Polyamide 11 Composites with Surface-Activated Intact Mica Structures for Advanced Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
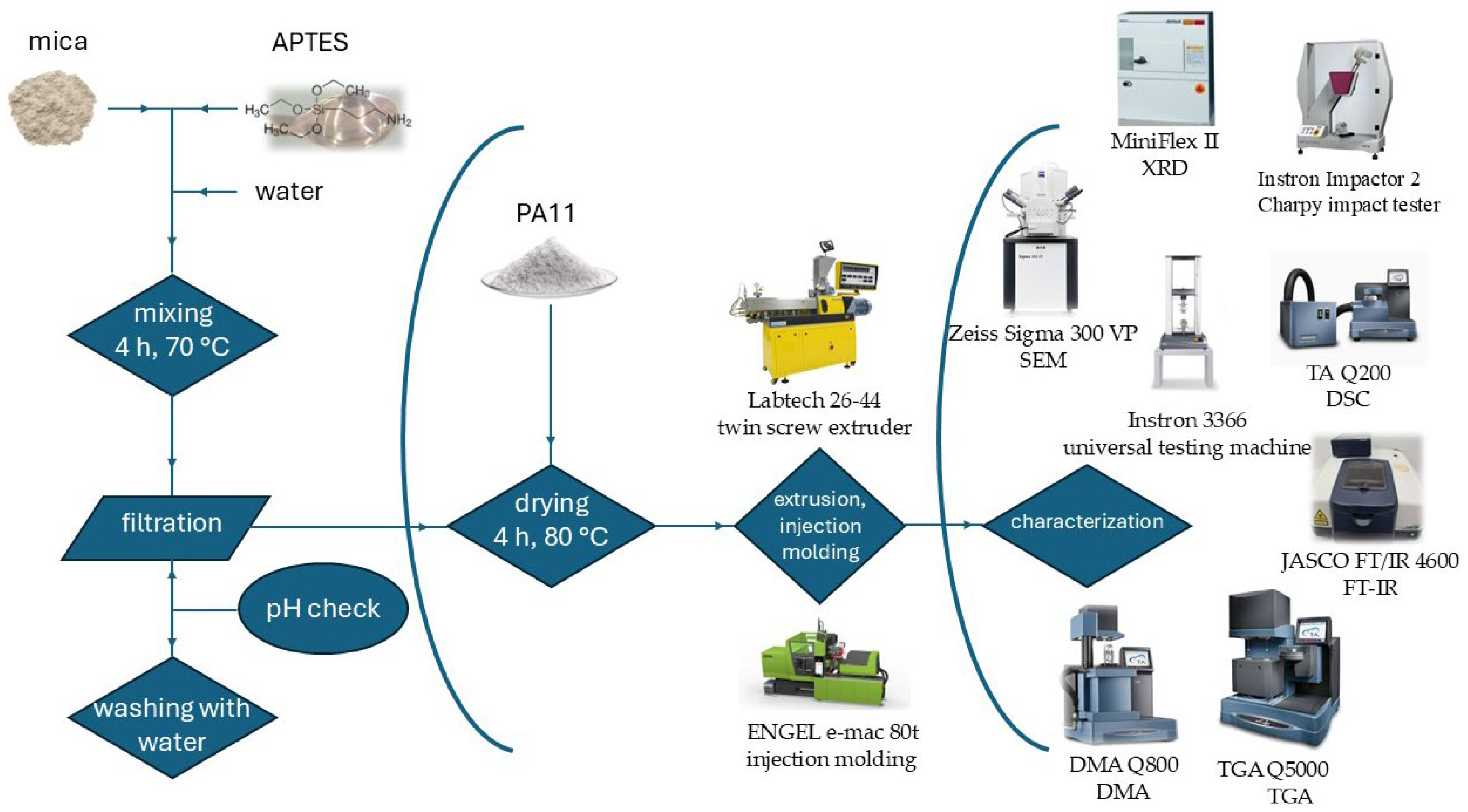
2.2. Preparation of the Composites
2.3. Characterization
2.3.1. Density
2.3.2. Immersion Water Uptake
2.3.3. Fourier Transform Infrared Spectroscopy (FT-IR Spectroscopy)
2.3.4. Impact Strength
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Scanning Electron Microscopy (SEM)
2.3.7. Dynamic Mechanical Analysis (DMA)
2.3.8. Tensile and Flexural Tests
2.3.9. X-Ray Diffraction (XRD)
3. Results and Discussion
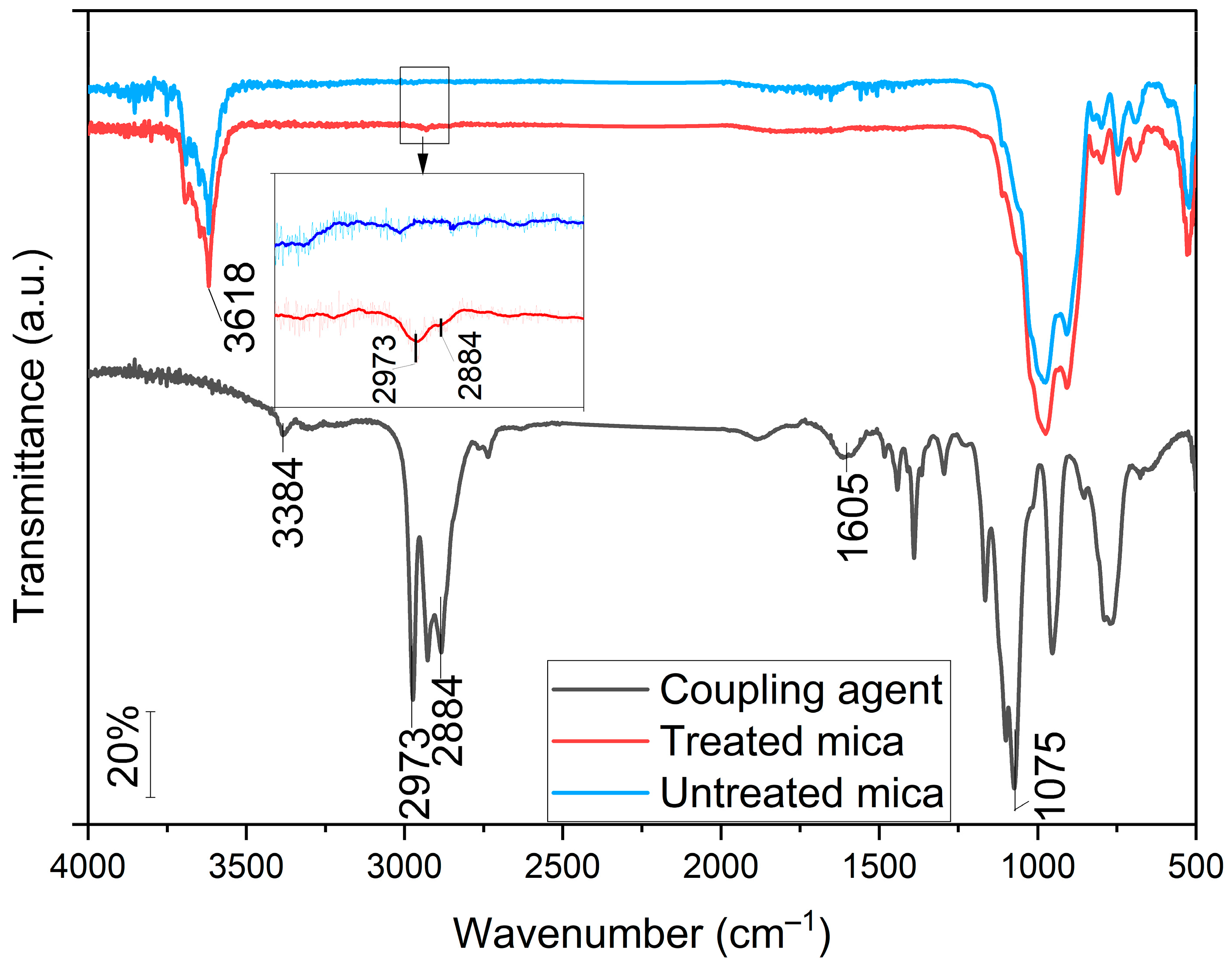
3.1. FT-IR Analysis of Mica
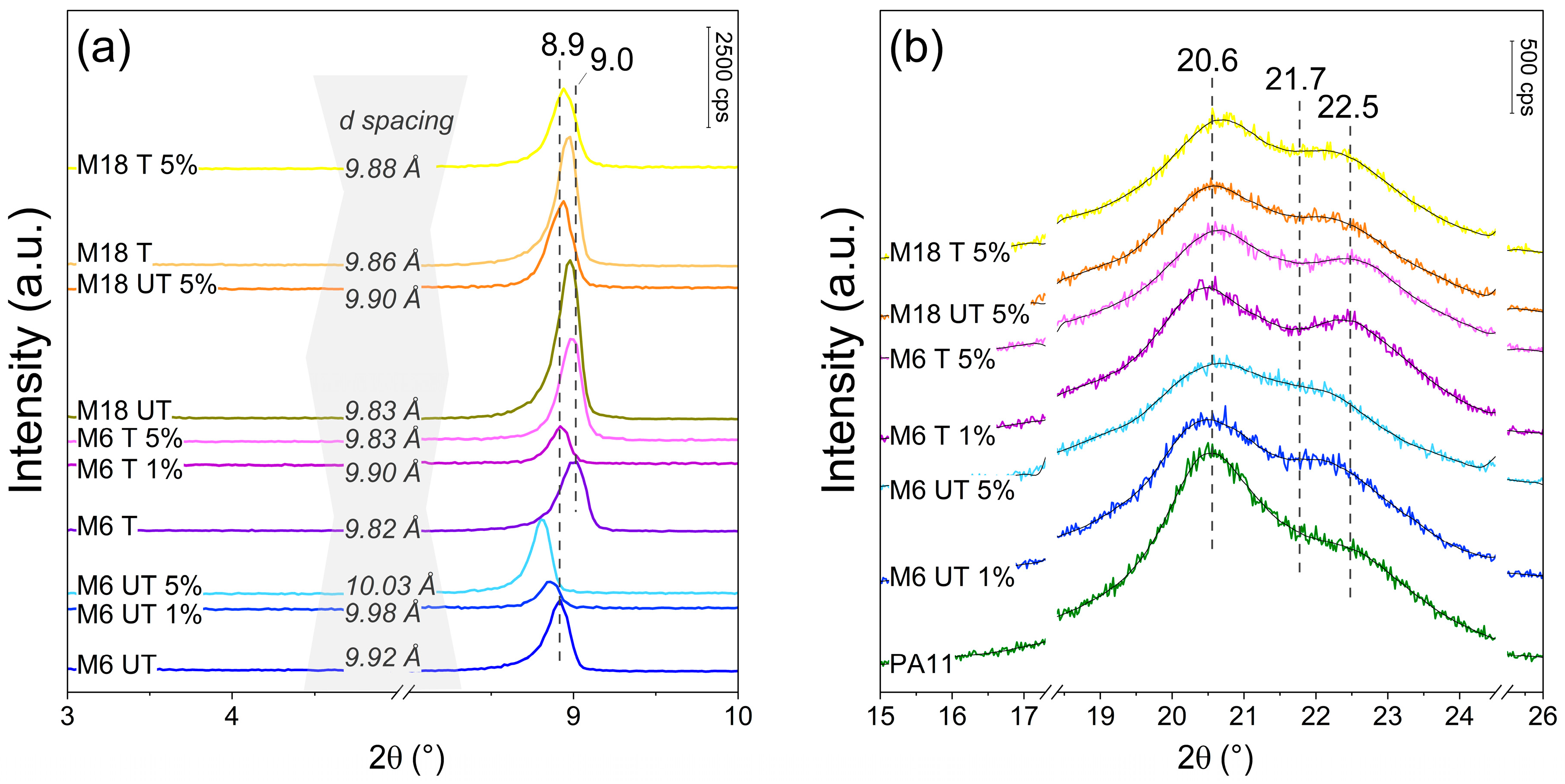
3.2. X-Ray Diffraction
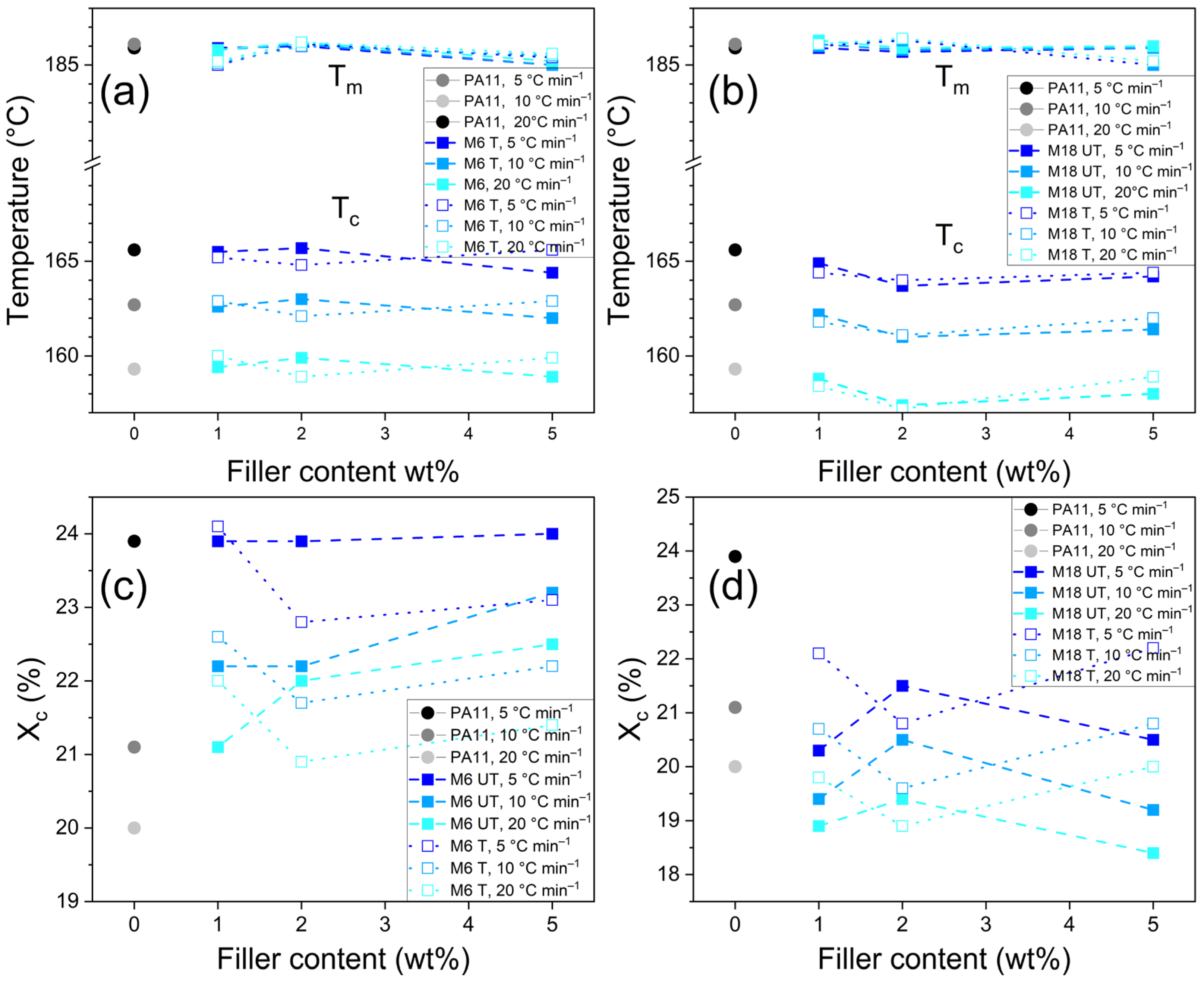
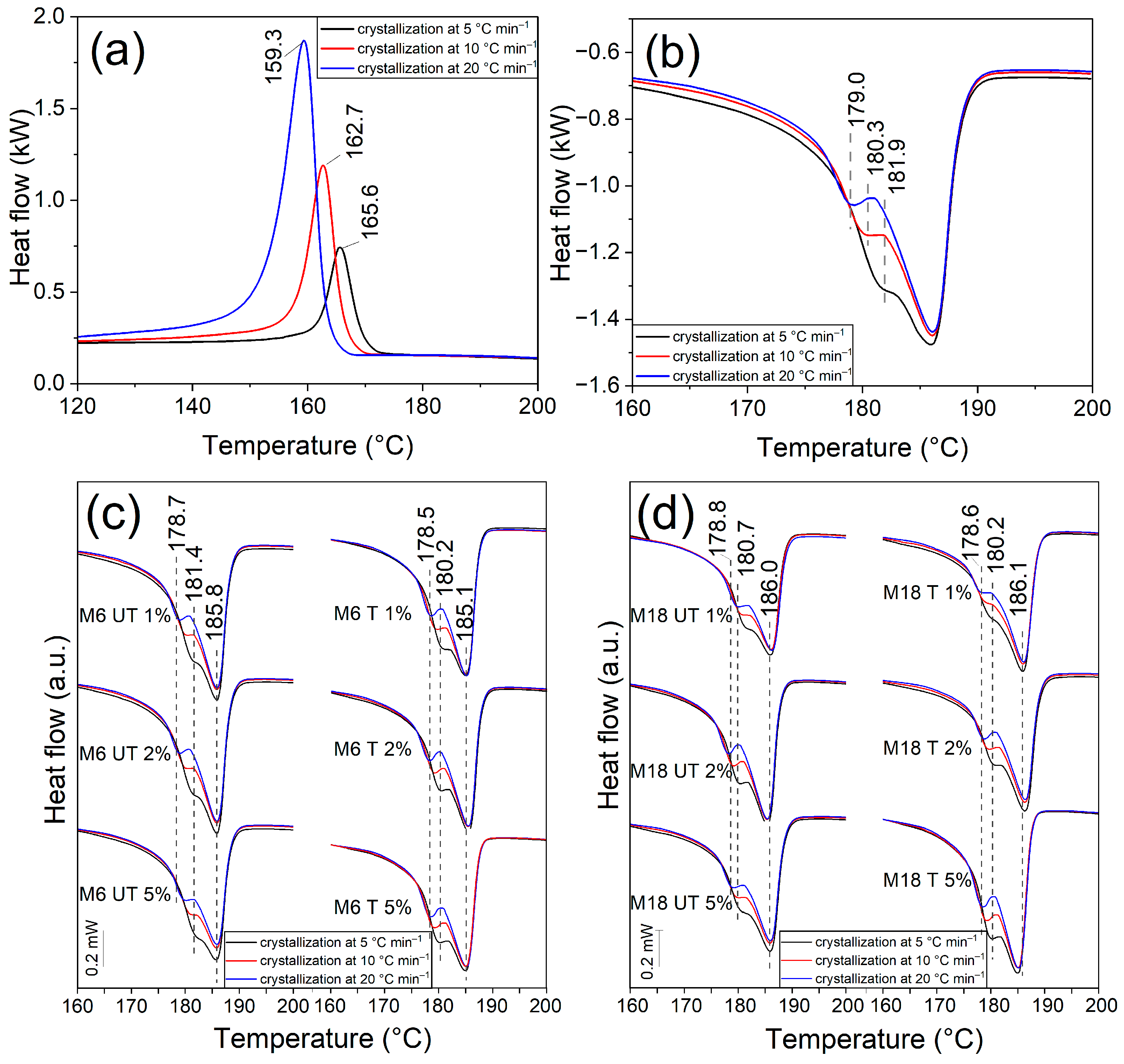
3.3. Differential Scanning Calorimetry
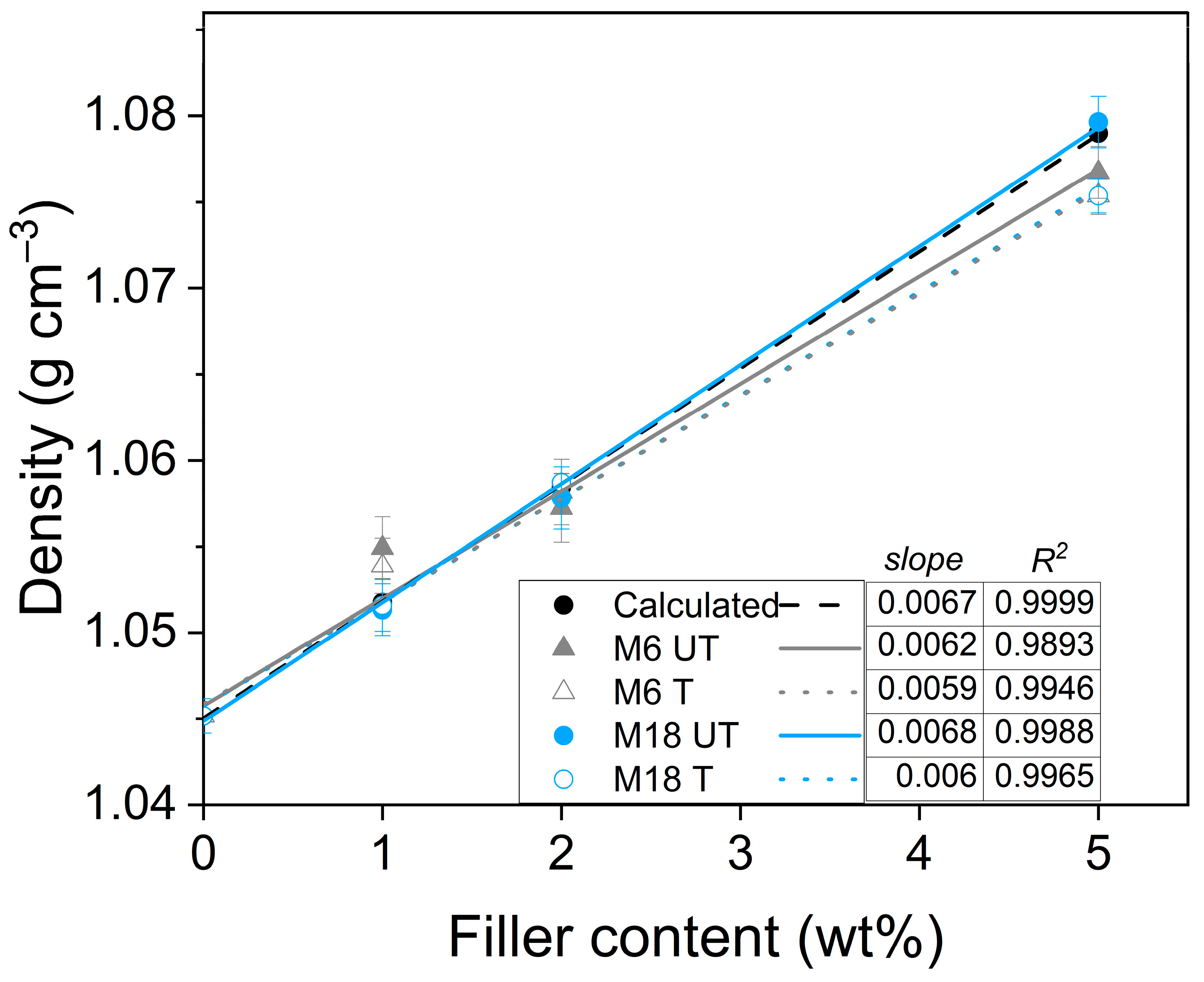
3.4. Densities and Water Uptake
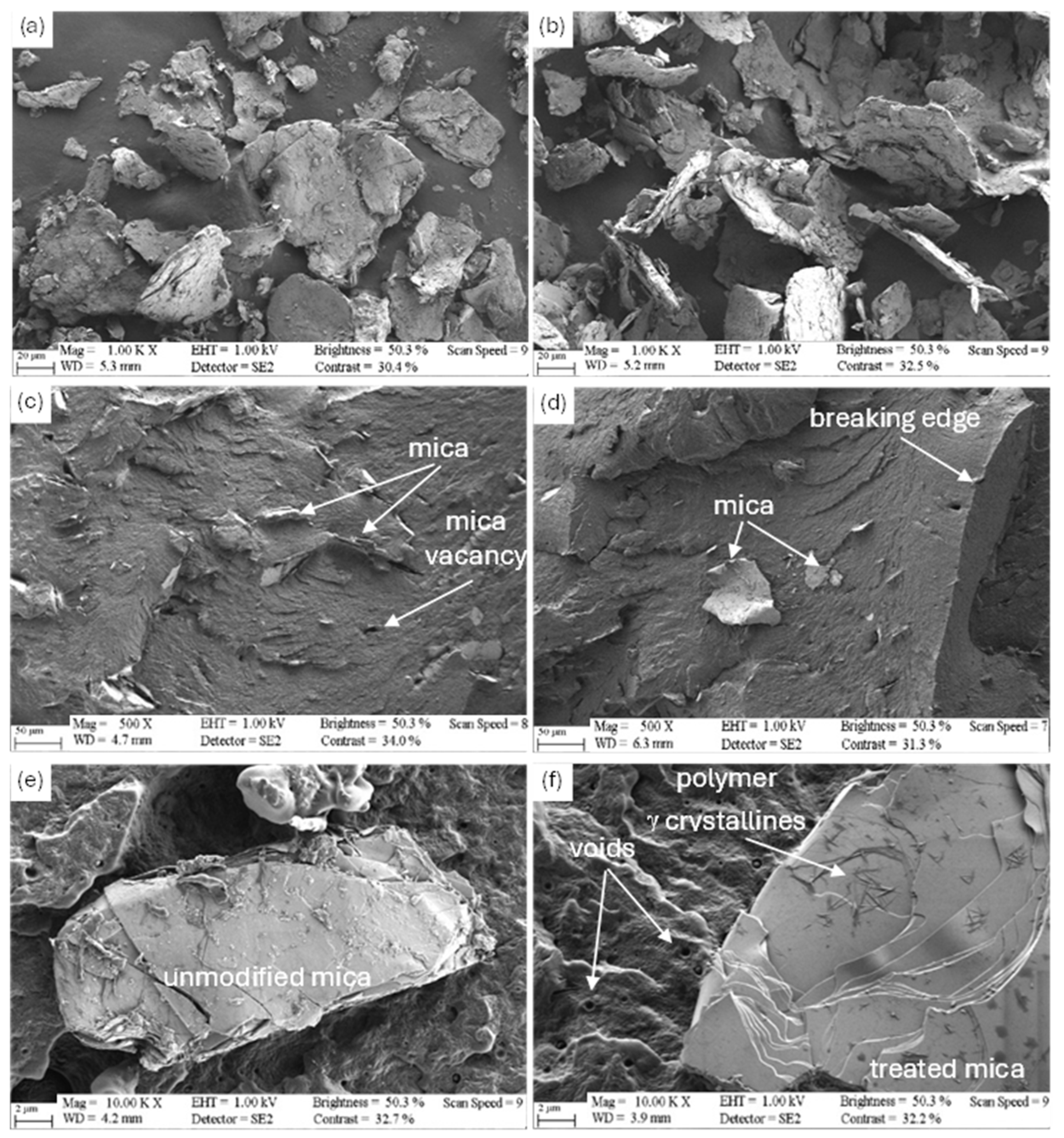
3.5. Scanning Electron Microscopic Analysis
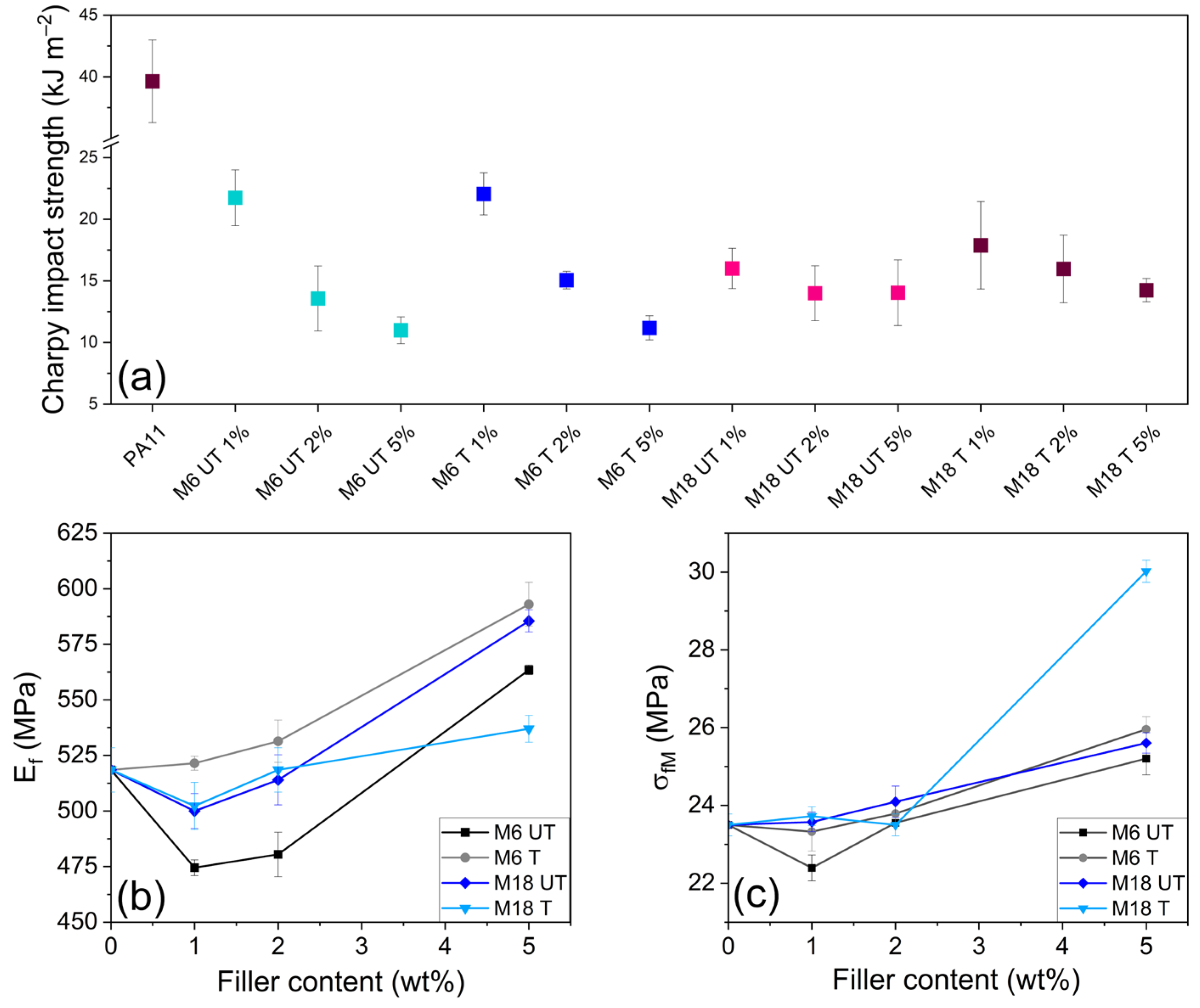
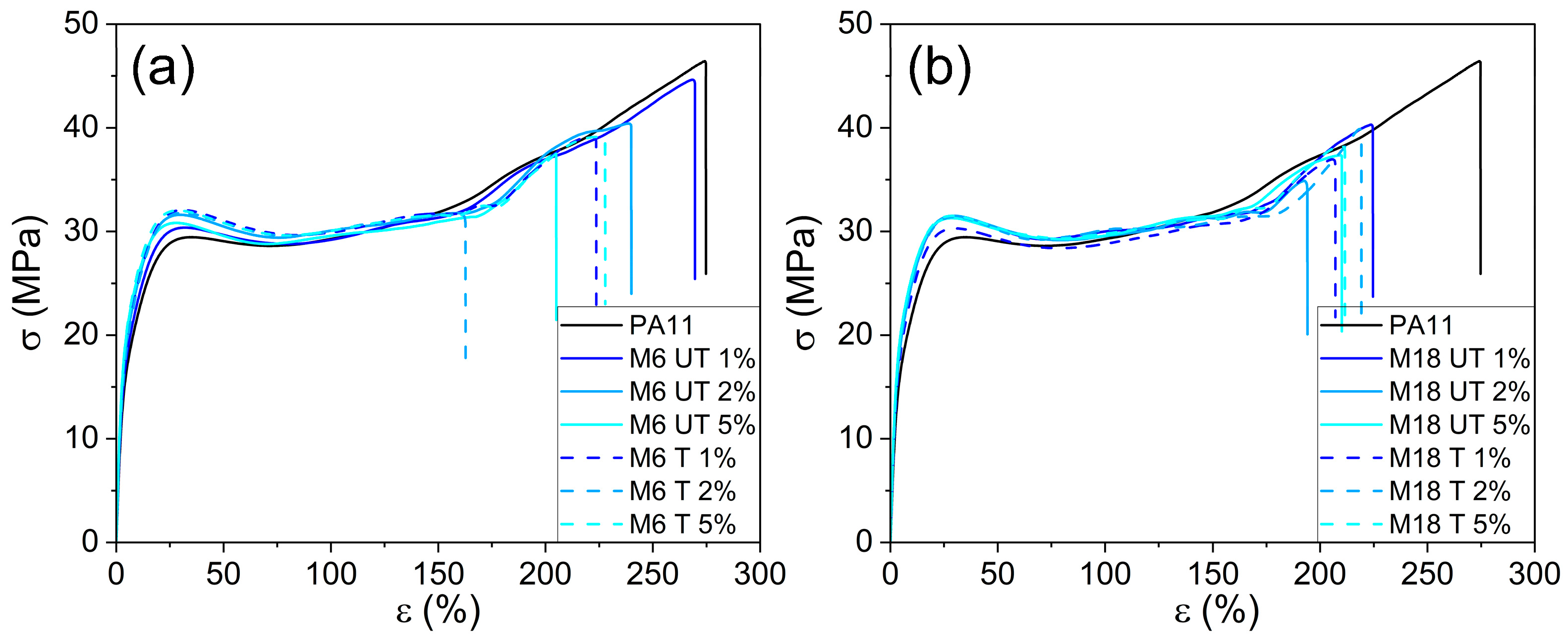
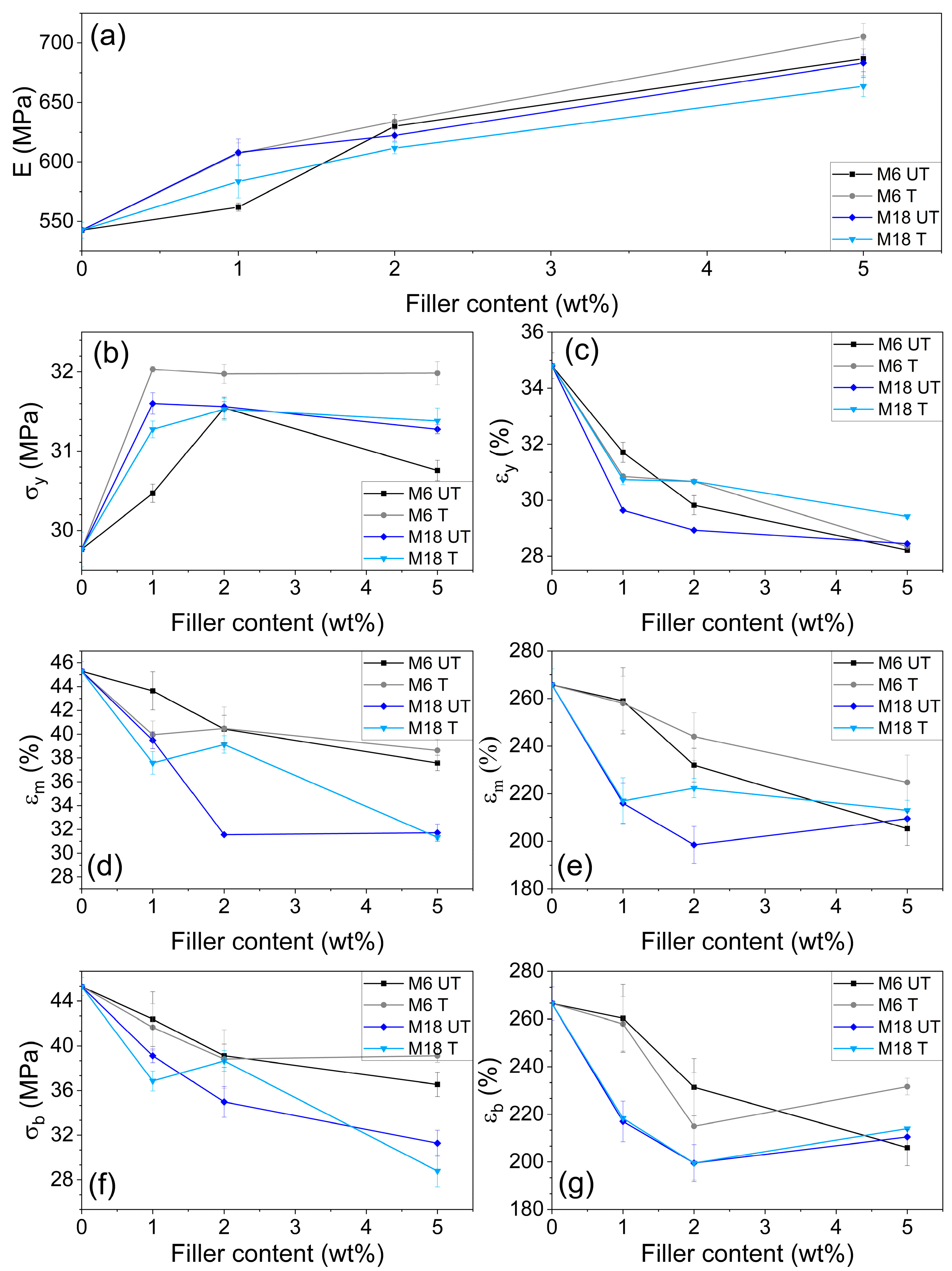
3.6. Mechanical Testing
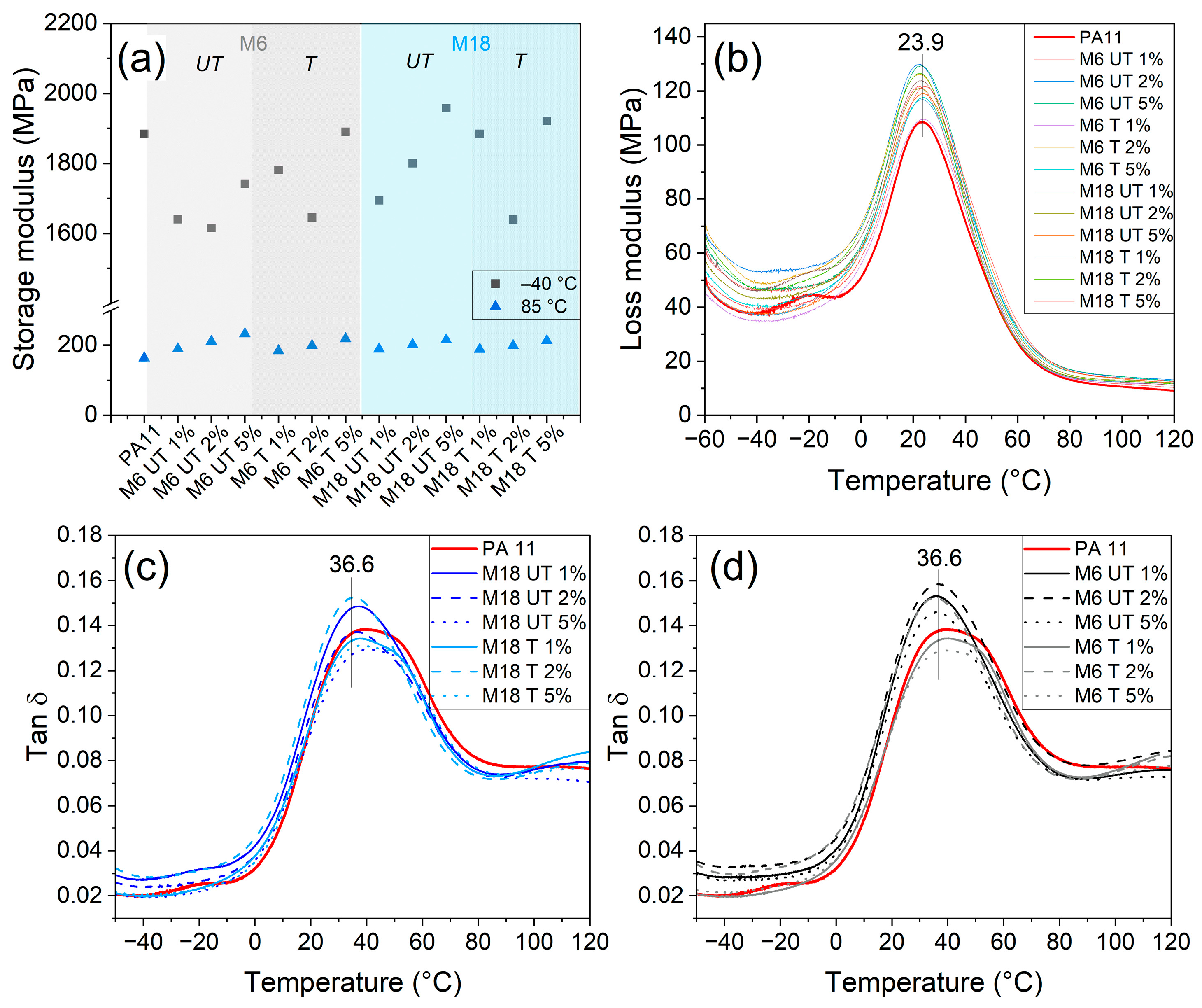
3.7. Thermomechanical Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSC | Differential Scanning Calorimetry |
| DMA | Dynamic Mechanical Analysis |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscopy |
| MMT | Montmorillonite |
| XRD | X-ray Diffraction |
| PA | Polyamide |
References
- Pervaiz, M.; Faruq, M.; Jawaid, M.; Sain, M. Polyamides: Developments and Applications Towards Next-Generation Engineered Plastics. Curr. Org. Synth. 2017, 14, 146–155. [Google Scholar] [CrossRef]
- Kondo, M.Y.; Montagna, L.; Morgado, G.; Castilho, A.; Batista, L.; Botelho, E.; Costa, M.; Passador, F.; Rezende, M.C.; Ribeiro, M. Recent Advances in the Use of Polyamide-Based Materials for the Automotive Industry. Polímeros 2022, 32, e2022023. [Google Scholar] [CrossRef]
- Sonawane, S.S.; Mishra, S.; Shimpi, N.G. Polyamide Nanocomposites: Investigation of Mechanical, Thermal and Morphological Characteristics. Polym.-Plast. Technol. Eng. 2009, 48, 1055–1061. [Google Scholar] [CrossRef]
- Cruz, P.; Shoemake, E.D.; Adam, P.; Leachman, J. Tensile Strengths of Polyamide Based 3D Printed Polymers in Liquid Nitrogen. IOP Conf. Ser. Mater. Sci. Eng. 2015, 102, 012020. [Google Scholar] [CrossRef]
- Gijsman, P.; Meijers, G.; Vitarelli, G. Comparison of the UV-Degradation Chemistry of Polypropylene, Polyethylene, Polyamide 6 and Polybutylene Terephthalate. Polym. Degrad. Stab. 1999, 65, 433–441. [Google Scholar] [CrossRef]
- Song, J.; Ehrenstein, G.W. The Influence of Water Absorption on the Properties of Polyamide. Kunstst. Ger. Plast. 1990, 80, 722–726. [Google Scholar]
- Jariyavidyanont, K.; Focke, W.; Androsch, R. Thermal Properties of Biobased Polyamide 11. In Thermal Properties of Bio-Based Polymers; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-39961-0. [Google Scholar]
- Bahrami, M.; Abenojar, J.; Martínez, M.A. Comparative Characterization of Hot-Pressed Polyamide 11 and 12: Mechanical, Thermal and Durability Properties. Polymers 2021, 13, 3553. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Granda, L.A.; Espinach, F.X.; Delgado-Aguilar, M.; Duran, J.; Mutjé, P. Stiffness of Bio-Based Polyamide 11 Reinforced with Softwood Stone Ground-Wood Fibres as an Alternative to Polypropylene-Glass Fibre Composites. Eur. Polym. J. 2016, 84, 481–489. [Google Scholar] [CrossRef]
- Surisetty, J.; Shahroodi, Z.; Lucyshyn, T.; Oreski, G. The Effect of Liquid Organic Hydrogen Carrier on the Physico-Mechanical and Rheological Properties of Injection-Molded High-Density Polyethylene and Polyketone. Results Eng. 2025, 25, 103918. [Google Scholar] [CrossRef]
- Gyawali, B.; Haghnazar, R.; Akula, P.; Alba, K.; Nasir, V. A Review on 3D Printing with Clay and Sawdust/Natural Fibers: Printability, Rheology, Properties, and Applications. Results Eng. 2024, 24, 103024. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Méndez, J.A.; Espinach, F.X.; Tarrés, Q.; Ardanuy, M.; Mutjé, P. Impact Strength and Water Uptake Behaviors of Fully Bio-Based PA11-SGW Composites. Polymers 2018, 10, 717. [Google Scholar] [CrossRef]
- Yu, M.; Qi, L.; Cheng, L.; Min, W.; Mei, Z.; Gao, R.; Sun, Z. The Effect of Cooling Rates on Thermal, Crystallization, Mechanical and Barrier Properties of Rotational Molding Polyamide 11 as the Liner Material for High-Capacity High-Pressure Vessels. Molecules 2023, 28, 2425. [Google Scholar] [CrossRef]
- Lao, S.; Wu, C.; Moon, T.; Koo, J.; Morgan, A.; Pilato, L.; Wissler, G. Flame-Retardant Polyamide 11 and 12 Nanocomposites: Thermal and Flammability Properties. J. Compos. Mater. 2009, 43, 1803–1818. [Google Scholar] [CrossRef]
- Mancic, L.; Osman, R.F.M.; Costa, A.M.L.M.; d’Almeida, J.R.M.; Marinkovic, B.A.; Rizzo, F.C. Thermal and Mechanical Properties of Polyamide 11 Based Composites Reinforced with Surface Modified Titanate Nanotubes. Mater. Des. 2015, 83, 459–467. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, L.; Yang, H.; Wang, M.; Liu, T.; Liang, T.; Zhang, J. Nanoindentation Studies on Nylon 11/Clay Nanocomposites. Polym. Test. 2006, 25, 492–497. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bastos, I.; Abud Kappel, M.A.; Nascimento, C.; Ferreira, L.; Silva, A. Micromechanical Property Study of Nylon 11 and Organoclay Systems for Offshore Flexible Pipe. Fibers Polym. 2021, 22, 3172–3182. [Google Scholar] [CrossRef]
- Kotek, J.; Baldrian, J.; Slouf, M. Deformation and Fracture Behavior of Polyamide Nanocomposites: The Effect of Clay Dispersion. J. Appl. Polym. Sci. 2008, 110, 3752–3757. [Google Scholar] [CrossRef]
- Chow, W.S. Mechanical, Morphological and Rheological Properties of Polyamide 6/Organo-Montmorillonite Nanocomposites. Express Polym. Lett. 2007, 1, 77–83. [Google Scholar] [CrossRef]
- Kelnar, I.; Rotrekl, J.; Kotek, J.; Kaprálková, L.; Hromádková, J. Effect of Montmorillonite on Structure and Properties of Nanocomposite with PA6/PS/Elastomer Matrix. Eur. Polym. J. 2009, 45, 2760–2766. [Google Scholar] [CrossRef]
- Kaci, M.; Dehouche, N.; Focke, W.; van der Merwe, E. A Degradation Study of Polyamide 11/Vermiculite Nanocomposites under Accelerated UV Test. Polym. Eng. Sci. 2019, 59, 2449–2457. [Google Scholar] [CrossRef]
- Stoclet, G.; Sclavons, M.; Devaux, J. Relations between Structure and Property of Polyamide 11 Nanocomposites Based on Raw Clays Elaborated by Water-assisted Extrusion. J. Appl. Polym. Sci. 2013, 127, 4809–4824. [Google Scholar] [CrossRef]
- Zhu, Q.; Chua, M.H.; Ong, P.J.; Cheng Lee, J.J.; Le Osmund Chin, K.; Wang, S.; Kai, D.; Ji, R.; Kong, J.; Dong, Z.; et al. Recent Advances in Nanotechnology-Based Functional Coatings for the Built Environment. Mater. Today Adv. 2022, 15, 100270. [Google Scholar] [CrossRef]
- Malik, T.M. Morphological and Mechanical Studies of Surface Treated Mica Reinforced High Density Polyethylene. Polym. Bull. 1991, 26, 709–714. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Sun, P.; Yu, F.; Li, B.; Dun, L. Study on the Performance and Mechanism of Modified Mica for Improving Polypropylene Composites. Int. J. Low-Carbon Technol. 2022, 17, 176–184. [Google Scholar] [CrossRef]
- Jung, W.-Y.; Cho, S.-W.; Jang, K.-S. Effect of Surface-Modified Mica in Hybrid Filler Systems on the Curing and Mechanical Behavior of Ethylene–Propylene–Diene Monomer (EPDM)/Butadiene Rubber (BR) Blend. Polymers 2025, 17, 2250. [Google Scholar] [CrossRef]
- Halim, K.A.A.; Farrell, J.B.; Kennedy, J.E. Preparation and Characterisation of Polyamide 11/Montmorillonite (MMT) Nanocomposites for Use in Angioplasty Balloon Applications. Mater. Chem. Phys. 2013, 143, 336–348. [Google Scholar] [CrossRef]
- Ismail, M.A.; Yousef, E.A.; Nasr, G.M. Surface Modification of Montmorillonite—MMT Nanofiller: How It Affects Both the Rigid Amorphous Fraction (RAF) and the Physical Properties of Polyamide 66. Polym. Bull. 2025, 82, 3553–3611. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Ahmad, Z.; Ishaq, M.; Sarwar, M.I. Aromatic–Aliphatic Polyamide/Montmorillonite Clay Nanocomposite Materials: Synthesis, Nanostructure and Properties. Mater. Sci. Eng. A 2009, 525, 30–36. [Google Scholar] [CrossRef]
- Li, X.; Dong, C.; Liu, Y.; Li, J.; Bin, G.; Zhou, C.; Han, W. Study on the Effect of Hydrogen Cycle Pressure Relief Time on the Hydrogen Permeability and Mechanical Properties of Polyamide Liner Materials for Type IV Hydrogen Storage Cylinders of HFCVs. Int. J. Hydrogen Energy 2024, 95, 993–1003. [Google Scholar] [CrossRef]
- Cheng, L.; Qi, L.; Tang, X.; Li, X.; Chen, L.; Min, W.; Mei, Z.; Gao, R.; Sun, M.; Xiao, J.; et al. Effects of Hydrogen Cycling on the Performance of 70 MPa High-Pressure Hydrogen Storage Tank Liners Formed by Different Processes. Int. J. Hydrogen Energy 2024, 83, 499–511. [Google Scholar] [CrossRef]
- Qi, L.; Gao, R.; Mei, Z.; Cheng, L.; Min, W.; Kang, D.; Yu, M.; Sun, Z. An Investigation on Enhancing the Bonding Properties of PA11-CFRP Interface in Type IV High Pressure Hydrogen Storage Vessel through Nanosecond Pulsed Laser Treatment and Failure Mechanism Research. Int. J. Hydrogen Energy 2024, 139, 895–907. [Google Scholar] [CrossRef]
- Qi, L.; Min, W.; Gao, R.; Li, Z.; Yu, M.; Sun, Z. Optimization of Interfacial Bonding Properties between Thermoplastic Liners and Carbon Fiber-reinforced Composites by Atmospheric-pressure Plasma and Failure Mechanism Study. Polym. Compos. 2023, 44, 2361–2378. [Google Scholar] [CrossRef]
- Feki, I.; Shirinbayan, M.; Nouira, S.; Bi, R.T.; Maeso, J.-B.; Thomas, C.; Fitoussi, J. Composites in High-Pressure Hydrogen Storage: A Review of Multiscale Characterization and Mechanical Behavior. Compos. Part C Open Access 2025, 16, 100555. [Google Scholar] [CrossRef]
- Balasooriya, W.; Clute, C.; Schrittesser, B.; Pinter, G. A Review on Applicability, Limitations, and Improvements of Polymeric Materials in High-Pressure Hydrogen Gas Atmospheres. Polym. Rev. 2022, 62, 175–209. [Google Scholar] [CrossRef]
- Kis, D.I.; Kókai, E. A Review on the Factors of Liner Collapse in Type IV Hydrogen Storage Vessels. Int. J. Hydrogen Energy. 2024, 50, 236–253. [Google Scholar] [CrossRef]
- Tamura, K.; Uno, H.; Yamada, H. Synthesis and Characterization of Exfoliated Natural Mica/Polymer Nanocomposites. Mater. Sci. Forum 2007, 539–543, 948–955. [Google Scholar] [CrossRef]
- McNally, T.; Raymond Murphy, W.; Lew, C.Y.; Turner, R.J.; Brennan, G.P. Polyamide-12 Layered Silicate Nanocomposites by Melt Blending. Polymer 2003, 44, 2761–2772. [Google Scholar] [CrossRef]
- Fuse, N.; Kozako, M.; Tanaka, T.; Ohki, Y. Effects of Mica Fillers on Dielectric Properties of Polyamide Nanocomposites. In Proceedings of the CEIDP ’05. 2005 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, 2005, Nashville, TN, USA, 16–19 October 2005; Volume 2005, p. 151, ISBN 0-7803-9257-4. [Google Scholar]
- Pramoda, K.P.; Liu, T.; Liu, Z.; He, C.; Sue, H.-J. Thermal Degradation Behavior of Polyamide 6/Clay Nanocomposites. Polym. Degrad. Stab. 2003, 81, 47–56. [Google Scholar] [CrossRef]
- Dintcheva, N.; Filippone, G.; Arrigo, R.; La mantia, F. paolo Low-Density Polyethylene/Polyamide/Clay Blend Nanocomposites: Effect of Morphology of Clay on Their Photooxidation Resistance. J. Nanomater. 2017, 2017, 3549475. [Google Scholar] [CrossRef]
- Varga, E.; Palásti, F.; Bata, A.; Kovács, P.I. Comparative Study of Plasma, Laser, and Flame Induced Activation of HDPE Liner Surfaces of Type 4 Hydrogen Vessels. J. Adhes. 2024, 101, 909–929. [Google Scholar] [CrossRef]
- ISO 527-2; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization: Geneva, Switzerland, 2025.
- ISO 179-1; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. International Organization for Standardization: Geneva, Switzerland, 2023.
- Oliver-Ortega, H.; Méndez, J.A.; Mutjé, P.; Tarrés, Q.; Espinach, F.X.; Ardanuy, M. Evaluation of Thermal and Thermomechanical Behaviour of Bio-Based Polyamide 11 Based Composites Reinforced with Lignocellulosic Fibres. Polymers 2017, 9, 522. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J. Effect of Treated Mica on Rheological, Cure, Mechanical, and Dielectric Properties of Ethylene Propylene Diene Monomer (EPDM)/Barium Titanate (BaTiO 3)/Mica: ARTICLE. J. Appl. Polym. Sci. 2017, 134, 44833. [Google Scholar] [CrossRef]
- Tang, C.; Yan, H.; Li, M.; Lv, Q. A Novel Phosphorus-Containing Polysiloxane for Fabricating High Performance Electronic Material with Excellent Dielectric and Thermal Properties. J. Mater. Sci. Mater. Electron. 2018, 29, 195–204. [Google Scholar] [CrossRef]
- Beran, A. Infrared Spectroscopy of Micas. Rev. Mineral. Geochem. 2002, 46, 351–369. [Google Scholar] [CrossRef]
- Kawsihan, A.; Dissanayake, S.; Chandrakumara, G.T.D.; Mantilaka, P.; Rajapakse, R.; Pitawala, H.M.T.G.; de Silva, P. Akaganeite Nanorices Deposited Muscovite Mica Surfaces as Sunlight Active Green Photocatalyst. R. Soc. Open Sci. 2019, 6, 182212. [Google Scholar] [CrossRef]
- Gauvin, F.; Robert, M. Durability Study of Vinylester/Silicate Nanocomposites for Civil Engineering Applications. Polym. Degrad. Stab. 2015, 121, 359–368. [Google Scholar] [CrossRef]
- Lee, W.P.C.; Wu, S.; Anariba, F.; Wu, P. Breaking New Ground in Mica Exfoliation: Harnessing Biaxial Straining Principles through H2 and N2 Intercalation for Enhanced Layer Separation. Mater. Today Adv. 2023, 19, 100406. [Google Scholar] [CrossRef]
- Franceschi, G.; Brandstetter, S.; Balajka, J.; Sokolović, I.; Pavelec, J.; Setvin, M.; Schmid, M.; Diebold, U. Interaction of Surface Cations of Cleaved Mica with Water in Vapor and Liquid Forms. Faraday Discuss. 2023, 249, 84–97. [Google Scholar] [CrossRef]
- Cruz, B.; Tienne, L.; Gondim, F.; Candido, L.; Marques, M.; Chaves, E. Influence of the Addition of Multi-walled Carbon Nanotubes on the Thermal and Mechanical Properties of Polyamide-11 before and after Aging Tests. J. Appl. Polym. Sci. 2020, 138, 50071. [Google Scholar] [CrossRef]
- Panaitescu, D.; Gabor, R.; Frone, A.; Vasile, E. Influence of Thermal Treatment on Mechanical and Morphological Characteristics of Polyamide 11/Cellulose Nanofiber Nanocomposites. J. Nanomater. 2015, 2015, 136204. [Google Scholar] [CrossRef]
- Yoon, K.; Polk, M.; Min, B.; Schiraldi, D. Structure and Property Study of Nylon-6/Clay Nanocomposite Fiber. Polym. Int. 2004, 53, 2072–2078. [Google Scholar] [CrossRef]
- Varga, E.; Tóth, L.; Ádám, B.; Tajti, F.; Hansághy, P. Novel Insights into the Morphological Effects of Micron-Scale Inorganic Fillers on Polyethylene Composites. Compos. Commun. 2025, 56, 102394. [Google Scholar] [CrossRef]
- Wu, T.-M.; Wu, J.-Y. Structural Analysis of Polyamide/Clay Nanocomposites. J. Macromol. Sci. Part B 2002, 41, 17–31. [Google Scholar] [CrossRef]
- Venkataramani, S.; Lee, J.H.; Park, M.G.; Kim, S.C. Structure and Properties of Polyamide-6 & 6/66 Clay Nanocomposites. J. Macromol. Sci. Part A 2008, 46, 65–73. [Google Scholar] [CrossRef]
- Uno, H.; Tamura, K.; Yamada, H.; Umeyama, K.; Hatta, T.; Moriyoshi, Y. Preparation and Mechanical Properties of Exfoliated Mica-Polyamide 6 Nanocomposites Using Sericite Mica. Appl. Clay Sci. 2009, 46, 81–87. [Google Scholar] [CrossRef]
- Dobrosielska, M.; Dobrucka, R.; Brząkalski, D.; Kozera, P.; Martyła, A.; Gabriel, E.; Kurzydłowski, K.J.; Przekop, R.E. Polyamide 11 Composites Reinforced with Diatomite Biofiller—Mechanical, Rheological and Crystallization Properties. Polymers 2023, 15, 1563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, M.; Fu, Q. Crystal Morphology and Crystallization Kinetics of Polyamide-11/Clay Nanocomposites. Polym. Int. 2004, 53, 1941–1949. [Google Scholar] [CrossRef]
- Verkinderen, O.; Baeten, D.; Van Puyvelde, P.; Goderis, B. The Crystallization of PA11, PA12, and Their Random Copolymers at Increasing Supercooling: From Eutectic Segregation to Mesomorphic Solid Solutions. Polym. Cryst. 2021, 4, e10216. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Focke, W.; Androsch, R. Crystallization Kinetics of Polyamide 11 in the Presence of Sepiolite and Montmorillonite Nanofillers. Colloid Polym. Sci. 2016, 294, 1143–1151. [Google Scholar] [CrossRef]
- Kolesov, I.; Androsch, R.; Mileva, D.; Lebek, W.; Benhamida, A.; Kaci, M.; Focke, W. Crystallization of a Polyamide 11/Organo-Modified Montmorillonite Nanocomposite at Rapid Cooling. Colloid Polym. Sci. 2013, 291, 2541–2549. [Google Scholar] [CrossRef]
- Sayah, N.; Smith, D.E. Effect of Process Parameters on Void Distribution, Volume Fraction, and Sphericity within the Bead Microstructure of Large-Area Additive Manufacturing Polymer Composites. Polymers 2022, 14, 5107. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Liu, T. Effect of Moisture on the Dynamic Mechanical Relaxation of Polyamide-6/Clay Nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1823–1830. [Google Scholar] [CrossRef]
- Majka, T.; Majka, M. The Influence of Maintenance Liquids\’ Absorbence Used in the Automotive Industry on the Mechanical Properties of Polyamide-6/Montmorillonite Nanocomposites. In Proceedings of the 16th International Electronic Conference on Synthetic Organic Chemistry session Polymer and Supramolecular Chemistry, Virtual Conference, 1–30 November 2012; MDPI: Basel, Switzerland, 2012. [Google Scholar] [CrossRef]
- Vlasveld, D.P.N.; Groenewold, J.; Bersee, H.E.N.; Picken, S.J. Moisture Absorption in Polyamide-6 Silicate Nanocomposites and Its Influence on the Mechanical Properties. Polymer 2005, 46, 12567–12576. [Google Scholar] [CrossRef]
- Taha, Z.T.; Bata, A.; Molnár, B.; Ronkay, F. Impact of Montmorillonite Reinforcement on the Physical Recyclability of Biobased and Petroleum-Based Polyesters. Heliyon 2025, 11, e43022. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Mai, Y.-W. Transcrystalline Regions in the Vicinity of Nanofillers in Polyamide-6. Macromolecules 2007, 40, 123–130. [Google Scholar] [CrossRef]
- Anoukou, K.; Zaïri, F.; Naït-Abdelaziz, M.; Zaoui, A.; Qu, Z.; Gloaguen, J.M.; Lefebvre, J.M. A Micromechanical Model Taking into Account the Contribution of α- and γ-Crystalline Phases in the Stiffening of Polyamide 6-Clay Nanocomposites: A Closed-Formulation Including the Crystal Symmetry. Compos. Part B Eng. 2014, 64, 84–96. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, Y.; Ke, W.; Zhang, Q.; Du, R.; Fu, Q. Effects of Matrix Molecular Weight on Structure and Reinforcement of High Density Polyethylene/Mica Composites. Chin. J. Polym. Sci. 2011, 29, 377–389. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Structure and Properties of Nanocomposites Based on Nylon-11 and -12 Compared with Those Based on Nylon-6. Macromolecules 2004, 37, 7698–7709. [Google Scholar] [CrossRef]
- Ünal, H.; Mimaroglu, A. Mechanical and Morphological Properties of Mica and Short Glass Fiber Reinforced Polyamide 6 Composites. Int. J. Polym. Mater. 2012, 61, 834–846. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.H.; Ellis, T.S.; Shi, J. Crystal Structures and Their Effects on the Properties of Polyamide 12/Clay and Polyamide 6–Polyamide 66/Clay Nanocomposites. J. Appl. Polym. Sci. 2006, 100, 4782–4794. [Google Scholar] [CrossRef]
- Masenelli-Varlot, K.; Reynaud, E.; Vigier, G.; Varlet, J. Mechanical Properties of Clay-reinforced Polyamide. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 272–283. [Google Scholar] [CrossRef]
- Mahanwar, P.A.; Bose, S.; Tirumalai, A.V. The Influence of Interfacial Adhesion on the Predicted Young’s Modulus of Mica-Reinforced Nylon-6. Polym.-Plast. Technol. Eng. 2006, 45, 597–600. [Google Scholar] [CrossRef]
- Ono, H. Micromechanical Analysis for Effective Elastic Moduli and Thermal Expansion Coefficient of Composite Materials Containing Ellipsoidal Fillers Oriented Randomly. Compos. Part C Open Access 2024, 14, 100482. [Google Scholar] [CrossRef]
- Wang, N.; Pan, X.; Wang, P.; Wang, Y.; He, H.; Zeng, Y.-J.; Zhang, L.; Li, Y.; Wang, F.; Lu, B.; et al. Is All Epitaxy on Mica van Der Waals Epitaxy? Mater. Today Nano 2022, 20, 100255. [Google Scholar] [CrossRef]
- Liu, T.; Ping Lim, K.; Chauhari Tjiu, W.; Pramoda, K.P.; Chen, Z.-K. Preparation and Characterization of Nylon 11/Organoclay Nanocomposites. Polymer 2003, 44, 3529–3535. [Google Scholar] [CrossRef]
- Risite, H.; El Mabrouk, K.; Bousmina, M.; Fassi-Fehri, O. Role of Polyamide 11 Interaction with Clay and Modifier on Thermal, Rheological and Mechanical Properties in Polymer Clay Nanocomposites. J. Nanosci. Nanotechnol. 2016, 16, 7584–7593. [Google Scholar] [CrossRef]
- Bureau, M.; Denault, J.; Cole, K.; Enright, G. The Role of Crystallinity and Reinforcement in the Mechanical Behavior of Polyamide-6/Clay Nanocomposites. Polym. Eng. Sci. 2002, 42, 1897–1906. [Google Scholar] [CrossRef]
- Kis, D.I.; Bata, A.; Takács, J.; Kókai, E. Mechanical Properties of Clay-Reinforced Polyamide 6 Nanocomposite Liner Materials of Type IV Hydrogen Storage Vessels. Nanomaterials 2024, 14, 1385. [Google Scholar] [CrossRef]
- Bata, A.; Gerse, P.; Slezák, E.; Ronkay, F. Time- and Temperature-Dependent Mechanical and Rheological Behaviours of Injection Moulded Biodegradable Organoclay Nanocomposites. Adv. Ind. Eng. Polym. Res. 2023, 7, 482–496. [Google Scholar] [CrossRef]
- Masenelli-Varlot, K.; Vigier, G.; Vermogen, A.; Gauthier, C.; Cavaillé, J.-Y. Quantitative Structural Characterization of Polymer–Clay Nanocomposites and Discussion of an “Ideal” Microstructure, Leading to the Highest Mechanical Reinforcement. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1243–1251. [Google Scholar] [CrossRef]
- Vlasveld, D.P.N.; Jong, M.; Bersee, H.; Gotsis, A.D.; Picken, S.J. The Relation between Rheological and Mechanical Properties of PA6 Nano- and Micro-Composites. Polymer 2005, 46, 10279–10289. [Google Scholar] [CrossRef]
- da Cruz, B.d.S.M.; Tienne, L.G.P.; Gondim, F.F.; da Silva Candido, L.; Chaves, E.G.; Marques, M.d.F.V.; da Luz, F.S.; Monteiro, S.N. Graphene Nanoplatelets Reinforced Polyamide-11 Nanocomposites Thermal Stability and Aging for Application in Flexible Pipelines. J. Mater. Res. Technol. 2022, 18, 1842–1854. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, E.; Palásti, F.; Bata, A.; Kis, D.I.; Tajti, F. Polyamide 11 Composites with Surface-Activated Intact Mica Structures for Advanced Applications. Polymers 2025, 17, 2861. https://doi.org/10.3390/polym17212861
Varga E, Palásti F, Bata A, Kis DI, Tajti F. Polyamide 11 Composites with Surface-Activated Intact Mica Structures for Advanced Applications. Polymers. 2025; 17(21):2861. https://doi.org/10.3390/polym17212861
Chicago/Turabian StyleVarga, Erika, Ferenc Palásti, Attila Bata, Dávid István Kis, and Ferenc Tajti. 2025. "Polyamide 11 Composites with Surface-Activated Intact Mica Structures for Advanced Applications" Polymers 17, no. 21: 2861. https://doi.org/10.3390/polym17212861
APA StyleVarga, E., Palásti, F., Bata, A., Kis, D. I., & Tajti, F. (2025). Polyamide 11 Composites with Surface-Activated Intact Mica Structures for Advanced Applications. Polymers, 17(21), 2861. https://doi.org/10.3390/polym17212861






