Review of Plasma-Synthesized/Modified Polymer and Metal Nanoparticles for Biomedical Applications Using Cold Atmospheric Pressure Plasma
Abstract
1. Introduction
2. Plasma Process
2.1. Plasma Synthesis of Polymer Films for Biomedical Applications
2.2. Plasma Surface Treatment of Polymer Films for Biomedical Applications
3. Plasma Synthesis and Surface Treatment of Metal NPs for Biomedical Applications
4. Recent Research Trends of CAP-Based APPJ Technique
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| Al | Aluminum |
| APP | Atmospheric pressure plasma |
| AP | Atmospheric pressure |
| AgNPs/CS | Silver nanoparticles/chitosan |
| APM | AP microplasma |
| Ar | Argon |
| AP–PP–lim | AP plasma–polymerized D–limonene |
| APPJ | Atmospheric pressure plasma jet |
| AFM | Atomic force microscope |
| AAc | Acrylic acid |
| AAm | Allyl amine |
| AAOH | Allyl alcohol |
| AAP | Acetic acid plasma |
| AC | Alternating current |
| APM | Atmospheric pressure microplasma |
| APTES | 3–aminopropyl)triethoxysilane |
| APTD | Atmospheric pressure Townsend–like discharge |
| APS | Ammonium persulfate |
| AuQDs | Gold quantum dots |
| BM | Bone marrow |
| BMSCs | Bone marrow–derived stem cells |
| CAP | Cold atmospheric pressure plasma |
| CCHM | Coherence–controlled holographic microscopy |
| CFU | Colony–forming units |
| CPA | Cyclopropylamine |
| Cu | Copper |
| COC | Cyclic olefin copolymer |
| CS | Chitosan |
| DI | Deionized water |
| DBD | Dielectric barrier discharge |
| DCSBD | Diffuse coplanar surface barrier discharge |
| DS | Downstream |
| DCC | Dicyclohexylcarbodiimide |
| dECM | Decellularized extracellular matrix |
| dECMf | Decellularized extracellular matrix film |
| E. coli | Escherichia coli |
| ES | Embryonic stem |
| EGDMA | Ethylene glycol dimethacrylate |
| FCC | Face-centered cubic |
| FE–SEM | Field emission scanning electron microscopy |
| FT–IR | Fourier–transform infrared |
| FMA | Furfuryl methacrylate |
| GND | Ground |
| GO | Graphene oxide |
| HADSCs | Human adipose–derived stem cells |
| HDFs | Human dermal fibroblasts |
| He | Helium |
| HFF | Human foreskin fibroblast |
| H[AuCl4]·3H2O | Gold(III) chloroauric acid trihydrate |
| HEMA | 2–hydroxyethyl methacrylate |
| H2O2 | Hydrogen peroxide |
| HMDSO | Hexamethyldisiloxane |
| HAp | Hydroxyapatite |
| HV | High voltage |
| HPP | High–power plasma |
| OH | Hydroxyl radical |
| IPP | Ion–assisted plasma polymerization |
| LDPE | Low density polyethylene |
| LP | Low pressure |
| LPP | Low–power plasma |
| lPM | Liters per minute |
| LECs | Lens epithelial cells |
| LA–APPiP | Liquid–assisted atmospheric pressure plasma induced polymerization |
| M–cresol | 3–methylphenol |
| MgO | Magnesium oxide |
| MP | Medium pressure |
| MA | Maleic anhydride |
| MSCs | Mesenchymal stem cells |
| MSCs | Mesenchymal stromal cells |
| NHDF | Normal human dermal fibroblast |
| NPs | Nanoparticles |
| NTCP | Non–thermal cold plasma |
| Na2S | Sodium sulfide |
| N2 | Nitrogen |
| NO | Nitric oxide |
| NO2 | Nitrogen dioxide |
| NaOH | Sodium hydroxide |
| O2 | Oxygen |
| OES | Optical emission spectroscopy |
| OD | 1,7, octadiene |
| PBS | Phosphate buffered saline |
| PRP | Platelet-rich plasma |
| ppHEPTYL–HE–PS | Plasma polymerized–heptylamine |
| PC | Polycarbonate |
| PCL | Poly–ε caprolactone |
| P(3HB) | Poly(3–hydroxybutyrate) |
| PDA | Polydopamine |
| PET | Polyethylene terephthalate |
| PE–CVD | Plasma enhanced chemical vapor deposition |
| PI | Propidium iodide |
| PLA | Polylactic acid |
| PP | Plasma polymer |
| PEO | Polyethylene oxide |
| POx | Poly(2–oxazolines) |
| PMEOx | Poly(2–methyl–2–oxazoline)–stat–(2–(3–butenyl)–2–oxazoline) |
| PPOx | Polyoxazoline |
| PEGMA | Poly(ethylene glycol) methacrylate |
| PLGA | Poly(DL–lactide–co–glycolide) |
| PR | Photoresist |
| PS | Polystyrene |
| Pt | Platinum |
| PVA | Polyvinyl alcohol |
| PTFE | Polytetrafluoroethylene |
| RF | Radio frequency |
| RT | Room temperature |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| RONS | Reactive oxygen and nitrogen species |
| Ra | Average roughness |
| Rq | Root mean square roughness |
| Ag | Silver |
| S. aureus | Staphylococcus aureus |
| SPR | Surface plasmon resonance |
| SEM | Scanning electron microscope |
| SLM | Standard liter per minute |
| ToF–SIMS | Time–of–flight secondary ion mass spectrometry |
| TEM | Transmission electron microscope |
| TCPS | Tissue culture PS |
| Ti | Titanium |
| UT–PS | Untreated PS |
| Vp | Peak voltage |
| Vp–p | Peak–to–peak voltage |
| WCA | Water contact angle |
| XPS | X–ray photoelectron spectroscopy |
| XRD | X–ray diffraction |
| ZnS NPs | Zinc sulfide NPs |
| ZnS | Zinc sulfide |
| Zn(NO3)2 | Zinc nitrate |
| ZnCl2 | Zinc chloride |
| ZnSO4 | Zinc sulfate |
| ZOI | Zone of inhibition |
| 1D | One–dimensional |
| 2D | Two–dimensional |
References
- Liu, P.; Wang, G.; Ruan, Q.; Tang, K.; Chu, P.K. Plasma–activated interfaces for biomedical engineering. Bioact. Mater. 2021, 6, 2134–2143. [Google Scholar] [CrossRef]
- Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; De Abreu, G.M.A.; Lima, G.D.M.G.; Figueira, L.W.; Koga-Ito, C.Y. Applications of cold atmospheric pressure plasma in dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Xu, M.; Chen, H.; Lu, X.; Ostrikov, K. Cold atmospheric pressure plasmas in dermatology: Sources, reactive agents, and therapeutic effects. Plasma Processes Polym. 2020, 17, e1900218. [Google Scholar] [CrossRef]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G. Plasma–activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B. Molecular mechanisms of the efficacy of cold atmospheric pressure plasma (CAP) in cancer treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef]
- Naujokat, H.; Harder, S.; Schulz, L.Y.; Wiltfang, J.; Flörke, C.; Açil, Y. Surface conditioning with cold argon plasma and its effect on the osseointegration of dental implants in miniature pigs. J. Cranio–Maxillofac. Surg. 2019, 47, 484–490. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lim, D.J.; Lee, M.Y.; Lee, W.J.; Chang, S.E.; Won, C.H. Prospective, Comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci. Rep. 2021, 11, 14461. [Google Scholar] [CrossRef]
- Yan, D.; Malyavko, A.; Wang, Q.; Ostrikov, K.; Sherman, J.H.; Keidar, M. Multi–modal biological destruction by cold atmospheric plasma: Capability and mechanism. Biomedicines 2021, 9, 1259. [Google Scholar] [CrossRef]
- Dai, X.; Bazaka, K.; Thompson, E.; Ostrikov, K. Cold atmospheric plasma: A promising controller of cancer cell states. Cancers 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Cold atmospheric plasma as an interface biotechnology for enhancing surgical implants. Crit. Rev. Biotechnol. 2021, 41, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Controlling stem cell fate using cold atmospheric plasma. Stem Cell Res. Ther. 2020, 11, 368. [Google Scholar] [CrossRef]
- Sainz-García, E.; López, M.; Múgica-Vidal, R.; Rojo-Bezares, B.; Lozano, C.; González-Marcos, A.; Toledano, P.; Muro-Fraguas, I.; Sainz-García, A.; Sáenz, Y.; et al. Promotion of biofilm production via atmospheric–pressure plasma–polymerization for biomedical applications. Appl. Surf. Sci. 2022, 581, 152350. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Lee, H.J.; Kim, G.C.; Kim, S.S.; Han, S.; Song, K. Non–thermal atmospheric pressure plasma is an excellent tool to activate proliferation in various mesoderm–derived human adult stem cells. Free Radic. Biol. Med. 2019, 134, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Gamaleev, V.; Shimizu, N.; Hori, M. Nanosecond–scale impulse generator for biomedical applications of atmospheric–pressure plasma technology. Rev. Sci. Instrum. 2022, 93, 053503. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B. Cold atmospheric pressure plasma in wound healing and cancer treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Rutkowski, R.; Daeschlein, G.; von Woedtke, T.; Smeets, R.; Gosau, M.; Metelmann, H.R. Long–term risk assessment for medical application of cold atmospheric pressure plasma. Diagnostics 2020, 10, 210. [Google Scholar] [CrossRef]
- Eggers, B.; Marciniak, J.; Memmert, S.; Kramer, F.J.; Deschner, J.; Nokhbehsaim, M. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast–like cells in vitro. Odontology 2020, 108, 607–616. [Google Scholar] [CrossRef]
- Xu, Z.; Lan, Y.; Ma, J.; Shen, J.; Han, W.; Hu, S.; Ye, C.; Xi, W.; Zhang, Y.; Yang, C. Applications of atmospheric pressure plasma in microbial inactivation and cancer therapy: A brief review. Plasma Sci. Technol. 2020, 22, 103001. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Emmert, S.; Metelmann, H.R.; Rupf, S.; Weltmann, K.D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Phys. Plasmas 2020, 27, 070601. [Google Scholar] [CrossRef]
- Mazánková, V.; Sťahel, P.; Matoušková, P.; Brablec, A.; Čech, J.; Prokeš, L.; Buršíková, V.; Stupavská, M.; Lehocký, M.; Ozaltin, K.; et al. Atmospheric pressure plasma polymerized 2–ethyl–2–oxazoline based thin films for biomedical purposes. Polymers 2020, 12, 2679. [Google Scholar] [CrossRef]
- Ji, H.W.; Kim, H.; Kim, H.W.; Yun, S.H.; Park, J.E.; Choi, E.H.; Kim, S.J. Genome–wide comparison of the target genes of the reactive oxygen species and non–reactive oxygen species constituents of cold atmospheric plasma in cancer cells. Cancers 2020, 12, 2640. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Privat-Maldonado, A.; Smits, E.; Bogaerts, A. Cold atmospheric plasma increases temozolomide sensitivity of three–dimensional glioblastoma spheroids via oxidative stress–mediated DNA damage. Cancers 2021, 13, 1780. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, A.; Toyoda, H.; Orita, K.; Hirakawa, Y.; Aoki, K.; Oh, J.S.; Shirafuji, T.; Nakamura, H. In vivo study on the healing of bone defect treated with non–thermal atmospheric pressure gas discharge plasma. PLoS ONE 2021, 16, e0255861. [Google Scholar] [CrossRef]
- Turicek, J.; Ratts, N.; Kaltchev, M.; Masoud, N. Surface treatment of ultra–high molecular weight polyethylene (UHMWPE) by cold atmospheric plasma (CAP) for biocompatibility enhancement. Appl. Sci. 2021, 11, 1703. [Google Scholar] [CrossRef]
- Saito, K.; Toyoda, H.; Okada, M.; Oh, J.S.; Nakazawa, K.; Ban, Y.; Orita, K.; Shimatani, A.; Yao, H.; Shirafuji, T. Fracture healing on non–union fracture model promoted by non-thermal atmospheric–pressure plasma. PLoS ONE 2024, 19, e0298086. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Wang, Y.; Zhang, S.; Shui, R.; Chen, J. Plasma dermatology: Skin therapy using cold atmospheric plasma. Front. Oncol. 2022, 12, 918484. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-Q.; Li, Y.-H.; Zhou, T.; Lu, C.; Li, D.; Xiong, Z.-L.; Deng, Y.-H. Effect of an atmospheric plasma jet on the differentiation of melanoblast progenitor. Curr. Med. Sci. 2022, 42, 629–634. [Google Scholar] [CrossRef]
- Bekeschus, S.; Kramer, A.; Schmidt, A. Gas plasma–augmented wound healing in animal models and veterinary medicine. Molecules 2021, 26, 5682. [Google Scholar] [CrossRef]
- Mirpour, S.; Fathollah, S.; Mansouri, P.; Larijani, B.; Ghoranneviss, M.; Mohajeri Tehrani, M.; Amini, M.R. Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: A randomized clinical trial. Sci. Rep. 2020, 10, 10440. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, J.; Li, X.; Liu, D.; Lu, X. On the dose of plasma medicine: Equivalent total oxidation potential (ETOP). Phys. Plasmas 2020, 27, 063514. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.-X.; He, T.; Dong, W.-W.; Yuan, Y.; Zhao, X.; Chen, X.-Y.; Zhang, N.; Zou, Z.-F.; Zhang, Y.; et al. A novel method for estimating the dosage of cold atmospheric plasmas in plasma medical applications. Appl. Sci. 2021, 11, 11135. [Google Scholar] [CrossRef]
- Wu, E.; Song, K.; Pei, X.; Nie, L.; Mesbah, A.; Lu, X. Real–time control of plasma dose delivery in plasma medicine using deep reinforcement learning. Appl. Phys. Lett. 2025, 127, 053701. [Google Scholar] [CrossRef]
- Sun, D.; Turner, J.; Jiang, N.; Zhu, S.; Zhang, L.; Falzon, B.G.; McCoy, C.P.; Maguire, P.; Mariotti, D.; Sun, D. Atmospheric pressure microplasma for antibacterial silver nanoparticle/CS nanocomposites with tailored properties. Compos. Sci. Technol. 2020, 186, 107911. [Google Scholar] [CrossRef]
- Nolan, H.; Sun, D.; Falzon, B.G.; Chakrabarti, S.; Padmanaba, D.B.; Maguire, P.; Mariotti, D.; Yu, T.; Jones, D.; Andrews, G.; et al. Metal nanoparticle–hydrogel nanocomposites for biomedical applications–An atmospheric pressure plasma synthesis approach. Plasma Processes Polym. 2018, 15, e1800112. [Google Scholar] [CrossRef]
- Masood, A.; Ahmed, N.; Mohd Razip Wee, M.F.; Patra, A.; Mahmoudi, E.; Siow, K.S. Atmospheric pressure plasma polymerisation of D–limonene and its antimicrobial activity. Polymers 2023, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Bryant, C.; Krasowska, M.; Cowin, A.J.; Whittle, J.D.; MacNeil, S.; Short, R.D. Haptotatic plasma polymerized surfaces for rapid tissue regeneration and wound healing. ACS Appl. Mater. Interfaces 2016, 8, 32675–32687. [Google Scholar] [CrossRef]
- Sťahel, P.; Mazánková, V.; Tomečková, K.; Matoušková, P.; Brablec, A.; Prokeš, L.; Jurmanová, J.; Buršíková, V.; Přibyl, R.; Lehocký, M.; et al. Atmospheric pressure plasma polymerized oxazoline–based thin films—antibacterial properties and cytocompatibility performance. Polymers 2019, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Kaushik, N.; Bhartiya, P.; Gurmessa, S.K.; Kim, H.-J.; Nguyen, L.Q.; Kaushik, N.K.; Choi, E.H. Plasma–synthesized mussel–inspired gold nanoparticles promote autophagy–dependent damage–associated molecular pattern release to potentiate immunogenic cancer cell death. J. Ind. Eng. Chem. 2021, 100, 99–111. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kaushik, N.; Lamichhane, P.; Mumtaz, S.; Paneru, R.; Bhartiya, P.; Kwon, J.S.; Mishra, Y.K.; Nguyen, L.Q.; Kaushik, N.K.; et al. In situ plasma–assisted synthesis of polydopamine functionalized gold nanoparticles for biomedical applications. Green Chem. 2020, 22, 6588–6599. [Google Scholar] [CrossRef]
- Doherty, K.G.; Oh, J.-S.; Unsworth, P.; Sheridan, C.M.; Weightman, P.; Bradley, J.W.; Williams, R.L. Plasma polymerization using helium atmospheric–pressure plasma jet with heptylamine monomer. Plasma Processes Polym. 2019, 16, e1800185. [Google Scholar] [CrossRef]
- Liao, S.-C.; Chen, K.-S.; Chien, J.-L.; Chen, S.-C.; Lin, W.-L. Acetic–acid plasma–polymerization on polymeric substrates for biomedical application. Nanomaterials 2019, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Teske, M.; Wulf, K.; Fink, J.; Brietzke, A.; Eickner, T.; Arbeiter, D.; Senz, V.; Grabow, N.; Illner, S. Controlled biodegra dation of metallic biomaterials by plasma polymer coatings using hexamethyldisiloxane and allylamine monomers. Curr. Dir. Biomed. Eng. 2019, 5, 315–318. [Google Scholar] [CrossRef]
- Štrbková, L.; Manakhov, A.; Zajíčková, L.; Stoica, A.; Veselý, P.; Chmelík, R. The adhesion of normal human dermal fibroblasts to the cyclopropylamine plasma polymers studied by holographic microscopy. Surf. Coat. Technol. 2016, 295, 70–77. [Google Scholar] [CrossRef]
- Gheorghiu, A.A.; Muguet, I.; Chakiris, J.; Chan, K.M.; Priest, C.; Macgregor, M. Plasma deposited polyoxazoline films integration into spiral microfluidics for the targeted capture of size selected cells. Front. Chem. 2021, 9, 690781. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri, S.; Abdijahed, S.; Farivar, S.; Hosseini, S.I.; Rezaei, F.; Ardeshirylajimi, A.; Shokri, B. Study on physio–chemical properties of plasma polymerization in C2H2/N2 plasma and their impact on COL X. Sci. Rep. 2017, 7, 9149. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Zhai, C.; Hung, J.; Shineh, G.; Stewart, C.A.C.; Fadzil, A.A.; Ionescu, M.; Gan, Y.; Wise, S.G.; Akhavan, B. Mechanically robust nitrogen–rich plasma polymers: Biofunctional interfaces for surface engineering of biomedical implants. Mater. Today Adv. 2021, 12, 100188. [Google Scholar] [CrossRef]
- Van Guyse, J.F.R.; Cools, P.; Egghe, T.; Asadian, M.; Vergaelen, M.; Rigole, P.; Yan, W.; Benetti, E.M.; Jerca, V.-V.; Declercq, H.; et al. Influence of the aliphatic side chain on the near atmospheric pressure plasma polymerization of 2–Alkyl–2-oxazolines for biomedical applications. ACS Appl. Mater. Interfaces 2019, 11, 31356–31366. [Google Scholar] [CrossRef]
- Hartl, H.; Li, W.; Michl, T.D.; Anangi, A.; Speight, R.; Vasilev, K.; Ostrikov, K.K.; MacLeod, J. Antimicrobial adhesive films by plasma–enabled polymerization of m–cresol. Sci. Rep. 2022, 12, 7560. [Google Scholar] [CrossRef]
- Sardella, E.; Gristina, R.; Fanelli, F.; Veronico, V.; Ponte, G.D.; Kroth, J.; Fracassi, F.; Favia, P. How to confer a permanent bio-repelling and bio–adhesive character to biomedical naterials through cold plasmas. Appl. Sci. 2020, 10, 9101. [Google Scholar] [CrossRef]
- Sardella, E.; Salama, R.A.; Waly, G.H.; Habib, A.N.; Favia, P.; Gristina, R. Improving internal cell colonization of porous scaffolds with chemical gradients produced by plasma assisted approaches. ACS Appl. Mater. Interfaces 2017, 9, 4966–4975. [Google Scholar] [CrossRef]
- Armenise, V.; Gristina, R.; Favia, P.; Cosmai, S.; Fracassi, F.; Sardella, E. Plasma–assisted deposition of magnesium–containing coatings on porous scaolds for bone tissue engineering. Coatings 2020, 10, 356. [Google Scholar] [CrossRef]
- Hodásová, Ľ.; Quintana, R.; Czuba, U.; del Valle, L.J.; Fargas, G.; Alemán, C.; Armelin, E. Atmospheric pressure plasma liquid assisted deposition of polydopamine/acrylate copolymer on zirconia (Y–TZP) ceramics: A biocompatible and adherent nanofilm. RSC Adv. 2021, 11, 17360–17368. [Google Scholar] [CrossRef]
- Solovieva, A.; Miroshnichenko, S.; Kovalskii, A.; Permyakova, E.; Popov, Z.; Dvořáková, E.; Kiryukhantsev-Korneev, P.; Obrosov, A.; Polčak, J.; Zajíčková, L.; et al. Immobilization of platelet–rich plasma onto COOH plasma–coated PCL nanofibers boost viability and proliferation of human mesenchymal stem cells. Polymers 2017, 9, 736. [Google Scholar] [CrossRef]
- Shirazi, H.S.; Rogers, N.; Michelmore, A.; Whittle, J.D. Furfuryl methacrylate plasma polymers for biomedical applications. Biointerphases 2016, 11, 031014. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Jin, C.; Yuan, Y.; Zhang, Y. Plasma deposited APTES: A potential film for biomedical application. Mater. Lett. 2020, 264, 127350. [Google Scholar] [CrossRef]
- Czuba, U.; Quintana, R.; Pauw-Gillet, M.D.; Bourguignon, M.; Moreno-Couranjou, M.; Alexandre, M.; Detrembleur, C.; Choquet, P. Atmospheric plasma deposition of methacrylate layers containing catechol/quinone groups: An alternative to polydopamine bioconjugation for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1701059. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, A.; Kedroňová, E.; Medalová, J.; Černochová, P.; Obrusník, A.; Michlíček, M.; Shtansky, D.V.; Zajíčková, L. Carboxyl–anhydride and amine plasma coating of PCL nanofibers to improve their bioactivity. Mater. Des. 2017, 132, 257–265. [Google Scholar] [CrossRef]
- Šrámková, P.; Zahoranová, A.; Kelar, J.; Tučeková, Z.K.; Stupavská, M.; Krumpolec, R.; Jurmanová, J.; Kováčik, D.; Černák, M. Cold atmospheric pressure plasma: Simple and efficient strategy for preparation of poly(2–oxazoline)–based coatings designed for biomedical applications. Sci. Rep. 2020, 10, 9478. [Google Scholar] [CrossRef]
- Zarei, M.; Sayedain, S.S.; Askarinya, A.; Sabbaghi, M.; Alizadeh, R. Improving physio–mechanical and biological properties of 3D–printed PLA scaffolds via in–situ argon cold plasma treatment. Sci. Rep. 2023, 13, 14120. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzam, N.; Alazzam, A. Micropatterning of cells via adjusting surface wettability using plasma treatment and graphene oxide deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar] [CrossRef]
- Han, I.; Rana, J.N.; Kim, J.-H.; Choi, E.H.; Kim, Y.S. A non–thermal biocompatible plasma–modified CS scaffold enhances osteogenic differentiation in bone marrow stem cells. Pharmaceutics 2022, 14, 465. [Google Scholar] [CrossRef]
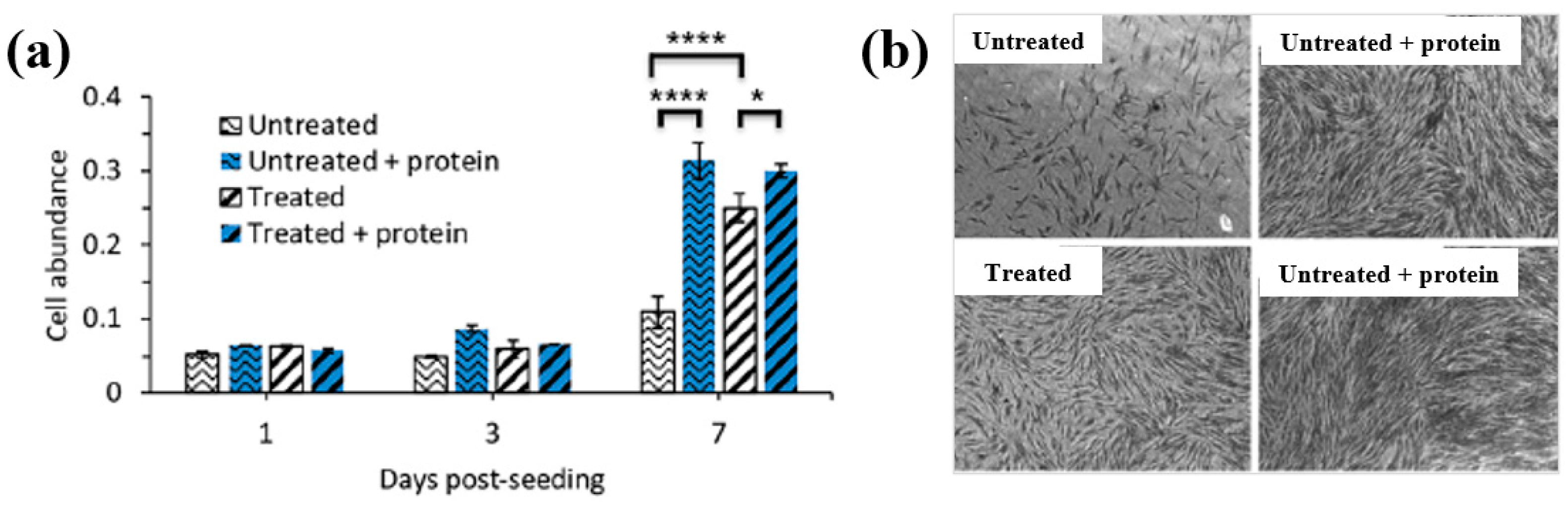
- Sabrin, S.; Hong, S.-H.; KC, S.K.; Oh, J.-S.; Derrick-Roberts, A.; Karmokar, D.K.; Habibullah, H.; Short, R.D.; Ghimire, B.; Fitridge, R.; et al. Electrochemically enhanced antimicrobial action of plasma–activated poly(vinyl alcohol) hydrogel dressings. Adv. Funct. Mater. 2024, 34, 2314345. [Google Scholar] [CrossRef]
- Teske, M.; Sternberg, K. Surface functionalization of poly(ε–caprolactone) and poly(3–hydroxybutyrate) with VEGF. BioNanoMaterials 2017, 18, 20170001. [Google Scholar] [CrossRef]
- Cools, P.; Asadian, M.; Nicolaus, W.; Declercq, H.; Morent, M.; De Geyter, N. Surface treatment of PEOT/PBT (55/45) with a dielectric barrier discharge in air, helium, argon and nitrogen at medium pressure. Materials 2018, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.E.; Mariscotti, V.; Chevallier, P.; Copes, F.; Boccafoschi, F.; Sarkissian, A.; Mantovani, D. Effects of cold plasma treatment on the biological performances of decellularized bovine pericardium extracellular matrix–based Films for biomedical applications. Explor. BioMat–X 2024, 1, 84–99. [Google Scholar] [CrossRef]
- Liao, S.-C.; Wu, Y.-D.; Siao, J.-K. Atmospheric–pressure plasma jet–induced graft polymerization of composite hydrogel on 3D–printed polymer surfaces for biomedical application. Coatings 2023, 13, 367. [Google Scholar] [CrossRef]
- Lotz, O.; Zhang, A.; Zhianmanesh, M.; Coffi Dit Gleize, K.; McKenzie, D.R.; Bilek, M.M.M.; Akhavan, B. Reagent–free biomolecule functionalization of atmospheric pressure plasma–activated polymers for biomedical applications: Path ways for covalent attachment. Appl. Surf. Sci. 2024, 662, 160101. [Google Scholar] [CrossRef]
- Alavi, S.K.; Lotz, O.; Akhavan, B.; Yeo, G.; Walia, W.; McKenzie, D.R.; Bilek, M.M. Atmospheric pressure plasma jet treatment of polymers enables reagent–free covalent attachment of biomolecules for bioprinting. ACS Appl. Mater. Interfaces 2020, 12, 38730–38743. [Google Scholar] [CrossRef]
- Paneru, R.; Ki, S.H.; Lamichhane, P.; Nguyen, L.N.; Adhikari, B.C.; Jeong, I.J.; Mumtaz, S.; Choi, J.; Kwon, J.S.; Choi, E.H. Enhancement of antibacterial and wettability performances of polyvinyl alcohol/chitosan film using non–thermal atmospheric pressure plasma. Appl. Surf. Sci. 2020, 532, 1147339. [Google Scholar] [CrossRef]
- Bitar, R.; Asadian, M.; Vrekhem, S.V.; Cools, P.; Declercq, H.; Morent, R.; De Geyter, N. Local plasma activation of PS films with a defined design for biomedical use. Surf. Coat. Technol. 2018, 350, 985–996. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Li, J.; Wang, X.; Chen, Q.; Yang, S.; Chen, Z.; Bazaka, K.; Ostrikov, K. Synergistic effect of atmospheric pressure plasma and TiO2 photocatalysis on inactivation of Escherichia coli cells in aqueous media. Sci. Rep. 2016, 6, 39552. [Google Scholar] [CrossRef]
- Ananth, A.; Han, I.; Akter, M.; Boo, J.-H.; Choi, E.H. Handy soft jet plasma as an effective technique for tailored preparation of ZnS nanomaterials and shape dependent antibacterial performance of ZnS. J. Ind. Eng. Chem. 2020, 90, 389–398. [Google Scholar] [CrossRef]
- Ananth, A.; Dharaneedharan, S.; Seo, H.-J.; Heo, M.-S.; Boo, J.-H. Soft jet plasma–assisted synthesis of zinc oxide nano–materials: Morphology controls and antibacterial activity of ZnO. Chem. Eng. J. 2017, 322, 742–751. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Wahab, R.; Bhartiya, P.; Linh, N.N.; Khan, F.; Al-Khedhairy, A.A.; Choi, E.H. Cold atmos-pheric plasma and gold quantum dots exert dual cytotoxicity mediated by the cell receptor–activated apoptotic pathway in glio–blastoma cells. Cancers 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Jung, E.Y.; Parsons, T.; Tae, H.-S.; Park, C.-S. A review of plasma synthesis methods for polymer films and nanoparticles under atmospheric pressure conditions. Polymers 2021, 13, 2267. [Google Scholar] [CrossRef]
- Jung, E.Y.; Suleiman, H.O.; Tae, H.-S.; Park, C.-S. A review of plasma-synthesized and plasma surface–modified piezoelectric polymer films for nanogenerators and sensors. Polymers 2024, 16, 1548. [Google Scholar] [CrossRef]
- Karthik, C.; Sarngadharan, S.C.; Thomas, V. A low–temperature plasma techniques in biomedical applications and therapeutics: An overview. Int. J. Mol. Sci. 2024, 25, 524. [Google Scholar] [CrossRef]
- Waskow, A.; Avino, F.; Howling, A.; Furno, I. Entering the plasma agriculture field: An attempt to standardize protocols for plasma treatment of seeds. Plasma Processes Polym. 2022, 19, e2100152. [Google Scholar] [CrossRef]
- Radetić, M.; Marković, D. A review on the role of plasma technology in the nano–finishing of textile materials with metal and metal oxide nanoparticles. Plasma Processes Polym. 2022, 19, e2100197. [Google Scholar] [CrossRef]
- Förster, F. Atmospheric Pressure Plasma in Industrial Applications: Surface treatment of thermally sensitive polymers. Plasma Processes Polym. 2022, 19, e2100240. [Google Scholar] [CrossRef]
- Loesch-Zhang, A.; Geissler, A.; Biesalski, M. Plasma polymerization of biogenic precursors. Plasma Processes Polym. 2023, 20, e2300016. [Google Scholar] [CrossRef]
- Bertin, M.; Leitao, E.M.; Bickerton, S.; Reinhard Verbeek, C.J. A review of polymer surface modification by cold plasmas toward bulk functionalization. Plasma Processes Polym. 2024, 21, e2300208. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Asrafali, S.P.; Periyasamy, T. Synthesis, morphology, and biomedical applications of plasma–based polymers: Recent trends and advances. Polymers 2024, 16, 2701. [Google Scholar] [CrossRef]
- O’Neill, F.; O’Neill, L.; Bourke, P. Recent developments in the use of plasma in medical applications. Polymers 2024, 7, 284–299. [Google Scholar] [CrossRef]
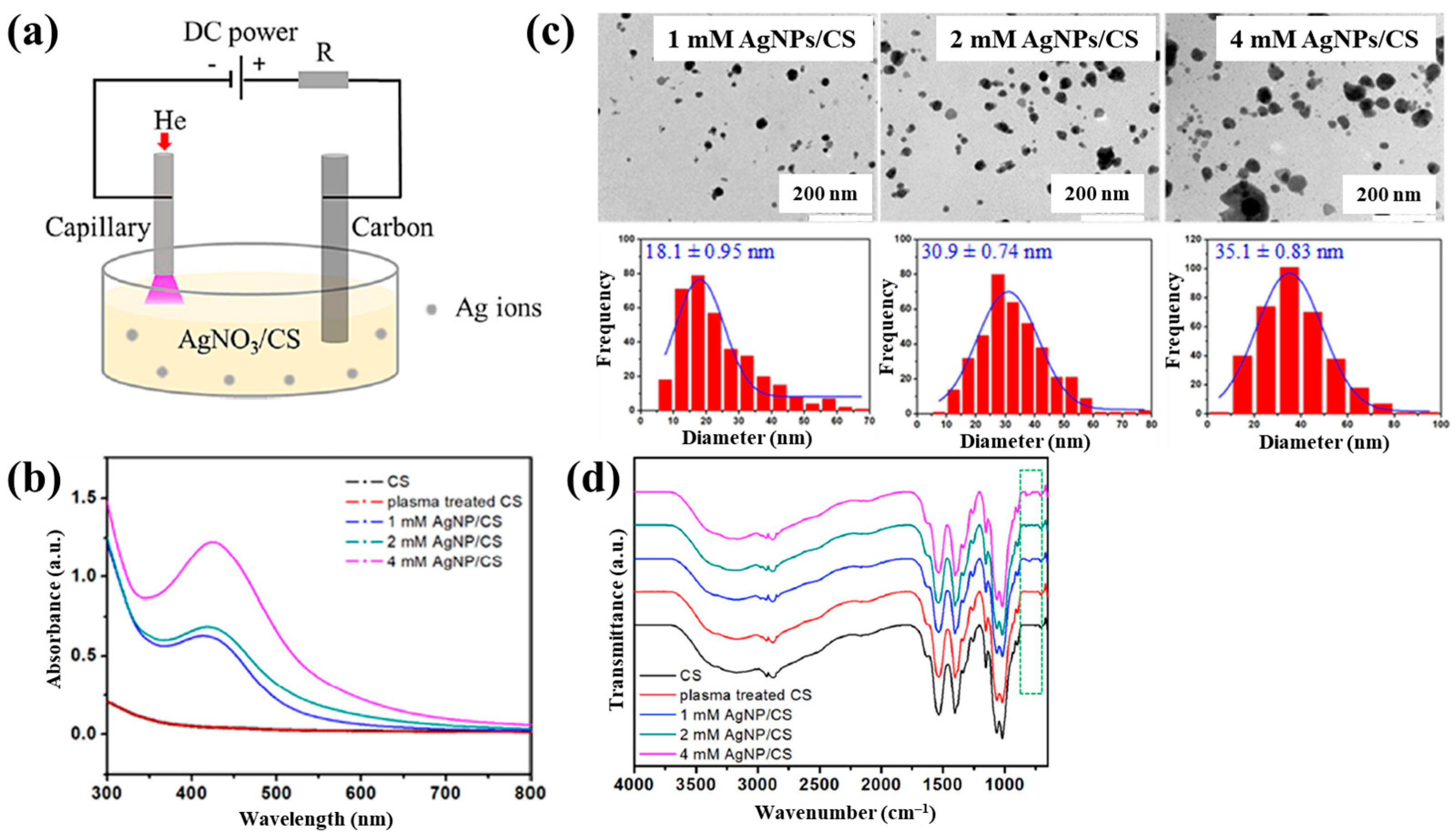
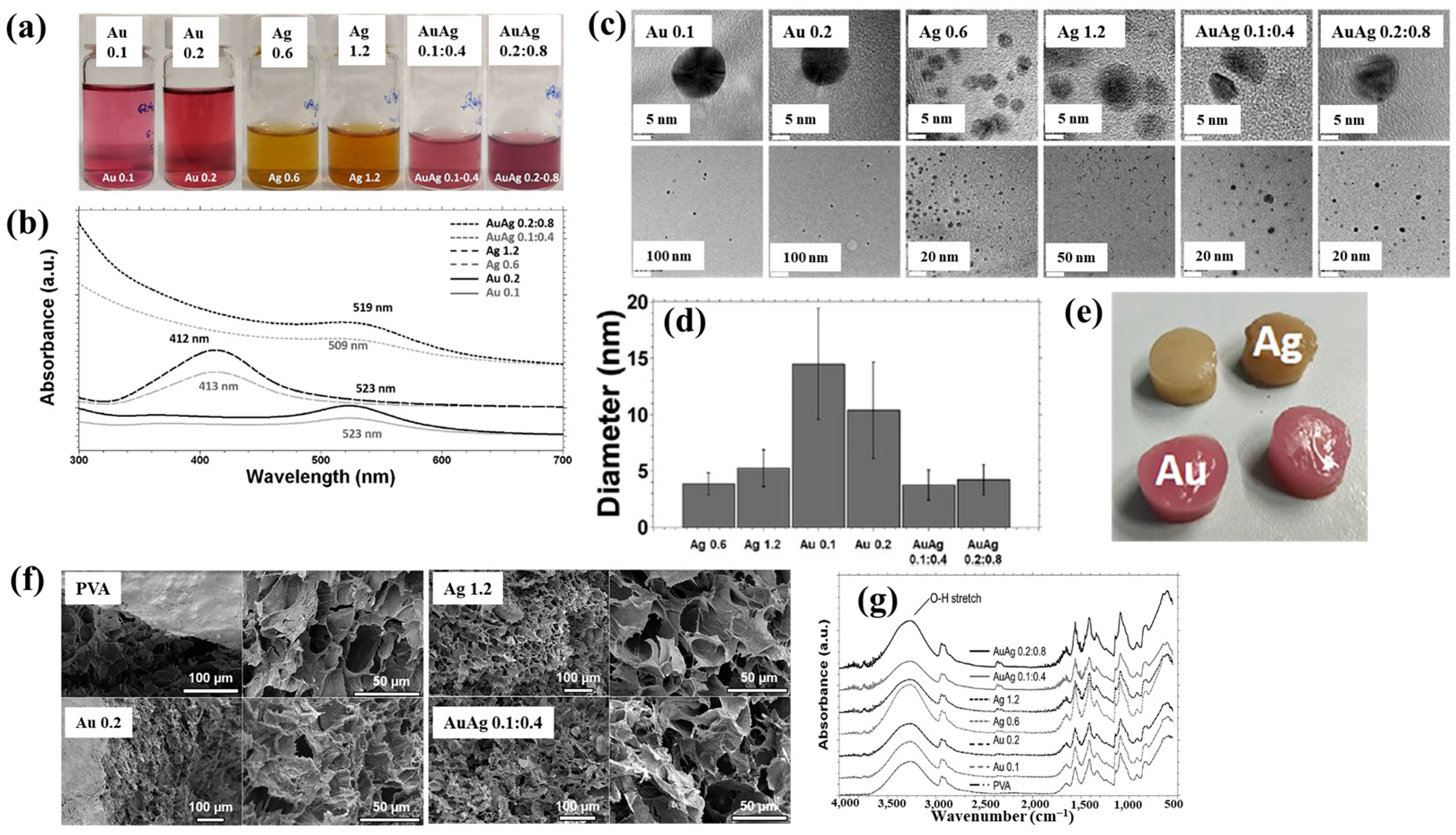
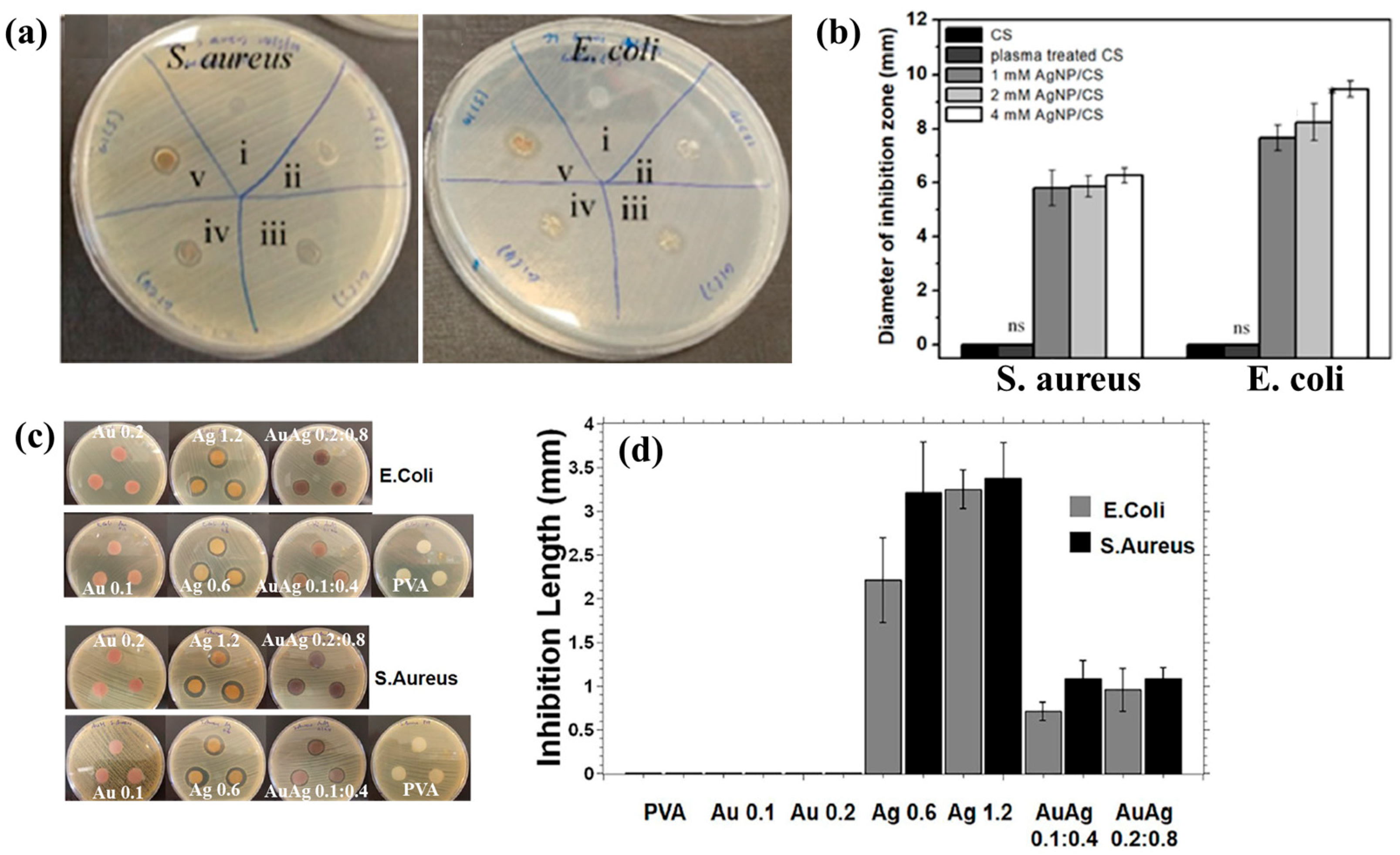
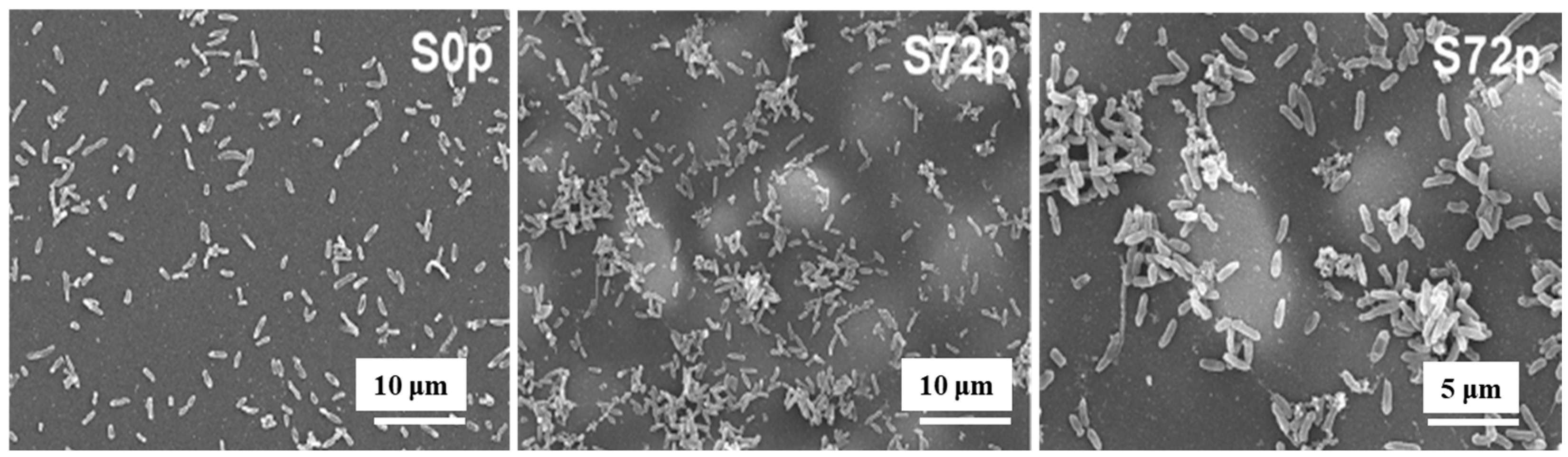
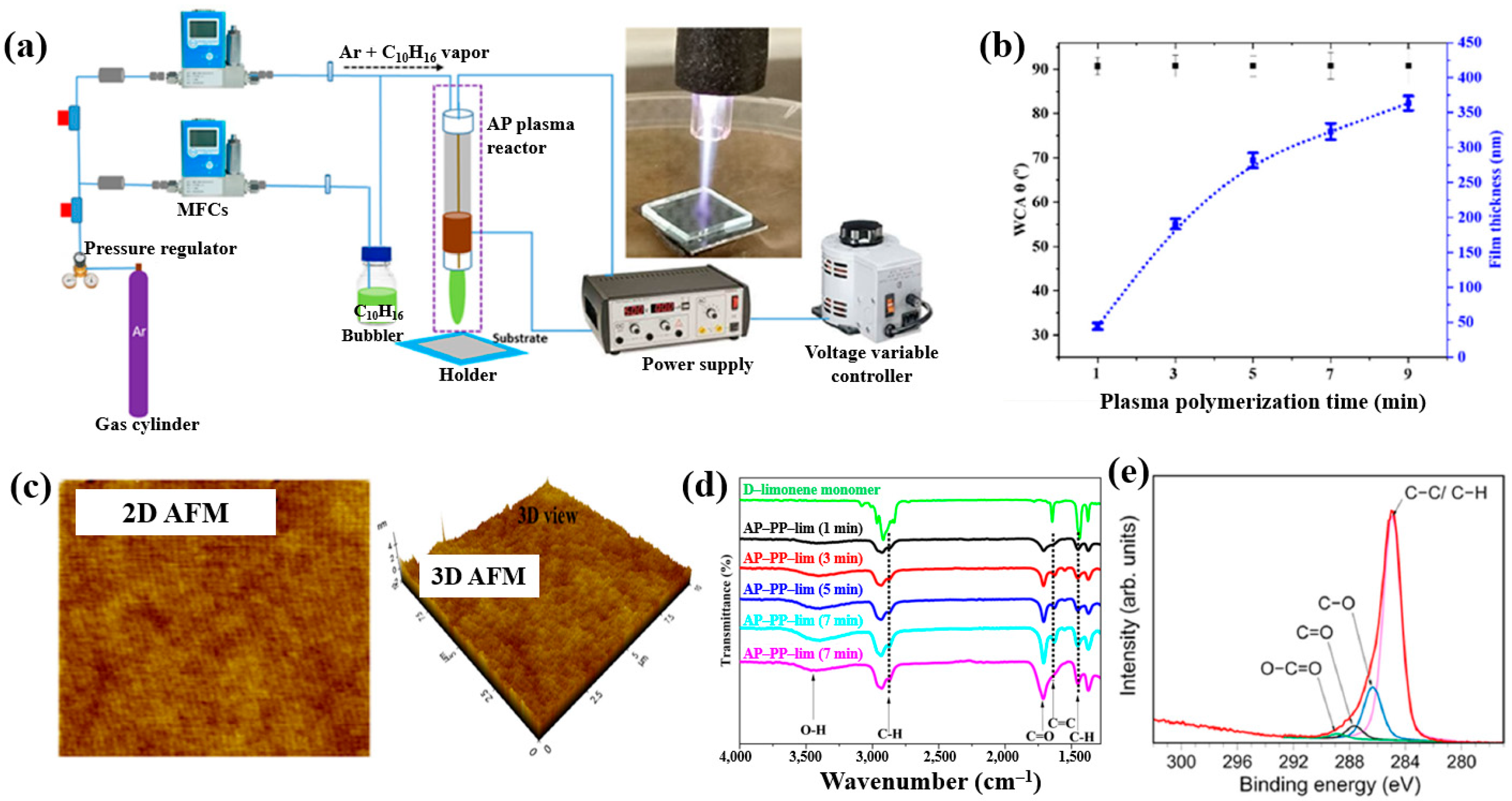

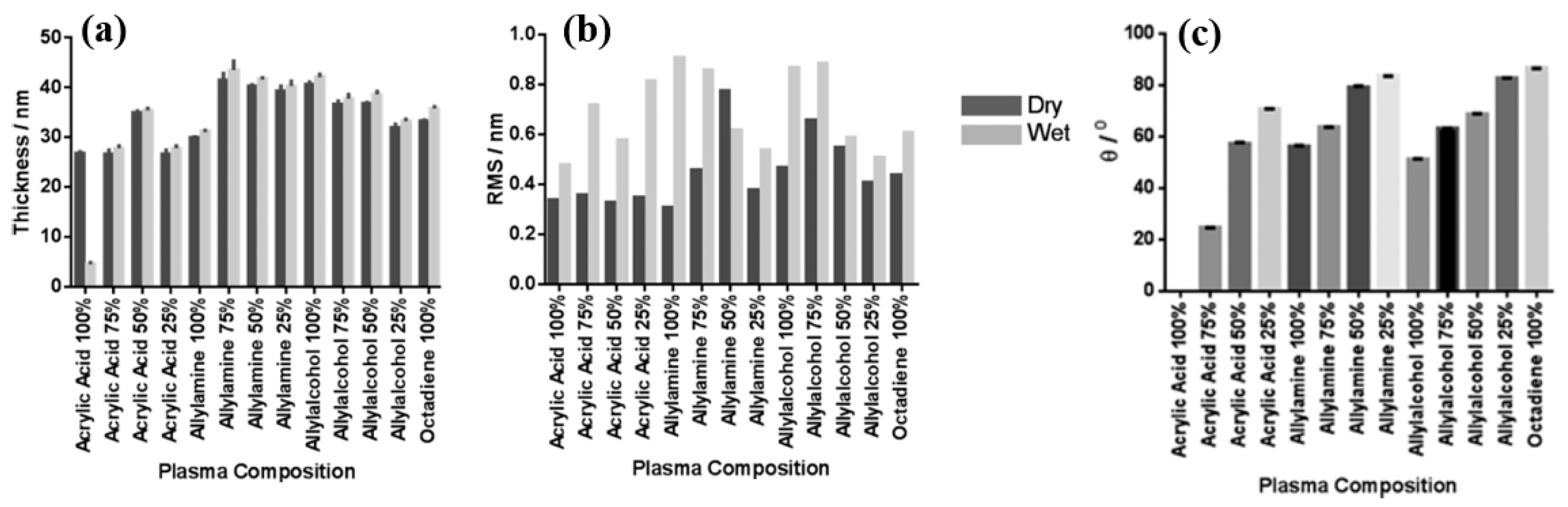


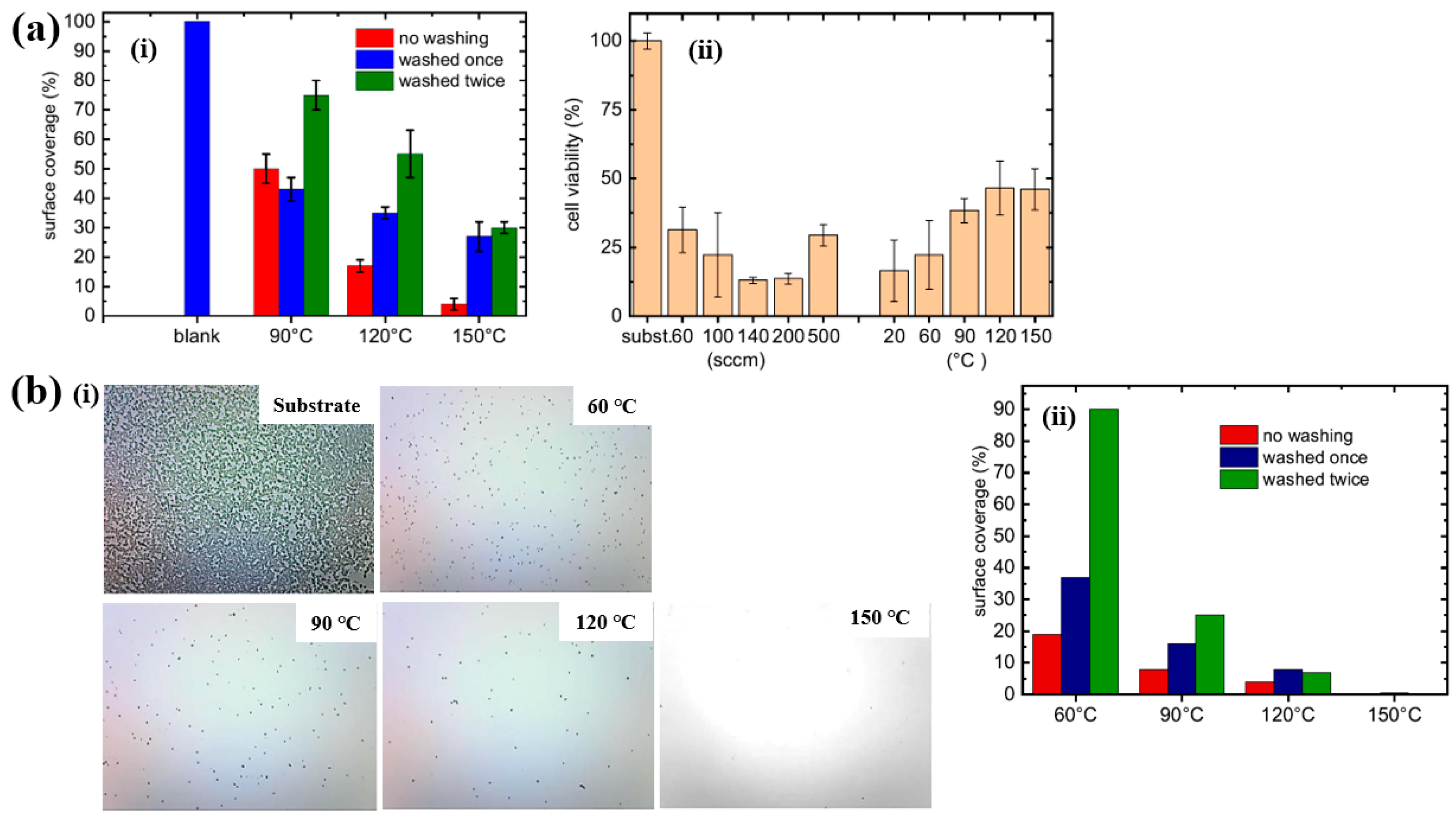




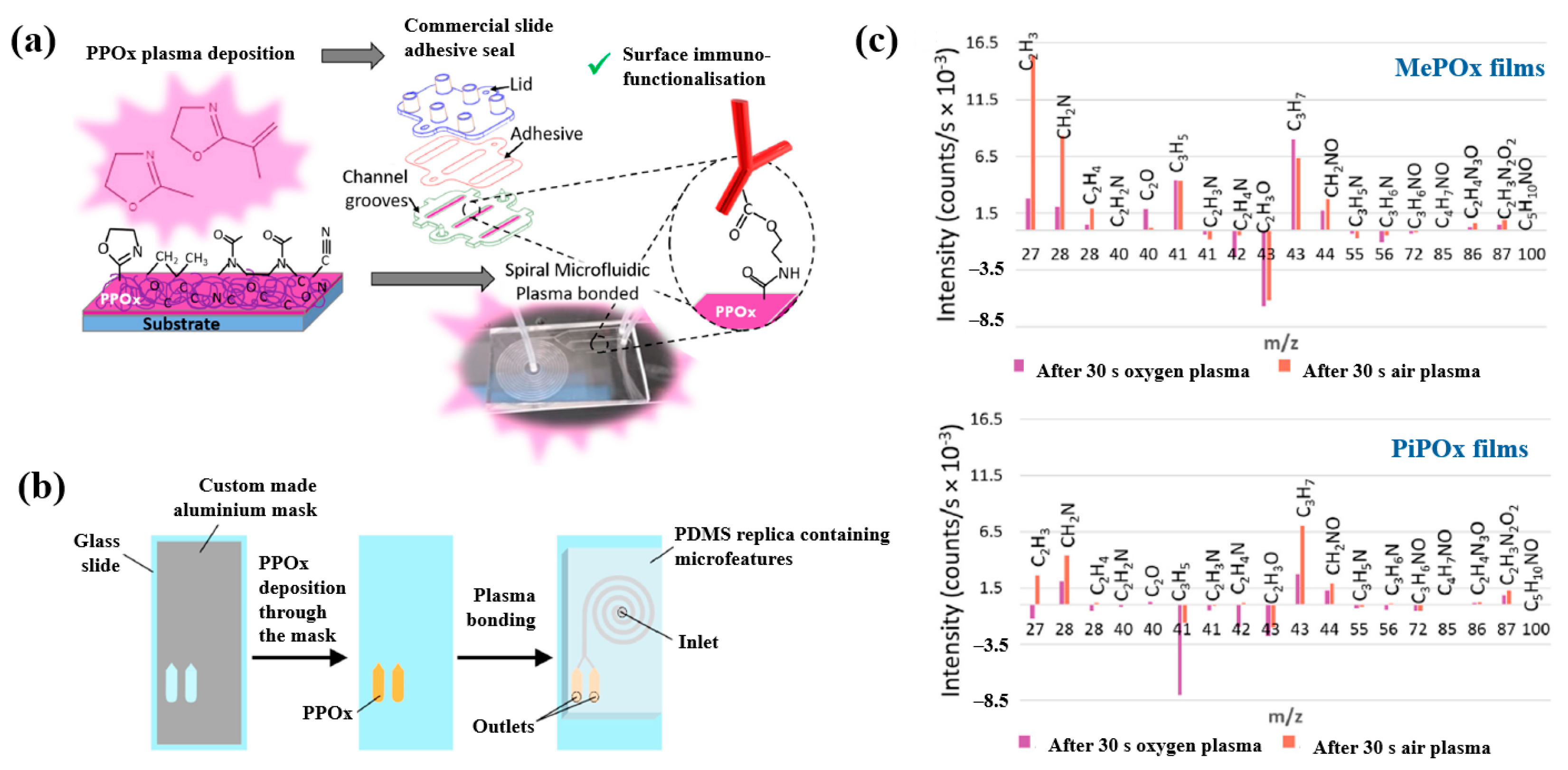
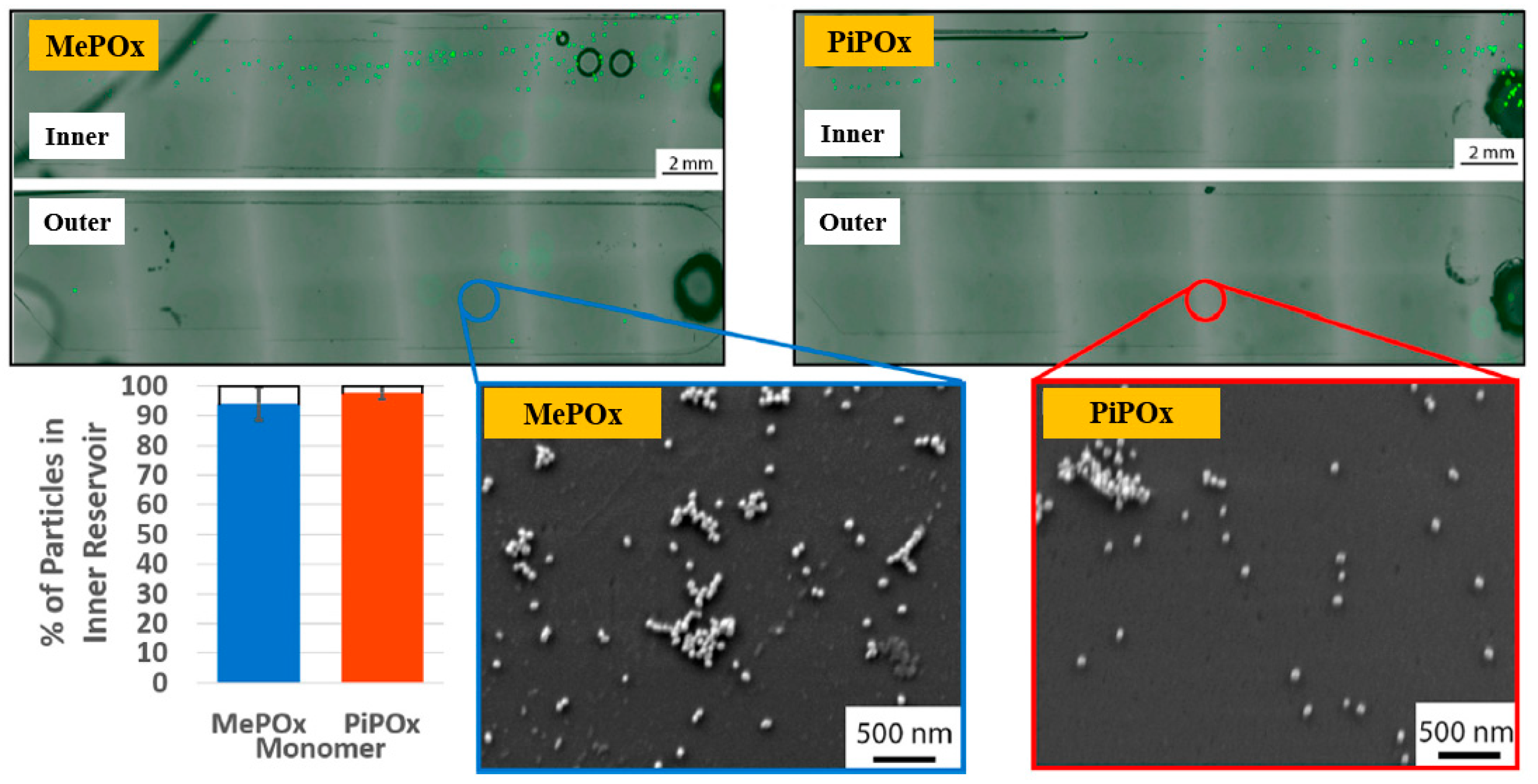
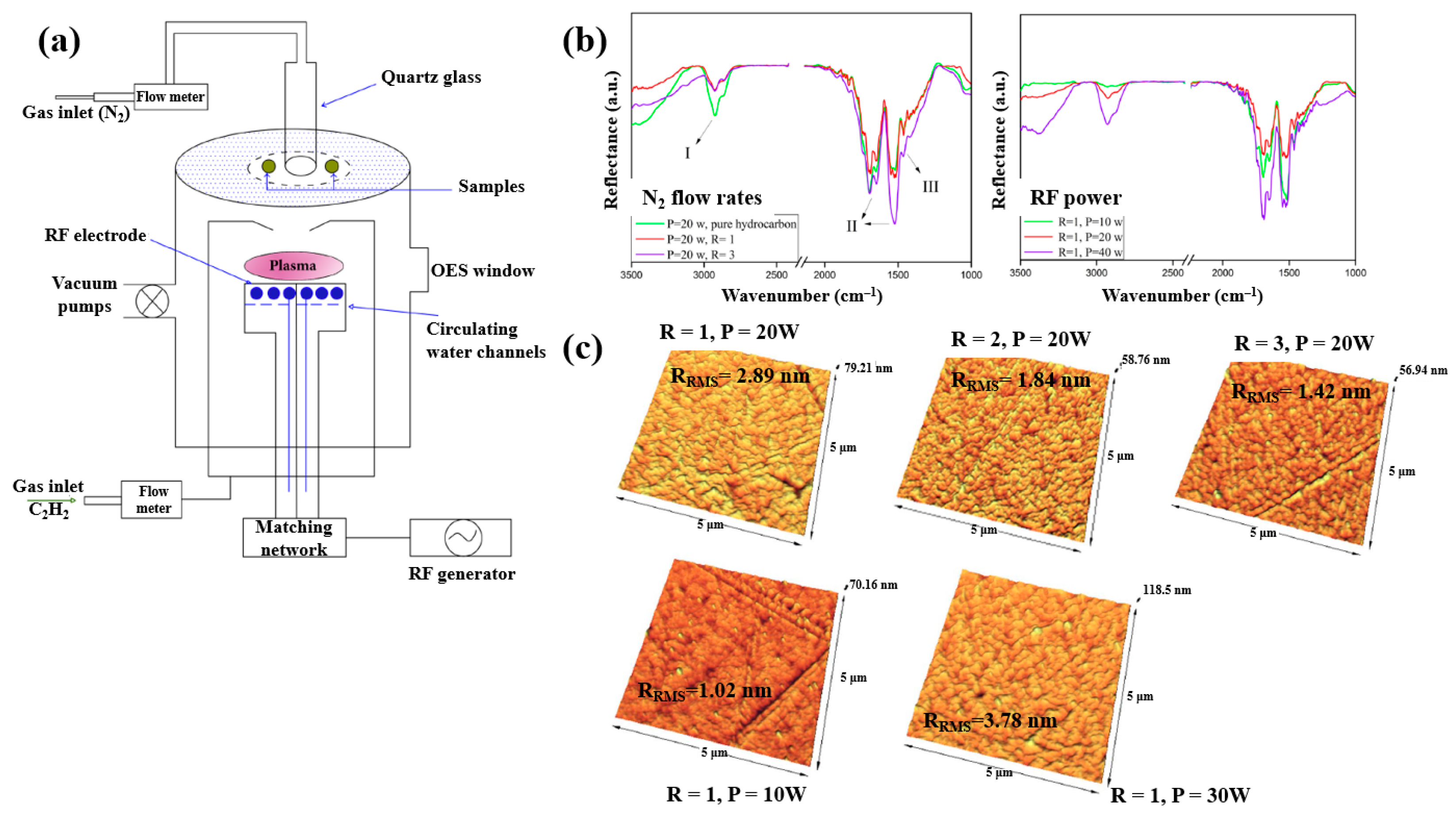
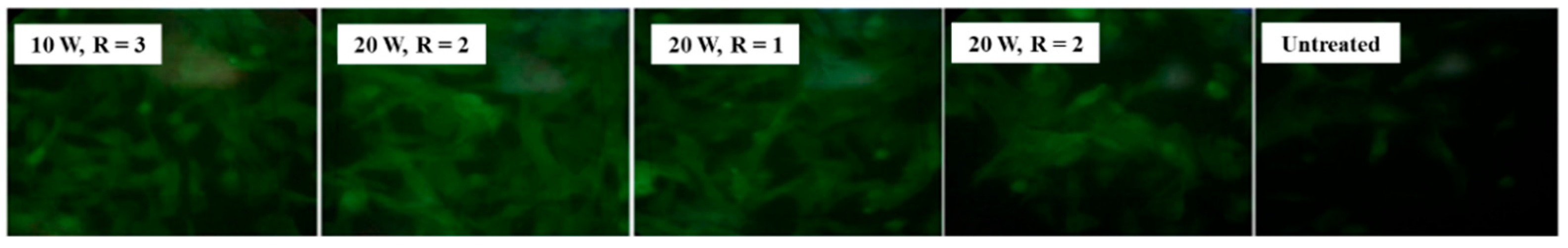



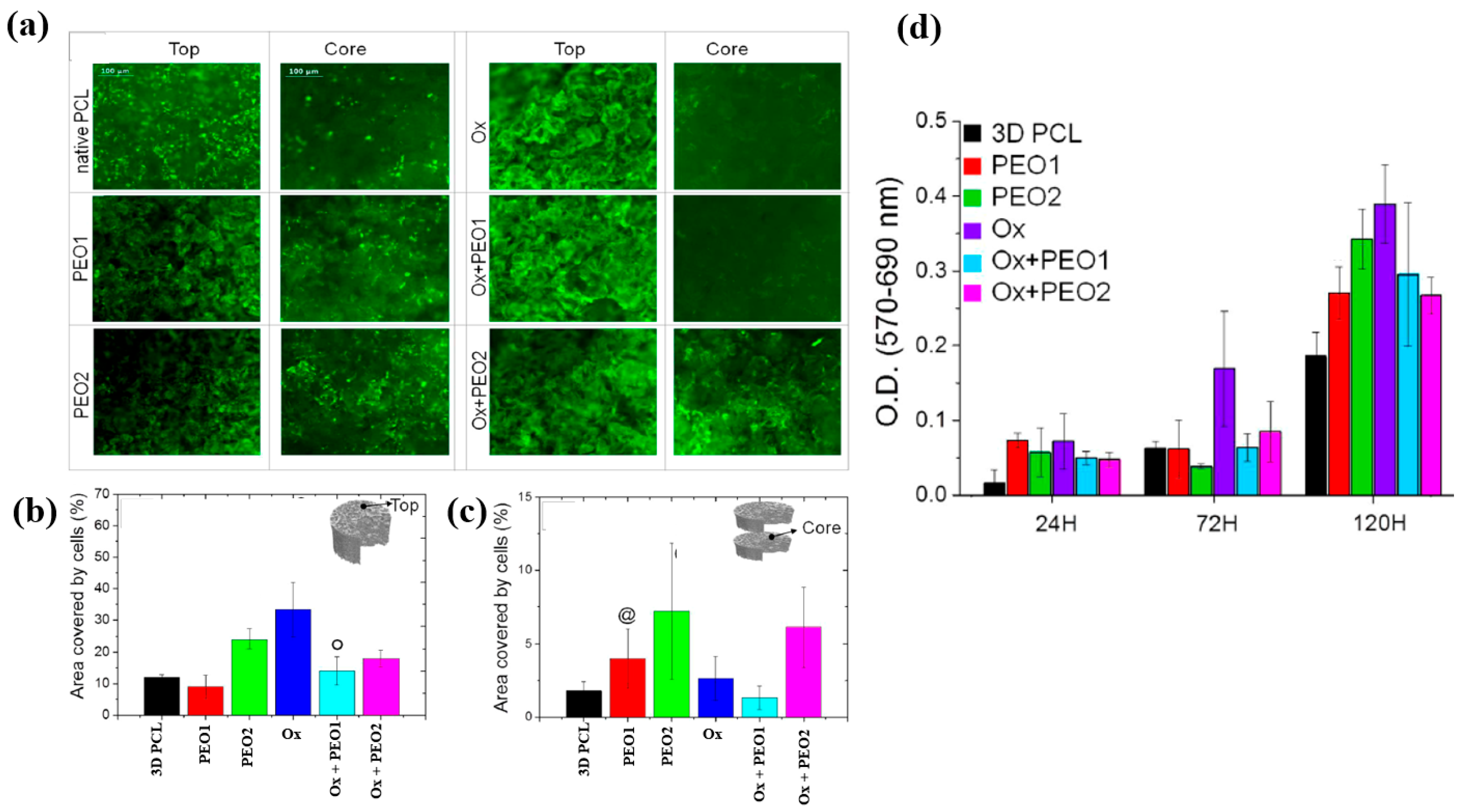
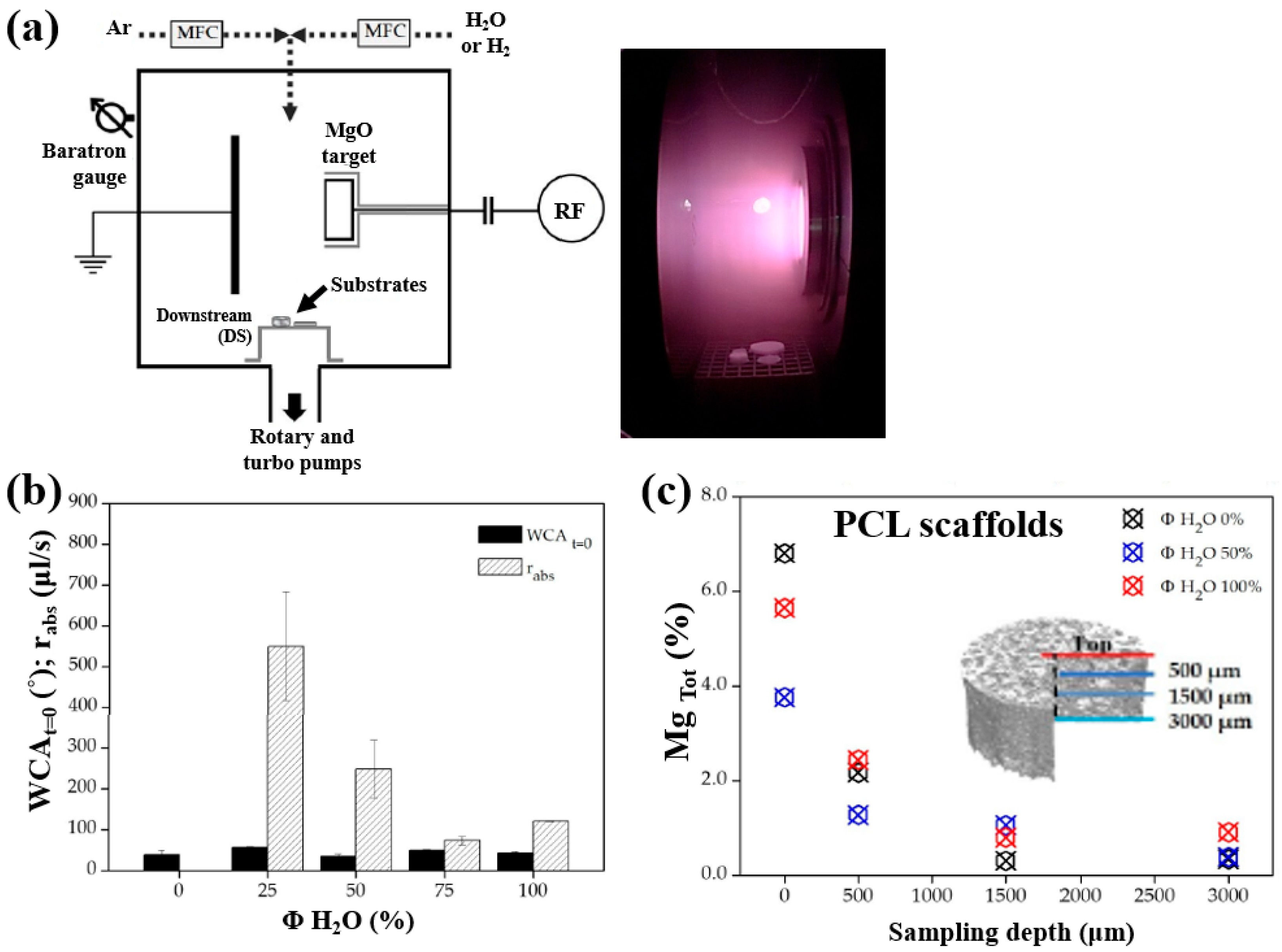
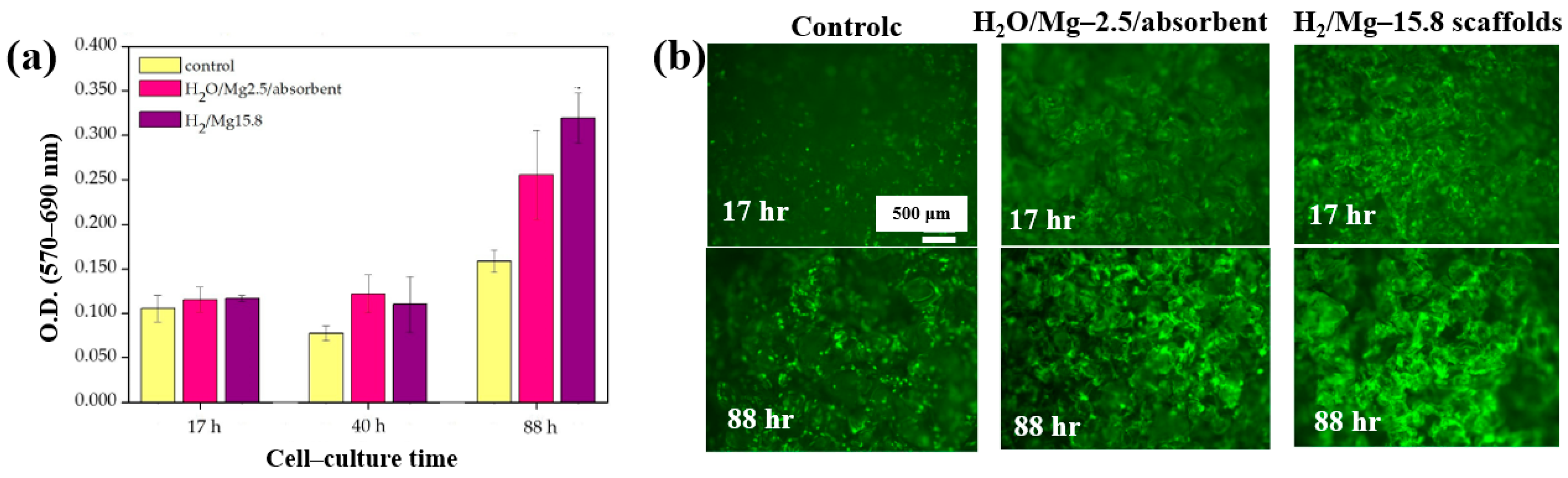
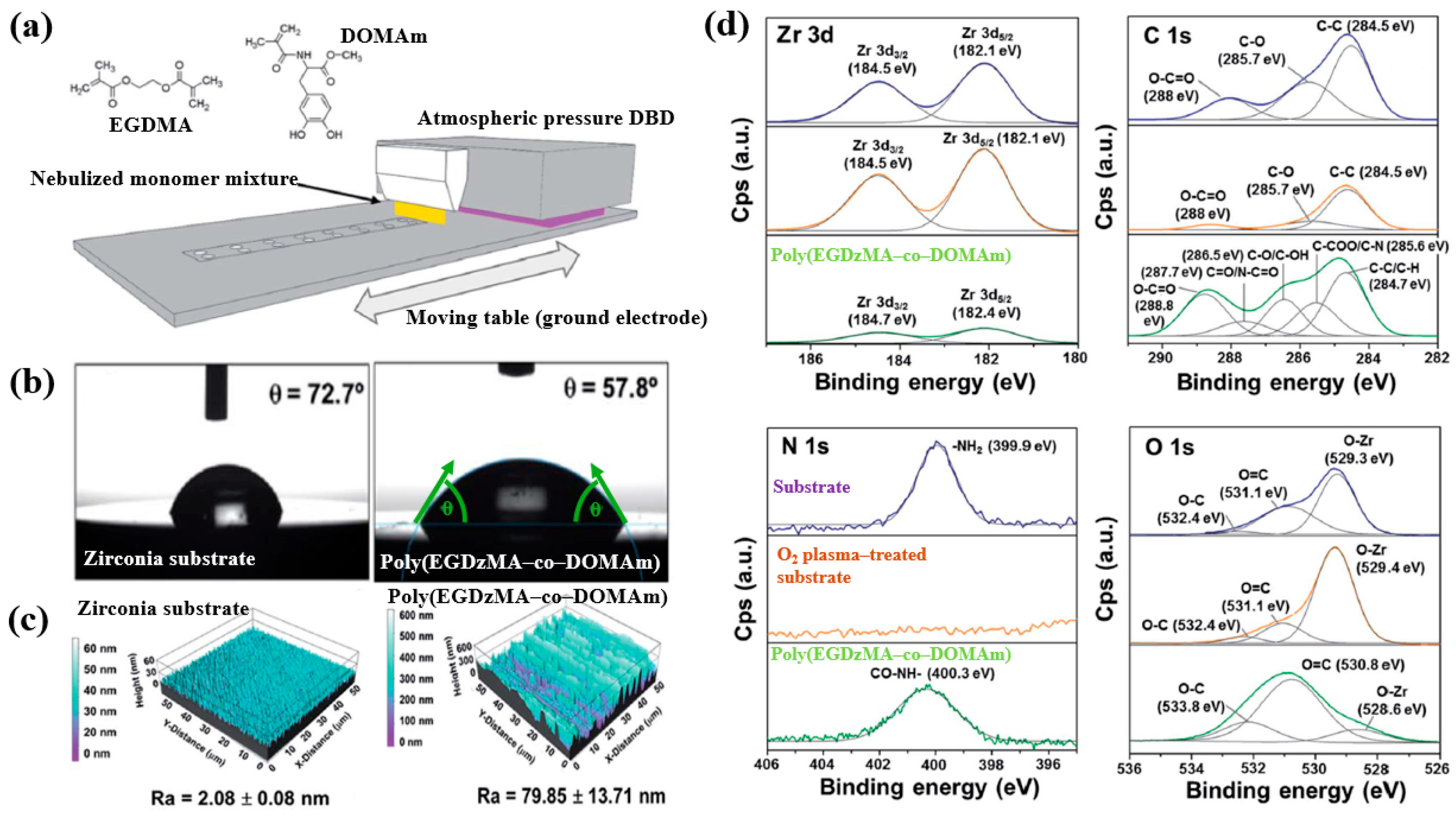
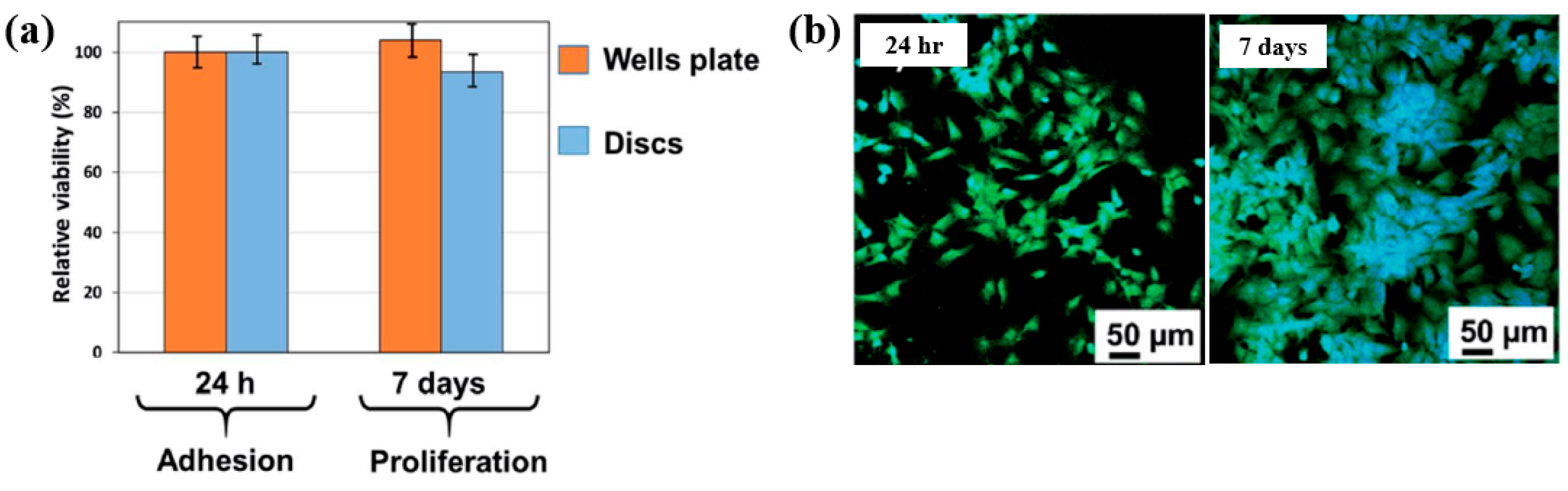
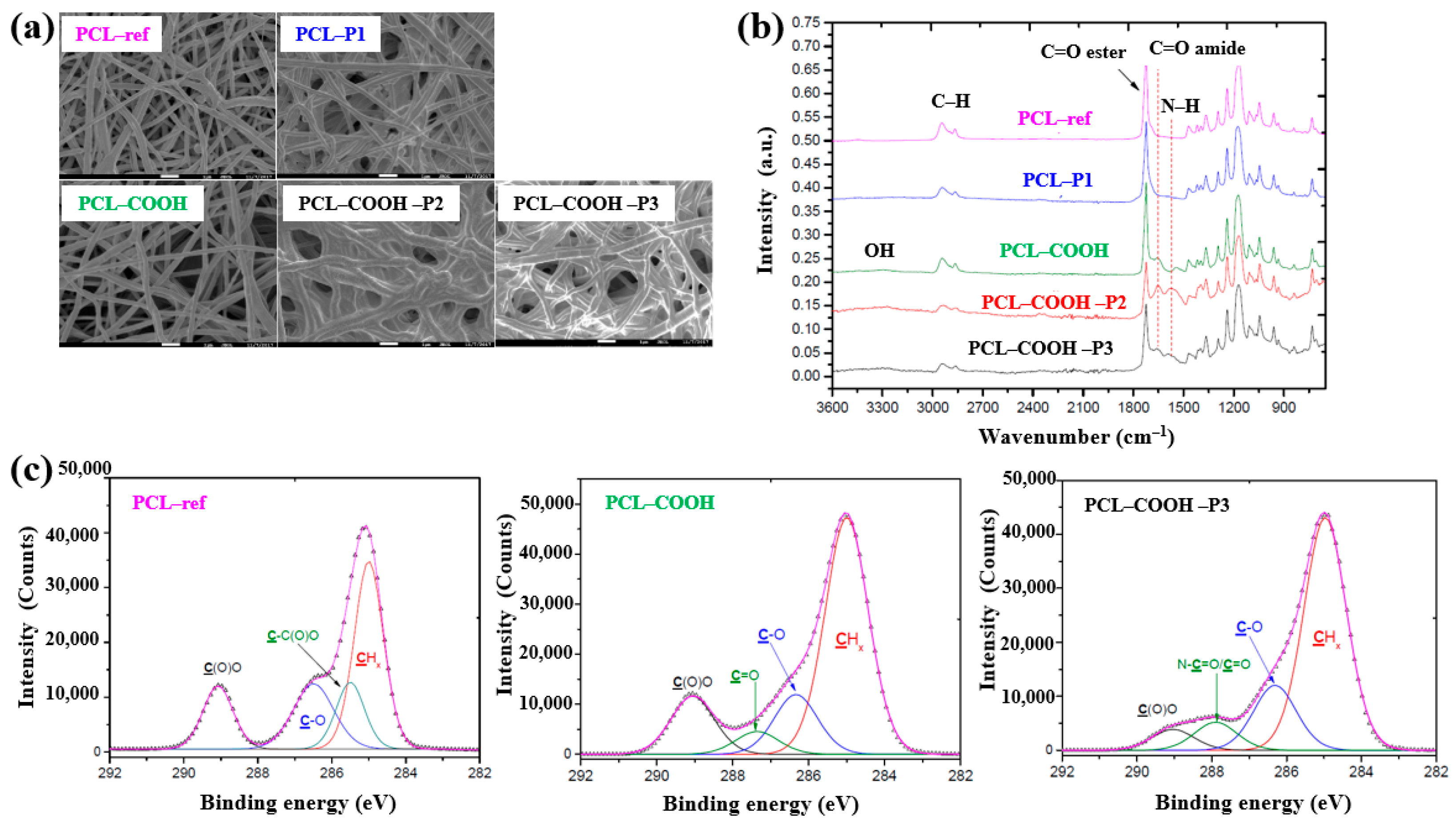
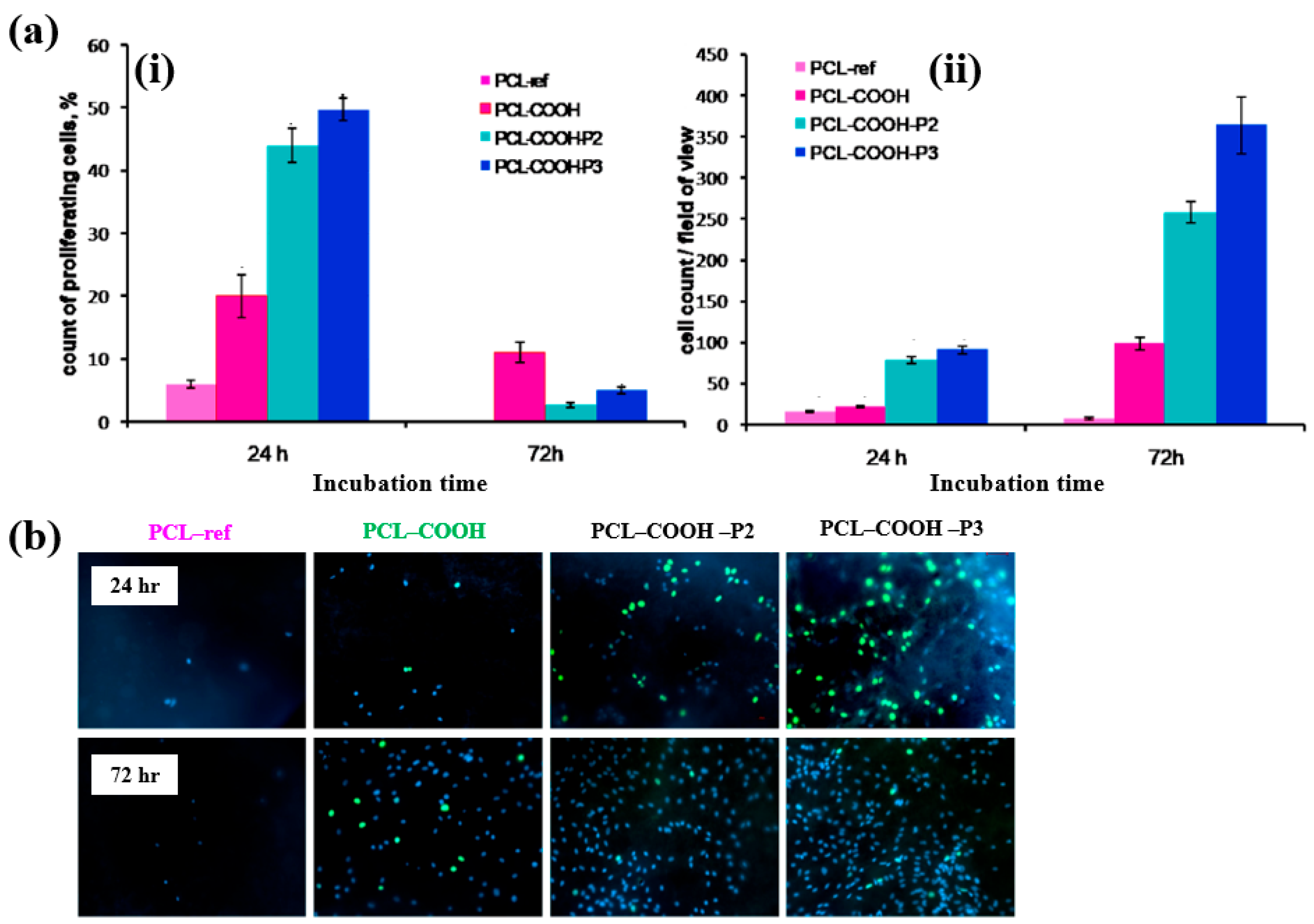
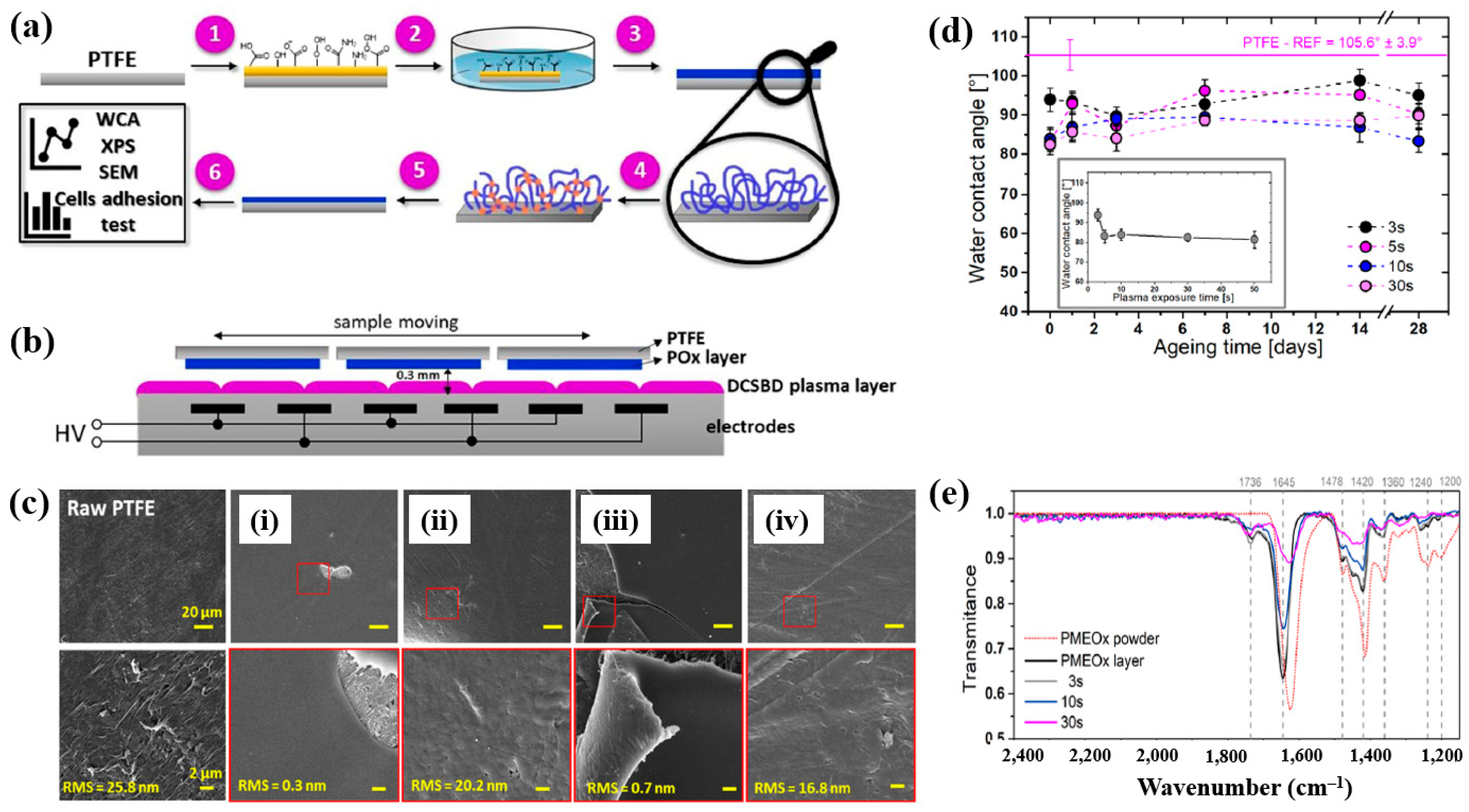


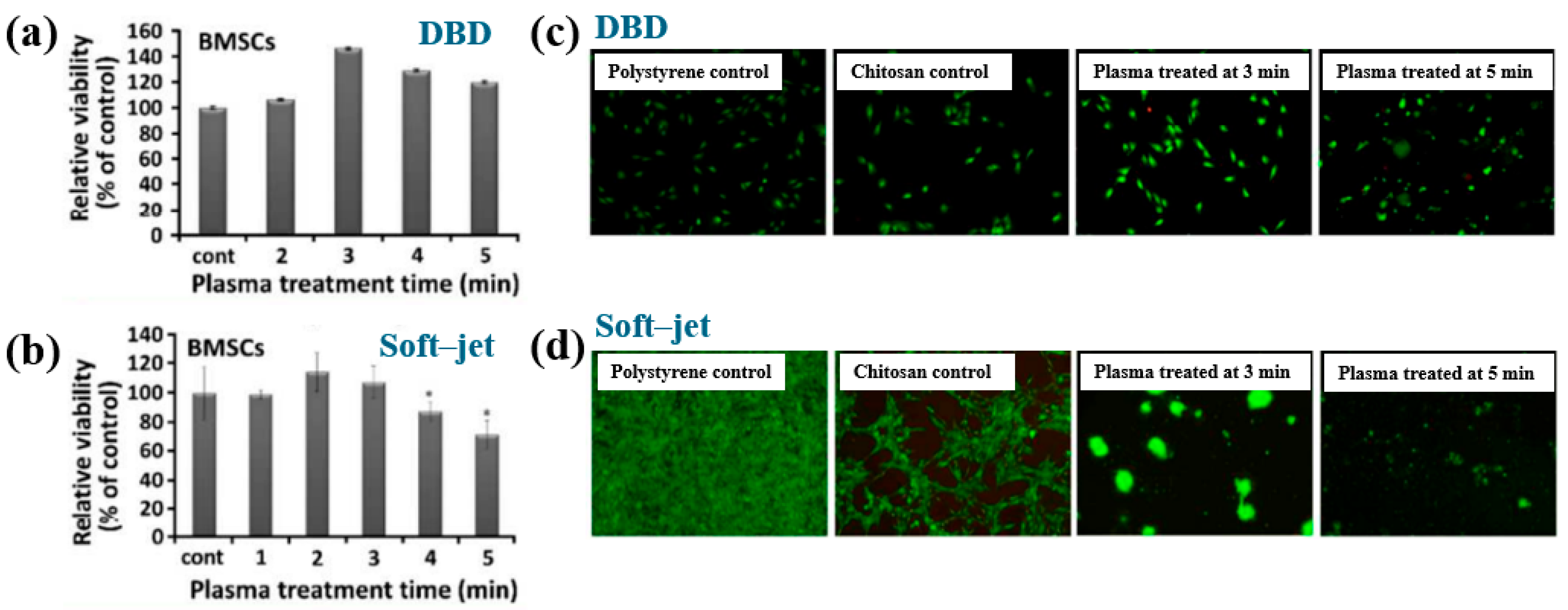
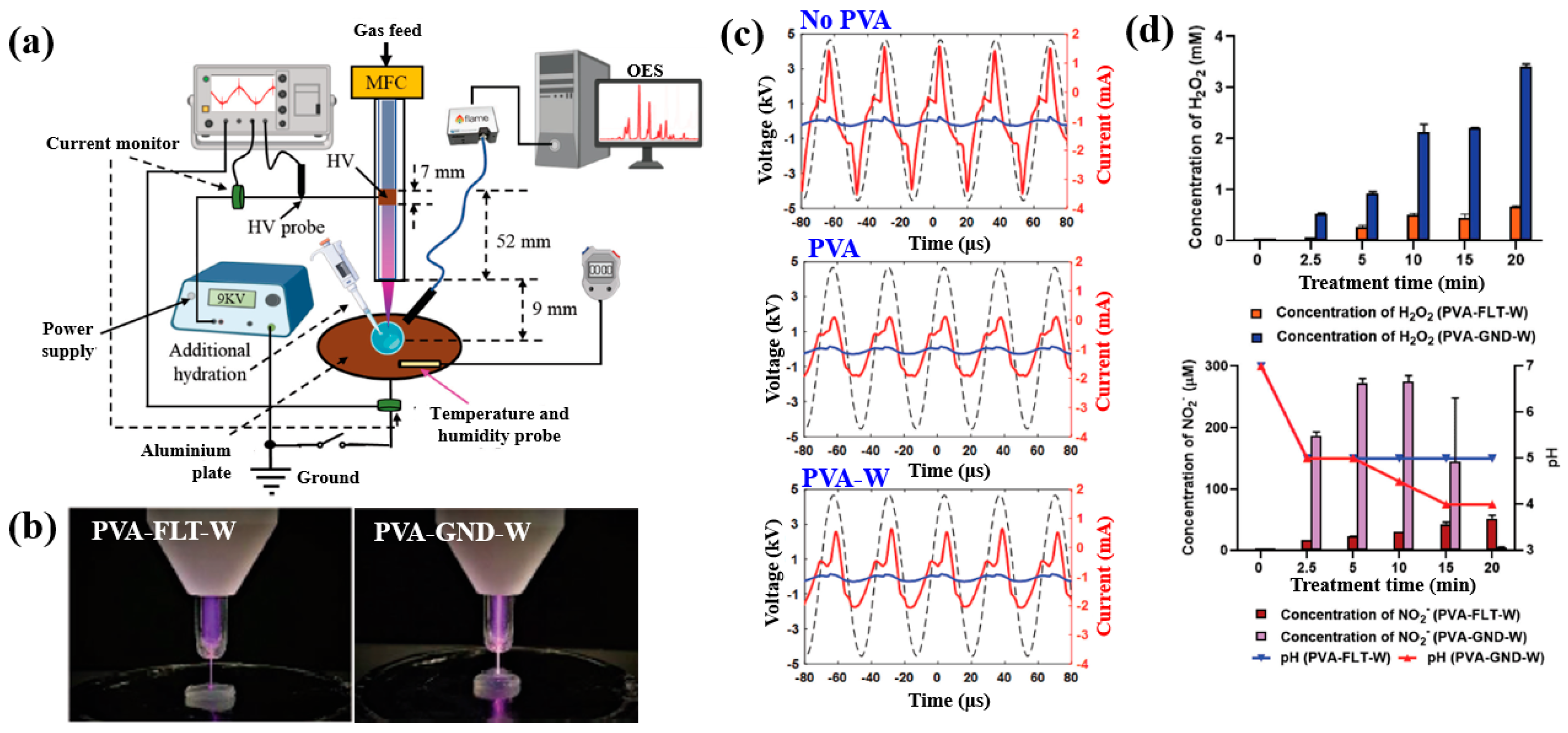



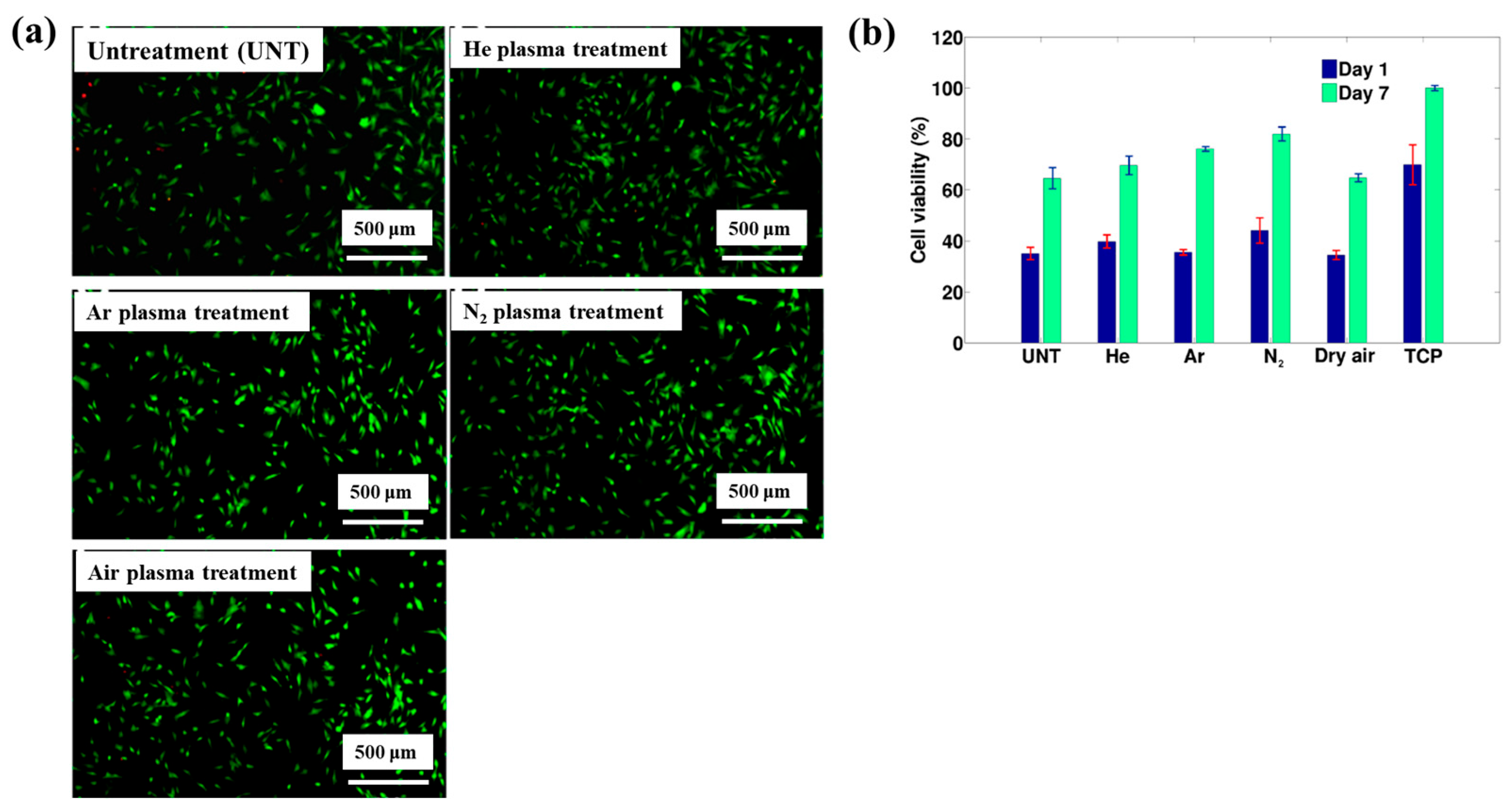
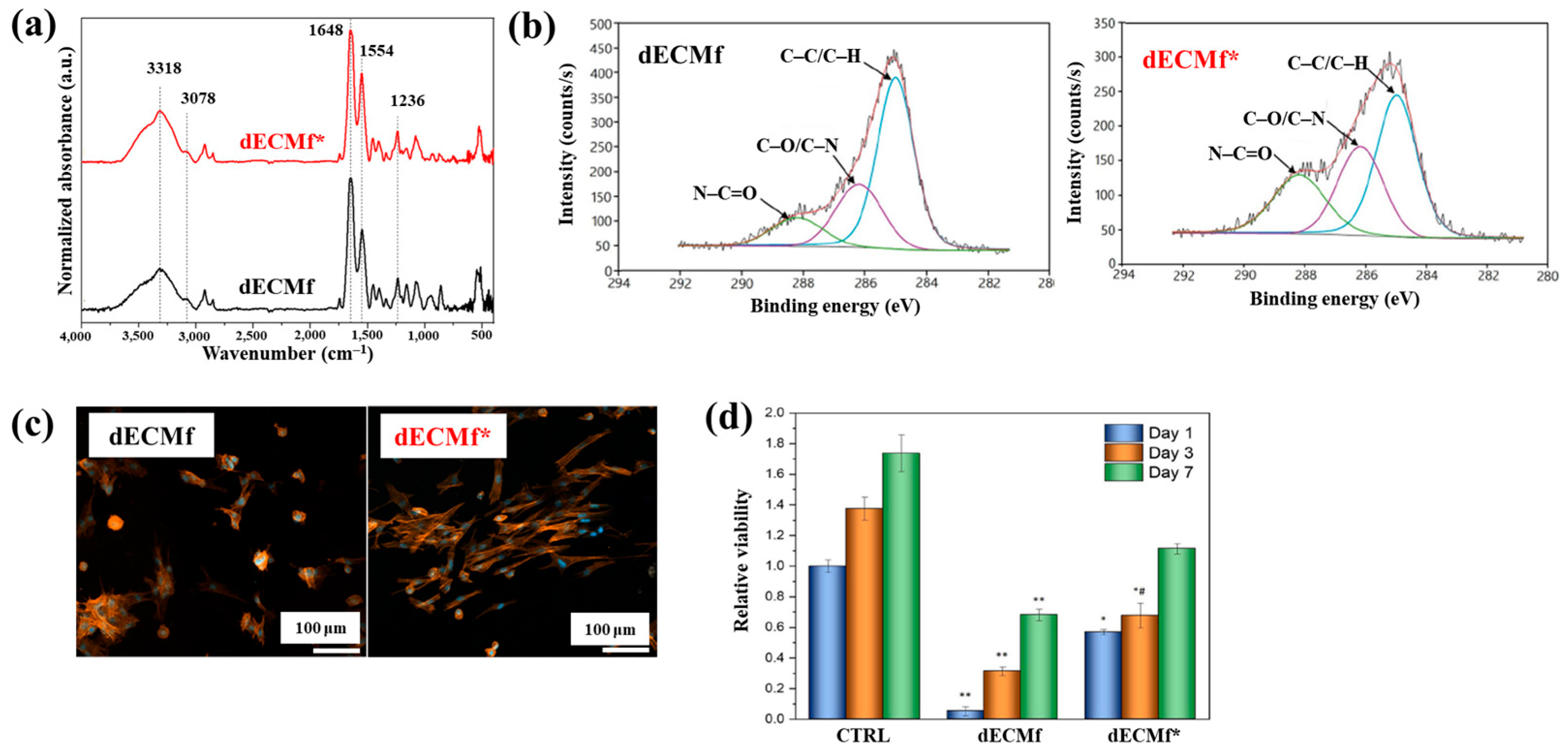



| Precursor | Mixture with CS powder, 2% acetic acid solution, and AgNO3 (10 mM) |
| Synthesis method | APM on the solution |
| Applied voltage (kV) | 4 |
| Gas type | He |
| Gas flow (sccm) | 25 |
| Plasma process time (min) | 10 |
| AgNP/CS film fabrication | Drop-casting |
| Functional group of AgNP/CS | N–H (amid), ROS (–OH, C–O) |
| Bacteria type | E. coli, S. aureus |
| Inhibition zone | 9 mm at 4 mM AgNPs/CS under E.coli 6 mm at 4 mM AgNPs/CS under S.aureus |
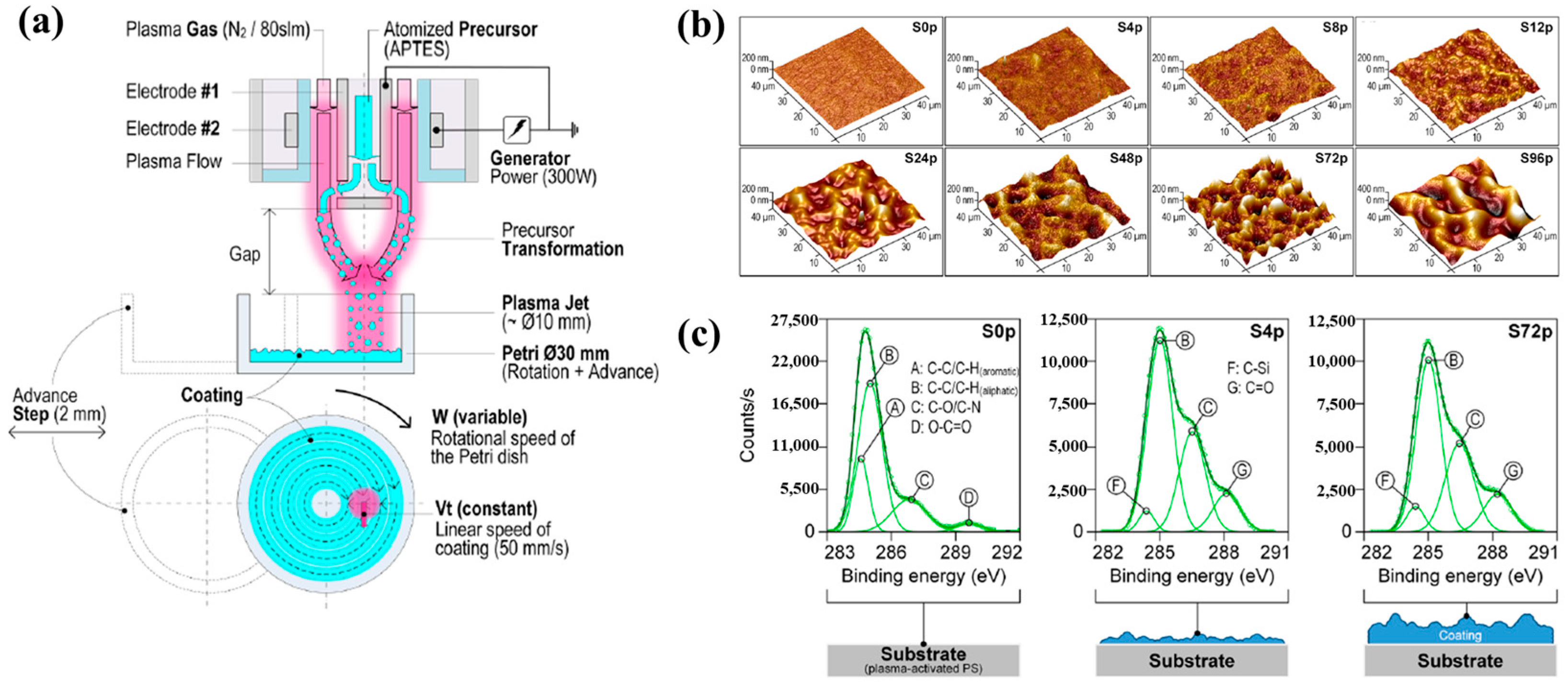
| Precursor | 3-(Aminopropyl)triethoxysilane (APTES) |
| Chemical formula | (CH3CH2O)3–Si–CH2CH2CH2NH2 |
| Synthesis method | APPJ |
| Plasma power (W) | 300 |
| Frequency (kHz) | 68 |
| Plasma gas | N2 |
| Plasma gas flow (SLM) | 80 |
| Precursor carrier gas | N2 |
| Precursor carrier gas flow (SLM) | 1.5 |
| Number of plasma passes | 0, 4, 8, 12, 24, 48, 72, 96 |
| Substrate speed (mm/s) | 50 |
| Functional group of pAPTES film | Amine (NH2), and oxygenated carbon groups |
| Roughness (Ra, nm) | S0p (4.8) and S72p (55.1) |
| WCA (°) | S0p (54.7) and S72p (56.6) |
| Bacterial type | P. aeruginosa |
| Bacterial inoculum (CFU/mL) | 106 |
| Precursor | Essential oil (D–limonene, C10H16) |
| Synthesis method | APPJ |
| Applied voltage (kV) | 3 |
| Plasma gas | Ar |
| Plasma gas flow (sccm) | 130 |
| Plasma deposition time (min) | 1, 3, 5, 7, 7, 9 |
| Deposition rate (nm/s) | 0.8 |
| Functional group of AP–PP–lim thin film | Oxygenated carbon groups (C–O, C=O, O–C=O) |
| Roughness (Rq, nm) | 0.27 |
| Roughness (Ra, nm) | 0.23 |
| WCA (°) | 90 at 1 min |
| Bacterial type | Gram-negative (E. coli) |
| Bacterial inoculum (CFU/mL) | 105 |
| Number of te attached bacteria | 4.3 × 105 (substrate (control)) 2.4 × 104 (AP–PP–lim film) |
| Precursor | AAc, AAm, AAOH, and OD |
| Synthesis method | LPP |
| Plasma power (W) | 3 (ppAAc, ppAAOH, ppOD), 5 (ppAAm) |
| Precursor gas flow (sccm) | 4 (ppAAc, ppAAOH, ppOD), 5 (ppAAm) |
| Plasma deposition time (min) | 20 (ppAAc, ppAAOH), 35 (ppAAm) |
| Deposition rate (nm/min) | 1.3 (ppAAc), 1 (ppAAm), 2 (ppAAOH) |
| Functional group of PP films | COOH/R (carboxyl or ester) at ppAAc COH/R (alcohol/ether) at ppAAOH |
| WCA (°) | 72 (ppAAc at 25%AAc), 56 (ppAAm), 51 (ppAAOH) |
| Cell type | keratinocytes, fibroblasts, and endothelial cells |
| Ref. [21] | Ref. [38] | |
|---|---|---|
| Precursor | 2–ethyl–2–oxazoline (C5H9NO) | |
| Synthesis method | APTD (DBD–type) | |
| Plasma power (W) | 55 | |
| Frequency (kHz) | 6 | |
| Plasma gas | N2 | |
| Plasma gas flow (sccm) | 500 | |
| Precursor gas flow (sccm) | 50–500 | |
| Plasma deposition time (min) | 23 | |
| Functional group of POx film | N–H bonds | |
| POx film thickness (μm) | 0.6 (150 °C), 1.2 (500 sccm) | |
| Rq and Ra roughness (nm) of POx film | 3.2, 2.1 | 4.8, 3.7 |
| WCA (°) | 33.4 (bare substrate), 24.1 (POx film) | |
| Bacterial type | S. aureus, E. coli | |
| Number of injected bacterial (CFU/mL) | 1.7 × 105 (S. aureus) 2.4 × 106 (E. coli) | 8.9 × 105 (S. aureus) 3.4 × 106 (E. coli) |
| Number of attached bacteria (CFU/cm2) at 150 °C, 500 sccm | 1.3 × 106 (substrate) 1.7 (S. aureus) 3.3 (E. coli) | 2.0 × 105 (substrate) 1.6 (S. aureus) 5.4 (E. coli) |
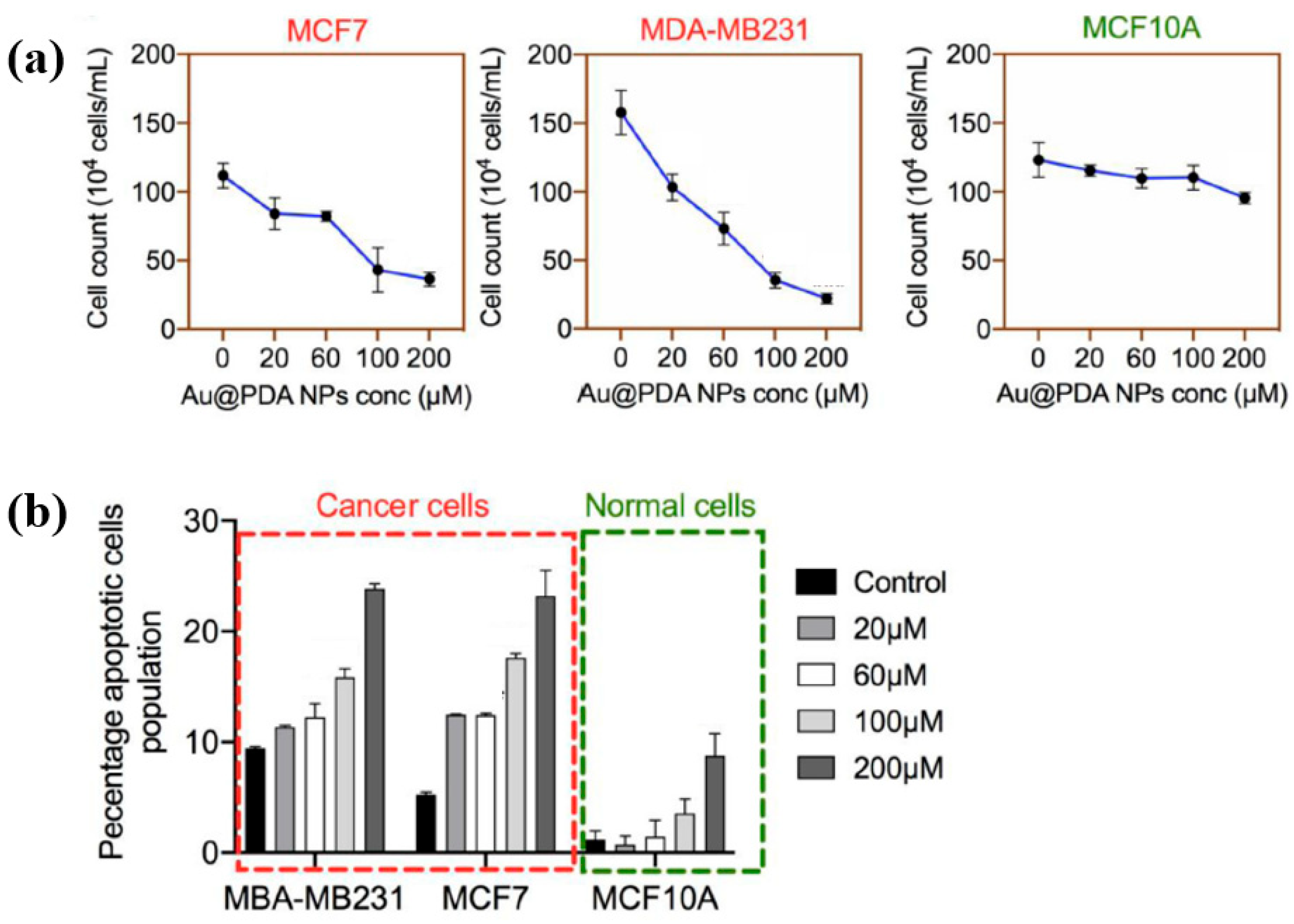
| Precursor | Mixture solution (2 mL) of 0.2 mM H[AuCl4] and 0.05 mM PDA |
| Synthesis method | AP solution plasma |
| Applied voltage (kV) | 4.8 |
| Frequency (kHz) | 28 |
| Plasma gas | Ar |
| Plasma gas flow (sccm) | 700 |
| Plasma synthesis time (min) | 5 |
| Size of PDA coated Au NPs (nm) | 42 |
| Thickness of PDA coating (nm) | 4 |
| Cell type | Breast carcinoma cells |
| Number of cells (×104 cells/mL) | 100–150 (before treatment) <20 (after treatment at 200 μM) |
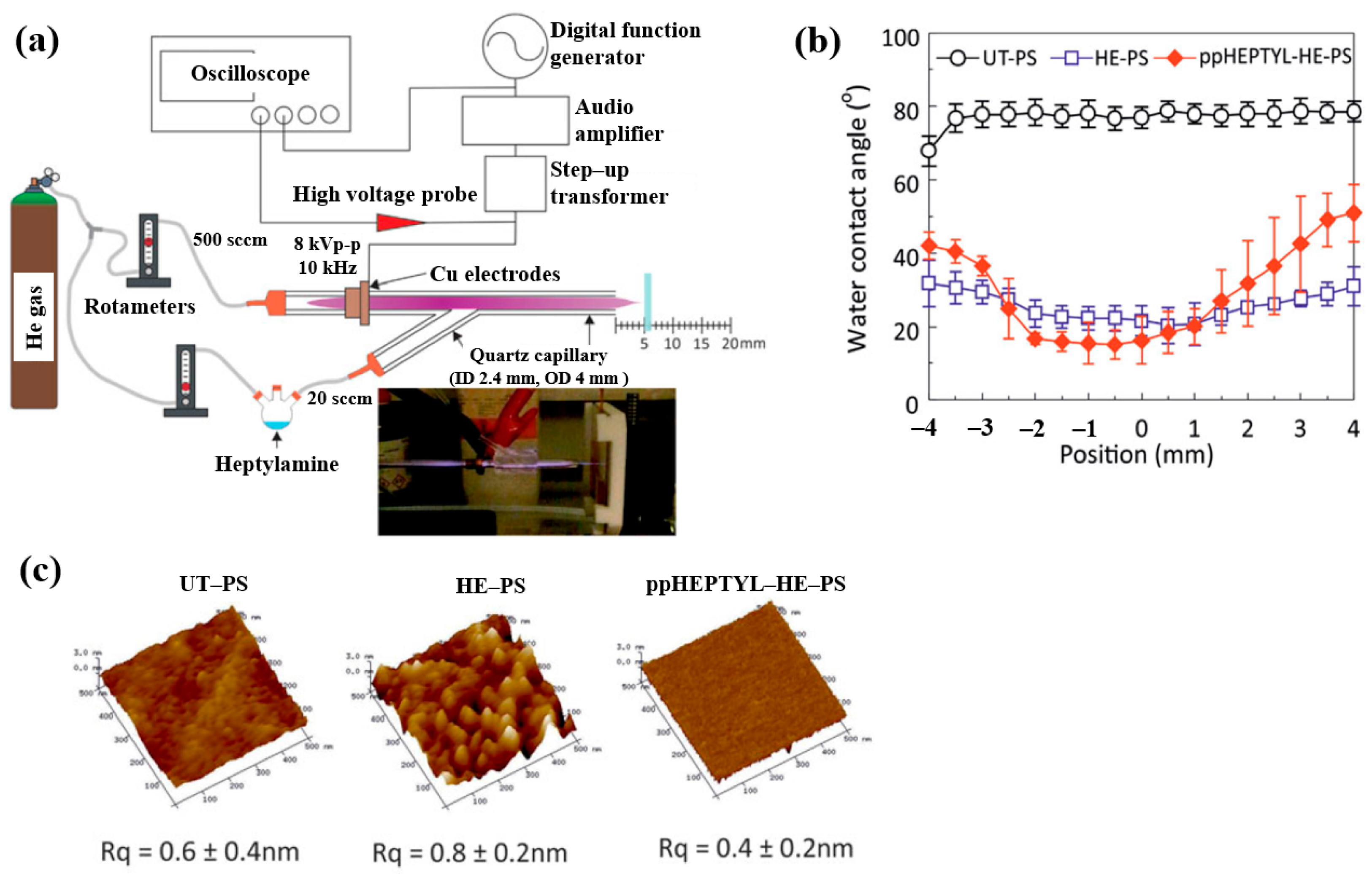
| Precursor | Heptylamine (H3C(CH2)5CH2NH2) |
| Synthesis method | APPJ |
| Applied voltage (kVp–p) | 8 |
| Frequency (kHz) | 10 |
| Plasma gas | He |
| Plasma gas flow (sccm) | 500 |
| Precursor vapor gas flow (sccm) | 20, 50, 100 by He gas |
| Plasma synthesis time (min) | 10 |
| WCA (°) | 77.2 (UT–PS) >40 (edge) or <20 (center) for ppHEPTYL–HE–PS |
| Roughness (Rq, nm) | 0.6 (UT–PS), 0.4 (ppHEPTYL–HE–PS) |
| Cell type | Human LEC line |
| Number of cells (cells/cm2) | 1 × 104 |
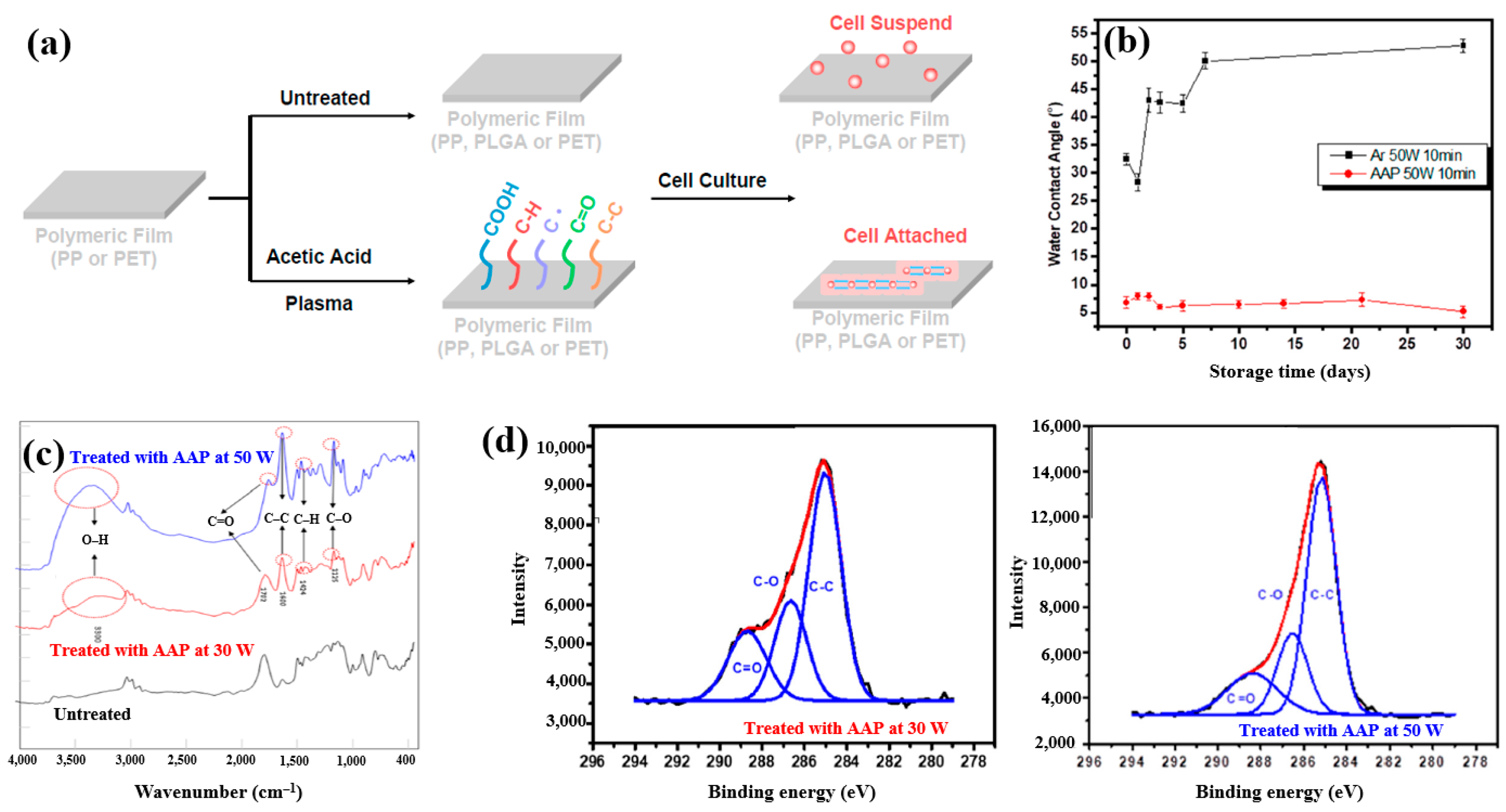
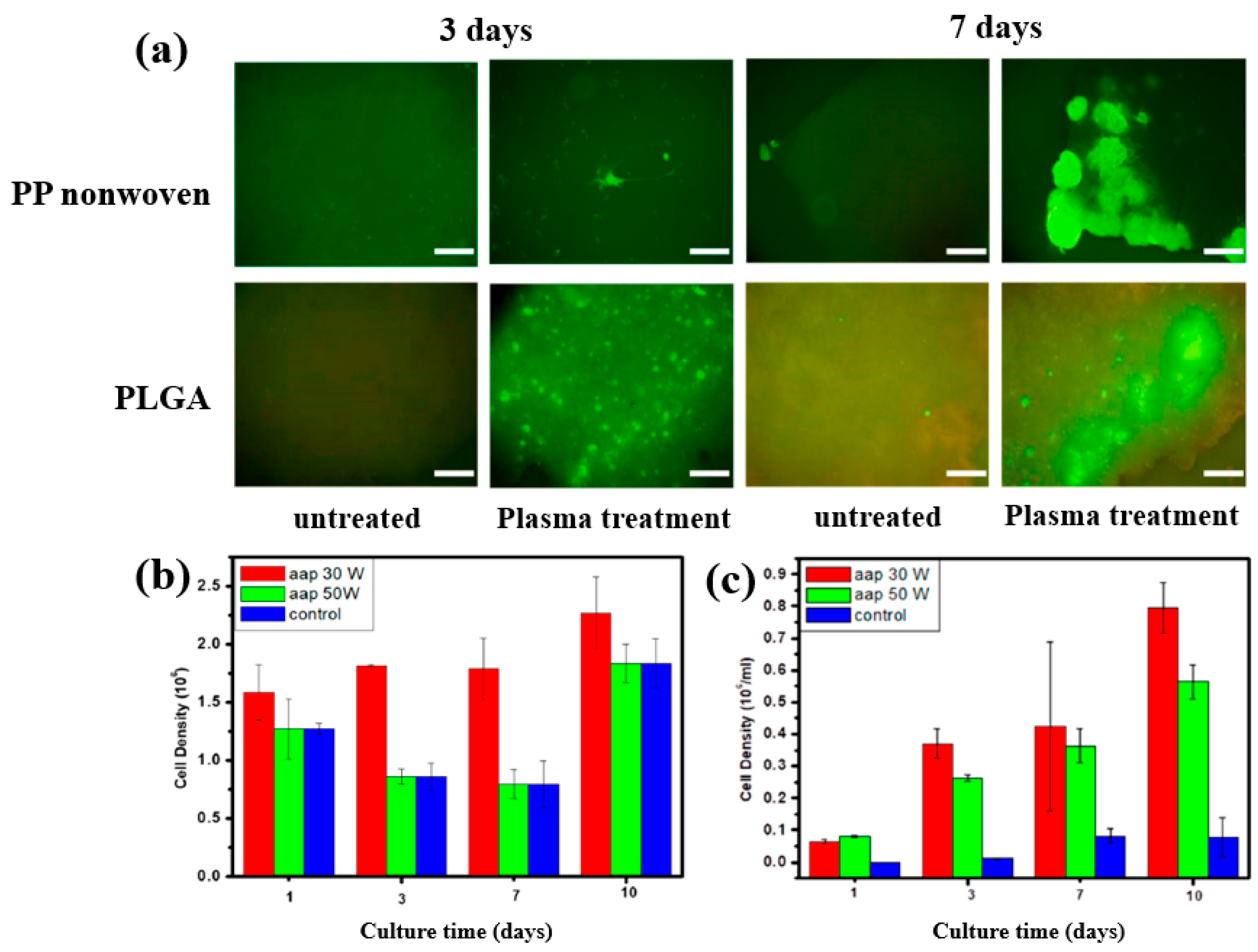
| Precursor | Acetic acid |
| Substrate | PP, PET, PLGA |
| Synthesis method | LPP |
| Plasma power (W) | 10, 30, 50 |
| Frequency (MHz) | 13.56 |
| Plasma synthesis time (min) | 10 |
| Functional groups of PP film | C–H, C=O, C–C |
| WCA (°) | 85.5 (Untreated PP), 58.0 (Untreated PLGA) 12.1 (PP with AAP), 28.8 (PLGA with AAP) |
| Cell type | Mouse embryonic stem (ES) |
| Number of incubated cells (μL) | 100 |
| Precursor | Allylamine, hexamethyldisiloxane (HMDSO) |
| Synthesis method | PECVD |
| Substrate | Metal—Al foil |
| Plasma powder (W) | 10 (HMDSO), 100 (allyamine) |
| Frequency (MHz) | 13.56 |
| Plasma synthesis time (min) | 20 |
| Cell type | Mouse fibroblasts |
| Precursor | Cyclopropylamine (CPA, C3H7N) |
| Synthesis method | LPP–RF capacitively coupled plasma |
| Plasma power (W) | 100 |
| Frequency (MHz) | 13.56 |
| Plasma discharge mode | Pulse (CPA40, CPA43), continuous (CPA42) |
| Plasma gas | Ar |
| Plasma gas flow (sccm) | 28 |
| Plasma synthesis time (min) | 60 |
| Functional groups of PP film | Amide (N–C=O, C=N), imine/nitride (C=N) |
| Cell type | Human dermal fibroblasts (HDFs), endothelial cells |
| Number of incubated cells (cells/mm2) | 15 |
| Precursor | 2–methyl–2–oxazoline (C4H7NO) |
| Synthesis method | LPP |
| Plasma gas | Air |
| Plasma deposition time (s) | 30, 50 |
| Gas for plasma treatment | O2, air |
| Plasma treatment time (s) | 30 |
| Functional groups of POx film | Oxygen containing polar groups (COOH) |
| Cell type | Prostate carcinoma |
| Precursor | Acetylene (C2H2) |
| Synthesis method | LPP RF–PECVD |
| Plasma power (W) | 10, 20, 30 |
| Frequency (MHz) | 13.56 |
| Plasma gas | N2 |
| Plasma gas flow (sccm) | 40 |
| Precursor vapor gas flow (sccm) | 20 |
| Functional groups of L–PPA:N film | Amine (NH2), alkyl, carboxyl |
| Roughness (Rq, nm) of L–PPA:N film | 1.0 (10 W), 2.9 (20 W), 3.8 (30 W) |
| Cell type | Mesenchymal stem cells (MSCs) |
| Precursor | Acetylene (C2H2) |
| Synthesis method | RF plasma |
| Plasma power (W) | 50 |
| Substrate | Titanium foil |
| Plasma gas | Ar |
| Plasma gas flow (sccm) | 15 |
| N2/C2H2 gas flow ratio | 0, 0.5, 1, 2, 5 |
| Functional groups of IPP film | Amide |
| Cell type | Osteoblast cells (mouse long bones) |
| Number of incubated cells (cells/well) | 103∼104 |
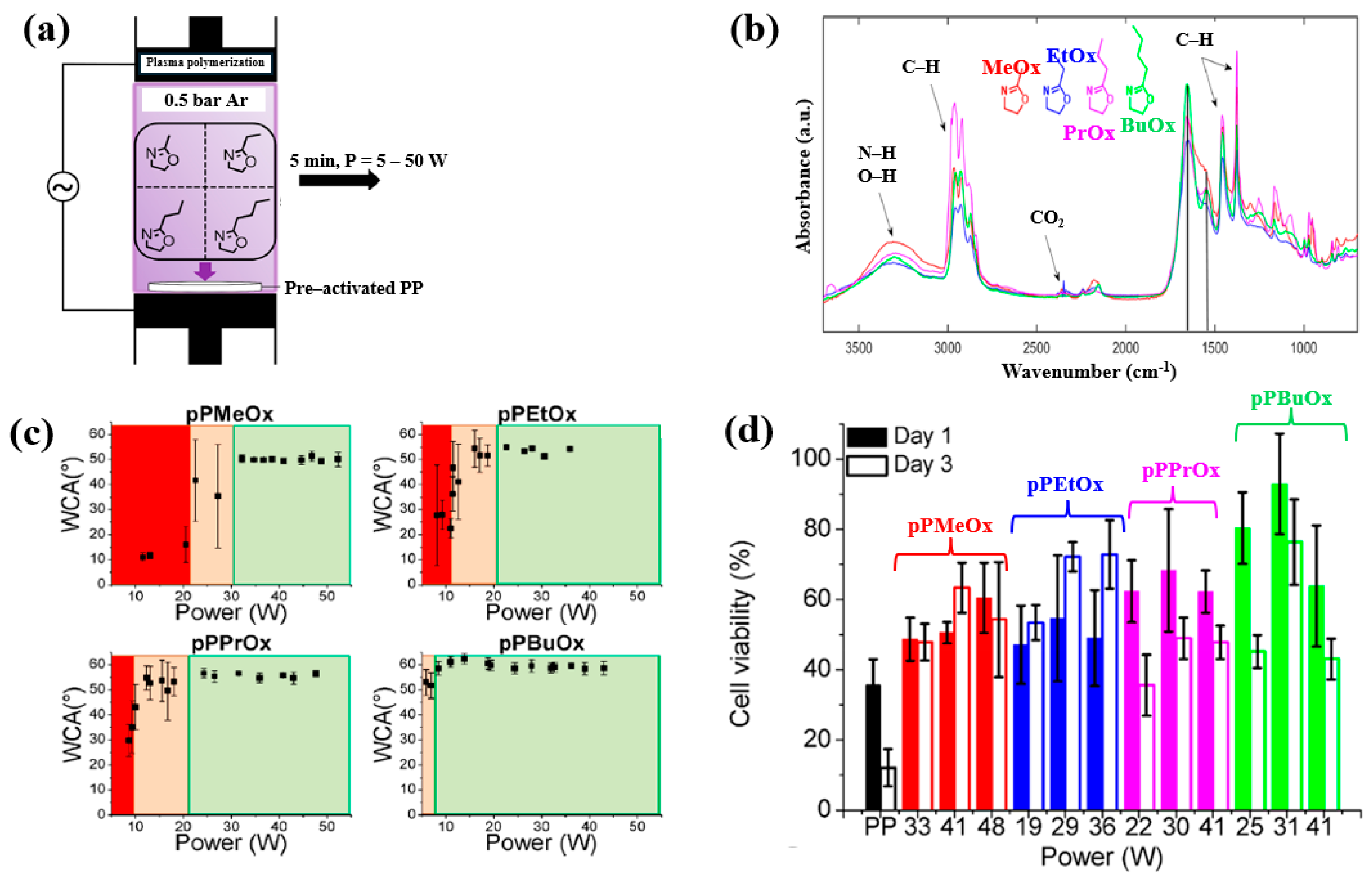
| Polymer materials | pPMeOx, pPEtOx, pPPrOx, and pPBuOx |
| Precursor | Four 2–alkyl–2–oxazolines (MeOx, EtOx, PrOx, and BuOx) |
| Applied peak voltage (kV) | 9.5 |
| Substrate | Polypropylene (PP) |
| Plasma gas | Ar |
| Plasma gas flow (SLM) | 3 |
| Vapor gas flow for monomer (SLM) | 7 |
| Plasma treatment time (min) | 5 |
| Functional groups of polymer film | Amide, imine |
| Cell type | Human foreskin fibroblasts (HFFs) |
| Number of incubated cells (cells/1000 μL) | 10.000 |
| Precursor | 3–methylphenol (M–cresol, C7H8O) |
| Synthesis method | DBD–plasma |
| Plasma voltage (Vp–p, kV) | 30 |
| Frequency (kHz) | 40 |
| Substrate | Silicon wafer |
| Plasma gas | Open air |
| Plasma synthesis time (s) | 30 |
| Functional groups of polymer film | Benzene ring with a hydroxyl functional group |
| Cell type | E. coli |
| Number of incubated cells (μL) | 10 |
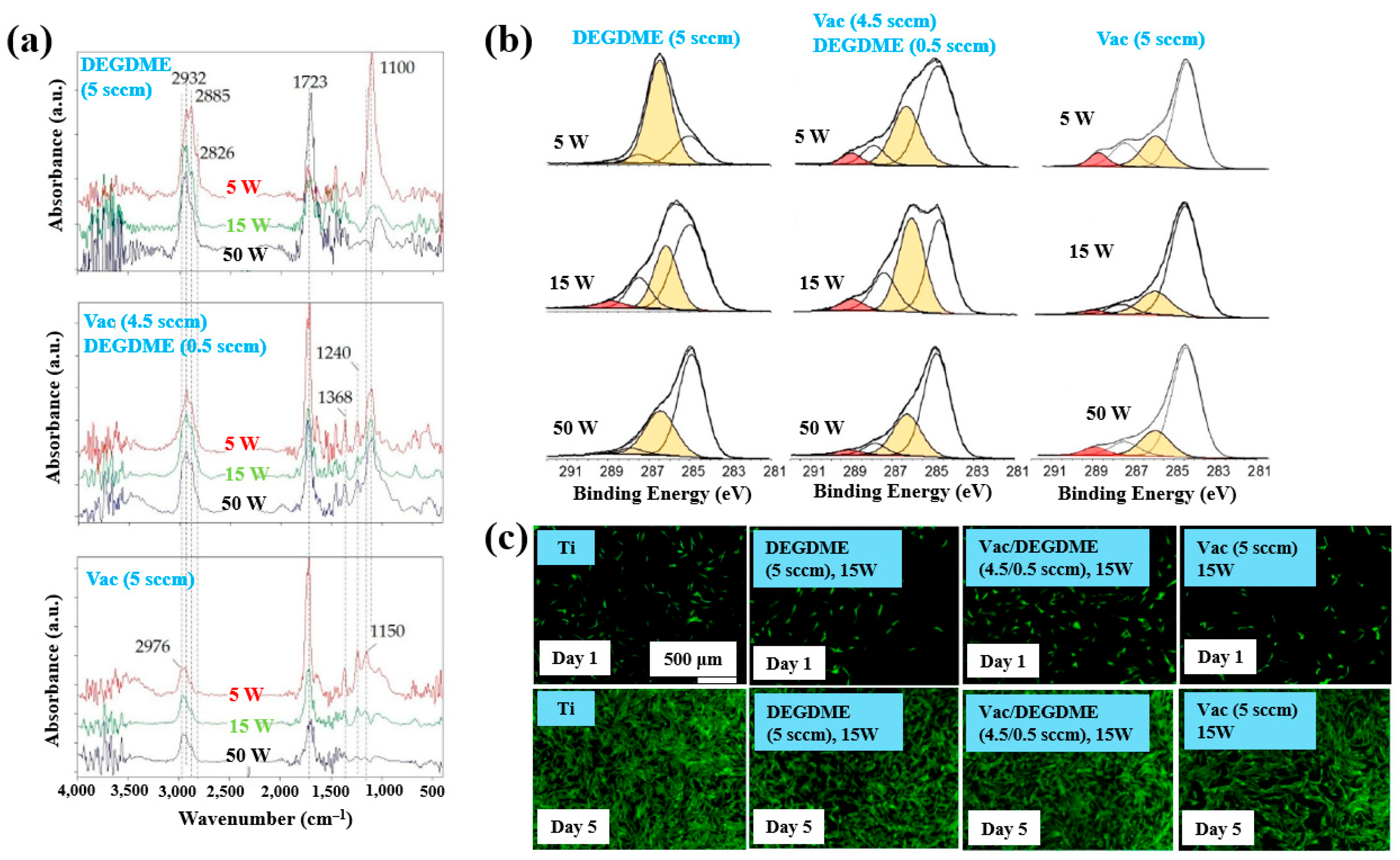
| Deposition Step | Sample Notification | C2H4 (sccm) | He–TEGDME (SLM) | He (SLM) | Voltage (kVp–p) | Frequency (kHz) | Deposition (s) |
|---|---|---|---|---|---|---|---|
| Step 1 | 8 | 8 | 8.5 | 16, 26 | 60 | ||
| Step 2 | 8 | 3.15 | 4.85 | 8.5 | 16, 26 | 10 | |
| Step 3 | PEOA PEOB | 3.15 3.15 | 4.85 6.85 | 6.5, 8.5 6.5, 8.5 | 16, 26 26 | 300 300 |
| Precursor | Vinyl acetate (VAc), Diethylene glycol dimethyl ether (DEGDME) |
| Synthesis method | LP and AP PE–CVD |
| Substrate | Ti, polycarbonate (PC) |
| Plasma gas | He |
| Functional groups of polymer film | C–OH, Ester/carboxyl groups (–COOR (H)) |
| Cell type | Human dermal fibroblasts (HDFs), osteoblasts |
| Number of incubated cells (cells/mL) | 1 × 105 |
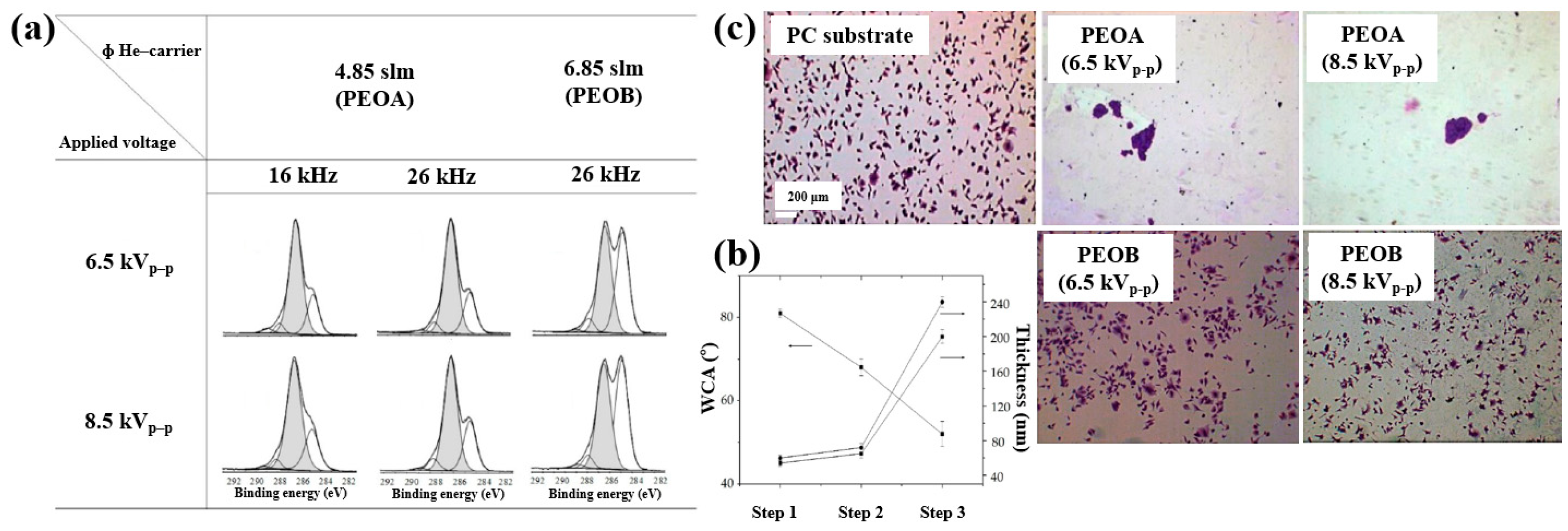
| Sample Notification | DEGDME (sccm) | Ar (sccm) | O2 (mTorr) | Power (W) | Deposition (min) | |
|---|---|---|---|---|---|---|
| PEO1 | 0.4 | 5 | 5 | 60 | ||
| PEO2 | 0.4 | 5 | 10 | 60 | ||
| Ox | 100 | 20 | 200 | 15 | ||
| Ox+PEO1 | Step 1 | 100 | 20 | 200 | 15 | |
| Step 2 | 0.4 | 5 | 5 | 60 | ||
| Ox+PEO2 | Step 1 | 100 | 20 | 200 | 15 | |
| Step 2 | 0.4 | 5 | 10 | 60 |
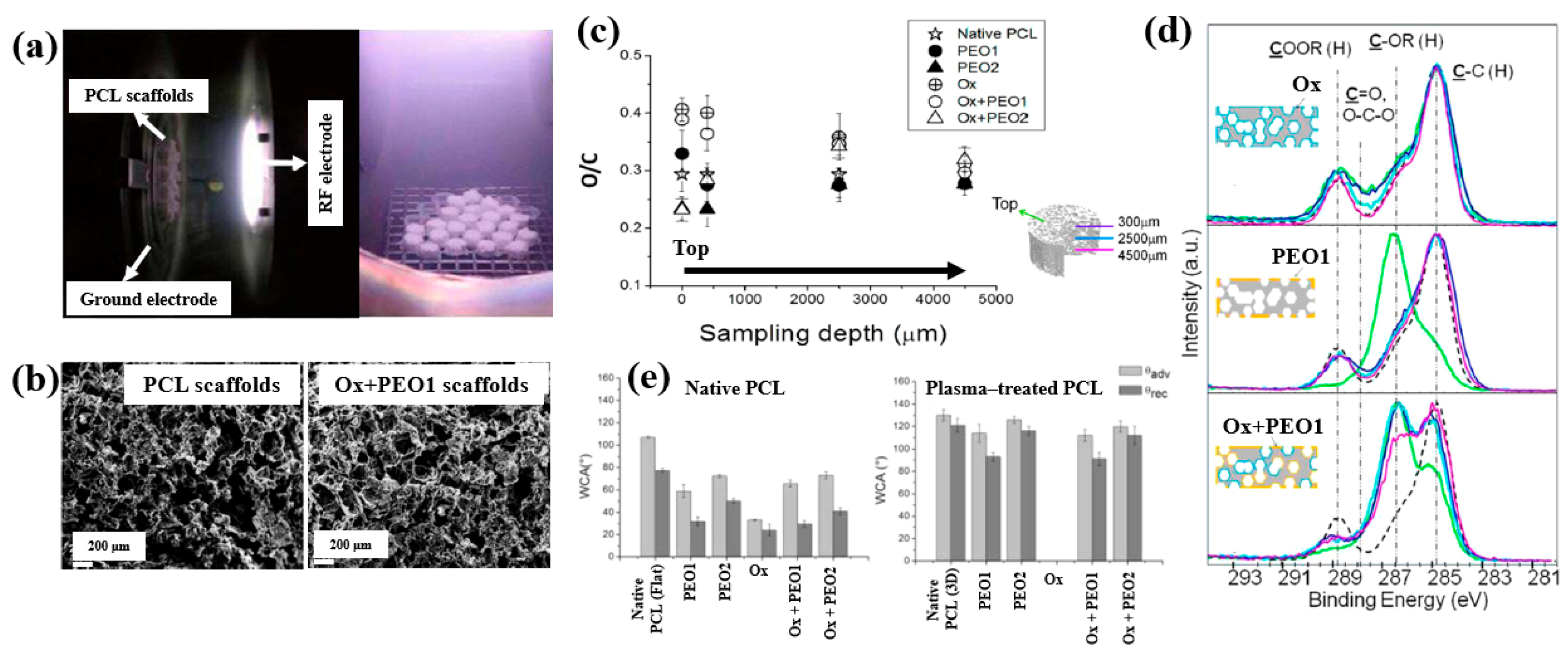
| Polymer | PEO |
| Synthesis method | LPP–RF plasma |
| Plasma power (W) | 5 (PEO 1), 10 (PEO 2) |
| Plasma gas | Ar, O2 |
| Substrate | PCL scaffolds |
| Functional groups of polymer | Oxygen-containing functionalities |
| Cell type | Saos–2 osteoblast cells |
| Number of incubated cells (cells/well) | 5 × 104 |
| Polymer | Mg contained 3D–PCL scaffolds |
| Synthesis method | LPP–RF sputtering |
| Plasma power (W) | 50 |
| Plasma gas | Ar, H2O, H2, Ar/H2O, and Ar/H2 |
| Total gas flow (sccm) | 20 |
| Deposition time (min) | 60 |
| Cell type | Saos–2 osteoblast cells |
| Number of incubated cells (cells/well) | 5 × 104 |
| Precursor | EGDMA, DOMAm monomers |
| Polymer | Poly(EGDMA–co–DOMAm) copolymer |
| Synthesis method | APP–DBD plasma |
| Discharge waveform | Sinusoidal pulse |
| Frequency (kHz) | 10 |
| Plasma power (W/cm2) | 1.6 |
| Substrate | Zirconia |
| Plasma gas | Ar |
| Deposition time (s) | 8 |
| Plasma gas flow (SLM) | 20 |
| Functional groups of PP film | Amide (–CONH), Ester group (–COO–) |
| Cell type | Human osteoblast–like MG–63 cells |
| Number of incubated cells (cells/well) | 2 × 104 |
| Identification | Sample Conditions |
|---|---|
| PCL–ref | Electrospinning PCL NFs |
| PCL–COOH | Plasma polymer coating on PCL–ref |
| PCL–P1 | PRP treatment on PCL–ref |
| PCL–COOH–P2 | PRP treatment on PCL–COOH |
| PCL–COOH–P3 | DCC treatment on PCL–COOH |
| Precursor | Acetylene (C2H2) |
| Synthesis method | LPP–RF plasma |
| Plasma power (W) | 500 |
| Substrate | Polycaprolactone nanofibers (PCL NFs) |
| Plasma gas | Ar/CO2/C2H4 |
| Plasma gas flow (sccm) | 50/16.2/6.2 |
| Deposition time (min) | 15 |
| Functional groups of PP film | Amides (N–C=O), –COOH, C=O |
| Cell type | Bone marrow (BM) mesenchymal stromal cells (MSCs) |
| Number of incubated cells (cells/well) | 5 × 103 |
| No | Object | Plasma Source | Application | Year | Author Reference |
|---|---|---|---|---|---|
| 1 | Furfuryl methacrylate (FMA) | LPP—RF plasma (13.56 MHz) | Cell Adhesion | 2016 | Shirazi et al. [57] |
| 2 | APTES | APP–DBD Ar plasma | Cell Adhesion | 2020 | Chen et al. [58] |
| 3 | Polydopamine (PDA) | APP–PECVD | Cell Adhesion | 2018 | Czuba et al. [59] |
| 4 | Maleic anhydride (MA) and cetylene | APP–DBD RF plasma (13.56 MHz) | Cell Adhesion | 2017 | Manakhov et al. [60] |
| No | Object | Plasma Source | Application | Year | Author Reference |
|---|---|---|---|---|---|
| 1 | AgNP/CS nanocomposite | APP solution plasma | Antibacterial | 2020 | Sun et al. [34] |
| 2 | Au, AgNP/PVA nanocomposite | APP solution plasma | Antibacterial | 2018 | Nolan et al. [35] |
| 3 | 3–(Aminopropyl)triethoxysilane (APTES) | APPJ | Cell adhesion | 2022 | Sainz–García et al. [12] |
| 4 | PP–lim | APPJ | Antibacterial | 2023 | Masood et al. [36] |
| 5 | ppAAc, ppAAm, ppAAOH | APP plasma RF (13.56 MHz) | Cell adhesion | 2016 | Smith et al. [37] |
| 6 | Polyoxazoline (pPOx) | APP–DBD plasma, (6 kHz, 55 W) | Antibacterial | 2020 | Mazánková et al. [21] |
| 7 | Polyoxazoline (pPOx) | APP–DBD plasma, (6 kHz, 55 W) | Antibacterial | 2019 | St’ahel et al. [38] |
| 8 | Au NP/PDA nanocomposite | APP solution plasma | Medical | 2021 | Nguyen et al. [39] |
| 9 | Au NP/PDA nanocomposite | APP solution plasma | Medical | 2020 | Nguyen et al. [40] |
| 10 | PpHEPTYL | APPJ (8 kVp–p, 10 kHz) | Cell adhesion | 2019 | Doherty et al. [41] |
| 11 | Acetic acid | LPP plasma RF (13.56 MHz) | Cell adhesion | 2019 | Liao et al. [42] |
| 12 | polyHMDSO, polyallyamine | PECVD RF (13.56 MHz) | Cell adhesion | 2019 | Teske et al. [43] |
| 13 | Cyclopropylamine (CPA) | LPP–RF plasma (13.56 MHz) | Cell adhesion | 2016 | Štrbková et al. [44] |
| 14 | Polyoxazoline (pPOx) thin films | LPP–RF plasma (13.56 MHz) | Medical | 2021 | Gheorghiu et al. [45] |
| 15 | L–PPA:N film | LPP–PECVD (13.56 MHz) | Cell adhesion | 2017 | Ghafouri et al. [46] |
| 16 | N2–rich acetylene polymer film | LPP–RF plasma (13.56 MHz) | Cell adhesion | 2021 | Sharifahmadian et al. [47] |
| 17 | pPMeOx, pPEtOx, pPPrOx, and pPBuOx | LPP–DBD Ar plasma (50 kHz) | Cell adhesion | 2019 | Van Guyse et al. [48] |
| 18 | M–cresol | APP–DBD plasma | Antibacterial | 2022 | Hartl et al. [49] |
| 19 | Polyethylene oxide (PEO) | LPP, APP–PECVD (13.56 MHz) | Cell adhesion | 2020 | Sardella et al. [50] |
| 20 | Polyethylene oxide (PEO) scaffolds | LPP–DBD RF plasma (13.56 MHz) | Cell adhesion | 2017 | Sardella et al. [51] |
| 21 | Mg coated poly-ε caprolactone (PCL) | LPP–DBD RF plasma (13.56 MHz) | Cell adhesion | 2020 | Armenise et al. [52] |
| 22 | Polydopamine (PDA)/acrylate copolymer | APP–PECVD | Cell adhesion | 2021 | Hod’asov’a et al. [53] |
| 23 | Polycaprolactone (PCL) NFs | LPP–RF plasma (13.56 MHz) | Cell Adhesion | 2017 | Solovieva et al. [54] |
| 24 | Furfuryl methacrylate (FMA) | LPP–RF plasma (13.56 MHz) | Cell Adhesion | 2016 | Shirazi et al. [55] |
| 25 | 3–(Aminopropyl)triethoxysilane (APTES) | APP–DBD Ar plasma | Cell Adhesion | 2020 | Chen et al. [56] |
| 26 | Polydopamine (PDA) | APP–PECVD | Cell Adhesion | 2018 | Czuba et al. [57] |
| 27 | Maleic anhydride (MA) and acetylene | APP–DBD RF plasma (13.56 MHz) | Cell Adhesion | 2017 | Manakhov et al. [58] |
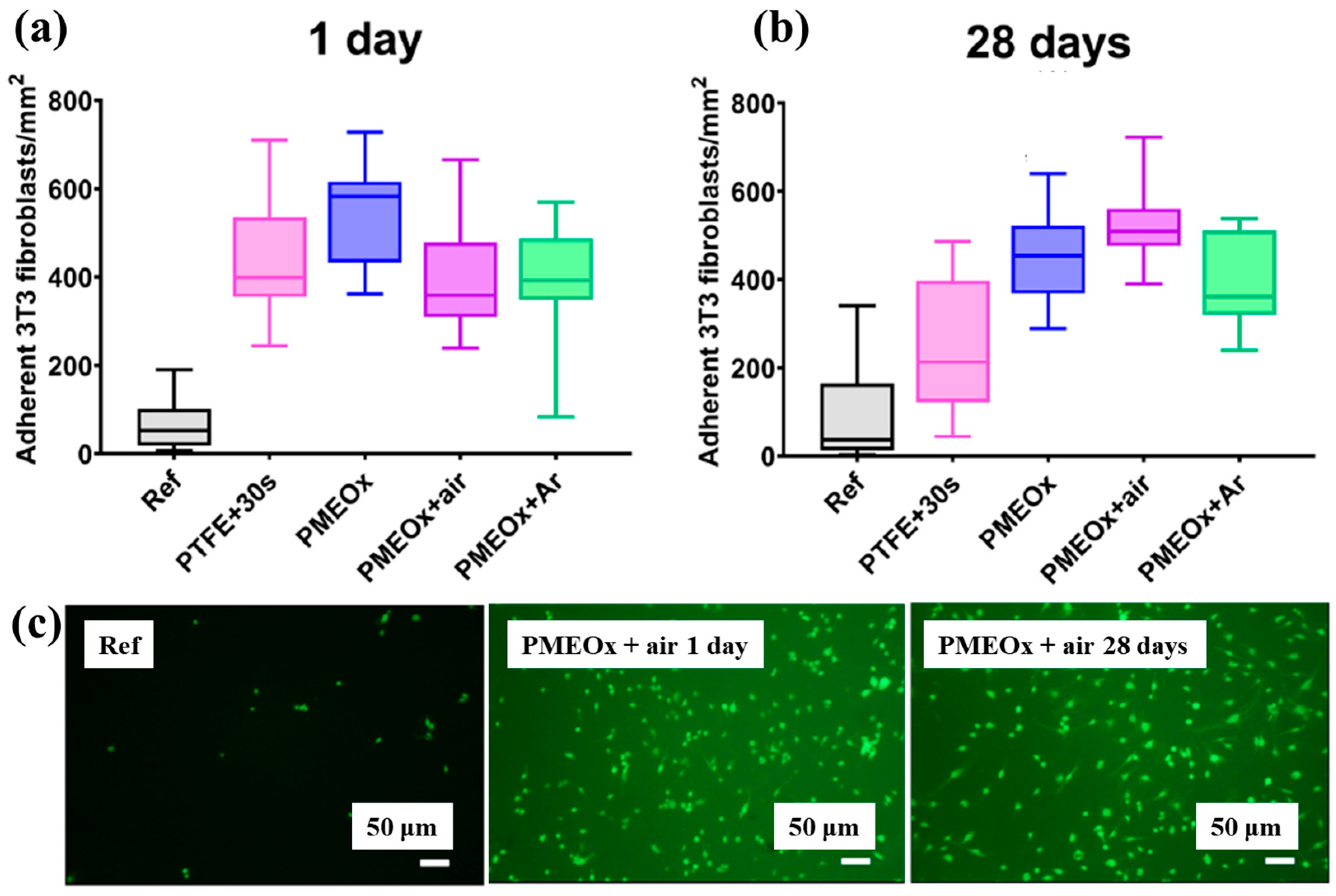
| Polymer | POx |
| Surface treatment method | APP–DCSBD |
| Applied voltage (kV) | 20 |
| Frequency (kHz) | 15 |
| Plasma gas | Air, Ar |
| Plasma gas flow rate (L/min) | 1 |
| Functional groups of POx film | Amines, ester, carbonyl and amide |
| Roughness (nm) | 0.7 (PMEOx–air), 25.8 (after PMEOx deposition) |
| WCA (°) | 59.5 (PMEOx–air), 61.4 (PMEOx–Ar) |
| Cell type | Mammalian (mice fibroblasts) cells |
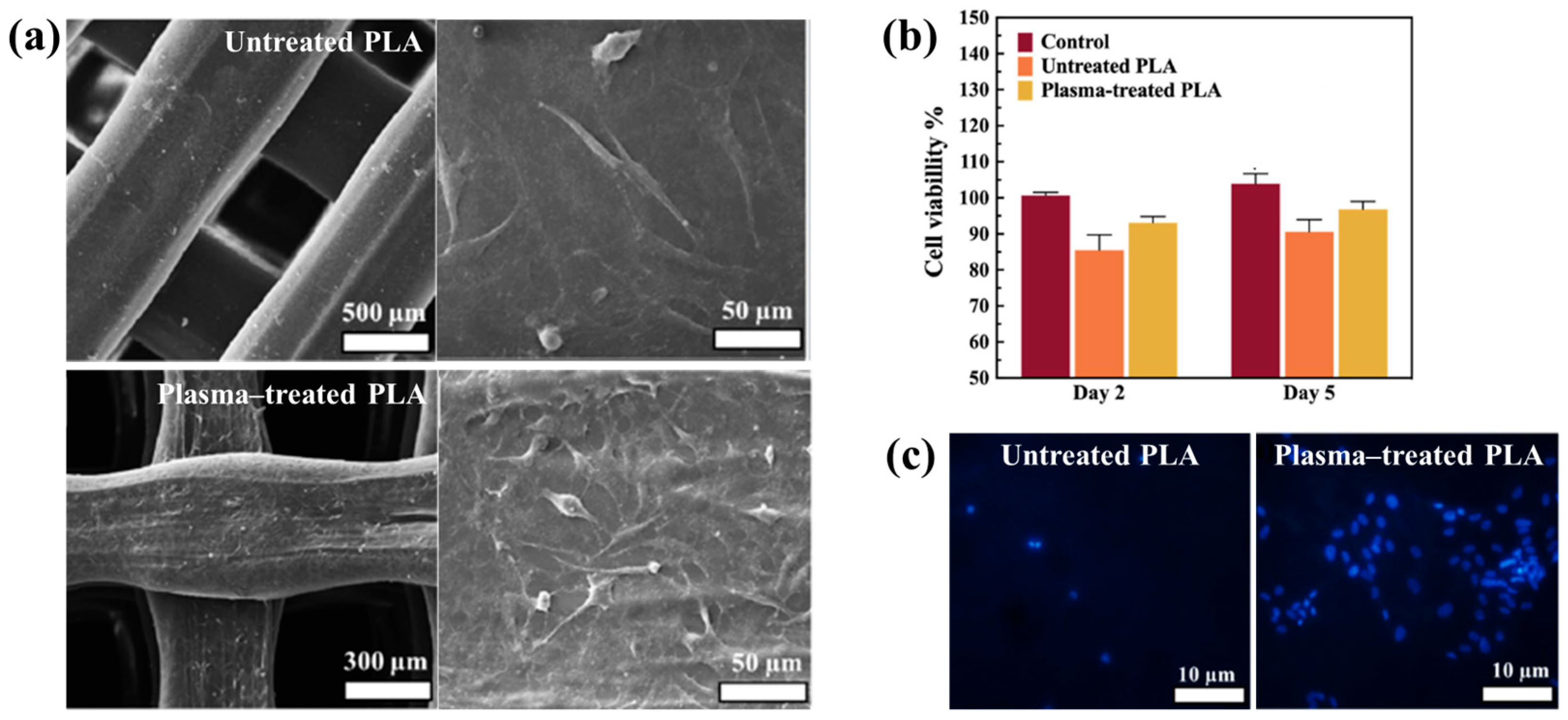
| Polymer | PLA |
| Surface treatment method | In situ APP (DBD type) with 3D printing technique |
| Plasma voltage (kV) | 15 |
| Plasma gas | Ar |
| Plasma gas flow rate (L/min) | 1.5 |
| Functional groups of 3D-printed PLA | C=O, C–O bonds |
| Roughness (Rq) of PLA (nm) | 1.5 (untreated), 70 (Ar–treated) |
| WCA (°) | 92.5 (untreated), 42.2 (Ar–treated) |
| Cell type | Human adipose-derived stem cells (hADSCs) |
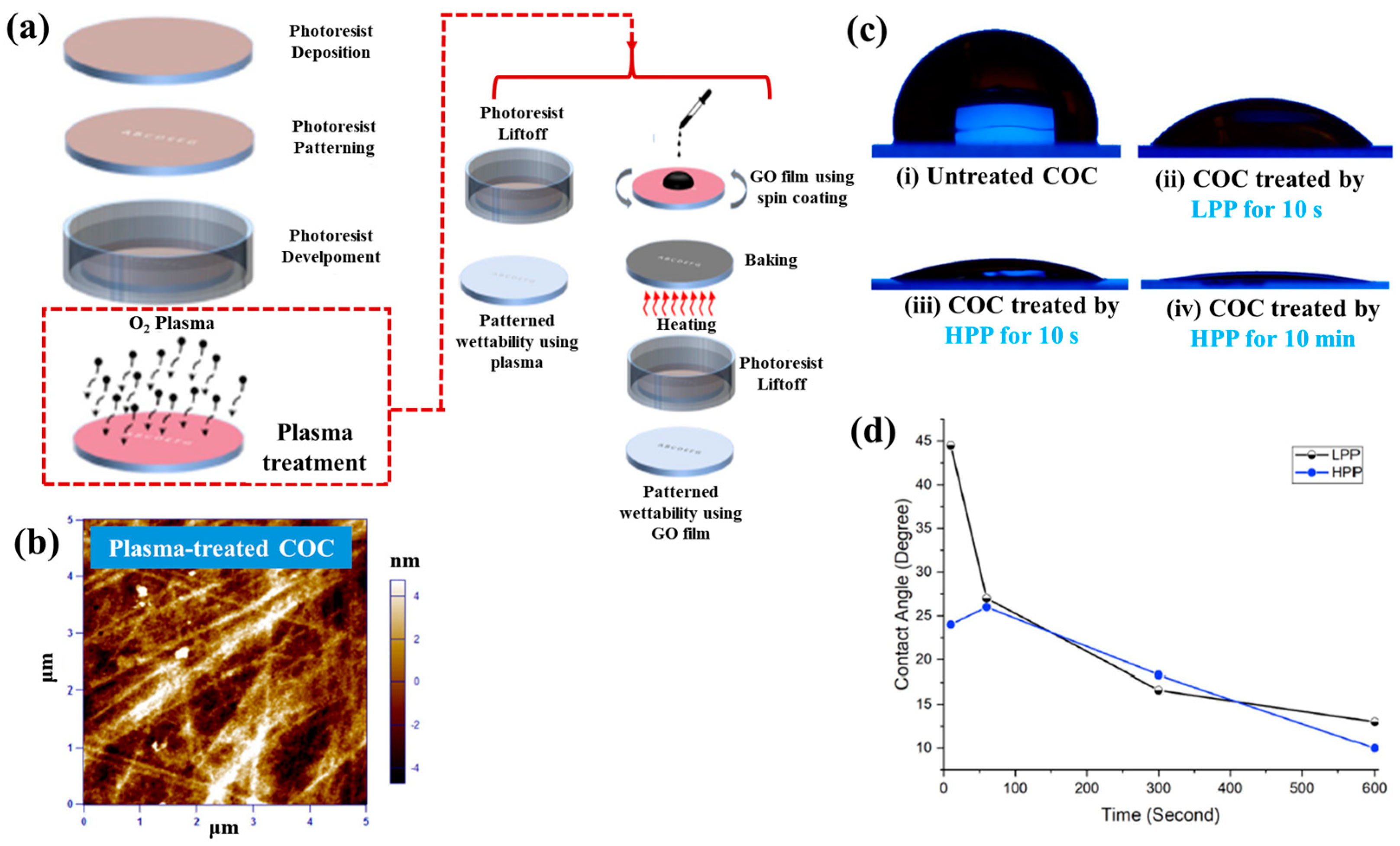
| Polymer | Cyclic olefin copolymer (COC) |
| Surface treatment method | LPP, HPP inductive plasma |
| Plasma power (W) | 7.2 (LPP), 29.6 (HPP) |
| Plasma gas | O2 |
| Plasma treatment time (s, min) | 10 s (LPP), 10 s, 10 min (HPP) |
| Roughness (Rq) of COC (nm) | 2.5 (O2–treated) |
| WCA (°) | 110 (untreated), 20 (O2-treated) |
| Cell type | Human breast cancer cells |
| Number of incubated cells | 10 mL containing 5 × 105 cells |
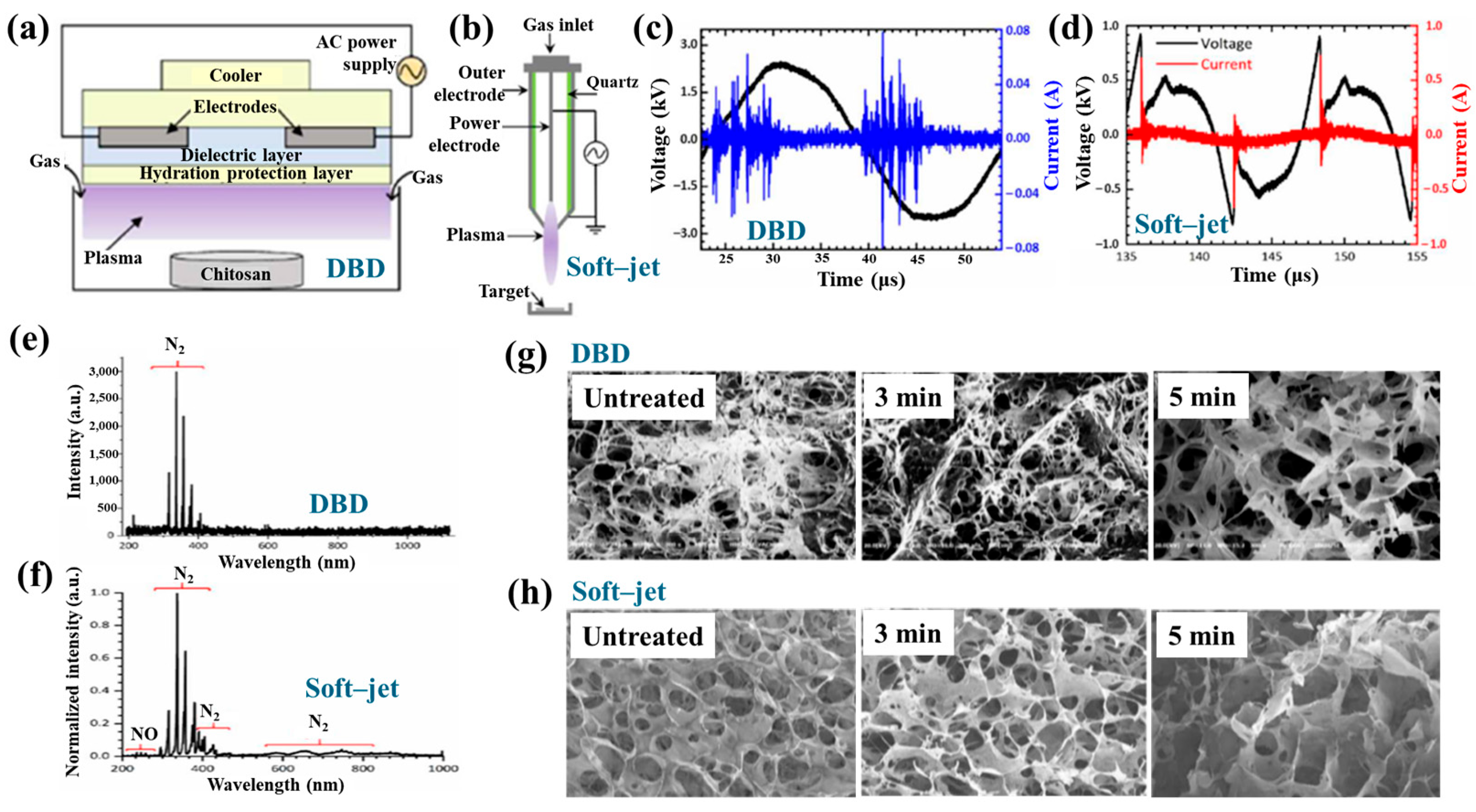
| Precursor | CS scaffolds |
| Surface treatment method | DBD, soft–jet plasma (APPJ) |
| Applied peak voltage (Vp, kV) | 2 (DBD), 0.5 (soft–jet) |
| Plasma gas | N2 |
| Plasma gas flow (lpm) | 1.5 |
| Plasma treatment time (min) | 5 |
| Functional groups of CS scaffolds | Amino and hydroxyl groups |
| Cell type | Bone marrow (BM)–derived stem cells (BMSCs) |
| Number of incubated cells (cells) | 1 × 105 |
| Material | Poly(vinyl alcohol) (PVA) |
| Plasma method | DBD–APPJ |
| Discharge voltage (kV) | 9 Vp–p |
| Frequency (kHz) | 30 |
| Plasma gas | He |
| Plasma gas flow (SLM) | 0.5 |
| Plasma treatment time (min) | 20 |
| Functional groups of PVA film | RNS, H2O2 |
| Cell type | E. coli, P. Aeruginosa, and S. aureus |
| Number of incubated cells (cells/mL) | 1 × 106 |
| Zone of inhibition (ZOI) (mm) | >10 (He plasma treatment at 20 min) |
| Material | PCL, P(3HB) |
| Plasma gas | O2, CO2 |
| Functional groups of PCL and P(3HB) | Amino, oxygen group |
| WCA (°) | PCL: 71 (untreated), 50 (CO2), 51 (O2) P(3HB): 69 (untreated), 44 (CO2), 44 (O2) |
| Cell type | Mouse fibroblast cells (L929) |
| Cell viability (%) | PCL:98 (CO2), 107 (O2) P(3HB): 114 (CO2), 76 (O2) |
| Polymer material | PEO–PEOT/PBT thin film |
| Plasma treatment method | MP–DBD |
| Plasma power (W) | 3 (Ar, Dry air), 6 (He, N2) |
| Frequency (kHz) | 50 |
| Plasma gas | Ar, He, N2, Dry air |
| Plasma gas flow (SLM) | 3 |
| Plasma treatment time (min) | 3 |
| Functional groups | C–O, C–N |
| WCA (°) | 59 (Untreated) 45 (Ar), 31 (N2) |
| Cell type | Human foreskin fibroblast (HFF) |
| Number of incubated cells (cells/mL) | 40,000 |
| Polymer material | dECM thin film |
| Plasma method | Microwave–LPP |
| Plasma power (kW) | 100 |
| Plasma gas | N2, H2 |
| Plasma gas pressure (mTorr) | 300 |
| Plasma treatment time (s) | 60 |
| Functional groups | Amine and amide groups (C–O/C–N, –N–C=O) |
| WCA (°) | 92.4 (Untreated), 86.1 (plasma–treated) |
| Cell type | Human dermal fibroblasts (HDFs) |
| Number of incubated cells (cells/cm2) | 5 × 104 |
| Sample Label | APPJ Treatment | UV–Graftrf Hydrogels | |
|---|---|---|---|
| Time (s) | HEMA (mL) | PEGMA (mL) | |
| Untreated | |||
| Treatment A | 60 s | ||
| Treatment B | 90 s | ||
| Treatment A—H1 | 60 s | 15 | 15 |
| Treatment A—H2 | 60 s | 10 | 20 |
| Treatment A—H3 | 60 s | 20 | 10 |
| Treatment B—H1 | 90 s | 15 | 15 |
| Treatment B—H2 | 90 s | 10 | 20 |
| Treatment B—H3 | 90 s | 20 | 10 |
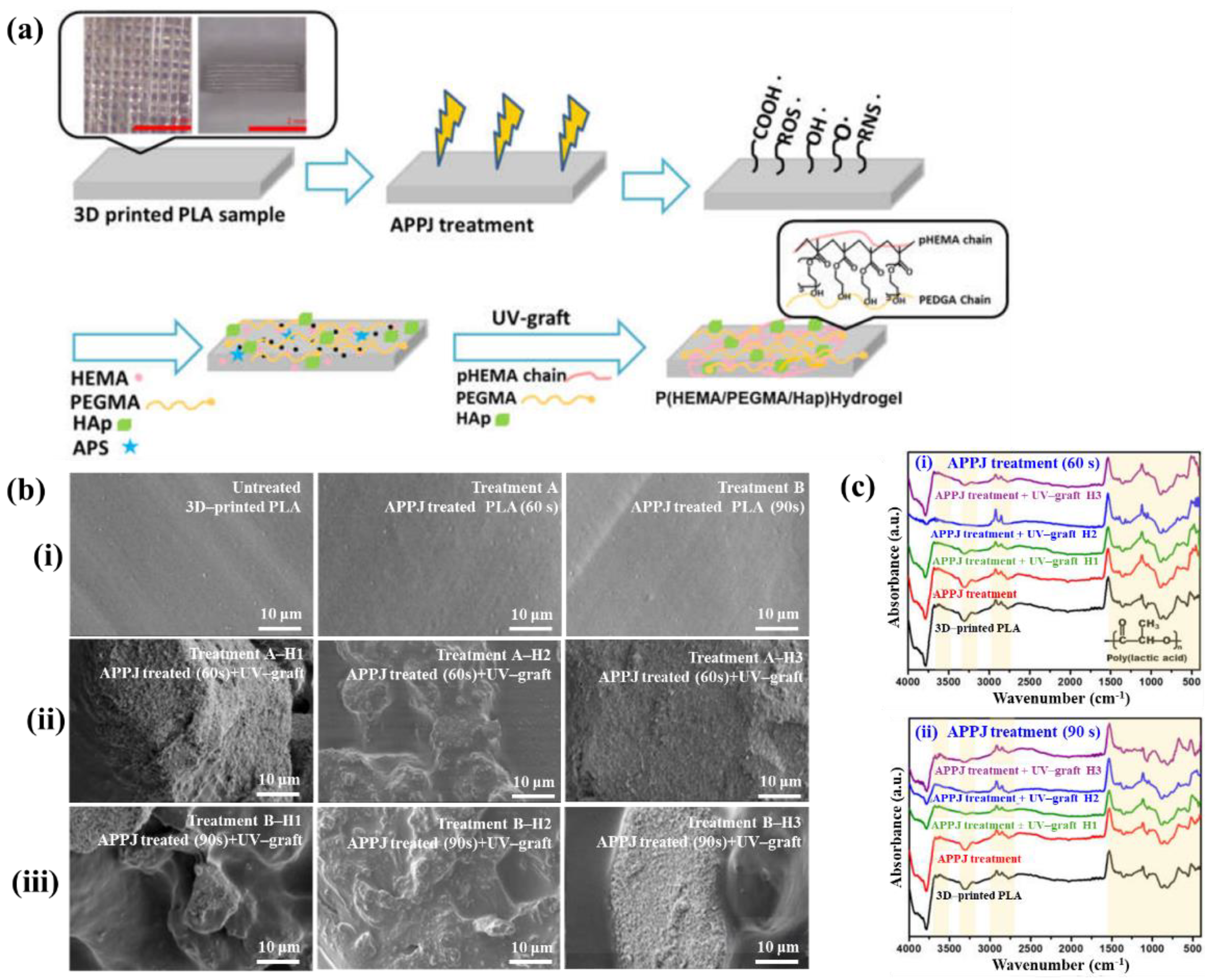
| Polymer | Poly(lactic acid) (PLA), HEMA, PEGMA |
| Plasma treatment method | APPJ |
| Plasma power (W) | 600 |
| Plasma gas | Ar |
| Plasma gas flow (SLM) | 20 |
| Plasma treatment time (s) | 60, 90 |
| Functional groups of PP film | C–O–C, carbonyl peaks (C=O) |
| Cell type | Osteoblast MG63 cells |
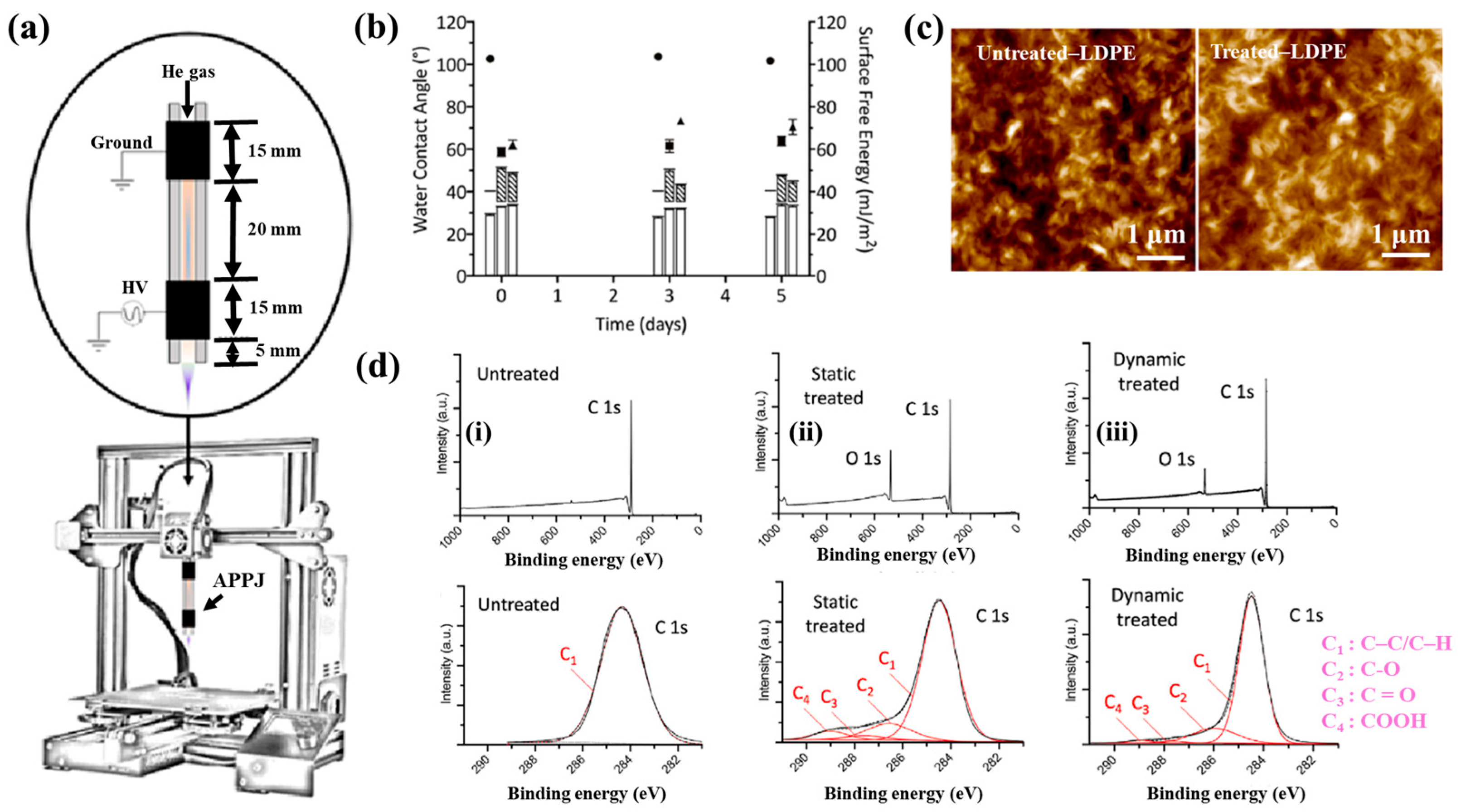
| Polymer | Low–density polyethylene (LDPE) film |
| Plasma treatment method | APPJ plasma |
| Voltage waveform | AC sinusoidal signal |
| Applied voltage (kVp–p) | 9 |
| Frequency (kHz) | 36 |
| Plasma gas | He |
| Plasma gas flow (L/min) | 8.1 |
| Plasma treatment time (s) | 10 |
| Functional groups of PP film | C–OH/R, C–O, COOH, and C–O–O–C |
| Cell type | DNA |
| Number of incubated cells (μL) | 160 |
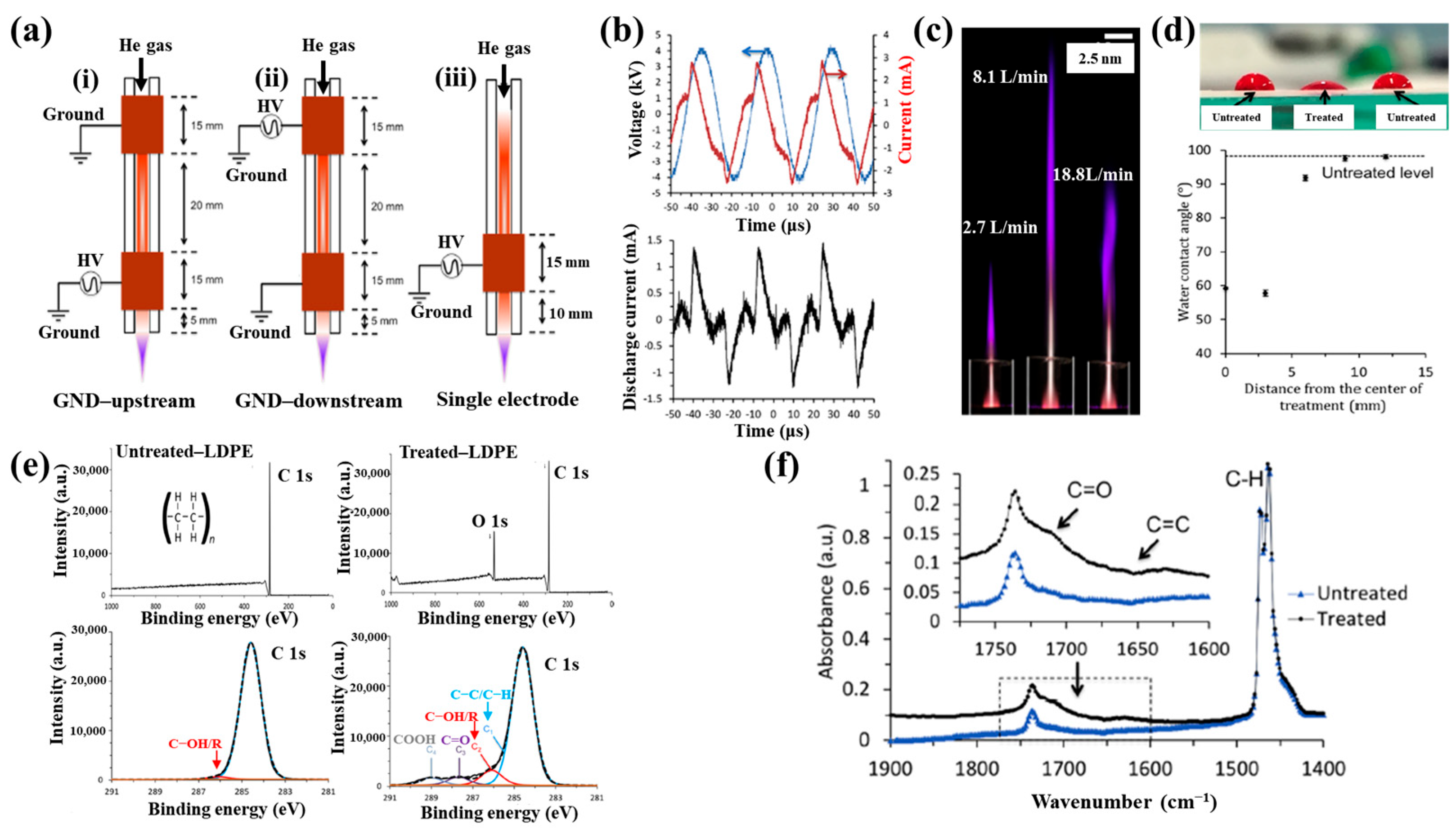
| Polymer | LDPE, poly–ε–caprolactone (PCL) |
| Plasma treatment method | APPJ plasma |
| Voltage waveform | AC sinusoidal signal |
| Average plasma power (W) | 3.2 |
| Frequency (kHz) | 31.1 |
| Plasma gas | He |
| Plasma gas flow (L/min) | 1.9 |
| Plasma treatment time (s) | 5 |
| Functional groups of PP film | C=O, C=C |
| Cell type | GM3348 human dermal fibroblast cells |
| Number of incubated cells (cells/cm2) | 5000 |
| No | Object | Plasma Source | Application | Year | Author Reference |
|---|---|---|---|---|---|
| 1 | Polyvinyl alcohol/chitosan (PVA/Cs) films | APP–DBD Ar plasma | Antibacterial | 2020 | Paneru et al. [70] |
| 2 | Polystyrene (PS) films | DBD Air plasma (APPJ) | Cell adhesion | 2018 | Bitara et al. [71] |
| No | Object | Plasma Source | Application | Year | Author Reference |
|---|---|---|---|---|---|
| 1 | PTFE, POx coating layer | APP plasma | Cell adhesion | 2020 | Šrámková et al. [59] |
| 2 | PLA scaffolds | In situ APP plasma | Cell adhesion | 2023 | Zarei et al. [60] |
| 3 | Cyclic olefin copolymer (COC) | LPP plasma | Cell adhesion | 2022 | Al-Azzam et al. [61] |
| 4 | CS scaffolds | DBD, soft jet plasma (APPJ) | Cell adhesion | 2022 | Han et al. [62] |
| 5 | PVA hydrogel film | APPJ (He, 9 kVp–p, 30 kHz) | Antibacterial | 2024 | Sabrin et al. [63] |
| 6 | PCL, P(3HB) film | CO2, O2 plasma | Antibacterial | 2017 | Teske et al. [64] |
| 7 | PEOT/PBT copolymer film | DBD plasma (50 kHz) | Cell adhesion | 2018 | Cools et al. [65] |
| 8 | dECMfs | LPP microwave plasma (100 kW) | Antibacterial | 2024 | Lombardo et al. [66] |
| 9 | 3D-printed poly(lactic acid) (PLA) | APPJ | Cell adhesion | 2023 | Liao et al. [67] |
| 10 | Low-density polyethylene (LDPE) | APPJ | Cell adhesion | 2024 | Lotz et al. [68] |
| 11 | Low-density polyethylene (LDPE) Poly–ε caprolactone (PCL) | APPJ | Cell adhesion | 2020 | Alavi et al. [69] |
| 12 | Polyvinyl alcohol/chitosan (PVA/Cs) films | APP–DBD Ar plasma | Antibacterial | 2020 | Paneru et al. [70] |
| 13 | Polystyrene (PS) films | DBD Air plasma (APPJ) | Cell adhesion | 2018 | Bitara et al. [71] |
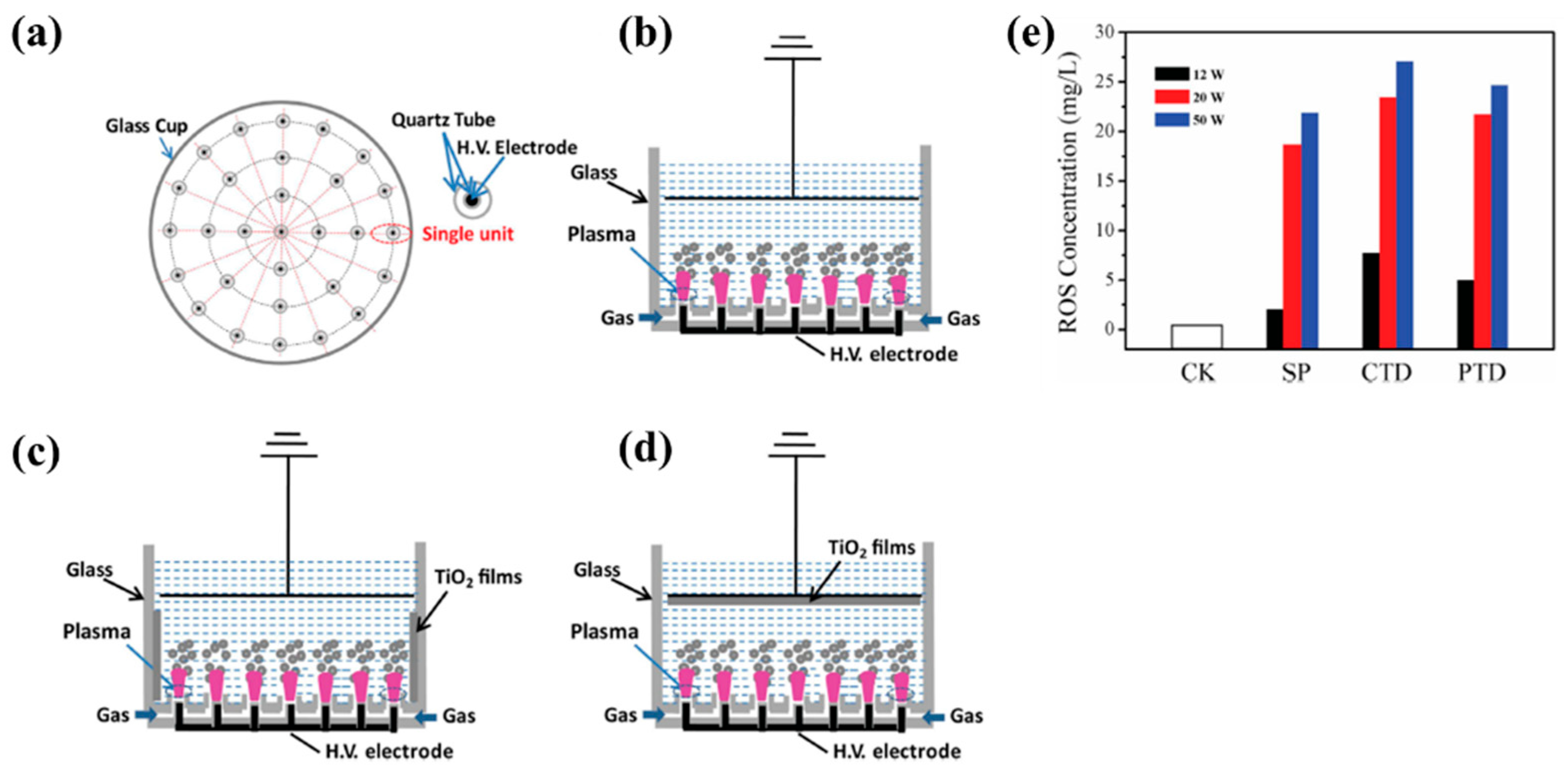
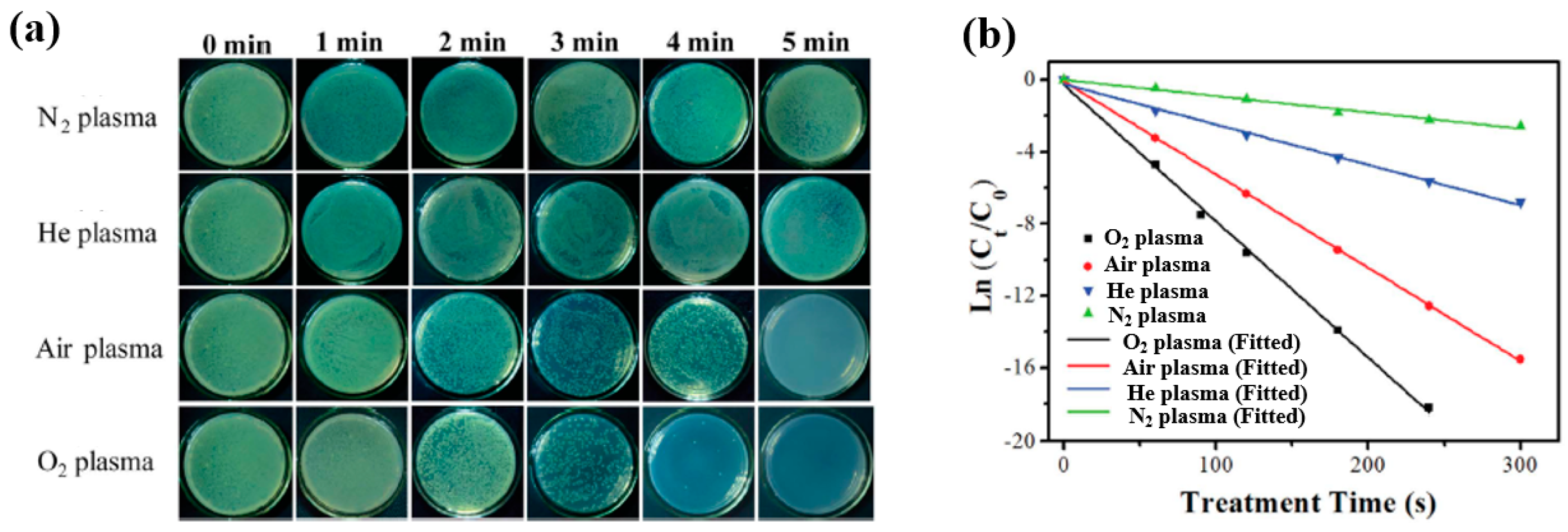
| Material | TIO2 NPs film |
| Plasma method | APP |
| Applied voltage (kV) | 4.5 |
| Frequency (kHz) | 9 |
| Plasma gas | O2, Air, He, N2 |
| Plasma gas flow (SLM) | 2 |
| Plasma treatment time (min) | 0, 1, 2, 3, 4, and 5 |
| Cell type | E. coli |
| Number of incubated cells (CFU/mL) | 106 |
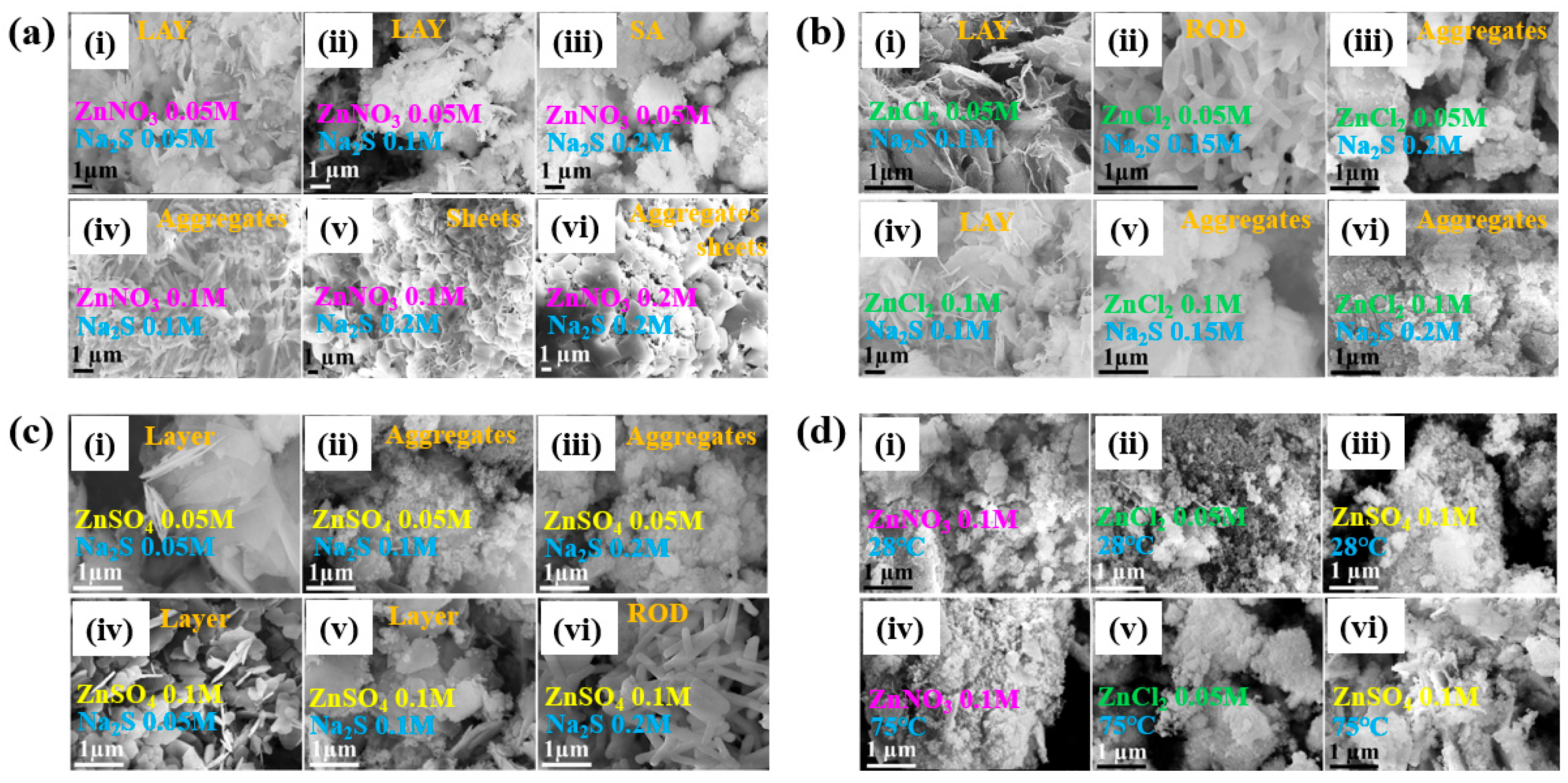
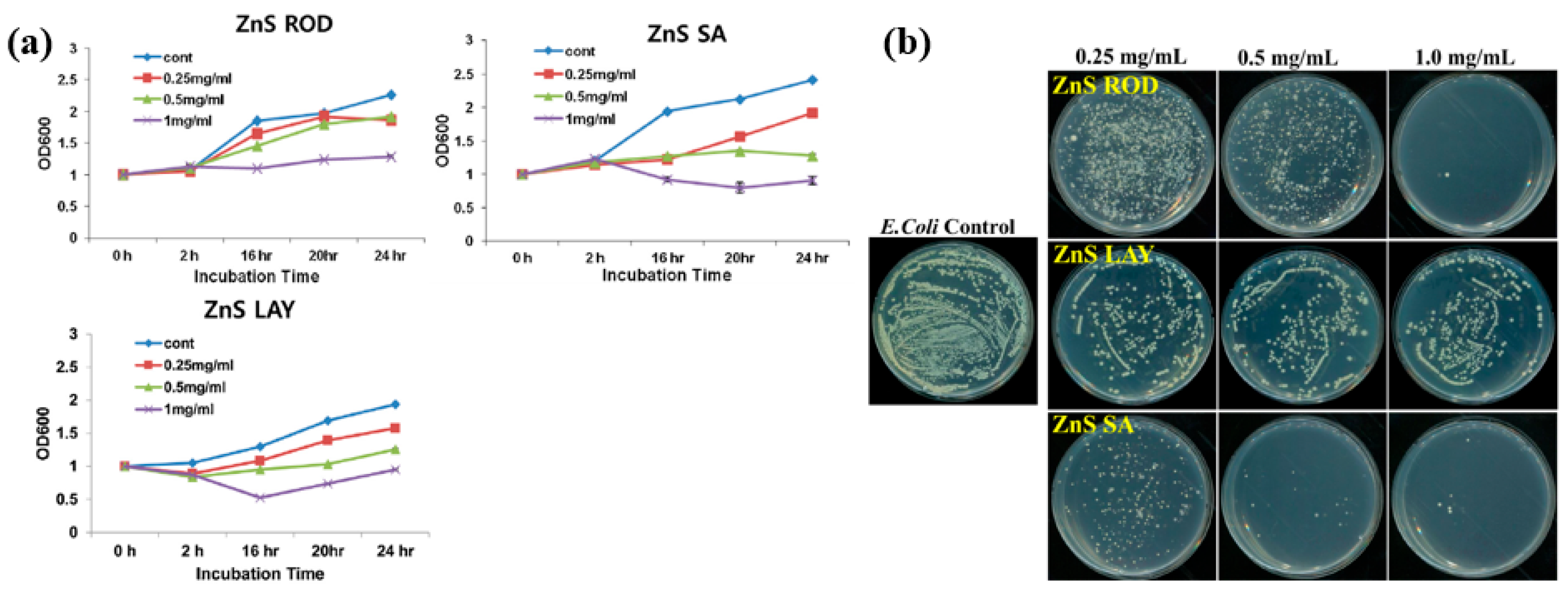
| Precursor | Zn(NO3)2, ZnCl2, ZnSO4, and Na2S |
| Synthesis method | APPJ |
| Plasma synthesis time (h) | 1 |
| Cell type | E. coli, S. aureus |
| Number of incubated cells (CFU/mL) | 106 |
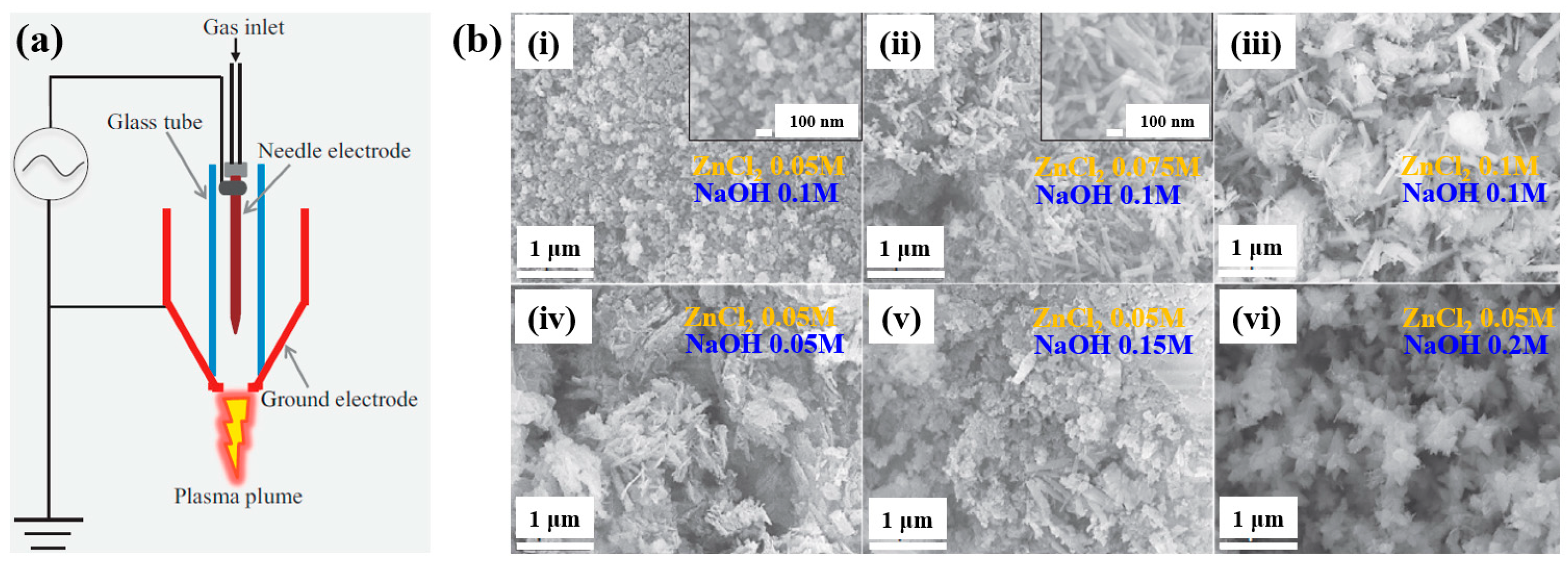
| Precursor | ZnCl2, NaOH |
| Synthesis method | AP soft jet (APPJ) |
| Applied voltage (kV) | 0.5 |
| Current (A) | 0.1 |
| Plasma gas | Air |
| Plasma gas flow (L/min) | 2 |
| Plasma synthesis time (hr) | 1 |
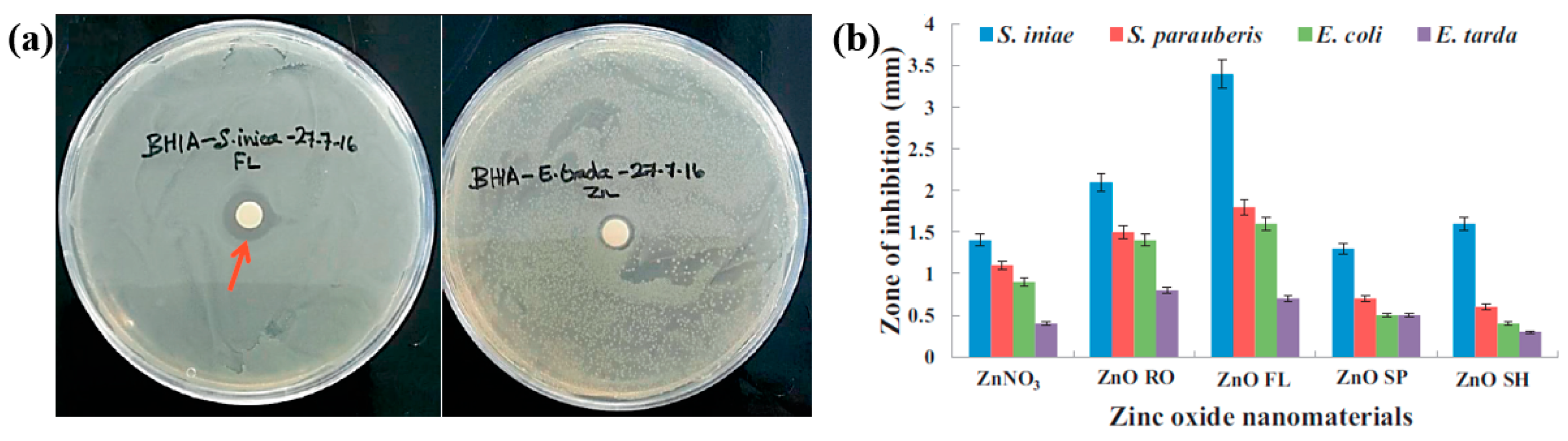
| Cell type | E. coli, S. iniae, S. parauberis, and E. tarda |
| Number of incubated cells (CFU/mL) | 106 |
| Length of ZOI (mm) | 3.4 (ZnO RO at S. iniae) |
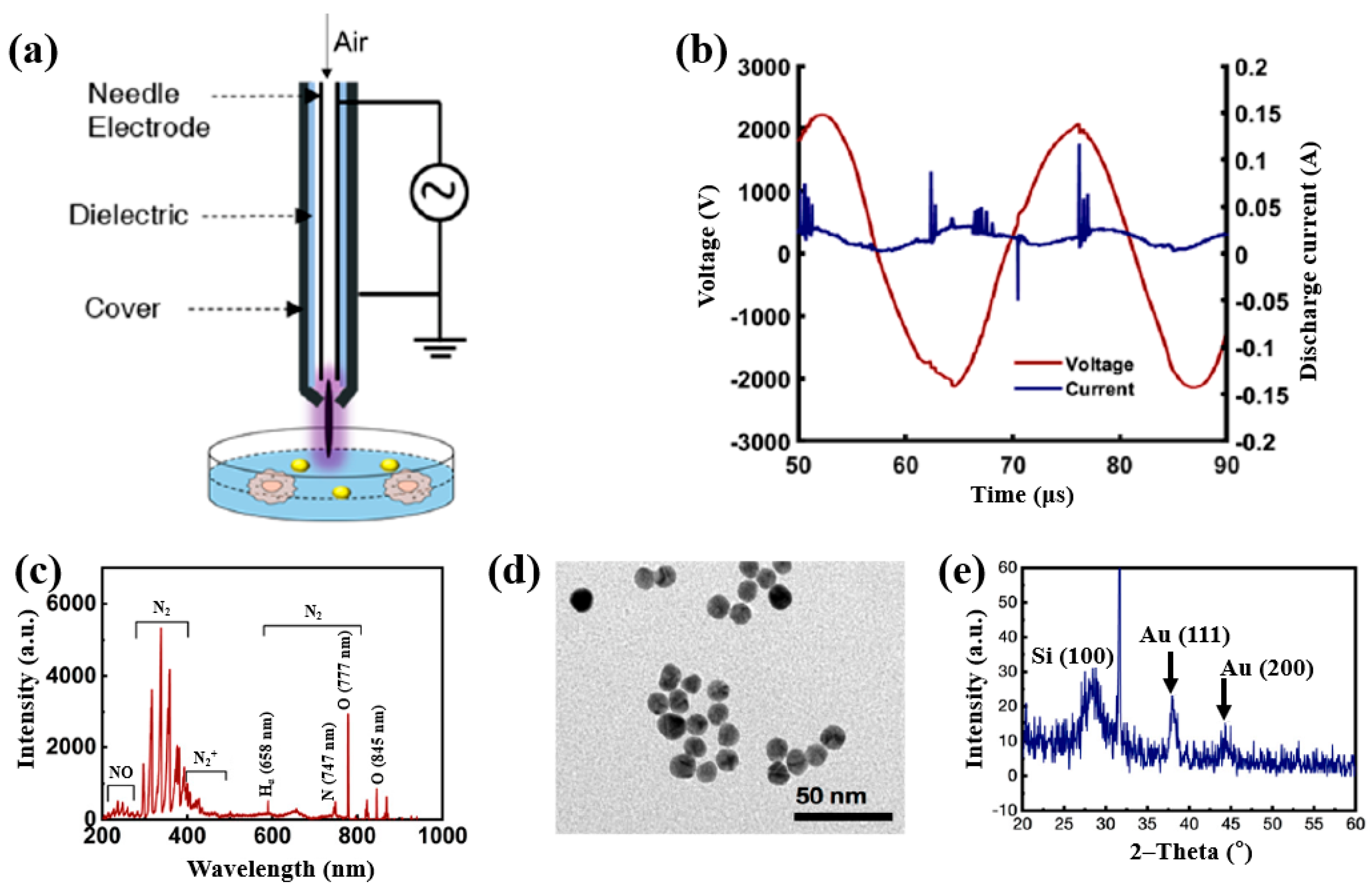
| Precursor | Chloroauric acid trihydrate (H[AuCl4]·3H2O) |
| Plasma synthesis | AP soft jet plasma (APPJ) |
| Applied voltage (kV) | 2.2 |
| Frequency (kHz) | 42 |
| Plasma gas | Air |
| Plasma gas flow (L/min) | 1 |
| Plasma treatment time (s) | 200 |
| Functional groups | Free radicals (RONS, H2O2) |
| Cell type | Brain cancer cells (U373) |
| Number of incubated cells (cells/mL) | 5 × 104 |
| No | Object | Plasma Source | Plasma Process | Application | Year | Author Reference |
|---|---|---|---|---|---|---|
| 1 | TiO2 NPs | APP solution plasma | Plasma surface treatments | Antibacterial | 2016 | Zhou et al. [72] |
| 2 | ZnS NPs | Soft jet plasma (APPJ) | Plasma synthesis | Antibacterial | 2020 | Ananth et al. [73] |
| 3 | ZnO NPs | Soft jet plasma (APPJ) | Plasma synthesis | Antibacterial | 2017 | Ananth et al. [74] |
| 4 | AuQDs | APP soft jet plasma (APPJ) | Plasma synthesis | Medical | 2020 | Kaushik et al. [75] |
| No | Object | Plasma Process | Year | Author Reference |
|---|---|---|---|---|
| 1 | Synthesis of polymer film and NPs | CAP, APPJ | 2021 | Jang et al. [76] |
| 2 | Plasma synthesis and surface treatment of polymer film and NPs for nanogenerators and sensors | CAP, APPJ | 2024 | Jung et al. [77] |
| 3 | Plasma techniques in biomedical field | CAP, APPJ | 2024 | Karthik et al. [78] |
| 4 | Plasma treatment in agriculture field | CAP, APPJ | 2022 | Waskow et al. [79] |
| 5 | Plasma techniques for NP synthesis | CAP | 2022 | Radetić et al. [80] |
| 6 | Plasma surface technology of polymer in industrial field | CAP | 2022 | Förster et al. [81] |
| 7 | Plasma polymerization of biogenic precursors | CAP, APPJ | 2023 | Loesch–Zhang et al. [82] |
| 8 | Plasma surface treatment of polymer | APPJ | 2024 | Bertin et al. [83] |
| 9 | Plasma synthesis of polymer in biomedical field | APPJ | 2024 | Rahman Khan et al. [84] |
| 10 | Plasma techniques in medical field | CAP, APPJ | 2024 | O’Neill et al. [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, E.Y.; Shin, B.J.; Suleiman, H.O.; Tae, H.-S.; Park, C.-S. Review of Plasma-Synthesized/Modified Polymer and Metal Nanoparticles for Biomedical Applications Using Cold Atmospheric Pressure Plasma. Polymers 2025, 17, 2856. https://doi.org/10.3390/polym17212856
Jung EY, Shin BJ, Suleiman HO, Tae H-S, Park C-S. Review of Plasma-Synthesized/Modified Polymer and Metal Nanoparticles for Biomedical Applications Using Cold Atmospheric Pressure Plasma. Polymers. 2025; 17(21):2856. https://doi.org/10.3390/polym17212856
Chicago/Turabian StyleJung, Eun Young, Bhum Jae Shin, Habeeb Olaitan Suleiman, Heung-Sik Tae, and Choon-Sang Park. 2025. "Review of Plasma-Synthesized/Modified Polymer and Metal Nanoparticles for Biomedical Applications Using Cold Atmospheric Pressure Plasma" Polymers 17, no. 21: 2856. https://doi.org/10.3390/polym17212856
APA StyleJung, E. Y., Shin, B. J., Suleiman, H. O., Tae, H.-S., & Park, C.-S. (2025). Review of Plasma-Synthesized/Modified Polymer and Metal Nanoparticles for Biomedical Applications Using Cold Atmospheric Pressure Plasma. Polymers, 17(21), 2856. https://doi.org/10.3390/polym17212856









