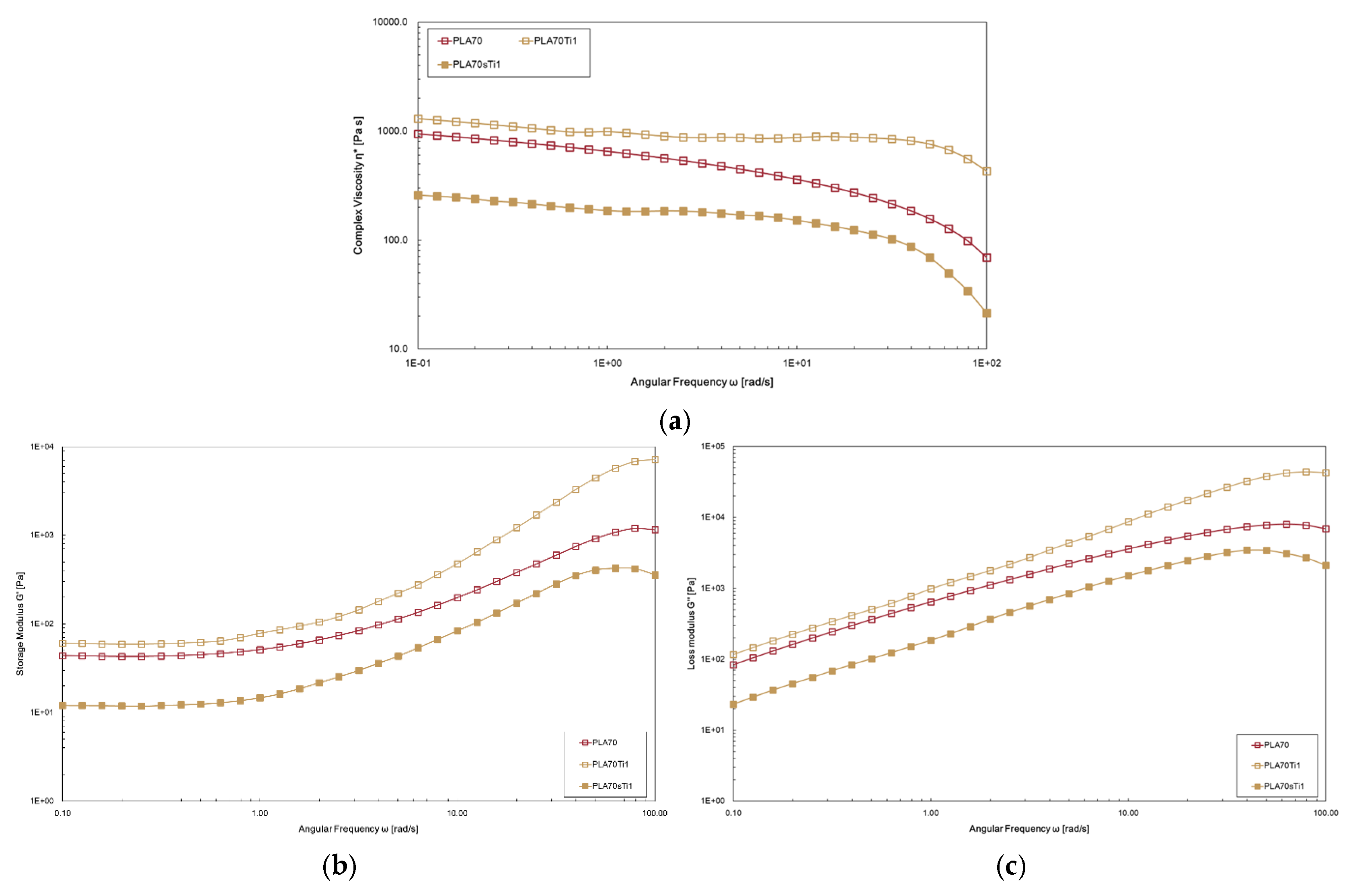
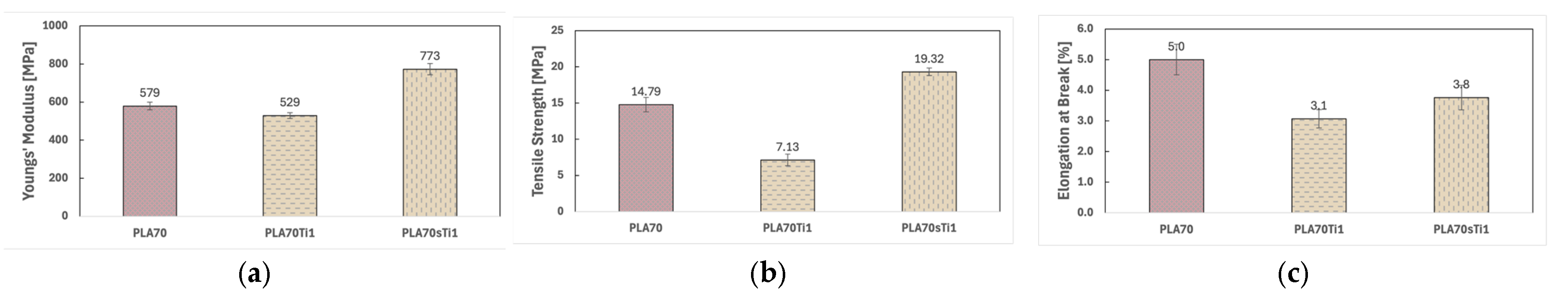
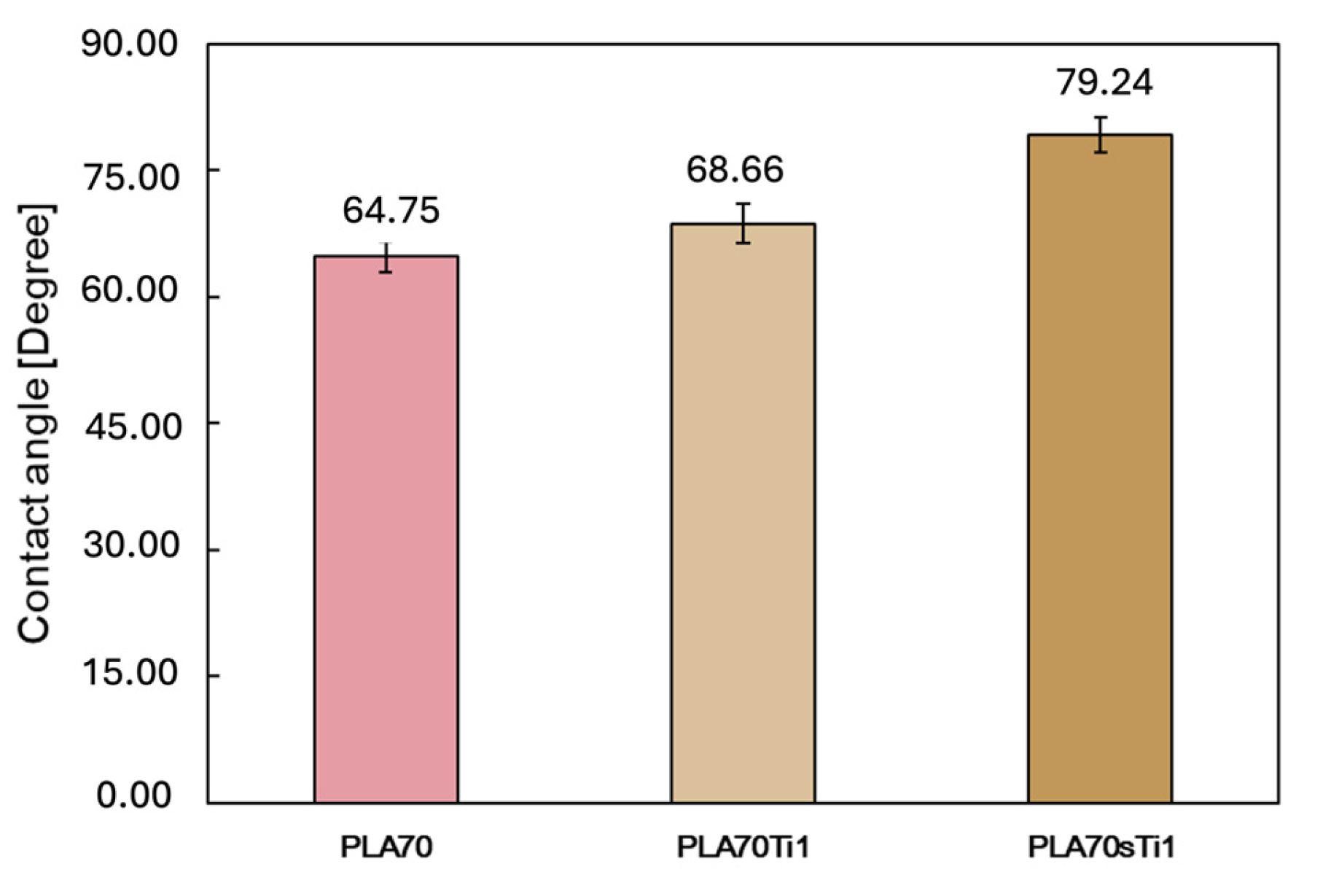
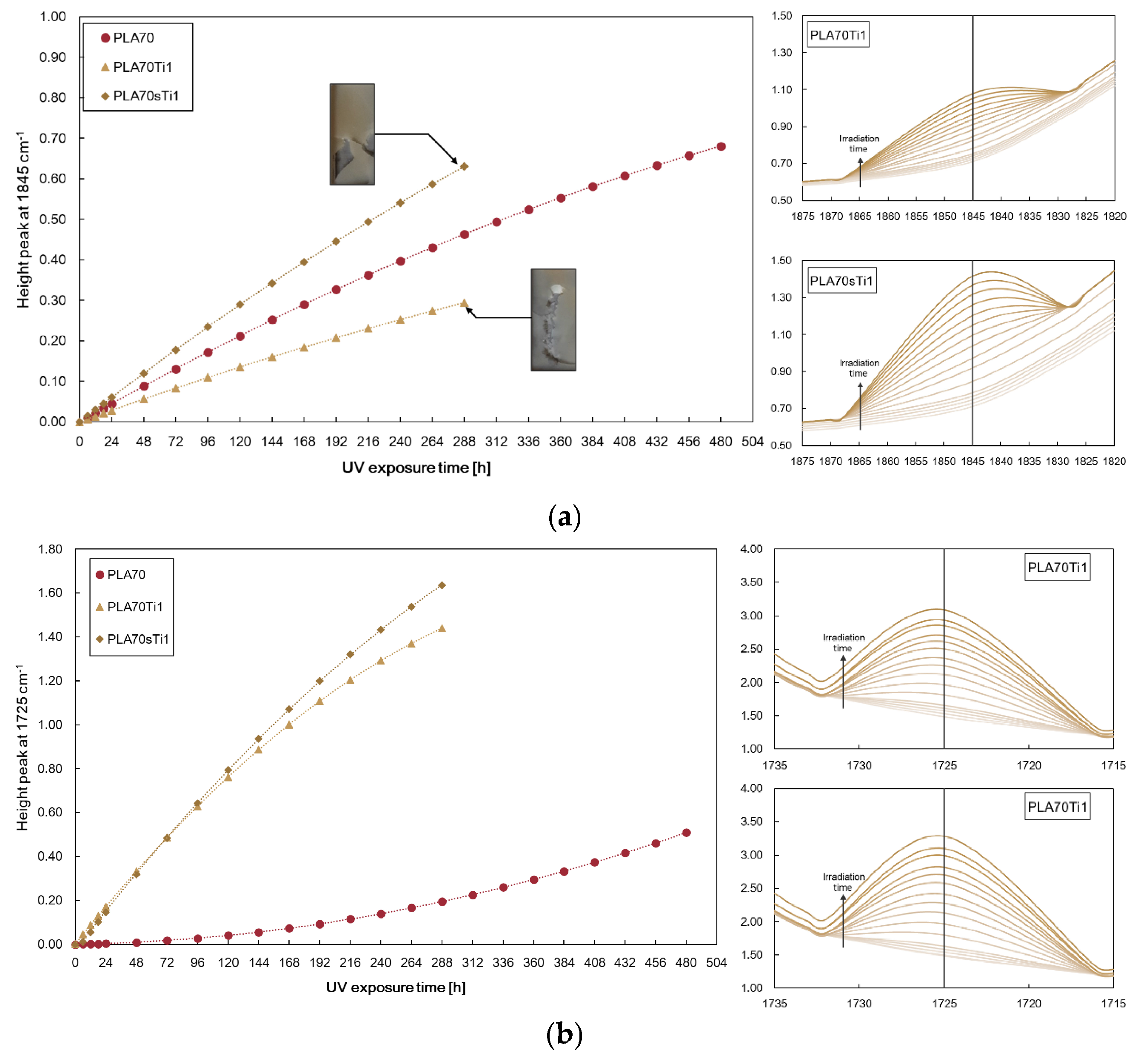
3.2. Characterisations of Biopolymer Blend Containing s-TiO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Murariu, M.; Dubois, P. PLA Composites: From Production to Properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT Bionanocomposites with Antimicrobial Natural Rosin for Green Packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Elkholy, H.M.; Hamdani, S.S.; Alghaysh, M.O.; Wyman, I.; Duncan, E.; Wang, Y.; Li, K.; Rabnawaz, M. Design of Carboxylic Acid-Functionalized Poly(Butylene Adipate-Co-Terephthalate) for Recyclable and Biodegradable Zero-Waste Paper Packaging. Adv. Sustain. Syst. 2024, 9, 2400621. [Google Scholar] [CrossRef]
- Kozłowska, A.; Gorący, K.; El Fray, M. Biodegradable Polymer Composites Based on Poly(Butylene Succinate) Copolyesters and Wood Flour. Polymers 2025, 17, 883. [Google Scholar] [CrossRef] [PubMed]
- Infurna, G.; Botta, L.; Ingargiola, I.; Maniscalco, M.; Caputo, G.; Dintcheva, N.T. Biochar from Digestate Pyrolysis as a Filler for Biopolymer Blends: Effect of Blend Composition. J. Polym. Environ. 2023, 32, 1921–1936. [Google Scholar] [CrossRef]
- Surendren, A.; Mohanty, A.K.; Liu, Q.; Misra, M. A Review of Biodegradable Thermoplastic Starches, Their Blends and Composites: Recent Developments and Opportunities for Single-Use Plastic Packaging Alternatives. Green Chem. 2022, 24, 8606–8636. [Google Scholar] [CrossRef]
- Averous, L.; Boquillon, N. Biocomposites Based on Plasticized Starch: Thermal and Mechanical Behaviours. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Masmoudi, F.; Bessadok, A.; Dammak, M.; Jaziri, M.; Ammar, E. Biodegradable Packaging Materials Conception Based on Starch and Polylactic Acid (PLA) Reinforced with Cellulose. Environ. Sci. Pollut. Res. 2016, 23, 20904–20914. [Google Scholar] [CrossRef]
- Nuzzo, A.; Bilotti, E.; Peijs, T.; Acierno, D.; Filippone, G. Nanoparticle-Induced Co-Continuity in Immiscible Polymer Blends—A Comparative Study on Bio-Based PLA-PA11 Blends Filled with Organoclay, Sepiolite, and Carbon Nanotubes. Polymer 2014, 55, 4908–4919. [Google Scholar] [CrossRef]
- Rasselet, D.; Caro-Bretelle, A.-S.; Taguet, A.; Lopez-Cuesta, J.-M. Reactive Compatibilization of PLA/PA11 Blends and Their Application in Additive Manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Zhang, Z.; Peng, S.; Chen, H.; Zhao, X. High-Performance Fully Bio-Based Poly(Lactic Acid)/ Polyamide11 (PLA/PA11) Blends by Reactive Blending with Multi-Functionalized Epoxy. Polym. Test. 2019, 78, 105980. [Google Scholar] [CrossRef]
- Puglisi, R.; Scamporrino, A.A.; Dintcheva, N.T.; Filippone, G.; Bruno, E.; Scarfato, P.; Cerruti, P.; Carroccio, S.C. Photo- and Water-Degradation Phenomena of ZnO Bio-Blend Based on Poly(Lactic Acid) and Polyamide 11. Polymers 2023, 15, 1434. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.; Coiai, S.; Carroccio, S.C.; Dintcheva, N.T.; Gambarotti, C.; Filippone, G. Heat-Resistant Fully Bio-Based Nanocomposite Blends Based on Poly(Lactic Acid). Macromol. Mater. Eng. 2014, 299, 31–40. [Google Scholar] [CrossRef]
- Morici, E.; Pecoraro, G.; Carroccio, S.C.; Bruno, E.; Scarfato, P.; Filippone, G.; Dintcheva, N.T. Understanding the Effects of Adding Metal Oxides to Polylactic Acid and Polylactic Acid Blends on Mechanical and Rheological Behaviour, Wettability, and Photo-Oxidation Resistance. Polymers 2024, 16, 922. [Google Scholar] [CrossRef] [PubMed]
- Infurna, G.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Dintcheva, N.T. Effect of Different Processing Techniques and Presence of Antioxidant on the Chitosan Film Performance. Vinyl Addit. Technol. 2022, 28, 343–351. [Google Scholar] [CrossRef]
- Infurna, G.; Caruso, G.; Dintcheva, N.T. Sustainable Materials Containing Biochar Particles: A Review. Polymers 2023, 15, 343. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable Compatibilized Polymer Blends for Packaging Applications: A Literature Review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Cho, H.S.; Moon, H.S.; Kim, M.; Nam, K.; Kim, J.Y. Biodegradability and Biodegradation Rate of Poly(Caprolactone)-Starch Blend and Poly(Butylene Succinate) Biodegradable Polymer under Aerobic and Anaerobic Environment. Waste Manag. 2011, 31, 475–480. [Google Scholar] [CrossRef]
- Filippi, S.; Dintcheva, N.T.; Scaffaro, R.; La Mantia, F.P.; Polacco, G.; Magagnini, P. Effects of Organoclay on Morphology and Properties of Nanocomposites Based on LDPE/PA-6 Blends without and with SEBS-g-MA Compatibilizer. Polym. Eng. Sci. 2009, 49, 1187–1197. [Google Scholar] [CrossRef]
- Arrigo, R.; Dintcheva, N.T.; Guenzi, M.; Gambarotti, C.; Filippone, G.; Coiai, S.; Carroccio, S. Thermo-Oxidative Resistant Nanocomposites Containing Novel Hybrid-Nanoparticles Based on Natural Polyphenol and Carbon Nanotubes. Polym. Degrad. Stab. 2015, 115, 129–137. [Google Scholar] [CrossRef]
- Trabelsi, A.; Mostafa, A.; Alkallas, F.; Elsharkawy, W.; Al-Ahmadi, A.; Ahmed, H.; Nafee, S.; Pashameah, R.; Mwafy, E. Effect of CuO Nanoparticles on the Optical, Structural, and Electrical Properties in the PMMA/PVDF Nanocomposite. Micromachines 2023, 14, 1195. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO Nanoparticles Stabilized with Gelatin for Potential Use in Food Packaging Applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eddine, L.S.; Souhaila, M.; Hasan, G.G.; Kir, I.; Abdullah, J.A.A. Green Synthesis of SnO2 Nanoparticles from Laurus nobilis L. Extract for Enhanced Gelatin-Based Films and CEF@SnO2 for Efficient Antibacterial Activity. Food Bioprocess Technol. 2024, 17, 1364–1382. [Google Scholar] [CrossRef]
- Zagloul, H.; Dhahri, M.; Bashal, A.H.; Khaleil, M.M.; Habeeb, T.H.; Khalil, K.D. Multifunctional Ag2O/Chitosan Nanocomposites Synthesized via Sol-Gel with Enhanced Antimicrobial, and Antioxidant Properties: A Novel Food Packaging Material. Int. J. Biol. Macromol. 2024, 264, 129990. [Google Scholar] [CrossRef]
- Amini, M.; Ashrafi, M. Photocatalytic Degradation of Some Organic Dyes under Solar Light Irradiation Using TiO2 and ZnO Nanoparticles. Nanochem. Res. 2016, 1, 79–86. [Google Scholar]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S.; Pawar, S.H. Developments in Photocatalytic Antibacterial Activity of Nano TiO2: A Review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/Metal Oxide Nanocomposites for Bactericidal Effect: A Review. Chemosphere 2021, 272, 128607. [Google Scholar] [CrossRef]
- Xie, W.; Li, J.; Sun, F.; Dong, W. Ultrasonication Favors TiO2 Nano-Particles Dispersion in PVDF Ultrafiltration Membrane to Effectively Enhance Membrane Hydrophilicity and Anti-Fouling Capability. Env. Sci. Pollut Res. 2020, 27, 9503–9519. [Google Scholar] [CrossRef]
- Bekat, T.; Öner, M. Effects of Surface Modification and Ultrasonic Agitation on the Properties of PHBV/ZnO Nanocomposites. Pure Appl. Chem. 2016, 88, 1027–1035. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2018. [CrossRef]
- Narwal, M.; Jeevanandam, P. Surface Modification of ZnO Nanorods Using Stearic Acid and Their Self-cleaning and Photocatalytic Applications. ChemistrySelect 2023, 8, e202301835. [Google Scholar] [CrossRef]
- Moreira, A.P.D.; Souza, B.S.; Teixeira, A.M.R.F. Monitoring of Calcium Stearate Formation by Thermogravimetry. J. Therm. Anal. Calorim. 2009, 97, 647–652. [Google Scholar] [CrossRef]
- Morici, E.; Infurna, G.; Dintcheva, N.T. Ecofriendly Biopolymer-Based Nanocomposite Films with Improved Photo-Oxidative Resistance. Materials 2022, 15, 5778. [Google Scholar] [CrossRef]
- Motzkus, C.; Macé, T.; Vaslin-Reimann, S.; Ausset, P.; Maillé, M. Characterization of Manufactured TiO2 Nanoparticles. J. Phys. Conf. Ser. 2013, 429, 012012. [Google Scholar] [CrossRef]

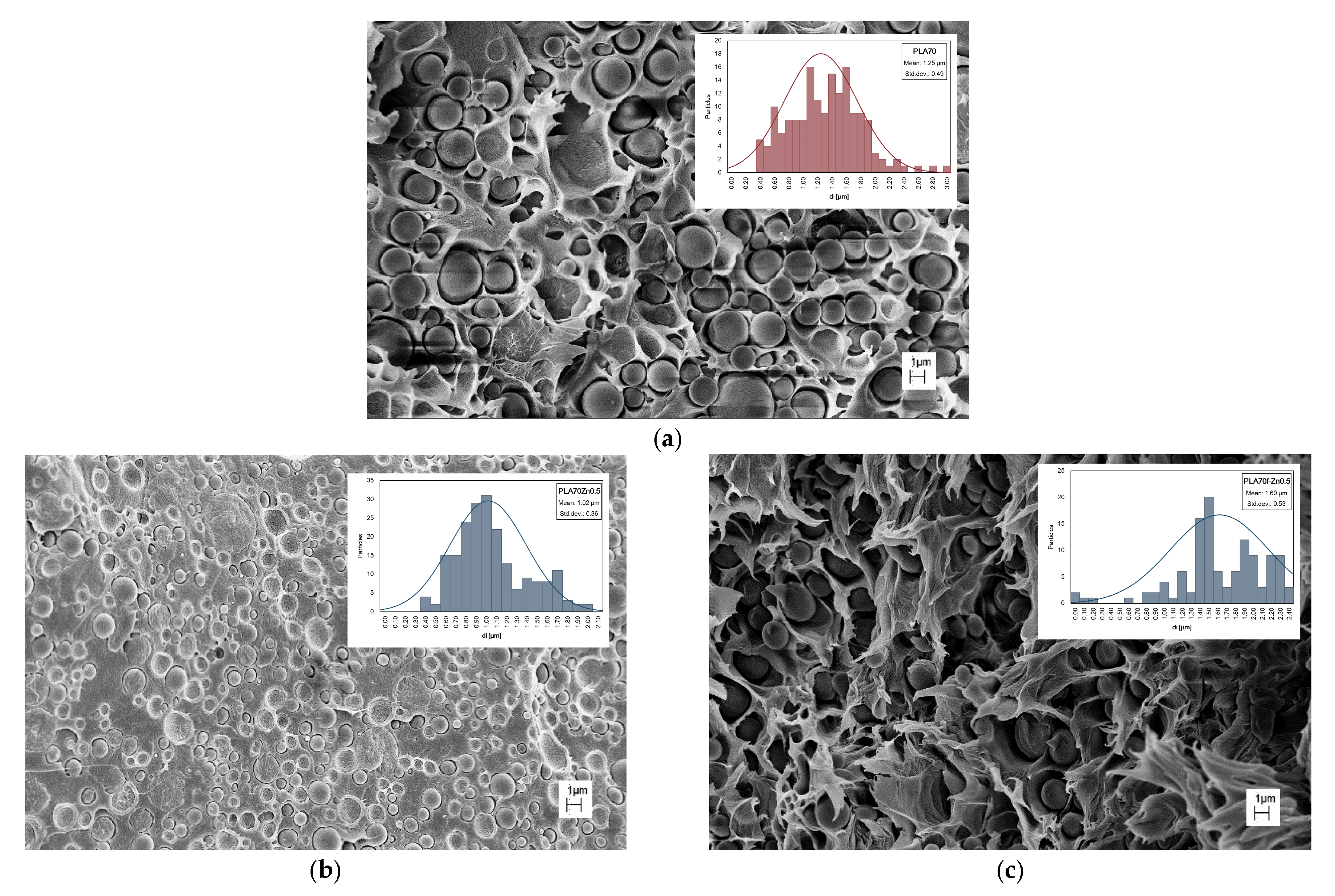



| Samples | Σni | ||
|---|---|---|---|
| PLA70 | 166 | 1.35 ± 0.11 | 1.41 |
| PLA70 Zn0.5 | 204 | 1.02 ± 0.08 | 1.36 |
| PLA70 f-Zn0.5 | 118 | 1.69 ± 0.14 | 1.13 |
| Samples | Tg [°C] | Tc1 [°C] | Tc2 [°C] | Tm1 [°C] | Tm2 [°C] | Xc1 [%] | Xc2 [%] |
|---|---|---|---|---|---|---|---|
| PLA | 62.23 | 96.18 | - | 174.54 | - | 14.59 | - |
| PA11 | - | - | 146.27 | 190.10 | - | - | |
| PLA70 | 61.78 | 97.04 | 147.16 | 167.76 | 183.29 | 8.92 | 2.71 |
| PLA70 Zn0.5 | 61.73 | 97.66 | 147.23 | 174.11 | 189.38 | 10.90 | 3.56 |
| PLA70 f-Zn0.5 | 61.51 | 97.98 | 148.62 | 173.67 | 188.82 | 8.68 | 2.27 |
| Samples | Σni | ||
|---|---|---|---|
| PLA70 | 166 | 1.35 ± 0.11 | 1.41 |
| PLA70TiO2-1 | 132 | 1.57 ± 0.13 | 1.50 |
| PLA70 s-TiO2-1 | 138 | 1.67 ± 0.18 | 1.55 |
| Samples | Tg [°C] | Tc,1 [°C] | Tc,2 [°C] | Tm,1 [°C] | Tm,2 [°C] | Xc,1 [%] | Xc,2 [%] |
|---|---|---|---|---|---|---|---|
| PLA70 | 61.78 | 97.04 | 147.16 | 167.76 | 183.29 | 8.92 | 2.71 |
| PLA70 TiO2-1 | 61.79 | 96.3 | 152.46 | 173.49 | 188.33 | 10.43 | 1.01 |
| PLA70 s-TiO2-1 | 61.29 | 92.33 | 150.64 | 173.38 | 188.36 | 9.01 | 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infurna, G.; Scamporrino, A.A.; Morici, E.; Bruno, E.; Pecoraro, G.; Dintcheva, N.T. Performance and Durability of Biopolymer Blends Containing Modified Metal Oxide Particles. Polymers 2025, 17, 3000. https://doi.org/10.3390/polym17223000
Infurna G, Scamporrino AA, Morici E, Bruno E, Pecoraro G, Dintcheva NT. Performance and Durability of Biopolymer Blends Containing Modified Metal Oxide Particles. Polymers. 2025; 17(22):3000. https://doi.org/10.3390/polym17223000
Chicago/Turabian StyleInfurna, Giulia, Andrea Antonino Scamporrino, Elisabetta Morici, Elena Bruno, Giuseppe Pecoraro, and Nadka Tz. Dintcheva. 2025. "Performance and Durability of Biopolymer Blends Containing Modified Metal Oxide Particles" Polymers 17, no. 22: 3000. https://doi.org/10.3390/polym17223000
APA StyleInfurna, G., Scamporrino, A. A., Morici, E., Bruno, E., Pecoraro, G., & Dintcheva, N. T. (2025). Performance and Durability of Biopolymer Blends Containing Modified Metal Oxide Particles. Polymers, 17(22), 3000. https://doi.org/10.3390/polym17223000










