Abstract
This study offers an integrated synthesis of polymeric materials in biomedical engineering, revealing four major and interlinked research domains: tissue engineering and regenerative medicine, drug delivery and nanomedicine, wound healing and antimicrobial applications, and advanced fabrication through 3D/4D printing and bioprinting. Across these areas, hydrogels, biodegradable composites, and stimuli-responsive polymers emerge as the most influential material classes. The analysis highlights substantial progress in extracellular matrix–mimetic scaffolds, smart drug delivery systems with controlled release, multifunctional wound dressings integrating antimicrobial and healing functions, and patient-specific constructs produced via additive manufacturing. Despite these advances, recurring challenges persist in long-term biocompatibility and safety, scalable and reproducible fabrication, and regulatory standardisation. The results point toward a convergence of bioactivity, manufacturability, and clinical translation, with hybrid natural–synthetic systems and personalised polymeric designs defining the next phase of biomedical polymer innovation.
1. Introduction
Biomaterials are substances engineered to interact with biological systems for the purpose of evaluating, treating, augmenting, or replacing any tissue, organ, or bodily function [1]. The history of biomaterials can be categorised into generations based on their functional objectives. The earliest, first-generation materials were designed to be bio-inert, simply coexisting with the host without eliciting a toxic response [2]. Over time, the field evolved, leading to a third generation of materials that are not merely inert but are designed to actively promote or inhibit specific biological activities, such as cell adhesion and tissue regeneration [2,3]. This shift has been catalysed by the increasing demand for sophisticated medical interventions that not only repair but also restore physiological function.
Within this evolving landscape, polymeric materials have emerged as the basis of modern biomedical engineering. Their ascendancy is a result of their distinct advantages over traditional materials, such as metals and ceramics. Polymers offer remarkable versatility, with the ability to be synthesised with customised chemical and physical properties that suit a wide range of applications [1]. They are also lightweight and possess a lower coefficient of thermal expansion than metals, which can be particularly beneficial for load-bearing applications, as it minimises stress on surrounding tissues [4]. The ability to specifically modify their properties for different parts of the body has made them a centre of innovation and commercial development [5].
The foundational material science of polymeric biomaterials is rooted in a fundamental choice between two distinct classes of materials: natural and synthetic polymers. This choice presents an essential trade-off between intrinsic biological compatibility and mechanical or chemical properties.
Natural polymers are derived from living organisms, including plants, animals, and microorganisms [6,7]. Their primary advantage lies in their inherent biological compatibility and their structural similarity to the native extracellular matrix (ECM) of the human body [7,8]. This biomimicry enables them to be recognised by the body, thereby minimising the risk of chronic inflammatory reactions, toxicity, or immunological rejection [7,8]. Examples of natural polymers widely used in biomedical applications include proteins such as collagen, fibrin, and silk, as well as polysaccharides like chitosan and hyaluronic acid [7]. Despite these benefits, they are mechanically inferior to synthetic alternatives and are susceptible to batch-to-batch variability [7]. Furthermore, since they are derived from biological sources, they carry a potential risk of disease transmission or immunogenicity [1].
In contrast, synthetic polymers are artificially created in laboratories, offering a high degree of control over their properties [7,9]. They can be produced under controlled conditions with predictable and reproducible characteristics, including mechanical strength, degradation rate, and chemical composition. This tunability allows researchers to engineer materials with properties tailored for specific functions, such as the tensile strength required for a suture or the elasticity needed for a cardiovascular graft [1]. Widely studied examples include polylactic acid (PLA), poly(glycolic acid) (PGA), poly(caprolactone) (PCL), and their copolymer PLGA [10]. The primary disadvantage of synthetic polymers is their potential for poor biocompatibility and a general lack of cell adhesion sites, which often necessitates chemical modifications or surface treatments to enhance their integration with biological tissue [7].
Table 1 presents a comparison between natural and synthetic polymers used for biomedical applications.

Table 1.
Comparison between natural and synthetic polymers used for biomedical applications.
The distinct advantages and disadvantages of natural and synthetic polymers have created a clear and persistent dilemma in the field. Natural polymers are inherently well-tolerated by the body but lack the robust and reproducible mechanical properties required for many applications. Synthetic polymers offer unparalleled mechanical and chemical control, but they also pose risks of adverse biological reactions. The logical progression of research has been to bridge this gap by developing hybrid systems that combine both polymer types [15,16]. This approach is not a simple mixture but a sophisticated engineering strategy that leverages the mechanical strength and reproducibility of synthetic polymers while benefiting from the superior bioactivity and biocompatibility of natural ones [17,18]. The creation of these synergistic, hybrid materials represents a fundamental shift away from the earlier paradigm of single-material biomaterials. The most promising future materials are therefore not pure but are intelligently designed composites that actively integrate the strengths of both classes to meet the complex demands of biomedical applications.
The economic significance of this field is substantial, reflecting its rapid growth. In 2013, the global market for implantable biomaterials was valued at nearly 75.1 billion USD and was projected to reach 109.5 billion USD by 2019, growing at a compound annual growth rate (CAGR) of 6.7% [1,19]. The polymeric biomaterials sector was identified as a key driver of this expansion, with a projected CAGR of 22.1% [1,19]. More recent data reinforce this trend, showing an astonishing growth surge with the polymer biomaterial market escalating from 79.06 billion USD in 2024 to 94.98 billion USD in 2025, a CAGR of 20.1% [20]. Projections indicate that this market will continue its robust expansion, potentially reaching 169.88 billion USD by 2029 [20]. This growth is geographically concentrated, with North America and the Asia-Pacific region anticipated to dominate the market, reflecting a high concentration of research and healthcare investment [21].
Given the rapid evolution and growing complexity of this field, a comprehensive synthesis of the existing literature is warranted. This paper presents a review of highly cited review articles to provide a high-level, authoritative synthesis of the current state of knowledge. The aim is to identify key findings and knowledge gaps and to outline future directions in the role of polymers in biomedical applications, thereby providing a foundational reference for researchers and industry professionals.
2. Applications and Innovations: A New Frontier for Healthcare
Polymeric materials are not merely substitutes for other materials; they are enablers of new medical technologies. Their versatility has led to transformative applications across three primary domains: tissue engineering, controlled drug delivery, and advanced medical devices.
Table 2 presents some examples of polymers and their specific medical applications.

Table 2.
Specific medical applications of polymers.
2.1. Tissue Engineering and Regenerative Medicine
The core function of polymers in tissue engineering (TE) is to design and construct scaffolds that provide a three-dimensional environment for tissue regeneration [40,41,42,43]. These scaffolds are bio-mimetic structures that emulate the natural ECM, offering mechanical, spatial, and biological cues that guide cellular responses, including adhesion, proliferation, and differentiation [44,45]. A key design objective is to ensure that the scaffold degrades at a rate commensurate with new tissue formation, allowing the new tissue to gradually assume mechanical load as the polymer recedes [46,47,48,49].
The field has advanced from simple scaffolds to complex, patient-specific structures. For example, in spinal cord regeneration, scaffolds loaded with neural progenitor cells (NPCs) have been bioprinted to accelerate axon regeneration and improve motor function in animal models [50,51]. Similarly, for cartilage repair, 3D printed scaffolds can be designed with complex, irregular shapes that mimic the natural geometry of tissue, allowing for the reconstruction of structures such as auricular or tracheal cartilage [50,52,53]. Bone regeneration has also seen significant advances with the development of composites. The addition of hydroxyapatite (Hap) to polymeric scaffolds has been shown to improve bioactivity and promote the growth of a mineral layer that closely mimics natural bone [54,55,56,57,58].
2.2. Controlled Drug Delivery Systems (DDS)
Polymers are indispensable for controlled drug delivery, serving as a primary means to manage medication release, protect drugs from physiological degradation, and enhance patient compliance by reducing the frequency of dosing [59,60,61,62].
The stealth effect enhances nanomaterial pharmacokinetics by improving blood circulation and tissue targeting, yet most systems still show rapid clearance after administration—a phenomenon termed the pseudo-stealth effect. Achieving true stealth behaviour requires a holistic surface design, where overall structure, charge, and hydrophobicity are optimised rather than relying solely on single polymer coatings such as PEGylation [63]. A significant innovation within this area is the development of “smart” or stimuli-responsive polymers [64]. These materials are designed to change their physical or chemical properties in response to specific environmental triggers [61]. These triggers can be internal, such as the acidic pH of a tumour microenvironment, the presence of specific enzymes, or changes in glucose levels [65,66,67,68]. Alternatively, they can be external, like light, ultrasound, or magnetic fields [65,66,67,68,69]. This responsiveness enables precise, site-specific drug delivery, thereby maximising therapeutic efficacy while minimising off-target effects and systemic toxicity. This level of control is fundamental to the advancement of personalised medicine, where treatments can be tailored to an individual’s unique physiological or pathological state [70].
2.3. Innovations in Medical Devices and Implants
Polymers are used in a vast spectrum of medical devices, from simple, single-use items to complex, long-term implants [71,72,73]. Their utility extends to every aspect of healthcare, from surgical tools and wound dressings to advanced prosthetic devices. For instance, ultrahigh-molecular-weight polyethylene (UHMWPE) is the material of choice for load-bearing components in hip and knee replacements [74]. Polyurethanes (PU) are prized for their high biocompatibility and hemocompatibility, making them ideal for applications such as artificial hearts [75,76,77,78,79,80], catheters [81,82,83], and wound dressings [84,85,86]. Poly(ether ether ketone) (PEEK) is increasingly employed in long-term orthopaedic implants [28,87,88,89].
The convergence of two major trends—customisation and adaptability—is shaping the next generation of these devices. Additive manufacturing, specifically 3D printing and bioprinting, enables the creation of patient-specific implants and scaffolds derived from medical imaging data [90,91,92,93,94,95,96,97]. Simultaneously, the development of smart polymers allows for materials that are adaptive to a patient’s unique physiological state, such as fluctuating glucose levels or the acidic pH of a cancerous tumour. This convergence of customisation and adaptability is poised to usher in a new era of dynamic, responsive systems that are fully integrated into an individual’s biology, marking an actual realisation of the promise of personalised medicine.
3. Materials and Methods
This study was designed as a bibliometric mapping combined with a structured synthesis of highly cited review papers on polymeric materials in biomedical engineering. The rationale for focusing on review articles was to consolidate established evidence and consensus knowledge, providing a reliable foundation for identifying research trends, methodological advances, and persisting challenges. While this design ensures a comprehensive and validated overview of the field, it may also introduce a degree of bias toward mature or well-explored topics, potentially underrepresenting emerging or niche research areas not yet reflected in major reviews. Acknowledging this trade-off, the analysis emphasised the interpretative depth of the selected reviews rather than the sheer volume of data, aiming to distil the most significant findings shaping the field.
First, a bibliometric analysis was conducted using the Web of Science Core Collection, selected for its rigorous indexing and broad coverage of biomedical research.
Second, a synthesis of the most cited review articles (≥100 citations) was performed, to highlight the dominant themes, methodological advances, and persistent challenges reported across the literature. By analysing these highly influential works, the study aimed to identify knowledge gaps and outline future research trajectories.
The search was conducted using the query: (“polymeric materials” OR “biomedical polymers” OR “polymeric biomaterials”) AND (“biomedical” OR “medical” OR “healthcare” OR “tissue engineering” OR “drug delivery” OR “implant”) AND (“Review” OR “Systematic Review”). This search returned 1095 records.
The results were refined by restricting the document type to reviews, which yielded 844 articles. The search was then restricted to the period 2016–2025, which reduced our dataset to 628 articles. No duplicates were found. Then titles and abstracts were screened to confirm alignment with the research objective. Finally, the full texts of the remaining articles were examined, with inclusion limited to those reviews that addressed biomedical applications of polymers.
By manual scanning, 39 articles were excluded because their field of study did not align with the scope of this review, which focuses on biomedical applications of polymeric materials.
More specifically, the excluded articles belonged to the following broader domains:
- Fundamental and synthetic chemistry (15 articles);
- Energy, environment, and sustainability (7 articles);
- Plant- and marine-derived biopolymers (3 articles);
- Nanomaterials and coatings for non-medical applications (5 articles);
- Engineering and additive manufacturing outside biomedical focus (4 articles) *;
- Electronics and supramolecular nanostructures (3 articles);
- Miscellaneous non-biomedical topics (2 articles): where the emphasis was on interdisciplinary areas without substantive medical or healthcare relevance.
* Reviews were excluded if they addressed polymeric materials used exclusively for non-medical purposes, such as industrial coatings or packaging. In addition, reviews focused on engineering and additive manufacturing were included only when these technologies were applied to biomedical contexts. For instance, studies centred on mechanical optimisation of 3D printing parameters for structural polymers, without reference to biocompatibility or medical devices, were excluded.
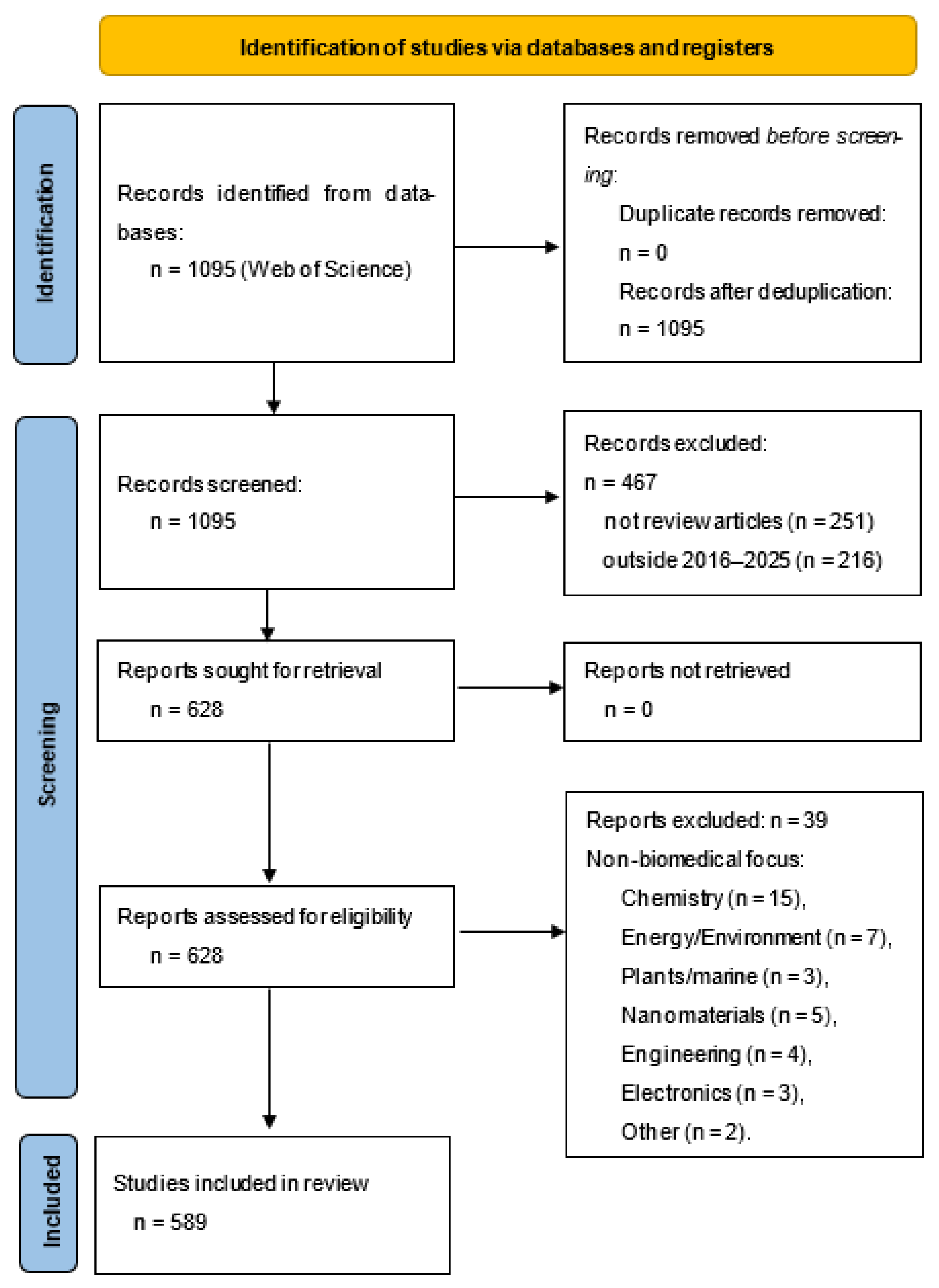
Ultimately, the research encompassed 589 review papers. The study selection process is summarised in the PRISMA flow diagram (Figure 1), which details the identification, screening, eligibility, and inclusion stages of the review (Table S1 in the Supplementary).
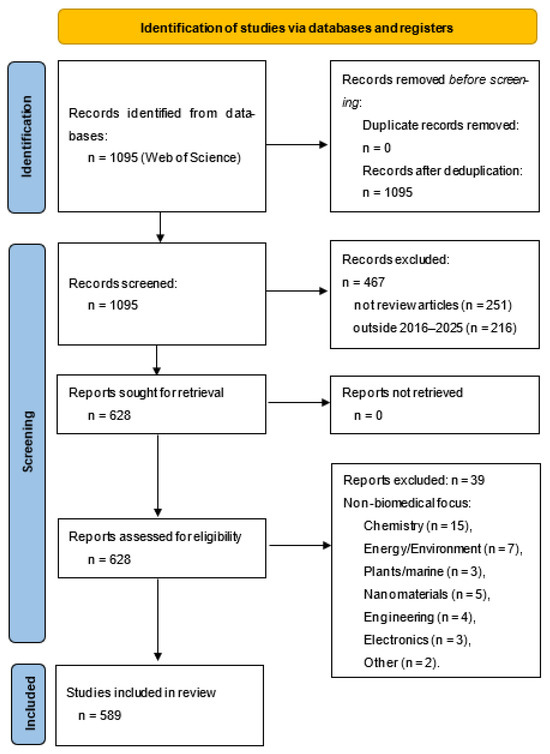
Figure 1.
PRISMA flow for bibliometric selection.
The bibliometric analysis was performed using VOSviewer (version 1.6.20), a software tool designed to map and visualise scientific literature. Co-occurrence networks of keywords, bibliographic coupling among sources, and international co-authorship maps were generated. Both overlay and density visualisations were used to illustrate temporal and structural patterns.
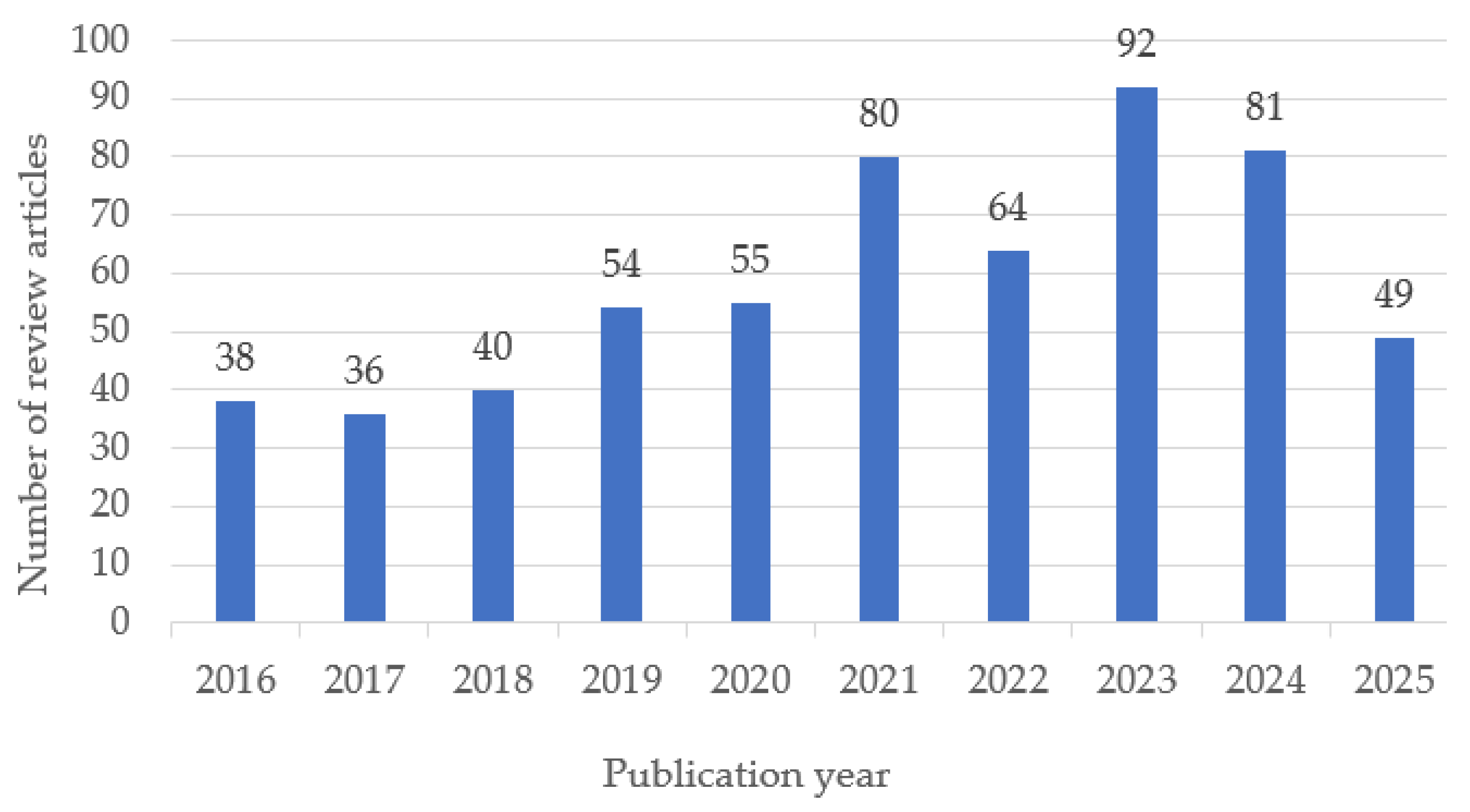
The temporal distribution of the selected review articles shows a steady growth in publications on biomedical polymers between 2016 and 2025, with a peak in 2023 (Figure 2). This trend reflects the increasing scientific interest and diversification of research topics in recent years.
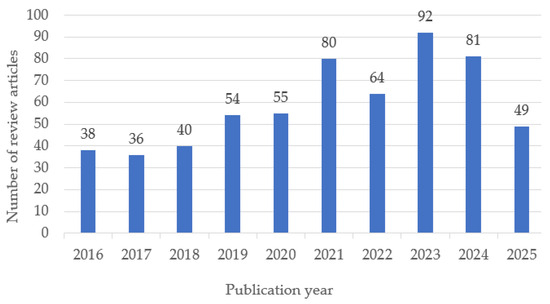
Figure 2.
Annual distribution of review articles on biomedical polymers included in the study (2016–2025). Note: Data for 2025 are partial, as the year is ongoing; therefore, the apparent decline reflects incomplete indexing rather than a decrease in publication activity.
4. Bibliometric Analysis of Polymeric Materials in Biomedical Engineering
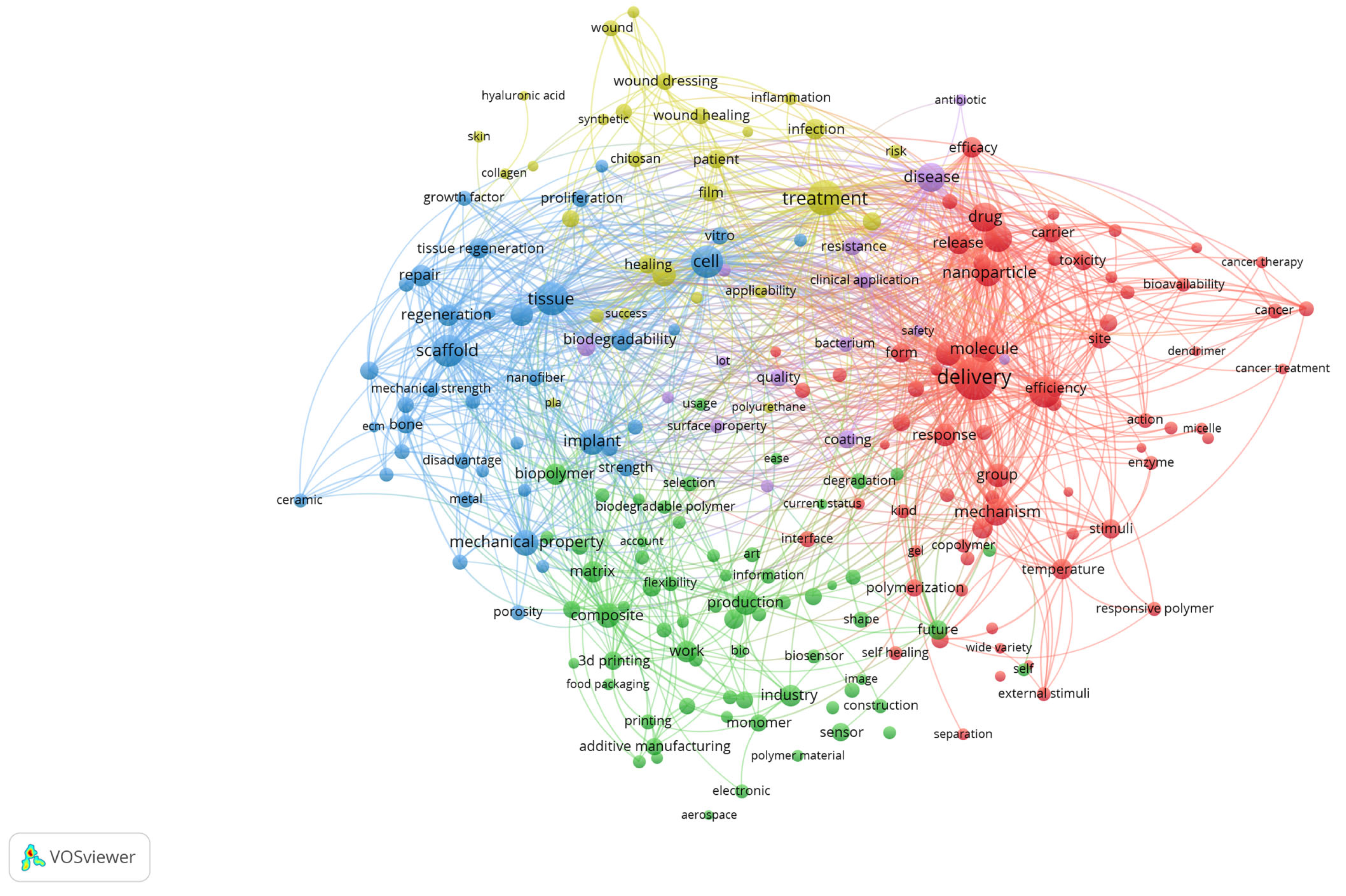
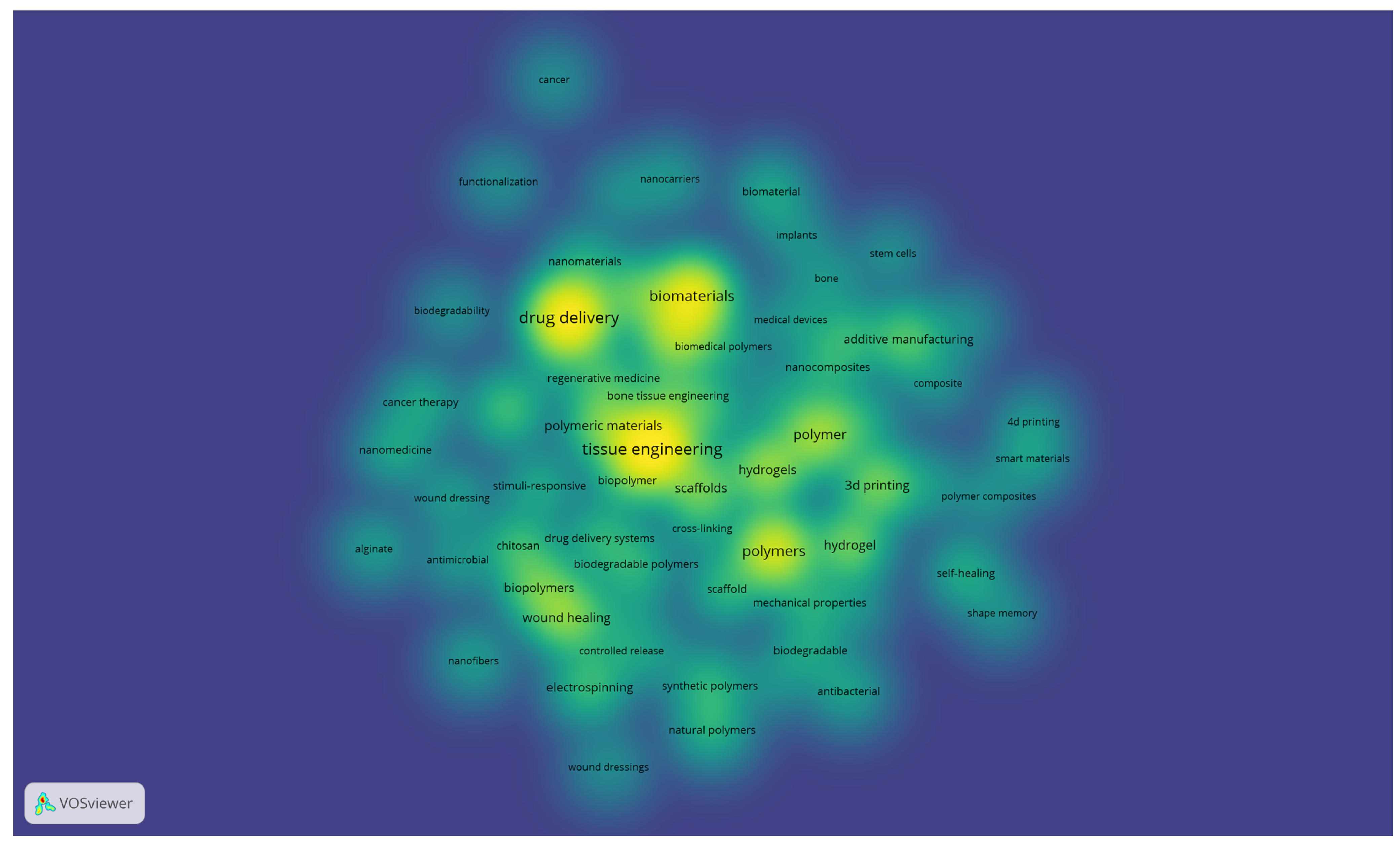
A co-occurrence analysis of terms derived from the titles and abstracts of the included review articles was performed using VOSviewer with binary counting, which considers the presence of a term within a document regardless of its frequency of occurrence (Figure 3). The generated map visualises the structural relationships among recurrent concepts and reveals the main thematic directions in biomedical polymer research.
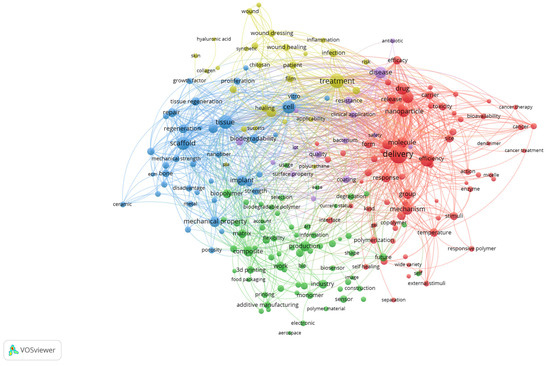
Figure 3.
Keyword co-occurrence network of biomedical polymer research, based on text data.
The red cluster, situated on the right side of the map, is centred around terms such as “drug,” “delivery,” “nanoparticle,” “cancer,” and “toxicity”. This cluster emphasises the extensive exploration of polymeric systems as nanocarriers, particularly in oncology, where controlled release, targeting strategies, and responsiveness to biological stimuli represent dominant research trends. The strong linkages within this cluster highlight the convergence of drug delivery science, cancer therapy, and polymer chemistry.
The blue cluster, positioned on the left, brings together terms such as scaffold, tissue, regeneration, bone, repair, implant and cell. This cluster underscores the importance of tissue engineering and regenerative medicine, where polymeric scaffolds, both natural and synthetic, are evaluated for their ability to replicate extracellular matrix properties and promote cell proliferation, differentiation, and tissue repair. The interconnections with terms like implant and biodegradability indicate a focus on clinical translation.
The green cluster, located in the lower part of the map, comprises terms including production, composite, 3D printing, additive manufacturing, and industry. This group highlights the importance of material science and engineering aspects of biomedical polymers, where optimising mechanical strength, porosity, and processing techniques is crucial for ensuring functionality in clinical devices. The inclusion of additive manufacturing terms indicates the growing importance of advanced fabrication methods in customising polymer-based biomaterials.
The yellow cluster, located in the upper part of the visualisation, is characterised by terms such as healing, treatment, infection, film, wound dressing, and patient. This cluster captures the translation of polymeric materials into clinical solutions for wound management and infection control. The presence of both synthetic and natural polymers within this cluster suggests ongoing efforts to strike a balance between cost-effectiveness, biodegradability, and therapeutic performance in real-world healthcare applications.
Taken together, the map demonstrates that the field of biomedical polymers is structured along two complementary axes: (i) the development of advanced materials and fabrication strategies, and (ii) their translation into biomedical applications ranging from targeted drug delivery and cancer therapy to tissue regeneration and wound healing. The overlaps among clusters underscore the interdisciplinary nature of the field, where chemistry, engineering, and clinical sciences converge to shape emerging research frontiers.
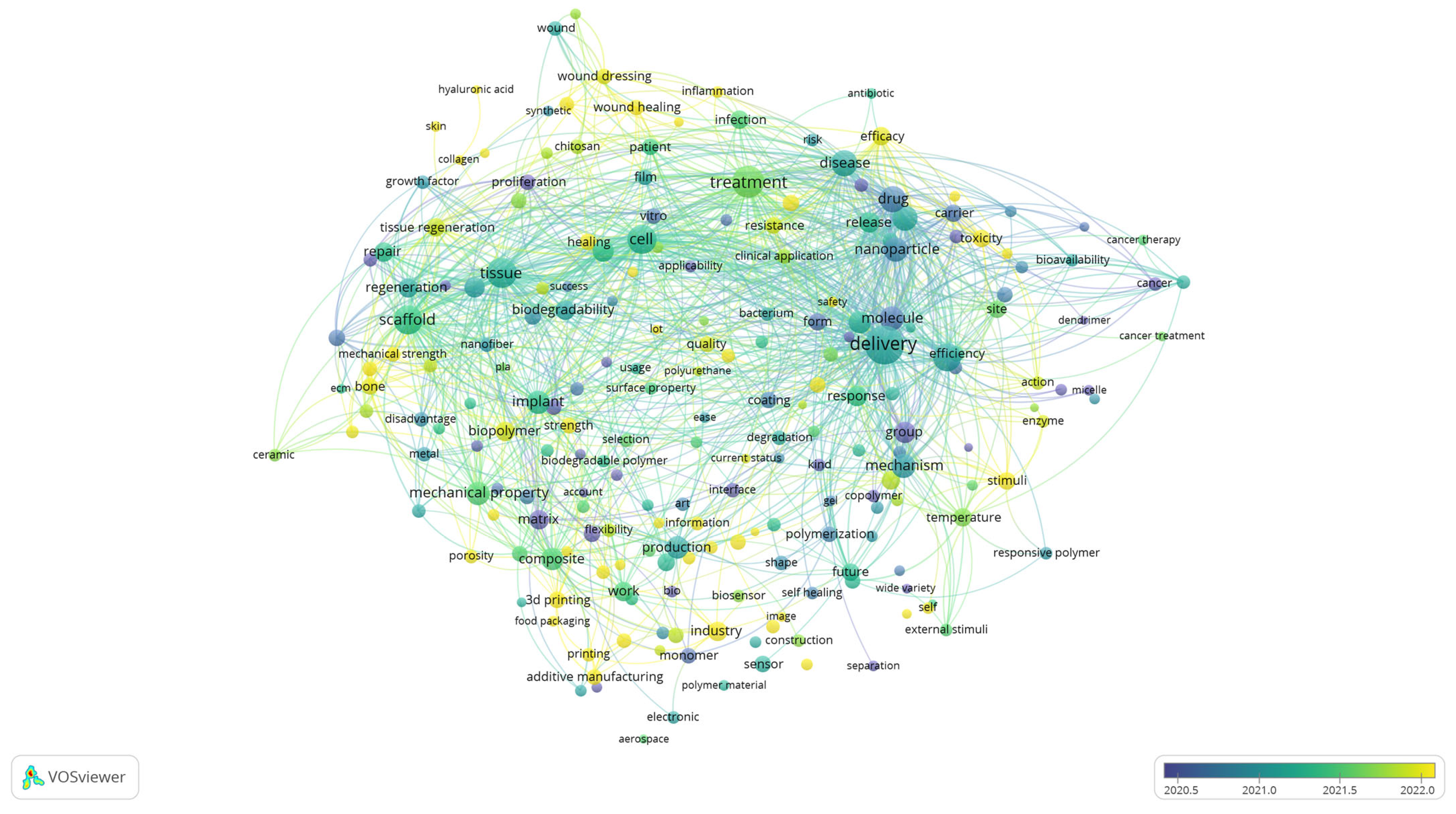
While the cluster visualisation highlights the structural composition of the research field by grouping related terms into thematic clusters, the overlay visualisation provides a complementary perspective by incorporating the temporal dimension. By colouring terms according to their average year of occurrence, Figure 4 enables the tracing of how the focus of biomedical polymer research has shifted over time. In this way, the analysis identifies the dominant thematic areas and reveals which topics are well-established and which ones are emerging more recently.
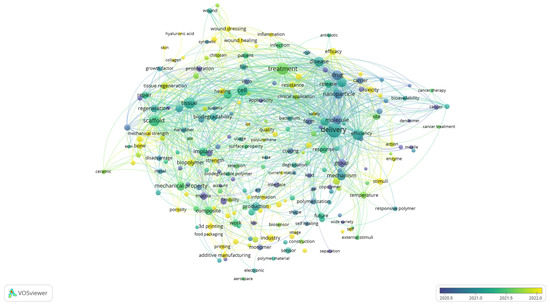
Figure 4.
Overlay visualisation of keyword co-occurrence, showing temporal evolution of research themes, based on text data.
Although the scale displayed in the figure emphasises the most active period from 2020 to 2022, the underlying dataset encompasses the entire decade. Earlier contributions (shown in blue and green) are associated with terms such as “molecule, “drug,” “in vitro,” “tissue,” and “cancer”, reflecting the foundational focus on drug design, experimental validation, and the early application of polymers in biomedical and oncological contexts. Terms positioned in the central spectrum (greenish tones), including delivery, nanoparticle, biodegradability, and treatment, represent themes that have remained consistently relevant across the years, particularly in drug delivery systems, nanomedicine, and biocompatibility assessment. More recent developments (yellow shades) highlight emerging directions such as wound healing, 3D printing, biopolymer, industry, and innovation, pointing to a growing emphasis on translational research, advanced manufacturing strategies, and the integration of polymer science into clinical and industrial applications.
This temporal distribution suggests that the field has progressed from a predominantly fundamental orientation, focused on molecular design, in vitro validation, and early biomedical applications, toward more application-driven and translational research.
Based on the bibliographical data, additional visualisations were generated to provide deeper insights into the structural composition and thematic development of the field. A co-occurrence analysis of author keywords was performed in VOSviewer using complete counting with a resolution parameter set at 0.70.
The resulting density visualisation (Figure 5) highlights the most frequently used terms, with brighter areas indicating higher occurrence and stronger interconnections. Central concepts, such as tissue engineering, drug delivery, biomaterials, and polymers, emerge as dominant research fronts, surrounded by related themes including hydrogels, 3D printing, wound healing, and nanomaterials. This distribution reflects the interplay between foundational topics in polymer science and their translation into clinical and technological applications, offering a representation of the knowledge structure in biomedical polymer research.
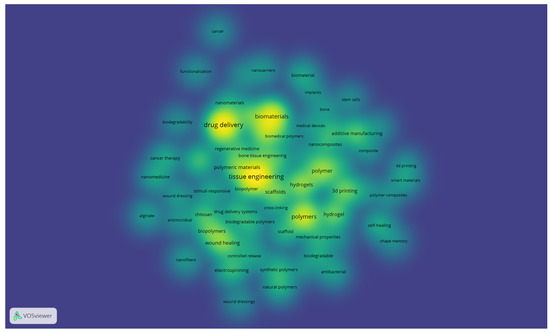
Figure 5.
Density visualisation of author keywords in biomedical polymer research (VOSviewer, resolution = 0.70, full counting).
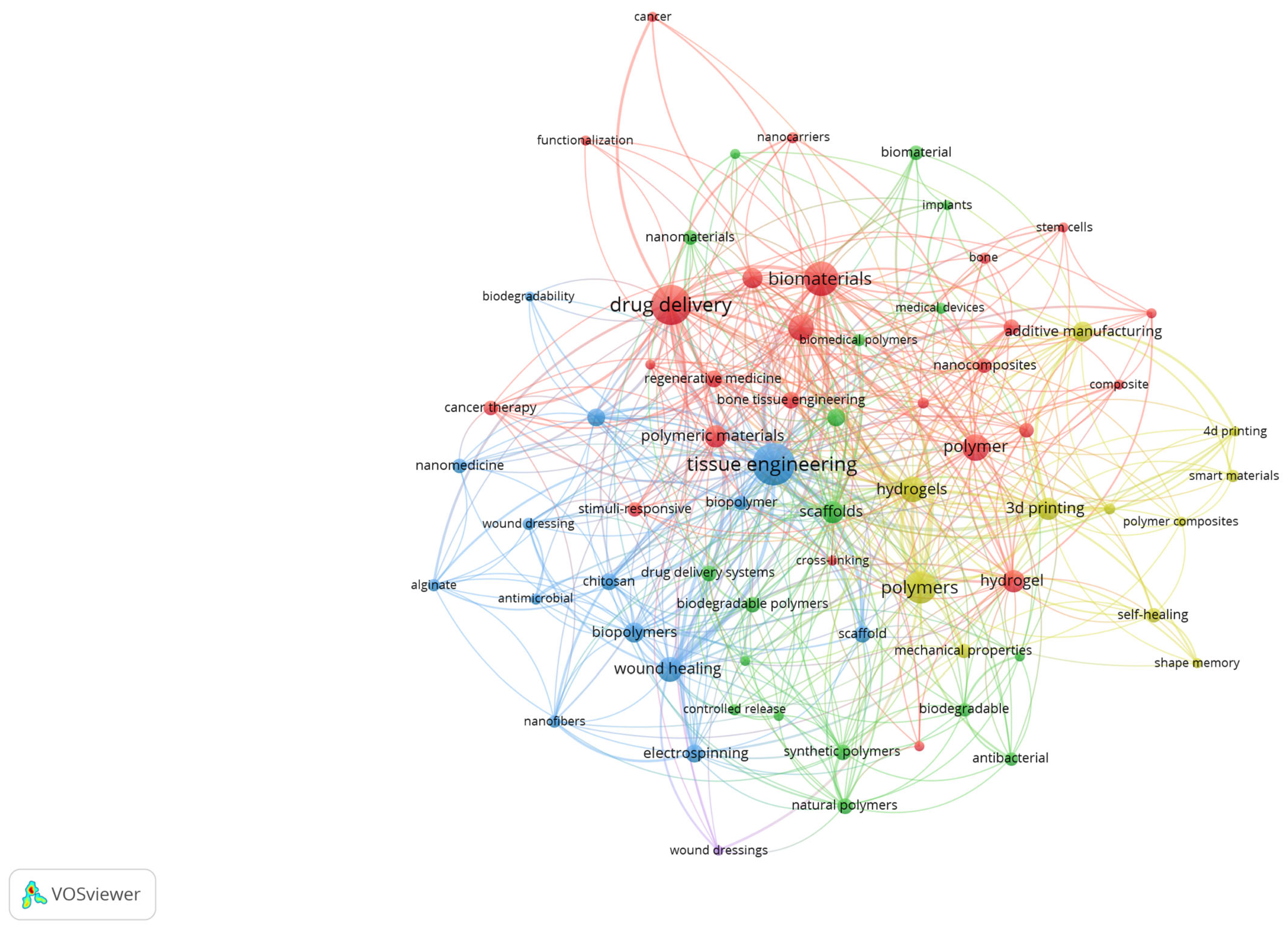
To better illustrate the structural relationships among the most relevant terms, a network visualisation of author keywords was also generated. Figure 6 presents the co-occurrence clusters, where nodes represent keywords and their size reflects the frequency, while the links indicate the strength of co-occurrence. The colour-coded clusters highlight distinct thematic areas, such as tissue engineering, drug delivery, biomaterials, and additive manufacturing, emphasising the interdisciplinary connections that characterise biomedical polymer research.
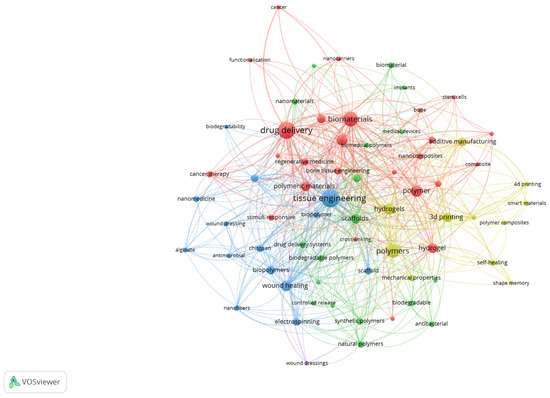
Figure 6.
Network visualisation of author keywords in biomedical polymer research.
The red cluster is centred on drug delivery and biomaterials, emphasising the extensive exploration of polymer-based carriers for controlled release, cancer therapy, and the design of biocompatible systems. The blue cluster groups terms such as tissue engineering, scaffold, wound healing, and nanofibers, reflecting the intense focus on regenerative medicine and the development of polymeric scaffolds that mimic extracellular matrices. The green cluster includes keywords related to biomedical polymers, biodegradability, nanomaterials, and natural polymers, indicating the increasing interest in sustainable, bio-inspired materials with advanced functionalities. Finally, the yellow cluster brings together 3D printing, additive manufacturing, smart materials, and hydrogels, highlighting the emerging role of additive manufacturing and advanced fabrication strategies in tailoring polymer properties for clinical applications. Together, these clusters illustrate both the consolidation of established research directions and the diversification toward novel, application-driven topics.
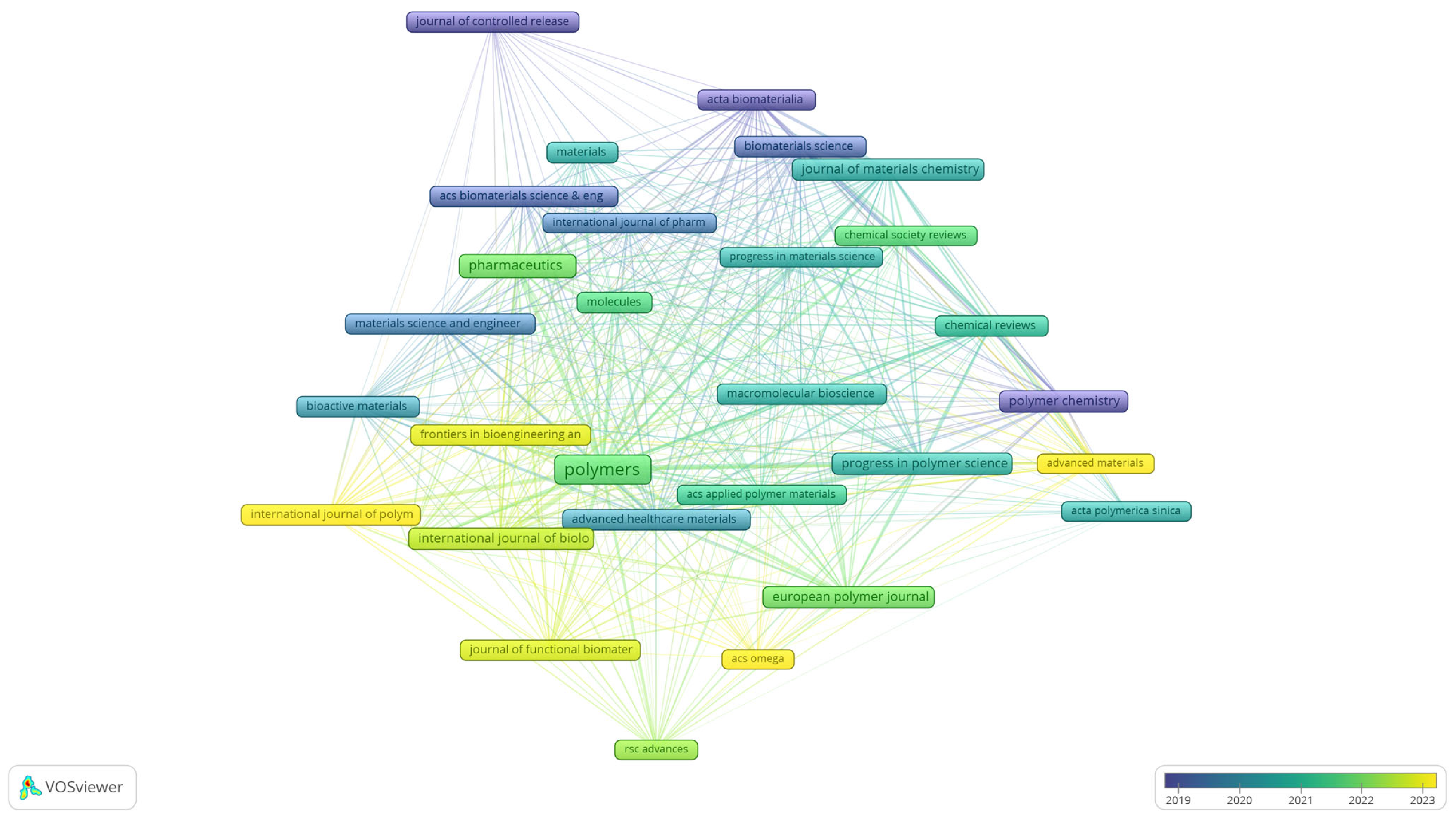
To complement the keyword analyses, the bibliographical dataset was further examined through bibliographic coupling of sources, providing an overview of the journals that shape the field of biomedical polymer research. Figure 7 presents the overlay visualisation of this network, where node size reflects the number of documents, the thickness of the links indicates the strength of shared references, and the colour scale corresponds to the average year of publication.
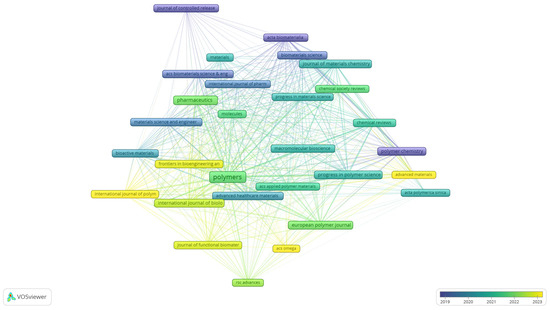
Figure 7.
Overlay visualisation of bibliographic coupling among sources in biomedical polymer research.
The map indicates that long-established journals, such as the Journal of Controlled Release, Acta Biomaterialia, and Biomaterials Science (represented by blue to green tones), have consistently provided a foundation for the field, with a focus on drug delivery, biomaterials development, and materials chemistry. More recent contributions are concentrated around open-access and interdisciplinary outlets, including Polymers, Pharmaceutics, and Chemical Reviews, as well as the Journal of Materials Chemistry (green to yellow), reflecting the expansion of the field toward broader dissemination and increased accessibility. The emerging presence of titles such as RSC Advances, ACS Omega, and Advanced Materials indicates a diversification of publication venues in recent years. This distribution highlights the coexistence of high-impact, specialised journals with newer, more inclusive platforms, underscoring both continuity and renewal in the dissemination of biomedical polymer research.
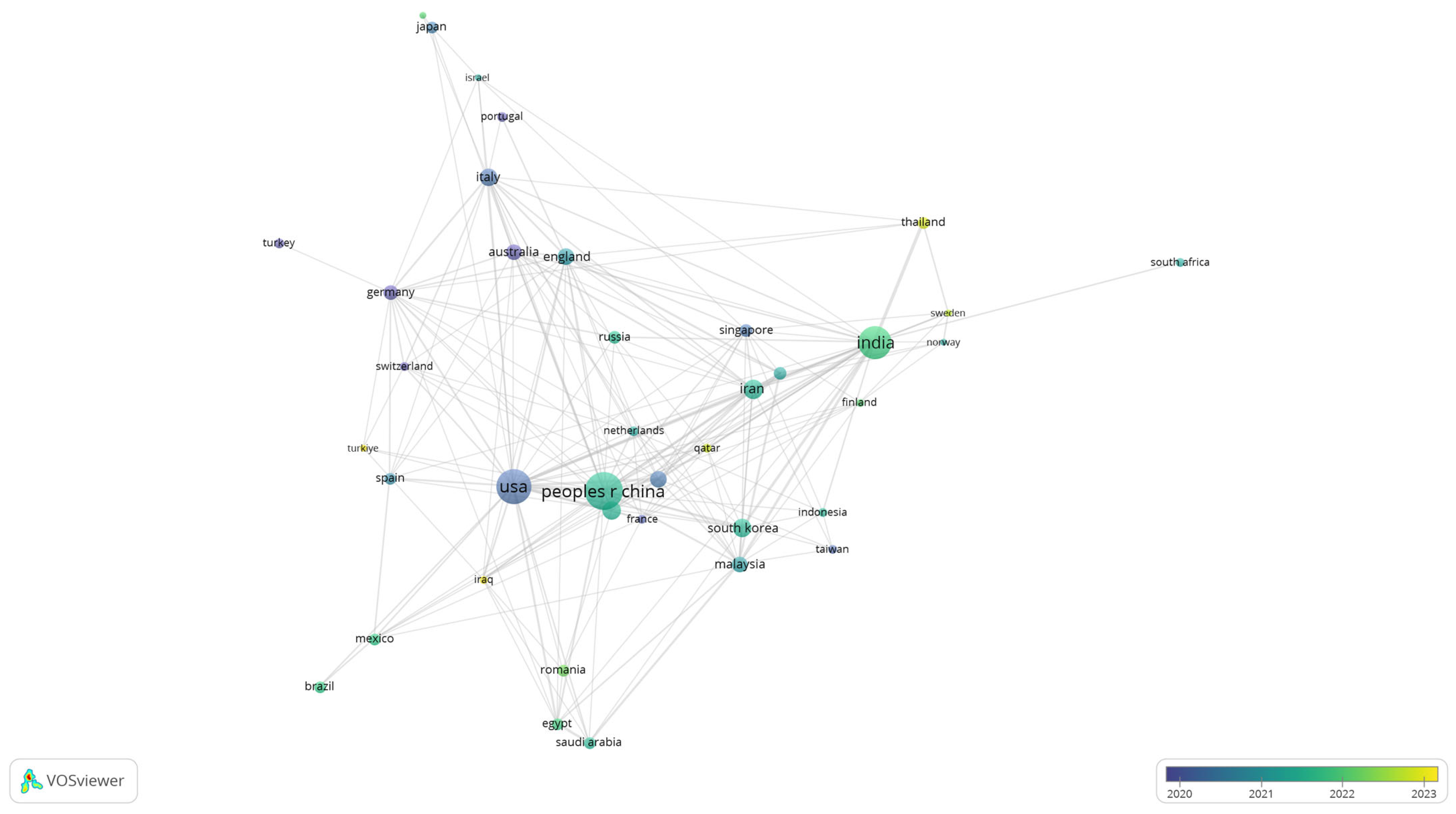
To further explore patterns of collaboration, a co-authorship analysis was conducted at the country level. Figure 8 displays the network of international cooperation in biomedical polymer research, where node size corresponds to the number of documents and the colour scale reflects the average year of publication.
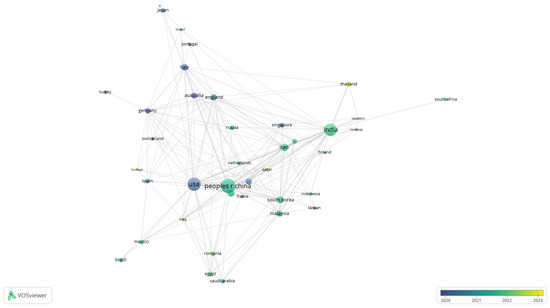
Figure 8.
Overlay visualisation of international co-authorship in biomedical polymer research.
The visualisation highlights the central role of leading contributors, including the United States, China, and India, which not only dominate in terms of publication volume but also establish strong collaborative links with European and Asian partners. Countries like Germany, England, and Australia appear as important bridges in the network.
Established contributors with earlier average years include the USA, China, Germany, England, Italy, Japan and the Netherlands (blue to green), reflecting long-standing output and dense collaboration ties. By contrast, more recent and growing contributors—most visibly India, Sweden, Iraq, Turkey, Thailand and Romania (yellow hues)—signal increased engagement during 2022–2023.
Overall, the bibliometric mapping highlights both the consolidation of established domains—such as tissue engineering, drug delivery, and wound healing—and the emergence of newer directions, including additive manufacturing, smart polymers, and bio-inspired biomaterials. The temporal overlay visualisations confirmed a shift from fundamental, chemistry-driven studies to more application-oriented and translational research, while the density and network maps emphasised the centrality of hydrogels, scaffolds, and nanomaterials in the current discourse. The coupling analysis of journals demonstrated a balance between long-established, high-impact outlets and newer open-access platforms. In contrast, the co-authorship networks revealed the increasing globalisation of the field, with substantial contributions from the United States, China, India, and an expanding presence of European partners. Taken together, these results provide a structured framework that guided the subsequent qualitative synthesis of the most highly cited review articles, allowing for a deeper understanding of how research fronts are shaped and where future opportunities may lie.
5. Analysis of the Most Cited Review Articles
To complement the bibliometric mapping, a qualitative analysis was conducted on the most cited review articles within the dataset. Articles with over 100 citations were selected as representative of the field’s most influential contributions. This approach allowed for the identification of dominant themes, methodological advances, and knowledge gaps reported across highly visible works, providing additional depth to the quantitative mapping results.
Out of the 589 review papers, 90 reached this threshold of more than 100 citations. Table 3 presents these articles, including information on authorship, research area, and primary focus, together with a concise description of their key findings and the number of times they have been cited.

Table 3.
The most cited review articles on polymeric materials in biomedical engineering (>100 citations).
The synthesis of the 90 most cited review articles highlights the diversity and maturity of research on polymeric biomaterials. While the individual studies cover a wide spectrum of applications, several dominant thematic lines emerge across the literature. These include tissue engineering and regenerative medicine, controlled drug delivery and nanomedicine, wound healing and antimicrobial strategies, as well as the adoption of advanced fabrication methods such as 3D printing and bioprinting. At the same time, recurring methodological concerns—such as reproducibility, scalability, and long-term clinical validation—are repeatedly emphasised, highlighting gaps that remain insufficiently addressed despite the field’s rapid expansion.
Based on the distribution of the most cited reviews, the literature can be broadly grouped into four major thematic domains: (i) tissue engineering and regenerative medicine, (ii) drug delivery and nanomedicine, (iii) wound healing and antimicrobial applications, and (iv) advanced fabrication strategies such as 3D printing and bioprinting. Each of these domains reflects both the consolidation of established research fronts and the emergence of novel directions that shape the field of polymeric biomaterials.
(i) Tissue engineering and regenerative medicine
A substantial proportion of the highly cited reviews focus on polymer-based scaffolds designed to mimic the extracellular matrix and support cell growth, differentiation, and tissue repair. Hydrogels, composite materials, and bio-inspired scaffolds dominate this category, reflecting the centrality of tissue regeneration in biomedical polymer research. Reviews such as those by Nikolova & Chavali [99], Reddy et al. [9], and Islam et al. [103] emphasise advances in scaffold fabrication, electrospinning techniques, and the incorporation of bioactive molecules to enhance regeneration. Additional contributions, including those by Bai et al. [104] and Boni et al. [110], discuss hydrogels for bone and neural tissue applications. Meanwhile, Kennedy et al. [131] and Abbasian et al. [134] highlight cell–matrix interactions and the potential of natural macromolecules in scaffold design. Despite these advances, persistent challenges remain in achieving controlled degradation, mechanical stability, and full integration into host tissue, underlining tissue engineering as a long-standing but still evolving frontier.
(ii) Drug delivery and nanomedicine
Another major cluster of reviews centres on polymeric nanoparticles and stimuli-responsive polymers for targeted drug delivery. Begines et al. [100], Karimi et al. [108], and Tang et al. [112] highlight how smart polymer systems enable controlled release profiles, responsiveness to biological stimuli, and enhanced therapeutic efficacy in oncology and chronic diseases. Other reviews, such as Bernard et al. [119], Bagheri et al. [118], and Khan et al. [129], extend the discussion to biocompatibility, lanthanide-doped systems, and brain-targeted nanoformulations. Similarly, Essa et al. [130], Elmowafy et al. [153], and Shrimal et al. [170] analyse PLGA-based carriers, bioactive natural agents, and novel nanoparticle preparation methods. Across these studies, concerns regarding systemic toxicity, large-scale production, and regulatory hurdles are consistently reported, suggesting that while laboratory-scale innovations are promising, their translation into standardised clinical applications remains incomplete.
(iii) Wound healing and antimicrobial applications
Hydrogels and polymer-based coatings for wound care and infection control constitute a third recurring theme. Reviews by Zhang et al. [98], Varaprasad et al. [102], and Sharma et al. [136] illustrate the potential of catechol-functionalized hydrogels, chitosan-based composites, and nitric oxide-releasing polymers to accelerate healing and provide antimicrobial protection. Complementary perspectives are provided by Cho et al. [109], Sánchez-Cid et al. [123], and Mushtaq et al. [124], who report on adhesive hydrogels, multifunctional gelatin-based systems, and hybrid composites. More recent works, including those by Cai et al. [154], Kaniuk & Stachewicz [155], and Gnanasekar [160], suggest the use of electrospun fibres, PHBV-based biodegradable scaffolds, and engineered polymers for the photodynamic inactivation of pathogens. Despite encouraging laboratory results, unresolved issues related to cost-effectiveness, long-term safety, and comparative clinical performance continue to limit translation into routine healthcare.
(iv) 3D printing, bioprinting, and advanced fabrication
Several of the most cited reviews—such as those by González-Henríquez et al. [106], Culmone et al. [113], and Nouri et al. [144]—position additive manufacturing as a transformative trend in the field. Patient-specific implants, complex scaffolds, and 4D-printed smart polymers are repeatedly emphasised as enabling technologies for personalised medicine. Reviews by Alghamdi [115], Arif et al. [150], and Arif et al. [164] further detail the role of sustainable biomaterials, multifunctional drug-loaded constructs, and the prospects of 4D bioprinting. More specialised perspectives, such as those of Chen et al. [145] on oncology-related applications and Marco-Dufort & Tibbitt [126] on mouldable hydrogels, underscore how advanced manufacturing techniques intersect with biomedical requirements. Across these studies, reproducibility, material standardisation, and regulatory acceptance are consistently identified as barriers, despite the transformative potential of additive manufacturing in bridging the gap between polymer science and clinical practice.
The four major thematic domains align one-to-one with what the VOS maps already show. In Figure 3 text, the blue cluster groups scaffold, tissue, regeneration, bone, repair, implant and cell, which supports the tissue-engineering strand; the red cluster concentrates drug, delivery, nanoparticle, cancer and toxicity, which anchors drug delivery and nanomedicine; the yellow cluster highlights healing, treatment, infection, wound dressing and patient, which maps to wound healing and antimicrobial applications; and the green cluster contains production, composite, 3D printing and additive manufacturing, which substantiates the advanced-fabrication strand. The keyword density and network visualisations reinforce this structure by placing tissue engineering, drug delivery, hydrogels, 3D printing, wound healing and nanomaterials among the most central and tightly connected terms.
By systematically analysing the most influential reviews, we developed an overview of the progress, challenges, and future directions in polymeric biomaterials, complementing the bibliometric mapping and fulfilling the dual objective of mapping and synthesis, as presented in Table 4.

Table 4.
Progress, challenges, and future directions in polymeric biomaterials.
Beyond the descriptive categorization, the synthesis of the 90 most-cited reviews reveals an evolutionary pattern across the decade analysed. Early reviews (2016–2018) focused predominantly on biocompatibility, degradation kinetics, and proof-of-concept demonstrations, reflecting the field’s preclinical orientation. Mid-period studies (2019–2021) progressively shifted toward hybrid and stimuli-responsive systems, where scalability and reproducibility emerged as dominant concerns. The most recent reviews (2022–2024) highlight translational priorities, including clinical validation, manufacturing standardisation, and cost-effectiveness—indicating a maturing field moving from experimental innovation toward clinical implementation.
Interestingly, while there is broad consensus on the importance of hybrid natural–synthetic systems for improving mechanical and biological performance, divergences persist regarding regulatory feasibility and long-term safety standards, especially in smart and degradable polymer systems.
When integrated with the bibliometric overlay map (Figure 4), these qualitative patterns align with the temporal emergence of terms such as wound healing, 3D printing, biopolymer, and innovation (highlighted in yellow), corresponding to the “Future Directions” cluster in Table 4. This convergence underscores that the topics now gaining bibliometric visibility—such as additive manufacturing, responsive hydrogels, and patient-specific biopolymers—are the very directions identified qualitatively as defining the next decade of research.
Taken together, the evidence summarised in Table 4 integrates the quantitative mapping into a coherent narrative of the field. The pattern suggests a gradual shift from materials discovery to application-driven, clinically oriented engineering, while highlighting persistent bottlenecks at the interfaces of biology, manufacturing, and regulation. These insights frame the implications developed next, namely the need for design-for-clinic criteria, harmonised evaluation and reporting practices, and credible pathways to scale up adoption across healthcare settings, which we distil in the Conclusions.
6. Conclusions
This article combined bibliometric mapping with a review of the most cited articles to provide a consolidated perspective on the role of polymeric materials in biomedical engineering. The bibliometric analysis demonstrated the rapid expansion and diversification of the field between 2016 and 2025, with clusters converging around tissue engineering, drug delivery, smart polymers, wound healing, and additive manufacturing. International collaboration maps also revealed the increasing global scope of this research, with the United States, China, and India as dominant contributors, complemented by emerging involvement from European and developing countries.
By integrating bibliometric mapping with a structured synthesis of reviewed literature, this study offers a comprehensive reference for researchers, clinicians, and industry stakeholders. The findings underline the need for interdisciplinary strategies that bridge material science, engineering, and clinical practice.
This study has several limitations that should be acknowledged. First, the search was conducted exclusively in the Web of Science Core Collection, which may have excluded relevant reviews indexed in other databases such as Scopus or PubMed. Second, although the inclusion criteria were restricted to the years 2016–2025, some earlier influential reviews might not have been captured. Finally, the bibliometric mapping reflects only the structural and temporal patterns of the selected dataset; therefore, the interpretation of thematic trends should be understood as indicative rather than exhaustive.
The combined use of bibliometric mapping and structured synthesis reveals a clear transition from materials discovery toward application-driven, clinically oriented engineering. Four interlinked research fronts—tissue regeneration, polymer-based drug delivery, wound management, and advanced fabrication—have converged, demonstrating growing translational momentum. Synthesising insights from the 90 most-cited reviews, several recurring solutions emerge: aligning polymer degradation kinetics with tissue healing rates, ensuring sterilisation and imaging compatibility, harmonising validation protocols, and integrating early health-economic assessment. Industrial scalability remains challenged by variability in polymer synthesis, reproducibility of additive-manufactured structures, and the absence of unified regulatory frameworks for hybrid materials.
In summary, the findings suggest that future progress depends on embedding “design-for-clinic” principles within polymer development and strengthening the interface between material innovation, validation, and manufacturing. These integrative pathways provide a concise roadmap for advancing polymeric biomaterials from laboratory innovation to clinical and industrial translation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym17212886/s1, Table S1: PRISMA 2020 Checklist.
Author Contributions
Conceptualization, C.V., M.T. and D.-A.S.; methodology, C.V.; software, C.V.; validation, C.V., M.T. and D.-A.S.; formal analysis, C.V. and D.-A.S.; investigation, C.V.; resources, M.T.; data curation, D.-A.S.; writing—original draft preparation, C.V., M.T. and D.-A.S.; writing—review and editing, C.V., M.T. and D.-A.S.; visualisation, D.-A.S.; supervision, M.T.; project administration, M.T.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
ChatGPT by OpenAI, 2025 version was used to assist in the linguistic refinement of the manuscript. Its use was strictly limited to stylistic editing, without generating scientific content.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Narayan, R.J. The next Generation of Biomaterial Development. Philos. Trans. R. Soc. A 2010, 368, 1831–1837. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, H.L.; Monticeli, F.M.; Agnol, L.D. A Review on Polymers for Biomedical Applications on Hard and Soft Tissues and Prosthetic Limbs. Polymers 2023, 15, 4034. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liang, W.; Zhang, Y.; Fu, Q. Nanoencapsulation in Polymeric Materials: Weaving Magical Coats for Microorganisms. Nano Today 2023, 52, 101973. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. ISBN 978-0-12-396983-5. [Google Scholar]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications—A Review of the Literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Socci, M.C.; Rodríguez, G.; Oliva, E.; Fushimi, S.; Takabatake, K.; Nagatsuka, H.; Felice, C.J.; Rodríguez, A.P. Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering 2023, 10, 218. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent Concepts in Biodegradable Polymers for Tissue Engineering Paradigms: A Critical Review. Int. Mater. Rev. 2019, 64, 91–126. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and Biocompatible Polymers for Tissue Engineering Application: A Review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Chapa, C. Hybrid Polymeric Systems: Design, Biomedical Applications, Opportunities, Challenges, and Future Perspectives. In Hybrid Polymeric Systems for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2025; pp. 1–18. ISBN 978-0-443-15564-2. [Google Scholar]
- Grethe, T. Biodegradable Synthetic Polymers in Textiles—What Lies Beyond PLA and Medical Applications? A Review. Tekstilec 2021, 64, 32–46. [Google Scholar] [CrossRef]
- Chang, C.; Ginn, B.; Livingston, N.K.; Yao, Z.; Slavin, B.; King, M.W.; Chung, S.; Mao, H.-Q. Medical Fibers and Biotextiles. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 575–600. ISBN 978-0-12-816137-1. [Google Scholar]
- BCC Research. Global Markets for Implantable Biomaterials: AVM118A. Available online: https://www.bccresearch.com/market-research/advanced-materials/implantable-biomaterials-markets-report.html (accessed on 27 August 2025).
- 2025 Polymer Biomaterial Industry Trends Report: Long-Term. Available online: https://www.openpr.com/news/4151135/2025-polymer-biomaterial-industry-trends-report-long-term (accessed on 27 August 2025).
- Global Polymeric Biomaterials Market Size & Forecast, 2032. Available online: https://www.coherentmarketinsights.com/industry-reports/global-polymeric-biomaterials-market (accessed on 27 August 2025).
- Al Salloum, H.; Saunier, J.; Dazzi, A.; Vigneron, J.; Etcheberry, A.; Marlière, C.; Aymes-Chodur, C.; Herry, J.M.; Bernard, M.; Jubeli, E.; et al. Characterization of the Surface Physico-Chemistry of Plasticized PVC Used in Blood Bag and Infusion Tubing. Mater. Sci. Eng. C 2017, 75, 317–334. [Google Scholar] [CrossRef]
- Lu, X.; Khanna, A.; Luzinov, I.; Nagatomi, J.; Harman, M. Surface Modification of Polypropylene Surgical Meshes for Improving Adhesion with Poloxamine Hydrogel Adhesive. J. Biomed. Mater. Res. 2019, 107, 1047–1055. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tone, S.; Naito, Y.; Sudo, A. Ultra-High-Molecular-Weight Polyethylene in Hip and Knee Arthroplasties. Materials 2023, 16, 2140. [Google Scholar] [CrossRef]
- Hadasha, W.; Bezuidenhout, D. Poly(Lactic Acid) as Biomaterial for Cardiovascular Devices and Tissue Engineering Applications. In Industrial Applications of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Advances in Polymer Science; Springer International Publishing: Cham, Switzerland, 2017; Volume 282, pp. 51–77. ISBN 978-3-319-75458-1. [Google Scholar]
- Miri, Z.; Farè, S.; Ma, Q.; Haugen, H.J. Updates on Polyurethane and Its Multifunctional Applications in Biomedical Engineering. Prog. Biomed. Eng. 2023, 5, 042001. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Zhu, X.; Huang, H.; Xu, C. Performance of Polydopamine Complex and Mechanisms in Wound Healing. Int. J. Mol. Sci. 2021, 22, 10563. [Google Scholar] [CrossRef]
- Mbogori, M.; Vaish, A.; Vaishya, R.; Haleem, A.; Javaid, M. Poly-Ether-Ether-Ketone (PEEK) in Orthopaedic Practice-A Current Concept Review. J. Orthop. Rep. 2022, 1, 3–7. [Google Scholar] [CrossRef]
- Shiroud Heidari, B.; Dodda, J.M.; El-Khordagui, L.K.; Focarete, M.L.; Maroti, P.; Toth, L.; Pacilio, S.; El-Habashy, S.E.; Boateng, J.; Catanzano, O.; et al. Emerging Materials and Technologies for Advancing Bioresorbable Surgical Meshes. Acta Biomater. 2024, 184, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, P.A.; Pascale, M.S.; Huegel, M.O. Early Experience with the GORE-TEX Polytetrafluoroethylene Anterior Cruciate Ligament Prosthesis. Am. J. Sports Med. 1989, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, Z.; Horný, L.; Chlup, H.; Kohan, M.; Hudák, R.; Valášek, M. Nonlinearly Elastic and Anisotropic Constitutive Model for ePTFE Vascular Graft Based on Tensile and Inflation Experiments. Mech. Adv. Mater. Struct. 2025, 1–11. [Google Scholar] [CrossRef]
- Pramanik, S.; Aggarwal, A.; Kadi, A.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Koul, K.; Deepak, A.; Bellucci, S. Chitosan Alchemy: Transforming Tissue Engineering and Wound Healing. RSC Adv. 2024, 14, 19219–19256. [Google Scholar] [CrossRef]
- Kaniuk, Ł.; Berniak, K.; Lichawska-Cieślar, A.; Jura, J.; Karbowniczek, J.E.; Stachewicz, U. Accelerated Wound Closure Rate by Hyaluronic Acid Release from Coated PHBV Electrospun Fiber Scaffolds. J. Drug Deliv. J. Drug Deliv. Sci. Technol. 2022, 77, 103855. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, Q.; Zhu, Z.; Shi, S.; Xu, C.; Xie, R.; Li, Y. Hyaluronic Acid-Based Dynamic Hydrogels for Cartilage Repair and Regeneration. Gels 2024, 10, 703. [Google Scholar] [CrossRef]
- Rao, L.; Zhou, H.; Li, T.; Li, C.; Duan, Y.Y. Polyethylene Glycol-Containing Polyurethane Hydrogel Coatings for Improving the Biocompatibility of Neural Electrodes. Acta Biomater. 2012, 8, 2233–2242. [Google Scholar] [CrossRef]
- Fattahi, N.; Reed, J.; Heronemus, E.; Fernando, P.; Hansen, R.; Parameswaran, P. Polyethylene Glycol Hydrogel Coatings for Protection of Electroactive Bacteria against Chemical Shocks. Bioelectrochemistry 2024, 156, 108595. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef]
- Rihani, R.; Tasnim, N.; Javed, M.; Usoro, J.O.; D’Souza, T.M.; Ware, T.H.; Pancrazio, J.J. Liquid Crystalline Polymers: Opportunities to Shape Neural Interfaces. Neuromodul. Technol. Neural Interface 2022, 25, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Hyun Bae, S.; Min, K.S.; Seo, J.-M.; Chung, H.; June Kim, S. A Miniaturized, Eye-Conformable, and Long-Term Reliable Retinal Prosthesis Using Monolithic Fabrication of Liquid Crystal Polymer (LCP). IEEE Trans. Biomed. Eng. 2015, 62, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J.T. Design and Development of Three-Dimensional Scaffolds for Tissue Engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Tonti, O.R.; Larson, H.; Lipp, S.N.; Luetkemeyer, C.M.; Makam, M.; Vargas, D.; Wilcox, S.M.; Calve, S. Tissue-Specific Parameters for the Design of ECM-Mimetic Biomaterials. Acta Biomater. 2021, 132, 83–102. [Google Scholar] [CrossRef]
- Liu, H.; Xing, F.; Yu, P.; Lu, R.; Ma, S.; Shakya, S.; Zhou, X.; Peng, K.; Zhang, D.; Liu, M. Biomimetic Fabrication Bioprinting Strategies Based on Decellularized Extracellular Matrix for Musculoskeletal Tissue Regeneration: Current Status and Future Perspectives. Mater. Des. 2024, 243, 113072. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Farazin, A.; Darghiasi, S.F. Advanced Polymeric Scaffolds for Bone Tissue Regeneration. Explor. BioMat-X 2025, 2, 101340. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold Degradation in Bone Tissue Engineering: An Overview. Int. Biodeterior. Biodegrad. 2023, 180, 105599. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Qin, C.; Wu, C. 3D Printing of Cell-Delivery Scaffolds for Tissue Regeneration. Regen. Biomater. 2023, 10, rbad032. [Google Scholar] [CrossRef] [PubMed]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-Printed Scaffolds for Spinal Cord Injury Repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.M.; Ju, Y.M.; Jung, J.W.; Kang, H.-W.; Lee, S.J.; Yoo, J.J.; Kim, S.W.; Kim, S.H.; Cho, D.-W. A Novel Tissue Engineered Trachea with a Mechanical Behavior Similar to Native Trachea. Biomaterials 2015, 62, 106–115. [Google Scholar] [CrossRef]
- Dong, X.; Askinas, C.; Kim, J.; Sherman, J.E.; Bonassar, L.J.; Spector, J.A. Efficient Engineering of Human Auricular Cartilage through Mesenchymal Stem Cell Chaperoning. J. Tissue Eng. Regen. Med. 2022, 16, 825–835. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Fendi, F.; Abdullah, B.; Suryani, S.; Usman, A.N.; Tahir, D. Development and Application of Hydroxyapatite-Based Scaffolds for Bone Tissue Regeneration: A Systematic Literature Review. Bone 2024, 183, 117075. [Google Scholar] [CrossRef]
- Liu, W.; Cheong, N.; He, Z.; Zhang, T. Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review. J. Funct. Biomater. 2025, 16, 127. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Mysore, T.H.M.; Patil, A.Y.; Hegde, C.; Sudeept, M.A.; Kumar, R.; Soudagar, M.E.M.; Fattah, I.M.R. Apatite Insights: From Synthesis to Biomedical Applications. Eur. Polym. J. 2024, 209, 112842. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Langer, R. Polymer-Controlled Drug Delivery Systems. Acc. Chem. Res. 1993, 26, 537–542. [Google Scholar] [CrossRef]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart Polymers for the Controlled Delivery of Drugs—A Concise Overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and Pseudo-Stealth Nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Cheng, Q.; Bardelang, D.; Wang, R. Challenges and Opportunities of Functionalized Cucurbiturils for Biomedical Applications. JACS Au 2023, 3, 2356–2377. [Google Scholar] [CrossRef]
- Zhang, A.; Jung, K.; Li, A.; Liu, J.; Boyer, C. Recent Advances in Stimuli-Responsive Polymer Systems for Remotely Controlled Drug Release. Prog. Polym. Sci. 2019, 99, 101164. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Prajapati, B.G.; Singh, S. A Critical Review on the Dissemination of PH and Stimuli-Responsive Polymeric Nanoparticular Systems to Improve Drug Delivery in Cancer Therapy. Crit. Rev. Oncol./Hematol. 2023, 185, 103961. [Google Scholar] [CrossRef]
- Jin, Z.; Al Amili, M.; Guo, S. Tumor Microenvironment-Responsive Drug Delivery Based on Polymeric Micelles for Precision Cancer Therapy: Strategies and Prospects. Biomedicines 2024, 12, 417. [Google Scholar] [CrossRef]
- Karunakar, K.K.; Cheriyan, B.V.; Anandakumar, R.; Murugathirumal, A.; Senthilkumar, A.; Nandhini, J.; Kataria, K.; Yabase, L. Stimuli-Responsive Smart Materials: Bridging the Gap between Biotechnology and Regenerative Medicine. Bioprinting 2025, 48, e00415. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Galaev, I.; Mattiasson, B. (Eds.) Smart Polymers for Bioseparation and Bioprocessing; CRC Press: London, UK, 2001; ISBN 978-0-367-80369-8. [Google Scholar]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable Polymers in Biomedical Applications: A Review—Developments, Perspectives and Future Challenges. Int. J. Mol. Sci. 2023, 24, 16952. [Google Scholar] [CrossRef]
- Modjarrad, K.; Ebnesajjad, S. Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Chadds Ford, PA, USA, 2013; ISBN 978-0-323-22169-6. [Google Scholar]
- Shenoy, S.R.; Wagdarikar, M.J.; Desai, N.D. Polymers in Medical Devices and Pharmaceutical Packaging. In Polymers for Pharmaceutical and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 333–382. ISBN 978-0-323-95496-9. [Google Scholar]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Sabatini, L.; Aprato, A.; Bracco, P.; Bellare, A. Ultra-High Molecular Weight Polyethylene (UHMWPE) for Hip and Knee Arthroplasty: The Present and the Future. J. Orthop. 2021, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Shi, X.; Yang, H. Recent Advances in Polyurethane for Artificial Vascular Application. Polymers 2024, 16, 3528. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.S.; Yuan, B.I.; Bishop, D.; Topaz, S.; Griensven, J.V.; Hofma, S.; Swier, P.; Klinkmann, J.; Kolff, J.; Kolff, W.J. New Polyurethane Valves in New Soft Artificial Hearts. ASAIO Trans 1989, 35, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Crago, M.; Lee, A.; Farajikhah, S.; Oveissi, F.; Fletcher, D.F.; Dehghani, F.; Winlaw, D.S.; Naficy, S. The Evolution of Polyurethane Heart Valve Replacements: How Chemistry Translates to the Clinic. Mater. Today Commun. 2022, 33, 104916. [Google Scholar] [CrossRef]
- Boffito, M.; Sartori, S.; Mattu, C.; Ciardelli, G. Polyurethanes for Cardiac Applications. In Advances in Polyurethane Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 387–416. ISBN 978-0-08-100614-6. [Google Scholar]
- Szycher, M.; Poirier, V.L. Synthetic Polymers in Artificial Hearts: A Progress Report. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 588–593. [Google Scholar] [CrossRef]
- Navas-Gómez, K.; Valero, M.F. Why Polyurethanes Have Been Used in the Manufacture and Design of Cardiovascular Devices: A Systematic Review. Materials 2020, 13, 3250. [Google Scholar] [CrossRef]
- Tokhadzé, N.; Chennell, P.; Pereira, B.; Mailhot-Jensen, B.; Sautou, V. Critical Drug Loss Induced by Silicone and Polyurethane Implantable Catheters in a Simulated Infusion Setup with Three Model Drugs. Pharmaceutics 2021, 13, 1709. [Google Scholar] [CrossRef]
- Roth, Y.; Lewitus, D.Y. The Grafting of Multifunctional Antithrombogenic Chemical Networks on Polyurethane Intravascular Catheters. Polymers 2020, 12, 1131. [Google Scholar] [CrossRef]
- Dias, L.D.; Duarte, L.S.; Naves, P.L.F.; Napolitano, H.B.; Bagnato, V.S. Self-Disinfecting Urethral Catheter to Overcome Urinary Infections: From Antimicrobial Photodynamic Action to Antibacterial Biochemical Entities. Microorganisms 2022, 10, 2484. [Google Scholar] [CrossRef]
- Liang, W.; Ni, N.; Huang, Y.; Lin, C. An Advanced Review: Polyurethane-Related Dressings for Skin Wound Repair. Polymers 2023, 15, 4301. [Google Scholar] [CrossRef]
- Morales-González, M.; Díaz, L.E.; Dominguez-Paz, C.; Valero, M.F. Insights into the Design of Polyurethane Dressings Suitable for the Stages of Skin Wound-Healing: A Systematic Review. Polymers 2022, 14, 2990. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Y.; Ma, J.; Miao, H.; Chen, S.; Gao, S.; Rong, H.; Deng, L.; Zhang, J.; Dong, A.; et al. Preparation and Characterization of a Polyurethane-Based Sponge Wound Dressing with a Superhydrophobic Layer and an Antimicrobial Adherent Hydrogel Layer. Acta Biomater. 2024, 181, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Senra, M.R.; Marques, M.D.F.V.; Monteiro, S.N. Poly (Ether-Ether-Ketone) for Biomedical Applications: From Enhancing Bioactivity to Reinforced-Bioactive Composites—An Overview. Polymers 2023, 15, 373. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Chen, M.; Bai, J.; Xu, Y.; Wang, M.; Geng, D.; Pan, G. Recent Advances in Orthopedic Polyetheretherketone Biomaterials: Material Fabrication and Biofunction Establishment. Smart Mater. Med. 2022, 3, 20–36. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.; Zhu, W.; Weng, X. Polyetheretherketone Development in Bone Tissue Engineering and Orthopedic Surgery. Front. Bioeng. Biotechnol. 2023, 11, 1207277. [Google Scholar] [CrossRef]
- Kumar, R.; Mehdi, H.; Bhati, S.S.; Arunkumar, M.; Mishra, S.; Lohumi, M.K. A Comprehensive Review of Advancements in Additive Manufacturing for 3D Printed Medical Components Using Diverse Materials. Discov. Mater. 2025, 5, 152. [Google Scholar] [CrossRef]
- Vickram, A.S.; Infant, S.S.; Manikandan, S.; Sowndharya, B.B.; Gulothungan, G.; Chopra, H. 3D Bio-Printed Scaffolds and Smart Implants: Evaluating Functional Performance in Animal Surgery Models. Ann. Med. Surg. 2025, 87, 3618–3634. [Google Scholar] [CrossRef]
- Nizam, M.; Purohit, R.; Taufik, M. Role of 3D Printing in Healthcare: A Comprehensive Review on Treatment and Training. Proc. Inst. Mech. Eng. Part H 2025, 239, 239–265. [Google Scholar] [CrossRef]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Santos, J.D.; Atayde, L.; Alves, N.; Maurício, A.C. Three-Dimensional Printing/Bioprinting and Cellular Therapies for Regenerative Medicine: Current Advances. J. Funct. Biomater. 2025, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Zhang, H. Innovative 3D Printing Technologies and Advanced Materials Revolutionizing Orthopedic Surgery: Current Applications and Future Directions. Front. Bioeng. Biotechnol. 2025, 13, 1542179. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, M.H.; Islam, M.A.; Hossain, N.; Al Mahmud, M.Z.; Rayhan, M.T.; Nishi, N.J.; Chowdhury, M.A. Recent Advances of Additive Manufacturing in Implant Fabrication—A Review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Dong, C.; Petrovic, M.; Davies, I.J. Applications of 3D Printing in Medicine: A Review. Ann. 3D Print. Med. 2024, 14, 100149. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Abid, A.S.; Munna, M.S.; Dutta, M.; Rimon, M.I.H. Additive Manufacturing in Biomedical: Applications, Challenges, and Prospects. Hybrid Adv. 2025, 10, 100467. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.X.; Sun, Z.M.; Zhu, X.W.; Zhao, Q.; Zhang, T.F.; Cholewinski, A.; Yang, F.; Zhao, B.X.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Aranda, M.; Merinero, M.; Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application: A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodríguez-Hernández, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S.F. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Asl, H.G.; Mandieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.Y.; Oh, D.X.; Park, J. Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: Boronic/boronate esters, borax, and benzoxaborole. J. Mater. Chem. A 2021, 9, 14630–14655. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and Novel Polymeric Biomaterials for Neural Tissue Engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Martins, C.; Sousa, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Healthc. Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef]
- Tang, Z.H.; He, C.L.; Tian, H.Y.; Ding, J.X.; Hsiao, B.S.; Chu, B.; Chen, X.S. Polymeric Nanostructured Materials for Biomedical Applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef]
- Culmone, C.; Smit, G.; Breedveld, P. Additive Manufacturing of Medical Instruments: A State-of-the-Art Review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Liao, G.F.; He, F.; Li, Q.; Zhong, L.; Zhao, R.Z.; Che, H.N.; Gao, H.Y.; Fang, B.Z. Emerging Graphitic Carbon Nitride-Based Materials for Biomedical Applications. Prog. Mater. Sci. 2020, 109, 100666. [Google Scholar] [CrossRef]
- Alghamdi, S.S. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Su, L.; Feng, Y.L.; Wei, K.C.; Xu, X.Y.; Liu, R.Y.; Chen, G.S. Carbohydrate-Based Macromolecular Biomaterials. Chem. Rev. 2021, 121, 10950–11029. [Google Scholar] [CrossRef]
- Bagheri, A.; Arandiyan, H.; Boyer, C.; Lim, M. Lanthanide-Doped Upconversion Nanoparticles: Emerging Intelligent Light-Activated Drug Delivery Systems. Adv. Sci. 2016, 3, 1500437. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices—Regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Wo, Y.Q.; Brisbois, E.J.; Bartlett, R.H.; Meyerhoff, M.E. Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: Just say yes to nitric oxide (NO). Biomater. Sci. 2016, 4, 1161–1183. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer-metal composites for drug delivery and biomedical applications. Drug Discov. Today 2017, 22, 625–637. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Cid, P.; Rosado, M.; Romero, A.; Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 210, 879–911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Li, Y.J.; Kang, W.B.; Liu, X.G.; Wang, Q. Current advances and future perspectives of additive manufacturing for functional polymeric materials and devices. SusMat 2021, 1, 63–83. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Tibbitt, M.W. Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters. Mater. Today Chem. 2019, 12, 16–33. [Google Scholar] [CrossRef]
- Wang, D.; Liu, P.F.; Luo, D. Putting DNA to Work as Generic Polymeric Materials. Angew. Chem. Int. Ed. 2022, 61, e202110666. [Google Scholar] [CrossRef]
- Kenry; Liu, B. Recent Advances in Biodegradable Conducting Polymers and Their Biomedical Applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.Y.; Fu, M.F.; Zhai, G.X. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Bhaw-Luximon, A.; Jhurry, D. Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance. Acta Biomater. 2017, 50, 41–55. [Google Scholar] [CrossRef]
- Long, R.; Hui, C.Y.; Gong, J.P.; Bouchbinder, E. The Fracture of Highly Deformable Soft Materials: A Tale of Two Length Scales. Annu. Rev. Condens. Matter Phys. 2021, 12, 71–94. [Google Scholar] [CrossRef]
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P.A. Superabsorbent polymers in agriculture and other applications: A review. Polym.-Plast. Technol. Mater. 2020, 59, 975–995. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical Review of Biodegradable and Bioactive Polymer Composites for Bone Tissue Engineering and Drug Delivery Applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Zhao, W.L.; Yu, J.M.; Li, Y.; Zhao, C. Recent Development of pH-Responsive Polymers for Cancer Nanomedicine. Molecules 2019, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 164, 3886–3908. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Chenab, K.K.; Taheri-Ledari, R.; Mosafer, J.; Hashemi, S.M.; Mokhtarzadeh, A.; Maleki, A.; Hamblin, M.R. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110267. [Google Scholar] [CrossRef]
- Esrafili, A.; Wagner, A.; Inamdar, S.; Acharya, A.P. Covalent Organic Frameworks for Biomedical Applications. Adv. Healthc. Mater. 2021, 10, 2002090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q. From plant phenols to novel bio-based polymers. Prog. Polym. Sci. 2022, 125, 101473. [Google Scholar] [CrossRef]
- Nouri, A.; Shirvan, A.R.; Li, Y.C.; Wen, C.E. Additive manufacturing of metallic and polymeric load-bearing biomaterials using laser powder bed fusion: A review. J. Mater. Sci. Technol. 2021, 79, 149–168. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, Q.W.; Chen, Q.; Cui, C.Y.; Duan, S.; Kang, Y.Y.; Liu, Y.; Liu, Y.; Muhammad, W.; Shao, S.Q.; et al. Biomedical polymers: Synthesis, properties, and applications. Sci. China Chem. 2022, 65, 1050–1122. [Google Scholar] [CrossRef]
- Dias, J.R.; Granja, P.L. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.C.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef]
- Dziadek, M.; Stodolak-Zych, E.; Cholewa-Kowalska, K. Biodegradable ceramic-polymer composites for biomedical applications: A review. Mater. Sci. Eng. C 2017, 71, 1175–1191. [Google Scholar] [CrossRef]
- Mann, J.L.; Yu, A.C.; Agmon, G.; Appel, E.A. Supramolecular polymeric biomaterials. Biomater. Sci. 2017, 6, 10–37. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Hossain, M.; Shi, H.H.; Tariq, A.; Ramakrishna, S.; Umer, R. Additive manufacturing of sustainable biomaterials for biomedical applications. Asian J. Pharm. Sci. 2023, 18, 100812. [Google Scholar] [CrossRef]
- Peng, Z.L.; Zhao, T.S.; Zhou, Y.Q.; Li, S.H.; Li, J.J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.Q.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–38. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Cai, L.L.; Liu, S.; Guo, J.W.; Jia, Y.G. Polypeptide-based self-healing hydrogels: Design and biomedical applications. Acta Biomater. 2020, 113, 84–100. [Google Scholar] [CrossRef]
- Kaniuk, L.; Stachewicz, U. Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications. Acs Biomater. Sci. Eng. 2021, 7, 5339–5362. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, M.A.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 4. [Google Scholar] [CrossRef]
- Cook, A.B.; Perrier, S. Branched and Dendritic Polymer Architectures: Functional Nanomaterials for Therapeutic Delivery. Adv. Funct. Mater. 2020, 30, 1901001. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skrzynski, S.; Smiechowski, K.; Kolodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Rother, M.; Nussbaumer, M.G.; Renggli, K.; Bruns, N. Protein cages and synthetic polymers: A fruitful symbiosis for drug delivery applications, bionanotechnology and materials science. Chem. Soc. Rev. 2016, 45, 6213–6249. [Google Scholar] [CrossRef]
- Gnanasekar, S. Recent advances in engineered polymeric materials for efficient photodynamic inactivation of bacterial pathogens. Bioact. Mater. 2023, 20, 477–489. [Google Scholar] [CrossRef]
- Naikwadi, A.T.; Sharma, B.K.; Bhatt, K.D.; Mahanwar, P.A. Gamma Radiation Processed Polymeric Materials for High Performance Applications: A Review. Front. Chem. 2022, 10, 837111. [Google Scholar] [CrossRef]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Versino, F. Biobased composites from agro-industrial wastes and by-products. Emergent Mater. 2022, 5, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Zolfagharian, A.; Bodaghi, M. 4D bioprinting of smart polymers for biomedical applications: Recent progress, challenges, and future perspectives. React. Funct. Polym. 2022, 173, 105374. [Google Scholar] [CrossRef]
- Rong, F.; Tang, Y.Z.; Wang, T.J.; Feng, T.; Song, J.; Li, P.; Huang, W. Nitric Oxide-Releasing Polymeric Materials for Antimicrobial Applications: A Review. Antioxidants 2019, 8, 556. [Google Scholar] [CrossRef]
- Su, W.; Li, W.Y.; Wu, Z.Q.; Che, H. Chemical Surface Modification of Polymeric Biomaterials for Biomedical Applications. Macromol. Rapid Commun. 2020, 41, 1900430. [Google Scholar] [CrossRef]
- Tan, Y.J. Progress and Roadmap for Intelligent Self-Healing Materials in Autonomous Robotics. Adv. Mater. 2021, 33, 2002800. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, D.Y.; Li, L.; Sun, K.X. Charge reversal nano-systems for tumor therapy. J. Nanobiotechnol. 2022, 20, 8. [Google Scholar] [CrossRef]
- Scognamiglio, F. Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef]
- Shrimal, P.; Jadeja, G.; Patel, S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Review of polymer MEMS micromachining. J. Micromech. Microeng. 2016, 26, 013001. [Google Scholar] [CrossRef]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giann, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. CeO2 Nanoparticle-Containing Polymers for Biomedical Applications: A Review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Seaweed Polysaccharide-Based Nanoparticles: Preparation and Applications for Drug Delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Paino, M.; Bonilla, A. Antimicrobial Polymers in the Nano-World. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.A.; Kandasubramanian, B. Functionalized polylysine biomaterials for advanced medical applications: A review. Eur. Polym. J. 2021, 146, 110248. [Google Scholar] [CrossRef]
- Cross, L.M.; Thakur, A.; Jalili, N.A.; Detamore, M.; Gaharwar, A.K. Nanoengineered biomaterials for repair and regeneration of orthopedic tissue interfaces. Acta Biomater. 2016, 42, 2–17. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef]
- Olmos, D.; Benito, J. Polymeric Materials with Antibacterial Activity: A Review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef]
- Mohamadhoseini, M.; Mohamadnia, Z. Supramolecular self-healing materials via host-guest strategy between cyclodextrin and specific types of guest molecules. Coord. Chem. Rev. 2021, 429, 213711. [Google Scholar] [CrossRef]
- Arif, M.M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Khan, R.U.; Awais, S.M.; Butt, M.A. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef]
- Chahal, S.; Kumar, A.; Hussian, F.S.J. Development of biomimetic electrospun polymeric biomaterials for bone tissue engineering: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 1308–1344. [Google Scholar] [CrossRef]
- Hussain, Z. Recent Advances in Polymer-based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-art. Curr. Drug Targets 2018, 19, 527–536. [Google Scholar] [CrossRef]
- Rahimi, M. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021, 123, 31–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).