Advanced Biocompatible and Biodegradable Polymers: A Review of Functionalization, Smart Systems, and Sustainable Applications
Abstract
1. Introduction
2. Key Properties and Classification of Biopolymers
2.1. Key Physicochemical and Biological Properties
2.1.1. Degradation Mechanisms
2.1.2. Biocompatibility Metrics
2.1.3. Mechanical and Thermal Characteristics
2.2. Natural and Synthetic Polymers
2.2.1. Natural Polymers
- Starch
| Polymer | Mechanical Strength | Degradation Rate | Biocompatibility and Biodegradability | Key Limitations | References |
|---|---|---|---|---|---|
| Starch | Poor mechanical strength; reinforced with fiber matrix or by chemical or physical modification | Fast; temperature dependent | Biocompatible and biodegradable | Moisture absorbance and mechanical failure | [10,27] |
| Cellulose | Mechanical property weakened by moisture absorption; improved by chemical modification | Fast; temperature and environmental factors dependent | Biocompatible and biodegradable | Moisture absorbance and deterioration of mechanical properties | [10,27] |
| Polyhydroxyalkanoates (PHAs) | Weak mechanical property. Brittle. | Relatively fast, depending on HV content | Biocompatible and biodegradable | Commercially available PHAs are still brittle | [27,34,35] |
| Chitosan | Moderate mechanical properties, 10–60 MPa | Tunable degradation rate, Days-Months | Biocompatible and biodegradable | Poor water resistance, non-thermoplastic | [7,36,37,38] |
| Silk protein | 0.74–1.65 GPa tensile strength (native silk fibers) | Weeks–months; tunable | Biocompatible | Brittle when dry, recombinant yield is low | [39,40,41] |
| Collagen | 50–150 MPa tensile strength, 0.3–1.2 GPa Young’s modulus | Weeks–months (native); days–weeks (marine) | fully biodegradable and highly biocompatible | May be immunogenic and pathogens contaminated | [42,43,44,45,46,47] |
| Alginate | Poor mechanical properties; may be improved by crosslinking with multivalent cations. | Fast at high temperatures | Biocompatible and biodegradable | Poor stability and mechanical properties. Difficulty in customization. | [48] |
- Cellulose
- Polyhydroxyalkanoates (PHAs)
- Chitosan
- Silk Protein
- Collagen
- Alginate
2.2.2. Synthetic Polymers
- Polylactic acid (PLA)
| Polymer | Mechanical Strength | Degradation Rate | Biocompatibility and Biodegradability | Key Limitations | References |
|---|---|---|---|---|---|
| Polylactic acid (PLA) | Satisfactory; depends on stereoisomer distribution. | Accelerated at high temperature and humidity | Biocompatible and biodegradable | Weak mechanical properties | [11] |
| Polyglycolic acid (PGA) | ~31 MPa (pure PGA), 4–5% elongation | 90% mass loss in 20 days (70 °C, water) | Biocompatible and biodegradable | High crystallinity leads to low elongation (≈4–5%), limiting flexibility. | [27,87] |
| Poly (lactide-co-glycolide) (PLGA) | High (up to 2× with HA/β-TCP); load bearing | Tunable (1–6 months) | Biocompatible and biodegradable | Burst release, acidic microenvironment, brittleness | [10,20,54] |
| Polycaprolactone (PCL) | ~23 MPa (bulk), 2.5 MPa (electrospun), ~700% elongation | 4.8% mass loss (32 weeks), full resorption ≈14 months | Biocompatible and biodegradable | Slow degradation and hydrophobicity | [27,88,89,90] |
| Polyethylene glycol (PEG) | Flexible and elastic, 1.24–1.44 Mpa (PVA-PEG-CNF hydrogel) | Very slow; partial degradation in blends after 35–45 days | Biocompatible | Poor biodegradability, moisture instability, and MW variability | [91,92] |
| Polyurethane (PU) | 10–40 MPa (bio-based PU); up to 1000% elongation | Weeks to months (depending on structure and environment) | Biocompatible and biodegradable | Isocyanate toxicity, poor biodegradability, and recycling limits | [93,94,95] |
| Polyvinyl Alcohol | High (adjustable via crosslinking and FT cycles) | Weeks–months; accelerated by ester copolymerization | Biocompatible | Non-degradability, swelling, process sensitivity | [96,97,98] |
- Polyglycolic acid (PGA)
- Poly (lactide-co-glycolide) (PLGA)
- Polycaprolactone (PCL)
- Polyethylene Glycol (PEG)
- Polyurethane (PU)
- Polyvinyl Alcohol (PVA)
- Glycerol-based polyesters
3. Recent Technological Advancements
3.1. Surface Modification and Functionalization
3.1.1. PEGylation
3.1.2. Chemical and Plasma Treatments
- Chemical treatments
- Plasma treatment
3.2. Stimuli-Responsive Polymers
3.2.1. Temperature-Responsive Polymers
3.2.2. pH-Responsive Polymers
3.2.3. Redox-Sensitive Systems
3.3. Bio-Based and Green Synthesis
3.3.1. Renewable Sources
3.3.2. Green Solvents and Catalysis
3.3.3. Eco-Friendly Processing Techniques
4. Applications in Key Sectors
4.1. Biomedical Applications
4.1.1. Drug Delivery Vehicles and Diagnostics
4.1.2. Tissue Engineering Scaffolds
- Bone Tissue Engineering
- Cartilage Tissue Engineering
- Neural Tissue Engineering
- Skin and Wound Healing
- Vascular and Cardiac Tissue Engineering
- Other Soft Tissue Applications
- Smart and Stimuli-Responsive Scaffolds
4.1.3. Temporary Implants and Wound-Healing Materials
- Temporary Implants
- Wound-Healing Materials
4.2. Environmental and Industrial Uses
4.2.1. Sustainable Packaging Alternatives
- Natural Biopolymers for Packaging
- Synthetic Biodegradable Polyesters
- Blends, Composites, and Compatibilization
- Active, Antioxidant, and Smart Packaging
- Industrial Applications and Cross-Sector Relevance
4.2.2. Agricultural Applications
- Biodegradable Mulch Films and Crop Covers
- Controlled- and Slow-Release Fertilizers
4.3. Additive Manufacturing and 3D Printing
4.3.1. Custom Implants
4.3.2. Dental and Craniofacial Applications
4.3.3. Tissue Models and Hydrogel Bioinks
4.3.4. Advanced Additive Manufacturing Technologies
4.3.5. Stimuli-Responsive and 4D Printing
| Biopolymer (Family) | Key Property | Typical Forms | Representative Application | References |
|---|---|---|---|---|
| Medicine | ||||
| PLGA, PEG, nanogels, PEGylated carriers | Biocompatible, biodegradable, tunable degradation and surface chemistry, stimuli-responsive | Nanoparticles, micelles, dendrimers, nanogels | Drug delivery and theranostics (cancer, ocular, pulmonary, neurological) | [200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215] |
| Collagen, gelatin, hyaluronic acid, chitosan, alginate (natural); PLA, PGA, PCL, PLGA, PU, polyphosphazenes (synthetic) | Support cell adhesion, tunable degradation, and mechanical strength | Hydrogels, electrospun fibers, 3D-printed scaffolds, nanocomposites | Tissue engineering (bone, cartilage, neural, skin, vascular, cardiac) | [216,217,219,220,221,222,223] |
| PLA, PLLA, PGA, PLGA, PCL, PGS, PGSuc, short-chain diol-dicarboxylic acid polyesters (PBS, PESu, PBA) | Biodegradable and biocompatible; tunable stiffness and elasticity; hydrolytic degradation into non-toxic by-products | Composite scaffolds, fixation plates and screws, nanocomposites, elastomeric films | Temporary implants (orthopedic, dental) and hard/soft-tissue scaffolds (bone, cartilage, cardiac) | [113,224,225,226,227,228,229,230,231,232,233,234,235,236] |
| PGS, PGSuc, PU, collagen, chitosan, gelatin, alginate, silk fibroin, PVA composites | Elastomeric, bioactive, hemostatic, flexible; tunable cross-linking and hydroxyl-functional surfaces | Hydrogels, foams, nanofibrous mats, bio adhesives, and elastomeric membranes | Wound healing and soft-tissue regeneration (skin, vascular, cardiac, bladder, neural) | [113,237,238,239,240,241,242,243] |
| Starch, cellulose, chitosan, alginate, carrageenan, gelatin, casein, PESu, PBS blends | Renewable, biodegradable, edible, antimicrobial; tunable crystallinity and processability | Films, coatings, TPS blends, edible composites | Sustainable food packaging (active, antioxidant, smart films) | [113,244,245,246,247,248,249,250,251,252,253,254,255,256,257] |
| PLA, PCL, PBAT, PHAs, PBS, PESu, and blends | Biodegradable synthetic polyesters with tunable mechanics and barrier properties | Blends, composites, and nanocomposites for thermal and mechanical stability | Packaging (eco-friendly, industrial, agricultural, pharmaceutical) | [7,113,258,259,260,261,262,263,264,265,266,267,268,269,270] |
| Starch, cellulose, PVA, PLA, chitosan, gelatin, gum Arabic, PU, PCL coatings | Moisture retention, nutrient-controlled release, soil biodegradability | Mulch films, hydrogels, coatings, nanocarriers | Agricultural films, controlled-release fertilizers, pesticide carriers, biosorbents | [272,273,274,275,276,277,278,279,280,281,283,284,285,286,287,288,289,290,291,292,293,294,295,296,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362] |
| PLA, PCL, PLGA, PGS, PGSuc, PBS, GelMA, alginate, collagen, PEG derivatives | Printable, biocompatible, degradable; photo-/thermo-crosslinkable | FDM filaments, hydrogel bioinks, composites for 3D/4D printing | Additive manufacturing (implants, dental, tissue models, 4D printing) | [227,273,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332] |
| Environment | ||||
| Chitosan | Cationic, chelating, antimicrobial | Beads, powders, membranes | Waste water treatment (dyes, heavy metals), antimicrobial food wraps | [401] |
| Alginate | Gel-forming, non-toxic, high water absorption | Beads, films, hydrogels | Heavy metal removal, encapsulation of microbes, food packaging | [402,403] |
| Starch-based blends, | Biodegradable, renewable, adhesive | Films, foams, hydrogels, composites | Controlled-release fertilizers/urea, compostable packaging, bioplastic blends | [404] |
| Silk fibroin | Amphiphilic, High mechanical strength | Membrane, films, gels, films, composites | Heavy-metal adsorption membranes; water purification | [405] |
| PHA, PCL | Fully biodegradable, thermoplastic | Films, fibers, molded parts | Biodegradable packaging, soil-biodegradable plastics, mulch films | [406,407] |
| Industry | ||||
| Starch/PVA | Adhesive, high tensile strength, hydrophobicity, flexibility | Pastes, glues | Paper, food packaging adhesives, corrugation | [408,409,410] |
| PLA | Thermoplastic, compostable, high tensile strength | Films, fibers, molded parts | Packaging, bioplastic, 3D printing filaments | [411,412] |
| PHA | Versatile thermoplastics | Films, fibers, composites | Bioplastics, single-use items, paper coatings | [413] |
| Cellulose derivatives | Renewable, water-soluble | Films, fibers, membranes, composites | Packaging, textile finishing, paper coatings | [414,415] |
| Polyurethane | Adhesive, flexible, high mechanical strength | Foams, composites | Coatings, furniture | [416,417] |
5. Challenges and Limitations
5.1. Mechanical Property Enhancement
5.2. Degradation Kinetics and By-Products
5.3. Scalability and Cost
5.4. Regulatory and Standardization Issues
- D5209 [ASTM D5209-92; Standard Test Method for Determining the Aerobic Biodegradation of Plastic Materials in the Presence of Municipal Sewage Sludge (Withdrawn 2004). ASTM International: West Conshohocken, PA, USA, 1996-2025],
- D5338 [ASTM D5338-21; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions. ASTM International: West Conshohocken, PA, USA, 2021],
- D6002 [Standard Guide for Assessing the Compostability of Environmentally Degradable Plastics. ASTM International: West Conshohocken, PA, USA, 1996],
- D5988-03 [Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil of Plastic Materials or Residual Plastic Materials After Composting. ASTM International: West Conshohocken, PA, USA, 2003], and
- D6954 [Standard Guide for Exposing and Testing Plastics That Degrade in the Environment by a Combination of Oxidation and Biodegradation. ASTM International: West Conshohocken, PA, USA, 2018].Comparable frameworks have been developed by the Bureau of Indian Standards (BIS) and the International Organization for Standardization (ISO), with specifications adopted across countries to harmonize evaluation methods [438]:
- ISO 14851 [Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Measuring the Oxygen Demand in a Closed Respirometer. International Organization for Standardization: Geneva, Switzerland, 2019],
- ISO 14852 [Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Analysis of Evolved Carbon Dioxide. International Organization for Standardization: Geneva, Switzerland, 2021],
- ISO 14853 [Determination of the Ultimate Anaerobic Biodegradability of Plastic Materials in an Aqueous System—Method by Measurement of Biogas Production. International Organization for Standardization: Geneva, Switzerland, 2016], and
- EN 13432 [EN 13432:2000; Packaging—Requirements for packaging recoverable through composting and biodegradation—Test scheme and evaluation criteria for the final acceptance of packaging. European Committee for Standardization: Brussels, Belgium, 2000.]. Despite these advances, implementation remains inconsistent across regions. These gaps highlight that even when test methods exist, enforcement and harmonization lag behind scientific progress. However, third-party auditing and dissemination of guidelines through both policy and academic channels are now the strategy used to ensure compliance and public accountability [439].
6. Future Prospects
6.1. Smart and Multi-Responsive Polymers
6.2. Integration with Bioelectronics and Sensors
6.3. Clinical Translation and Industrial Scalability
6.4. Circular Economy and Recyclability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe: Enabling a Sustainable Future. Available online: https://plasticseurope.org/ (accessed on 24 September 2025).
- Booth, J.-P.; Mozetič, M.; Nikiforov, A.; Oehr, C. Foundations of Plasma Surface Functionalization of Polymers for Industrial and Biological Applications. Plasma Sources Sci. Technol. 2022, 31, 103001. [Google Scholar] [CrossRef]
- Plastic Pollution Is Growing Relentlessly as Waste Management and Recycling Fall Short, Says OECD. Available online: https://www.oecd.org/en/about/news/press-releases/2022/02/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.html (accessed on 24 September 2025).
- Andanje, M.N.; Mwangi, J.W.; Mose, B.R.; Carrara, S. Biocompatible and Biodegradable 3D Printing from Bioplastics: A Review. Polymers 2023, 15, 2355. [Google Scholar] [CrossRef]
- Maurya, A.K.; de Souza, F.M.; Dawsey, T.; Gupta, R.K. Biodegradable Polymers and Composites: Recent Development and Challenges. Polym. Compos. 2024, 45, 2896–2918. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical Carbon Dioxide as a Green Solvent for Processing Polymer Melts: Processing Aspects and Applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable Polymers in Biomedical Applications: A Review—Developments, Perspectives and Future Challenges. Int. J. Mol. Sci. 2023, 24, 16952. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Varghese, D.; Krishna, J.S.; Boominathan, T.; Rakeshkumar, R.; Dineshkumar, S.; Brahmananda Rao, C.V.S.; Sivaramakrishna, A. Recent Advances in Biodegradable Polymers—Properties, Applications and Future Prospects. Eur. Polym. J. 2023, 192, 112068. [Google Scholar] [CrossRef]
- Nanda, H.S.; Yang, L.; Hu, J.; Jiang, S.; Mao, H. Editorial: Biodegradable Polymers for Biomedical Applications-Volume II. Front. Mater. 2023, 10, 1231445. [Google Scholar] [CrossRef]
- Yao, X.; Yang, X.; Lu, Y.; Qiu, Y.; Zeng, Q. Review of the Synthesis and Degradation Mechanisms of Some Biodegradable Polymers in Natural Environments. Polymers 2024, 17, 66. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A Review on Degradation Mechanisms of Polylactic Acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef]
- Rosa, V.; Silikas, N.; Yu, B.; Dubey, N.; Sriram, G.; Zinelis, S.; Lima, A.F.; Bottino, M.C.; Ferreira, J.N.; Schmalz, G.; et al. Guidance on the Assessment of Biocompatibility of Biomaterials: Fundamentals and Testing Considerations. Dent. Mater. 2024, 40, 1773–1785. [Google Scholar] [CrossRef]
- Chen, Y.; Su, Y.-C.; Roffler, S.R. Polyethylene Glycol Immunogenicity in Nanomedicine. Nat. Rev. Bioeng. 2025, 3, 742–760. [Google Scholar] [CrossRef]
- Liu, R.; He, T.; Li, R.; Wang, S.; Lai, C.; Zhang, K. Comparison of Different Types of Poly-L-Lactic Acid Microspheres In Vitro and In Vivo Studies. Aesthetic Surg. J. Open Forum 2024, 6, ojae091. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Oz, F.; Khan, M.H.; Roy, S.; Esatbeyoglu, T.; Pratap-Singh, A. Thermal Properties of Biopolymer Films: Insights for Sustainable Food Packaging Applications. Food Eng. Rev. 2024, 16, 497–512. [Google Scholar] [CrossRef]
- Kalva, S.N.; Zakaria, Y.; Velasquez, C.A.; Koç, M. Tailoring the Mechanical and Degradation Properties of 3DP PLA/PCL Scaffolds for Biomedical Applications. Rev. Adv. Mater. Sci. 2025, 64, 20250098. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L. PLGA Implants for Controlled Drug Delivery and Regenerative Medicine: Advances, Challenges, and Clinical Potential. Pharmaceuticals 2025, 18, 631. [Google Scholar] [CrossRef]
- Arruda, T.R.; Machado, G.D.O.; Marques, C.S.; Souza, A.L.D.; Pelissari, F.M.; Oliveira, T.V.D.; Silva, R.R.A. An Overview of Starch-Based Materials for Sustainable Food Packaging: Recent Advances, Limitations, and Perspectives. Macromol 2025, 5, 19. [Google Scholar] [CrossRef]
- Chouhan, A.; Tiwari, A. Production of Polyhydroxyalkanoate (PHA) Biopolymer from Crop Residue Using Bacteria as an Alternative to Plastics: A Review. RSC Adv. 2025, 15, 11845–11862. [Google Scholar] [CrossRef] [PubMed]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Biduski, B.; Silva, W.M.F.D.; Colussi, R.; Halal, S.L.D.M.E.; Lim, L.-T.; Dias, Á.R.G.; Zavareze, E.D.R. Starch Hydrogels: The Influence of the Amylose Content and Gelatinization Method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Perez-Rea, D.; Bergenståhl, B.; Nilsson, L. Development and Evaluation of Methods for Starch Dissolution Using Asymmetrical Flow Field-Flow Fractionation. Part I: Dissolution of Amylopectin. Anal. Bioanal. Chem. 2015, 407, 4315–4326. [Google Scholar] [CrossRef] [PubMed]
- Modjarrad, K.; Ebnesajjad, S.; Plastics Design Library (Eds.) Handbook of Polymer Applications in Medicine and Medical Devices; Plastics Design Library Handbook Series; Elsevier Science: Oxford, UK, 2014; ISBN 978-0-323-22169-6. [Google Scholar]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Karnwal, A.; Rauf, A.; Jassim, A.Y.; Selvaraj, M.; Al-Tawaha, A.R.M.S.; Kashyap, P.; Kumar, D.; Malik, T. Advanced Starch-Based Films for Food Packaging: Innovations in Sustainability and Functional Properties. Food Chem. X 2025, 29, 102662. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Elfaghi, A.M.; Khattak, M.A.; Ilyas, R.A.; Sapuan, S.M. Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films. Polymers 2022, 15, 63. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of Natural Antioxidants from Rice Straw into Renewable Starch Films. Int. J. Biol. Macromol. 2020, 146, 976–986. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Yang, Y.; Zhang, D.; Hao, G. Multifunctional Natural Starch-Based Hydrogels: Critical Characteristics, Formation Mechanisms, Various Applications, Future Perspectives. Carbohydr. Polym. 2025, 357, 123458. [Google Scholar] [CrossRef]
- Alipournazari, P.; Pourmadadi, M.; Abdouss, M.; Rahdar, A.; Pandey, S. Enhanced Delivery of Doxorubicin for Breast Cancer Treatment Using pH-Sensitive Starch/PVA/g-C3N4 Hydrogel. Int. J. Biol. Macromol. 2024, 265, 130901. [Google Scholar] [CrossRef]
- Koshenaj, K.; Ferrari, G. A Comprehensive Review on Starch-Based Hydrogels: From Tradition to Innovation, Opportunities, and Drawbacks. Polymers 2024, 16, 1991. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, M.L.; Maia, A.L.C.; Poletto, F.; De Andrade, F.V.; Soares, D.C.F. Poly(-3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV): Current Advances in Synthesis Methodologies, Antitumor Applications and Biocompatibility. J. Drug Deliv. Sci. Technol. 2019, 51, 115–126. [Google Scholar] [CrossRef]
- Jin, A.; Del Valle, L.J.; Puiggalí, J. Copolymers and Blends Based on 3-Hydroxybutyrate and 3-Hydroxyvalerate Units. Int. J. Mol. Sci. 2023, 24, 17250. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Cuenca, V.; Salaris, V.; Muñoz-Gimena, P.F.; Agüero, Á.; Peltzer, M.A.; Montero, V.A.; Arrieta, M.P.; Sempere-Torregrosa, J.; Pavon, C.; Samper, M.D.; et al. Bio-Based and Biodegradable Polymeric Materials for a Circular Economy. Polymers 2024, 16, 3015. [Google Scholar] [CrossRef]
- Shi, B.; Hao, Z.; Du, Y.; Jia, M.; Xie, S. Mechanical and Barrier Properties of Chitosan-Based Composite Film as Food Packaging: A Review. BioResources 2024, 19, 4001–4014. [Google Scholar] [CrossRef]
- Zafar, W.; Tabassum, M.; Jia, X.; Yang, B.; Liu, H.; Xu, G.; Zafar, M.N. Chitosan-Based Bionanocomposites for Elimination of Hazardous Environmental Contaminants and Food Packaging: A Comprehensive Review. Sustain. Mater. Technol. 2025, 45, e01524. [Google Scholar] [CrossRef]
- Liu, Y.; Gilchrist, A.E.; Heilshorn, S.C. Engineered Protein Hydrogels as Biomimetic Cellular Scaffolds. Adv. Mater. 2024, 36, 2407794. [Google Scholar] [CrossRef]
- Johari, N.; Moroni, L.; Samadikuchaksaraei, A. Tuning the Conformation and Mechanical Properties of Silk Fibroin Hydrogels. Eur. Polym. J. 2020, 134, 109842. [Google Scholar] [CrossRef]
- Jung, D.; Lee, J.; Park, T.Y.; Yang, Y.J.; Cha, H.J. Diverse Silk and Silk-like Proteins Derived from Terrestrial and Marine Organisms and Their Applications. Acta Biomater. 2021, 136, 56–71. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen Blended with Natural Polymersv: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Sabarees, G.; Vishvaja, S.; Raghuraman, S.; Velmurugan, V.; Alagarsamy, V.; Raja Solomon, V.; Padmini Tamilarasi, G. Collagen-Based Nanofibers: Revolutionizing Therapeutics for Impaired Wound Healing. Int. J. Polym. Mater. Polym. Biomater. 2025, 74, 1128–1156. [Google Scholar] [CrossRef]
- Farooq, S.; Ahmad, M.I.; Zheng, S.; Ali, U.; Li, Y.; Shixiu, C.; Zhang, H. A Review on Marine Collagen: Sources, Extraction Methods, Colloids Properties, and Food Applications. Collagen Leather 2024, 6, 11. [Google Scholar] [CrossRef]
- Furtado, M.; Chen, L.; Chen, Z.; Chen, A.; Cui, W. Development of Fish Collagen in Tissue Regeneration and Drug Delivery. Eng. Regen. 2022, 3, 217–231. [Google Scholar] [CrossRef]
- Ding, C.; Yi, Y.; Cheng, K.; Wang, Y.; Wang, S.; Zhang, M. Full Life Cycle Green Preparation of Collagen-Based Food Packaging Films Using Halocynthia Roretzi as Raw Material. Food Chem. 2024, 455, 139943. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, L.; Yang, Y. A Review of Sodium Alginate-Based Hydrogels: Structure, Mechanisms, Applications, and Perspectives. Int. J. Biol. Macromol. 2025, 292, 139151. [Google Scholar] [CrossRef] [PubMed]
- Kadri, H.J.; Ahmed, F.; Rahman, M.H.; Mondal, M.I.H. Synthesis and Characterisation of Starch-g-Polyacrylamide-Co-Polylactic Acid Hydrogel for the Potential Wound Dressing Application. Polym. Bull. 2025, 82, 7917–7941. [Google Scholar] [CrossRef]
- Costa, T.B.; Matias, P.M.C.; Sharma, M.; Murtinho, D.; Rosa, D.S.; Valente, A.J.M. Recent Advances on Starch-Based Adsorbents for Heavy Metal and Emerging Pollutant Remediation. Polymers 2024, 17, 15. [Google Scholar] [CrossRef]
- Junka, A.; Bartoszewicz, M.; Dziadas, M.; Szymczyk, P.; Dydak, K.; Żywicka, A.; Owczarek, A.; Bil-Lula, I.; Czajkowska, J.; Fijałkowski, K. Application of Bacterial Cellulose Experimental Dressings Saturated with Gentamycin for Management of Bone Biofilm In Vitro and Ex Vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 30–37. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Pawase, P.A.; Rout, S.; Tripathy, S.; Pathare, A.M.; Srivastav, P.P.; Bashir, O.; Panghal, A. Recent Advances in Cellulose, Chitosan, and Protein-Based Edible Films for Sustainable Food Packaging: A Comprehensive Review. Int. J. Biol. Macromol. 2025, 321, 146172. [Google Scholar] [CrossRef]
- Shah, T.V.; Vasava, D.V. A Glimpse of Biodegradable Polymers and Their Biomedical Applications. e-Polymers 2019, 19, 385–410. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers- A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Marinho, E. Cellulose: A Comprehensive Review of Its Properties and Applications. Sustain. Chem. Environ. 2025, 11, 100283. [Google Scholar] [CrossRef]
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’Connor, K. A Review on Biological Synthesis of the Biodegradable Polymers Polyhydroxyalkanoates and the Development of Multiple Applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, K. Bioplastics—Classification, Production and Their Potential Food Applications. J. Hill Agric. 2017, 8, 118. [Google Scholar] [CrossRef]
- Lalonde, J.N.; Pilania, G.; Marrone, B.L. Materials Designed to Degrade: Structure, Properties, Processing, and Performance Relationships in Polyhydroxyalkanoate Biopolymers. Polym. Chem. 2025, 16, 235–265. [Google Scholar] [CrossRef]
- Gautam, S.; Gautam, A.; Pawaday, J.; Kanzariya, R.K.; Yao, Z. Current Status and Challenges in the Commercial Production of Polyhydroxyalkanoate-Based Bioplastic: A Review. Processes 2024, 12, 1720. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tachibana, Y.; Kasuya, K. Biodegradability of Poly(3-Hydroxyalkanoate) and Poly(ε-Caprolactone) via Biological Carbon Cycles in Marine Environments. Polym. J. 2021, 53, 47–66. [Google Scholar] [CrossRef]
- Wang, C.; Venditti, R.A.; Zhang, K. Tailor-Made Functional Surfaces Based on Cellulose-Derived Materials. Appl. Microbiol. Biotechnol. 2015, 99, 5791–5799. [Google Scholar] [CrossRef]
- Perveen, K.; Masood, F.; Hameed, A. Preparation, Characterization and Evaluation of Antibacterial Properties of Epirubicin Loaded PHB and PHBV Nanoparticles. Int. J. Biol. Macromol. 2020, 144, 259–266. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent Advances in the Development of Biodegradable PHB-Based Toughening Materials: Approaches, Advantages and Applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Moliner, C.; Badia, J.D.; Bosio, B.; Arato, E.; Lagazzo, A.; Capurro, M.; Ribes-Greus, A. Mechanical and Thermal Performance of Pla and Phbv-Based Biopolymers as Potential Alternatives to Pet. Chem. Eng. Trans. 2017, 57, 1417–1422. [Google Scholar] [CrossRef]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of Polyhydroxyalkanoate (PHA) Polymers Family as Substitutes of Petroleum Based Polymers for Packaging Applications and Solutions Brought by Their Composites to Form Barrier Materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Zairy, E.M.R. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; InTechOpen: Rijeka, Croatia, 2015; ISBN 978-953-51-2232-6. [Google Scholar]
- Morrow, B.H.; Payne, G.F.; Shen, J.K. Titration Properties and pH-Dependent Aggregation of Chitosan. Biophys. J. 2015, 108, 488a. [Google Scholar] [CrossRef]
- Castro, J.I.; Valencia-Llano, C.H.; Valencia Zapata, M.E.; Restrepo, Y.J.; Mina Hernandez, J.H.; Navia-Porras, D.P.; Valencia, Y.; Valencia, C.; Grande-Tovar, C.D. Chitosan/Polyvinyl Alcohol/Tea Tree Essential Oil Composite Films for Biomedical Applications. Polymers 2021, 13, 3753. [Google Scholar] [CrossRef]
- Chandarana, C.; Bonde, S.; Sonwane, S.; Prajapati, B. Chitosan-Based Packaging: Leading Sustainable Advancements in the Food Industry. Polym. Bull. 2025, 82, 5431–5462. [Google Scholar] [CrossRef]
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan Modifications for Adsorption of Pollutants—A Review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, B.; Greco, G.; Pessatti, T.B.; Sonavane, S.; Langwallner, V.; Arndt, T.; Rising, A. Strategies for Making High-Performance Artificial Spider Silk Fibers. Adv. Funct. Mater. 2024, 34, 2305040. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk Fibroin for Skin Injury Repair: Where Do Things Stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef]
- Senthilkumar, D.; Don, T.-M.; Liao, Y.-J.; Kuo, C.-Y. The Art of Biodegradable Polymer Design for the Treatments against Osteomyelitis. Int. J. Biol. Macromol. 2025, 285, 138347. [Google Scholar] [CrossRef]
- Kanoujia, J.; Raina, N.; Kishore, A.; Kaurav, M.; Tuli, H.S.; Kumar, A.; Gupta, M. Revealing the Promising Era of Silk-Based Nanotherapeutics: A Ray of Hope for Chronic Wound Healing Treatment. Naunyn. Schmiedebergs Arch. Pharmacol. 2025, 398, 6617–6641. [Google Scholar] [CrossRef]
- You, C.; Wang, C.; Ma, Z.; Yu, Q.; Liu, S. Review on Application of Silk Fibroin Hydrogels in the Management of Wound Healing. Int. J. Biol. Macromol. 2025, 298, 140082. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, Q.; Xu, X.; Liu, Z.; Cheng, G.; Long, D.; Cheng, L.; Dai, F. Recent Advances in Silk Fibroin-Based Composites for Bone Repair Applications: A Review. Polymers 2025, 17, 772. [Google Scholar] [CrossRef]
- Altman, G.H.; Farrell, B.D. Sericulture as a Sustainable Agroindustry. Clean. Circ. Bioecon. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Puerta, M.; Gomez-Maldonado, D.; Restrepo-Osorio, A.; Peresin, M.S. Self-Assembled Green Composites of Silk Fibroin and Microfibrillated Cellulose. MRS Bull. 2025, 50, 846–855. [Google Scholar] [CrossRef]
- Wosicka-Frąckowiak, H.; Poniedziałek, K.; Woźny, S.; Kuprianowicz, M.; Nyga, M.; Jadach, B.; Milanowski, B. Collagen and Its Derivatives Serving Biomedical Purposes: A Review. Polymers 2024, 16, 2668. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Q.; Xu, W.; Yang, M.; Guo, W.; He, S.; Liu, W. Alginate-Based Hydrogels Mediated Biomedical Applications: A Review. Int. J. Biol. Macromol. 2024, 279, 135019. [Google Scholar] [CrossRef]
- Lai, J.; Azad, A.K.; Sulaiman, W.M.A.W.; Kumarasamy, V.; Subramaniyan, V.; Alshehade, S.A. Alginate-Based Encapsulation Fabrication Technique for Drug Delivery: An Updated Review of Particle Type, Formulation Technique, Pharmaceutical Ingredient, and Targeted Delivery System. Pharmaceutics 2024, 16, 370. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Metha, C.; Pawar, S.; Suvarna, V. Recent Advancements in Alginate-Based Films for Active Food Packaging Applications. Sustain. Food Technol. 2024, 2, 1246–1265. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, J.; Lun, P.; Wu, Z.; Deng, W.; Sun, P. Dynamic Cross-Linking, Self-Healing, Antibacterial Hydrogel for Regenerating Irregular Cranial Bone Defects. ACS Appl. Mater. Interfaces 2024, 16, 39035–39050. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kim, H.; Lee, J.; Sadeghi, K.; Seo, J. PBAT/PGA Blend Films for Sustainable Packaging: Effect of PGA on Physicochemical and Morphological Changes during Abiotic Degradation. Food Packag. Shelf Life 2025, 51, 101589. [Google Scholar] [CrossRef]
- Firoozi, N.; Rezayan, A.H.; Tabatabaei Rezaei, S.J.; Mir-Derikvand, M.; Nabid, M.R.; Nourmohammadi, J.; Mohammadnejad Arough, J. Synthesis of Poly(ε-Caprolactone)-Based Polyurethane Semi-Interpenetrating Polymer Networks as Scaffolds for Skin Tissue Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 805–811. [Google Scholar] [CrossRef]
- Deshpande, M.V.; Girase, A.; King, M.W. Degradation of Poly(ε-Caprolactone) Resorbable Multifilament Yarn under Physiological Conditions. Polymers 2023, 15, 3819. [Google Scholar] [CrossRef]
- Gedik, B.; Erdem, M.A. Electrospun PCL Membranes for Localized Drug Delivery and Bone Regeneration. BMC Biotechnol. 2025, 25, 31. [Google Scholar] [CrossRef]
- Jahani, A.; Nassira, H. Polyethylene Glycol-Based Materials: Transformative Applications in Biomedicine and the Food Industry. Mater. Chem. Horiz. 2024, 3, 1074. [Google Scholar] [CrossRef]
- Jafri, N.H.S.; Jimat, D.N.; Wan Nawawi, W.M.F.; Ahmad Nor, Y.; Amid, A. Effect of Incorporating Cellulose Nanofibers and Lemongrass Essential Oil in Polyvinyl Alcohol-Polyethylene Glycol/Glycerin Hydrogel for Wound Dressing. IIUM Eng. J. 2024, 25, 99–115. [Google Scholar] [CrossRef]
- Cui, M.; Chai, Z.; Lu, Y.; Zhu, J.; Chen, J. Developments of Polyurethane in Biomedical Applications: A Review. Resour. Chem. Mater. 2023, 2, 262–276. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications—A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Jayalath, P.; Ananthakrishnan, K.; Jeong, S.; Shibu, R.P.; Zhang, M.; Kumar, D.; Yoo, C.G.; Shamshina, J.L.; Therasme, O. Bio-Based Polyurethane Materials: Technical, Environmental, and Economic Insights. Processes 2025, 13, 1591. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef]
- Hedayati, H.R.; Khorasani, M.; Ahmadi, M.; Ballard, N. Preparation of Well-Defined Poly(Vinyl Alcohol) by Hydrolysis of Poly(Vinyl Acetate) Synthesized by RAFT Suspension Polymerization. Polymer 2022, 246, 124674. [Google Scholar] [CrossRef]
- Hedir, G.; Stubbs, C.; Aston, P.; Dove, A.P.; Gibson, M.I. Synthesis of Degradable Poly(Vinyl Alcohol) by Radical Ring-Opening Copolymerization and Ice Recrystallization Inhibition Activity. ACS Macro Lett. 2017, 6, 1404–1408. [Google Scholar] [CrossRef]
- Vengadesan, E.; Morakul, S.; Muralidharan, S.; Pullela, P.K.; Alarifi, A.; Arunkumar, T. Enhancement of Polylactic Acid (PLA) with Hybrid Biomass-Derived Rice Husk and Biocarbon Fillers: A Comprehensive Experimental Study. Discov. Appl. Sci. 2025, 7, 161. [Google Scholar] [CrossRef]
- Pullarkad Bharathan, S.; Johnsy, G.; Raju, A.P.; Guthige, M.R.; Gowdahalli Mantelingachar, C.; Vasudevan, V.; Madan, R.; Kumar, R. Sustainable Antimicrobial Packaging Films: Effectiveness of Epsilon-Poly-L-Lysine in PLA/PBAT Blend Films. Sustain. Food Technol. 2025. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, J.; Ren, X. Natural Fiber-Based Biocomposites. In Green Biocomposites; Jawaid, M., Sapuan, S.M., Alothman, O.Y., Eds.; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2017; pp. 31–70. ISBN 978-3-319-46609-5. [Google Scholar]
- Ghanbarzadeh, B.; Almasi, H. Biodegradable Polymers. In Biodegradation—Life of Science; Chamy, R., Ed.; InTechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-1154-2. [Google Scholar]
- Chen, S.; Meng, X.; Xin, Z.; Gong, W.; Li, C.; Wen, W. Preparation of Nonlinear Structure Poly(Glycolic Acid) with High Toughness, Excellent Hydrolysis Stability, and Foaming Performance. Ind. Eng. Chem. Res. 2024, 63, 9058–9069. [Google Scholar] [CrossRef]
- Qiu, X.; Li, S.; Li, X.; Xiao, Y.; Li, S.; Fen, Q.; Kang, X.; Zhen, P. Experimental Study of β-TCP Scaffold Loaded with VAN/PLGA Microspheres in the Treatment of Infectious Bone Defects. Colloids Surf. B Biointerfaces 2022, 213, 112424. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Mai, S.; Shu, D.; Huang, Y.; Nie, Z.; Wang, Y.; Yang, W. Microfluidic-Based Preparation of PLGA Microspheres Facilitating Peptide Sustained-Release. Mater. Lett. 2024, 368, 136675. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J. Biodegradable Polymers and Polymer Blends. In Handbook of Biopolymers and Biodegradable Plastics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 109–128. ISBN 978-1-4557-2834-3. [Google Scholar]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Verma, V.S.; Pandey, A.; Jha, A.K.; Badwaik, H.K.R.; Alexander, A. Ajazuddin Polyethylene Glycol–Based Polymer-Drug Conjugates: Novel Design and Synthesis Strategies for Enhanced Therapeutic Efficacy and Targeted Drug Delivery. Appl. Biochem. Biotechnol. 2024, 196, 7325–7361. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W. Fabrication of Antibacterial Chitosan-PVA Blended Film Using Electrospray Technique for Food Packaging Applications. Int. J. Biol. Macromol. 2018, 107, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Trigui, K.; Magnin, A.; Putaux, J.-L.; Boufi, S. Poly(Vinyl Alcohol)/Oxidized Cellulose Nanofibril Composite Films with High Nanofiller Content for Enhanced Packaging Applications. J. Ind. Eng. Chem. 2025, 148, 602–613. [Google Scholar] [CrossRef]
- Narin, S.; Sahin, S.B.; Demir, E.; Cetinel, S. Electrospun Poly (Glycerol Sebacate) (PGS) Membranes for Corneal Tissue Engineering. Macromol. Mater. Eng. 2025, e00163. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Liu, L.; Mithieux, S.M.; Weiss, A.S. Polyglycerol Sebacate-Based Elastomeric Materials for Arterial Regeneration. J. Biomed. Mater. Res. A 2024, 112, 574–585. [Google Scholar] [CrossRef]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, Properties and Biomedical Applications of Poly(Glycerol Sebacate) (PGS): A Review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Andrä-Żmuda, S.; Chaber, P.; Martinka Maksymiak, M.; Musioł, M.; Adamus, G. Poly(Glycerol Sebacate): A Comparative Study of Various Synthesis Methods. Biomacromolecules 2025. [Google Scholar] [CrossRef]
- Risley, B.B.; Ding, X.; Chen, Y.; Miller, P.G.; Wang, Y. Citrate Crosslinked Poly(Glycerol Sebacate) with Tunable Elastomeric Properties. Macromol. Biosci. 2021, 21, 2000301. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zeng, G.; Xu, J.; Han, M.; Wang, Z.; Li, T.; Long, M.; Wang, L.; Huang, W.; Wu, Y. Development of Poly(Glycerol Sebacate) and Its Derivatives: A Review of the Progress over the Past Two Decades. Polym. Rev. 2023, 63, 613–678. [Google Scholar] [CrossRef]
- Martín-Cabezuelo, R.; Vilariño-Feltrer, G.; Vallés-Lluch, A. Influence of Pre-Polymerisation Atmosphere on the Properties of Pre- and Poly(Glycerol Sebacate). Mater. Sci. Eng. C 2021, 119, 111429. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.; Gama, N.; Ferreira, A. Different Methods of Synthesizing Poly(Glycerol Sebacate) (PGS): A Review. Front. Bioeng. Biotechnol. 2022, 10, 1033827. [Google Scholar] [CrossRef]
- Ning, Z.; Lang, K.; Xia, K.; Linhardt, R.J.; Gross, R.A. Lipase-Catalyzed Synthesis and Characterization of Poly(Glycerol Sebacate). Biomacromolecules 2022, 23, 398–408. [Google Scholar] [CrossRef]
- Yousefi Talouki, P.; Tamimi, R.; Zamanlui Benisi, S.; Goodarzi, V.; Shojaei, S.; Hesami tackalou, S.; Samadikhah, H.R. Polyglycerol Sebacate (PGS)-Based Composite and Nanocomposites: Properties and Applications. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1360–1374. [Google Scholar] [CrossRef]
- Martín Cabezuelo, R. Monitoring of the Parameters of Synthesis of Poly(Glycerol Sebacate) and Influence on the Physicochemical and Biological Properties of Its Elastomer. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2020. [Google Scholar]
- Shukla, K.; Arunagiri, A.; Muthukumar, K. Synthesis and Hydrolytic Degradation of Poly (Glycerol Succinate) Based Polyesters. J. Indian Chem. Soc. 2023, 100, 100841. [Google Scholar] [CrossRef]
- Godinho, B.; Nogueira, R.; Gama, N.; Ferreira, A. Synthesis and Characterization of Poly(Glycerol Sebacate), Poly(Glycerol Succinate) and Poly(Glycerol Sebacate-Co-Succinate). J. Polym. Environ. 2024, 32, 4330–4347. [Google Scholar] [CrossRef]
- Nakiou, E.A.; Lazaridou, M.; Pouroutzidou, G.K.; Michopoulou, A.; Tsamesidis, I.; Liverani, L.; Arango-Ospina, M.; Beketova, A.; Boccaccini, A.R.; Kontonasaki, E.; et al. Poly(Glycerol Succinate) as Coating Material for 1393 Bioactive Glass Porous Scaffolds for Tissue Engineering Applications. Polymers 2022, 14, 5028. [Google Scholar] [CrossRef] [PubMed]
- Kolbuk, D.; Jeznach, O.; Wrzecionek, M.; Gadomska-Gajadhur, A. Poly(Glycerol Succinate) as an Eco-Friendly Component of PLLA and PLCL Fibres towards Medical Applications. Polymers 2020, 12, 1731. [Google Scholar] [CrossRef]
- Hetemi, D.; Pinson, J. Surface Functionalisation of Polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef] [PubMed]
- Aditya, T.; Allain, J.P.; Jaramillo, C.; Restrepo, A.M. Surface Modification of Bacterial Cellulose for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 610. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Wu, Z.; Chen, H. Chemical Surface Modification of Polymeric Biomaterials for Biomedical Applications. Macromol. Rapid Commun. 2020, 41, 1900430. [Google Scholar] [CrossRef]
- Gao, Y.; Joshi, M.; Zhao, Z.; Mitragotri, S. PEGylated Therapeutics in the Clinic. Bioeng. Transl. Med. 2024, 9, e10600. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The Impact of PEGylation on Biological Therapies. BioDrugs 2012, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Santhanakrishnan, K.R.; Koilpillai, J.; Narayanasamy, D. PEGylation in Pharmaceutical Development: Current Status and Emerging Trends in Macromolecular and Immunotherapeutic Drugs. Cureus 2024, 16, e66669. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(Ethylene Glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Kurul, F.; Doruk, B.; Topkaya, S.N. Principles of Green Chemistry: Building a Sustainable Future. Discov. Chem. 2025, 2, 68. [Google Scholar] [CrossRef]
- Godinho, B.M.D.C.; Ogier, J.R.; Quinlan, A.; Darcy, R.; Griffin, B.T.; Cryan, J.F.; O’Driscoll, C.M. PEGylated Cyclodextrins as Novel siRNA Nanosystems: Correlations between Polyethylene Glycol Length and Nanoparticle Stability. Int. J. Pharm. 2014, 473, 105–112. [Google Scholar] [CrossRef]
- Carcao, M.D.; Chelle, P.; Clarke, E.; Kim, L.; Tiseo, L.; Morfini, M.; Hossain, T.; Rand, M.L.; Brown, C.; Edginton, A.N.; et al. Comparative Pharmacokinetics of Two Extended Half-life FVIII Concentrates (Eloctate and Adynovate) in Adolescents with Hemophilia A: Is There a Difference? J. Thromb. Haemost. 2019, 17, 1085–1096. [Google Scholar] [CrossRef]
- Makharadze, D.; del Valle, L.J.; Katsarava, R.; Puiggalí, J. The Art of PEGylation: From Simple Polymer to Sophisticated Drug Delivery System. Int. J. Mol. Sci. 2025, 26, 3102. [Google Scholar] [CrossRef]
- Robella, M.; Vaira, M.; Argenziano, M.; Spagnolo, R.; Cavalli, R.; Borsano, A.; Gentilli, S.; De Simone, M. Exploring the Use of Pegylated Liposomal Doxorubicin (Caelyx®) as Pressurized Intraperitoneal Aerosol Chemotherapy. Front. Pharmacol. 2019, 10, 669. [Google Scholar] [CrossRef]
- Demirel, E.; Karaca, E.; Yuksel Durmaz, Y. Effective PEGylation Method to Improve Biocompatibility of Graphene Derivatives. Eur. Polym. J. 2020, 124, 109504. [Google Scholar] [CrossRef]
- Zaky, M.F.; Hammady, T.M.; Gad, S.; Alattar, A.; Alshaman, R.; Hegazy, A.; Zaitone, S.A.; Ghorab, M.M.; Megahed, M.A. Influence of Surface-Modification via PEGylation or Chitosanization of Lipidic Nanocarriers on In Vivo Pharmacokinetic/Pharmacodynamic Profiles of Apixaban. Pharmaceutics 2023, 15, 1668. [Google Scholar] [CrossRef]
- Kesharwani, P.; Kumar, V.; Goh, K.W.; Gupta, G.; Alsayari, A.; Wahab, S.; Sahebkar, A. PEGylated PLGA Nanoparticles: Unlocking Advanced Strategies for Cancer Therapy. Mol. Cancer 2025, 24, 205. [Google Scholar] [CrossRef]
- Nemani, K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.; Abdelaal, A.; Sojoudi, H. Surface Modification: Surface Modification of Polymers: Methods and Applications (Adv. Mater. Interfaces 24/2018). Adv. Mater. Interfaces 2018, 5, 1870121. [Google Scholar] [CrossRef]
- Kurhade, R.R.; Shaikh, M.S.; Nagulwar, V.; Kale, M.A. Advancements in Carboxymethyl Cellulose (CMC) Modifications and Their Diverse Biomedical Applications: A Comprehensive Review. Int. J. Polym. Mater. Polym. Biomater. 2025, 74, 1043–1067. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X. Introducing Hydroxyl Groups as Cellulose-Binding Sites into Polymeric Solid Acids to Improve Their Catalytic Performance in Hydrolyzing Cellulose. Carbohydr. Polym. 2021, 261, 117895. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Garcia, E.S.; Zimmerman, S.C. Intramolecularly Cross-Linked Polymers: From Structure to Function with Applications as Artificial Antibodies and Artificial Enzymes. Acc. Chem. Res. 2020, 53, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.A.; Bello, A.; Adams, S.M.; Onwualu, A.P.; Anye, V.C.; Bello, K.A.; Obianyo, I.I. Nano-Enhanced Polymer Composite Materials: A Review of Current Advancements and Challenges. Polymers 2025, 17, 893. [Google Scholar] [CrossRef]
- Sharma, H.; Arora, G.; Singh, M.K.; Rangappa, S.M.; Bhowmik, P.; Kumar, R.; Debnath, S.; Siengchin, S. From Composition to Performance: Structural Insights into Polymer Composites. Next Mater. 2025, 8, 100852. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Torchio, A.; Cassino, C.; Chiono, V.; Ciardelli, G. Plasma Treatment of Polymer Powder as an Effective Tool to Functionalize Polymers: Case Study Application on an Amphiphilic Polyurethane. Polymers 2019, 11, 2109. [Google Scholar] [CrossRef]
- Primc, G.; Mozetič, M. Surface Modification of Polymers by Plasma Treatment for Appropriate Adhesion of Coatings. Materials 2024, 17, 1494. [Google Scholar] [CrossRef]
- Bhatt, P.; Kumar, V.; Subramaniyan, V.; Nagarajan, K.; Sekar, M.; Chinni, S.V.; Ramachawolran, G. Plasma Modification Techniques for Natural Polymer-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 2066. [Google Scholar] [CrossRef]
- Koodaryan, R.; Hafezeqoran, A. Surface Modification of Dental Polymers by Plasma Treatment: A Review. Biomed. Pharmacol. J. 2016, 9. [Google Scholar] [CrossRef]
- Pidhatika, B.; Widyaya, V.T.; Nalam, P.C.; Swasono, Y.A.; Ardhani, R. Surface Modifications of High-Performance Polymer Polyetheretherketone (PEEK) to Improve Its Biological Performance in Dentistry. Polymers 2022, 14, 5526. [Google Scholar] [CrossRef]
- Sundriyal, P.; Sahu, M.; Prakash, O.; Bhattacharya, S. Long-Term Surface Modification of PEEK Polymer Using Plasma and PEG Silane Treatment. Surf. Interfaces 2021, 25, 101253. [Google Scholar] [CrossRef]
- Hu, L.; Wan, Y.; Zhang, Q.; Serpe, M.J. Harnessing the Power of Stimuli-Responsive Polymers for Actuation. Adv. Funct. Mater. 2020, 30, 1903471. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. pH-Sensitive Polymers: Classification and Some Fine Potential Applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Bratek-Skicki, A. Towards a New Class of Stimuli-Responsive Polymer-Based Materials—Recent Advances and Challenges. Appl. Surf. Sci. Adv. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Lower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Yuan, Y.; Raheja, K.; Milbrandt, N.B.; Beilharz, S.; Tene, S.; Oshabaheebwa, S.; Gurkan, U.A.; Samia, A.C.S.; Karayilan, M. Thermoresponsive Polymers with LCST Transition: Synthesis, Characterization, and Their Impact on Biomedical Frontiers. RSC Appl. Polym. 2023, 1, 158–189. [Google Scholar] [CrossRef]
- Sharker, K.K.; Shigeta, Y.; Ozoe, S.; Damsongsang, P.; Hoven, V.P.; Yusa, S. Upper Critical Solution Temperature Behavior of pH-Responsive Amphoteric Statistical Copolymers in Aqueous Solutions. ACS Omega 2021, 6, 9153–9163. [Google Scholar] [CrossRef] [PubMed]
- Kotsuchibashi, Y. Recent Advances in Multi-Temperature-Responsive Polymeric Materials. Polym. J. 2020, 52, 681–689. [Google Scholar] [CrossRef]
- Halligan, E.; Zhuo, S.; Colbert, D.M.; Alsaadi, M.; Tie, B.S.H.; Bezerra, G.S.N.; Keane, G.; Geever, L.M. Modulation of the Lower Critical Solution Temperature of Thermoresponsive Poly(N-Vinylcaprolactam) Utilizing Hydrophilic and Hydrophobic Monomers. Polymers 2023, 15, 1595. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Lin, Y.; Qin, Z.; Su, T.; Xie, X.; Ji, H. Temperature-Responsive Gating Chitosan-Based Microcapsules for Controlled Release of Urea Fertilizers. Carbohydr. Polym. 2025, 348, 122929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, Y.; Inomata, K. Lower Critical Solution Temperature Behavior of Poly(2-Chloroethyl Vinyl Ether-Alt-Maleic Anhydride) in Organic Media. Polym. J. 2010, 42, 901–904. [Google Scholar] [CrossRef][Green Version]
- Concilio, M.; Beyer, V.P.; Becer, C.R. Thermoresponsive Polymers in Non-Aqueous Solutions. Polym. Chem. 2022, 13, 6423–6474. [Google Scholar] [CrossRef]
- Korpanty, J.; Wang, C.; Gianneschi, N.C. Upper Critical Solution Temperature Polymer Assemblies via Variable Temperature Liquid Phase Transmission Electron Microscopy and Liquid Resonant Soft X-Ray Scattering. Nat. Commun. 2023, 14, 3441. [Google Scholar] [CrossRef]
- Le, M.; Huang, W.; Chen, K.-F.; Lin, C.; Cai, L.; Zhang, H.; Jia, Y.-G. Upper Critical Solution Temperature Polymeric Drug Carriers. Chem. Eng. J. 2022, 432, 134354. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Raczkowska, J.; Harhay, K.; Gajos, K.; Melnyk, Y.; Dąbczyński, P.; Shevtsova, T.; Budkowski, A. Temperature-Responsive and Multi-Responsive Grafted Polymer Brushes with Transitions Based on Critical Solution Temperature: Synthesis, Properties, and Applications. Colloid Polym. Sci. 2020, 299, 363–383. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Q.-Y.; Li, T.-T.; Lou, C.-W.; Hung, C.; Lin, J.-H. Recent Developments and Applications of pH-Responsive Polymers. Text. Res. J. 2025, 95, 2248–2272. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Y.; Zou, Y.; Lu, C.; Li, N.; Shi, Z.; Li, X.; Lai, X. Ultra-Sensitive pH Responsive Hydrogels with Injectable and Self-Healing Performance for Controlled Drug Delivery. Int. J. Pharm. X 2025, 9, 100334. [Google Scholar] [CrossRef]
- Twal, S.; Jaber, N.; Al-Remawi, M.; Hamad, I.; Al-Akayleh, F.; Alshaer, W. Dual Stimuli-Responsive Polymeric Nanoparticles Combining Soluplus and Chitosan for Enhanced Breast Cancer Targeting. RSC Adv. 2024, 14, 3070–3084. [Google Scholar] [CrossRef]
- Kim, T.M.; Subba, S.H.; Hwang, Y.K.; Kim, S.G.; Park, J.; Jin, E.-J.; Park, S.Y. Electrical and Fluorescence in Situ Monitoring of Tumor Microenvironment-Based pH-Responsive Polymer Dot Coated Surface. Talanta 2025, 281, 126840. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Iftikhar, I.; Barkat, K.; Badshah, S.F.; Ashraf, M.U.; Mehmood, Y.; Anjum, I.; Shazly, G.A.; Metouekel, A.; Younous, Y.A.; Bourhia, M. Formulation of pH Responsive Polymeric Hydrogels for Prolonged Delivery of Famciclovir with Biosafety Evaluation. Sci. Rep. 2025, 15, 18686. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, T.; Liu, Y.; Chen, L.; Liao, S.; Gong, X.; Bello, M.G.; Zhu, W.; Huang, S.; Zhang, X. Loading Curcumin on Hyperbranched Polymers Functionalized Zein via the Phenol-Yne Click Reaction as pH-Responsive Drug Delivery System for Chemotherapy and Photodynamic Therapy. Int. J. Biol. Macromol. 2025, 293, 139750. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, P.; Wang, W.; Guo, G.; Wu, C.; Ge, R. Development and Characterization of a Poly(Amino Acid)-Based pH-Responsive Dual-Mode Antibacterial Nanoparticles Dental Restorative Resin. J. Appl. Polym. Sci. 2025, 142, e57570. [Google Scholar] [CrossRef]
- Jiao, X.; Chong, X.; Du, H.; Yang, M.; Zhu, Z.; Ma, Z.; Wen, Y. Development of pH and Enzyme Dual Responsive Chitosan/Polyaspartic Acid Nanoparticle-Embedded Nanofibers for Fruit Preservation. Int. J. Biol. Macromol. 2025, 297, 139903. [Google Scholar] [CrossRef]
- Shin, S.; Kim, S.; Hong, S.; Kim, N.; Kang, J.; Jeong, J. Tuning the Swelling Behavior of Superabsorbent Hydrogels with a Branched Poly(Aspartic Acid) Crosslinker. Gels 2025, 11, 161. [Google Scholar] [CrossRef]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-Responsive Polymers for Drug Delivery: From Molecular Design to Applications. Polym. Chem. 2014, 5, 1519–1528. [Google Scholar] [CrossRef]
- Xu, L.; Cao, Y.; Xu, Y.; Li, R.; Xu, X. Redox-Responsive Polymeric Nanoparticle for Nucleic Acid Delivery and Cancer Therapy: Progress, Opportunities, and Challenges. Macromol. Biosci. 2024, 24, 2300238. [Google Scholar] [CrossRef] [PubMed]
- Kilic Boz, R.; Aydin, D.; Kocak, S.; Golba, B.; Sanyal, R.; Sanyal, A. Redox-Responsive Hydrogels for Tunable and “On-Demand” Release of Biomacromolecules. Bioconjug. Chem. 2022, 33, 839–847. [Google Scholar] [CrossRef]
- Dabas, R.; Kamaly, N. Redox-Responsive Nanogels for Precision Protein Delivery. Eur. Polym. J. 2024, 215, 113183. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Du, J.; Chen, X.; Xue, Y.; Zhang, J.; Yang, X.; Chen, X.; Xie, J.; Ju, S. Redox-Responsive Polymer Micelles Co-Encapsulating Immune Checkpoint Inhibitors and Chemotherapeutic Agents for Glioblastoma Therapy. Nat. Commun. 2024, 15, 1118. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Dai, C.; Xie, Z.; You, X.; Li, K.; Wu, J.; Huang, H. Redox Responsive Polymeric Nanoparticles Enhance the Efficacy of Cyclin Dependent Kinase 7 Inhibitor for Enhanced Treatment of Prostate Cancer. Chin. Chem. Lett. 2024, 35, 109170. [Google Scholar] [CrossRef]
- Cherri, M.; Stergiou, P.S.; Ahmadian, Z.; Povolotsky, T.L.; Thongrom, B.; Fan, X.; Mohammadifar, E.; Haag, R. Redox-Responsive Hydrogels Loaded with an Antibacterial Peptide as Controlled Drug Delivery for Healing Infectious Wounds. Adv. Healthc. Mater. 2024, 13, 2401289. [Google Scholar] [CrossRef]
- Meng, X.; Shen, Y.; Zhao, H.; Lu, X.; Wang, Z.; Zhao, Y. Redox-Manipulating Nanocarriers for Anticancer Drug Delivery: A Systematic Review. J. Nanobiotechnol. 2024, 22, 587. [Google Scholar] [CrossRef]
- Kaur, R.; Pathak, L.; Vyas, P. Biobased Polymers of Plant and Microbial Origin and Their Applications—A Review. Biotechnol. Sustain. Mater. 2024, 1, 13. [Google Scholar] [CrossRef]
- Serra Sampaio, M.; Wojcieszak, R.; Itabaiana Junior, I. Synthesis of Bio-Based Polymers and Adjuvants through Biomass Valorization: Challenges and Opportunities. ChemCatChem 2024, 16, e202301126. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Erasmus, M. Green Synthesis of Bioplastics from Microalgae: A State-of-the-Art Review. Polymers 2024, 16, 1322. [Google Scholar] [CrossRef] [PubMed]
- Beena Unni, A.; Muringayil Joseph, T. Enhancing Polymer Sustainability: Eco-Conscious Strategies. Polymers 2024, 16, 1769. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef]
- Stavila, E.; Yuliati, F.; Adharis, A.; Arya Laksmono, J.; Iqbal, M. Recent Advances in Synthesis of Polymers Based on Palm Oil and Its Fatty Acids. RSC Adv. 2023, 13, 14747–14775. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, S.; Zhang, J.; Cheng, Z.; Song, L.; Hu, Y. Innovative Design and Green Synthesis of Bio-Based Non-Isocyanate Polyurethanes: Efficient Combination of Cardanol and Carbon Dioxide with High Fire Safety and Robust Adhesion. Chem. Eng. J. 2024, 482, 148846. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Zhao, Z.; Wang, J.; Chen, Y.; Qian, L.; Fang, Z.; Song, R.; Song, P. Green Synthesis of Bio-Based Flame Retardant/Natural Rubber Inorganic-Organic Hybrid and Its Flame Retarding and Toughening Effect for Polylactic Acid. Int. J. Biol. Macromol. 2024, 256, 128378. [Google Scholar] [CrossRef]
- Post, C.; Maniar, D.; Jongstra, J.A.; Parisi, D.; Voet, V.S.D.; Folkersma, R.; Loos, K. Enzymatic Bulk Synthesis, Characterization, Rheology, and Biodegradability of Biobased 2,5-Bis(Hydroxymethyl)Furan Polyesters. Green Chem. 2024, 26, 8744–8757. [Google Scholar] [CrossRef]
- Almustafa, W.; Grishchuk, S.; Redel, M.; Schubert, D.W.; Grun, G. Solvent-Free Processing of i-P3HB Blends: Enhancing Processability and Mechanical Properties for Sustainable Applications. Polymers 2025, 17, 2231. [Google Scholar] [CrossRef]
- Ramezani, M.; Getya, D.; Gitsov, I.; Browning Monroe, M.B. Solvent-Free Synthesis of Biostable Segmented Polyurethane Shape Memory Polymers for Biomedical Applications. J. Mater. Chem. B 2024, 12, 1217–1231. [Google Scholar] [CrossRef]
- Pellis, A.; Weinberger, S.; Gigli, M.; Guebitz, G.M.; Farmer, T.J. Enzymatic Synthesis of Biobased Polyesters Utilizing Aromatic Diols as the Rigid Component. Eur. Polym. J. 2020, 130, 109680. [Google Scholar] [CrossRef]
- Herrlé, C.; Fadlallah, S.; Toumieux, S.; Wadccccccccouachi, A.; Allais, F. Sustainable Mechanosynthesis of Diamide Tetraols Monomers and Their Enzymatic Polymerization. Green Chem. 2024, 26, 1462–1470. [Google Scholar] [CrossRef]
- Kimura, H.; Ogura, Y. Biodegradable Polymers for Ocular Drug Delivery. Ophthalmologica 2001, 215, 143–155. [Google Scholar] [CrossRef]
- Ha, C.-S.; Gardella, J.A. Surface Chemistry of Biodegradable Polymers for Drug Delivery Systems. Chem. Rev. 2005, 105, 4205–4232. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Q.; Wang, C.-C. Biodegradable Smart Nanogels: A New Platform for Targeting Drug Delivery and Biomedical Diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel Carrier Design for Targeted Drug Delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” Mechanical Properties of Three-dimensional Polyester Porous Scaffolds. J. Biomed. Mater. Res. A 2006, 76A, 264–271. [Google Scholar] [CrossRef]
- Giram, P.S.; Wang, J.T.-W.; Walters, A.A.; Rade, P.P.; Akhtar, M.; Han, S.; Faruqu, F.N.; Abdel-Bar, H.M.; Garnaik, B.; Al-Jamal, K.T. Green Synthesis of Methoxy-Poly(Ethylene Glycol)- Block -Poly( L -Lactide- Co -Glycolide) Copolymer Using Zinc Proline as a Biocompatible Initiator for Irinotecan Delivery to Colon Cancer in Vivo. Biomater. Sci. 2021, 9, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kataoka, K. Chemo-Physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and Pseudo-Stealth Nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Domb, A.J.; Khan, W. Biodegradable Polymers for Targeted Delivery of Anti-Cancer Drugs. Expert Opin. Drug Deliv. 2016, 13, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, Functionalization Strategies and Biomedical Applications of Targeted Biodegradable/Biocompatible Polymer-Based Nanocarriers for Drug Delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Pan, J.; Liu, Y.; Feng, S.-S. Multifunctional Nanoparticles of Biodegradable Copolymer Blend for Cancer Diagnosis and Treatment. Nanomedicine 2010, 5, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Sendil, D.; Bonney, I.M.; Carr, D.B.; Lipkowski, A.W.; Wise, D.L.; Hasirci, V. Antinociceptive Effects of Hydromorphone, Bupivacaine and Biphalin Released from PLGA Polymer after Intrathecal Implantation in Rats. Biomaterials 2003, 24, 1969–1976. [Google Scholar] [CrossRef]
- Ozeki, T.; Kaneko, D.; Hashizawa, K.; Imai, Y.; Tagami, T.; Okada, H. Improvement of Survival in C6 Rat Glioma Model by a Sustained Drug Release from Localized PLGA Microspheres in a Thermoreversible Hydrogel. Int. J. Pharm. 2012, 427, 299–304. [Google Scholar] [CrossRef]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele Di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry Powders Based on PLGA Nanoparticles for Pulmonary Delivery of Antibiotics: Modulation of Encapsulation Efficiency, Release Rate and Lung Deposition Pattern by Hydrophilic Polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Tahara, K.; Miyazaki, Y.; Kawashima, Y.; Kreuter, J.; Yamamoto, H. Brain Targeting with Surface-Modified Poly(d,l-Lactic-Co-Glycolic Acid) Nanoparticles Delivered via Carotid Artery Administration. Eur. J. Pharm. Biopharm. 2011, 77, 84–88. [Google Scholar] [CrossRef]
- Lloyd, A.W. Interfacial Bioengineering to Enhance Surface Biocompatibility. Med. Device Technol. 2002, 13, 18–21. [Google Scholar]
- Huang, S.; Chen, H.-J.; Deng, Y.-P.; You, X.; Fang, Q.; Lin, M. Preparation of Novel Stable Microbicidal Hydrogel Films as Potential Wound Dressing. Polym. Degrad. Stab. 2020, 181, 109349. [Google Scholar] [CrossRef]
- Bačáková, L.; Novotná, K.; Pařízek, M. Polysaccharides as Cell Carriers for Tissue Engineering: The Use of Cellulose in Vascular Wall Reconstruction. Physiol. Res. 2014, 63, S29–S47. [Google Scholar] [CrossRef]
- Song, Y.; Xu, L.; Xu, L.; Deng, L. Radiation Cross-Linked Gelatin/Sodium Alginate/Carboxymethylcellulose Sodium Hydrogel for the Application as Debridement Glue Paste. Polym. Bull. 2022, 79, 725–742. [Google Scholar] [CrossRef]
- Oh, G.-W.; Kim, S.-C.; Kim, T.-H.; Jung, W.-K. Characterization of an Oxidized Alginate-Gelatin Hydrogel Incorporating a COS-Salicylic Acid Conjugate for Wound Healing. Carbohydr. Polym. 2021, 252, 117145. [Google Scholar] [CrossRef]
- Ozdil, D.; Aydin, H.M. Polymers for Medical and Tissue Engineering Applications: Polymers for Medical and Tissue Engineering Applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef]
- Ma, P.X.; Zhang, R. Synthetic Nano-Scale Fibrous Extracellular Matrix. J. Biomed. Mater. Res. 1999, 46, 60–72. [Google Scholar] [CrossRef]
- Schofer, M.D.; Fuchs-Winkelmann, S.; Gräbedünkel, C.; Wack, C.; Dersch, R.; Rudisile, M.; Wendorff, J.H.; Greiner, A.; Paletta, J.R.J.; Boudriot, U. Influence of Poly(L-Lactic Acid) Nanofibers and BMP-2–Containing Poly(L-Lactic Acid) Nanofibers on Growth and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Sci. World J. 2008, 8, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Liu, B.; Liu, C.; Chou, H.; Ho, M.; Liu, H.; Wang, D.; Hou, L. Bone Tissue Engineering with Novel rhBMP2-PLLA Composite Scaffolds. J. Biomed. Mater. Res. A 2007, 81A, 771–780. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Q.; Xie, W.; Ye, W.; Zhong, L.; Huge, J.; Wang, Y. Performance of 3D Printed PCL/PLGA/HA Biological Bone Tissue Engineering Scaffold. Polym. Compos. 2021, 42, 3593–3602. [Google Scholar] [CrossRef]
- Lin, C.-C.; Fu, S.-J.; Lin, Y.-C.; Yang, I.-K.; Gu, Y. Chitosan-Coated Electrospun PLA Fibers for Rapid Mineralization of Calcium Phosphate. Int. J. Biol. Macromol. 2014, 68, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Kinoshita, Y.; Sato, E.; Maeda, H.; Ozono, S.; Negishi, H.; Kawase, T.; Hiraoka, Y.; Takamoto, T.; Tabata, Y.; et al. Proliferation and Differentiation of Rat Bone Marrow Stromal Cells on Poly(Glycolic Acid)–Collagen Sponge. Tissue Eng. 2005, 11, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, M.; Shalumon, K.T.; Mitha, M.K.; Ganesan, K.; Epple, M. Effect of Hydroxyapatite on the Biodegradation and Biomechanical Stability of Polyester Nanocomposites for Orthopaedic Applications. Acta Biomater. 2010, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Zamora, I.; Alfonso Morales, G.; Castro, J.I.; Ruiz Rojas, L.M.; Valencia-Llano, C.H.; Mina Hernandez, J.H.; Valencia Zapata, M.E.; Grande-Tovar, C.D. Chitosan (CS)/Hydroxyapatite (HA)/Tricalcium Phosphate (β-TCP)-Based Composites as a Potential Material for Pulp Tissue Regeneration. Polymers 2023, 15, 3213. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical Electrospinning and 3D Printing Scaffold Design for Bone Regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Liu, P.S. Mechanical Relation for Porous Metal Foams under Complex Loads of Triaxial Tension and Compression. Mater. Des. 2010, 31, 2264–2269. [Google Scholar] [CrossRef]
- Jiang, N.; Qi, B.; Fan, X.; Yao, L.; Wang, Y.; Zhao, Z.; Xu, Y.; Razali, M.H. Fabrication of Biocompatible and Biodegradable Polyvinyl Alcohol/Sodium Alginate Blend Polymers Incorporating Ca2+ Doped TiO2 Nanocomposite 3D Scaffold for Biomedical Applications. J. Saudi Chem. Soc. 2023, 27, 101758. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Power, R.N.; Genoud, K.J.; Matheson, A.; González-Vázquez, A.; Costard, L.; Eichholz, K.; Pitacco, P.; Hallegouet, T.; Chen, G.; et al. A Multifunctional Scaffold for Bone Infection Treatment by Delivery of microRNA Therapeutics Combined with Antimicrobial Nanoparticles. Adv. Mater. 2024, 36, 2307639. [Google Scholar] [CrossRef]
- Müller, W.E.; Neufurth, M.; Wang, S.; Tolba, E.; Schröder, H.C.; Wang, X. Morphogenetically Active Scaffold for Osteochondral Repair (Polyphosphate/Alginate/N,O-Carboxymethyl Chitosan). Eur. Cell Mater. 2016, 31, 174–190. [Google Scholar] [CrossRef]
- Mahapatra, C.; Jin, G.-Z.; Kim, H.-W. Alginate-Hyaluronic Acid-Collagen Composite Hydrogel Favorable for the Culture of Chondrocytes and Their Phenotype Maintenance. Tissue Eng. Regen. Med. 2016, 13, 538–546. [Google Scholar] [CrossRef]
- González-González, A.M.; Cruz, R.; Rosales-Ibáñez, R.; Hernández-Sánchez, F.; Carrillo-Escalante, H.J.; Rodríguez-Martínez, J.J.; Velasquillo, C.; Talamás-Lara, D.; Ludert, J.E. In Vitro and In Vivo Evaluation of a Polycaprolactone (PCL)/Polylactic-Co-Glycolic Acid (PLGA) (80:20) Scaffold for Improved Treatment of Chondral (Cartilage) Injuries. Polymers 2023, 15, 2324. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, J.M.; Krüger, J.P.; Böss, H.G.; Ketzmar, A.K.; Freymann, U.; Sittinger, M.; Notter, M.; Endres, M.; Kaps, C. Polyglycolic Acid-Hyaluronan Scaffolds Loaded with Bone Marrow-Derived Mesenchymal Stem Cells Show Chondrogenic Differentiation in Vitro and Cartilage Repair in the Rabbit Model. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- McCullen, S.D.; Autefage, H.; Callanan, A.; Gentleman, E.; Stevens, M.M. Anisotropic Fibrous Scaffolds for Articular Cartilage Regeneration. Tissue Eng. Part A 2012, 18, 2073–2083. [Google Scholar] [CrossRef]
- Pulat, G.; Gökmen, O.; Özcan, Ş.; Karaman, O. Collagen Binding and Mimetic Peptide-Functionalized Self-Assembled Peptide Hydrogel Enhance Chondrogenic Differentiation of Human Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2025, 113, e37786. [Google Scholar] [CrossRef]
- Biomaterials and Neural Regeneration: Neural Regeneration Research. Available online: https://journals.lww.com/nrronline/fulltext/2020/15070/biomaterials_and_neural_regeneration.8.aspx (accessed on 25 September 2025).
- Durgam, H.; Sapp, S.; Deister, C.; Khaing, Z.; Chang, E.; Luebben, S.; Schmidt, C.E. Novel Degradable Co-Polymers of Polypyrrole Support Cell Proliferation and Enhance Neurite Out-Growth with Electrical Stimulation. J. Biomater. Sci. Polym. Ed. 2010, 21, 1265–1282. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-Coated Electrospun PLGA Nanofibers for Neural Tissue Applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef]
- Tarus, D.; Hamard, L.; Caraguel, F.; Wion, D.; Szarpak-Jankowska, A.; Van Der Sanden, B.; Auzély-Velty, R. Design of Hyaluronic Acid Hydrogels to Promote Neurite Outgrowth in Three Dimensions. ACS Appl. Mater. Interfaces 2016, 8, 25051–25059. [Google Scholar] [CrossRef]
- Pinho, T.S.; Cunha, C.B.; Lanceros-Méndez, S.; Salgado, A.J. Electroactive Smart Materials for Neural Tissue Regeneration. ACS Appl. Bio Mater. 2021, 4, 6604–6618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Sun, C.; Hu, W.; Zhao, J.; Li, G.; Zhang, L.; Liu, M.; Liu, Y.; Ding, F.; et al. Chitosan Degradation Products Promote Nerve Regeneration by Stimulating Schwann Cell Proliferation via miR-27a/FOXO1 Axis. Mol. Neurobiol. 2016, 53, 28–39. [Google Scholar] [CrossRef]
- Jiang, M.; Cheng, Q.; Su, W.; Wang, C.; Yang, Y.; Cao, Z.; Ding, F. The Beneficial Effect of Chitooligosaccharides on Cell Behavior and Function of Primary Schwann Cells Is Accompanied by Up-Regulation of Adhesion Proteins and Neurotrophins. Neurochem. Res. 2014, 39, 2047–2057. [Google Scholar] [CrossRef]
- Jin, J.; Ji, Z.; Xu, M.; Liu, C.; Ye, X.; Zhang, W.; Li, S.; Wang, D.; Zhang, W.; Chen, J.; et al. Microspheres of Carboxymethyl Chitosan, Sodium Alginate, and Collagen as a Hemostatic Agent in Vivo. ACS Biomater. Sci. Eng. 2018, 4, 2541–2551. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Bhattarai, N.; Viswanathamurthi, P.; Yi, H.K.; Hwang, P.H.; Kim, H.Y. Hydrophilic Nanofibrous Structure of Polylactide; Fabrication and Cell Affinity. J. Biomed. Mater. Res. A 2006, 78, 247–257. [Google Scholar] [CrossRef]
- Sharifi, F.; Irani, S.; Zandi, M.; Soleimani, M.; Atyabi, S.M. Comparative of Fibroblast and Osteoblast Cells Adhesion on Surface Modified Nanofibrous Substrates Based on Polycaprolactone. Prog. Biomater. 2016, 5, 213–222. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Peng, C.; Huang, T.; Zhou, H.; Ou, B.; Chen, J.; Liu, Q.; He, S.; Cao, D.; et al. Fabrication and Physical Properties of Gelatin/Sodium Alginate/Hyaluronic Acid Composite Wound Dressing Hydrogel. J. Macromol. Sci. Part A 2014, 51, 318–325. [Google Scholar] [CrossRef]
- Haidari, H.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Bacteria-Activated Dual pH- and Temperature-Responsive Hydrogel for Targeted Elimination of Infection and Improved Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 51744–51762. [Google Scholar] [CrossRef] [PubMed]
- Soletti, L.; Hong, Y.; Guan, J.; Stankus, J.J.; El-Kurdi, M.S.; Wagner, W.R.; Vorp, D.A. A Bilayered Elastomeric Scaffold for Tissue Engineering of Small Diameter Vascular Grafts. Acta Biomater. 2010, 6, 110–122. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; An, Q.; Li, D.; Liu, P.; Zhu, W.; Mo, X. Electrospun Poly(l-Lactic Acid-Co-ε-Caprolactone) Fibers Loaded with Heparin and Vascular Endothelial Growth Factor to Improve Blood Compatibility and Endothelial Progenitor Cell Proliferation. Colloids Surf. B Biointerfaces 2015, 128, 106–114. [Google Scholar] [CrossRef]
- Radisic, M. Biomaterials for Cardiac Tissue Engineering. Biomed. Mater. 2015, 10, 030301. [Google Scholar] [CrossRef] [PubMed]
- Mihic, A.; Cui, Z.; Wu, J.; Vlacic, G.; Miyagi, Y.; Li, S.-H.; Lu, S.; Sung, H.-W.; Weisel, R.D.; Li, R.-K. A Conductive Polymer Hydrogel Supports Cell Electrical Signaling and Improves Cardiac Function After Implantation into Myocardial Infarct. Circulation 2015, 132, 772–784. [Google Scholar] [CrossRef]
- Navaei, A.; Moore, N.; Sullivan, R.; Truong, D.; Migrino, R.; Nikkhah, M. Electrically Conductive Hydrogel-Based Micro-Topographies for the Development of Organized Cardiac Tissues. RSC Adv. 2017, 7, 3302–3312. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, W.M. Heating/Solvent Responsive Shape-Memory Polymers for Implant Biomedical Devices in Minimally Invasive Surgery: Current Status and Challenge. Macromol. Biosci. 2020, 20, 2000108. [Google Scholar] [CrossRef]
- Sivaraman, S.; Amoroso, N.; Gu, X.; Purves, J.T.; Hughes, F.M.; Wagner, W.R.; Nagatomi, J. Evaluation of Poly (Carbonate-Urethane) Urea (PCUU) Scaffolds for Urinary Bladder Tissue Engineering. Ann. Biomed. Eng. 2019, 47, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.L.; Cook, W.D.; Thouas, G.A.; Chen, Q.Z. The Mechanical Characteristics and in Vitro Biocompatibility of Poly(Glycerol Sebacate)-Bioglass Elastomeric Composites. Biomaterials 2010, 31, 8516–8529. [Google Scholar] [CrossRef]
- Full, S.M.; Delman, C.; Gluck, J.M.; Abdmaulen, R.; Shemin, R.J.; Heydarkhan-Hagvall, S. Effect of Fiber Orientation of Collagen-Based Electrospun Meshes on Human Fibroblasts for Ligament Tissue Engineering Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 103, 39. [Google Scholar] [CrossRef]
- Sensini, A.; Gualandi, C.; Focarete, M.L.; Belcari, J.; Zucchelli, A.; Boyle, L.; Reilly, G.C.; Kao, A.P.; Tozzi, G.; Cristofolini, L. Multiscale Hierarchical Bioresorbable Scaffolds for the Regeneration of Tendons and Ligaments. Biofabrication 2019, 11, 035026. [Google Scholar] [CrossRef]
- Khosravimelal, S.; Mobaraki, M.; Eftekhari, S.; Ahearne, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Hydrogels as Emerging Materials for Cornea Wound Healing. Small 2021, 17, 2006335. [Google Scholar] [CrossRef]
- Ahadian, S.; Khademhosseini, A. Smart Scaffolds in Tissue Regeneration. Regen. Biomater. 2018, 5, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef]
- Pilgrim, T.; Muller, O.; Heg, D.; Roffi, M.; Kurz, D.J.; Moarof, I.; Weilenmann, D.; Kaiser, C.; Tapponnier, M.; Losdat, S.; et al. Biodegradable- Versus Durable-Polymer Drug-Eluting Stents for STEMI. JACC Cardiovasc. Interv. 2021, 14, 639–648. [Google Scholar] [CrossRef] [PubMed]
- The Development of Polymeric Biomaterials Inspired by the Extracellular Matrix. Available online: https://www.tandfonline.com/doi/epdf/10.1080/09205063.2017.1297285?needAccess=true (accessed on 25 September 2025).
- Patel, B.B.; Chakraborty, S. Biodegradable Polymers: Emerging Excipients for the Pharmaceutical and Medical Device Industries. J. Excip. Food Chem. 2013, 4, 126–157. [Google Scholar]
- Adeosun, S.O.; Lawal, G.I.; Gbenebor, O.P. Characteristics of Biodegradable Implants. J. Miner. Mater. Charact. Eng. 2014, 2, 88–106. [Google Scholar] [CrossRef]
- Charles, L.F.; Shaw, M.T.; Olson, J.R.; Wei, M. Fabrication and Mechanical Properties of PLLA/PCL/HA Composites via a Biomimetic, Dip Coating, and Hot Compression Procedure. J. Mater. Sci. Mater. Med. 2010, 21, 1845–1854. [Google Scholar] [CrossRef]
- Russias, J.; Saiz, E.; Nalla, R.K.; Gryn, K.; Ritchie, R.O.; Tomsia, A.P. Fabrication and Mechanical Properties of PLA/HA Composites: A Study of in Vitro Degradation. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2006, 26, 1289. [Google Scholar] [CrossRef]
- Kumar, A.; Mir, M.; Aldulijan, I.; Mahajan, A.; Anwar, A.; Leon, C.H.; Terracciano, A.; Zhao, X.; Su, T.-L.; Kalyon, D.M.; et al. Load-Bearing Biodegradable PCL-PGA-Beta TCP Scaffolds for Bone Tissue Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 193–200. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, Biodegradation and Excretion of Polylactic Acid (PLA) in Medical Implants and Theranostic Systems. Chem. Eng. J. 2018, 340, 9. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, Biodegradation and Biomedical Applications of Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Micro and Nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Pérez-Davila, S.; Garrido-Gulías, N.; González-Rodríguez, L.; López-Álvarez, M.; Serra, J.; López-Periago, J.E.; González, P. Physicochemical Properties of 3D-Printed Polylactic Acid/Hydroxyapatite Scaffolds. Polymers 2023, 15, 2849. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zheng, W.; Shi, K.; Wang, W.; Shao, Y.; Zhang, D. An in Vivo Evaluation of PLLA/PLLA-gHA Nano-Composite for Internal Fixation of Mandibular Bone Fractures. Biomed. Mater. 2015, 10, 065007. [Google Scholar] [CrossRef]
- Tang, Y.; Singh, J. Biodegradable and Biocompatible Thermosensitive Polymer Based Injectable Implant for Controlled Release of Protein. Int. J. Pharm. 2009, 365, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Koutserimpas, C.; Alpantaki, K.; Chatzinikolaidou, M.; Chlouverakis, G.; Dohm, M.; Hadjipavlou, A.G. The Effectiveness of Biodegradable Instrumentation in the Treatment of Spinal Fractures. Injury 2018, 49, 2111–2120. [Google Scholar] [CrossRef]
- Kukk, A.; Nurmi, J.T. A Retrospective Follow-up of Ankle Fracture Patients Treated with a Biodegradable Plate and Screws. Foot Ankle Surg. 2009, 15, 192–197. [Google Scholar] [CrossRef]
- Amini, A.R.; Wallace, J.S.; Nukavarapu, S.P. Short-Term and Long-Term Effects of Orthopedic Biodegradable Implants. J. Long-Term Eff. Med. Implants 2011, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Grayson, A.C.R.; Voskerician, G.; Lynn, A.; Anderson, J.M.; Cima, M.J.; Langer, R. Differential Degradation Rates in Vivo and in Vitro of Biocompatible Poly(Lactic Acid) and Poly(Glycolic Acid) Homo- and Co-Polymers for a Polymeric Drug-Delivery Microchip. J. Biomater. Sci. Polym. Ed. 2004, 15, 1281–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial; Semantic Scholar: Seattle, WA, USA, 2017. [Google Scholar]
- Gore, M. Evaluation of Safety and Efficacy of Barrier Foam Dressing in Patients with Exuding Wounds. Biomed. J. Sci. Tech. Res. 2021, 33, 26292–26297. [Google Scholar] [CrossRef]
- Haik, J.; Ullman, Y.; Gur, E.; Ad-El, D.; Egozi, D.; Kruchevsky, D.; Zissman, S.; Biros, E.; Nir, R.; Kornhaber, R.; et al. Advances in the Use of Electrospun Nanofibrous Polymeric Matrix for Dermal Healing at the Donor Site after the Split-Thickness Skin Graft Excision: A Prospective, Randomized, Controlled, Open-Label, Multicenter Study. J. Burn Care Res. 2021, 43, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Jamlang, J.; Marañon, M.T.; Rigor, G.J.; Tumolva, T. Developing a Physically Cross-Linked Hydroxyethyl Cellulose Hydrogel for Wound Dressing Applications. Mater. Sci. Forum 2019, 947, 3–12. [Google Scholar] [CrossRef]
- Abulayzied, D.; Alturki, A.; Alatawi, A.; Bousbih, R.; Alamlah, R.; Abomostafa, H.; El Komy, G.; Abdel Aziz Taha, M.; Youness, R.A. Production of Polyvinyl Alcohol/Natural Hydroxyapatite/Magnesia/Silicon Carbide Hybrid Composites for Use in Orthopedic Applications: Optical, Electrical, and Mechanical Propertie. Egypt. J. Chem. 2024, 67, 397–409. [Google Scholar] [CrossRef]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical Properties and in Vivo Behavior of a Biodegradable Synthetic Polymer Microfiber–Extracellular Matrix Hydrogel Biohybrid Scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef]
- Tripathi, S.; Dixit, P. Comparative Evaluation of Efficacy of Cyanoacrylate and Suture Material in The Management of Operative Skin Wounds. Asian J. Pharm. Clin. Res. 2022, 15, 59–61. [Google Scholar] [CrossRef]
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B. Alemtoshi PHA-Based Bioplastic: A Potential Alternative to Address Microplastic Pollution. Water Air Soil Pollut. 2023, 234, 21. [Google Scholar] [CrossRef]
- Kubowicz, S.; Booth, A.M. Biodegradability of Plastics: Challenges and Misconceptions. Environ. Sci. Technol. 2017, 51, 12058–12060. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of Biodegradable Materials in Food Packaging: A Review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Gupta, R.; Guha, P.; Srivastav, P. Natural Polymers in Bio-Degradable/Edible Film: A Review on Environmental Concerns, Cold Plasma Technology and Nanotechnology Application on Food Packaging—A Recent Trends. Food Chem. Adv. 2022, 1, 100135. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Drozłowska, E. Polysaccharide/Gelatin Blend Films as Carriers of Ascorbyl Palmitate—A Comparative Study. Food Chem. 2020, 333, 127465. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Z.S.; Chatta, A.M.; Shafi, J.; Waheed, K.N.; Saleem, S.; Hanif, M.M. The Effect of Natural Edible Coatings on Chemical, Microbial, and Sensory Quality of Tilapia during Frozen Storage. J. Food Qual. Hazards Control 2023, 10, 163–174. [Google Scholar] [CrossRef]
- Uzundağ, D.; Can, Ö.P.; Saraç, M.G. Extending the Shelf Life of Unsalted White Cheese Produced for Special Dietary Preferences: Role of Essential Oils and Coating. Harran Tarım Gıda Bilim. Derg. 2025, 29, 191–204. [Google Scholar] [CrossRef]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R. Optimizing the Mechanical and Physical Properties of Thermoplastic Starch via Tuning the Molecular Microstructure through Co-plasticization by Sorbitol and Glycerol. Polym. Int. 2017, 66, 809–819. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Oprea, O.-C.; Sonmez, M.; Ficai, A.; Motelica, L.; Ficai, D.; Georgescu, M.; Gurau, D.F. Structural and Thermal Characterization of Some Thermoplastic Starch Mixtures. Polysaccharides 2024, 5, 504–522. [Google Scholar] [CrossRef]
- González Carmona, E.; Schlapp-Hackl, I.; Jääskeläinen, S.; Järvinen, M.; Nieminen, K.; Sawada, D.; Hummel, M.; Sixta, H. Development of Cellulose Films by Means of the Ioncell® Technology, as an Alternative to Commercial Films. Cellulose 2023, 30, 11633–11648. [Google Scholar] [CrossRef]
- Yang, Q.; Saito, T.; Isogai, A. Transparent, Flexible, and High-strength Regenerated Cellulose/Saponite Nanocomposite Films with High Gas Barrier Properties. J. Appl. Polym. Sci. 2013, 130, 3168–3174. [Google Scholar] [CrossRef]
- Zhao, S.; Bian, Y.; Zhang, G.; Yang, G.; Hou, X.; Gui, J.; Mu, S.; Liu, S.; Fang, Y. Shelf-life Extension of Pacific White Shrimp (Litopenaeus Vannamei) Using Sodium Alginate/Chitosan Incorporated with Cell-free Supernatant of Streptococcus Thermophilus FUA 329 during Cold Storage. J. Food Sci. 2024, 89, 1976–1987. [Google Scholar] [CrossRef]
- Putranti, L.N.; Nugraheni, P.S. Effect of Carboxymethyl Cellulose Addition on the Characteristic of Chitosan-Based Bioplastic. IOP Conf. Ser. Earth Environ. Sci. 2023, 1289, 012038. [Google Scholar] [CrossRef]
- Raghav, P.T.; Narayanapurapu; de Bellevue, S.A. Effect of Composite Edible Coatings and Abiotic Stress on Post Harvest Quality of Fruits. Available online: https://www.semanticscholar.org/paper/EFFECT-OF-COMPOSITE-EDIBLE-COATINGS-AND-ABIOTIC-ON-Raghav-Narayanapurapu/5badccb98be6ef0c40ca4309c1d21df125943eeb (accessed on 25 September 2025).
- Tullo, A. A Biodegradable Polymer Hits the Big Time. CEN Glob. Enterp. 2021, 99, 26–27. [Google Scholar] [CrossRef]
- Aversa, C.; Barletta, M.; Cappiello, G.; Gisario, A. Compatibilization Strategies and Analysis of Morphological Features of Poly(Butylene Adipate-Co-Terephthalate) (PBAT)/Poly(Lactic Acid) PLA Blends: A State-of-Art Review. Eur. Polym. J. 2022, 173, 111304. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Chen, H.; Liu, H.; Chen, L.; Yu, L. Thermal Degradation and Combustion Properties of Most Popular Synthetic Biodegradable Polymers. Waste Manag. Res. J. Sustain. Circ. Econ. 2023, 41, 431–441. [Google Scholar] [CrossRef]
- Auras, R.; Singh, S.; Singh, J. Performance Evaluation of PLA against Existing PET and PS Containers. J. Test. Eval. 2006, 34, 530–536. [Google Scholar] [CrossRef]
- Ali, N. Effect of Polyethylene Terphalet (PET) on Mechanical and Optical Properties of Polylactic Acid (PLA) for Packaging Application. Available online: https://www.semanticscholar.org/paper/Effect-of-Polyethylene-Terphalet-(PET)-On-and-of-Ali/ad324e13added4d76d366a9c134b9df5d1172f29 (accessed on 25 September 2025).
- Qi, Y.; Zhai, H.; Sun, Y.; Xu, H.; Wu, S.; Chen, S. Electrospun Hybrid Nanofibrous Meshes with Adjustable Performance for Potential Use in Soft Tissue Engineering. Text. Res. J. 2021, 92, 1537–1549. [Google Scholar] [CrossRef]
- Klabukov, I.; Tenchurin, T.; Shepelev, A.; Baranovskii, D.; Mamagulashvili, V.; Dyuzheva, T.; Krasilnikova, O.; Balyasin, M.; Lyundup, A.; Krasheninnikov, M.; et al. Biomechanical Behaviors and Degradation Properties of Multilayered Polymer Scaffolds: The Phase Space Method for Bile Duct Design and Bioengineering. Biomedicines 2023, 11, 745. [Google Scholar] [CrossRef]
- Nagy, B.; Miskolczi, N.; Eller, Z. Evaluation of the Effect of Castor Oil-Based Experimental Additives on Pla/Starch Foils. Hung. J. Ind. Chem. 2023, 51, 35–41. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Zhao, W.; Huang, Z.; Liu, H.; Ma, L.; Yang, L. Preparation and Properties of Poly (Butylene-adipate-co-terephthalate) /Thermoplastic Hydroxypropyl Starch Films. Polym. Int. 2024, 73, 761–769. [Google Scholar] [CrossRef]
- Alothman, O.Y.; Shaikh, H.M.; Alshammari, B.A.; Jawaid, M. Structural, Morphological and Thermal Properties of Nano Filler Produced from Date Palm-Based Micro Fibers (Phoenix dactylifera L.). J. Polym. Environ. 2022, 30, 622–630. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dufresne, A. Sustainable Biodegradable Coffee Grounds Filler and Its Effect on the Hydrophobicity, Mechanical and Thermal Properties of Biodegradable PBAT Composites. J. Appl. Polym. Sci. 2017, 134, app.44498. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Jawaid, M.; Saba, N. Inamuddin Effect of Cellulose Nano Fibers and Nano Clays on the Mechanical, Morphological, Thermal and Dynamic Mechanical Performance of Kenaf/Epoxy Composites. Carbohydr. Polym. 2020, 239, 116248. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Studies on Mechanical, Thermal, and Barrier Properties of Carboxymethyl Cellulose Film Highly Filled with Nanocellulose. J. Thermoplast. Compos. Mater. 2019, 32, 995–1014. [Google Scholar] [CrossRef]
- Ma, F.; Chen, S.; Liu, P.; Geng, F.; Li, W.; Liu, X.; He, D.; Pan, D. Improvement of β-TCP/PLLA Biodegradable Material by Surface Modification with Stearic Acid. Mater. Sci. Eng. C 2016, 62, 407–413. [Google Scholar] [CrossRef]
- De La Rosa-Ramírez, H.; Aldas, M.; Ferri, J.M.; Pawlak, F.; López-Martínez, J.; Samper, M.D. Control of Biodegradability Under Composting Conditions and Physical Performance of Poly (Lactic Acid) Based Materials Modified with Phenolic-Free Rosin Resin. J. Polym. Environ. 2023, 31, 5462–5476. [Google Scholar] [CrossRef]
- Samorì, P.; Kinloch, I.A.; Feng, X.; Palermo, V. Graphene-Based Nanocomposites for Structural and Functional Applications: Using 2-Dimensional Materials in a 3-Dimensional World. 2D Mater. 2015, 2, 030205. [Google Scholar] [CrossRef]
- Drozdov, A.; Christiansen, J.; Klitkou, R. Volume Growth and Viscoplasticity of Polymer/Clay Nanocomposites. Available online: https://www.semanticscholar.org/paper/Volume-growth-and-viscoplasticity-of-polymer-clay-Drozdov-Christiansen/04a32ceebb37d07312ef4221fb2d24dae53776ad (accessed on 25 September 2025).
- Rezvani, M.B.; Atai, M.; Hamze, F.; Hajrezai, R. The Effect of Silica Nanoparticles on the Mechanical Properties of Fiber-Reinforced Composite Resins. J. Dent. Res. Dent. Clin. Dent. Prospect. 2016, 10, 112–117. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, N.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.; Eldesoukey, I.; Masoud, M. Graphene Oxide Decorated with Zinc Oxide Nanoflower, Silver and Titanium Dioxide Nanoparticles: Fabrication, Characterization, DNA Interaction, and Antibacterial Activity. RSC Adv. 2019, 9, 3704–3714. [Google Scholar] [CrossRef]
- Mandal, D.; Mualchin, M. Effects of Essential Oils on Post Harvest Quality and Shelf Life of Mango (Mangifera indica L.). Bangladesh J. Bot. 2021, 50, 1143–1149. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Trabalza-Marinucci, M.; Miraglia, D.; Roila, R.; Acuti, G.; Giusepponi, D.; Dal Bosco, A.; Ranucci, D. Effects of Olive Mill Vegetation Water Phenol Metabolites Transferred to Muscle through Animal Diet on Rabbit Meat Microbial Quality. Sustainability 2021, 13, 4522. [Google Scholar] [CrossRef]
- Miraglia, D.; Castrica, M.; Esposto, S.; Roila, R.; Selvaggini, R.; Urbani, S.; Taticchi, A.; Sordini, B.; Veneziani, G.; Servili, M. Quality Evaluation of Shrimp (Parapenaeus longirostris) Treated with Phenolic Extract from Olive Vegetation Water during Shelf-Life, before and after Cooking. Foods 2021, 10, 2116. [Google Scholar] [CrossRef]
- Martinelli, M.; Bruner, M. The Effect of Cinnamon Extract on the Prevention of Fruit Spoilage via Delay in Microbial Growth. J. Stud. Res. 2024, 13, 1–8. [Google Scholar] [CrossRef]
- Fiorentini, C.; Leni, G.; De Apodaca, E.D.; Fernández-de-Castro, L.; Rocchetti, G.; Cortimiglia, C.; Spigno, G.; Bassani, A. Development of Coated PLA Films Containing a Commercial Olive Leaf Extract for the Food Packaging Sector. Antioxidants 2024, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Bouaziz, M. Polylactide Films with the Addition of Olive Leaf Extract—Physico-Chemical Characterization. Materials 2021, 14, 7623. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.; Jafari, S. pH-Sensitive (Halochromic) Smart Packaging Films Based on Natural Food Colorants for the Monitoring of Food Quality and Safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Ma, L.; Long, T.; Yuan, S.; Qi, P.; Han, L.; Hao, J. A pH-Indicating Smart Tag Based on Porous Hydrogel as Food Freshness Sensors. J. Colloid Interface Sci. 2023, 647, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Y.; Zhao, L.; Wang, Y. Anthocyanin-Based pH-Sensitive Smart Packaging Films for Monitoring Food Freshness. J. Agric. Food Res. 2022, 9, 100340. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Padua, G.W. Review: Nanocomposites in Food Packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Saharan, N.; Wadhwa, N. A Comprehensive Review of Biodegradable Polymers in Sustainable Packaging Applications. Res. Rev. J. Food Sci. Technol. 2024, 13, 28–37. [Google Scholar] [CrossRef]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable Starch/Clay Nanocomposite Films for Food Packaging Applications. Food Chem. 2005, 93, 467–474. [Google Scholar] [CrossRef]
- Brodhagen, M.; Goldberger, J.R.; Hayes, D.G.; Inglis, D.A.; Marsh, T.L.; Miles, C. Considerations for Limiting Unintended Residual Plastic in Agricultural Soils. Environ. Sci. Policy 2017, 69, 81–84. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and Biodegradable Mulches for Agricultural Applications: A Review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Torquebiau, E.; Rosenzweig, C.; Chatrchyan, A.M.; Andrieu, N.; Khosla, R. Identifying Climate-Smart Agriculture Research Needs. Cah. Agric. 2018, 27, 26001. [Google Scholar] [CrossRef]
- Cirujeda, A.; Pueyo, J.; Moreno, M.M.; Moreno, C.; Villena, J.; López-Marín, J.; Romero-Muñoz, M.; Pardo, G. Weed Control in Perennial Crops Using Hydromulch Compositions Based on the Circular Economy: Field Trial Results. J. Crop Health 2024, 76, 1101–1116. [Google Scholar] [CrossRef]
- Alptekin, H.; Gürbüz, R. The Effect of Organic Mulch Materials on Weed Control in Cucumber (Cucumis sativus L.) Cultivation. J. Agric. 2022, 5, 68–79. [Google Scholar] [CrossRef]
- Li, Y.-C.; Li, H.-P.; Wang, Y.-X.; Sun, Y.-P.; Wang, K.-R.; Yang, Q.-X. Pollution Status and Control Countermeasures of Polyethylene Mulch Film Residue in Farmland Soils of Qingdao City, China. Available online: https://www.semanticscholar.org/paper/Pollution-Status-and-Control-Countermeasures-of-in-Yan-chao-Hai-ping/45db99d64c7d02aa82aff8a53abcdf2edcafa113 (accessed on 25 September 2025).
- Vargha, V.; Rétháti, G.; Heffner, T.; Pogácsás, K.; Korecz, L.; László, Z.; Czinkota, I.; Tolner, L.; Kelemen, O. Behavior of Polyethylene Films in Soil. Period. Polytech. Chem. Eng. 2016, 60, 60–68. [Google Scholar] [CrossRef]
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene Film Incorporation into the Horticultural Soil of Small Periurban Production Units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef]
- Halley, P.; Rutgers, R.; Coombs, S.; Kettels, J.; Gralton, J.; Christie, G.; Jenkins, M.; Beh, H.; Griffin, K.; Jayasekara, R.; et al. Developing Biodegradable Mulch Films from Starch-Based Polymers. Starch-Stärke 2001, 53, 362. [Google Scholar] [CrossRef]
- Barragán, D.H.; Pelacho, A.M.; Martin-Closas, L. Degradation of Agricultural Biodegradable Plastics in the Soil under Laboratory Conditions. Soil Res. 2016, 54, 216–224. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, Y.; He, S.; Leng, W.; Chen, Y.; Dai, J.; Sun, M.; Wu, Z.; Li, J. Biomass-Derived Transparent Bamboo Composite Films with Europium-Based Photoconversion for Energy-Efficient Smart Agriculture. Energy Environ. Mater. 2025, e70125. [Google Scholar] [CrossRef]
- Popović, S.; Hromiš, N.; Šuput, D.; Bulut, S.; Vitas, S.; Savić, M.; Lazić, V. Pumpkin Seed Oil Cake/Polyethylene Film as New Food Packaging Material, with Perspective for Packing under Modified Atmosphere. Packag. Technol. Sci. 2021, 34, 25–33. [Google Scholar] [CrossRef]
- Titova, L.M.; Nugmanov, A. Study of the Properties of Biodegradable Mulching Coating Obtained from Waste Water Treatment Waste. Oil Gas Technol. Environ. Saf. 2025, 2025, 35–42. [Google Scholar] [CrossRef]
- Sham, I.N.; Yap, C.K.; Nulit, R.; Peng, S.H.T.; Chai, E.W. Nutrient Leaching in Oil Palm Plantation: A Review on Special Reference to Fertilization Application. Pak. J. Life Soc. Sci. PJLSS 2024, 22, 855–877. [Google Scholar] [CrossRef]
- Albano, J.; Merhaut, D.; Blythe, E.; Newman, J. Nutrient Release from Controlled-Release Fertilizers in a Neutral-pH Substrate in an Outdoor Environment: II. Leachate Calcium, Magnesium, Iron, Manganese, Zinc, Copper, and Molybdenum Concentrations. Hortscience 2006, 41, 1674–1682. [Google Scholar] [CrossRef]
- Jin, S.; Wang, Y.; He, J.; Yang, Y.; Yu, X.; Yue, G. Preparation and Properties of a Degradable Interpenetrating Polymer Networks Based on Starch with Water Retention, Amelioration of Soil, and Slow Release of Nitrogen and Phosphorus Fertilizer. J. Appl. Polym. Sci. 2013, 128, 407–415. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N.; John, K.S.; Sreekumar, J. Cassava Starch Based Superabsorbent Polymer as Soil Conditioner: Impact on Soil Physico-Chemical and Biological Properties and Plant Growth. CLEAN–Soil Air Water 2014, 42, 1610–1617. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Tariq, Z.; Philips, B.; Sadiqa, A.; Ahmad, M.; Al-Ahmary, K.M.; Ali, I.; Ahmed, M. Nanocellulose/Wood Ash-Reinforced Starch–Chitosan Hydrogel Composites for Soil Conditioning and Their Impact on Pea Plant Growth. RSC Adv. 2024, 14, 8652–8664. [Google Scholar] [CrossRef]
- Akhter, M.; Shah, G.A.; Niazi, M.B.K.; Mir, S.; Jahan, Z.; Rashid, M.I. Novel Water-soluble Polymer Coatings Control NPK Release Rate, Improve Soil Quality and Maize Productivity. J. Appl. Polym. Sci. 2021, 138, 51239. [Google Scholar] [CrossRef]
- Nooeaid, P.; Chuysinuan, P.; Pitakdantham, W.; Aryuwananon, D.; Techasakul, S.; Dechtrirat, D. Eco-Friendly Polyvinyl Alcohol/Polylactic Acid Core/Shell Structured Fibers as Controlled-Release Fertilizers for Sustainable Agriculture. J. Polym. Environ. 2021, 29, 552–564. [Google Scholar] [CrossRef]
- Qiao, D.; Li, J.; Zhang, S.; Yang, X. Controlled Release Fertilizer with Temperature-Responsive Behavior Coated Using Polyether Polyol (PPG)/Polycaprolactone (PCL) Blend-Based Polyurethane Performs Smart Nutrient Release. Mater. Today Chem. 2022, 26, 101249. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Meena, S.; Janaki, P.; Karthikeyan, S.; Nalina, L. Effect of Addition of Biopolymer Coated DAP on Phosphorus Release Kinetics over an Incubation Time. Res. Crops 2022, 23, 781–786. [Google Scholar] [CrossRef]
- An, T.; Qin, Y.; Cheng, H.; Wu, J.; Su, W.; Meng, G.; Wei, H.; Sun, C.; Liu, Z.; Guo, X. TiO2-WO3 Activated Weathered Lignite Coating Phosphate Fertilizer to Improve Longitudinal Migration Efficiency. J. Clean. Prod. 2022, 351, 131549. [Google Scholar] [CrossRef]
- Firmanda, A.; Fahma, F.; Syamsu, K.; Suryanegara, L.; Wood, K. Controlled/Slow-Release Fertilizer Based on Cellulose Composite and Its Impact on Sustainable Agriculture: Review. Biofuels Bioprod. Biorefin. 2022, 16, 1909–1930. [Google Scholar] [CrossRef]
- Kontárová, S.; Přikryl, R.; Škarpa, P.; Kriška, T.; Antošovský, J.; Gregušková, Z.; Figalla, S.; Jašek, V.; Sedlmajer, M.; Menčík, P.; et al. Slow-Release Nitrogen Fertilizers with Biodegradable Poly(3-Hydroxybutyrate) Coating: Their Effect on the Growth of Maize and the Dynamics of N Release in Soil. Polymers 2022, 14, 4323. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Li, B.; Zhang, M.; Lu, Q.; Zhang, B.; Miao, Z.; Li, L.; Zheng, T.; Liu, P. Application and Development of Modern 3D Printing Technology in the Field of Orthopedics. BioMed Res. Int. 2022, 2022, 8759060. [Google Scholar] [CrossRef]
- Veeman, D.; Sai, M.S.; Sureshkumar, P.; Jagadeesha, T.; Natrayan, L.; Ravichandran, M.; Mammo, W.D. Additive Manufacturing of Biopolymers for Tissue Engineering and Regenerative Medicine: An Overview, Potential Applications, Advancements, and Trends. Int. J. Polym. Sci. 2021, 2021, 1–20. [Google Scholar] [CrossRef]
- Palaniappan, M.; Tirlangi, S.; Mohamed, M.J.S.; Moorthy, R.M.S.; Valeti, S.V.; Boopathi, S. Fused Deposition Modelling of Polylactic Acid (PLA)-Based Polymer Composites: A Case Study. In Advances in Chemical and Materials Engineering; Keshavamurthy, R., Tambrallimath, V., Davim, J.P., Eds.; IGI Global: Hershey, PA, USA, 2023; pp. 66–85. ISBN 978-1-6684-6009-2. [Google Scholar]
- Raveverma, P.; Ibrahim, M.; Sa’ude, N.; Yarwindran, M. Mechanical Behaviour and Compatibility Analysis of Thermoplastic Polyurethane/ Polycaprolactone-Based New Fused Deposition Modelling Filament Composite. J. Mech. Eng. 2018, SI 5, 116–128. [Google Scholar]
- Banothu, D.; Kumar, P.; Ali, S.G.M.; Reddy, R.; Gobinath, R.; Dhanapalan, S. Design, Fabrication, and in Vitro Evaluation of a 3D Printed, Bio-Absorbable PLA Tibia Bone Implant with a Novel Lattice Structure. Biomed. Phys. Eng. Express 2025, 11, 055015. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Chatzinikolaidou, M.; Michalakis, K.; Tsouknidas, A. Clinical Translation of Polycaprolactone-Based Tissue Engineering Scaffolds, Fabricated via Additive Manufacturing: A Review of Their Craniofacial Applications. Biomater. Adv. 2024, 162, 213902. [Google Scholar] [CrossRef]
- Shao, X.X.; Hutmacher, D.W.; Ho, S.T.; Goh, J.C.H.; Lee, E.H. Evaluation of a Hybrid Scaffold/Cell Construct in Repair of High-Load-Bearing Osteochondral Defects in Rabbits. Biomaterials 2006, 27, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chow, L.C.; Frukhtbeyn, S.A.; Ting, A.H.; Dong, Q.; Yang, M.; Mitchell, J.W. Improve the Strength of PLA/HA Composite through the Use of Surface Initiated Polymerization and Phosphonic Acid Coupling Agent. J. Res. Natl. Inst. Stand. Technol. 2011, 116, 785. [Google Scholar] [CrossRef]
- Leung, L.; DiRosa, A.; Naguib, H. Physical and Mechanical Properties of Poly(E-Caprolactone)–Hydroxyapatite Composites for Bone Tissue Engineering Applications. Available online: https://www.semanticscholar.org/paper/Physical-and-Mechanical-Properties-of-Composites-Leung-DiRosa/fa6093b2ab275c44d338143c8c390ddb234aa8dc (accessed on 25 September 2025).
- Bondarenko, S.; Filipenko, V.; Ashukina, N.; Maltseva, V.Y.; Ivanov, G.; Lazarenko, I.; Sereda, D.; Schwarzkopf, R. World Journal of Orthopedics. Available online: https://www.semanticscholar.org/paper/World-Journal-of-Orthopedics-Bondarenko-Filipenko/65d0fc3ba86a6c5e931286161621e65a87468b62 (accessed on 25 September 2025).
- Fujibayashi, S.; Takemoto, M.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Sasaki, K.; Mori, S.; Matsuda, S. Stand-Alone Anterior Cervical Discectomy and Fusion Using an Additive Manufactured Individualized Bioactive Porous Titanium Implant without Bone Graft: Results of a Prospective Clinical Trial. Asian Spine J. 2021, 15, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Ragone, V.; Canciani, E.; Arosio, M.; Olimpo, M.; Piras, L.A.; Von Degerfeld, M.M.; Augusti, D.; D’Ambrosi, R.; Dellavia, C. In Vivo Osseointegration of a Randomized Trabecular Titanium Structure Obtained by an Additive Manufacturing Technique. J. Mater. Sci. Mater. Med. 2020, 31, 17. [Google Scholar] [CrossRef]
- Åkerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D-Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef]
- Dawood, R.M.; Mahdee, A.F. Fabrication and Characterization of 3D -printed Polymeric-based Scaffold Coated with Bioceramic and Naringin for a Potential Use in Dental Pulp Regeneration (in Vitro Study). Int. Endod. J. 2025, 58, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Heo, D.N.; Godzik, K.P.; Sosnoski, D.; Mrowczynski, O.D.; Rizk, E.B.; Ozbolat, V.; Tucker, S.; Gerhard, E.; Dey, M.; et al. 3D Printing of Poly(ε-Caprolactone)/Poly(D,L-Lactide-Co-Glycolide)/Hydroxyapatite Composite Constructs for Bone Tissue Engineering. J. Mater. Res. 2018, 33, 1972–1986. [Google Scholar] [CrossRef]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J. Three-Dimensional Printed Multiphase Scaffolds for Regeneration of Periodontium Complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Im, G.-B.; Lin, R.-Z. Bioengineering for Vascularization: Trends and Directions of Photocrosslinkable Gelatin Methacrylate Hydrogels. Front. Bioeng. Biotechnol. 2022, 10, 1053491. [Google Scholar] [CrossRef]
- Usme-Duque, L.K.; León-Campos, M.I.; Oyervides-Guajardo, V.G.; Gomez-Galicia, D.S.; Morales, M.A.M.; Flores-Guía, T.E.; Cano-Salazar, L.F.; Cabrera-Munguia, D.A.; Claudio-Rizo, J.A. The Bio-Envelope Revolution: Encapsulation Strategies for Stem Cell-Based Therapies. Mediterr. J. Basic Appl. Sci. 2025, 9, 204–227. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A Biomimetic Extracellular Matrix for Cartilage Tissue Engineering Centered on Photocurable Gelatin, Hyaluronic Acid and Chondroitin Sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef]
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Wang, K.; Gu, X.; Luo, Y. Polymerization of Hydrogel Network on Microfiber Surface: Synthesis of Hybrid Water-Absorbing Matrices for Biomedical Applications. ACS Biomater. Sci. Eng. 2016, 2, 887–892. [Google Scholar] [CrossRef]
- Das, M.; Sharabani-Yosef, O.; Eliaz, N.; Mandler, D. Hydrogel-Integrated 3D-Printed Poly(Lactic Acid) Scaffolds for Bone Tissue Engineering. J. Mater. Res. 2021, 36, 3833–3842. [Google Scholar] [CrossRef]
- Basu, A.; Kunduru, K.R.; Doppalapudi, S.; Domb, A.J.; Khan, W. Poly(Lactic Acid) Based Hydrogels. Adv. Drug Deliv. Rev. 2016, 107, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, M.; Alkhader, M.; Abu-Nabah, B.A.; Abuzaid, W. A Low-Cost Process for Fabricating Reinforced 3D Printing Thermoplastic Filaments. Polymers 2023, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.-J.; Wen, C.; Huang, X.; Ling, T.-X.; Li, J.-B.; Huang, J.-G. Digital Light Processing 3D Printing of High-Fidelity and Versatile Hydrogels via in Situ Phase Separation. J. Mater. Chem. B 2025, 13, 4630–4640. [Google Scholar] [CrossRef]
- Viray, C.; Magill, B.V.; Zreiqat, H.; Ramaswamy, Y. Stereolithographic Visible-Light Printing of Poly(l-Glutamic Acid) Hydrogel Scaffolds. ACS Biomater. Sci. Eng. 2022, 8, 1115–1131. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, L.; Savalani, M.M.; Harris, R.A.; Tanner, K.E. Characterization and Dynamic Mechanical Analysis of Selective Laser Sintered Hydroxyapatite-filled Polymeric Composites. J. Biomed. Mater. Res. A 2008, 86A, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.A.; Saada, G.; Cooperstein, I.; Larush, L.; Jackman, J.A.; Tabaei, S.R.; Cho, N.-J.; Magdassi, S. High-Performance 3D Printing of Hydrogels by Water-Dispersible Photoinitiator Nanoparticles. Sci. Adv. 2016, 2, e1501381. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.D.; Todd, N.; Alsharhan, A.T.; Bigio, D.I.; Sochol, R.D. A 3D Printed Morphing Nozzle to Control Fiber Orientation during Composite Additive Manufacturing. Adv. Mater. Technol. 2021, 6, 2000829. [Google Scholar] [CrossRef]
- Oryan, A.; Hassanajili, S.; Sahvieh, S.; Azarpira, N. Effectiveness of Mesenchymal Stem Cell-Seeded onto the 3D Polylactic Acid/Polycaprolactone/Hydroxyapatite Scaffold on the Radius Bone Defect in Rat. Life Sci. 2020, 257, 118038. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Granier, F.; Therriault, D. Advances in Coaxial Additive Manufacturing and Applications. Adv. Mater. Technol. 2021, 6, 2100356. [Google Scholar] [CrossRef]
- Goyal, R.; Sahu, S.; Mitra, S.; Niranjan, R.; Priyadarshini, R.; Yadav, R.; Lochab, B. Nanocellulose-Reinforced 4D Printed Hydrogels: Thermoresponsive Shape Morphing and Drug Release. ACS Appl. Polym. Mater. 2024, 6, 1348–1361. [Google Scholar] [CrossRef]
- Li, H.; Darmawan, B.; Go, G.; Kim, S.; Nan, M.; Kang, B.; Kim, H.; Lee, S.B.; Bang, D.; Park, J.-O.; et al. Single-Layer 4D Printing System Using Focused Light: A Tool for Untethered Microrobot Applications. Chem. Mater. 2021, 33, 7703–7712. [Google Scholar] [CrossRef]
- Cui, J.; Azzaroni, O.; Del Campo, A. Polymer Brushes with Phototriggered and Phototunable Swelling and pH Response. Macromol. Rapid Commun. 2011, 32, 1699–1703. [Google Scholar] [CrossRef]
- Molina, B.G.; Ocón, G.; Silva, F.M.; Iribarren, J.I.; Armelin, E.; Alemán, C. Thermally-Induced Shape Memory Behavior of Polylactic Acid/Polycaprolactone Blends. Eur. Polym. J. 2023, 196, 112230. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D Printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.S.W.; Fatinathan, S. Adsorption Characterization of Pb(II) and Cu(II) Ions onto Chitosan-Tripolyphosphate Beads: Kinetic, Equilibrium and Thermodynamic Studies. J. Environ. Manag. 2010, 91, 958–969. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy Metal Sorption by Calcium Alginate Beads from Laminaria Digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Haliza, N.; Izzah, N.; Tahir, M.M.; Dirpan, A. Physical, Mechanical, Barrier, and Optical Properties of Sodium Alginate/Gum Arabic/Gluten Edible Films Plasticized with Glycerol and Sorbitol. Foods 2025, 14, 1219. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Naqvi, M.; Kok Keong, L.; Almesfer, M.K.; Al-Qodah, Z.; Naqvi, S.R.; Elboughdiri, N. Production and Characterization of Controlled Release Urea Using Biopolymer and Geopolymer as Coating Materials. Polymers 2020, 12, 400. [Google Scholar] [CrossRef]
- Shilpa, M.; Harisha, K.S.; Thripthi, S.; Arpitha, M.A.; Sangappa, Y. Eco-Friendly Magnetic Silk Fibroin Films for Heavy Metal Adsorption in Water Purification. Next Mater. 2025, 9, 101062. [Google Scholar] [CrossRef]
- Griffin-LaHue, D.; Ghimire, S.; Yu, Y.; Scheenstra, E.J.; Miles, C.A.; Flury, M. In-Field Degradation of Soil-Biodegradable Plastic Mulch Films in a Mediterranean Climate. Sci. Total Environ. 2022, 806, 150238. [Google Scholar] [CrossRef]
- Othman, N.A.F.; Selambakkannu, S.; Seko, N. Biodegradable Dual-Layer Polyhydroxyalkanoate (Pha)/Polycaprolactone (Pcl) Mulch Film for Agriculture: Preparation and Characterization. Energy Nexus 2022, 8, 100137. [Google Scholar] [CrossRef]
- Wu, Q.; Shao, W.; Xia, N.; Wang, P.; Kong, F. A Separable Paper Adhesive Based on the Starch―Lignin Composite. Carbohydr. Polym. 2020, 229, 115488. [Google Scholar] [CrossRef] [PubMed]
- Mansour, G.; Zoumaki, M.; Tsongas, K.; Tzetzis, D. Starch-Sandstone Materials in the Construction Industry. Results Eng. 2020, 8, 100182. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Rafiq, S.; Ahmad, T.; Sikander, U.; Javaid, F. Improving Functional Properties of PVA/Starch-Based Films as Active and Intelligent Food Packaging by Incorporating Propolis and Anthocyanin. Polym. Polym. Compos. 2021, 29, 1472–1484. [Google Scholar] [CrossRef]
- Alex, Y.; Divakaran, N.C.; Pattanayak, I.; Lakshyajit, B.; Ajay, P.V.; Mohanty, S. Comprehensive Study of PLA Material Extrusion 3D Printing Optimization and Its Comparison with PLA Injection Molding through Life Cycle Assessment. Sustain. Mater. Technol. 2025, 43, e01222. [Google Scholar] [CrossRef]
- Hughes, J.; Thomas, R.; Byun, Y.; Whiteside, S. Improved Flexibility of Thermally Stable Poly-Lactic Acid (PLA). Carbohydr. Polym. 2012, 88, 165–172. [Google Scholar] [CrossRef]
- Genovesi, A.; Aversa, C.; Barletta, M. Polyhydroxyalkanoates-Based Cast Film as Bio-Based Packaging for Fresh Fruit and Vegetables: Manufacturing and Characterization. J. Polym. Environ. 2023, 31, 4522–4532. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S. Optimized UV-Barrier Carboxymethyl Cellulose-Based Edible Coatings Reinforced with Green Synthesized ZnO-NPs for Food Packaging Applications. Polym. Bull. 2024, 81, 16733–16755. [Google Scholar] [CrossRef]
- Siew, Z.Z.; Chan, E.W.C.; Wong, C.W. Enhancing the Tearability and Barrier Properties of Cellulose Acetate Bioplastic Film with Polyethylene Glycol 1450 as an LDPE Replacement for Food Packaging. Food Bioprocess Technol. 2024, 17, 2265–2276. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, B.; Zhang, H.; Peng, J.; Pan, X.; Guo, M.; Luo, X.; Zhou, C.; Liu, Y. A Bio-Based Waterborne Polyurethane with High Toughness, Superior Wear Resistance, and Water Resistance Enabled by Sorbitol Monooleate. Prog. Org. Coat. 2023, 185, 107895. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H.; Oh, K.W. Bio-Based Polyurethane Foams with Castor Oil Based Multifunctional Polyols for Improved Compressive Properties. Polymers 2021, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Advances in Biodegradable Polymers and Biomaterials for Medical Applications—A Review. Molecules 2023, 28, 6213. [Google Scholar] [CrossRef]
- El-hadi, A.M. Increase the Elongation at Break of Poly (Lactic Acid) Composites for Use in Food Packaging Films. Sci. Rep. 2017, 7, 46767. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Zhan, R.; Li, X.-L.; Zheng, Y.; Zeng, X.-R.; Shi, L.-Y.; Yang, K.-K.; Wang, Y.-Z. Fabrication of High-Strength and Tough PLA/PBAT Composites via in-Situ Copolymer Formation Using an Adaptable Epoxy Extender. Int. J. Biol. Macromol. 2025, 302, 140530. [Google Scholar] [CrossRef]
- Wang, L.; Abenojar, J.; Martínez, M.A.; Santiuste, C. Degradation of Mechanical Properties of Flax/PLA Composites in Hygrothermal Aging Conditions. Polymers 2024, 16, 528. [Google Scholar] [CrossRef]
- Regazzi, A.; Corn, S.; Ienny, P.; Bénézet, J.-C.; Bergeret, A. Reversible and Irreversible Changes in Physical and Mechanical Properties of Biocomposites during Hydrothermal Aging. Ind. Crops Prod. 2018, 84, 358–365. [Google Scholar] [CrossRef]
- Al Abdallah, H.; Abu-Jdayil, B.; Iqbal, M.Z. Improvement of Mechanical Properties and Water Resistance of Bio-Based Thermal Insulation Material via Silane Treatment. J. Clean. Prod. 2022, 346, 131242. [Google Scholar] [CrossRef]
- Li, F.; Chen, C.; Chen, X. Tremendous Advances, Multifaceted Challenges and Feasible Future Prospects of Biodegradable Medical Polymer Materials. RSC Adv. 2024, 14, 32267–32283. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Ma, S.; Feng, X.; Liu, F.; Wang, B.; Zhang, H.; Niu, X. The Pro-inflammatory Response of Macrophages Regulated by Acid Degradation Products of Poly(Lactide-co-glycolide) Nanoparticles. Eng. Life Sci. 2021, 21, 709–720. [Google Scholar] [CrossRef]
- Kim, J.-K.; Go, E.-J.; Ko, K.-W.; Oh, H.-J.; Han, J.; Han, D.K.; Park, W. PLGA Microspheres Containing Hydrophobically Modified Magnesium Hydroxide Particles for Acid Neutralization-Mediated Anti-Inflammation. Tissue Eng. Regen. Med. 2021, 18, 613–622. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Mansoor, Z.; Tchuenbou-Magaia, F.; Kowalczuk, M.; Adamus, G.; Manning, G.; Parati, M.; Radecka, I.; Khan, H. Polymers Use as Mulch Films in Agriculture—A Review of History, Problems and Current Trends. Polymers 2022, 14, 5062. [Google Scholar] [CrossRef]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Schick, S.; Heindel, J.; Groten, R.; Seide, G.H. Overcoming Challenges in the Commercialization of Biopolymers: From Research to Applications—A Review. Polymers 2024, 16, 3498. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hobby, A.M.; Chen, Y.; Chio, A.; Jenkins, B.M.; Zhang, R. Techno-Economic Analysis on an Industrial-Scale Production System of Polyhydroxyalkanoates (PHA) from Cheese By-Products by Halophiles. Processes 2021, 10, 17. [Google Scholar] [CrossRef]
- Faheem, M.; Khan, K.A. Harnessing Sustainable Biocomposites: A Review of Advances in Greener Materials and Manufacturing Strategies. Polym. Bull. 2025, 82, 8827–8870. [Google Scholar] [CrossRef]
- Wrzecionek, M.; Kryszak, B.; Gadomska-Gajadhur, A. The Smart Way of Simultaneous Scaling up and Optimizing the Synthesis of PGS for Further Rapid Thermal Cross-Linking with the Use of Response Surface Methodology. Polymer 2025, 336, 128862. [Google Scholar] [CrossRef]
- Dallaev, R.; Papež, N.; Allaham, M.M.; Holcman, V. Biodegradable Polymers: Properties, Applications, and Environmental Impact. Polymers 2025, 17, 1981. [Google Scholar] [CrossRef]
- Sharma, V.P.; Singh, R.L.; Singh, R.P. Degradable Polymers and Plastics of the Future: Steps Toward Environmental Sustainability, Regulations, and Safety Aspects. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Singh, R.L., Ed.; Springer: Singapore, 2017; pp. 467–487. ISBN 978-981-10-1865-7. [Google Scholar]
- Li, J.; Stachowski, M.; Zhang, Z. Application of Responsive Polymers in Implantable Medical Devices and Biosensors. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 259–298. ISBN 978-0-85709-713-2. [Google Scholar]
- Patel, S.; Sharma, S.; Suthar, J.; Suryawanshi, M. Regulatory Concern for Biomaterial and Its Toxicity. In Design and Processing of Green Materials; Faiyazuddin, M., Suryawanshi, M., Eds.; Biomaterials, Bioengineering and Sustainability; Springer Nature: Cham, Switzerland, 2025; Volume 4, pp. 433–458. ISBN 978-3-031-91789-9. [Google Scholar]
- Xia, B.; Liu, Y.; Xing, Y.; Shi, Z.; Pan, X. Biodegradable Medical Implants: Reshaping Future Medical Practice. Adv. Sci. 2025, 12, e08014. [Google Scholar] [CrossRef]
- Pappalardo, D.; Mathisen, T.; Finne-Wistrand, A. Biocompatibility of Resorbable Polymers: A Historical Perspective and Framework for the Future. Biomacromolecules 2019, 20, 1465–1477. [Google Scholar] [CrossRef]
- Azeem, B. Stimuli-Responsive Starch-Based Biopolymer Coatings for Smart and Sustainable Fertilizers. Gels 2025, 11, 681. [Google Scholar] [CrossRef]
- Yu, J.; Chen, R.; Liu, X.; Hileuskaya, K.; Kraskouski, A.; Shao, P. Advance of Stimulus-Responsive Materials in Food Packaging and Targeted Delivery of Bioactive Compounds: Insight of Discoloration and Deformation Mechanisms. Trends Food Sci. Technol. 2024, 153, 104732. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Balcerak-Woźniak, A.; Dzwonkowska-Zarzycka, M.; Kabatc-Borcz, J. A Comprehensive Review of Stimuli-Responsive Smart Polymer Materials—Recent Advances and Future Perspectives. Materials 2024, 17, 4255. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, Z.; Gillies, E.R. Designing Polymers with Stimuli-Responsive Degradation for Biomedical Applications. Curr. Opin. Biomed. Eng. 2023, 25, 100437. [Google Scholar] [CrossRef]
- Tariq, A.; Arif, Z.U.; Khalid, M.Y.; Hossain, M.; Rasool, P.I.; Umer, R.; Ramakrishna, S. Recent Advances in the Additive Manufacturing of Stimuli-Responsive Soft Polymers. Adv. Eng. Mater. 2023, 25, 2301074. [Google Scholar] [CrossRef]
- Jiang, M.; Fang, H.; Tian, H. Latest Advancements and Trends in Biomedical Polymers for Disease Prevention, Diagnosis, Treatment, and Clinical Application. J. Control. Release 2025, 380, 138–174. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Kong, D.; Yin, L. Recent Progress on Biodegradable Materials and Transient Electronics. Bioact. Mater. 2018, 3, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Vijjapu, M.T.; Neumaier, L.; Rauter, L.; Chakkunny, A.H.; Corzo, D.; Thoelen, R.; Picard, A.; Kosel, J.; Deferme, W. Convergence of Biocompatible Printed Electronics and Sensing in Wound Dressings: A Leap Forward in Sustainable Health Monitoring. Npj Flex. Electron. 2025, 9, 46. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, H.; Zhang, H.; Huang, P.; Liu, L.; Hu, H. Recent Advances in Biodegradable Electronics- from Fundament to the next-Generation Multi-Functional, Medical and Environmental Device. Sustain. Mater. Technol. 2023, 35, e00530. [Google Scholar] [CrossRef]
- Khan, T.; Vadivel, G.; Ramasamy, B.; Murugesan, G.; Sebaey, T.A. Biodegradable Conducting Polymer-Based Composites for Biomedical Applications—A Review. Polymers 2024, 16, 1533. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tran, H.; Feig, V.R.; Bao, Z. Biodegradable and Stretchable Polymeric Materials for Transient Electronic Devices. MRS Bull. 2020, 45, 96–102. [Google Scholar] [CrossRef]
- Pang, Q.; Lou, D.; Li, S.; Wang, G.; Qiao, B.; Dong, S.; Ma, L.; Gao, C.; Wu, Z. Smart Flexible Electronics-Integrated Wound Dressing for Real-Time Monitoring and On-Demand Treatment of Infected Wounds. Adv. Sci. 2020, 7, 1902673. [Google Scholar] [CrossRef]
- Dallaev, R. Smart and Biodegradable Polymers in Tissue Engineering and Interventional Devices: A Brief Review. Polymers 2025, 17, 1976. [Google Scholar] [CrossRef]
- Gorantla, A.; Hall, J.; Troidle, A.; Janjic, J. Biomaterials for Protein Delivery: Opportunities and Challenges to Clinical Translation. Micromachines 2024, 15, 533. [Google Scholar] [CrossRef]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef] [PubMed]
- Jani, K.; Kaushal, N. Clinical Translation of PLGA Nanoparticles into Market—From Benchside to Breakthrough Therapy. In Poly(Lactic-co-Glycolic Acid) (PLGA) Nanoparticles for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 433–456. ISBN 978-0-323-91215-0. [Google Scholar]
- Sternberg, J.; Sequerth, O.; Pilla, S. Green Chemistry Design in Polymers Derived from Lignin: Review and Perspective. Prog. Polym. Sci. 2021, 113, 101344. [Google Scholar] [CrossRef]
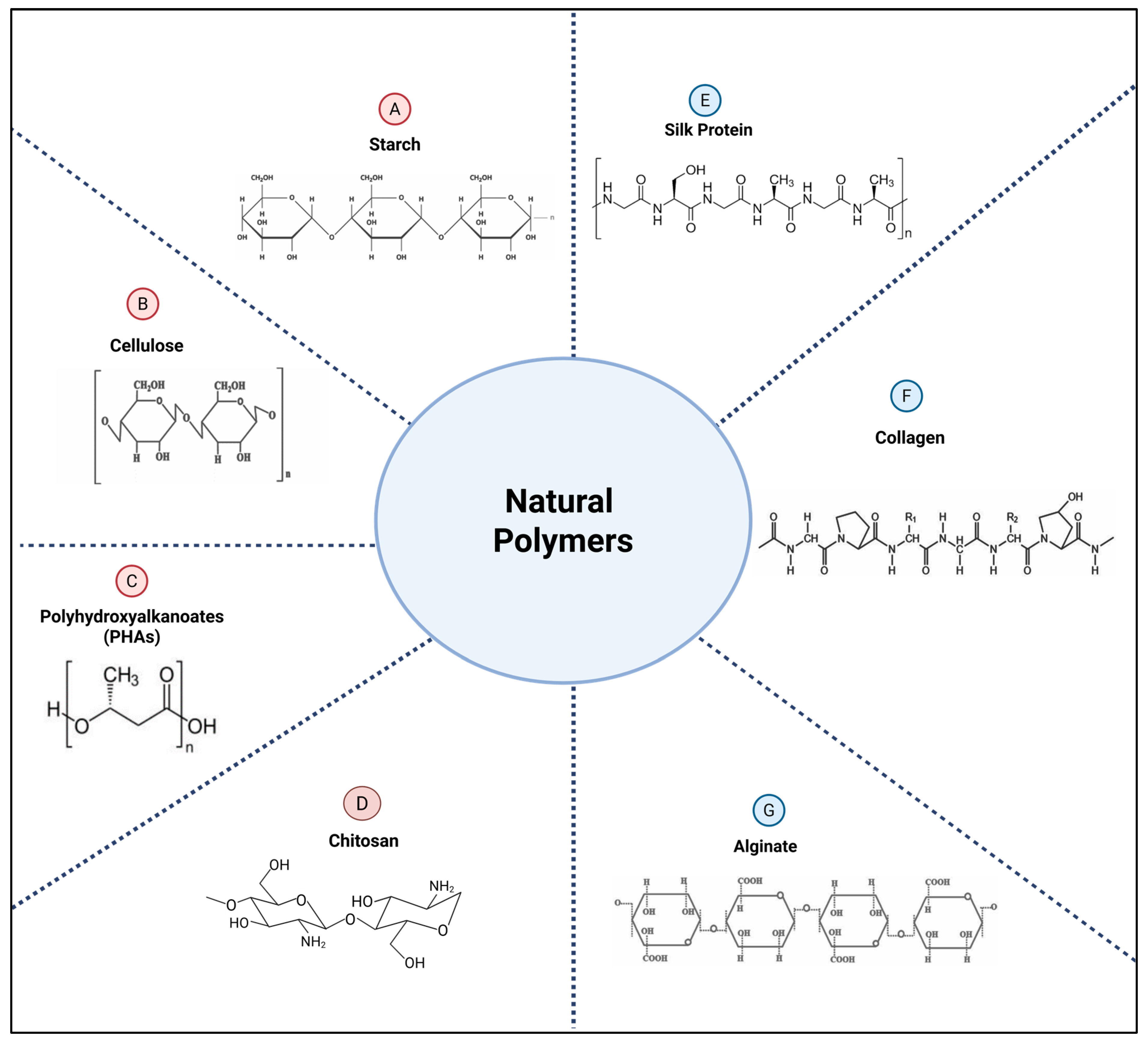
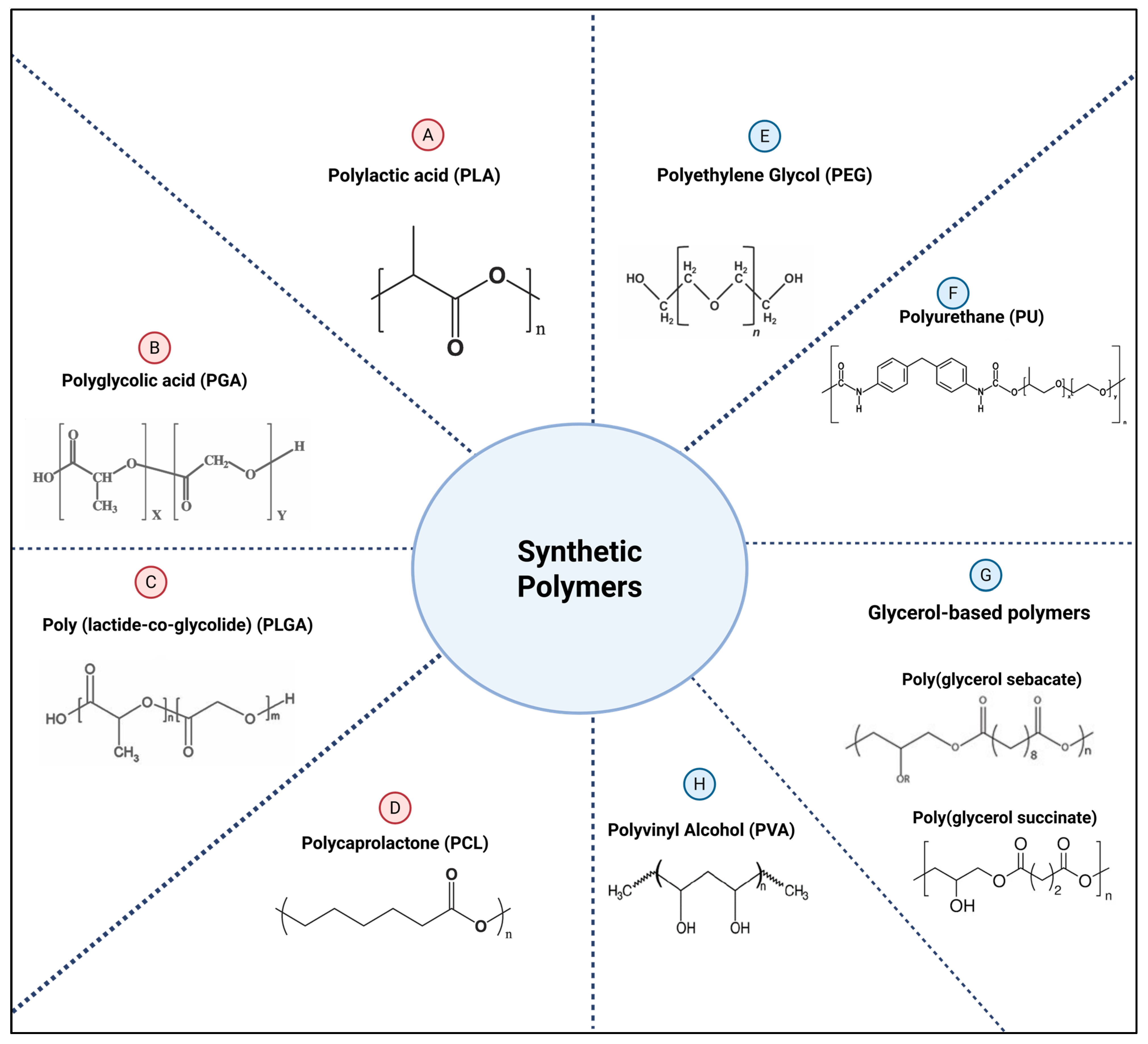
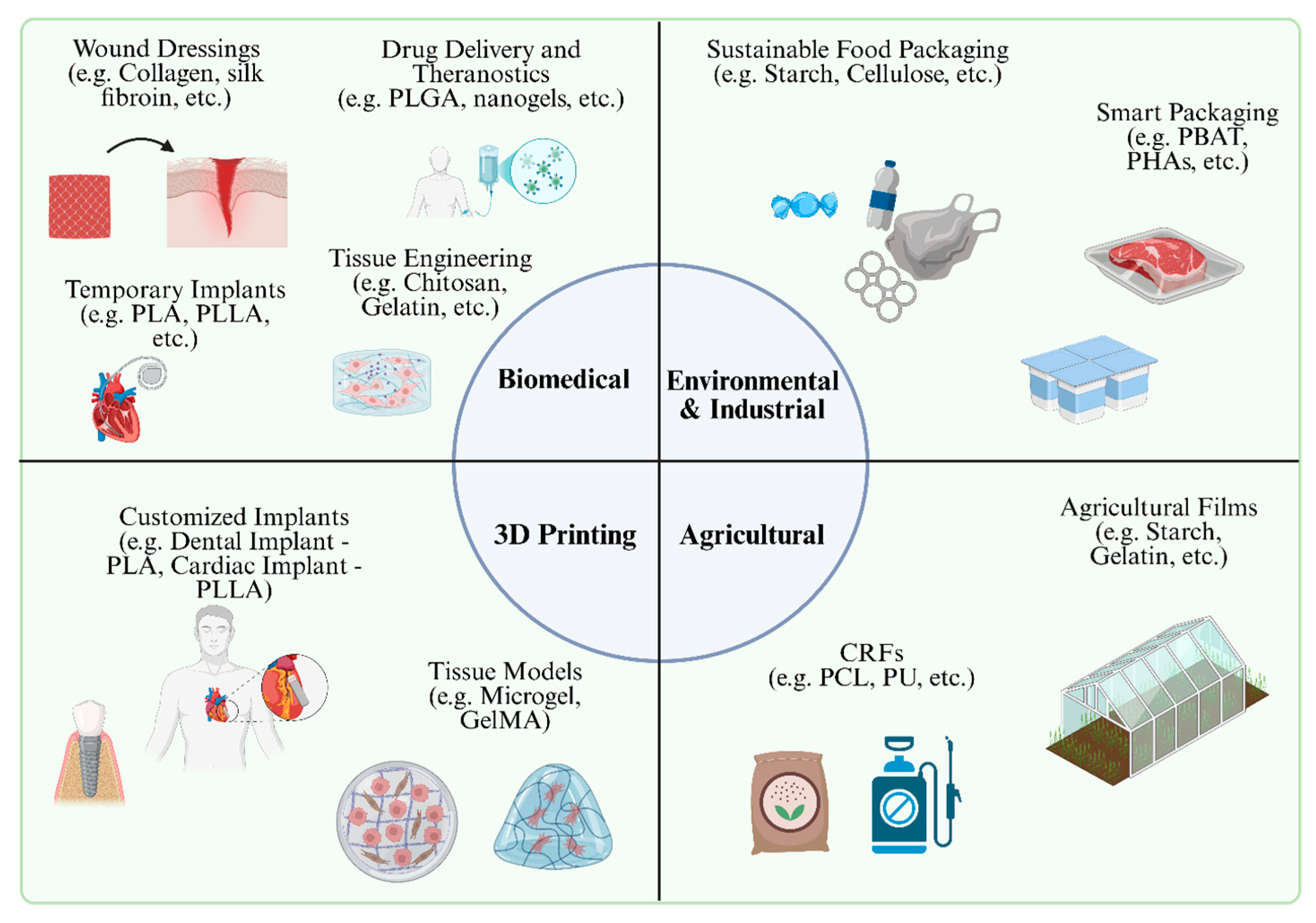
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulsalam, L.; Abubakar, S.; Permatasari, I.; Lawal, A.A.; Uddin, S.; Ullah, S.; Ahmad, I. Advanced Biocompatible and Biodegradable Polymers: A Review of Functionalization, Smart Systems, and Sustainable Applications. Polymers 2025, 17, 2901. https://doi.org/10.3390/polym17212901
Abdulsalam L, Abubakar S, Permatasari I, Lawal AA, Uddin S, Ullah S, Ahmad I. Advanced Biocompatible and Biodegradable Polymers: A Review of Functionalization, Smart Systems, and Sustainable Applications. Polymers. 2025; 17(21):2901. https://doi.org/10.3390/polym17212901
Chicago/Turabian StyleAbdulsalam, Latifat, Sadam Abubakar, Ikfa Permatasari, Anas Abdulwahab Lawal, Shihab Uddin, Saleem Ullah, and Irshad Ahmad. 2025. "Advanced Biocompatible and Biodegradable Polymers: A Review of Functionalization, Smart Systems, and Sustainable Applications" Polymers 17, no. 21: 2901. https://doi.org/10.3390/polym17212901
APA StyleAbdulsalam, L., Abubakar, S., Permatasari, I., Lawal, A. A., Uddin, S., Ullah, S., & Ahmad, I. (2025). Advanced Biocompatible and Biodegradable Polymers: A Review of Functionalization, Smart Systems, and Sustainable Applications. Polymers, 17(21), 2901. https://doi.org/10.3390/polym17212901










