Preparation and Performance Study of Silicone-Oligomer Composite-Modified Polyurethane Sealant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
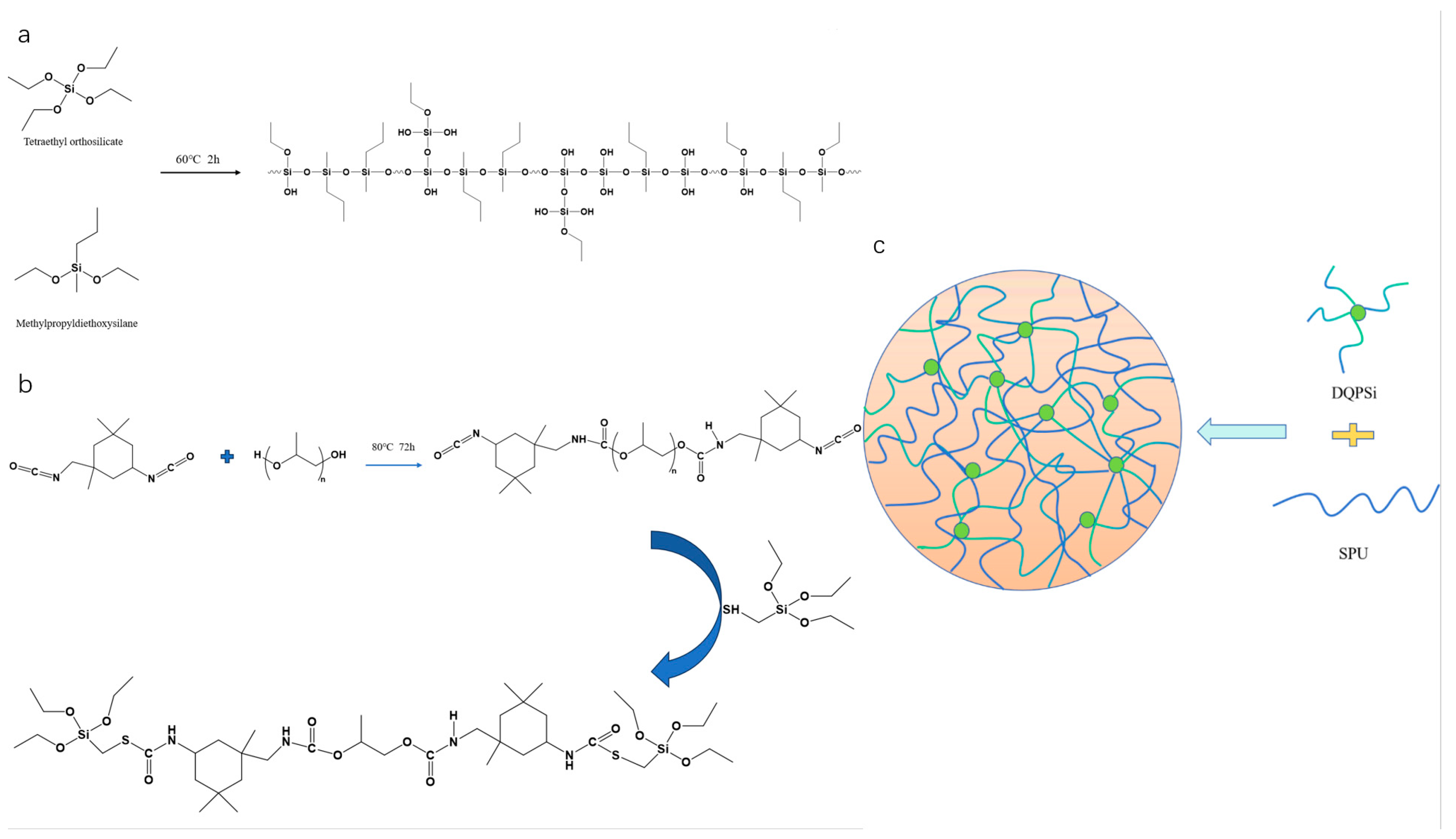
2.2. Synthesis of DQPSi Silicone Oligomer
2.3. Synthesis of Silane-Modified Polyurethane Prepolymer
2.4. Preparation and Optimization of Silane-Modified Polyurethane Sealant
2.5. Characterization
3. Results
3.1. Structural Characterization of DQPSi and SPU Prepolymer
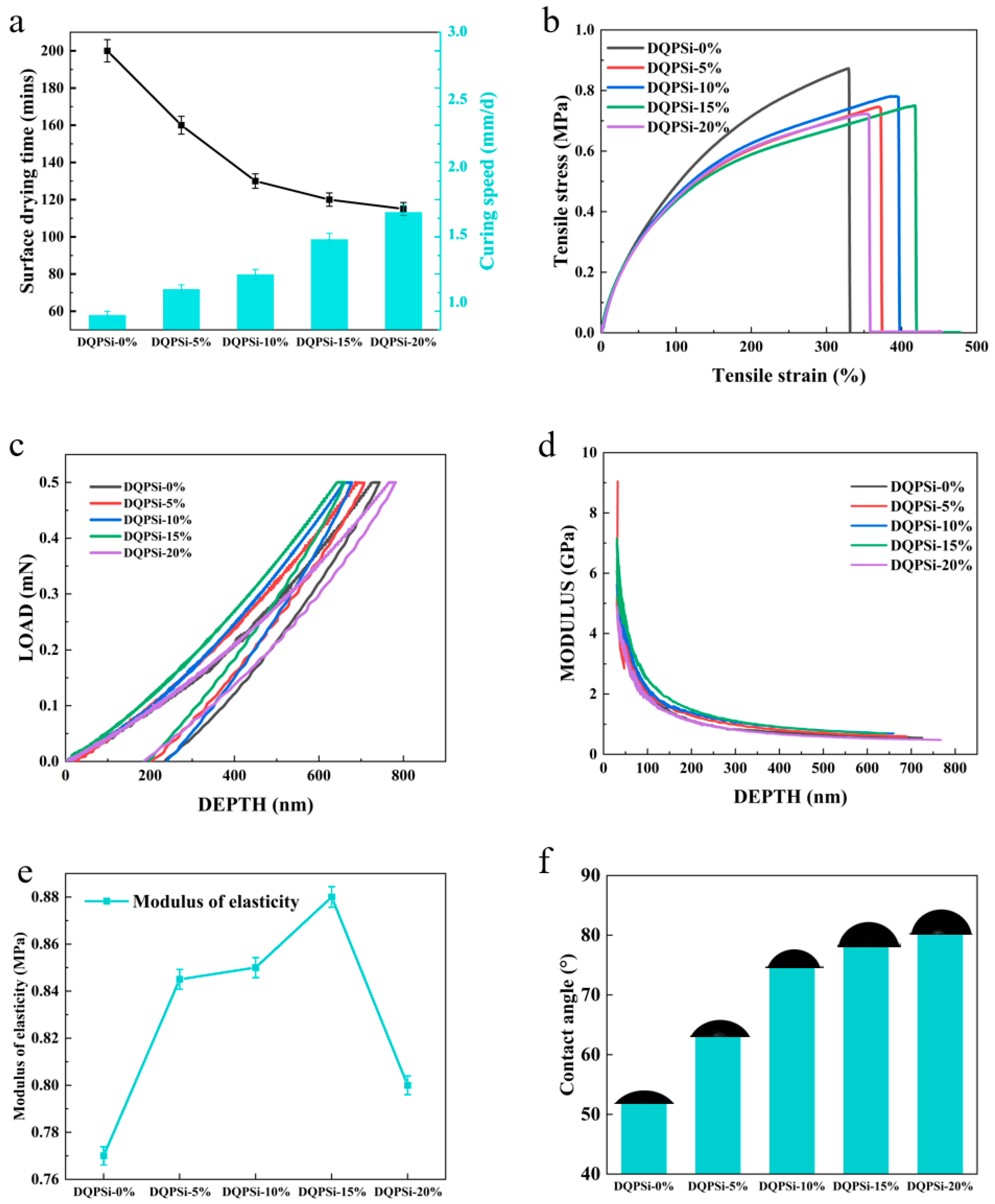
3.2. Effect of DQPSi on the Curing Behavior of Sealant
3.3. Effect of DQPSi on Mechanical Properties of Sealant
3.4. Effect of DQPSi on Hydrophobicity of Sealant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, K.A.; Yan, X.L.; Ai, T.; Niu, Y.H.; Jiang, S.Q. Study on properties and application of chloroprene rubber/polyurethane modified asphalt sealant. Constr. Build. Mater. 2023, 406, 133177. [Google Scholar] [CrossRef]
- Cassagnau, P.; Bounor-Legaré, V.; Fenouillot, F. Reactive processing of thermoplastic polymers: A review of the fundamental aspects. Int. Polym. Process. 2007, 22, 218–258. [Google Scholar] [CrossRef]
- Shi, S.; Ma, T.; Gu, L.H.; Zhang, Y.N. Thermal Behaviors, Interfacial Microstructure and Molecular Orientation of Shape Memory Polyurethane/SiO2 Based Sealant for Concrete Pavement. Polymers 2022, 14, 3336. [Google Scholar] [CrossRef]
- Chew, M.Y.L.; Zhou, X. Enhanced resistance of polyurethane sealants against cohesive failure under prolonged combination of water and heat. Polym. Test. 2002, 21, 187–193. [Google Scholar] [CrossRef]
- Valastro, S.; Calogero, G.; Smecca, E.; Arena, V.; Mannino, G.; Bongiorno, C.; Deretzis, I.; Fisicaro, G.; La Magna, A.; Galliano, S.; et al. Polyurethane-Encapsulated Mesoporous Carbon-Based Perovskite Solar Cells Resilient to Extreme Humidity and Mitigation of the Related Reversible J-V Bump. ACS Appl. Energy Mater. 2024, 7, 12069–12083. [Google Scholar] [CrossRef]
- Chew, M.Y.; Der Yi, L. Elastic recovery of sealants. Build. Environ. 1997, 32, 187–193. [Google Scholar] [CrossRef]
- Chew, M.Y.L. The effects of some chemical components of polyurethane sealants on their resistance against hot water. Build. Environ. 2003, 38, 1381–1384. [Google Scholar] [CrossRef]
- Ding, S.H.; Liu, D.Z. Durability evaluation of building sealants by accelerated weathering and thermal analysis. Constr. Build. Mater. 2006, 20, 878–881. [Google Scholar] [CrossRef]
- Shaafaey, M.; Bahrololoumi, A.; Mohammadi, H.; Alazhary, S.; Dargazany, R. Investigation of hygrothermal aging on the polyurethane-based (PUB) adhesive: Substantiating competition scenario between sub-aging thermo-oxidation and hydrolytic phenomena. J. Polym. Res. 2021, 28, 453. [Google Scholar] [CrossRef]
- Senichev, A.; Borisova, T.Y. One-component polyetherurethane sealing glues cured by air moisture. Polym. Sci. Ser. D 2016, 9, 273–275. [Google Scholar] [CrossRef]
- Hu, K.; Yuan, Y.; Yan, P.Y.; Zhou, C.L.; Lei, J.X. Green synthesis process and properties of polyurethane completely using ethanol as solvent. J. Polym. Res. 2017, 24, 80. [Google Scholar] [CrossRef]
- Kurapati, R.; Natarajan, U. Role of Chemical Linkage in Solvation of Polyurethanes in Organic Solvents Studied by Explicit Molecular Dynamics Simulations. Ind. Eng. Chem. Res. 2022, 61, 16883–16894. [Google Scholar] [CrossRef]
- Wu, J.H.; Wu, X.B.; Mu, C.D.; Wang, C.H.; Lin, W. Pickering emulsion approach: A novel strategy to fabricate waterborne polyurethane with enhanced abradability and water-resistance comparable to that of solvent-based coating. Prog. Org. Coat. 2024, 188, 108174. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Li, H.Q.; Li, J.; Yang, J.X.; Chen, Z.H. Preparation and characterization of solvent-free anti-corrosion polyurethane-urea coatings. Surf. Interfaces 2023, 36, 102504. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, H.R.; Han, L.; Zhou, H.B.; Chen, L.H.; Han, Q.Z.; Zong, C.Z. Preparation and properties of solvent-free and low temperature resistant polyurethane adhesive. React. Funct. Polym. 2025, 210, 106196. [Google Scholar] [CrossRef]
- Li, X.Q.; Mou, W.J.; Li, Y.R.; Zhou, C.L. TPU/EPDM-g-MAH/EPDM blends for elastomer sealing materials in hydrogen infrastructure: Enhanced hydrogen barrier and reduced hydrogen-induced damage. Int. J. Hydrogen Energy 2024, 87, 793–803. [Google Scholar] [CrossRef]
- Diao, M.Y.; Wang, D.F.; Wu, H.; Liu, L.; Lipponen, J.; Yao, J.M. Mechanically robust, waterproof, fast curing lignin-based waterborne polyurethane with hierarchical hydrogen bonding network. Ind. Crops Prod. 2024, 218, 119028. [Google Scholar] [CrossRef]
- He, J.M.; Li, M.; Li, D.X.; Bao, B.T.; Xue, M.J.; Huang, Y.Y.; Xu, Y.T.; Chen, G.R.; Dai, L.Z. Fabrication of azobenzene non-covalent bonding grafting graphene composite and its application in weathering and corrosion resistant polyurethane coating. Polym. Degrad. Stab. 2022, 206, 110157. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhang, L.Q.; Tan, T.W.; Fong, H. Nonisocyanate Biobased Poly(ester urethanes) with Tunable Properties Synthesized via an Environment-Friendly Route. ACS Sustain. Chem. Eng. 2016, 4, 2762–2770. [Google Scholar] [CrossRef]
- Petlin, I.; Sozonov, R.; Khakimullin, Y.N.; Kurkin, A. The effect of the molecular weight of polyester on the properties of silane terminated polyurethane sealants. Polym. Sci. Ser. D 2016, 9, 49–52. [Google Scholar] [CrossRef]
- Ren, C.L.; Niu, P.X.; Zhang, S.H.; Han, X.N.; Guo, Z.Q.; Zhang, Y.J.; Zhang, S.S.; Liu, X.J.; Wei, L.H. Silane-Modified Hyperbranched Polyurethane with High Strength, High Transparency, and Low Viscosity. ACS Appl. Polym. Mater. 2024, 6, 4060–4069. [Google Scholar] [CrossRef]
- Zhang, S.C.; Sun, Y.; Xu, J.T. (3-Mercaptopropyl)triethoxysilane-Modified Reduced Graphene Oxide-Modified Polyurethane Yarn Enhanced by Epoxy/Thiol Reactions for Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 34865–34876. [Google Scholar] [CrossRef]
- Wu, G.F.; Song, X.; Yang, Z.H.; Li, Y.C.; Zhang, H.X. Biodegradability of renewable waterborne polyurethane modified with vinyl-grafted gelatin by UV curing. Polym. Bull. 2022, 79, 9717–9740. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Shangguan, H.M.; Liu, Q.W.; Li, Y.H.; Liu, X.J.; Sun, A.L.; Li, Y.H.; Wei, L.H. Synthesis of hydroxyl silane coupling agent and its application in preparation of silane-modified polyurethane. Polym. Eng. Sci. 2023, 63, 2325–2335. [Google Scholar] [CrossRef]
- Pergal, M.V.; Dzunuzovic, J.V.; Poreba, R.; Steinhart, M.; Pergal, M.M.; Vodnik, V.V.; Spírková, M. Structure-Property Correlation Study of Novel Poly(urethane-ester-siloxane) Networks. Ind. Eng. Chem. Res. 2013, 52, 6164–6176. [Google Scholar] [CrossRef]
- Jiao, X.J.; Zhang, T.X.; Cheng, F.; Fan, Y.X.; Liu, J.L.; Lai, G.Q.; Wu, Y.F.; Yang, X.F. UV-Cured Coatings Prepared with Sulfhydryl-Terminated Branched Polyurethane and Allyl-Terminated Hyperbranched Polycarbosilane. Coatings 2020, 10, 350. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, R.H.; Song, D.Y. Preparation and performance study of carbodiimide-modified polyurethane-acrylic resin composite coatings with high transparency, strong adhesion, and excellent weathering performance. Prog. Org. Coat. 2025, 201, 109130. [Google Scholar] [CrossRef]
- Hong, Z.; Jiang, H.W.; Xue, M.S.; Ke, C.Y.; Luo, Y.D.; Yin, Z.Z.; Xie, C.; Zhang, F.; Xing, Y. SiC-enhanced polyurethane composite coatings with excellent anti-fouling, mechanical, thermal, chemical properties on various substrates. Prog. Org. Coat. 2022, 168, 106909. [Google Scholar] [CrossRef]
- Wang, L.; Han, X.N.; Wu, J.B.; Wu, L.Z.; Ren, C.L.; Hu, Y.C.; Zhang, W.Y.; Sun, A.L.; Liu, X.J. Silane coupling agent-modified environmentally friendly waterborne polyurethane with excellent mechanical properties and water resistance. Prog. Org. Coat. 2025, 208, 109499. [Google Scholar] [CrossRef]
- Liu, J.S.; Wu, S.P.; Dong, E. Effect of coupling agent as integral blend additive on silicone rubber sealant. J. Appl. Polym. Sci. 2013, 128, 2337–2343. [Google Scholar] [CrossRef]
- Comyn, J.; Day, J.; Shaw, S.J. Effect of surface treatment on the durability of aluminium joints bonded with a butadiene-acrylonitrile-epoxide sealant. Int. J. Adhes. Adhes. 2000, 20, 77–82. [Google Scholar] [CrossRef]
- Wang, H.M.; Zhang, H.; Liang, B.Q.; Tian, M.G.; Li, X.F.; Ding, T.; Liu, P.S.; Yang, Y.; Niu, L.Y.; Zhang, Z.J. Tri-functionally modified spherical silica for high-performance epoxy resin sealant. Compos. Commun. 2024, 51, 102081. [Google Scholar] [CrossRef]
- De Buyl, F.; Comyn, J.; Shephard, N.E.; Subramaniam, N.P. Kinetics of cure, cross link density and adhesion of water-reactive alkoxysilicone sealants. J. Adhes. Sci. Technol. 2002, 16, 1055–1071. [Google Scholar] [CrossRef]
- Senichev, V.Y.; Krasnosel’skikh, S.; Senichev, A. Fast-curing silanized polyetherurethane sealants. Polym. Sci. Ser. D 2018, 11, 56–59. [Google Scholar] [CrossRef]
- Jiang, H.M.; Zheng, Z.; Song, W.H.; Wang, X.L. Moisture-cured polyurethane/polysiloxane copolymers: Effects of the structure of polyester diol and NCO/OH ratio. J. Appl. Polym. Sci. 2008, 108, 3644–3651. [Google Scholar] [CrossRef]
- Yu, N.; Cheng, B.X.; Liu, Y.; Wu, W.; Li, R.K.Y.; Liang, Z.H.; Cheng, F.C.; Zhao, H. High-Strength and High-Toughness Supramolecular Materials for Self-Healing Triboelectric Nanogenerator. Small 2024, 20, e2405700. [Google Scholar] [CrossRef]
- Luo, G.; Guo, Y.K.; Yang, S.; Liu, Y.H.; Yang, C.; Miao, X.M.; Liu, S.J.; Bai, Y.D. Amphiphilic Janus Nanoparticles with Enhanced Sealing Properties as Follower Sealants. ACS Appl. Nano Mater. 2024, 7, 11704–11714. [Google Scholar] [CrossRef]
- Zhou, A.; Li, K.X.; Li, X.H.; Cui, W.; Que, Z.C.; Wang, X.; Wang, W.B.; Liu, T.J.; Zou, D.J.; Peng, X. Improving mechanical properties and durability of polyether sealant in prefabricated buildings with titanium dioxide and graphene. Constr. Build. Mater. 2023, 408, 133657. [Google Scholar] [CrossRef]
- Anoop, V.; Subramani, S.; Jaisankar, S.; Sohini, C.; Mary, N. Mechanical, dielectric, and thermal properties of polydimethylsiloxane/polysilsesquioxane nanocomposite for sealant application. J. Appl. Polym. Sci. 2019, 136, 47228. [Google Scholar] [CrossRef]
- Huang, L.Q.; Huang, H.X.; Yu, N.; Chen, C.Z.; Liu, Y.; Hu, G.H.; Du, J.; Zhao, H. High-Strength and Excellent Self-Healing Polyurethane Elastomer Based on Rigid Chain Segment Reinforcement. Macromolecules 2025, 58, 1425–1434. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wang, H.W.; Chiu, C.W.; Cheng, D.S.; Yen, M.Y.; Chiu, H.T. Low-temperature synthesis of transition metal nanoparticles from metal complexes and organopolysilane oligomers. Chem. Mater. 2002, 14, 4334–4338. [Google Scholar] [CrossRef]
- Pan, K.; Zeng, X.; Li, H.; Lai, X. Synthesis of silane oligomers containing vinyl and epoxy group for improving the adhesion of addition-cure silicone encapsulant. J. Adhes. Sci. Technol. 2016, 30, 1131–1142. [Google Scholar] [CrossRef]
- GB/T 528-2009; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. National Standardization Administration: Beijing, China, 2009.
- GB /T 13477.5-2002; Test Method for Building Sealants—Part 5: Determination for Tack-Free Time. National Standardization Administration: Beijing, China, 2002.
- GB/T 32369-2015; Determination of the Degree of Cure for Sealant. National Standardization Administration: Beijing, China, 2015.
- GB/T 13477.8-2017; Test Method for Building Sealants—Part 8: Determination of Tensile Properties. National Standardization Administration: Beijing, China, 2017.


| Level | A: Active Calcium Carbonate (phr) | B:PPG-2000 (phr) | C: KH-171 (phr) | D: KH-792 (phr) |
|---|---|---|---|---|
| 1 | 80 | 40 | 2 | 2 |
| 2 | 90 | 50 | 3 | 3 |
| 3 | 100 | 60 | 4 | 4 |
| Level | A: Active Calcium Carbonate (Phrase) | B:PPG-2000 (phr) | C: KH-171 (phr) | D: KH-792 (phr) | Tensile Strength (MPa) | Strain at Break (%) | Drying Time (Minutes) |
|---|---|---|---|---|---|---|---|
| First/Second/Third/Standard Deviation (MPa) | First/Second/Third/Standard Deviation (%) | First/Second/Third/Standard Deviation (min) | |||||
| 1 | 80 | 40 | 2 | 2 | 0.78/0.76/0.79/0.015 | 325/319/326/3.79 | 235/241/231/5.03 |
| 2 | 80 | 50 | 3 | 3 | 0.81/0.80/0.79/0.012 | 355/349/357/4.16 | 205/211/199/6.00 |
| 3 | 80 | 60 | 4 | 4 | 0.75/0.77/0.76/0.013 | 370/359/374/7.77 | 185/201/189/8.33 |
| 4 | 90 | 40 | 3 | 4 | 0.86/0.84/0.87/0.015 | 345/337/353/8.0 | 220/233/221/7.23 |
| 5 | 90 | 50 | 4 | 2 | 0.83/0.81/0.84/0.017 | 395/393/386/4.73 | 195/207/193/7.57 |
| 6 | 90 | 60 | 2 | 3 | 0.79/0.77/0.80/0.015 | 360/371/364/5.57 | 210/212/215/2.52 |
| 7 | 100 | 40 | 4 | 3 | 0.73/0/71/0.74/0.012 | 310/317/305/6.03 | 245/237/239/4.16 |
| 8 | 100 | 50 | 2 | 4 | 0.76/0.75/0.77/0.012 | 335/329/331/3.06 | 230/217/224/6.51 |
| 9 | 100 | 60 | 3 | 2 | 0.80/0.78/0.81/0/015 | 350/339/361/11.0 | 200/211/206/5.51 |
| Index | Factor | K1 | K2 | K3 | R (Range) | Order of Priority |
|---|---|---|---|---|---|---|
| Tensile strength (MPa) | A: Calcium carbonate | 0.780 | 0.827 | 0.763 | 0.064 | 1 |
| B: PPG | 0.790 | 0.800 | 0.780 | 0.020 | 4 | |
| C:KH-171 | 0.777 | 0.823 | 0.770 | 0.053 | 2 | |
| D:KH-792 | 0.803 | 0.777 | 0.790 | 0.026 | 3 |
| Index | Factor | K1 | K2 | K3 | R (Range) | Order of Priority |
|---|---|---|---|---|---|---|
| Elongation at break (%) | A: Calcium carbonate | 350.0 | 366.7 | 331.7 | 35.0 | 1 |
| B: PPG | 326.7 | 361.7 | 360.0 | 35.0 | 2 | |
| C:KH-171 | 340.0 | 350.0 | 358.3 | 18.3 | 3 | |
| D:KH-792 | 356.7 | 341.7 | 350.0 | 15.0 | 4 |
| Index | Factor | K1 | K2 | K3 | R (Range) | Order of Priority |
|---|---|---|---|---|---|---|
| Drying time (min) | A: Calcium carbonate | 208.3 | 208.3 | 225.0 | 16.7 | 2 |
| B: PPG | 233.3 | 210.0 | 198.3 | 35.0 | 1 | |
| C:KH-171 | 225.0 | 208.3 | 208.3 | 16.7 | 3 | |
| D:KH-792 | 210.0 | 220.0 | 211.7 | 10.0 | 4 |
| Factor | DQPSi-0% (phr) | DQPSi-5% (phr) | DQPSi-10% (phr) | DQPSi-15% (phr) | DQPSi-20% (phr) |
|---|---|---|---|---|---|
| Calcium carbonate (phr) | 90 | 90 | 90 | 90 | 90 |
| PPG-2000 (phr) | 50 | 50 | 50 | 50 | 50 |
| SPU (phr) | 100 | 100 | 100 | 100 | 100 |
| KH-171 (phr) | 3 | 3 | 3 | 3 | 3 |
| KH-192 (phr) | 3 | 3 | 3 | 3 | 3 |
| DQPSi (phr) | 0 | 13 | 27 | 43 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Chen, F.; Liu, Q.; Zhao, M.; Zhang, C.; Li, P.; Ma, X.; Zheng, J.; Zhang, Q. Preparation and Performance Study of Silicone-Oligomer Composite-Modified Polyurethane Sealant. Polymers 2025, 17, 2990. https://doi.org/10.3390/polym17222990
Li N, Chen F, Liu Q, Zhao M, Zhang C, Li P, Ma X, Zheng J, Zhang Q. Preparation and Performance Study of Silicone-Oligomer Composite-Modified Polyurethane Sealant. Polymers. 2025; 17(22):2990. https://doi.org/10.3390/polym17222990
Chicago/Turabian StyleLi, Ning, Feiyu Chen, Qing Liu, Ming Zhao, Cheng Zhang, Peizhe Li, Xueting Ma, Jiangye Zheng, and Qunchao Zhang. 2025. "Preparation and Performance Study of Silicone-Oligomer Composite-Modified Polyurethane Sealant" Polymers 17, no. 22: 2990. https://doi.org/10.3390/polym17222990
APA StyleLi, N., Chen, F., Liu, Q., Zhao, M., Zhang, C., Li, P., Ma, X., Zheng, J., & Zhang, Q. (2025). Preparation and Performance Study of Silicone-Oligomer Composite-Modified Polyurethane Sealant. Polymers, 17(22), 2990. https://doi.org/10.3390/polym17222990





