Structure–Property Relationships in Epoxy–Anhydride Systems: A Comprehensive Comparative Study of Cycloaliphatic, Novolac, and Aromatic Prepolymers
Abstract
1. Introduction
2. Materials and Methods
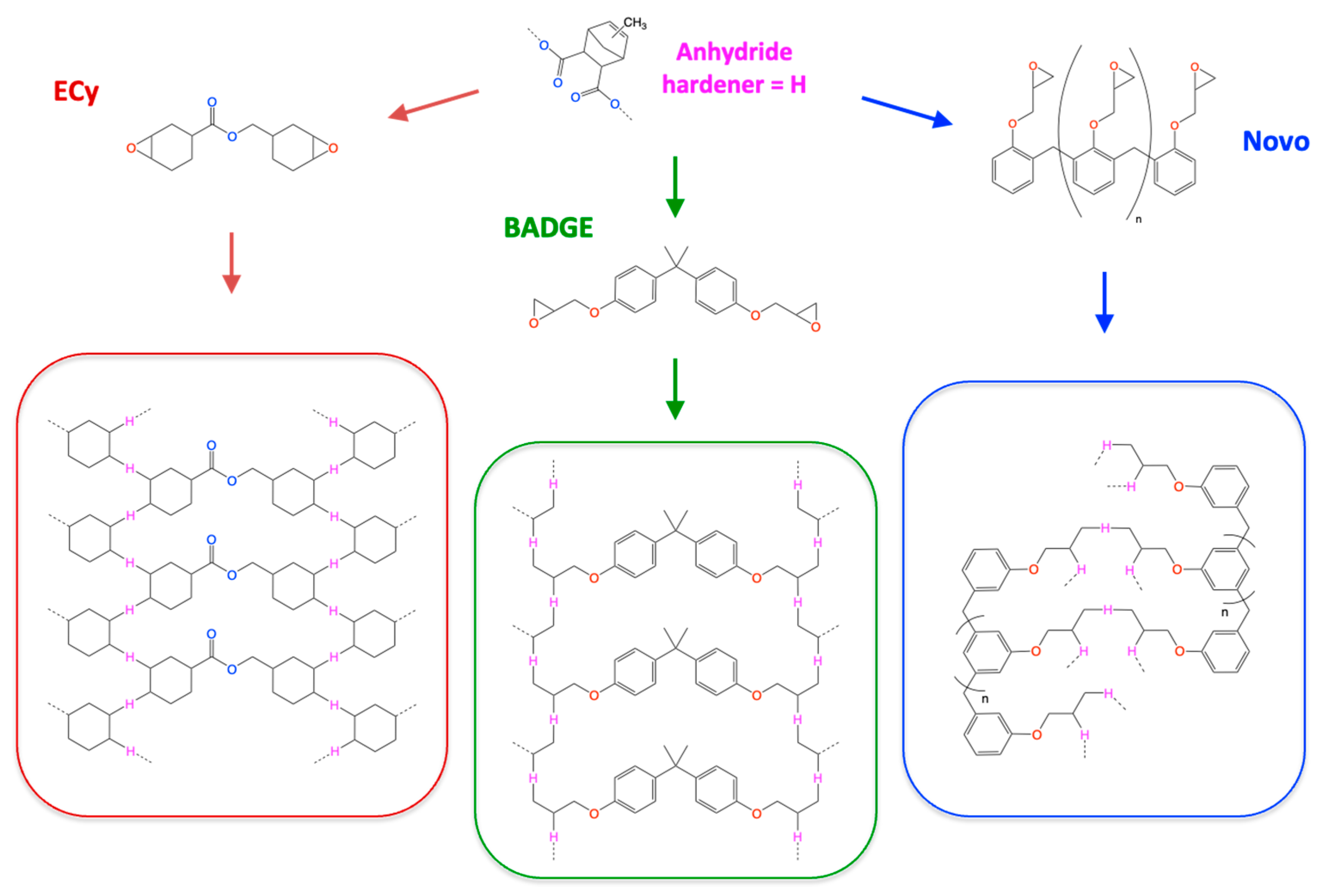
2.1. Chemicals
2.2. Preparation of Reactive Formulations
2.3. Preparation of Cured Samples
2.4. Differential Scanning Calorimetric Experiments (DSC)
2.5. Viscosimetric Measurements
2.6. Dynamic Rheometry
2.7. Thermogravimetric Analyses
3. Results and Discussion
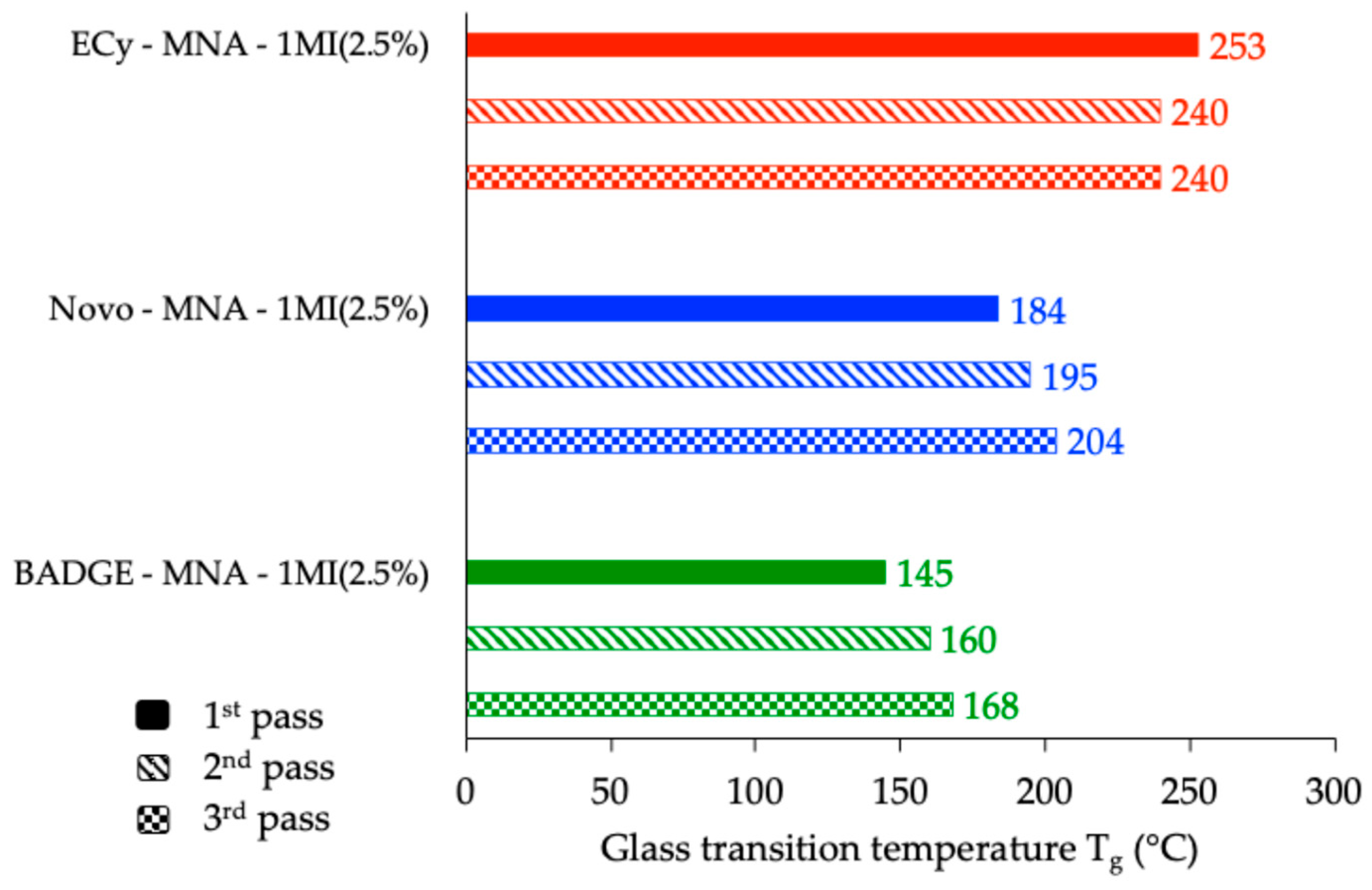
3.1. Optimization of the Stoichiometry of Different Formulations Using DSC
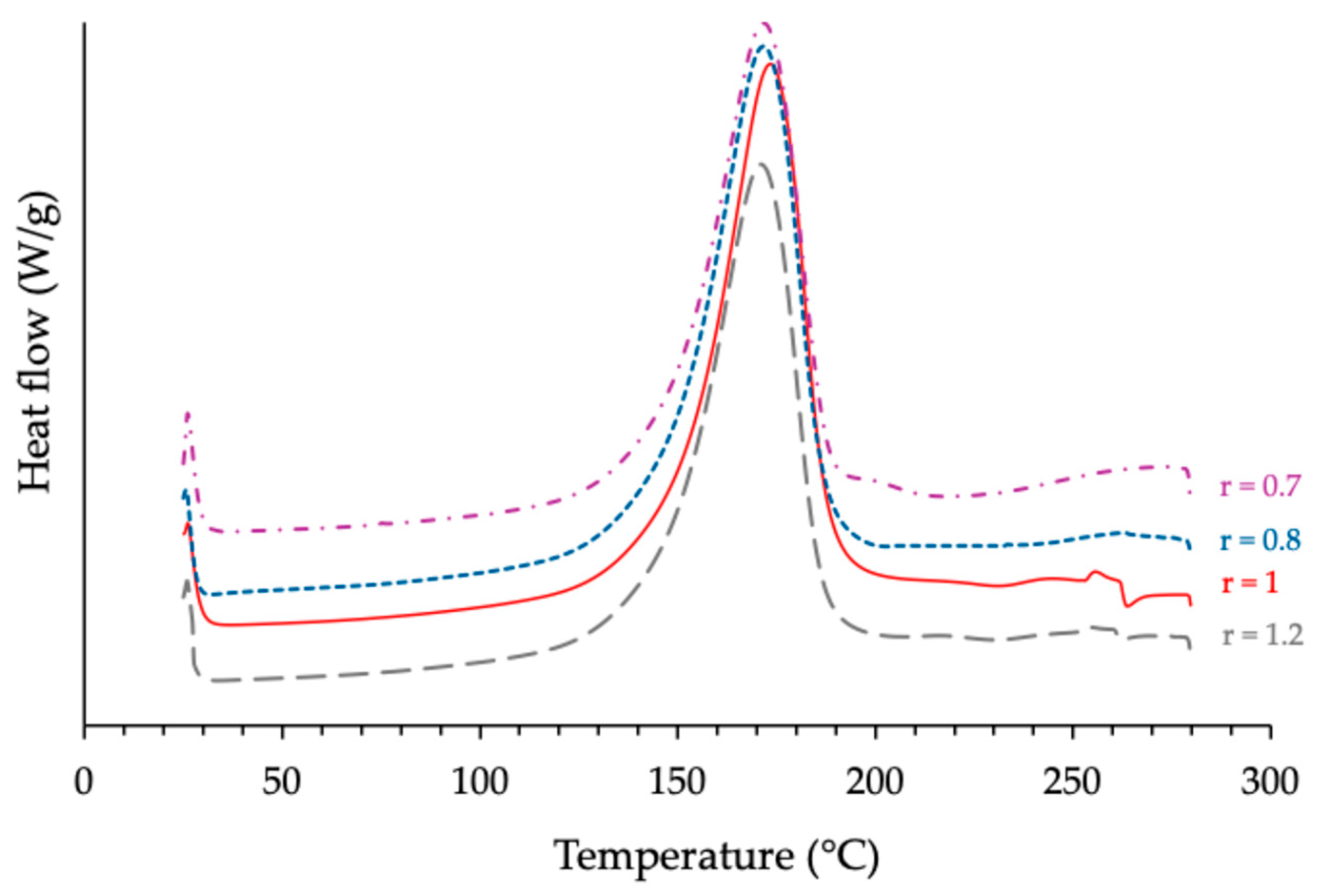
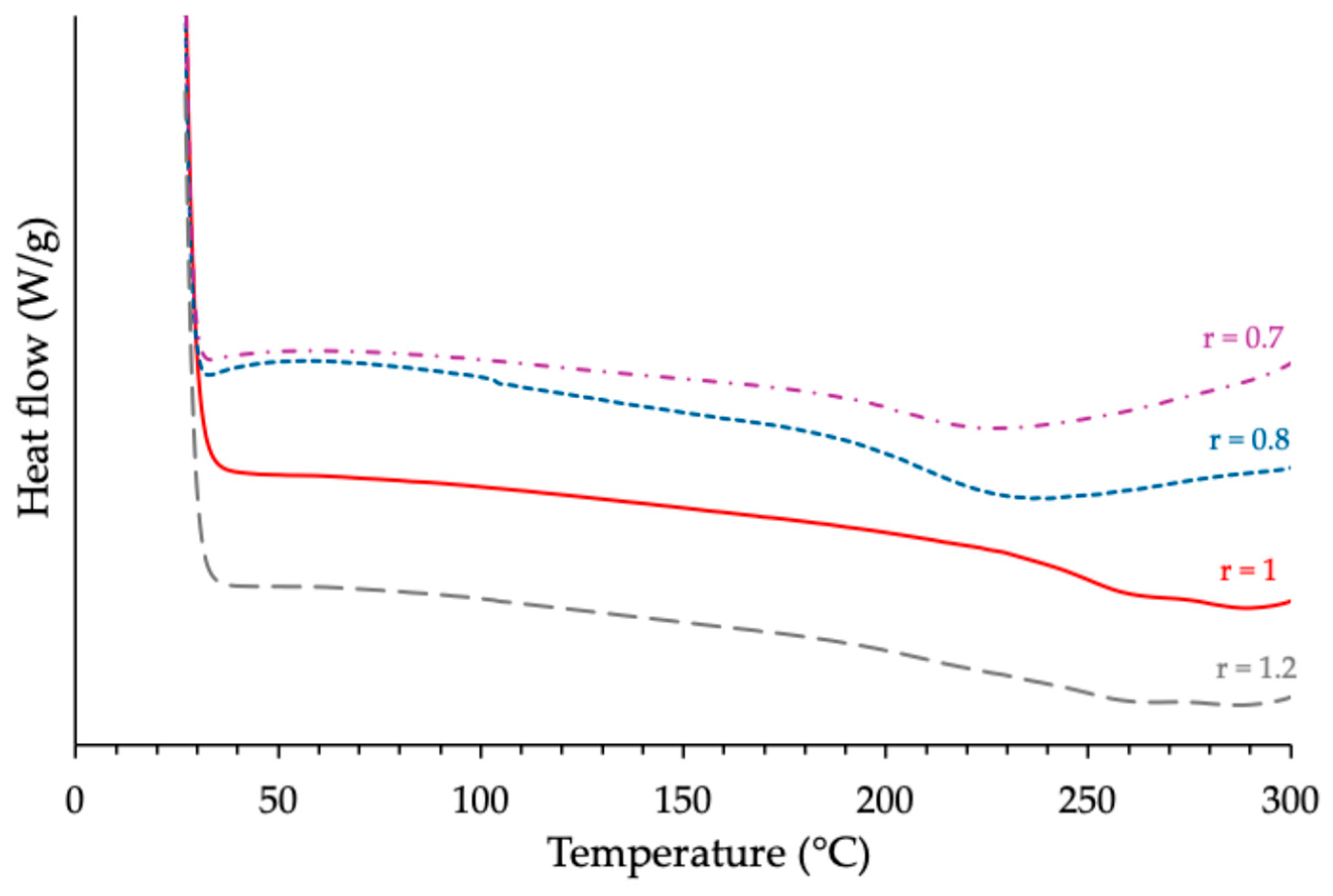
3.1.1. Investigation of the Effects Produced by the Anhydride/Epoxy Ratio
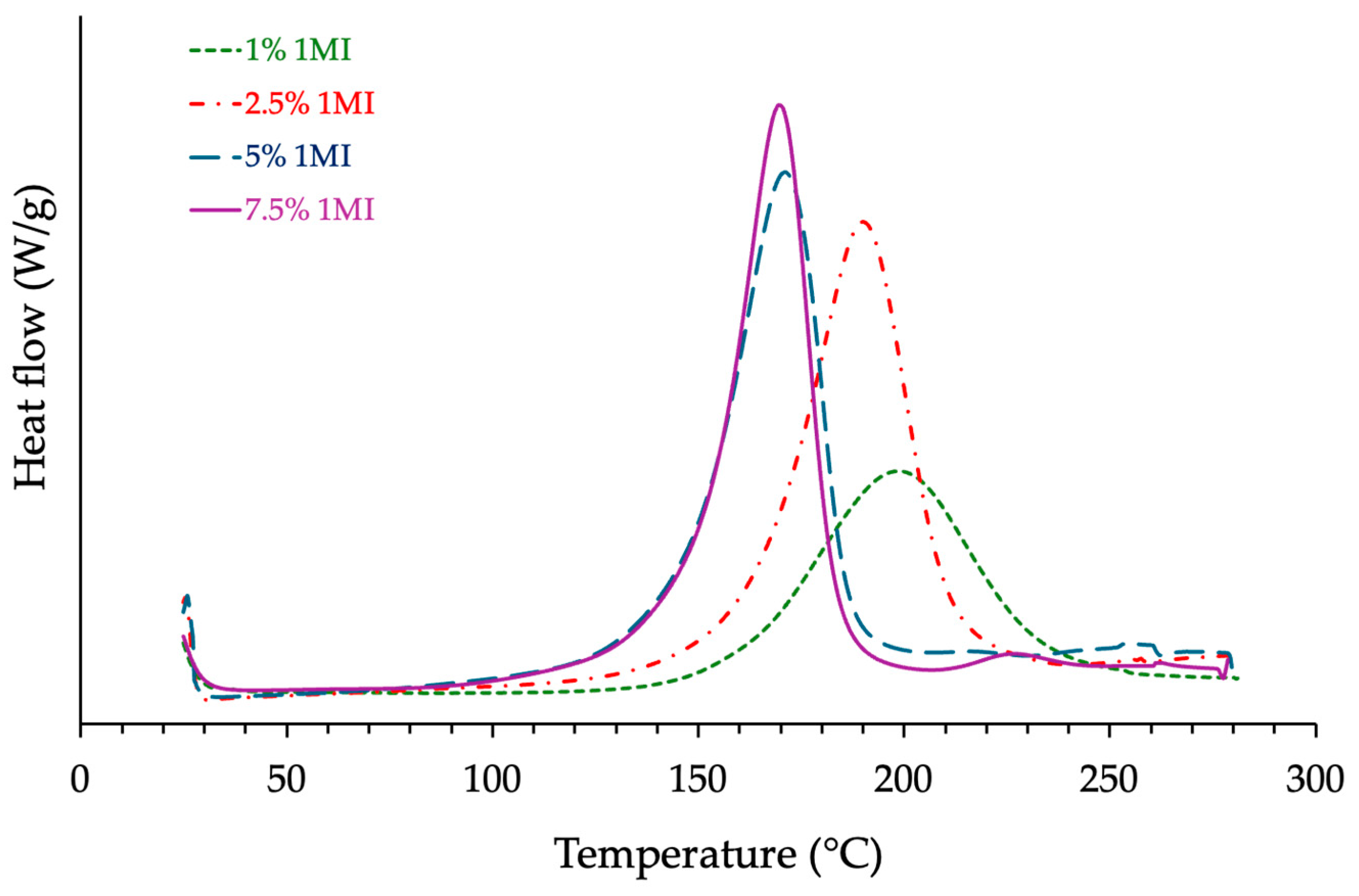
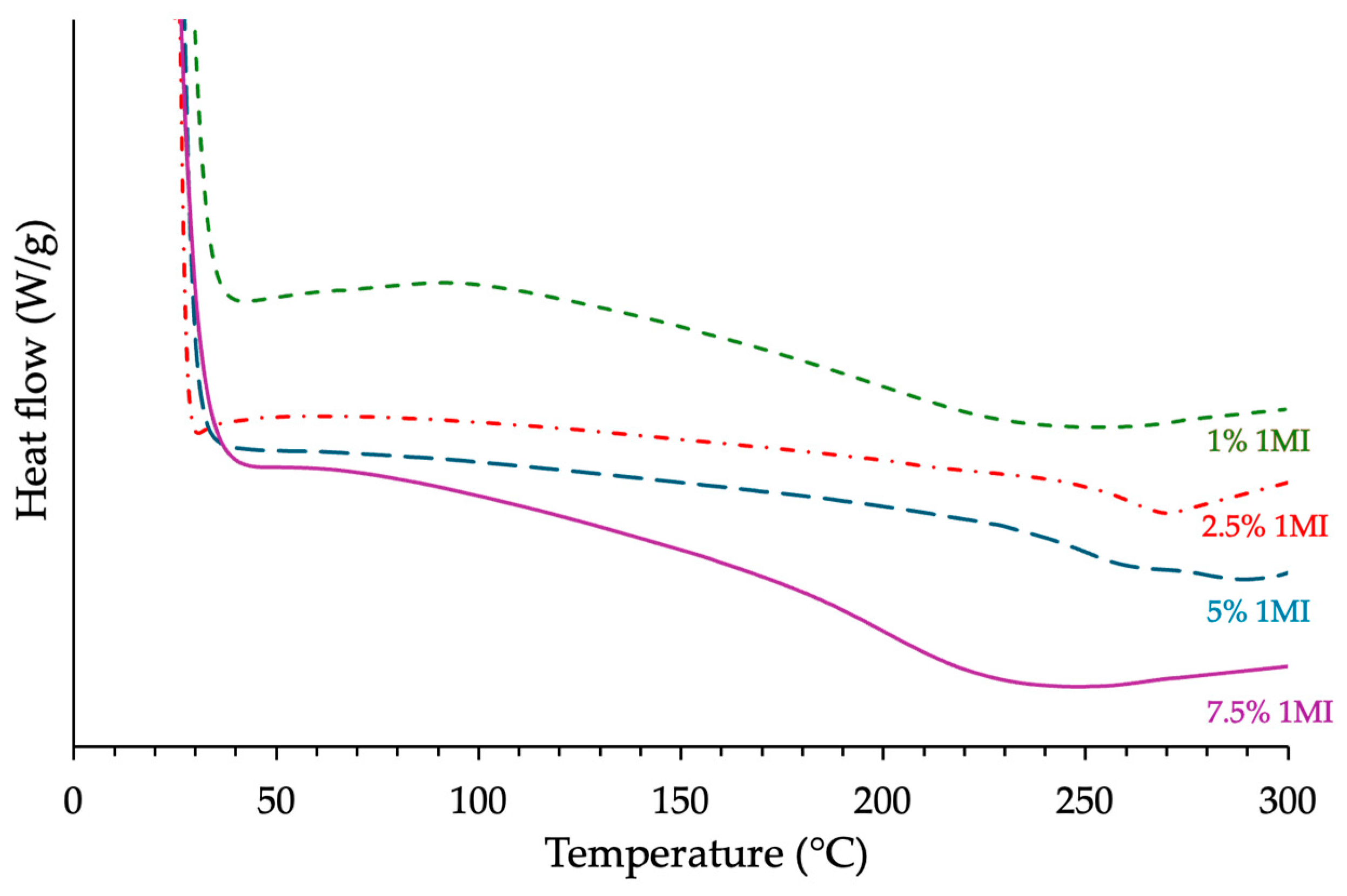
3.1.2. Influence of the Catalyst Content
| Formulation | x | Tp (°C) | ΔH (J/g) | ΔH (kJ/eq) * | Tg (°C) |
|---|---|---|---|---|---|
| ECy-MNA-1MI x% w/w | 1 2.5 5 7.5 | 199 191 173 169 | 268 378 329 389 | 81.6 115 100.1 118.4 | 178 260 250 199 |
| Novo-MNA-1MI x% w/w | 1 2.5 5 7.5 | 179 166 156 152 | 300 330 354 305 | 106 116.5 125 107.7 | 209 191 178 167 |
| BADGE-MNA-1MI x% w/w | 1 2.5 5 7.5 | 183 172 159 159 | 279 328 320 324 | 98.5 115.8 113 114.4 | 157 169 148 145 |
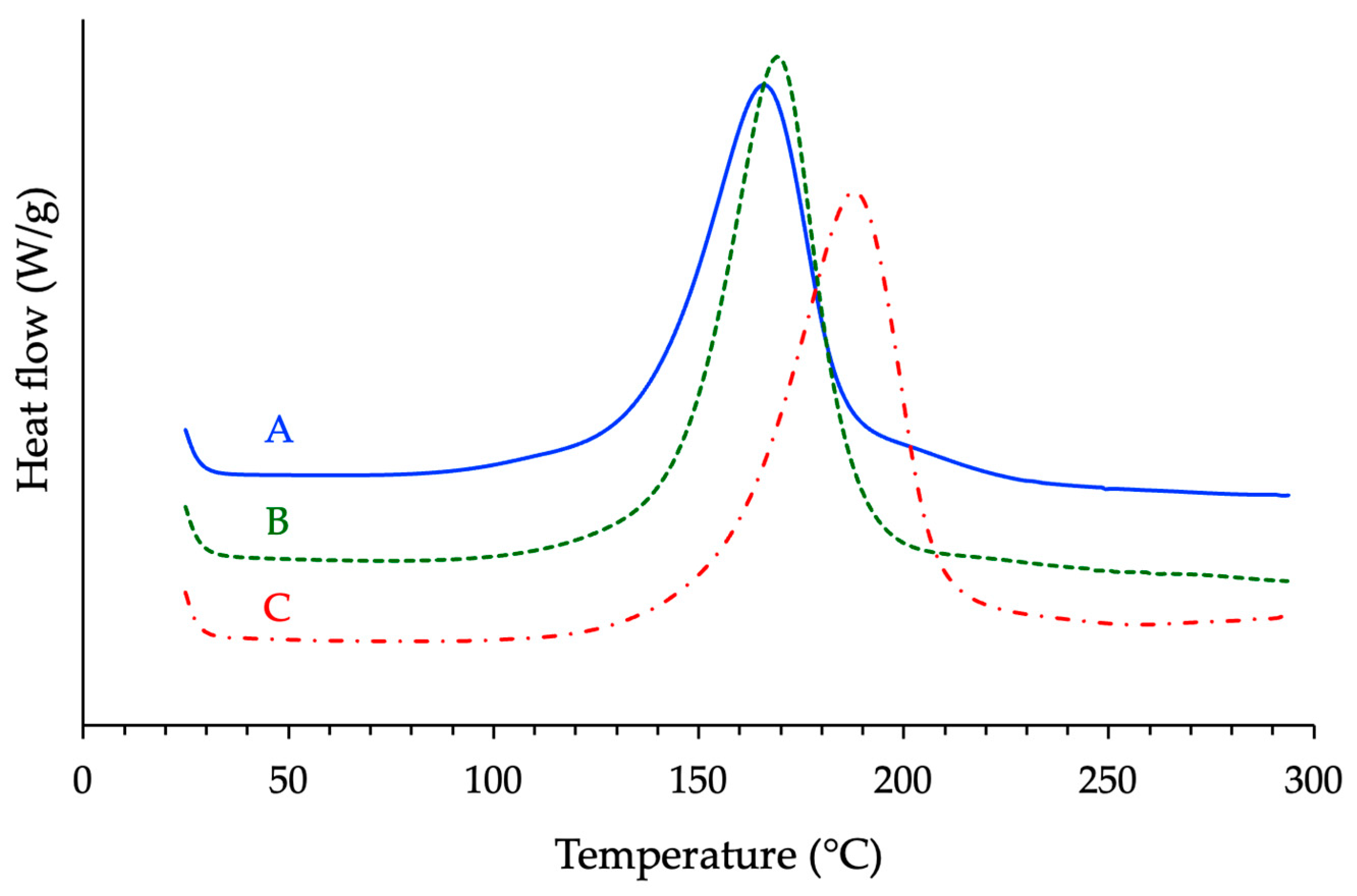
3.2. DSC Study of the Effects Produced by the Epoxy Prepolymer (r = 1 and 2.5% w/w 1MI)
3.3. Rheological Measurements on the Different Optimized Formulations
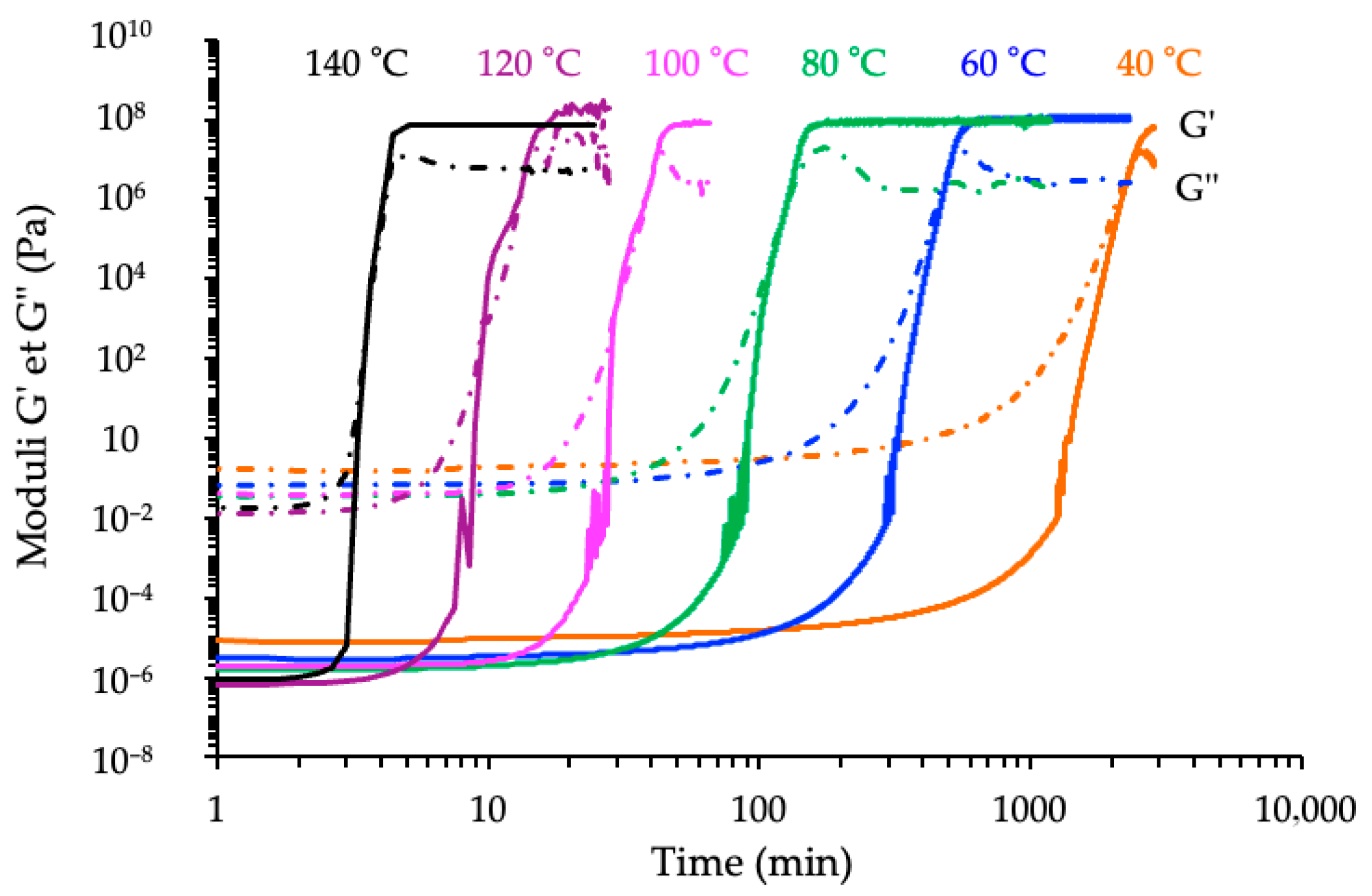
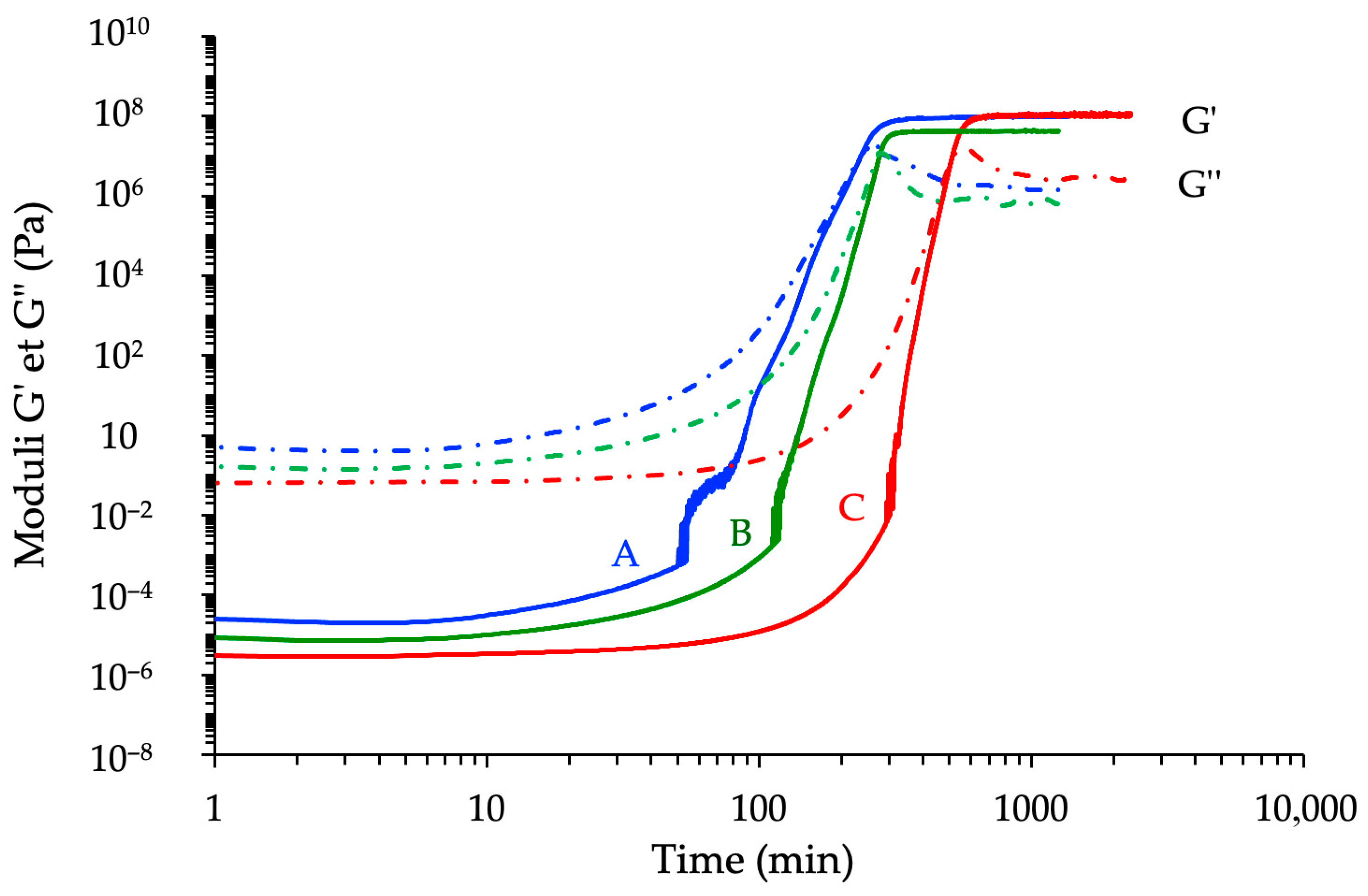
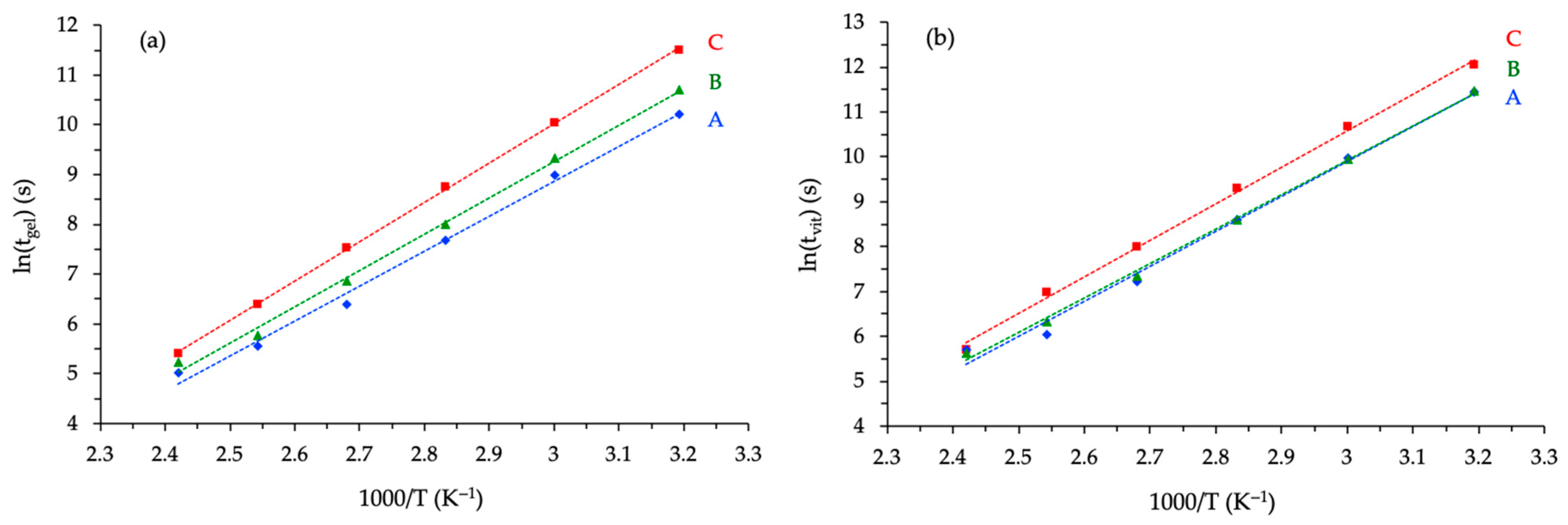
3.3.1. Kinetic Analyses
3.3.2. Correlation with Conversion Degree Determined by DSC
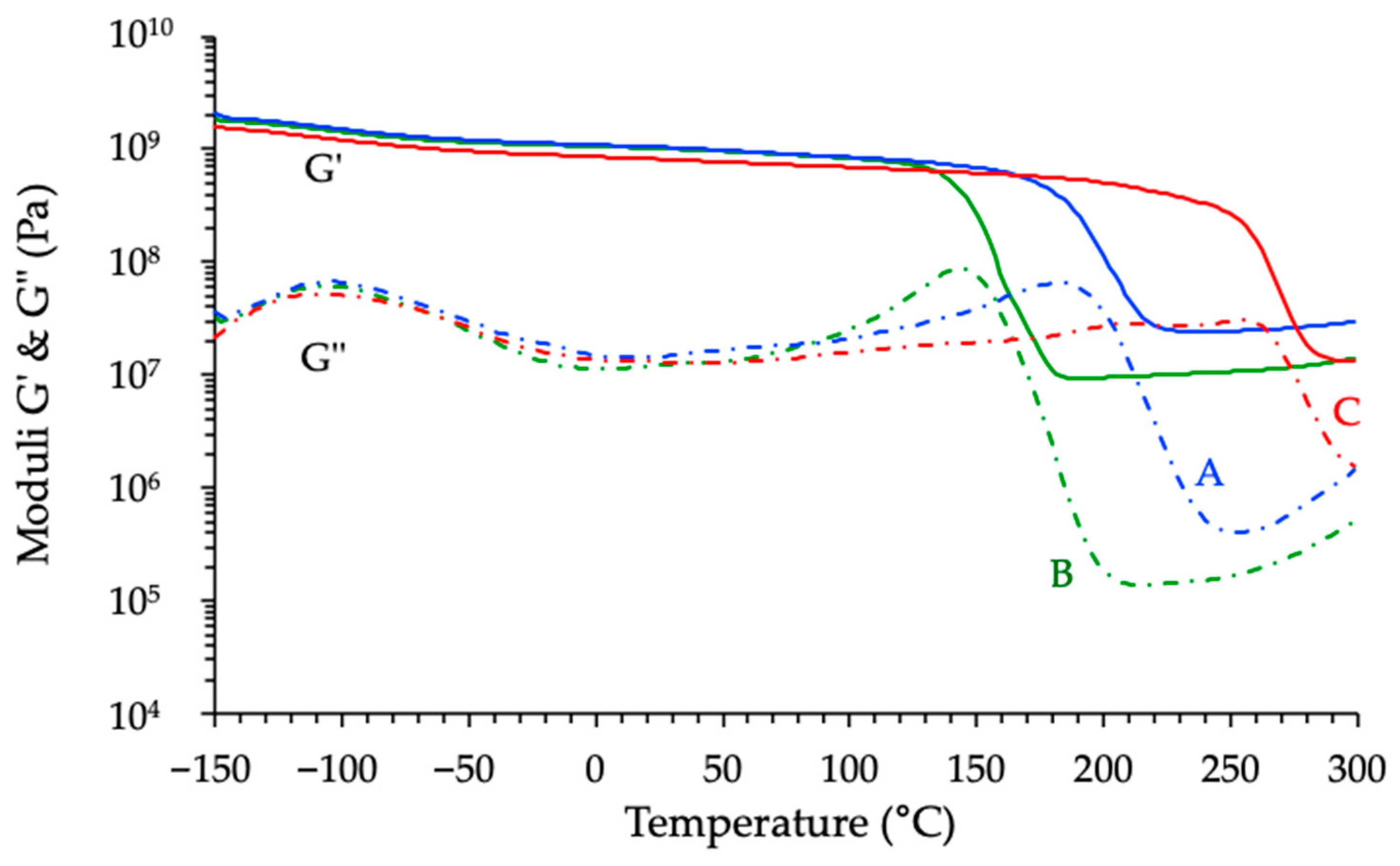
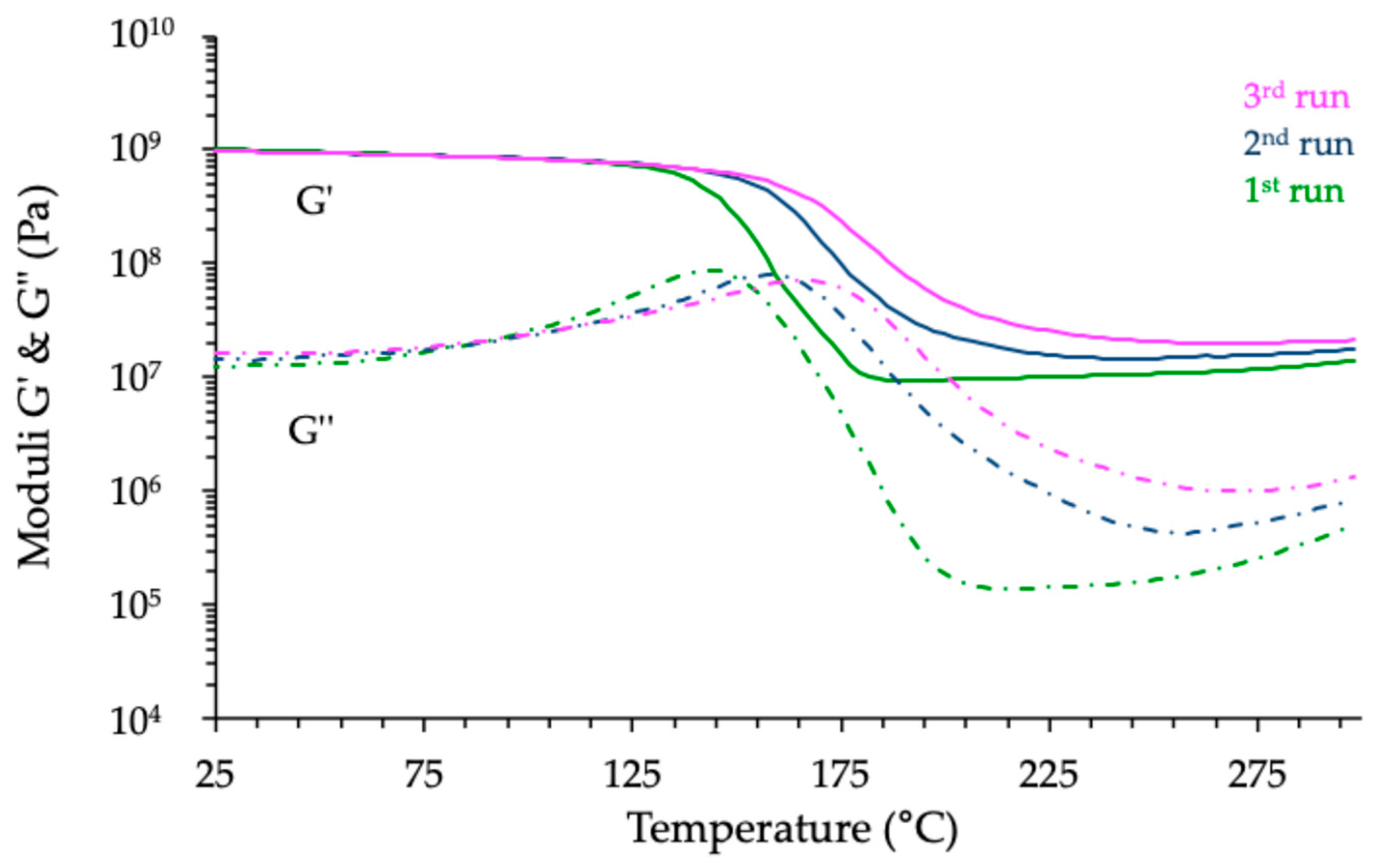
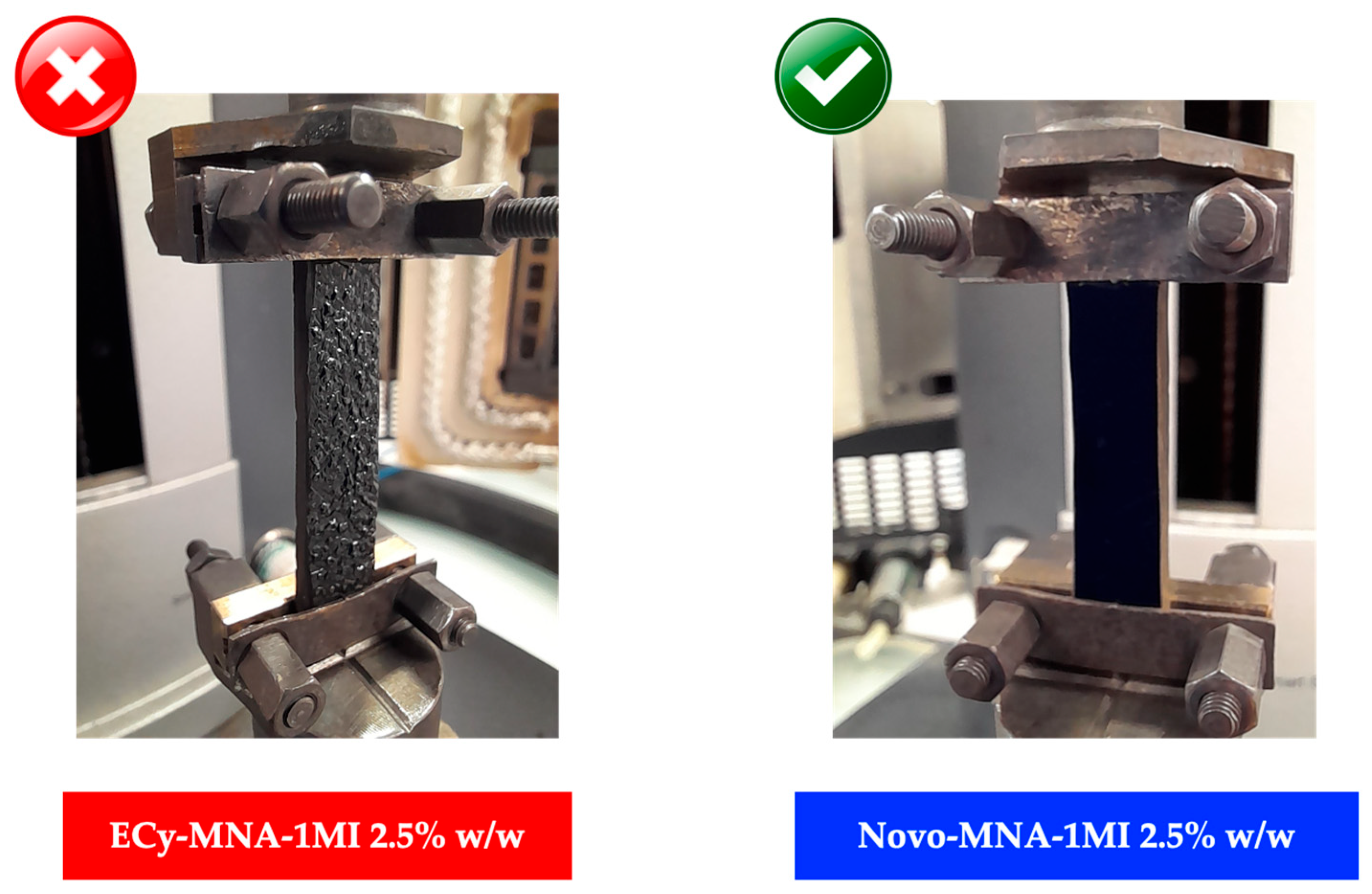
3.3.3. Thermomechanical Analyses of Cured Materials
3.4. Thermogravimetric Experiments
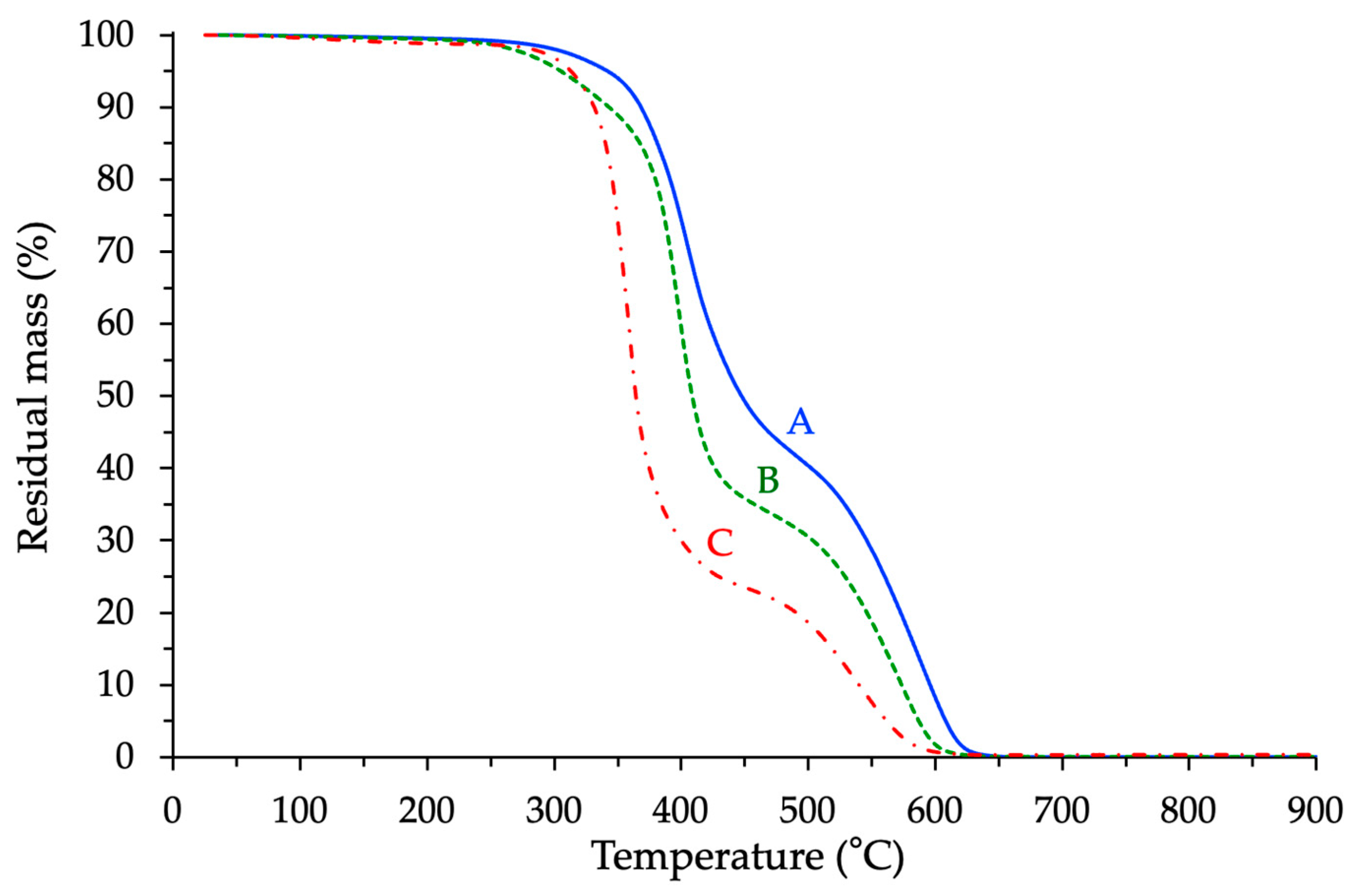
3.4.1. Determination of the Apparent Degradation Temperature (5 °C/min)
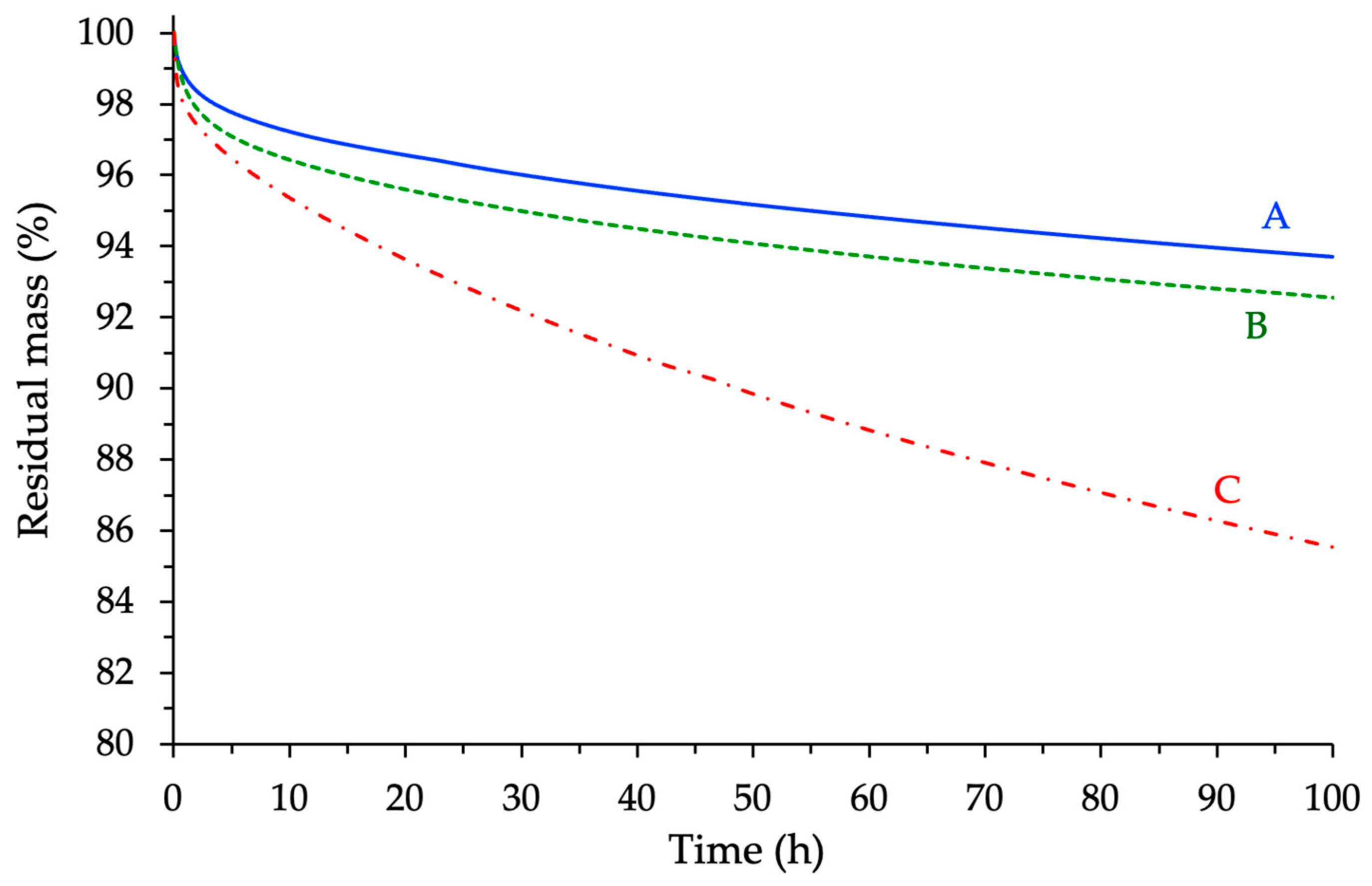
3.4.2. Isothermal TGA Analyses at T = 250 °C
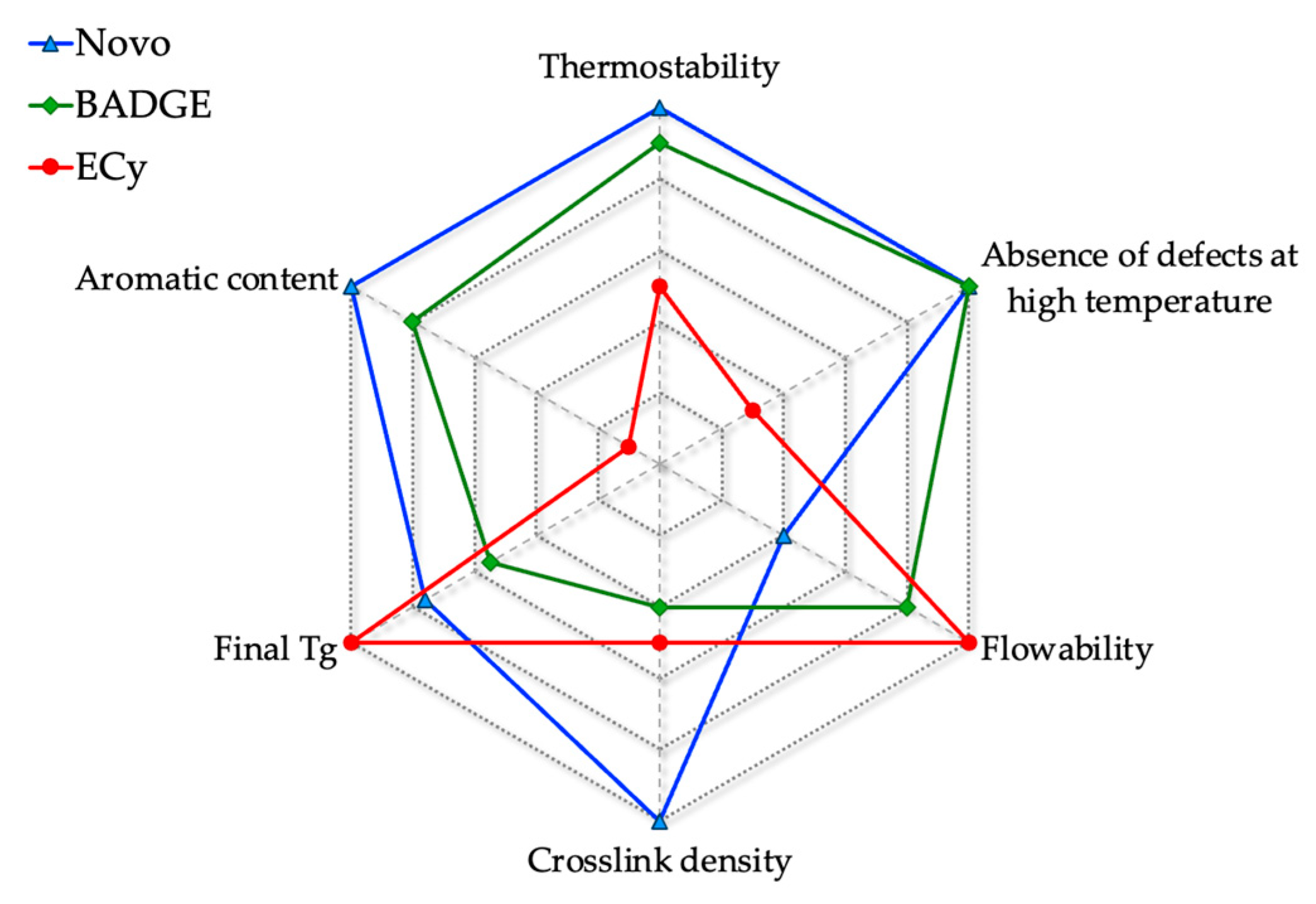
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEW | Anhydride Equivalent Weight |
| BADGE | Bisphenol A Diglycidyl Ether |
| BDMA | Benzyl dimethylamine |
| CAS | Chemical Abstracts Service |
| DMP-30 | Tris (dimethylaminomethyl) phenol |
| DSC | Differential Scanning Calorimetry |
| ECy | Cycloaliphatic epoxy prepolymer |
| EEW | Epoxy Equivalent Weight |
| MHHPA | Methylhexahydrophthalic Anhydride |
| MTHPA | Methyltetrahydrophthalic Anhydride |
| MNA | Methyl Nadic Anhydride |
| Novo | Novolac epoxy prepolymer |
| TCI | Tokyo Chemical Industry |
| TGA | Thermogravimetric analysis |
| Tp | Peak temperature |
| Tg | Glass transition temperature |
| T10 | Degradation temperature related to a mass loss of 10% w/w |
| 1MI | 1-methylimidazole |
References
- Pascault, J.-P.; Williams, R.J. Epoxy Polymers: New Materials and Innovations; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 3-527-62871-1. [Google Scholar]
- Pham, H.Q.; Marks, M.J. Epoxy Resins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- De Nograro, F.F.; Guerrero, P.; Corcuera, M.A.; Mondragon, I. Effects of Chemical Structure of Hardener on Curing Evolution and on the Dynamic Mechanical Behavior of Epoxy Resins. J. Appl. Polym. Sci. 1995, 56, 177–192. [Google Scholar] [CrossRef]
- Jain, R.; Choudhary, V.; Narula, A.K. Curing and Thermal Behavior of DGEBA in Presence of Dianhydrides and Aromatic Diamine. J. Appl. Polym. Sci. 2007, 105, 3804–3808. [Google Scholar] [CrossRef]
- Habas, J.-P.; Arrouy, J.-M.; Perrot, F. Effects of Electric Partial Discharges on the Rheological and Chemical Properties of Polymers Used in HV Composite Insulators after Railway Service. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1444–1454. [Google Scholar] [CrossRef]
- Palomo, B.; Habas-Ulloa, A.; Pignolet, P.; Quentin, N.; Fellmann, D.; Habas, J.P. Rheological and Thermal Study of the Curing Process of a Cycloaliphatic Epoxy Resin: Application to the Optimization of the Ultimate Thermomechanical and Electrical Properties. J. Phys. Appl. Phys. 2013, 46, 065301. [Google Scholar] [CrossRef]
- Gorur, R.S.; Montesinos, J. Electrical Performance of Cycloaliphatic Epoxy Materials and Insulators for Outdoor Use. IEEE Trans. Power Deliv. 2000, 15, 1274–1278. [Google Scholar] [CrossRef]
- Beisele, C.; Kultzow, B. Experiences with New Hydrophobic Cycloaliphatic Epoxy Outdoor Insulation Systems. IEEE Electr. Insul. Mag. 2001, 17, 33–39. [Google Scholar] [CrossRef]
- Sheng, Q.; Chen, Q.; Gu, W.; Wang, R.; Gu, X.; Liu, J.; Sun, T.; Chen, Y.; Sun, J.; Zhang, S. The Study of Curing Behavior and Thermo-Mechanical Properties of Epoxy Adhesives with Different Anhydrides. Polymer 2024, 307, 127342. [Google Scholar] [CrossRef]
- May, C. Epoxy Resins: Chemistry and Technology. Marcel Dekker: New York, NY, USA, 2018; ISBN 1-351-44996-6. [Google Scholar]
- Woo, E.M.; Seferis, J.C. Cure Kinetics of Epoxy/Anhydride Thermosetting Matrix Systems. J. Appl. Polym. Sci. 1990, 40, 1237–1256. [Google Scholar] [CrossRef]
- Altuna, F.I.; Riccardi, C.C.; Marín Quintero, D.C.; Ruseckaite, R.A.; Stefani, P.M. Effect of an Anhydride Excess on the Curing Kinetics and Dynamic Mechanical Properties of Synthetic and Biogenic Epoxy Resins. Int. J. Polym. Sci. 2019, 2019, 5029153. [Google Scholar] [CrossRef]
- Loban, O.I.; Olikhova, Y.V.; Gorbunova, I.Y.; Kostromina, N.V. Curing Rheokinetics of Epoxy-Amine Composition. Thermochim. Acta 2024, 740, 179825. [Google Scholar] [CrossRef]
- Laza, J.M.; Julian, C.A.; Larrauri, E.; Rodriguez, M.; Leon, L.M. Thermal Scanning Rheometer Analysis of Curing Kinetic of an Epoxy Resin: 2. An Amine as Curing Agent. Polymer 1999, 40, 35–45. [Google Scholar] [CrossRef]
- Ito, M.; Hata, H.; Kamagata, K. Thermal Properties of Epoxy Resin Cured with Imidazole. J. Appl. Polym. Sci. 1987, 33, 1843–1848. [Google Scholar] [CrossRef]
- ISO 11357-2; Plastics—Differential Scanning Calorimetry (DSC), Part 2: Determination of Glass Transition Temperature and Step Height. International Organization for Standardization: Geneva, Switzerland, 2020.
- Eloundou, J.P.; Ayina, O.; Noah Ngamveng, J. Comparative Rheological Analysis of Two Epoxy-Amine Systems near the Gel Point. Eur. Polym. J. 1998, 34, 1331–1340. [Google Scholar] [CrossRef]
- Boey, F.Y.C.; Qiang, W. Determining the Gel Point of an Epoxy-Hexaanhydro-4-Methylphthalic Anhydride (MHHPA) System. J. Appl. Polym. Sci. 2000, 76, 1248–1256. [Google Scholar] [CrossRef]
- Oberlin, C.; Patry, S.; Pochat-Bohatier, C.; Bechelany, M.; Habas, J.; Miele, P. Determination of the Formulation and Curing Conditions of Thermosetting Epoxy Resins for Optimizing Their Properties and Future Use in Gelcasting Process. J. Appl. Polym. Sci. 2022, 139, 52093. [Google Scholar] [CrossRef]
- Heise, M.; Martin, G. Curing Mechanism and Thermal Properties of Epoxy-Imidazole Systems. Macromolecules 1989, 22, 99–104. [Google Scholar] [CrossRef]
- Ham, Y.R.; Kim, S.H.; Shin, Y.J.; Lee, D.H.; Yang, M.; Min, J.H.; Shin, J.S. A Comparison of Some Imidazoles in the Curing of Epoxy Resin. J. Ind. Eng. Chem. 2010, 16, 556–559. [Google Scholar] [CrossRef]
- Turi, E. Thermal Characterization of Polymeric Materials; Academic Press: London, UK, 1981. [Google Scholar]
- Reynolds, J.L.; Turakhia, R.H.; Jacob, G.; Null, M.J. High Tg Epoxy Systems for Composite Applications. U.S. Patent 9315664, 19 April 2016. [Google Scholar]
- Fedoseev, M.S.; Gusev, V.Y.; Derzhavinskaya, L.F.; Antipin, V.E.; Tsvetkov, R.V. Heat-Resistant Epoxy Polymers of Anhydride Curing. Polym. Sci. Ser. D 2018, 11, 39–46. [Google Scholar] [CrossRef]
- Qian, H.; Badrinarayanan, P. High Tg Thermosets Based on Ultra-High Functionality Epoxy Novolacs (EPNs). SAMPE NeXus 2021. Available online: https://digitallibrarynasampe.org/data/pdfs/s2021_pdfs/TP21-0000000413.pdf (accessed on 20 October 2025).
- Sickfeld, J.; Mielke, W. Application of Thermal Analysis for the Investigation of Epoxy Resins. Prog. Org. Coat. 1984, 12, 27–116. [Google Scholar] [CrossRef]
- Bifulco, A.; Marotta, A.; Passaro, J.; Costantini, A.; Cerruti, P.; Gentile, G.; Ambrogi, V.; Malucelli, G.; Branda, F. Thermal and Fire Behavior of a Bio-Based Epoxy/Silica Hybrid Cured with Methyl Nadic Anhydride. Polymers 2020, 12, 1661. [Google Scholar] [CrossRef]
- Bouillon, N.; Pascault, J.; Tighzert, L. Influence of Different Imidazole Catalysts on Epoxy-anhydride Copolymerization and on Their Network Properties. J. Appl. Polym. Sci. 1989, 38, 2103–2113. [Google Scholar] [CrossRef]
- Galy, J.; Sabra, A.; Pascault, J. Characterization of Epoxy Thermosetting Systems by Differential Scanning Calorimetry. Polym. Eng. Sci. 1986, 26, 1514–1523. [Google Scholar] [CrossRef]
- Montserrat, S.; Flaque, C.; Calafell, M.; Andreu, G.; Malek, J. Influence of the Accelerator Concentration on the Curing Reaction of an Epoxy-Anhydride System. Thermochim. Acta 1995, 269, 213–229. [Google Scholar] [CrossRef]
- Belmonte, A.; Däbritz, F.; Ramis, X.; Serra, A.; Voit, B.; Fernández-Francos, X. Cure Kinetics Modeling and Thermomechanical Properties of Cycloaliphatic Epoxy-Anhydride Thermosets Modified with Hyperstar Polymers. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1227–1242. [Google Scholar] [CrossRef]
- Lu, M.; Liu, Y.; Du, X.; Zhang, S.; Chen, G.; Zhang, Q.; Yao, S.; Liang, L.; Lu, M. Cure Kinetics and Properties of High Performance Cycloaliphatic Epoxy Resins Cured with Anhydride. Ind. Eng. Chem. Res. 2019, 58, 6907–6918. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Lorenz, N.; Zawadzki, T.; Keller, L.; Fuchs, J.; Fischer, K.; Hopmann, C. Characterization and Modeling of an Epoxy Vitrimer Based on Disulfide Exchange for Wet Filament Winding Applications. Polym. Eng. Sci. 2024, 64, 3682–3702. [Google Scholar] [CrossRef]
- Ampudia, J.; Larrauri, E.; Gil, E.; Rodriguez, M.; León, L. Thermal Scanning Rheometric Analysis of Curing Kinetic of an Epoxy Resin. I. An Anhydride as Curing Agent. J. Appl. Polym. Sci. 1999, 71, 1239–1245. [Google Scholar] [CrossRef]
- Edwards, S.F. The Theory of Rubber Elasticity. Br. Polym. J. 1977, 9, 140–143. [Google Scholar] [CrossRef]
- Heinrich, G.; Straube, E.; Helmis, G. Rubber Elasticity of Polymer Networks: Theories. In Polymer Physics; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1988; Volume 85, pp. 33–87. ISBN 978-3-540-18484-3. [Google Scholar]
- Everaers, R.; Kremer, K. Test of the Foundations of Classical Rubber Elasticity. Macromolecules 1995, 28, 7291–7294. [Google Scholar] [CrossRef]
- Cristea, M.; Ibanescu, S.; Cascaval, C.N.; Rosu, D. Dynamic Mechanical Analysis of Polyurethane-Epoxy Interpenetrating Polymer Networks. High Perform. Polym. 2009, 21, 608–623. [Google Scholar] [CrossRef]
- Lopez, G.; Améduri, B.; Habas, J.-P. A Perfluoropolyether-Based Elastomers Library with on-Demand Thermorheological Features. Eur. Polym. J. 2017, 95, 207–215. [Google Scholar] [CrossRef]
- Hammami, N.; Majdoub, M.; Habas, J.-P. Structure-Properties Relationships in Isosorbide-Based Polyacetals: Influence of Linear or Cyclic Architecture on Polymer Physicochemical Properties. Eur. Polym. J. 2017, 93, 795–804. [Google Scholar] [CrossRef]
- Freeman, E.S.; Becker, A.J. Thermal Degradation of Nadic Methyl Anhydride-crosslinked Novolac Epoxy Resin. J. Polym. Sci. Part A-1 Polym. Chem. 1968, 6, 2829–2851. [Google Scholar] [CrossRef]
- Gac, N.; Spokes, G.; Benson, S. Thermal Degradation of Nadic Methyl Anhydride-cured Epoxy Novolac. J. Polym. Sci. Part A-1 Polym. Chem. 1970, 8, 593–608. [Google Scholar] [CrossRef]
- Fleming, G.J. Mechanisms for Initiating Thermal Degradation of Certain Anhydride-cured Epoxides. J. Appl. Polym. Sci. 1966, 10, 1813–1830. [Google Scholar] [CrossRef]
- Abiodun, S.; Krishnamoorti, R.; Bhowmick, A.K. Thermal Degradation of Polytetrafluoroethylene, Modified Polytetrafluoroethylene, and Their Nanocomposites with Boron Nitride Nanobarbs: Lifetime Predictions and Kinetic Analysis. J. Appl. Polym. Sci. 2025, 142, e56452. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Z.; Yue, D.; Belko, V.O.; Maksimenko, S.A.; Deng, J.; Sun, Y.; Yang, Z.; Fu, Q.; Liu, B.; et al. Recent Progress in Degradation and Recycling of Epoxy Resin. J. Mater. Res. Technol. 2024, 32, 2891–2912. [Google Scholar] [CrossRef]
- Jumahat, A.; Zamani, N.R.; Soutis, C.; Roseley, N.R.N. Thermogravimetry Analysis of Nanosilica-Filled Epoxy Polymer. Mater. Res. Innov. 2014, 18, S6-274–S6-279. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, D.; Li, T.; Zhang, A. Thermal Degradation Properties of Hyperbranched Poly (Trimellitic Anhydride Diethylene Glycol)/DGEBA Epoxy Hybrid Resin. Polym.-Plast. Technol. Eng. 2012, 51, 1149–1154. [Google Scholar] [CrossRef]
- Yang, Y.; Xian, G.; Li, H.; Sui, L. Thermal Aging of an Anhydride-Cured Epoxy Resin. Polym. Degrad. Stab. 2015, 118, 111–119. [Google Scholar] [CrossRef]
- Li, J.; Guo, P.; Kong, X.; Wang, Y.; Yang, Y.; Liu, F.; Du, B. Curing Kinetics and Dielectric Properties of Anhydride Cured Epoxy Resin with Different Accelerator Contents. IEEE Trans. Dielectr. Electr. Insul. 2022, 30, 20–30. [Google Scholar] [CrossRef]
- Datta, C.; Basu, D.; Banerjee, A. Mechanical and Dynamic Mechanical Properties of Jute Fibers–Novolac–Epoxy Composite Laminates. J. Appl. Polym. Sci. 2002, 85, 2800–2807. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P.; Thachil, E.T. Blends of Epoxy and Epoxidized Novolac Resins. J. Elastomers Plast. 2005, 37, 347–359. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P.; Thachil, E.T. Aging and Thermal Studies on Epoxy Resin Modified by Epoxidized Novolacs. Polym.-Plast. Technol. Eng. 2006, 45, 469–474. [Google Scholar] [CrossRef]
- Baby, M.; Pal, R.; Francis, N.; Sudhi, S. Novolac Epoxy Resin from 4,4′-dihydroxybenzophenone: Thermal, Thermomechanical, Interfacial, and Cure Kinetics with DGEBA/DICY Blend. J. Appl. Polym. Sci. 2018, 135, 46164. [Google Scholar] [CrossRef]



| Common Name in This Study | Abbreviation | CAS | Chemical Structure |
|---|---|---|---|
| Cycloaliphatic epoxy resin | ECy | 2386-87-0 | 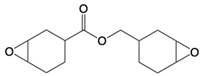 |
| Novolac epoxy resin | Novo | 28064-14-4 |  |
| Linear diaromatic epoxy | BADGE | 1675-54-3 | 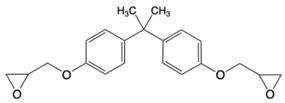 |
| Cyclic anhydride | MNA | 25134-21-8 |  |
| Catalyst | 1MI | 616-47-7 | 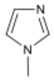 |
| Formulation | r | Tp (°C) | ΔH (J/g) | Tg (°C) |
|---|---|---|---|---|
| ECy-MNA-1MI 5% w/w | 0.7 0.8 1 1.2 | 171 172 173 172 | 327 338 350 335 | 206 212 250 234 |
| Novo-MNA-1MI 5% w/w | 0.7 0.8 1 1.2 | 156 156 156 157 | 384 366 354 312 | 175 183 178 175 |
| BADGE-MNA-1MI 5% w/w | 0.7 0.8 1 1.2 | 162 162 159 159 | 398 363 320 303 | 139 151 148 152 |
| Prepolymer Used in the Formulation | Gelation Time | Vitrification Time | ||
|---|---|---|---|---|
| Activation Energy (kJ/mol) | Value—T = 200 °C (s) | Activation Energy (kJ/mol) | Value—T = 200 °C (s) | |
| Novo | 58.2 | 14 | 64.9 | 20 |
| BADGE | 60.7 | 16 | 64.0 | 22 |
| ECy | 65.7 | 20 | 67.8 | 29 |
| Cured Formulation (r = 1) | Tg (°C) | (MPa) | ν (mol m−3) |
|---|---|---|---|
| ECy-MNA-1MI 2.5% w/w | 253 | 13.2 | 2768 |
| Novo-MNA-1MI 2.5% w/w | 184 | 24.5 | 5808 |
| BADGE-MNA-1MI 2.5% w/w | 145 | 9.5 | 2418 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patry, S.; Asseray, A.; Berne, M.; Loriot, V.; Loriot, L.; Habas, J.-P. Structure–Property Relationships in Epoxy–Anhydride Systems: A Comprehensive Comparative Study of Cycloaliphatic, Novolac, and Aromatic Prepolymers. Polymers 2025, 17, 2843. https://doi.org/10.3390/polym17212843
Patry S, Asseray A, Berne M, Loriot V, Loriot L, Habas J-P. Structure–Property Relationships in Epoxy–Anhydride Systems: A Comprehensive Comparative Study of Cycloaliphatic, Novolac, and Aromatic Prepolymers. Polymers. 2025; 17(21):2843. https://doi.org/10.3390/polym17212843
Chicago/Turabian StylePatry, Stephane, Alban Asseray, Mickaël Berne, Valéry Loriot, Luc Loriot, and Jean-Pierre Habas. 2025. "Structure–Property Relationships in Epoxy–Anhydride Systems: A Comprehensive Comparative Study of Cycloaliphatic, Novolac, and Aromatic Prepolymers" Polymers 17, no. 21: 2843. https://doi.org/10.3390/polym17212843
APA StylePatry, S., Asseray, A., Berne, M., Loriot, V., Loriot, L., & Habas, J.-P. (2025). Structure–Property Relationships in Epoxy–Anhydride Systems: A Comprehensive Comparative Study of Cycloaliphatic, Novolac, and Aromatic Prepolymers. Polymers, 17(21), 2843. https://doi.org/10.3390/polym17212843







