Investigation of Window Silicone Sealant Weathering Using Evolved Gas Analysis and Pyrolysis Gas Chromatography with Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Data Processing
3. Results and Discussion
3.1. Characterization of Sealants Using EGA-MS
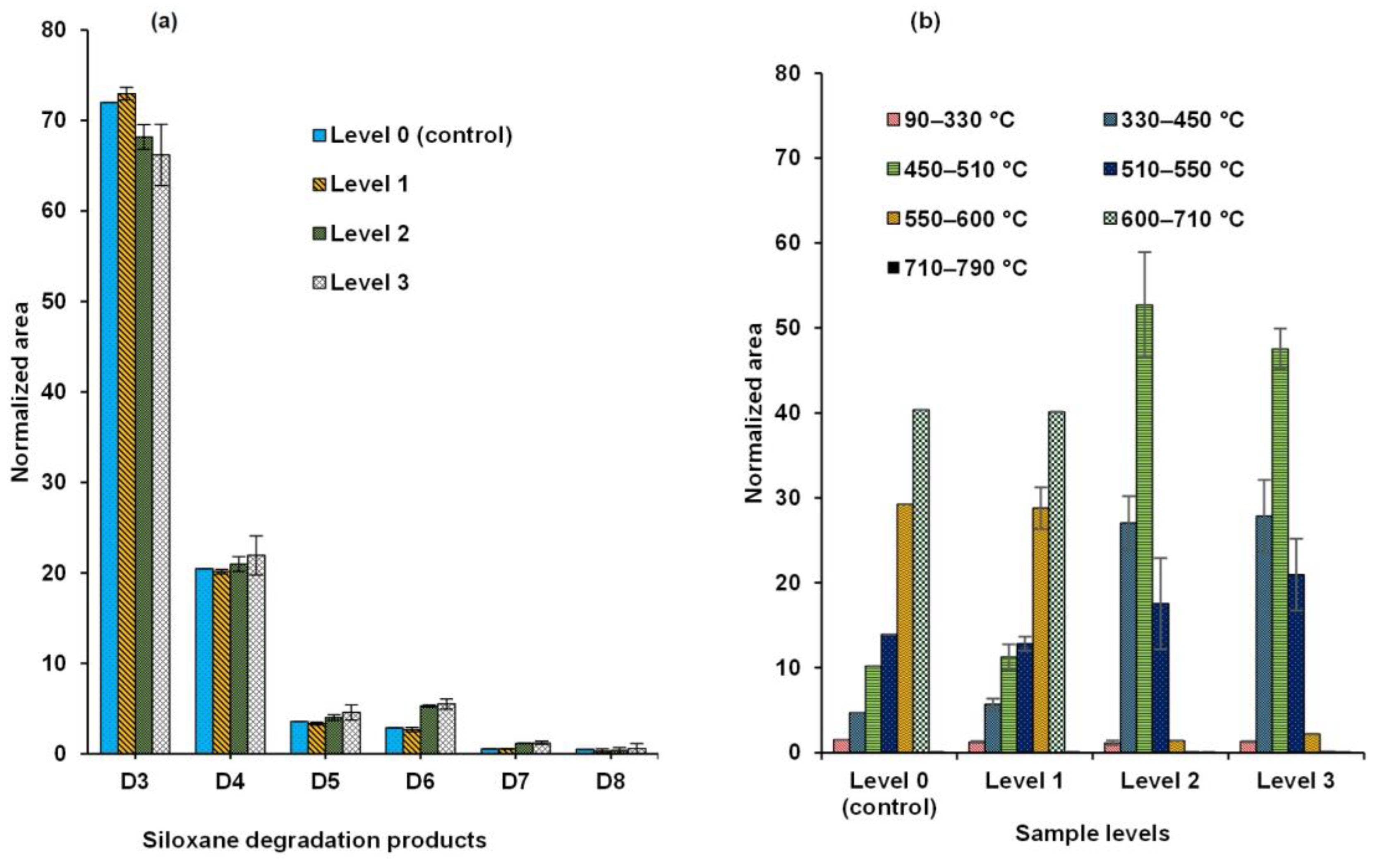
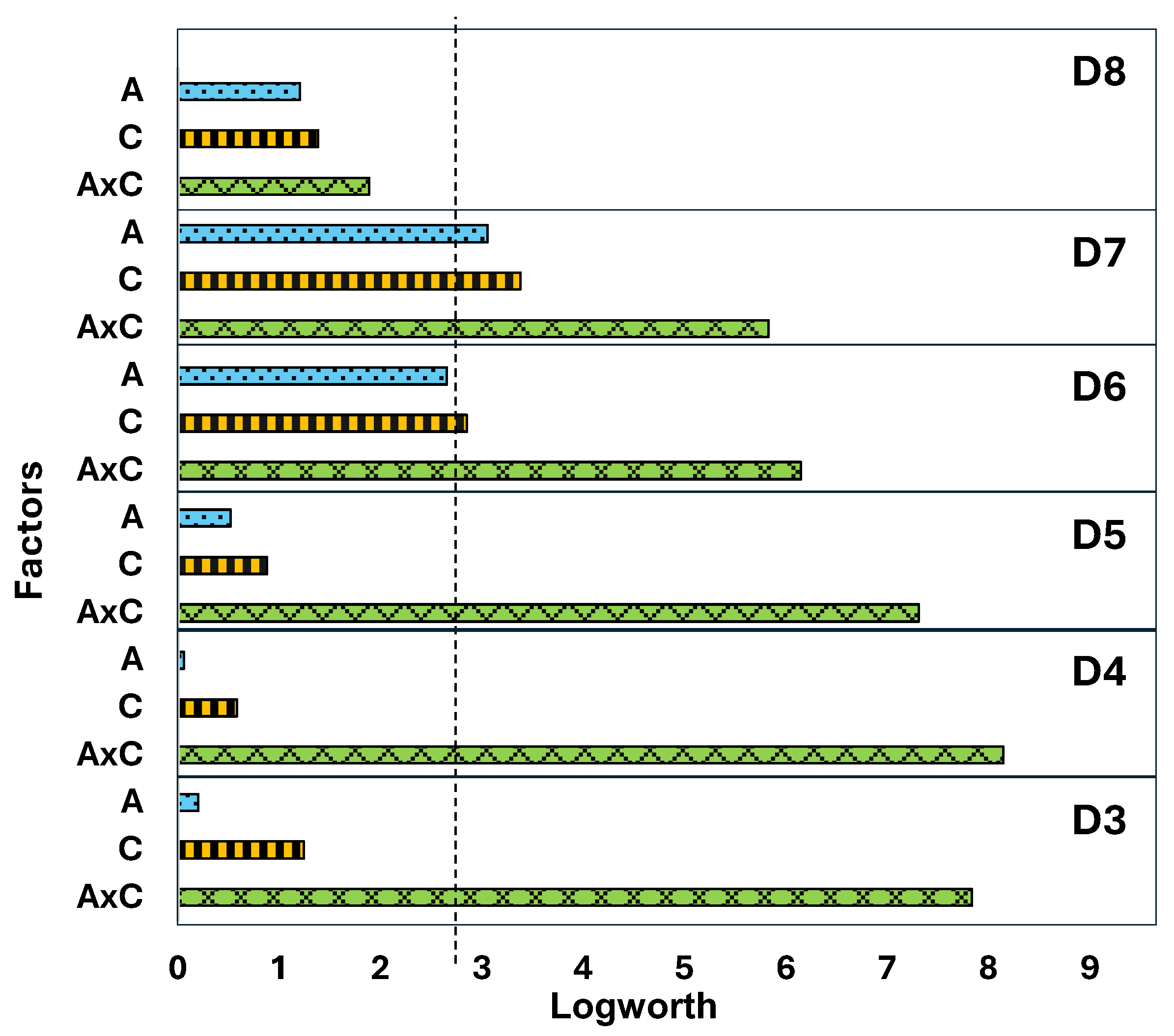
3.2. Distribution of Siloxanes Among the Sequential Pyrolyzer Temperature Steps Determined with Py-GC-MS
3.3. Weathering with Regard to Siloxane Speciation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Declaration of Use of Generative AI
Conflicts of Interest
Abbreviations
| Py-GC-MS | Pyrolysis gas chromatography mass spectrometry |
| EGA-MS | Evolved gas analysis and mass spectrometry |
| PDMS | Polydimethylsiloxane |
| TGA | Thermogravimetric analysis |
| TIC | Total ion current |
| ANOVA | Analysis of variance |
References
- Mark, J.E. Some interesting things about polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- De Buyl, F. Silicone sealants and structural adhesives. Int. J. Adhes. Adhes. 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Wolf, A.T. Concepts for Development of a Service Life Prediction Methodology for Sealed Building and Construction Joints-Review and Roadmap for Future Research; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- O’Lenick, A.J., Jr. Silicones—Basic chemistry and selected applications. J. Surfactants Deterg. 2000, 3, 229–236. [Google Scholar] [CrossRef]
- Ngolemasango, F.E.; Bennett, M.; Clarke, J. Degradation and life prediction of a natural rubber engine mount compound. J. Appl. Polym. Sci. 2008, 110, 348–355. [Google Scholar] [CrossRef]
- Paroli, R.M. Applications of TG-FTIR in the characterization of weathered sealants. Polym. Mater. Sci. Eng. 1993, 69, 139–140. [Google Scholar]
- Paroli, R.M. Applications of thermogravimetry in the characterization of silicone sealants. Polym. Mater. Sci. Eng. 1993, 68, 334–335. [Google Scholar]
- Paroli, R.M. Applications of thermogravimetry and PAS-FTIR in the characterization of silicone sealants. Can. J. Appl. Spectrosc. 1994, 39, 7–14. [Google Scholar]
- Klosowski, J.M.; George, A.G. The chemistry of silicone room temperature vulcanizing sealants. Org. Coat. Plast. Chem. 1979, 40, 319–323. [Google Scholar]
- Lacasse, M.A. Evaluating the durability of sealants. ASTM Spec. Tech. Publ. 1995, STP 1243, 29–48. [Google Scholar] [CrossRef]
- Paroli, R.M.; Cole, K.C.; Delgado, A.H.; Urban, M.W.; Provder, T. Evaluating the weatherability of polyurethane sealants. In Multidimensional Spectroscopy of Polymers: Vibrational, NMR, and Fluorescence Techniques; American Chemical Society: Washington, DC, USA, 1995; Volume 598, pp. 117–136. [Google Scholar]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, C.; Dittmann, D.; Eisentraut, P.; Wiesner, Y.; Schartel, B.; Klack, P.; Braun, U. Evaluation of thermoanalytical methods equipped with evolved gas analysis for the detection of microplastics in environmental samples. J. Anal. Appl. Pyrolysis 2020, 152, 104961. [Google Scholar] [CrossRef]
- Paroli, R.M.; Cole, K.C.; Delgado, A.H. Weatherability of Polyurethane Sealants: A Spectroscopic Approach; ResearchGate: Berlin, Germany, 1994; Volume 71, pp. 435–436. Available online: https://www.researchgate.net/publication/44049861_Weatherability_of_Polyurethane_Sealants_A_Spectroscopic_Approach (accessed on 23 October 2025).
- Lin, C.-W.; Chien, C.-H.; Tan, J.; Chao, Y.J.; Van Zee, J.W. Chemical degradation of five elastomeric seal materials in a simulated and an accelerated PEM fuel cell environment. J. Power Sources 2011, 196, 1955–1966. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Maxwell, R.S. Applications of Modern Pyrolysis Gas Chromatography for the Study of Degradation and Aging in Complex Silicone Elastomers; Graz University of Technology: Graz, Australia, 2014. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Albo, R.L.F.; Alviso, C.T.; Maxwell, R.S. Pyrolysis-gas chromatography/mass spectrometry for the forensic fingerprinting of silicone engineering elastomers. J. Anal. Appl. Pyrolysis 2013, 99, 85–91. [Google Scholar] [CrossRef]
- Maurer, J.; Buffaz, K.; Massonnet, G.; Roussel, C.; Burnier, C. Optimization of a Py-GC-MS method for silicone-based lubricants analysis. J. Anal. Appl. Pyrolysis 2020, 149, 104861. [Google Scholar] [CrossRef]
- Voeller, K.; Bílek, H.; Kreft, J.; Dostálková, A.; Kozliak, E.; Kubátová, A. Thermal carbon analysis enabling comprehensive characterization of lignin and its degradation products. ACS Sustain. Chem. Eng. 2017, 5, 10334–10341. [Google Scholar] [CrossRef]
- LaVallie, A.L.; Bilek, H.; Andrianova, A.; Furey, K.; Voeller, K.; Yao, B.; Kozliak, E.; Kubátová, A. Quantitative insights on de/repolymerization and deoxygenation of lignin in subcritical water. Bioresour. Technol. 2021, 342, 125974. [Google Scholar] [CrossRef]
- Karpovych, V.; Haiduk, N.; Oga, E.; Bala, N.; Kubátová, A.; Kozliak, E.; Sulkes, M. PAH induction upon pyrolysis of hydroxyl-terminated polybutadiene-based solid rocket fuels. J. Phys. Chem. A 2025, 129, 6356–6373. [Google Scholar] [CrossRef]
- Brzonova, I.; Kozliak, E.I.; Andrianova, A.A.; LaVallie, A.; Kubátová, A.; Ji, Y. Production of lignin-based insoluble polymers (anionic hydrogels) by C. versicolor. Sci. Rep. 2017, 7, 17507. [Google Scholar] [CrossRef]
- Bystritskaya, E.V.; Karpukhin, O.N.; Tsverava, V.G.; Nepovinnykh, V.I.; Rusin, M.Y. Thermal degradation of silicone sealant. Int. Polym. Sci. Eng. 2018, 39, 51–56. [Google Scholar] [CrossRef]
- Moseson, D.E.; Jordan, M.A.; Shah, D.D.; Corum, I.D.; Alvarenga, B.R., Jr.; Taylor, L.S. Application and limitations of thermogravimetric analysis to delineate the hot melt extrusion chemical stability processing window. Int. J. Pharm. 2020, 590, 119916. [Google Scholar] [CrossRef]
- Sabatini, F.; Nacci, T.; Degano, I.; Colombini, M.P. Investigating the composition and degradation of wool through EGA-MS and Py-GC-MS. J. Anal. Appl. Pyrolysis 2018, 135, 111–121. [Google Scholar] [CrossRef]
- Materazzi, S. Applications of evolved gas analysis. Talanta 2006, 68, 489–496. [Google Scholar] [CrossRef]
- Materazzi, S. Applications of evolved gas analysis. Part 2: EGA by mass spectrometry. Talanta 2006, 69, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Eiler, J. A Comprehensive Guide to Silicon Sealant. Available online: https://everkemproducts.com/comprehensive-guide-to-silicone-sealants/ (accessed on 20 September 2025).
- Silicone Sealant: Composition, Applications, Pros and Cons—A Complete Guide. Available online: https://lyzhuozhu.com/blog/silicone-sealant-composition-applications-pros-and-cons-a-complete-guide/ (accessed on 1 April 2025).
- Longkaew, K.; Gibaud, A.; Tessanan, W.; Daniel, P.; Phinyocheep, P. Spherical CaCO3: Synthesis, characterization, surface modification, and efficacy as a reinforcing filler in natural rubber composites. Polymers 2023, 15, 4287. [Google Scholar] [CrossRef]
- Lewicki, J.; Mayer, B.; Alviso, C.; Maxwell, R. Thermal degradation behavior and product speciation in model poly(dimethylsiloxane) networks. J. Inorg. Organomet. Polym. Mater. 2012, 22, 636–645. [Google Scholar] [CrossRef]
- Xiang, X.; Liu, N.; Xu, L.; Cai, Y. Review of recent findings on occurrence and fates of siloxanes in environmental compartments. Ecotoxicol. Environ. Saf. 2021, 224, 112631. [Google Scholar] [CrossRef] [PubMed]
- Tasic, A.M.; Pergal, V.; Antic, P.; Anti, V. Synthesis, structure, and thermogravimetric analysis of α,ω-telechelic polydimethylsiloxanes of low molecular weight. J. Serb. Chem. Soc. 2017, 82, 1395–1416. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymers 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Liu, S.; Zhao, J.; Li, D.; Yuan, Y. Thermal degradation behaviors of polydimethylsiloxane-graft-poly(methyl methacrylate). Thermochim. Acta 2013, 573, 32–38. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Lewicki, J.P.; Liggat, J.J.; Patel, M. The thermal degradation behavior of polydimethylsiloxane/montmorillonite nanocomposites. Polym. Degrad. Stab. 2009, 94, 1548–1557. [Google Scholar] [CrossRef]
- Chinn, S.C.; Alviso, C.T.; Berman, E.S.; Harvey, C.A.; Maxwell, R.S.; Wilson, T.S.; Cohenour, R.; Saalwachter, K.; Chasse, W. MQ NMR and SPME analysis of nonlinearity in the degradation of a filled silicone elastomer. J. Phys. Chem. B 2008, 114, 1520–6106. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, R.S.; Chinn, S.C.; Alviso, C.T.; Harvey, C.A.; Giuliani, J.R.; Wilson, T.S.; Cohenour, R. Quantification of radiation-induced crosslinking in a commercial, toughened silicone rubber, TR55 by 1H MQ-NMR. Polym. Degrad. Stab. 2009, 94, 456–464. [Google Scholar] [CrossRef]
- Ihemaguba, C.L.; Marossy, K. Combined thermal analysis of fluid plasticizers. J. Therm. Anal. Calorim. 2022, 147, 195–201. [Google Scholar] [CrossRef]




| Sample # | Sample Description: Number of Years Exposed | Weather Level Based on EGA-MS |
|---|---|---|
| Fresh (unweathered) sealant | ||
| 01 | Sealant 1 (Control) | L0 |
| 02 | Sealant 2 * | NA ** |
| Aged samples compared with Sealant 1 (Control L0) | ||
| 03 | 13 years | L2 |
| 04 | 12 years | L3 |
| 05 | 12 years | L3 |
| 06 | 12 years | L3 |
| 07 | 12 years | L2 |
| 08 | 11 years | L2 |
| 09 | 11 years | L2 |
| 10 | 9 years | L2 |
| 11 | 8 years | L1 |
| 12 | 3 years | L1 |
| 13 | 1 year | L1 |
| 14 | 1 year | L1 |
| Parameter | EGA-MS Mode | Py-GC-MS Mode |
|---|---|---|
| Carrier gas | Helium, 1.0 mL/min | Helium, 1.0 mL/min |
| Pyrolyzer temperature | 90–800 °C (ramp 20 °C/min, hold 1 min) | 7 steps (90–330 °C, 330–450 °C, 450–510 °C, 510–550 °C, 550–600 °C, 600–710 °C, 710–790 °C) at 20 °C/min |
| Injector | 300 °C, split 1:100 | 300 °C, split 1:100 |
| Cryogenic trapping | NA | Liquid nitrogen |
| GC oven temperature program | 300 °C | 40–300 °C, ramp 25 °C/min, hold 4 min |
| Column | Ultra ALLOY-DTM deactivated tube, 0.15 mm ID × 2.5 m, (Frontier Laboratories) | DB-5MS capillary column, 40 m × 250 μm ID × 0.25 µm film thickness (J&W Scientific, Folsom, CA, USA) |
| Transfer line temperature | 300 °C | 300 °C |
| Ionization source | EI (70 eV), 230 °C | EI (70 eV), 230 °C |
| MS Quad | 150 °C | 150 °C |
| MS scan range | 10–700 m/z | 10–700 m/z |
| Scan rate | scan/6 s | scan/2.66 s |
| Name | Hexamethylcyclo-trisiloxane | Octamethylcyclo-tetrasiloxane | Decamethylcyclo-pentasiloxane | Dodecamethylcyclo-hexasiloxane | Tetradecamethylcyclo-heptasiloxane | Hexadecamethylcyclooctasiloxane |
| Label * | D3 | D4 | D5 | D6 | D7 | D8 |
| Formula | C6H18O3Si3 | C8H24O4Si4 | C10H30O5Si5 | C12H36O6Si6 | C14H42O7Si7 | C16H18O8Si8 |
| tR (min) | 6.07 | 7.77 | 8.64 | 9.56 | 10.39 | 10.90 |
| m/z | 207 | 281 | 355 | 429 | 503 | 577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oga, E.; Bala, N.; Fisher, S.; Kozliak, E.; Kubátová, A. Investigation of Window Silicone Sealant Weathering Using Evolved Gas Analysis and Pyrolysis Gas Chromatography with Mass Spectrometry. Polymers 2025, 17, 2884. https://doi.org/10.3390/polym17212884
Oga E, Bala N, Fisher S, Kozliak E, Kubátová A. Investigation of Window Silicone Sealant Weathering Using Evolved Gas Analysis and Pyrolysis Gas Chromatography with Mass Spectrometry. Polymers. 2025; 17(21):2884. https://doi.org/10.3390/polym17212884
Chicago/Turabian StyleOga, Eugene, Nafisa Bala, Stephen Fisher, Evguenii Kozliak, and Alena Kubátová. 2025. "Investigation of Window Silicone Sealant Weathering Using Evolved Gas Analysis and Pyrolysis Gas Chromatography with Mass Spectrometry" Polymers 17, no. 21: 2884. https://doi.org/10.3390/polym17212884
APA StyleOga, E., Bala, N., Fisher, S., Kozliak, E., & Kubátová, A. (2025). Investigation of Window Silicone Sealant Weathering Using Evolved Gas Analysis and Pyrolysis Gas Chromatography with Mass Spectrometry. Polymers, 17(21), 2884. https://doi.org/10.3390/polym17212884







