The Use of Natural Rubber as an Initiator of LDPE Biodegradation in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
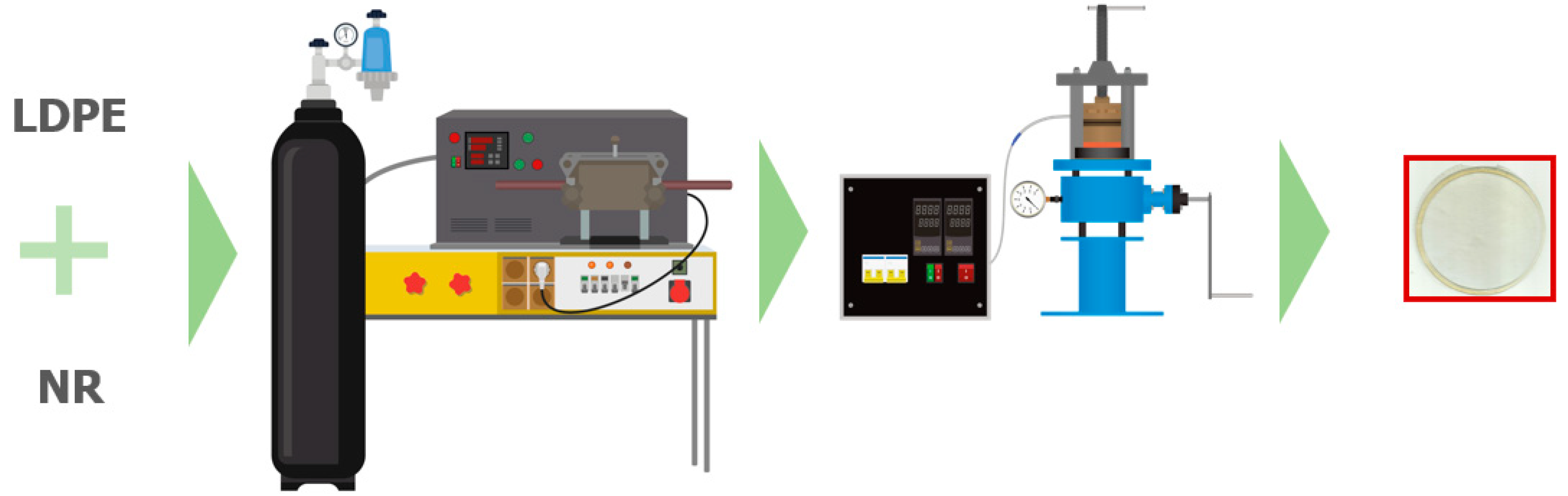
2.2. Methods
2.2.1. Optical Microscopy
2.2.2. Scanning Electron Microscopy
2.2.3. Atomic Force Microscopy
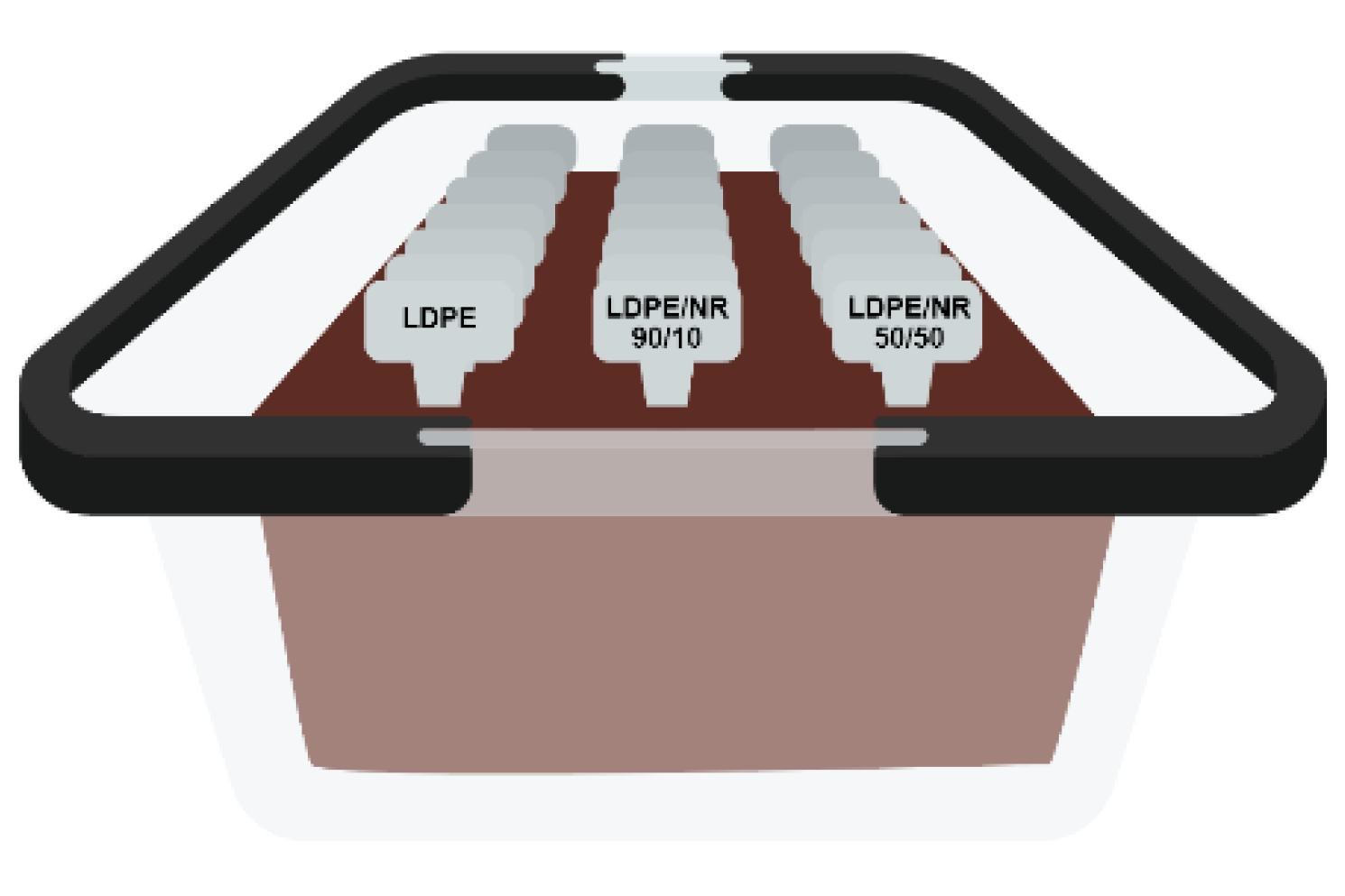
2.2.4. Ultrasonic Microscopy
2.2.5. Biodegradation
2.2.6. Differential Scanning Calorimetry
2.2.7. Mechanical Analysis After Exposure to Soil
2.2.8. Thermogravimetric Analysis
2.2.9. Electron Paramagnetic Resonance
2.2.10. Fourier Transform Infrared Spectroscopy
2.2.11. Gel-Permeation Chromatography
3. Results and Discussion
3.1. Study of the Structure of the Initial Samples
3.1.1. Microscopy
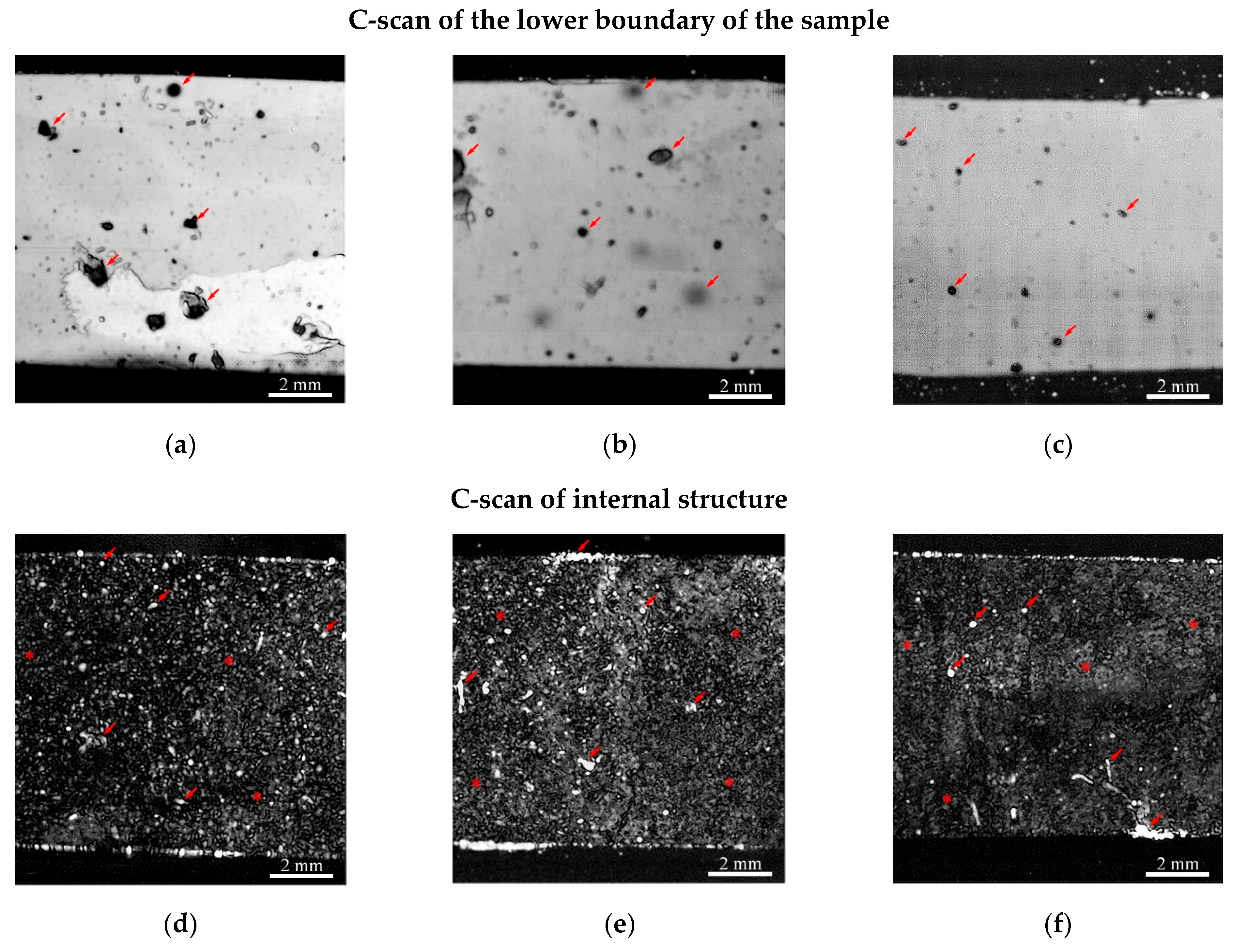
3.1.2. Ultrasonic Studies
3.1.3. SEM
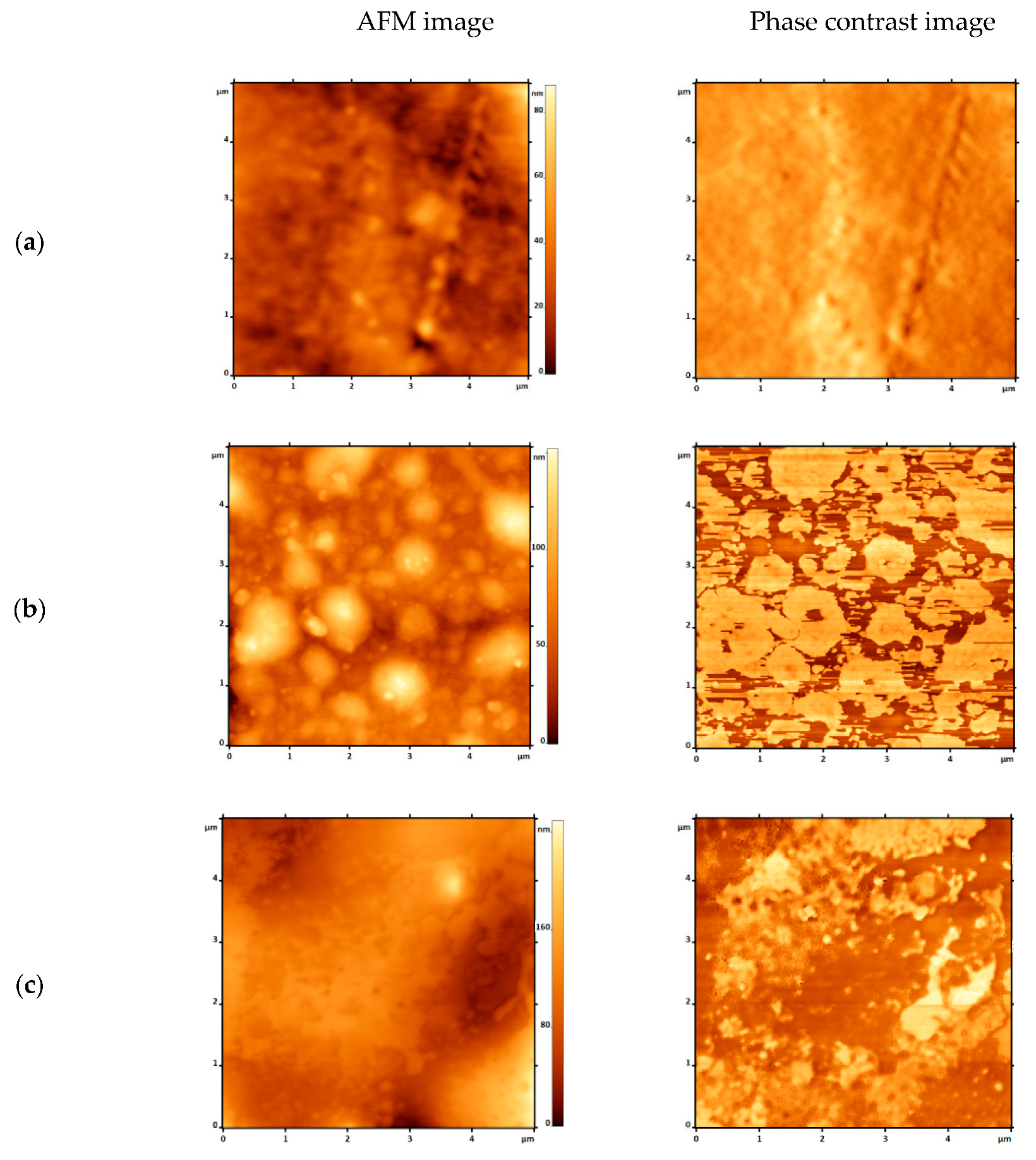
3.1.4. Atomic Force Microscopy (AFM)
3.2. Biodegradability Study
3.2.1. Laboratory Biodegradation Study
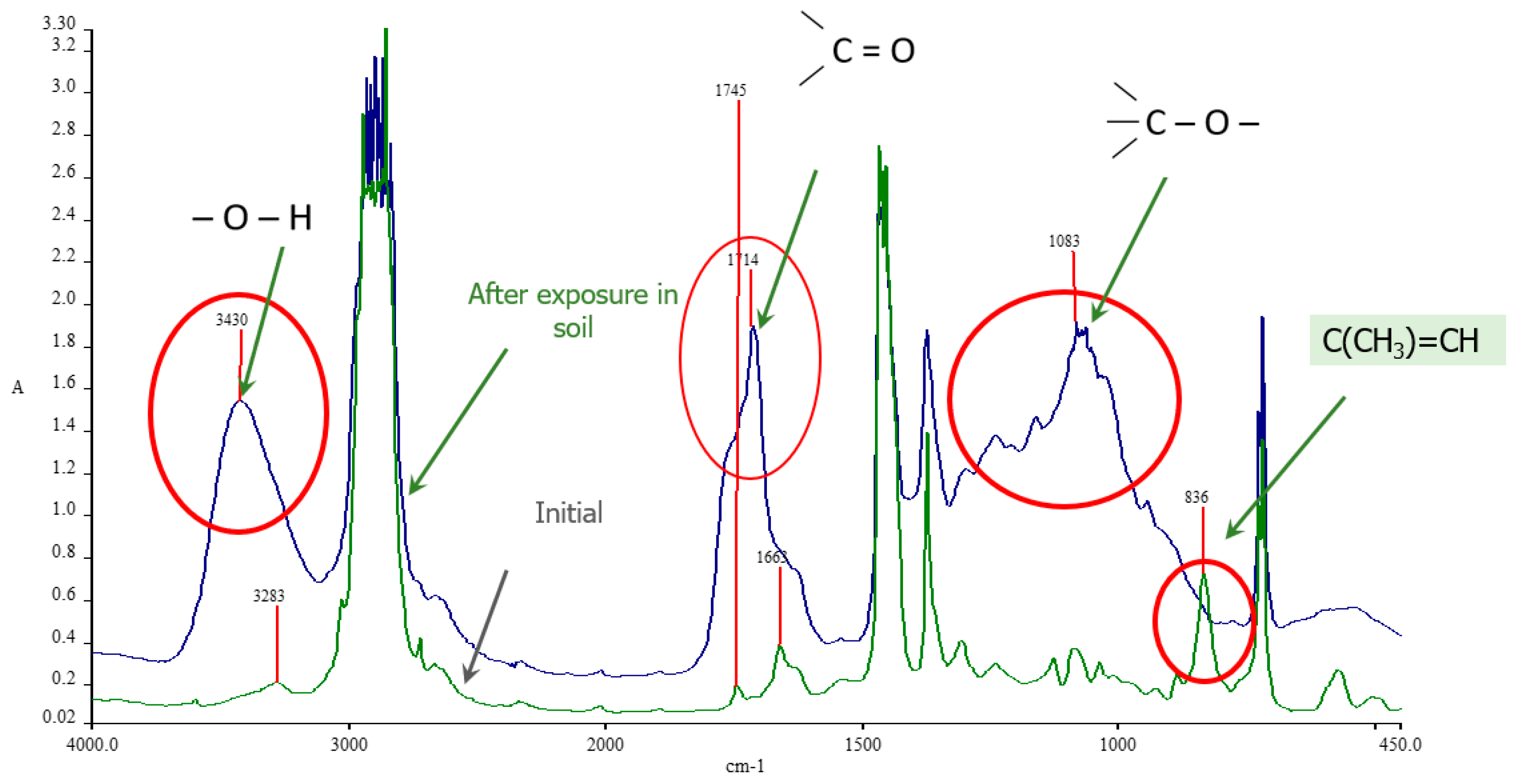
3.2.2. Investigation of Properties and Changes Using FTIR Spectroscopy
3.2.3. Physicomechanical Properties After Soil Exposure
3.2.4. Investigation of Thermophysical Characteristics by DSC
3.2.5. Investigation of LDPE/NR by EPR Method
3.2.6. Investigation of the Thermal Stability of the Samples
3.2.7. GPC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science (1979) 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Bour, A.; Köşker, A.R.; Walther, B.A.; Napierska, D.; Mihai, F.C.; Syberg, K.; Hansen, S.F.; Walker, T.R. Review of Microplastics and Chemical Risk Posed by Plastic Packaging on the Marine Environment to Inform the Global Plastics Treaty. Sci. Total Environ. 2024, 946, 174000. [Google Scholar] [CrossRef]
- Jubinville, D.; Esmizadeh, E.; Saikrishnan, S.; Tzoganakis, C.; Mekonnen, T. A Comprehensive Review of Global Production and Recycling Methods of Polyolefin (PO) Based Products and Their Post-Recycling Applications. Sustain. Mater. Technol. 2020, 25, e00188. [Google Scholar] [CrossRef]
- Burelo, M.; Hernández-Varela, J.D.; Medina, D.I.; Treviño-Quintanilla, C.D. Recent Developments in Bio-Based Polyethylene: Degradation Studies, Waste Management and Recycling. Heliyon 2023, 9, e21374. [Google Scholar] [CrossRef]
- Ritzen, L.; Sprecher, B.; Bakker, C.; Balkenende, R. Bio-Based Plastics in a Circular Economy: A Review of Recovery Pathways and Implications for Product Design. Resour. Conserv. Recycl. 2023, 199, 107268. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future Scenarios of Global Plastic Waste Generation and Disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Rong, Z.; Xu, X.W.; Wu, Y.H. Biodegradation of Low-Density Polyethylene Film by Two Bacteria Isolated from Plastic Debris in Coastal Beach. Ecotoxicol. Environ. Saf. 2024, 278, 116445. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in Terrestrial Ecosystems Research Shifts from Ecotoxicology to Ecosystem Effects and Earth System Feedbacks. Science (1979) 2020, 368, 1430–1431. [Google Scholar]
- Chae, Y.; An, Y.J. Current Research Trends on Plastic Pollution and Ecological Impacts on the Soil Ecosystem: A Review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Leontiadis, K.; Tsioptsias, C.; Messaritakis, S.; Terzaki, A.; Xidas, P.; Mystikos, K.; Tzimpilis, E.; Tsivintzelis, I. Optimization of Thermal and Mechanical Properties of Polypropylene-Wollastonite Composite Drawn Fibers Based on Surface Response Analysis. Polymers 2022, 14, 924. [Google Scholar] [CrossRef] [PubMed]
- Münstedt, H. Recoverable Extensional Flow of Polymer Melts and Its Relevance for Processing. Polymers 2020, 12, 1512. [Google Scholar] [CrossRef] [PubMed]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and Opportunities of Biodegradable Plastics: A Mini Review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A Comprehensive Review on Recent Advancements in Biodegradation and Sustainable Management of Biopolymers. Environ. Pollut. 2022, 307, 119600. [Google Scholar] [CrossRef]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Bordel, S.; Santos-Beneit, F.; Martínez-Mendoza, L.J.; Aragão Börner, R.; Börner, T.; Muñoz, R. Biodegradation of Bioplastics under Aerobic and Anaerobic Aqueous Conditions: Kinetics, Carbon Fate and Particle Size Effect. Bioresour. Technol. 2022, 344, 126265. [Google Scholar] [CrossRef]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Liu, D.; Wang, Q. Peculiarity of the Mechanism of Early Stages of Photo-Oxidative Degradation of Linear Low-Density Polyethylene Films in the Presence of Ferric Stearate. Polymers 2023, 15, 3672. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Zhong, S.; Zhang, L. AOPs-Based Remediation of Petroleum Hydrocarbons-Contaminated Soils: Efficiency, Influencing Factors and Environmental Impacts. Chemosphere 2020, 246, 125726. [Google Scholar] [CrossRef]
- Padermshoke, A.; An, Y.; Van Nguyen, T.; Kobayashi, Y.; Ito, H.; Takahara, A. Prooxidant-Based Polyolefins Exhibiting No Evidence of Biodegradation under Marine Environments. Mar. Pollut. Bull. 2025, 214, 117697. [Google Scholar] [CrossRef]
- Sciscione, F.; Hailes, H.C.; Miodownik, M. The Performance and Environmental Impact of Pro-Oxidant Additive Containing Plastics in the Open Unmanaged Environment—A Review of the Evidence. R. Soc. Open Sci. 2023, 10, 230089. [Google Scholar] [CrossRef]
- Heimowska, A. Environmental Degradation of Oxo-Biodegradable Polyethylene Bags. Water 2023, 15, 4059. [Google Scholar] [CrossRef]
- Hart, K.R.; Sottos, N.R.; White, S.R. Repeatable Self-Healing of an Epoxy Matrix Using Imidazole Initiated Polymerization. Polymer 2015, 67, 174–184. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the Aerobic Biodegradation of Biopolymers and the Corresponding Bioplastics: A Review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ahn, J.H.; Hur, H.G. Biodegradation of Polyethylene: A Brief Review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Iordanskii, A. Bio-Based and Biodegradable Plastics: From Passive Barrier to Active Packaging Behavior. Polymers 2020, 12, 1537. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-Based Antimicrobial Packaging Materials: A Mini-Review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of Liquid Natural Rubber via Oxidative Degradation of Natural Rubber. Polymers 2014, 6, 2928. [Google Scholar] [CrossRef]
- Mathew, A.P.; Packirisamy, S.; Thomas, S. Morphology, Mechanical Properties, and Failure Topography of Semi-Interpenetrating Polymer Networks Based on Natural Rubber and Polystyrene. J. Appl. Polym. Sci. 2000, 78, 2327–2344. [Google Scholar] [CrossRef]
- Jamil, M.S.; Ahmad, I.; Abdullah, I. Effects of Rice Husk Filler on the Mechanical and Thermal Properties of Liquid Natural Rubber Compatibilized High-Density Polyethylene/Natural Rubber Blends. J. Polym. Res. 2006, 13, 315–321. [Google Scholar] [CrossRef]
- Pischedda, A.; Tosin, M.; Degli-Innocenti, F. Biodegradation of Plastics in Soil: The Effect of Temperature. Polym. Degrad. Stab. 2019, 170, 109017. [Google Scholar] [CrossRef]
- Thew, C.X.E.; Lee, Z.S.; Srinophakun, P.; Ooi, C.W. Recent Advances and Challenges in Sustainable Management of Plastic Waste Using Biodegradation Approach. Bioresour. Technol. 2023, 374, 128772. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yan, W.; Cao, Z.; Ding, M.; Yuan, Y. Current Advances in the Biodegradation and Bioconversion of Polyethylene Terephthalate. Microorganisms 2022, 10, 39. [Google Scholar] [CrossRef]
- Filonov, A.; Yaminsky, I. Scanning Probe Microscopy Image Processing Software User’s Manual “FemtoScan”, Version 2.2.90; Advanced Technologies Center: Moscow, Russia, 2012; Volume 82.
- Morokov, E.; Yabbarov, N.; Sedush, N.; Bogachenkov, A.; Malykhin, A.; Demina, V.; Azarkevich, P.; Nikolskaya, E.; Chirkina, M.; Sokol, M. Observation of Discrepancy between the Degradation of Polymer Scaffolds in Vitro and in Vivo According to High-Resolution Ultrasound Technique. Eur. Polym. J. 2023, 195, 112248. [Google Scholar] [CrossRef]
- Poh, L.; Wu, Q.; Chen, Y.; Narimissa, E. Characterization of Industrial Low-Density Polyethylene: A Thermal, Dynamic Mechanical, and Rheological Investigation. Rheol. Acta 2022, 61, 701–720. [Google Scholar] [CrossRef]
- Sarkhel, G.; Banerjee, A.; Bhattacharya, P. Rheological and Mechanical Properties of LDPE/HDPE Blends. Polym.-Plast. Technol. Eng. 2006, 45, 713–718. [Google Scholar] [CrossRef]
- Das, B.; Sinha, S.; Gangopadhyay, T. Natural Rubber-Polystyrene Interpenetrating Networks. Morphology and Mechanical Properties. Eur. Polym. J. 1993, 29, 57–61. [Google Scholar] [CrossRef]
- Pillai, V.B.; Francis, D.J. Interpenetrating Polymer Networks Based on Liquid Natural Rubber. 1. Synthesis and Effect of Nco/Oh Ratio on Physical and Mechanical Properties. Die Angew. Makromol. Chem. 1994, 219, 67–76. [Google Scholar] [CrossRef]
- Artyukov, I.; Bellucci, S.; Kolesov, V.; Levin, V.; Morokov, E.; Polikarpov, M.; Petronyuk, Y. Studies of Fractal Microstructure in Nanocarbon Polymer Composite. Polymers 2024, 16, 1354. [Google Scholar] [CrossRef]
- Volodarsky, A.B.; Morokov, E.S.; Kokshaysky, A.I.; Odina, N.I.; Korobov, A.I. Effect of the Tensile Strain on the Propagation of Longitudinal Elastic Waves in the Volume of an Amorphous Polymer. JETP Lett. 2025, 121, 469–473. [Google Scholar] [CrossRef]
- Ishida, N. Atomic Force Microscopy. In Non-Destructive Material Characterization Methods; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Varyan, I.; Mastalygina, E.; Kolesnikova, N.; Popov, A. Physical-Mechanical Properties of Polyethylene-Natural Rubber Blends. J. Phys. Conf. Ser. 2018, 1129, 012036. [Google Scholar] [CrossRef]
- Scott, W.W.; Bhushan, B. Use of Phase Imaging in Atomic Force Microscopy for Measurement of Viscoelastic Contrast in Polymer Nanocomposites and Molecularly Thick Lubricant Films. Ultramicroscopy 2003, 97, 151–169. [Google Scholar] [CrossRef]
- Iwańczuk, A.; Kozłowski, M.; Łukaszewicz, M.; Jabłoński, S. Anaerobic Biodegradation of Polymer Composites Filled with Natural Fibers. J. Polym. Environ. 2015, 23, 277–282. [Google Scholar] [CrossRef]
- Ji, M.; Li, F.; Li, J.; Li, J.; Zhang, C.; Sun, K.; Guo, Z. Enhanced Mechanical Properties, Water Resistance, Thermal Stability, and Biodegradation of the Starch-Sisal Fibre Composites with Various Fillers. Mater. Des. 2021, 198, 109373. [Google Scholar] [CrossRef]
- Easton, Z.H.W.; Essink, M.A.J.; Rodriguez Comas, L.; Wurm, F.R.; Gojzewski, H. Acceleration of Biodegradation Using Polymer Blends and Composites. Macromol. Chem. Phys. 2023, 224, 2200421. [Google Scholar] [CrossRef]
- Nguyen, T.; Merna, J.; Kysor, E.; Kohlmann, O.; Levin, D.B. Bacterial Degradation of Low-Density Polyethylene Preferentially Targets the Amorphous Regions of the Polymer. Polymers 2024, 16, 2865. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Value |
|---|---|
| Total number of CH3 groups per 1000 carbon atoms | 21.6 |
| Number of terminal CH3 groups per 1000 carbon atoms | 4.5 |
| Ethyl branches | 14.4 |
| Total number of double bonds per 1000 carbon atoms, including: | 0.4–0.6 |
| vinyl double bonds (R-CH=CH2), % | 17 |
| Component | % |
|---|---|
| Rubber | 91.0–95.0 |
| Proteins | 2.37–3.76 |
| Ash | 0.10–0.90 |
| Acetone extract | 2.30–3.60 |
| Sugars | 0.30–0.35 |
| Water extract | 0.20–0.40 |
| Moisture | 0.18–0.90 |
| Sample | Speed of Sound, m/s |
|---|---|
| LDPE 100 | 2000 ± 10 |
| LDPE/NR 90/10 | 1900 ± 10 |
| LDPE/NR 50/50 | 1470 ± 10 |
| Sample | Ra, nm ±SD (n = 5) | Rq, nm ±SD (n = 5) |
|---|---|---|
| 0 | 30.52 ± 3 | 38.25 ± 4 |
| 10 | 18.88 ± 2 | 22.86 ± 2 |
| 50 | 18.14 ± 2 | 21.27 ± 2 |
| Parameter | Initial | Control | After Exposure in Soil |
|---|---|---|---|
| Relative elongation, %, (±10%) | 625 | 550 | 580 |
| Tensile strength, MPa (±0.5 MPa) | 15.2 | 10.4 | 10.9 |
| Modulus of elasticity, MPa (±0.5 MPa) | 87 | 60 | 55 |
| Parameter | Initial | Control | After Exposure in Soil |
|---|---|---|---|
| Relative elongation, %, (±10%) | 120 | 104 | 98 |
| Tensile strength, MPa (±0.5 MPa) | 6.3 | 5.8 | 5.3 |
| Modulus of elasticity, MPa (±0.5 MPa) | 62 | 60 | 55 |
| Parameter | Initial | Control | After Exposure in Soil |
|---|---|---|---|
| Relative elongation, %, (± 10%) | 450 | 250 | 45 |
| Tensile strength, MPa (±0.5 MPa) | 3.9 | 2.8 | 4.0 |
| Modulus of elasticity, MPa (±0.5 MPa) | 26 | 23 | 81 |
| Sample | Tonset, °C | T10 | T20 | T30 | T40 | T50 | Tmax, °C |
|---|---|---|---|---|---|---|---|
| NR | 220 | 363 | 349 | 369 | 378 | 383 | 380 |
| LDPE | 252 | 410 | 435 | 448 | 458 | 465 | 472 |
| LDPE/NR 90/10 | 303 | 417 | 441 | 452 | 460 | 472 | 476 |
| LDPE/NR 50/50 | 270 | 372 | 388 | 387 | 421 | 449 | 476 |
| Sample | Tonset, °C | T10 | T20 | T30 | T40 | T50 | Tmax, °C |
|---|---|---|---|---|---|---|---|
| LDPE | 252 | 405 | 423 | 441 | 450 | 459 | 467 |
| LDPE/NR 90/10 | 293 | 389 | 429 | 450 | 461 | 473 | 476 |
| LDPE/NR 50/50 | 271 | 371 | 383 | 397 | 418 | 448 | 478 |
| Sample | Tonset, °C | T10 | T20 | T30 | T40 | T50 | Tmax, °C |
|---|---|---|---|---|---|---|---|
| LDPE | 247 | 399 | 425 | 441 | 450 | 459 | 468 |
| LDPE/NR 90/10 | 200 | 435 | 457 | 465 | 480 | 483 | 482 |
| LDPE/NR 50/50 | 230 | 438 | 460 | 471 | 484 | 487 | 481 |
| Sample | Mp g/mol | Mn g/mol | Mw g/mol | Mz g/mol | Mv g/mol | PD |
|---|---|---|---|---|---|---|
| LDPE/NR 90/10 control | 51.2 × 103 | 17.0 × 103 | 142.6 × 103 | 1162.9 × 103 | 939.4 × 103 | 8.37 |
| LDPE/NR 90/10 after exposure | 54.4 × 103 | 17.7 × 103 | 129.7 × 103 | 998.4 × 103 | 802.35 × 103 | 7.32 |
| LDPE/NR 50/50 control | 30.9 × 103 | 16.0 × 103 | 108.2 × 103 | 927.7 × 103 | 738.6 × 103 | 6.76 |
| LDPE/NR 50/50 after exposure | 20.1 × 103 | 12.6 × 103 | 67.8 × 103 | 267.9 × 103 | 226.3 × 103 | 5.37 |
| Contents of Factions | Total Amount | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Less 1000 g/mol | 1000–10,000 g/mol | 10,000–50,000 g/mol | 50,000–100,000 g/mol | 100,000–1,000,000 g/mol | More 1,000,000 g/mol | PD |
| LDPE/NR 90/10 control | 0.3 | 14.1 | 38.1 | 18.6 | 26.4 | 2.5 | 100 |
| LDPE/NR 90/10 after exposure | 0.2 | 14.1 | 38.9 | 19.2 | 25.6 | 2.0 | 100 |
| LDPE/NR 50/50 control | 0.3 | 15.3 | 40.7 | 19.9 | 22.3 | 1.5 | 100 |
| LDPE/NR 50/50 after exposure | 0.2 | 13.2 | 24.1 | 15.5 | 16.7 | 1.0 | 70.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varyan, I.; Tyubaeva, P.; Poletto, M.; Morokov, E.S.; Bolshakova, A.V.; Karpova, S.G.; Kolesnikov, E.A.; Popov, A. The Use of Natural Rubber as an Initiator of LDPE Biodegradation in Soil. Polymers 2025, 17, 2885. https://doi.org/10.3390/polym17212885
Varyan I, Tyubaeva P, Poletto M, Morokov ES, Bolshakova AV, Karpova SG, Kolesnikov EA, Popov A. The Use of Natural Rubber as an Initiator of LDPE Biodegradation in Soil. Polymers. 2025; 17(21):2885. https://doi.org/10.3390/polym17212885
Chicago/Turabian StyleVaryan, Ivetta, Polina Tyubaeva, Matheus Poletto, Egor S. Morokov, Anastasia V. Bolshakova, Svetlana G. Karpova, Evgeny A. Kolesnikov, and Anatoly Popov. 2025. "The Use of Natural Rubber as an Initiator of LDPE Biodegradation in Soil" Polymers 17, no. 21: 2885. https://doi.org/10.3390/polym17212885
APA StyleVaryan, I., Tyubaeva, P., Poletto, M., Morokov, E. S., Bolshakova, A. V., Karpova, S. G., Kolesnikov, E. A., & Popov, A. (2025). The Use of Natural Rubber as an Initiator of LDPE Biodegradation in Soil. Polymers, 17(21), 2885. https://doi.org/10.3390/polym17212885









