Innovative Antimicrobial Fabrics Loaded with Nanocomposites from Chitosan and Black Mulberry Polysaccharide-Mediated Selenium Nanoparticles to Suppress Skin Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Black mulberry (Morus nigra L.) Polysaccharide
2.3. Phytosynthesis of SeNPs with MB
2.4. Preparation and Loading of Cht
2.5. Characterization of Nanomaterials/Nanocomposites
2.5.1. FTIR (“Fourier-Transform Infrared Spectroscopy”)
2.5.2. Zeta (ζ) Potential and Particles’ Size
2.5.3. Scanning Electron Microscopy (SEM)
2.5.4. Transmission Electron Microscopy (TEM)
2.6. Antimicrobial Testing Assays
2.6.1. Qualitative Assay
2.6.2. Minimum Inhibitory Concentration (MIC) Quantitative Assay
2.6.3. SEM Imaging of Challenged Microorganisms
2.7. Antimicrobial Textile Preparation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Cht/MB-SeNP Nanocomposites
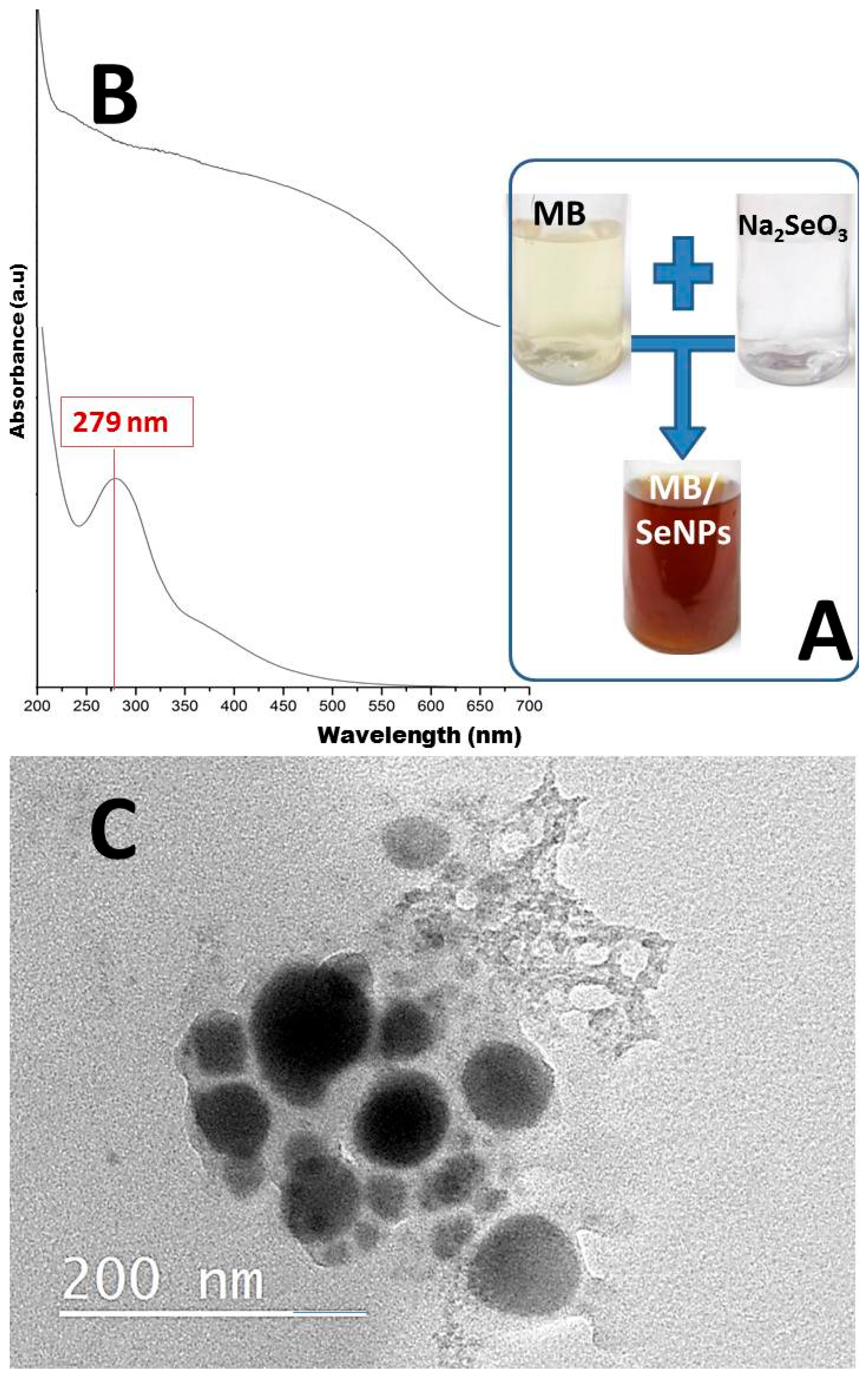
3.1.1. Optical Observation
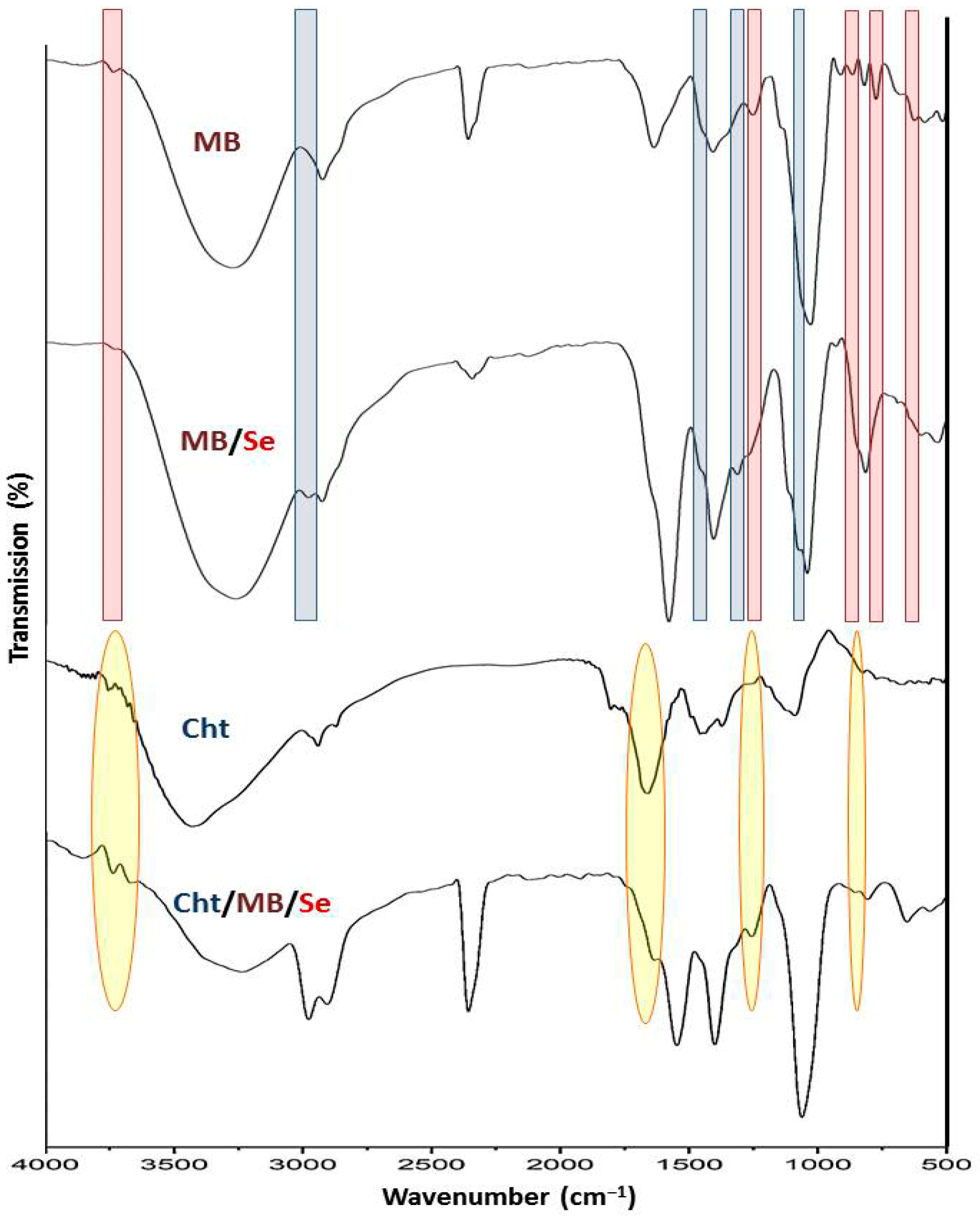
3.1.2. FTIR Analysis
3.1.3. Ps Distribution and NP Charge
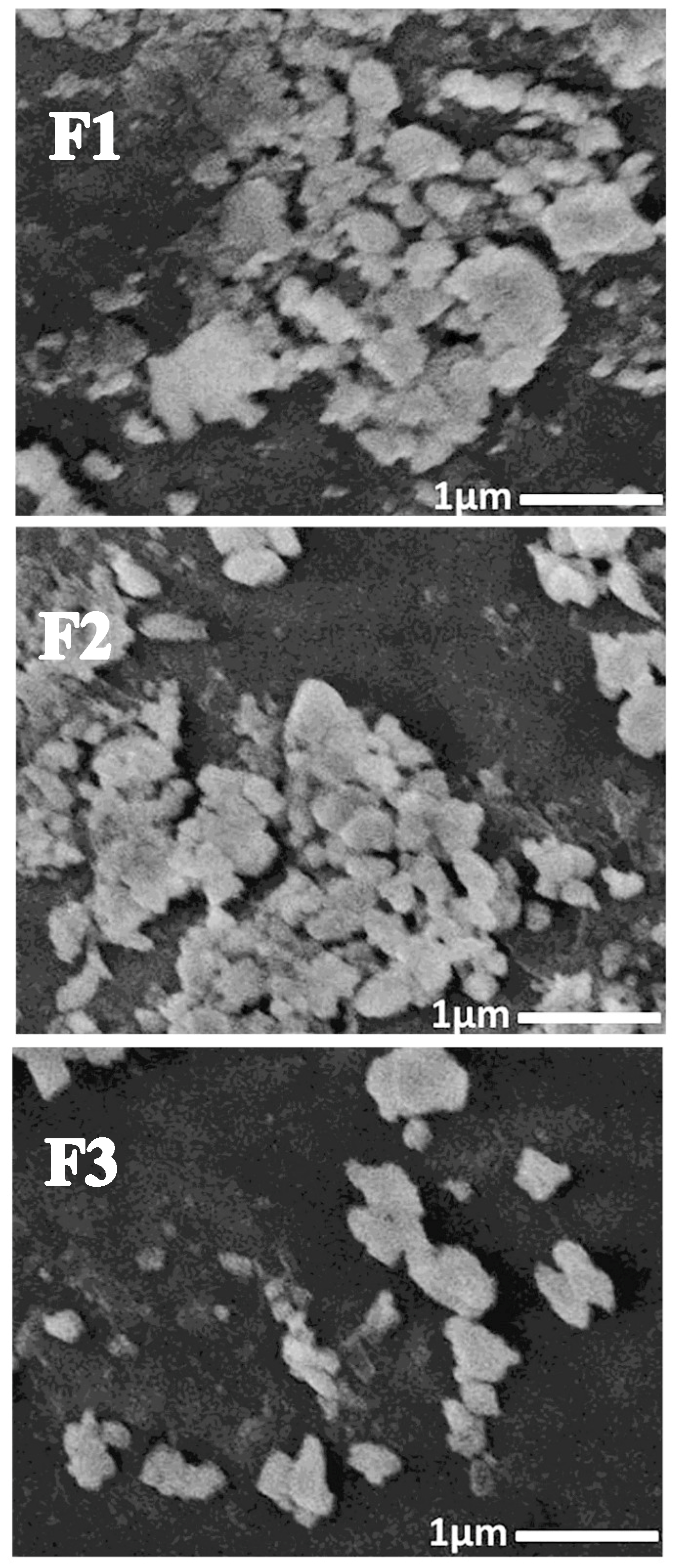
3.1.4. SEM Imaging
3.2. Antibacterial Assay
3.2.1. Qualitative and Quantitative Assays
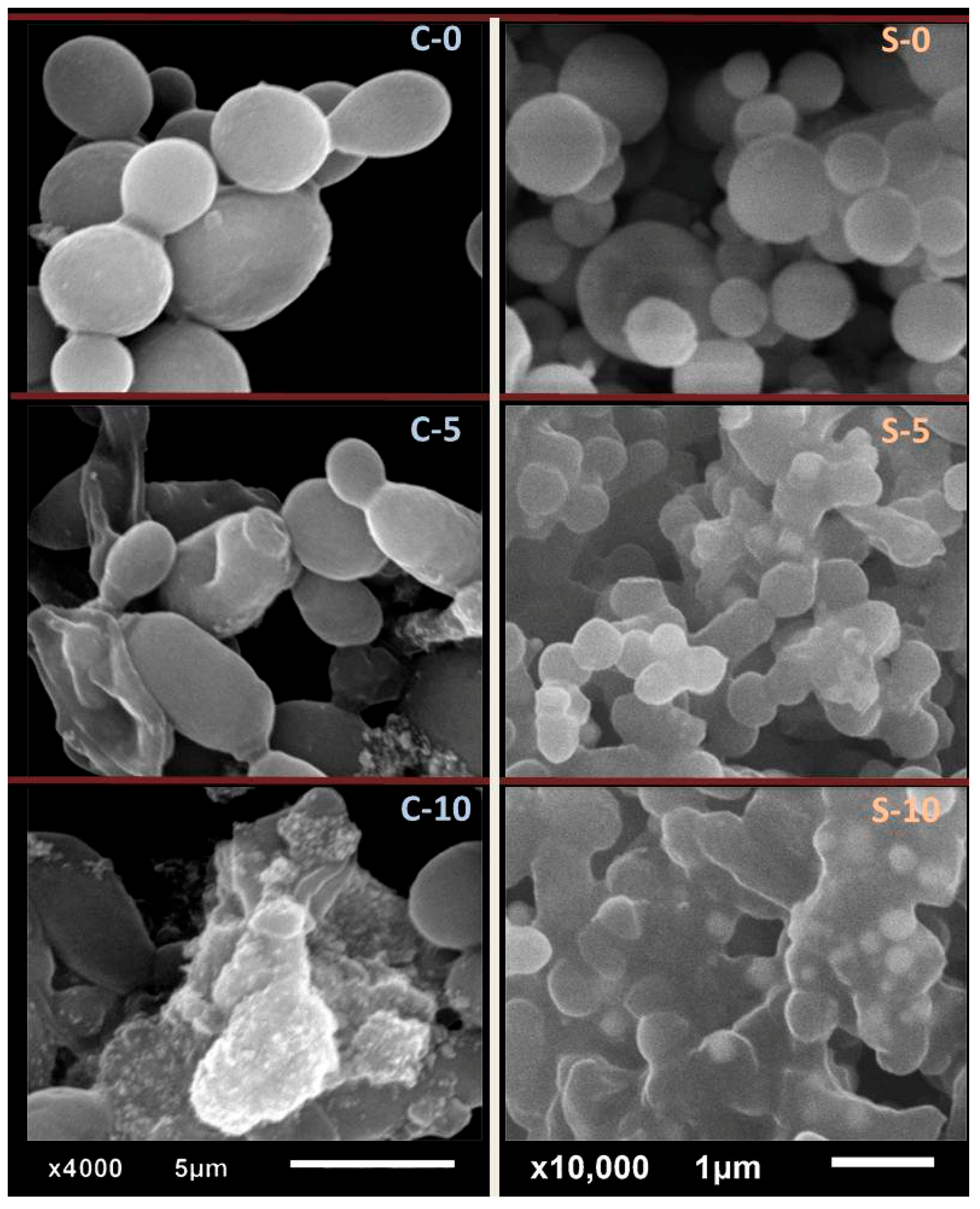
3.2.2. Antibacterial Elucidation via SEM
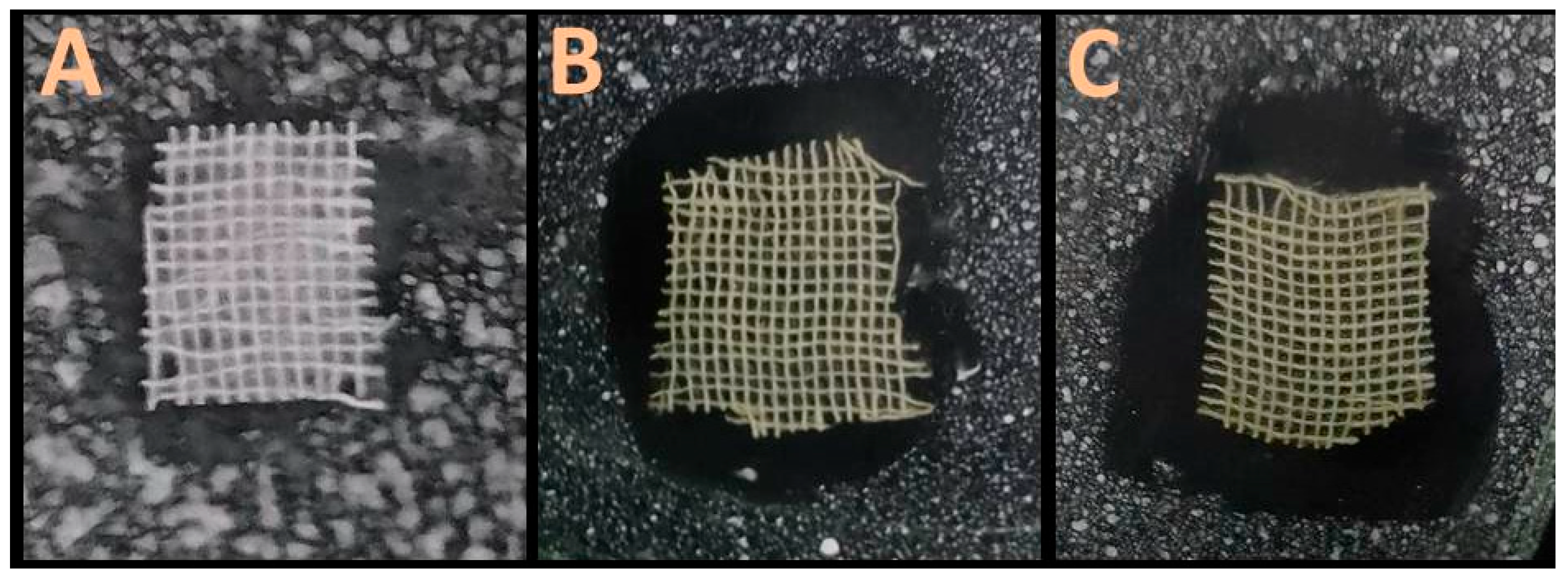
3.3. Antimicrobial Textile Preparation
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MB | Black mulberry polysaccharide |
| Cht | Chitosan |
| SeNPs | Selenium nanoparticles |
| PBS | Phosphate-buffered saline |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| DLS | Dynamic light scattering |
| FTIR | Fourier-transform infrared spectroscopy |
| ζ | Zeta potential |
| DDW | Double-distilled water |
| ZOI | Zone of inhibition |
| MIC | Minimum inhibitory concentration |
| RT | Room temperature |
References
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A. Polymeric nanoparticles for biomedical applications. Polymers 2024, 16, 249. [Google Scholar] [CrossRef]
- Osherov, A.; Prasad, R.; Chrzanowski, W.; New, E.J.; Brazaca, L.; Sadik, O.; Haynes, C.L.; Maine, E. Responsible nanotechnology for a sustainable future. One Earth 2023, 6, 763–766. [Google Scholar] [CrossRef]
- Ahire, S.A.; Bachhav, A.A.; Pawar, T.B.; Jagdale, B.S.; Patil, A.V.; Koli, P.B. The Augmentation of nanotechnology era: A concise review on fundamental concepts of nanotechnology and applications in material science and technology. Results Chem. 2022, 4, 100633. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of green synthesized metal nanoparticles—A review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef]
- Menon, S.; Agarwal, H.; Rajeshkumar, S.; Jacquline Rosy, P.; Shanmugam, V.K. Investigating the antimicrobial activities of the biosynthesized selenium nanoparticles and its statistical analysis. Bionanoscience 2020, 10, 122–135. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Selenium nanoparticles: Green synthesis and biomedical application. Molecules 2023, 28, 8125. [Google Scholar] [CrossRef]
- Rohela, G.K.; Shukla, P.; Muttanna; Kumar, R.; Chowdhury, S.R. Mulberry (Morus spp.): An ideal plant for sustainable development. Trees For. People 2020, 2, 100011. [Google Scholar] [CrossRef]
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Miere, F.; Venter, A.C.; Vicas, S.I. Phytochemical composition of different botanical parts of Morus species, health benefits and application in food industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Balart, R.; Garcia-Garcia, D.; Fombuena, V.; Quiles-Carrillo, L.; Arrieta, M.P. Biopolymers from natural resources. Polymers 2021, 13, 2532. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Li, X.; Hua, Y.; Yang, C.; Liu, S.; Tan, L.; Guo, J.; Li, Y. Polysaccharides extracted from mulberry fruits (Morus nigra L.): Antioxidant effect of ameliorating H2O2-induced liver injury in HepG2 cells. BMC Complement. Med. Ther. 2023, 23, 112. [Google Scholar] [CrossRef]
- Skender, A.; Kurtovic, M.; Becirspahic, D. Some Physicochemical Characteristics of Black and White Mulberry Genotypes from Bosnia and Herzegovina. Genetika 2019, 51, 1089–1101. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent advances in chitosan-based applications—A review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Reda Hussein, U.; Mahmoud, Z.H.; Abd Alaziz, K.M.; Alid, M.L.; Yasin, Y.; Ali, F.K.; Faisal, A.N.; Abd, A.N.; Kianfar, E. Antimicrobial finishing of textiles using nanomaterials. Braz. J. Biol. 2023, 84, e264947. [Google Scholar] [CrossRef]
- Phutane, P.; Telange, D.; Agrawal, S.; Gunde, M.; Kotkar, K.; Pethe, A. Biofunctionalization and applications of polymeric nanofibers in tissue engineering and regenerative medicine. Polymers 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Puertas, R.; Álvarez-Martínez, F.J.; Falco, A.; Barrajón-Catalán, E.; Mallavia, R. Phytochemical-based nanomaterials against antibiotic-resistant bacteria: An updated review. Polymers 2023, 15, 1392. [Google Scholar] [CrossRef]
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and metal nanoparticles for functional textiles: A review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef]
- Awlqadr, F.H.; Majeed, K.R.; Altemimi, A.B.; Hassan, A.M.; Qadir, S.A.; Saeed, M.N.; Faraj, A.M.; Salih, T.H.; Al-Manhel, A.J.A.; Najm, M.A.A.; et al. Nanotechnology-based herbal medicine: Preparation, synthesis, and applications in food and medicine. J. Agric. Food Res. 2025, 19, 101661. [Google Scholar] [CrossRef]
- Wang, H.; Huang, G. Extraction, purification, structural modification, activities and application of polysaccharides from different parts of mulberry. Food Funct. 2024, 15, 3939–3958. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, T.; Hu, X.; Ren, G.; Zhang, H.; Wang, Z.; Teng, Z.; Wu, R.; Wu, J. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (Murus alba L.) based on different extraction techniques with superfine grinding pretreatment. Int. J. Biol. Macromol. 2021, 183, 1774–1783. [Google Scholar] [CrossRef]
- Tayel, A.A.; Ebaid, A.M.; Otian, A.M.; Mahrous, H.; El Rabey, H.A.; Salem, M.F. Application of edible nanocomposites from chitosan/fenugreek seed mucilage/selenium nanoparticles for protecting lemon from green mold. Int. J. Biol. Macromol. 2024, 273, 133109. [Google Scholar] [CrossRef]
- Tayel, A.A.; Ghanem, R.A.; Moussa, S.H.; Fahmi, M.; Tarjam, H.M.; Ismail, N. Skin protectant textiles loaded with fish collagen, chitosan and oak galls extract composite. Int. J. Biol. Macromol. 2018, 117, 25–29. [Google Scholar] [CrossRef]
- Meshref, E.M.; Omar, A.A.E.; Moussa, S.H.; Alabdalall, A.H.; Al-Saggaf, M.S.; Alalawy, A.I.; Almutairi, F.M.; Gad, H.A.; Tayel, A.A. Antimicrobial Nanocomposites from Chitosan and Squash Synthesized Nano--Selenium Eradicate Skin Pathogens. ChemistrySelect 2024, 9, e202400881. [Google Scholar] [CrossRef]
- El-Sherbiny, M.M.; Orif, M.I.; El-Hefnawy, M.E.; Alhayyani, S.; Al-Goul, S.T.; Elekhtiar, R.S.; Mahrous, H.; Tayel, A.A. Fabrication of bioactive nanocomposites from chitosan, cress mucilage, and selenium nanoparticles with powerful antibacterial and anticancerous actions. Front. Microbiol. 2023, 14, 1210780. [Google Scholar] [CrossRef]
- Gad, H.A.; Tayel, A.A.; Al-Saggaf, M.S.; Moussa, S.H.; Diab, A.M. Phyto-fabrication of selenium nanorods using extract of pomegranate rind wastes and their potentialities for inhibiting fish-borne pathogens. Green Process. Synth. 2021, 10, 529–537. [Google Scholar] [CrossRef]
- AATCC-TM100; Test Method for Test Method for Antibacterial Finishes on Textile Material. American Association of Textile Chemists and Colorists: Durham, NC, USA, 2019.
- D’urso, G.; Mes, J.J.; Montoro, P.; Hall, R.D.; de Vos, R.C. Identification of bioactive phytochemicals in mulberries. Metabolites 2019, 10, 7. [Google Scholar] [CrossRef]
- Wani, M.Y.; Ganie, N.A.; Wani, D.M.; Wani, A.W.; Dar, S.Q.; Khan, A.H.; Khan, N.A.; Manzar, M.S.; Dehghani, M.H. The phenolic components extracted from mulberry fruits as bioactive compounds against cancer: A review. Phytother. Res. 2023, 37, 1136–1152. [Google Scholar] [CrossRef]
- Jeon, Y.N.; Ryu, S.J.; Lee, H.Y.; Kim, J.O.; Baek, J.S. Green synthesis of silver nanoparticle using black mulberry and characterization, phytochemical, and bioactivity. Antibiotics 2024, 13, 686. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Ma, Y.; Liang, L.; Jia, F.; Wang, Y.; Yu, L.; Huang, W. Extraction of flavonoids from black mulberry wine residues and their antioxidant and anticancer activity in vitro. Heliyon 2024, 10, e31518. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Abbasi, A.M.; Fu, X.; Liu, R.H.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2016, 7, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Yang, Z.; Liu, J.; Schols, H.A.; Battino, M.; Bao, B.; Tian, L.; Bai, W. Structural characterization and in vitro fermentation characteristics of enzymatically extracted black mulberry polysaccharides. J. Agric. Food Chem. 2022, 70, 3654–3665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Mu, D.; Yang, H.; Feng, Y.; Ji, R.; Wu, R.; Wu, J. Preparation, structural characterization and bioactivities of polysaccharides from mulberry (Mori Fructus). Food Biosci. 2022, 46, 101604. [Google Scholar] [CrossRef]
- Trak, D.; Arslan, Y. Synthesis of silver nanoparticles using dried black mulberry (Morus nigra L.) fruit extract and their antibacterial and effective dye degradation activities. Inorg. Nano-Met. Chem. 2023, 53, 1124–1136. [Google Scholar] [CrossRef]
- Tayel, A.A.; Elzahy, A.F.; Moussa, S.H.; Al-Saggaf, M.S.; Diab, A.M. Biopreservation of shrimps using composed edible coatings from chitosan nanoparticles and cloves extract. J. Food Qual. 2020, 2020, 8878452. [Google Scholar] [CrossRef]
- Amer, E.T.; Tayel, A.A.; El Maksoud, A.I.A.; Alsieni, M.; Gad, H.A.; Assas, M.A.; Abdella, A.; Elebeedy, D. Antibacterial potentialities of chitosan nanoparticles loaded with salvianolic acid B and tanshinone IIA. BioNanoScience 2024, 14, 594–604. [Google Scholar] [CrossRef]
- Zheng, T.; Tang, P.; Li, G. Development of a pH-sensitive film based on collagen/chitosan/ZnO nanoparticles and mulberry extract for pork freshness monitoring. Food Chem. 2023, 402, 134428. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; El-Sersy, Z.R.; Tayel, A.A.; Alsieni, M.A.; Abd El Maksoud, A.I. Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan. Green Process. Synth. 2021, 10, 811–823. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A. Corallina mediterranea Extract-Mediated Selenium Nanoparticles as Effectual Antibacterial Agent Against Food-Borne Pathogens. Egypt. J. Chem. 2025, 68, 267–277. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A. Antifungal Action of Edible Coating Comprising Artichoke-Mediated Nanosilver and Chitosan Nanoparticles for Biocontrol of Citrus Blue Mold. Polymers 2025, 17, 1671. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A. Antifungal Nanocomposites from Honeybee Chitosan and Royal Jelly-Mediated Nanosilver for Suppressing Biofilm and Hyphal Formation of Candida albicans. Polymers 2025, 17, 1916. [Google Scholar] [CrossRef]
| Biopolymer Composition | Chitosan–Mucilage Ratio | Particle Size Range (nm) | Particle Size Mean (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| Chitosan | 1:0 | ND | ND | +38.53 ± 1.41 |
| MB | 0:1 | ND | ND | −26.37 ± 1.09 |
| MB-SeNPs | 0:1 | 16.76–124.32 | 46.19 | −27.93 ± 0.62 |
| F-1 | 1:2 | 59.23–678.39 | 239.88 | −24.11 ± 0.73 |
| F-2 | 1:1 | 39.16–720.83 | 212.42 | −16.88 ± 0.84 |
| F-3 | 2:1 | 49.77–692.25 | 266.16 | +34.37 ± 1.36 |
| Nanocomposites | Skin Pathogens | |||
|---|---|---|---|---|
| Staphylococcus aureus | Candida albicans | |||
| ZOI (mm) * | MIC (µg/mL) | ZOI | MIC | |
| MB-SeNPs | 18.5 ± 1.4 a | 32.5 | 19.1 ± 1.5 a | 35.0 |
| F-1 | 22.4 ± 1.7 b | 27.5 | 24.2 ± 1.9 a | 30.0 |
| F-2 | 26.3 ± 2.2 c | 22.5 | 27.1± 2.5 b | 20.0 |
| F-3 | 24.8 ± 1.8 c | 25.0 | 25.2 ± 1.7 c | 25.0 |
| Antimicrobial Agent | Zone of Inhibition Toward Skin Pathogens * | |
|---|---|---|
| Candida albicans | Staphylococcus aureus | |
| Control (1.0% acetic) | Not Detected | Not Detected |
| Cht | 3.3 ± 0.8 a | 2.9 ± 0.6 a |
| MB-SeNPs | 4.5 ± 1.1 b | 3.3 ± 0.7 b |
| Cht/MB-SeNPs | 6.2 ± 1.6 c | 3.7 ± 0.8 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghuthaymi, M.A. Innovative Antimicrobial Fabrics Loaded with Nanocomposites from Chitosan and Black Mulberry Polysaccharide-Mediated Selenium Nanoparticles to Suppress Skin Pathogens. Polymers 2025, 17, 2902. https://doi.org/10.3390/polym17212902
Alghuthaymi MA. Innovative Antimicrobial Fabrics Loaded with Nanocomposites from Chitosan and Black Mulberry Polysaccharide-Mediated Selenium Nanoparticles to Suppress Skin Pathogens. Polymers. 2025; 17(21):2902. https://doi.org/10.3390/polym17212902
Chicago/Turabian StyleAlghuthaymi, Mousa Abdullah. 2025. "Innovative Antimicrobial Fabrics Loaded with Nanocomposites from Chitosan and Black Mulberry Polysaccharide-Mediated Selenium Nanoparticles to Suppress Skin Pathogens" Polymers 17, no. 21: 2902. https://doi.org/10.3390/polym17212902
APA StyleAlghuthaymi, M. A. (2025). Innovative Antimicrobial Fabrics Loaded with Nanocomposites from Chitosan and Black Mulberry Polysaccharide-Mediated Selenium Nanoparticles to Suppress Skin Pathogens. Polymers, 17(21), 2902. https://doi.org/10.3390/polym17212902







