The Influence of Zinc Stearate Complexes on the Sulfur Vulcanization of Ethylene–Propylene–Diene Monomer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zinc Complexes and Salts
2.3. Preparation of Rubber Compounds
2.4. Research Methods
3. Results and Discussion
3.1. Characterization of the Zinc Salts and Complexes
3.2. Characterization of the EPDM Composites Containing the Synthesized Zinc Salts and Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZnSt | Zinc stearate |
| EPDM | Ethylene–propylene–diene monomer |
| ZnO | Zinc oxide |
| StA | Stearic acid |
| LCA | Life Cycle Analysis |
| GHS | Globally Harmonized System of Classification and Labelling of Chemicals |
| CLD | Caprolactam disulfide |
| MBTS | 2,2’-benzothiazyl disulfide |
| CBS | N-cyclohexyl-2-benzothiazole sulfenamide |
| ZDTP | Zinc dialkyldithiophosphate |
| MDR | Moving Dye Rheometer |
| CS | Compression set |
| FTIR | Fourier Transform Infrared Spectroscopy |
| DSC | Differential Scanning Calorimetry |
| SEM | Scanning Electron Microscopy |
| TD-GC-MS | Thermal Desorption–Gas Chromatography–Mass Spectrometry |
| EDS | Energy-Dispersive X-ray Spectroscopy |
References
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc Oxide Particles: Synthesis, Properties and Applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Yan, M.F. Zinc Oxide. In Concise Encyclopedia of Advanced Ceramic Materials; Elsevier: Amsterdam, The Netherlands, 1991; pp. 523–525. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; van Baarle, B. Influence of Zinc Oxide during Different Stages of Sulfur Vulcanization. Elucidated by Model Compound Studies. J. Appl. Polym. Sci. 2005, 95, 1388–1404. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Wilaiwong, W.; Smitthipong, W. Study of Non-Negligible Chemical Reduction of ZnO in the Rubber Industry. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1234, 012014. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Macarrão, I.M.; Dutra, J.C. Sustainable Development Assessment of Zinc Oxide Production Focused on Agribusiness. J. Eng. Exact. Sci. 2020, 6, 577–584. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Grund, S.C.; van Genderen, E. Life Cycle Assessment and Product Carbon Footprint for SHG Zinc Production. J. Phys. Conf. Ser. 2024, 2738, 012038. [Google Scholar] [CrossRef]
- Buendia, E.; Tanabe, K.; Kranjc, A.; Jamsranjav, B.; Fukuda, M.; Ngarize, S.; Osako, A.; Pyrozhenko, Y.; Shermanau, P.; Federici, S. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Sreethu, T.K.; Naskar, K. Zinc Oxide with Various Surface Characteristics and Its Role on Mechanical Properties, Cure-Characteristics, and Morphological Analysis of Natural Rubber/Carbon Black Composites. J. Polym. Res. 2021, 28, 183. [Google Scholar] [CrossRef]
- Mostoni, S.; Milana, P.; Di Credico, B.; D’Arienzo, M.; Scotti, R. Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts 2019, 9, 664. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; van Baarle, B. Activators in Accelerated Sulfur Vulcanization. Rubber Chem. Technol. 2004, 77, 512–541. [Google Scholar] [CrossRef]
- Junkong, P.; Morimoto, R.; Miyaji, K.; Tohsan, A.; Sakaki, Y.; Ikeda, Y. Effect of Fatty Acids on the Accelerated Sulfur Vulcanization of Rubber by Active Zinc/Carboxylate Complexes. RSC Adv. 2020, 10, 4772–4785. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; Van Baarle, B. Various Ways to Reduce Zinc Oxide Levels in S-SBR Rubber Compounds. Macromol. Symp. 2006, 245, 657–667. [Google Scholar] [CrossRef]
- Przybyszewska, M.; Zaborski, M.; Jakubowski, B.; Zawadiak, J. Zinc Chelates as New Activators for Sulphur Vulcanization of Acrylonitrile-Butadiene Elastomer. Express Polym. Lett. 2009, 3, 256–266. [Google Scholar] [CrossRef]
- Ikeda, Y.; Yasuda, Y.; Ohashi, T.; Yokohama, H.; Minoda, S.; Kobayashi, H.; Honma, T. Dinuclear Bridging Bidentate Zinc/Stearate Complex in Sulfur Cross-Linking of Rubber. Macromolecules 2015, 48, 462–475. [Google Scholar] [CrossRef]
- Setianto, W.B.; Yohanes, H.; Astuti; Maisaroh; Atmaji, G. Synthesis of Palm Oil-Base Zinc Stearate and Its Application on Manufacture of Rubber Component. IOP Conf. Ser. Earth Environ. Sci. 2022, 963, 012028. [Google Scholar] [CrossRef]
- Bukhina, M.F.; Morozov, Y.L.; Ven, P.M.; Noordermeer, J.W.M. Mold Fouling of EPDM Rubber Compounds. Kautsch. Und Gummi Kunststoffe 2003, 56, 172–183. [Google Scholar]
- Kaczewiak, K.; Głąb, P.; Maciejewska, M. Vulcanization Temperature Impact on Cross-Linking Density and Compression Set of Sulfur-Cured Ethylene–Propylene–Diene Monomer. J. Appl. Polym. Sci. 2025, 142, e57188. [Google Scholar] [CrossRef]
- ISO 815-1:2019; Rubber, Vulcanized or Thermoplastic—Determination of Compression set, Part 1: At Ambient or Elevated Temperatures. International Organization for Standardization: Geneva, Switzerland, 2019.
- Konkoly-Thege, I.; Ruff, I.; Adeosun, S.O.; Sime, S.J. Properties of Molten Carboxylates Part 6. A Quantiative Differential Thermal Analysis Study of Phase Transitions in Some Zinc and Cadmium Carboxylates. Thermochim. Acta 1978, 24, 89–96. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, F.; Wang, Z.; Li, G.; Yang, S.; Feng, J. Comparative Investigation of the Structural Evolution of Zinc Stearate and Calcium Stearate in a Polypropylene Random Copolymer upon Heating and Cooling. Polymer 2023, 267, 125646. [Google Scholar] [CrossRef]
- Ishioka, T.; Maeda, K.; Watanabe, I.; Kawauchi, S.; Harada, M. Infrared and XAFS Study on Structure and Transition Behavior of Zinc Stearate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2000, 56, 1731–1737. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Li, L.; Zeng, Z.; Zhao, W.; Wang, G.; Guan, X. Simple and Green Fabrication of Super-Hydrophobic Surface by One-Step Immersion for Continuous Oil/Water Separation. J. Phys. Chem. A 2016, 120, 5617–5623. [Google Scholar] [CrossRef]
- Larkin, P.J.; Jackson, A. Interpretation of the Infrared Spectra of Metal-Stearate Salts. Appl. Spectrosc. Pract. 2024, 2, 27551857241253834. [Google Scholar] [CrossRef]
- Albarakaty, F.M.; Alzaban, M.I.; Alharbi, N.K.; Bagrwan, F.S.; Abd El-Aziz, A.R.M.; Mahmoud, M.A. Zinc oxide Nanoparticles, Biosynthesis, Characterization and Their Potent Photocatalytic Degradation, and Antioxidant Activities. J. King Saud. Univ. Sci. 2023, 35, 102434. [Google Scholar] [CrossRef]
- Choi, S.; Chung, H.; Joo, Y.; Yang, K.; Lee, S. Analysis of Whitening Phenomenon of EPDM Article by Humid Aging. J. Appl. Polym. Sci. 2012, 123, 2451–2457. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Roy Choudhury, N.; Ginic-Markovic, M.; Matisons, J.; Williams, D.R.G. Surface Studies on the Additive Migration and Diffusion in the Windowseal Rubber Component Influencing Adhesion to Coating. J. Adhes. Sci. Technol. 1998, 12, 1377–1390. [Google Scholar] [CrossRef]
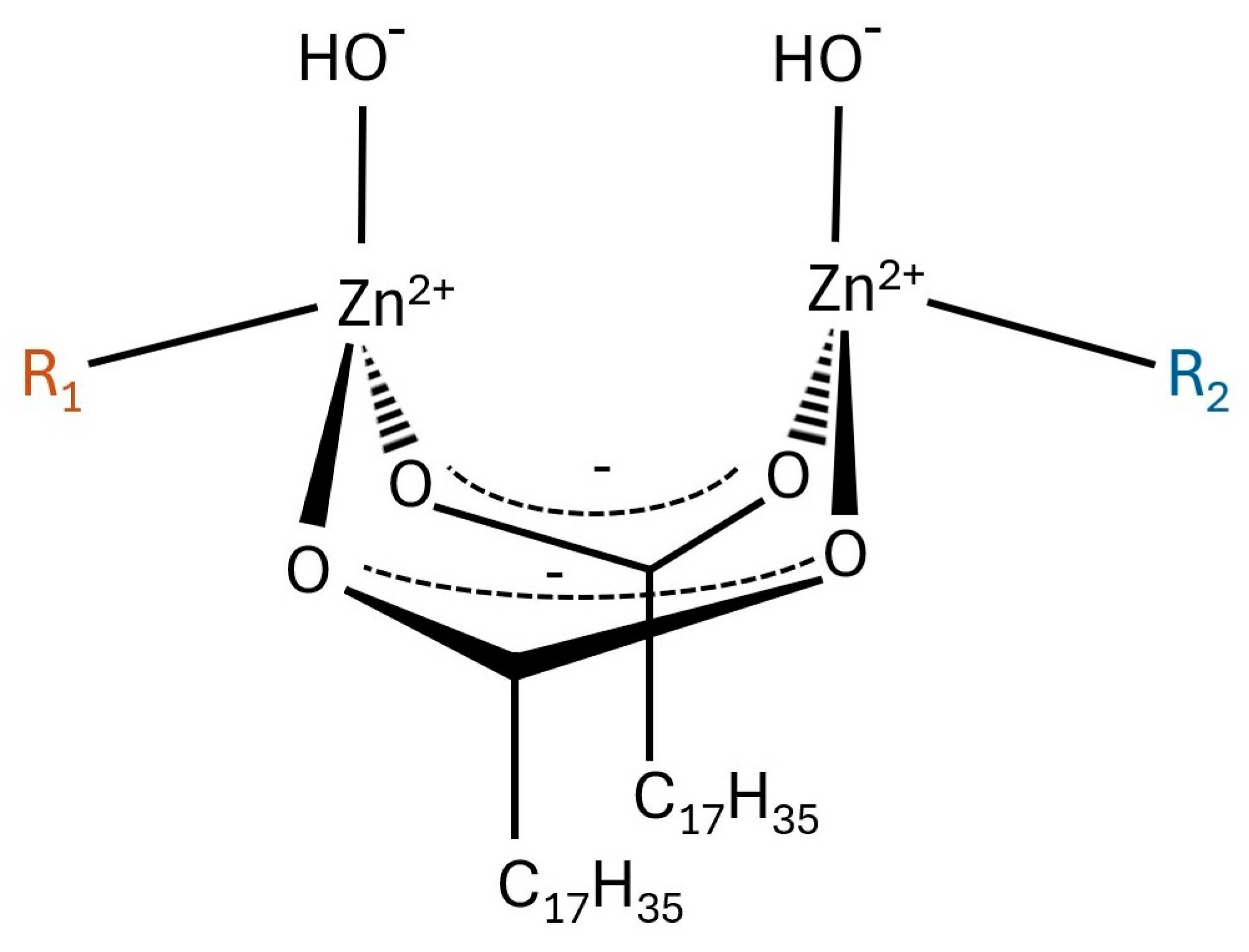

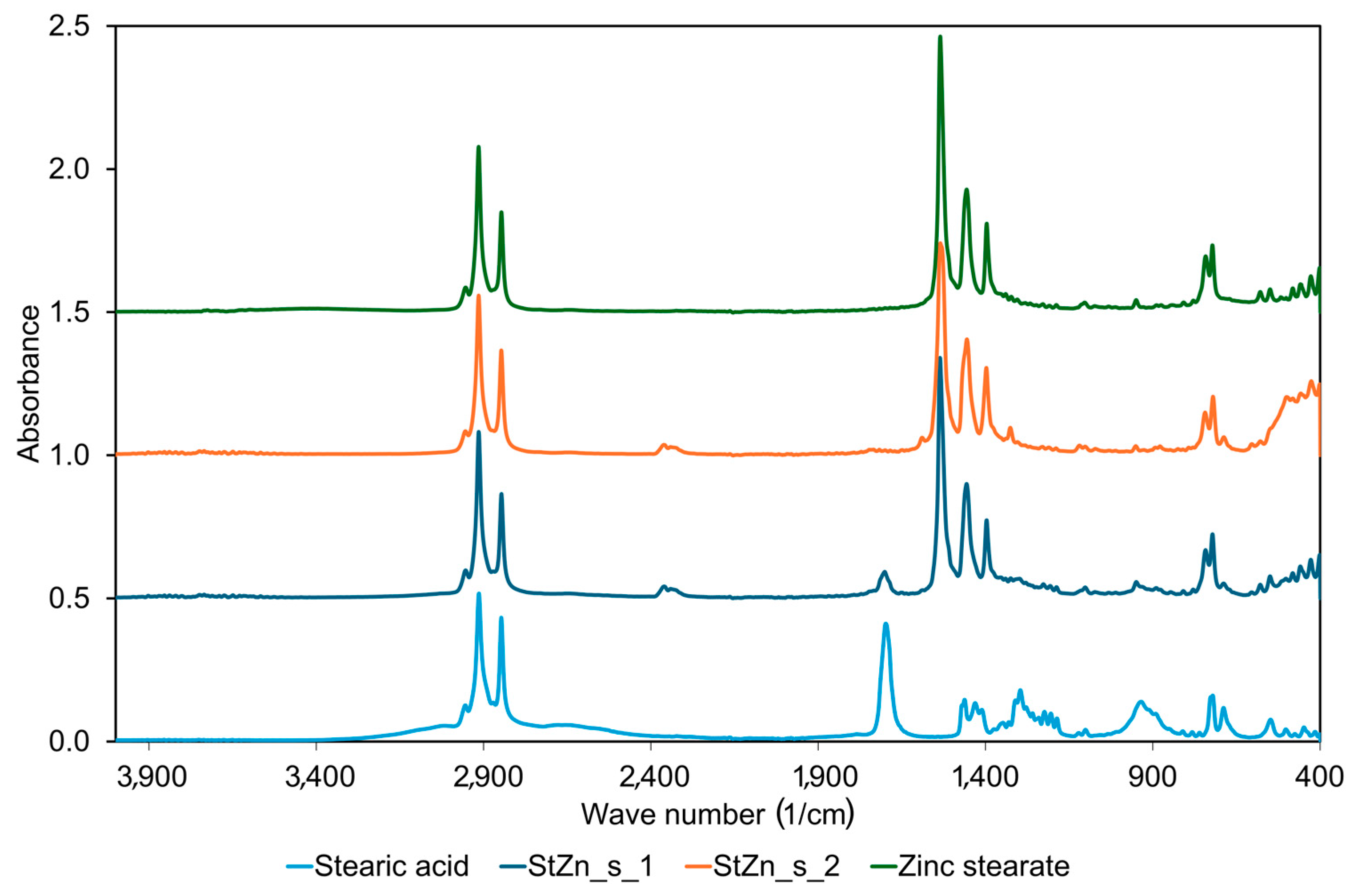
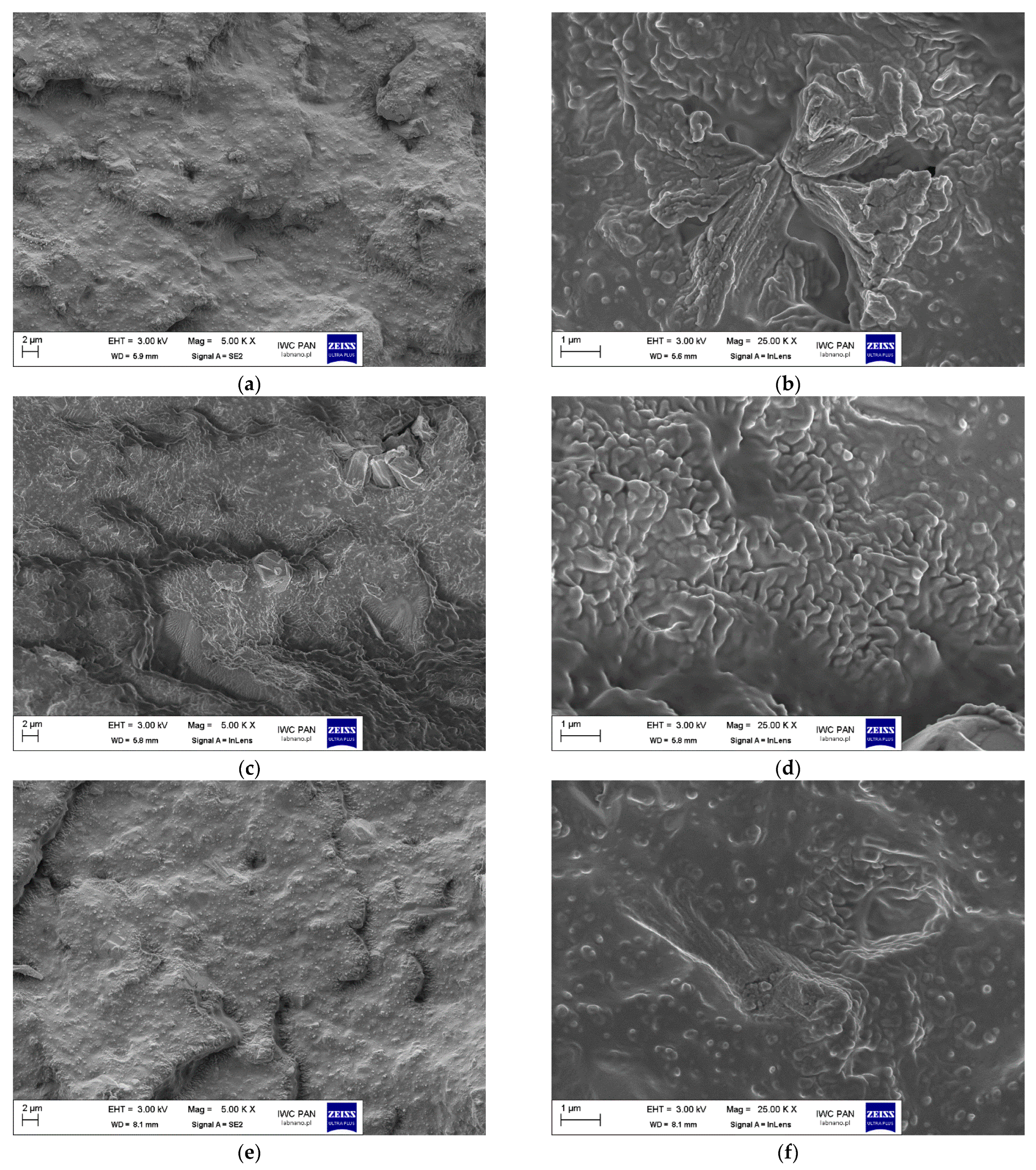
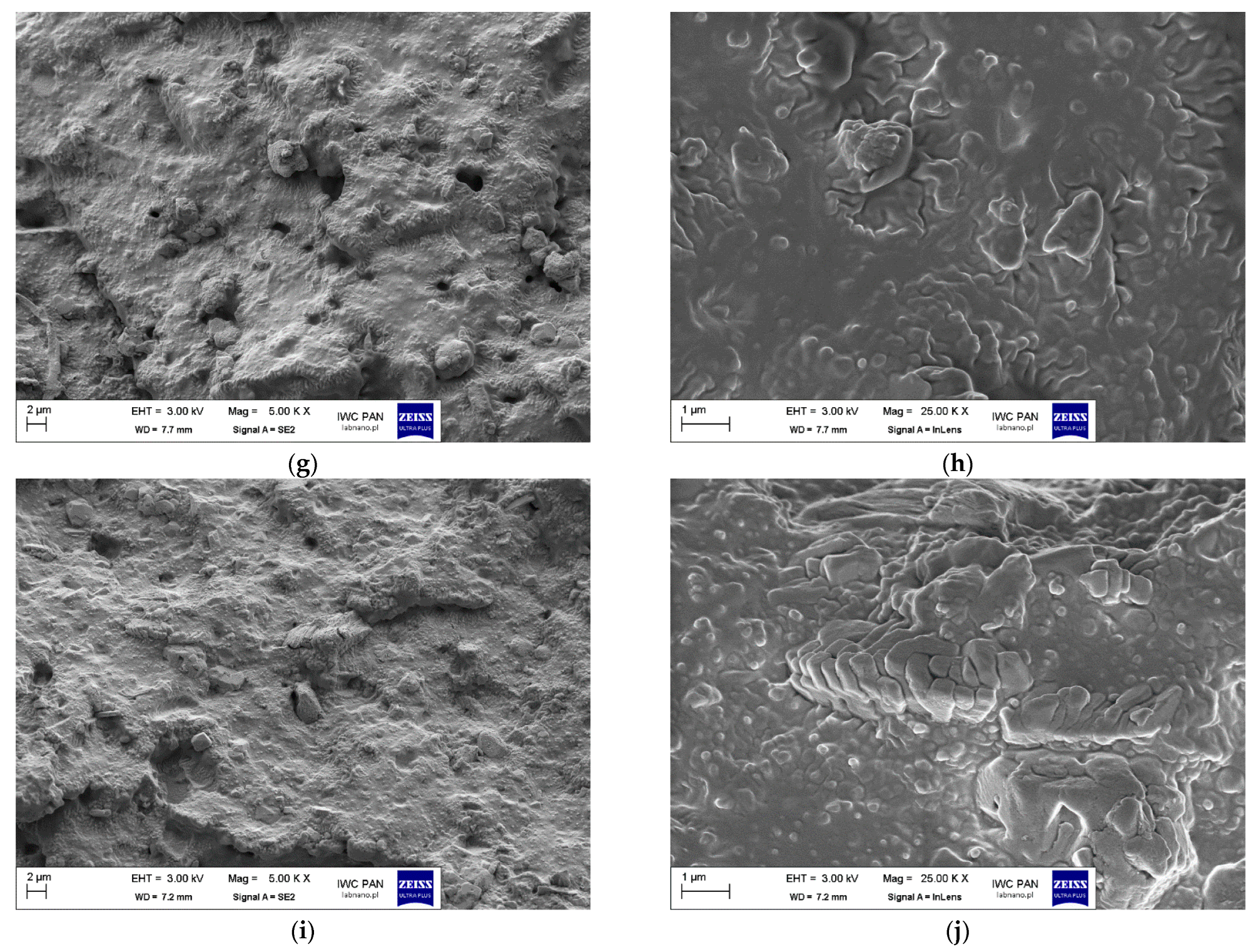


| No. | Sample Symbol | Stearic Acid (mole) | Zinc Oxide 1 (mole) | Sulfur 2 (mole) |
|---|---|---|---|---|
| 1 | StZn_s_1 | 2 | 1 | - |
| 2 | StZn_s_2 | 2 | 2 | - |
| 3 | StZnS_s_1 | 2 | 1 | 8 |
| 4 | StZnS_s_2 | 2 | 2 | 8 |
| 5 | StZn_a_1 | 2 | 1 A | - |
| 6 | StZn_a_2 | 2 | 2 A | - |
| 7 | StZnS_a_1_7 | 2 | 1 A | 8 |
| 8 | StZnS_a_2_8 | 2 | 2 A | 8 |
| 9 | StZnS_a_1_9 | 2 | 1 A | 8 |
| 10 | StZnS_a_2_10 | 2 | 2 A | 8 |
| Rubber Compound | Description | ZnO phr Equivalent | StA/Zn [mole/mole] |
|---|---|---|---|
| Ref0 | Reference compound with standard ZnO. | 3 | 0.15 |
| Ref1 | Reference compound with active ZnO. | 3 | 0.15 |
| Ref2 | Reference compound with active ZnO and sulfur thermally transformed (200 °C/5 min) in masterbatch before adding accelerator set—experiment following the previous study [13]. | 3 | 0.15 |
| Test1 | Rubber compound with StZn_a_1. Targeted to stoichiometric ZnSt. Amount of StZn_a_1 equivalent to StA concentration in reference. A total of 13.6 times less Zn than the reference. | 0.22 | 2 |
| Test2 | Rubber compound with StZn_a_1. Targeted to stoichiometric ZnSt. Amount of StZn_a_1 to obtain Zn equivalent 3 times less than the reference. | 1 | 2 |
| Test3 | Rubber compound with StZn_a_2. Targeted to ZnSt complex. Amount of StZn_a_2 equivalent to stearic acid concentration in reference. A total of 6.7 times less Zn than the reference. | 0.44 | 1 |
| Test4 | Rubber compound with StZn_a_2. Targeted to ZnSt complex. Amount of StZn_a_2 to obtain Zn equivalent 3 times less than the reference. | 1 | 1 |
| Test5 | Rubber compound with StZn_s_1. Targeted to stoichiometric ZnSt. Amount of StZn_s_1 equivalent to StA concentration in reference. A total of 13.6 times less Zn than the reference. | 0.22 | 2 |
| Test6 | Rubber compound with StZn_s_1. Targeted to stoichiometric ZnSt. Amount of StZn_s_1 to obtain Zn equivalent 3 times less than the reference. | 1 | 2 |
| Test7 | Rubber compound with StZn_s_2. Targeted to ZnSt complex. Amount of StZn_s_2 equivalent to StA concentration in reference. A total of 6.7 times less Zn than the reference. | 0.44 | 1 |
| Test8 | Rubber compound with StZn_s_2. Targeted to ZnSt complex. Amount of StZn_s_2 to obtain Zn equivalent 3 times less than in reference. | 1 | 1 |
| Test9 | Rubber compound with StZnS_a_1_7. Targeted to ZnSt/sulfur complex. Sulfur in amount equivalent to S8 per one Zn, added at the end. Amount of StZnS_a_1_7 equivalent to StA concentration in reference. A total of 13.6 times less Zn than the reference. 1 | 0.22 | 2 |
| Test10 | Rubber compound with StZnS_a_1_9. Targeted to ZnSt/sulfur complex. Sulfur in amount equivalent to S8 per one Zn, added before ZnO. Amount of StZnS_a_1_9 equivalent to StA concentration in reference. A total of 13.6 times less Zn than the reference. 1 | 0.22 | 2 |
| Test11 | Rubber compound with StZnS_a_1_7. Targeted to ZnSt/sulfur complex. Sulfur in amount equivalent to S8 per one Zn, added at the end. Amount of StZnS_a_1_7 equivalent to double StA concentration in reference. A total of 6.7 times less Zn than the reference. 1 | 0.44 | 2 |
| Test12 | Rubber compound with StZnS_a_2_8. Targeted to ZnSt/sulfur complex. Sulfur in amount equivalent to S8 per one Zn, added at the end. Amount of StZnS_a_2_8 equivalent to StA concentration in reference. A total of 6.7 times less Zn than the reference. 1 | 0.44 | 1 |
| Test13 | Rubber compound with StZnS_a_2_10. Targeted to ZnSt/sulfur complex. Sulfur in amount equivalent to S8 per one Zn, added before ZnO. Amount of StZnS_a_2_10 equivalent to StA concentration in reference. A total of 6.7 times less Zn than the reference. 1 | 0.44 | 1 |
| Test14 | Rubber compound with StZnS_s_1. Targeted to ZnSt/sulfur complex. Sulfur in amount equivalent to S8 per one Zn, added at the end. Amount of StZnS_s_1 equivalent to StA concentration in reference. A total of 13.6 times less Zn than the reference. 1 | 0.22 | 2 |
| Test15 | Rubber compound with StZnS_s_2. Targeted to ZnSt/sulfur complex. Sulfur in amount equivalent to S8 per one Zn, added at the end. Amount of StZnS_s_2 equivalent to stearic acid concentration in reference. A total of 6.7 times less Zn than the reference. 1 | 0.44 | 1 |
| Rubber Compound | Description | ZnO phr Equivalent | StA/Zn [mole/mole] |
|---|---|---|---|
| Ref3 | Reference compound with commercial ZnSt and 1 phr of StA. Amount of ZnSt to obtain Zn equivalent 3 times less than Ref1 (Table 2). | 1 | 2.28 |
| Test16 | Rubber compound with commercial ZnSt and 1 phr of StA premixed in Brabender with masterbatch. Amount of ZnSt to obtain Zn equivalent like in Ref3. | 1 | 2.28 |
| Test17 | Rubber compound with sulfur, ZnSt, and 1 phr of StA premixed in Brabender with masterbatch. Amount of ZnSt to obtain Zn equivalent like in Ref3. | 1 | 2.28 |
| Test18 | Rubber compound with StZn_a_1. Targeted to stoichiometric ZnSt. Amount of StZn_a_1 to obtain Zn equivalent 3 times less than the reference. Composition like Test2 (Table 2) but added 0.5 phr of stearic acid. Similar StA/Zn ratio like in Ref3. | 1 | 2.13 |
| Test19 | Rubber compound with StZn_a_2. Targeted to ZnSt complex. Amount of StZn_a_2 to obtain Zn equivalent 3 times less than the reference. Composition like Test4 (Table 2) but added 1 phr of stearic acid. Reduced StA/Zn ratio like in Ref3. | 1 | 1.29 |
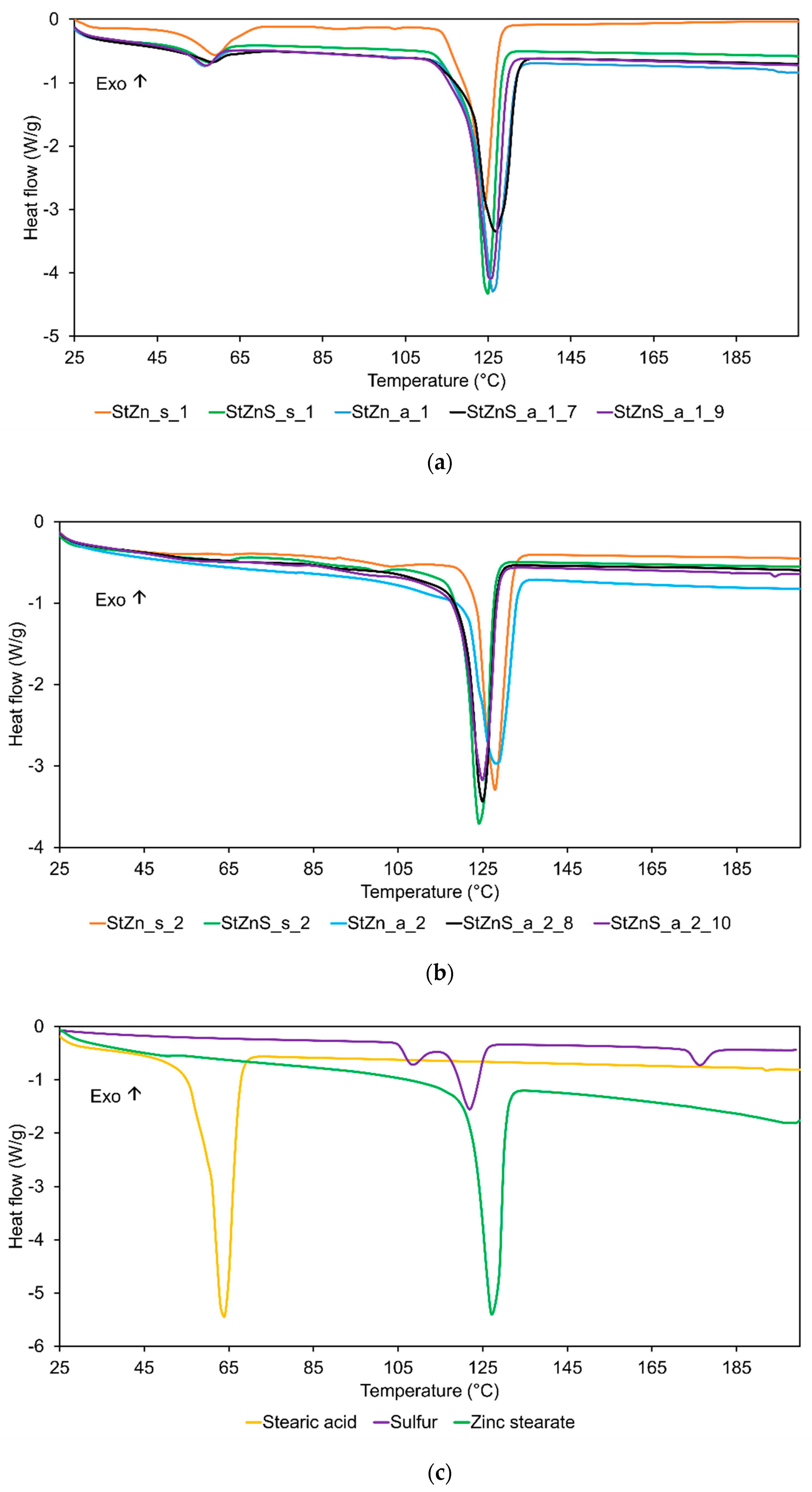
| Sample | Phase Change 1 | Phase Change 2 | ||
|---|---|---|---|---|
| Heat of Melting [J/g] | Peak [°C] | Heat of Melting [J/g] | Peak [°C] | |
| StZn_s_1 | −25.0 | 57.8 | −102.6 | 121 |
| StZn_s_2 | - | - | −95.9 | 126 |
| StZnS_s_1 | −12.7 | 57.8 | −134.2 | 123 |
| StZnS_s_2 | - | - | −108.5 | 122 |
| StZn_a_1 | −11.5 | 56.1 | −162.4 | 124 |
| StZn_a_2 | - | - | −121.9 | 127 |
| StZnS_a_1_7 | −6.3 | 57.7 | −142.3 | 125 |
| StZnS_a_2_8 | - | - | −104.9 | 123 |
| StZnS_a_1_9 | −8.9 | 56.6 | −149.6 | 123 |
| StZnS_a_2_10 | - | - | −104.9 | 123 |
| StA | −194.5 | 61.5 | - | - |
| S | −9.1 | 108.0 | −33.5 | 121 |
| ZnSt | - | - | −153.3 | 126 |
| Sample | Mass of DSC Sample [mg] | Mass of StA in DSC Sample [mg] | Initial Mass of StA in Sample [mg] | StA Reacted [mg] | ZnSt Yielded [mg] | Expected Heat of Melting [J/g] | Reacted StA [%] |
|---|---|---|---|---|---|---|---|
| StZn_s_1 | 11.8 | 1.52 | 10.3 | 8.8 | 9.8 | −127.6 | 85 |
| StZn_s_2 | 16.5 | 0.00 | 12.8 | 12.8 | 14.3 | −132.9 | 100 |
| StZn_a_1 | 13.6 | 0.80 | 11.9 | 11.1 | 12.3 | −139.4 | 93 |
| StZn_a_2 | 14.3 | 0.00 | 11.1 | 11.1 | 12.4 | −132.9 | 100 |
| Compound | MDR Results | Mooney Results | |||||
|---|---|---|---|---|---|---|---|
| ML [dNm] | MH [dNm] | MH–ML [dNm] | ts2 [min] | t90 [min] | ML [MU] | t5 [min] | |
| Ref0 | 1.54 | 7.79 | 6.25 | 0.97 | 1.79 | 32.8 | 14.0 |
| Ref1 | 0.96 | 9.05 | 8.09 | 0.93 | 1.94 | 26.1 | 12.4 |
| Ref2 | 1.05 | 9.53 | 8.48 | 0.93 | 2.01 | 28.3 | 11.8 |
| Test1 | 1.17 | 8.15 | 6.98 | 1.00 | 1.78 | 31.8 | 12.7 |
| Test2 | 1.05 | 9.21 | 8.16 | 1.21 | 2.88 | 26.1 | 18.6 |
| Test3 | 1.23 | 8.82 | 7.59 | 0.95 | 1.78 | 32.0 | 12.0 |
| Test4 | 1.10 | 8.95 | 7.85 | 1.10 | 2.26 | 28.4 | 17.2 |
| Test5 | 1.06 | 8.02 | 6.96 | 0.98 | 1.74 | 27.9 | 12.1 |
| Test6 | 0.93 | 8.11 | 7.18 | 1.13 | 2.52 | 23.4 | 17.9 |
| Test7 | 1.18 | 8.43 | 7.25 | 0.96 | 1.70 | 29.0 | 13.0 |
| Test8 | 1.06 | 7.47 | 6.41 | 1.10 | 1.88 | 26.4 | 16.7 |
| Test9 | 1.05 | 8.07 | 7.02 | 0.93 | 1.93 | 27.8 | 12.6 |
| Test10 | 0.99 | 7.99 | 7.00 | 0.88 | 1.62 | 27.0 | 11.4 |
| Test11 | 1.03 | 8.32 | 7.29 | 0.95 | 2.27 | 25.0 | 16.3 |
| Test12 | 1.11 | 8.43 | 7.32 | 0.90 | 1.73 | 27.9 | 13.4 |
| Test13 | 1.04 | 8.65 | 7.61 | 0.86 | 1.88 | 27.6 | 12.8 |
| Test14 | 1.08 | 8.11 | 7.03 | 0.95 | 1.80 | 28.3 | 12.5 |
| Test15 | 1.10 | 8.45 | 7.35 | 0.91 | 1.66 | 28.8 | 12.3 |
| Ref3 | 0.82 | 6.90 | 6.08 | 1.22 | 3.46 | 20.9 | 25.1 |
| Test16 | 0.98 | 7.60 | 6.62 | 1.32 | 2.66 | 22.9 | 24.1 |
| Test17 | 1.01 | 8.24 | 7.23 | 1.34 | 2.87 | 23.7 | 24.1 |
| Test18 | 0.83 | 6.71 | 5.88 | 0.89 | 2.20 | 21.9 | 14.7 |
| Test19 | 0.94 | 6.59 | 5.65 | 0.75 | 1.90 | 24.8 | 11.6 |
| Compound | Compression Set [%] | Equilibrium Swelling [%] | ||||
|---|---|---|---|---|---|---|
| Ref0 | 72.5 | +/− | 1.7 | 96.1 | +/− | 2.0 |
| Ref1 | 60.9 | +/− | 2.3 | 85.6 | +/− | 0.4 |
| Ref2 | 57.9 | +/− | 1.4 | 82.6 | +/− | 1.1 |
| Test1 | 73.7 | +/− | 1.1 | 103.9 | +/− | 1.9 |
| Test2 | 52.9 | +/− | 2.1 | 74.7 | +/− | 0.4 |
| Test3 | 66.8 | +/− | 1.7 | 95.2 | +/− | 0.5 |
| Test4 | 56.5 | +/− | 0.9 | 81.6 | +/− | 0.5 |
| Test5 | 74.9 | +/− | 1.6 | 105.9 | +/− | 1.4 |
| Test6 | 56.3 | +/− | 1.2 | 81.3 | +/− | 1.1 |
| Test7 | 69.3 | +/− | 1.1 | 100.2 | +/− | 2.0 |
| Test8 | 61.9 | +/− | 1.5 | 93.9 | +/− | 1.2 |
| Test9 | 70.4 | +/− | 0.8 | 102.1 | +/− | 2.5 |
| Test10 | 71.4 | +/− | 1.6 | 103.7 | +/− | 1.4 |
| Test11 | 68.4 | +/− | 0.8 | 92.0 | +/− | 1.1 |
| Test12 | 69.2 | +/− | 1.5 | 98.9 | +/− | 1.3 |
| Test13 | 69.2 | +/− | 0.2 | 97.8 | +/− | 1.3 |
| Test14 | 70.3 | +/− | 1.7 | 102.3 | +/− | 1.4 |
| Test15 | 67.9 | +/− | 1.6 | 97.1 | +/− | 0.5 |
| Ref3 | 44.5 | +/− | 0.7 | 82.6 | +/− | 1.7 |
| Test16 | 42.5 | +/− | 2.3 | 78.3 | +/− | 1.6 |
| Test17 | 41.5 | +/− | 0.8 | 77.3 | +/− | 1.4 |
| Test18 | 51.1 | +/− | 1.5 | 85.1 | +/− | 1.2 |
| Test19 | 52.7 | +/− | 0.8 | 97.1 | +/− | 1.6 |
| Compound | SE100 [MPa] | TS [MPa] | Eb [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref0 | 2.6 | +/− | 0.2 | 3.0 | +/− | 0.6 | 123 | +/− | 17 |
| Ref1 | 2.1 | +/− | 0.1 | 6.3 | +/− | 0.3 | 499 | +/− | 40 |
| Test2 | 2.2 | +/− | 0.1 | 7.9 | +/− | 0.7 | 421 | +/− | 55 |
| Test4 | 2.2 | +/− | 0.1 | 8.7 | +/− | 0.1 | 412 | +/− | 10 |
| Test6 | 2.6 | +/− | 0.1 | 9.0 | +/− | 0.1 | 415 | +/− | 12 |
| Test8 | 3.5 | +/− | 0.2 | 10.6 | +/− | 0.4 | 335 | +/− | 15 |
| Test9 | 2.4 | +/− | 0.2 | 7.6 | +/− | 0.1 | 445 | +/− | 69 |
| Test14 | 2.3 | +/− | 0.1 | 7.7 | +/− | 0.1 | 506 | +/− | 25 |
| Ref3 | 2.3 | +/− | 0.1 | 8.5 | +/− | 0.2 | 435 | +/− | 8 |
| Test16 | 2.7 | +/− | 0.1 | 8.5 | +/− | 0.2 | 352 | +/− | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczewiak, K.; Głąb, P.; Maciejewska, M. The Influence of Zinc Stearate Complexes on the Sulfur Vulcanization of Ethylene–Propylene–Diene Monomer. Polymers 2025, 17, 2875. https://doi.org/10.3390/polym17212875
Kaczewiak K, Głąb P, Maciejewska M. The Influence of Zinc Stearate Complexes on the Sulfur Vulcanization of Ethylene–Propylene–Diene Monomer. Polymers. 2025; 17(21):2875. https://doi.org/10.3390/polym17212875
Chicago/Turabian StyleKaczewiak, Krzysztof, Piotr Głąb, and Magdalena Maciejewska. 2025. "The Influence of Zinc Stearate Complexes on the Sulfur Vulcanization of Ethylene–Propylene–Diene Monomer" Polymers 17, no. 21: 2875. https://doi.org/10.3390/polym17212875
APA StyleKaczewiak, K., Głąb, P., & Maciejewska, M. (2025). The Influence of Zinc Stearate Complexes on the Sulfur Vulcanization of Ethylene–Propylene–Diene Monomer. Polymers, 17(21), 2875. https://doi.org/10.3390/polym17212875










