Micellization Studies of Block Copolymers of Poly(N-vinyl pyrrolidone) and n-Alkyl-Substituted Poly(vinyl esters) in Tetrahydrofuran
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Preparation of the Block Copolymers of NVP and VEs
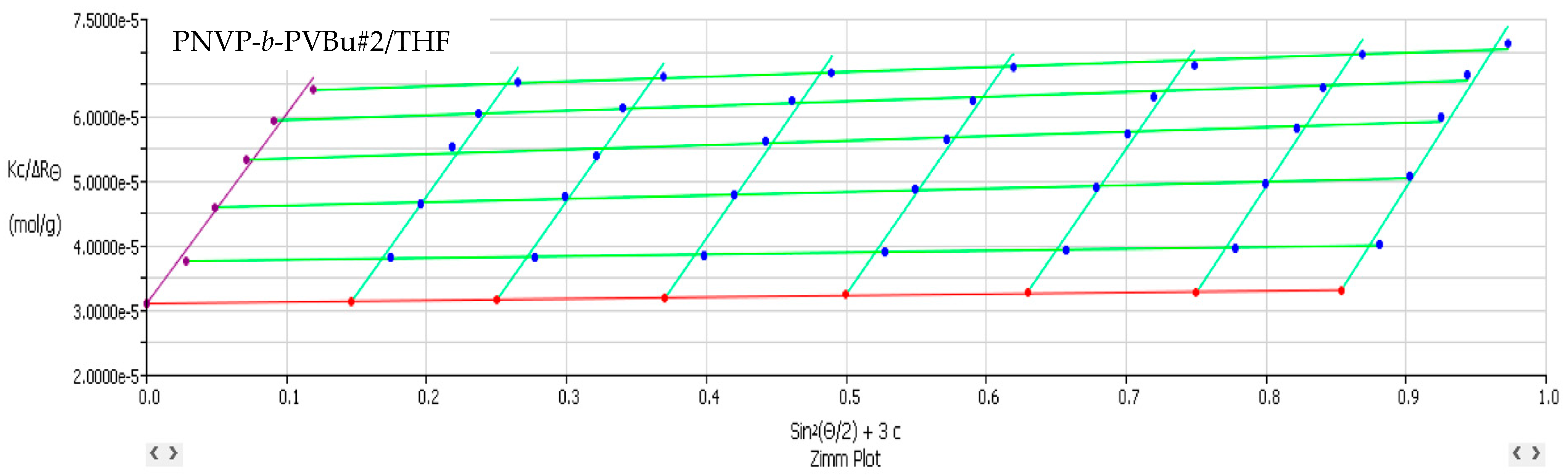
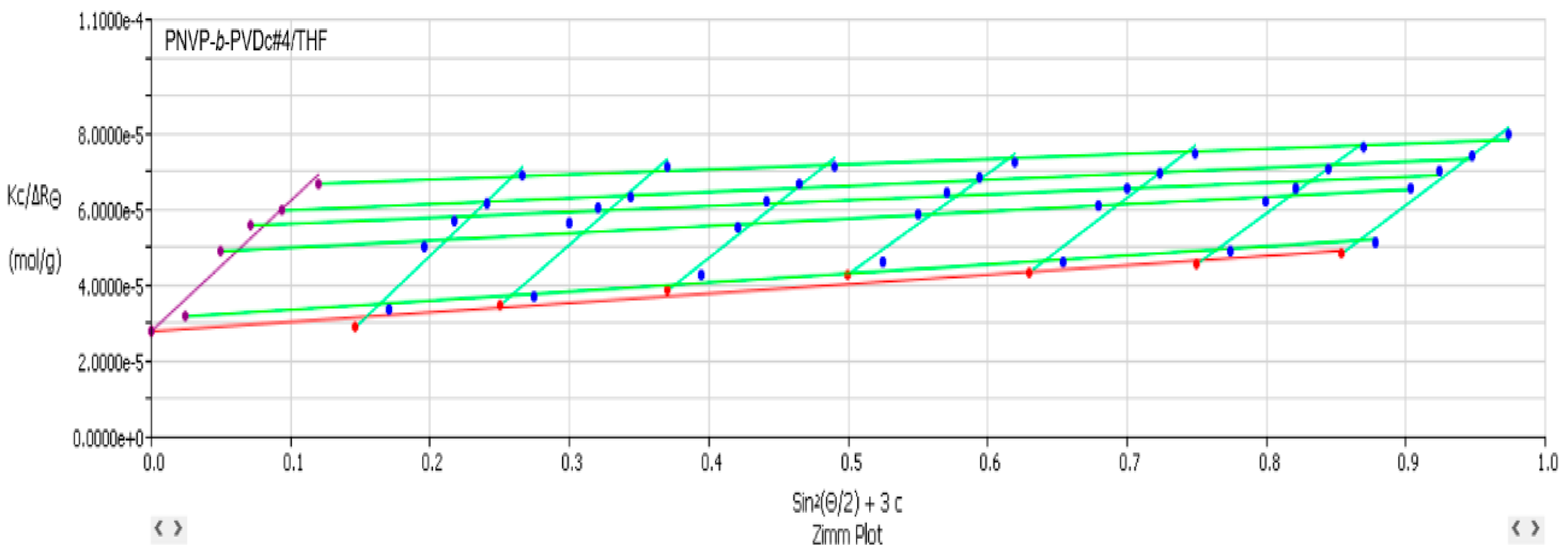
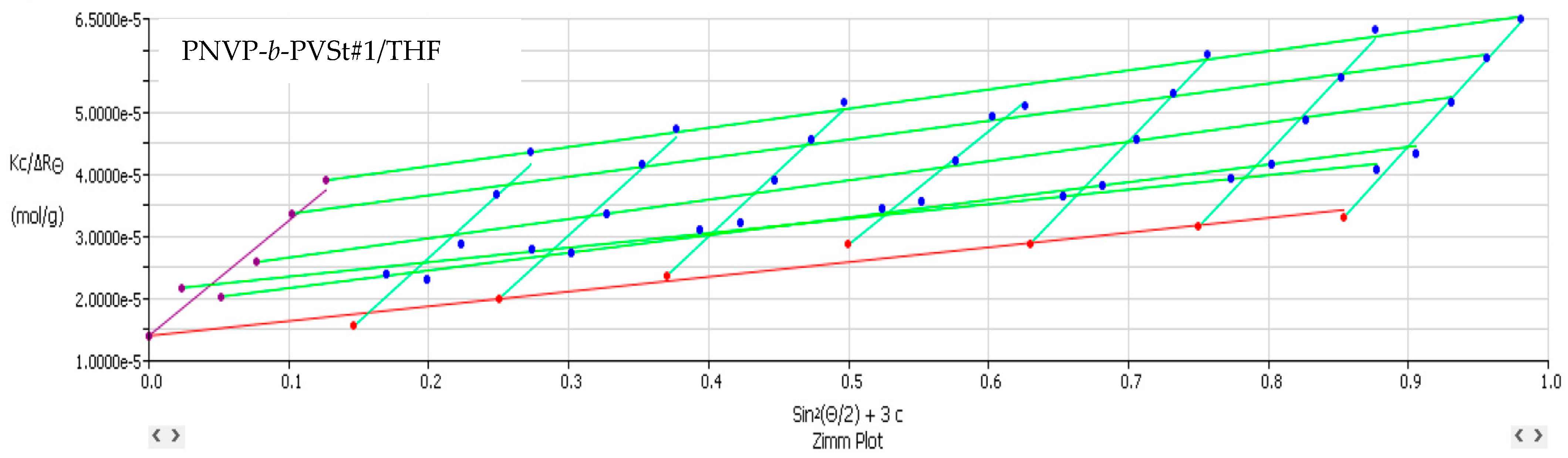
3.2. Self-Assembly Behavior of the PNVP-b-PVE Block Copolymers in THF Solutions by Static Light Scattering
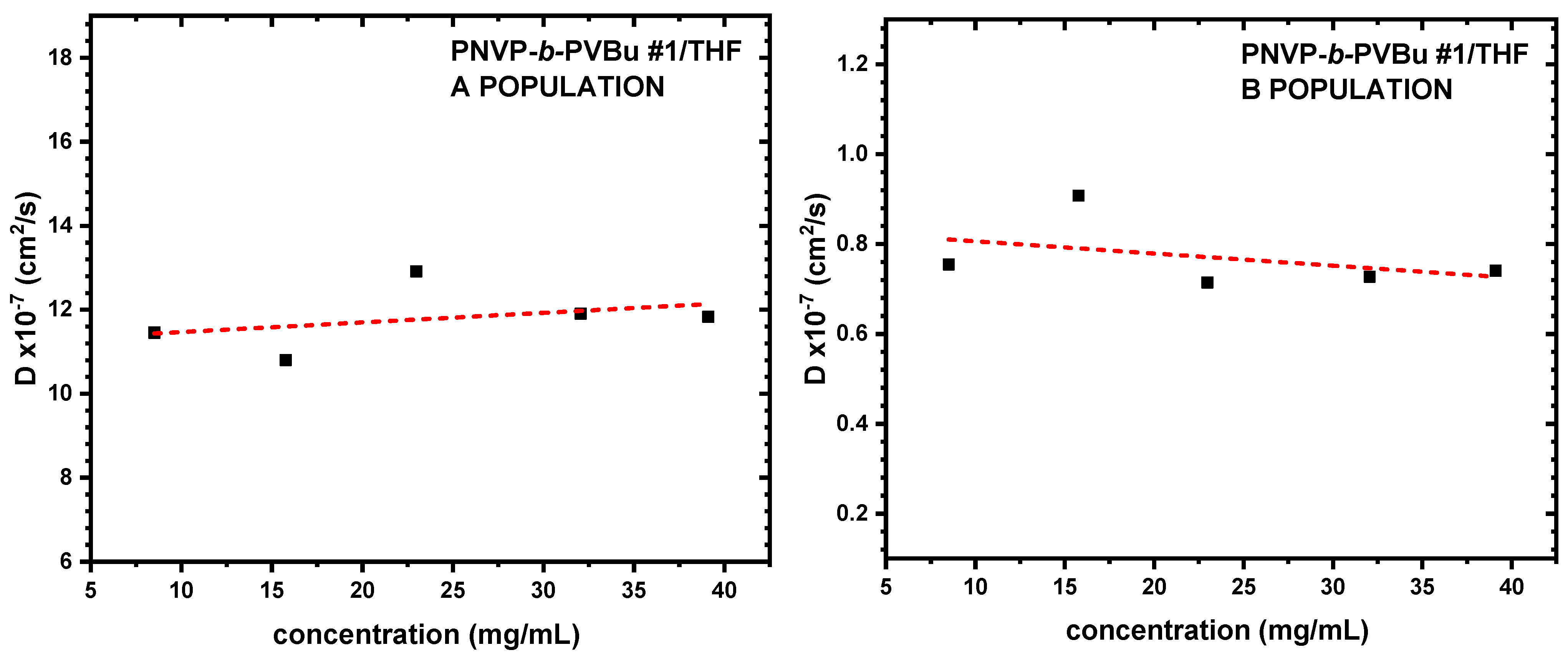
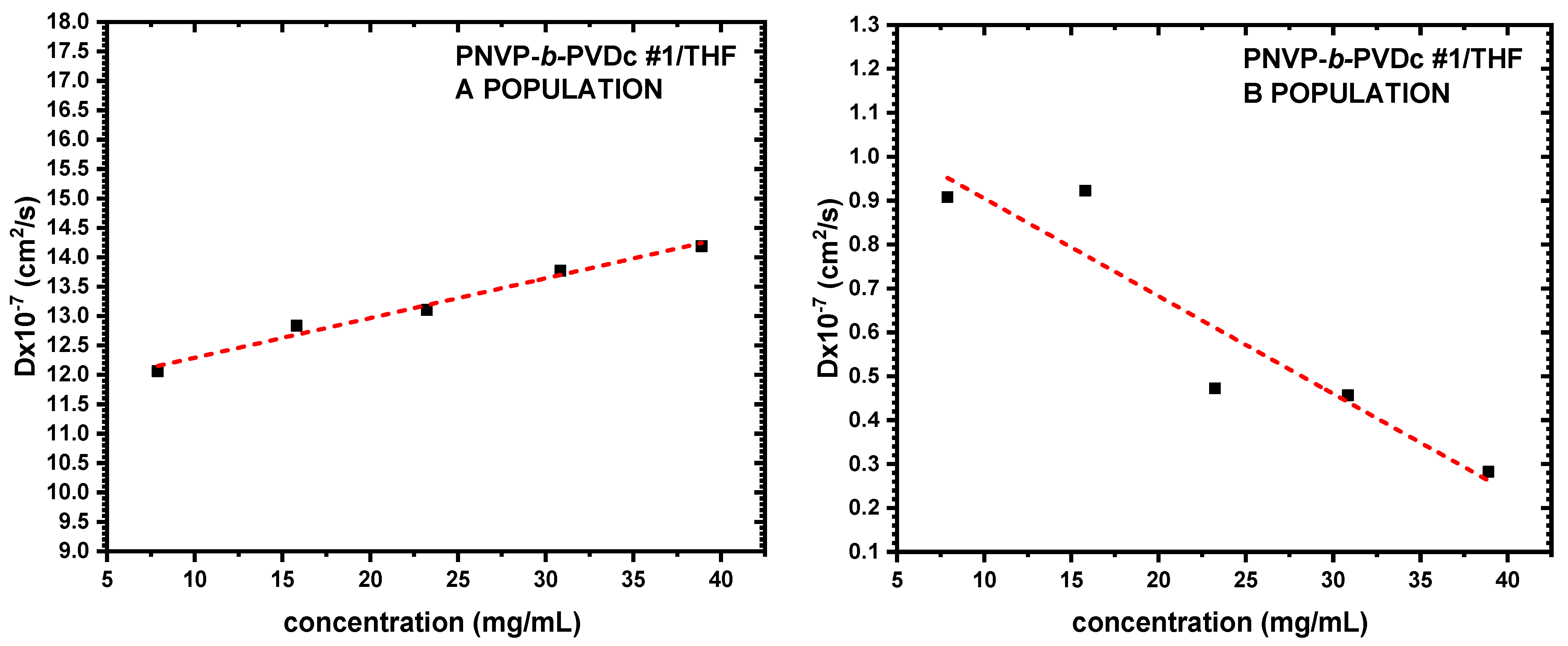
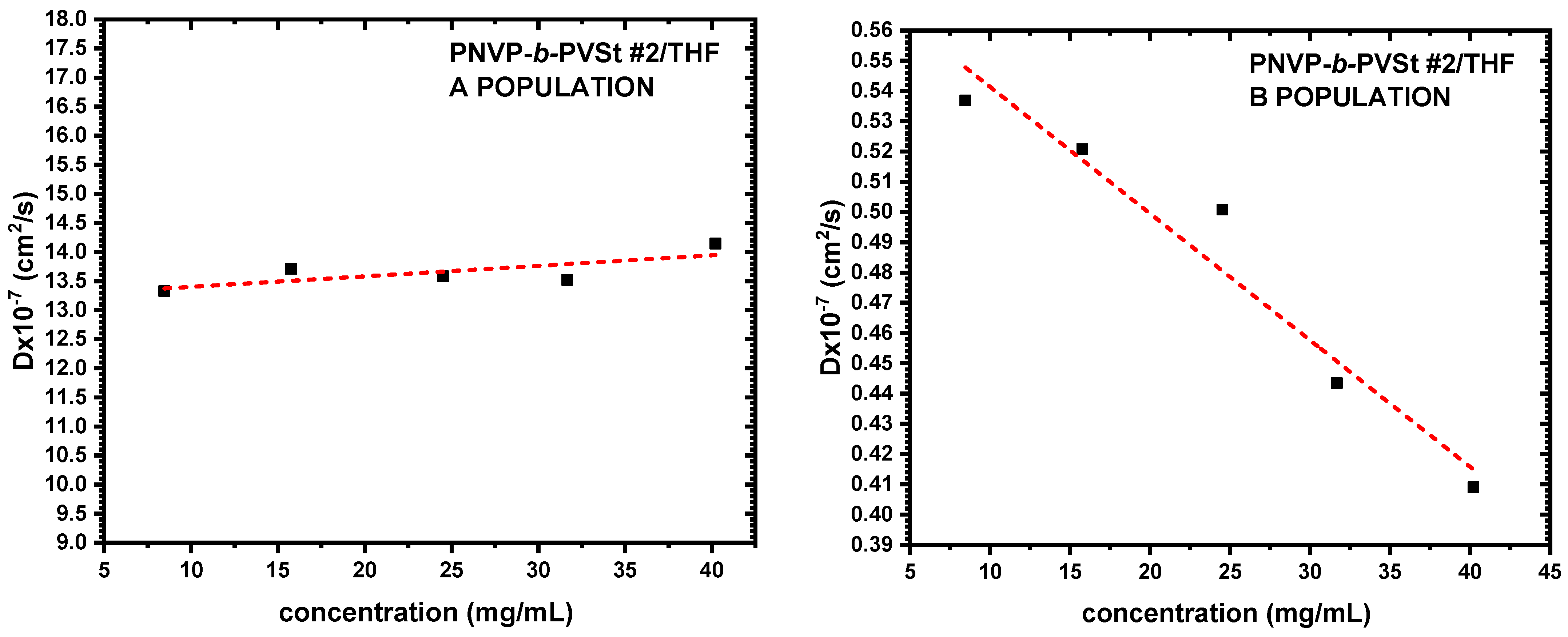
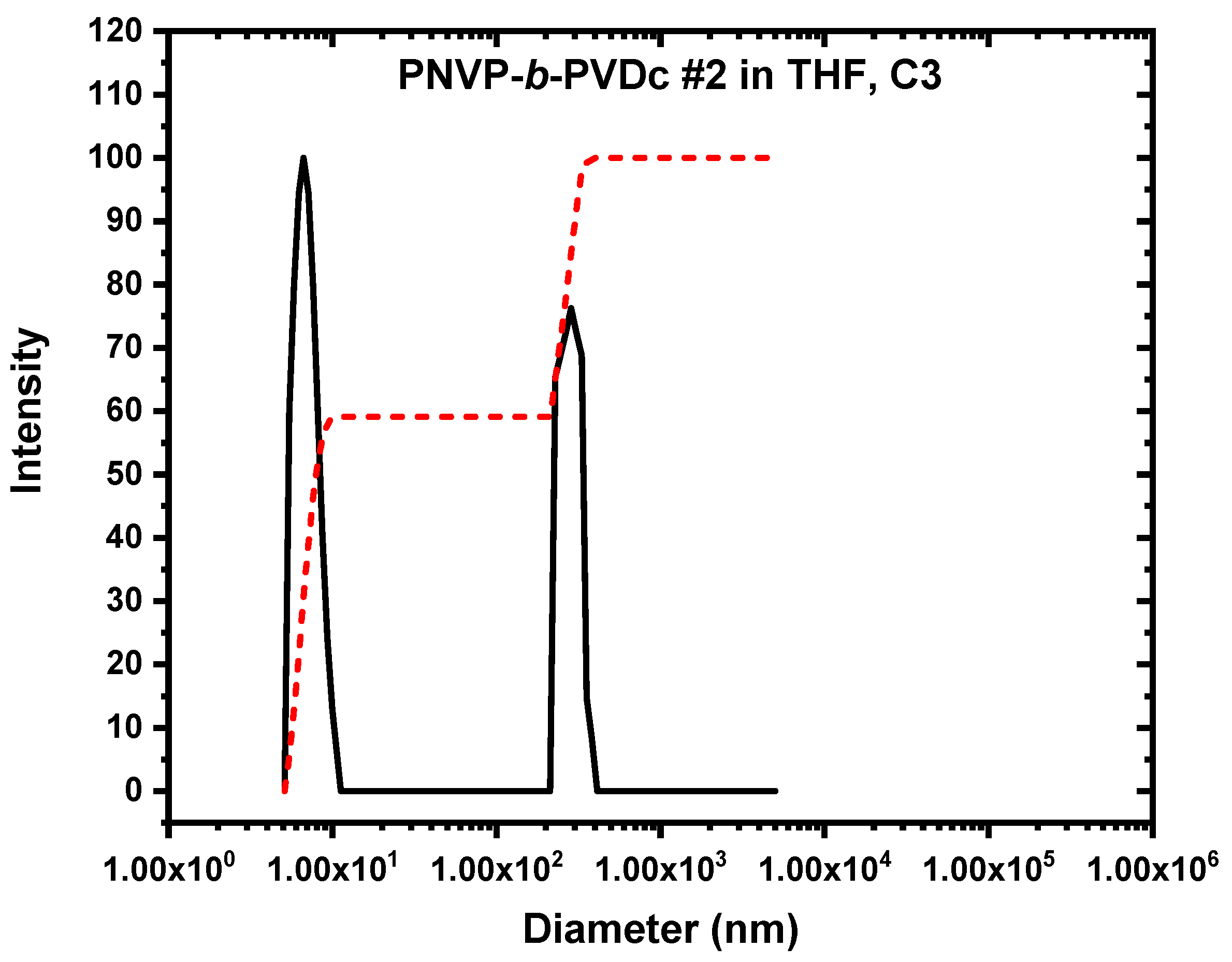
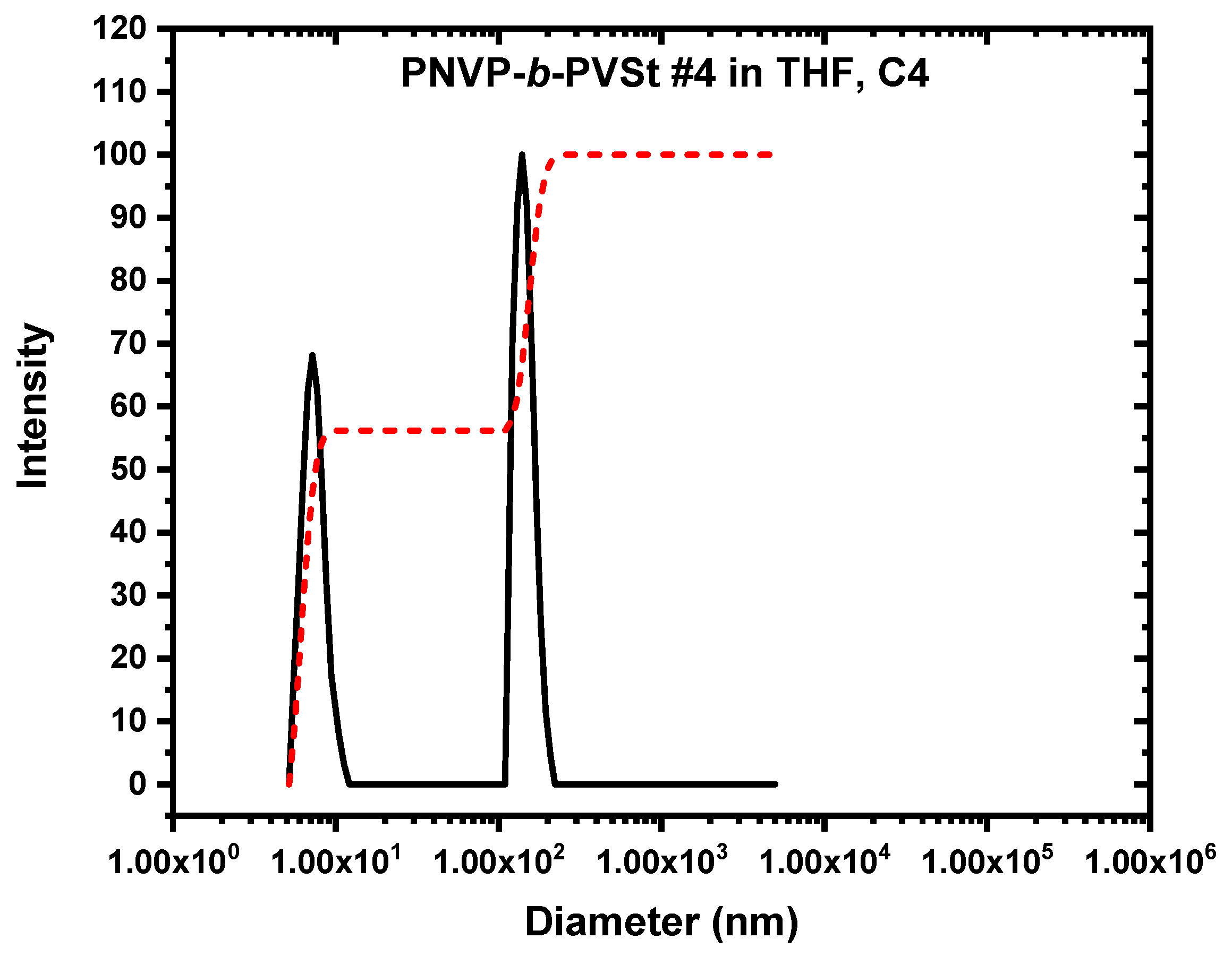
3.3. Self-Assembly Behavior of the PNVP-b-PVE Block Copolymers in THF Solutions by Dynamic Light Scattering
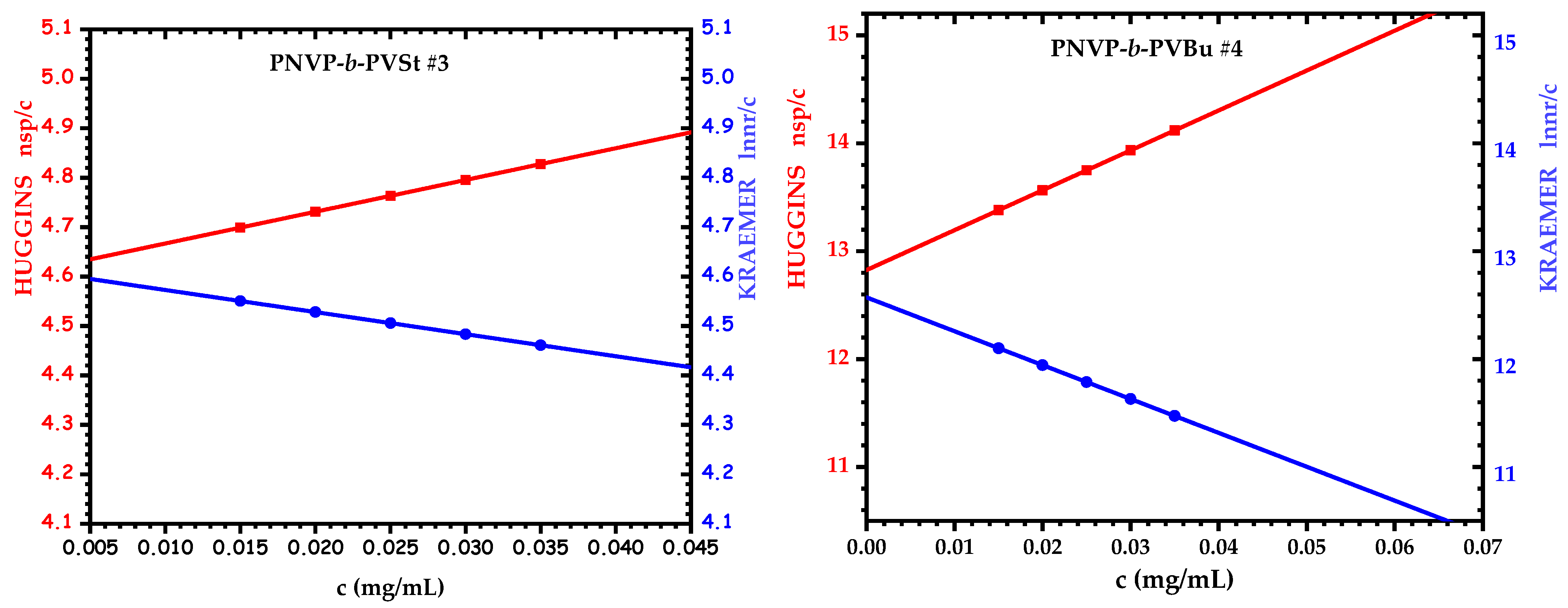
3.4. Self-Assembly Behavior of the PNVP-b-PVE Block Copolymers in THF Solutions by Dilute Solution Viscometry
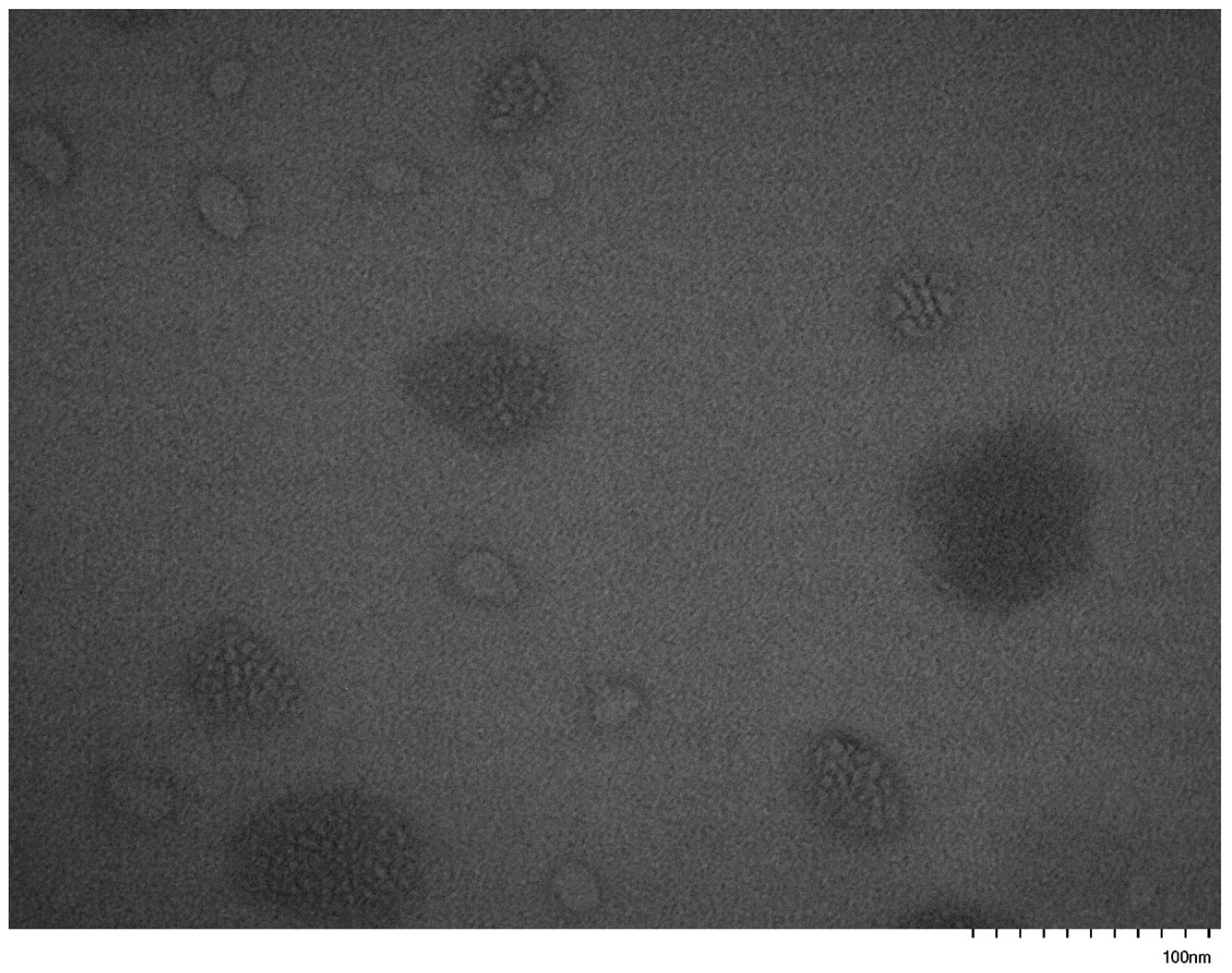
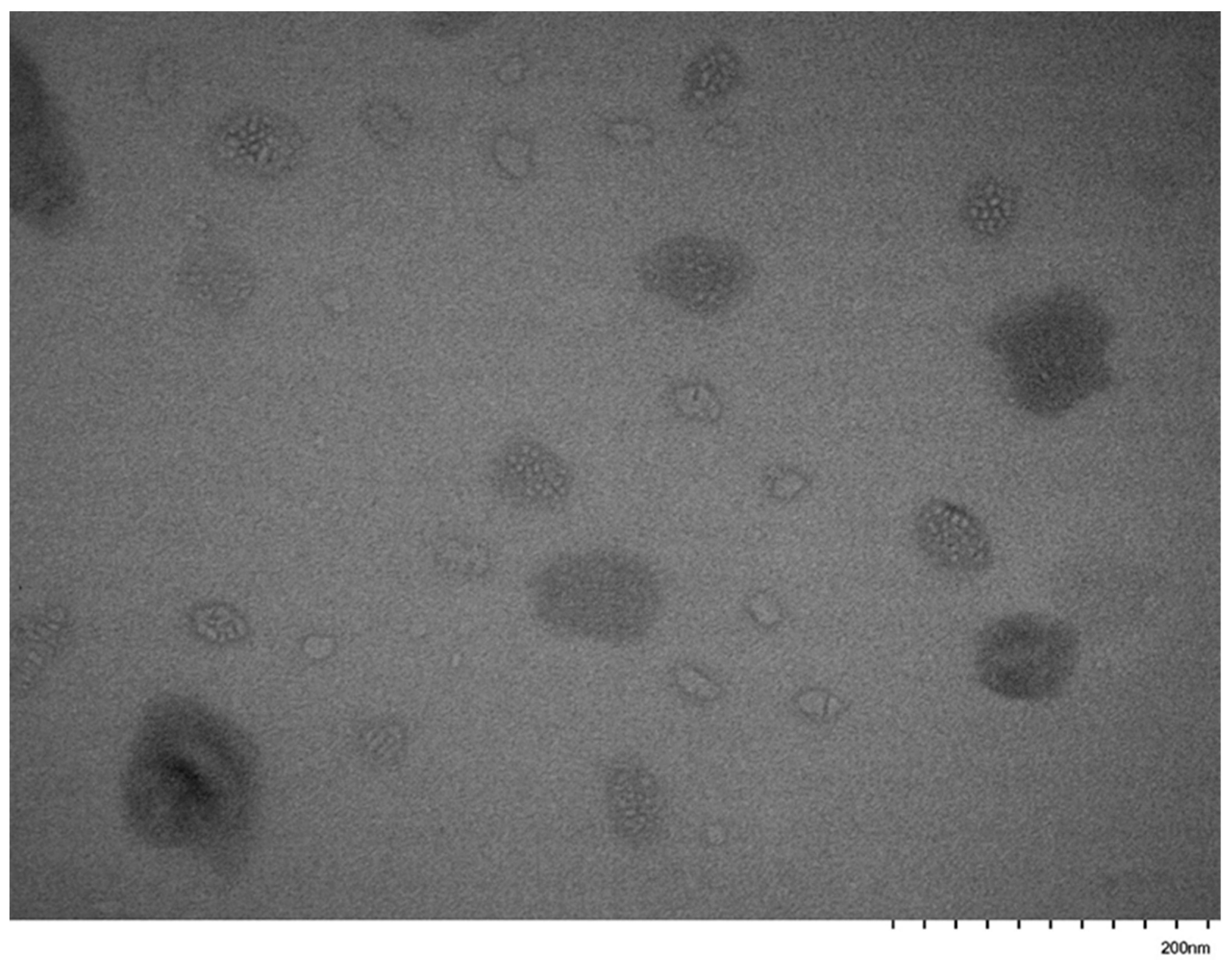
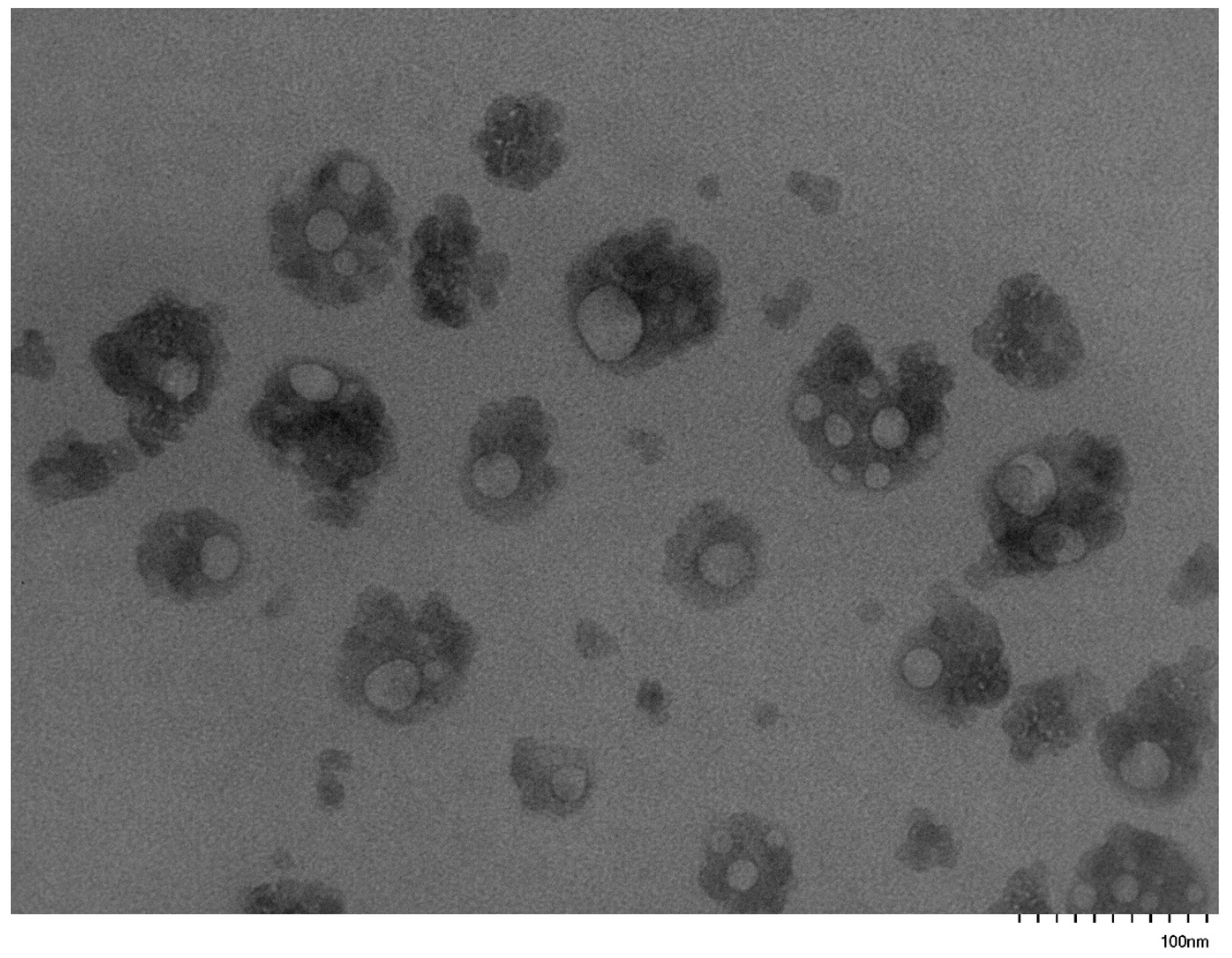
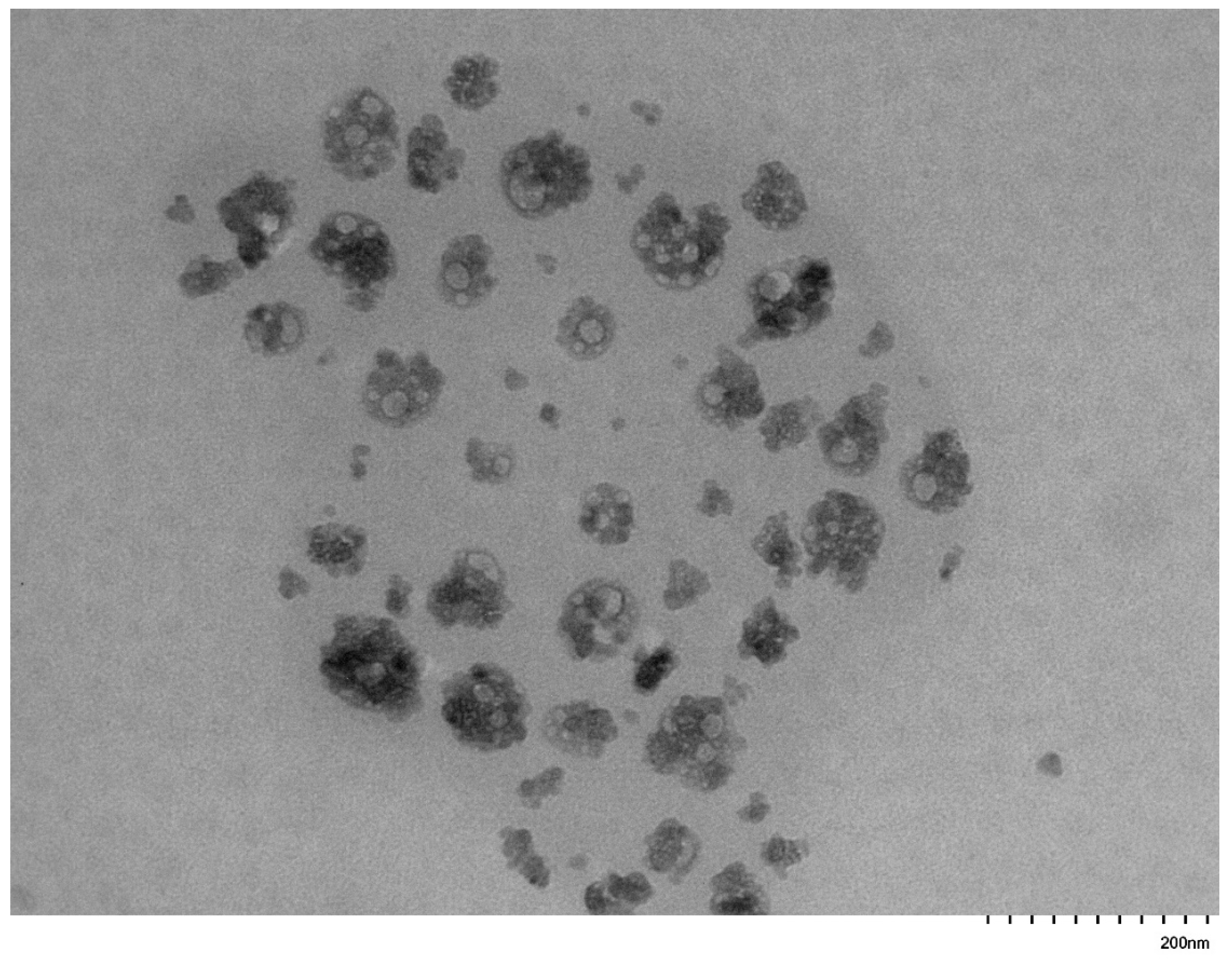
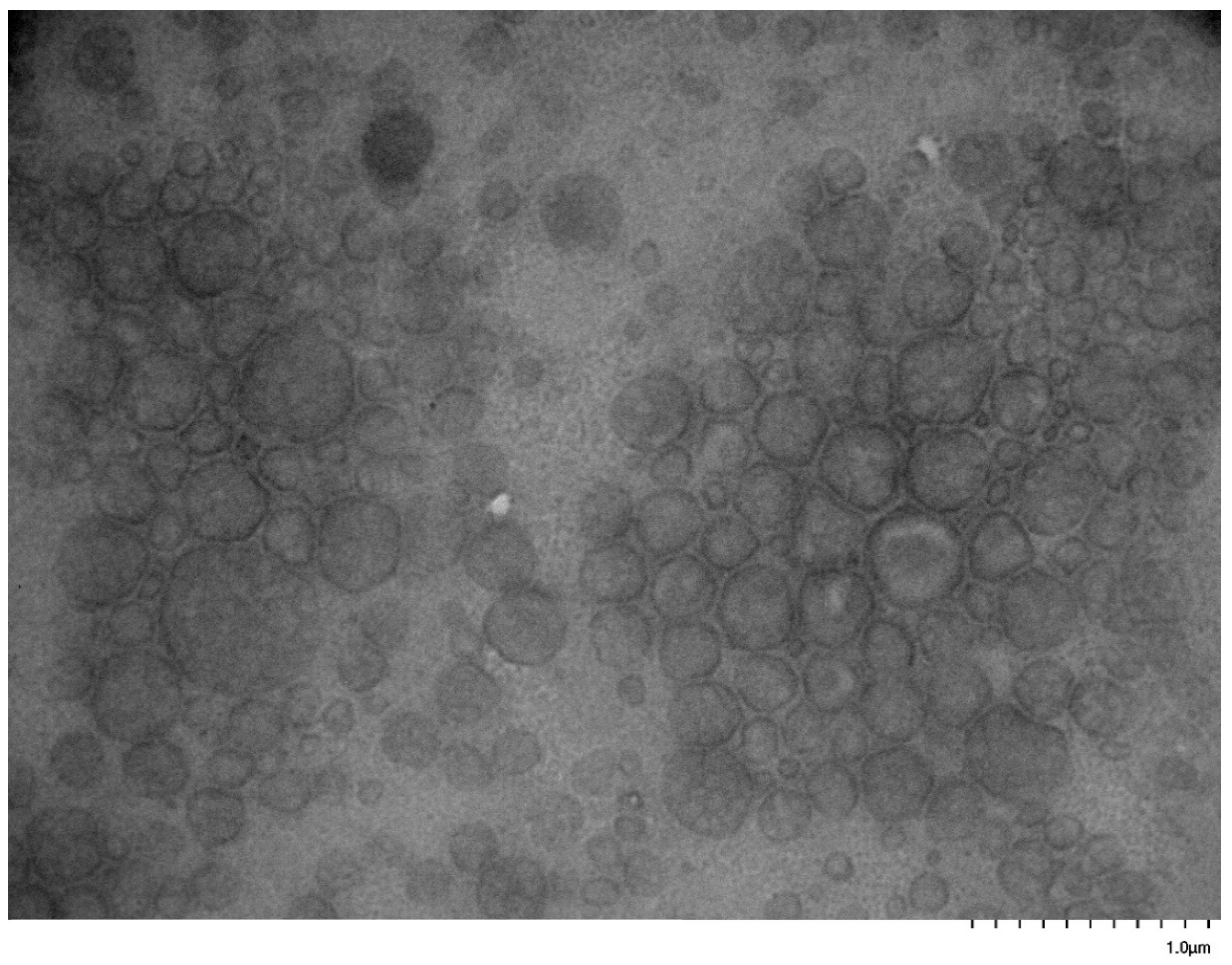
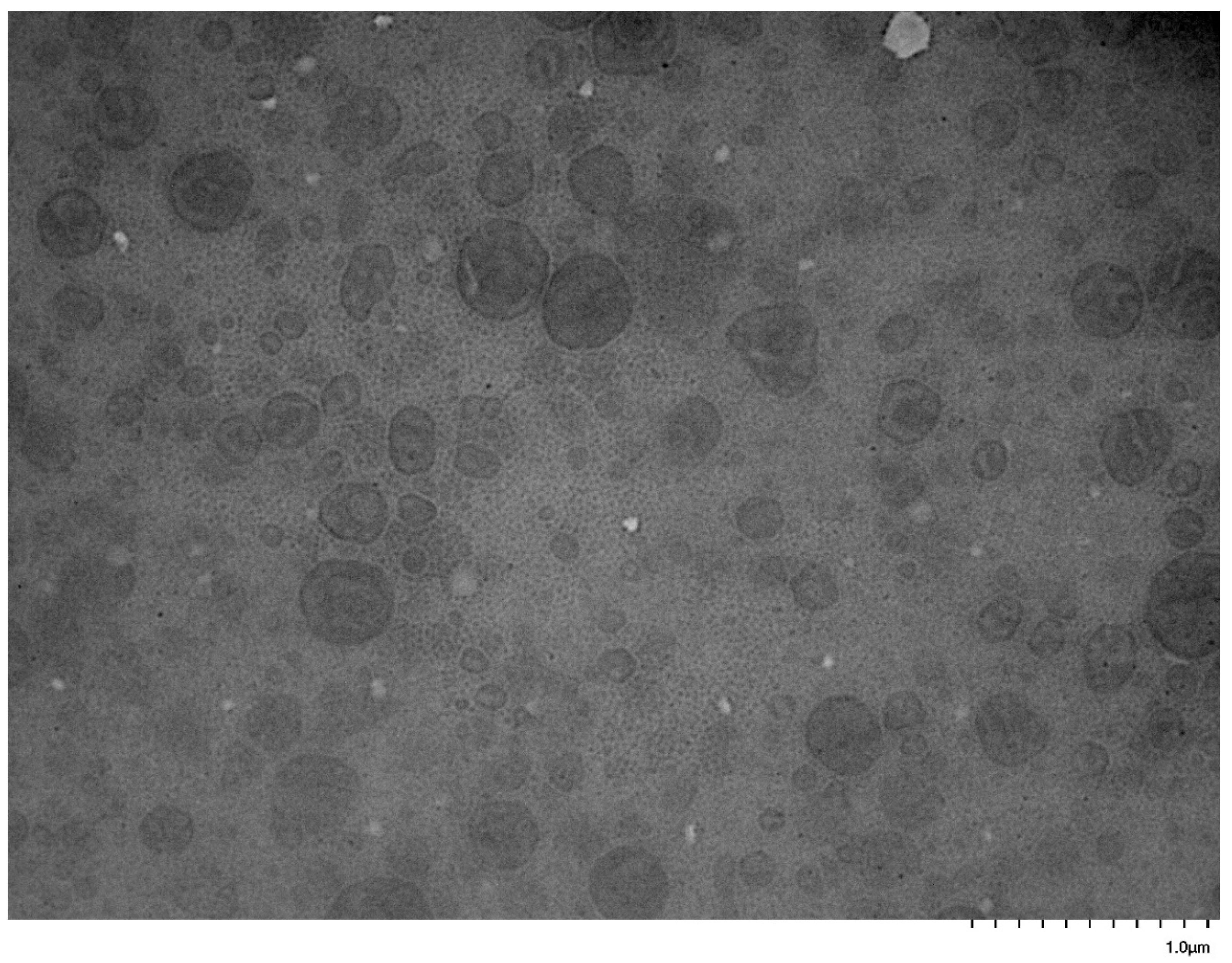
3.5. Self-Assembly Behavior of the PNVP-b-PVE Block Copolymers in THF Solutions by TEM Imaging
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadjichristidis, N.; Pispas, S.; Floudas, G.A. Block copolymers. In Synthetic Strategies, Physical Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Hamley, I.W. The Physics of Block Copolymers; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Hamley, I.W. (Ed.) Developments in Block Copolymer Science and Technology; Wiley: Chichester, UK, 2004. [Google Scholar]
- Hadjichristidis, N.; Hirao, A.; Tezuka, Y.; Du Prez, F. (Eds.) Complex Macromolecular Architectures: Synthesis, Characterization and Self-Assembly; John Wiley & Sons (Asia) Pte Ltd.: Singapore, 2011. [Google Scholar]
- Roka, N.; Kokkorogianni, O.; Kontoes-Georgoudakis, P.; Choinopoulos, I.; Pitsikalis, M. Recent Advances in the Synthesis of Complex Macromolecular Architectures Based on Poly(N-vinyl pyrrolidone) and the RAFT Polymerization Technique. Polymers 2022, 14, 701. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H. Synthesis of block copolymers. Adv. Polym. Sci. 2005, 189, 11–24. [Google Scholar]
- Theodosopoulos, G.; Pitsikalis, M. Block Copolymers: Recent Synthetic Routes and Developments. In Anionic Polymerization: Principles, Practice, Strength, Consequences and Applications; Hadjichristidis, N., Hirao, A., Tezuka, Y., Du Prez, F., Eds.; John Wiley & Sons (Asia) Pte Ltd.: Singapore, 2011. [Google Scholar]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architectures by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Hirao, A. (Eds.) Anionic Polymerization: Principles, Practice, Strength, Consequences and Applications; Springer: Tokyo, Japan; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015. [Google Scholar]
- Kennedy, J.P. Living cationic polymerization of olefins. How did the discovery come about? J. Polym. Sci. Part A Polym. Chem. 1999, 37, 2285–2293. [Google Scholar] [CrossRef]
- Webster, O.W. The discovery and commercialization of group transfer polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2855–2860. [Google Scholar] [CrossRef]
- Leitgeb, A.; Wappel, J.; Slugovc, C. The ROMP toolbox upgraded. Polymer 2010, 51, 2927–2946. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Davis, T.P. (Eds.) Handbook of Radical Polymerization; Wiley-Interscience: Hoboken, NJ, USA, 2002. [Google Scholar]
- Martin, L.; Gody, G.; Perrier, S. Preparation of complex multiblock copolymers via aqueous RAFT polymerization at room temperature. Polym. Chem. 2015, 6, 4875–4886. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J.W. Macromolecular architectures by living and controlled/living polymerizations. Progr. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Sakellariou, G. Macromolecular Architectures by Living and Controlled/living Polymerizations in Controlled and Living Polymerizations. From Mechanisms to Applications; Müller, A.H.E., Matyjaszewski, K., Eds.; Wiley VCH: Weinheim, Germany, 2009; pp. 343–443, Chapter 7. [Google Scholar]
- Dau, H.; Jones, G.R.; Tsogtgerel, E.; Nguyen, D.; Keyes, A.; Liu, Y.-S.; Rauf, H.; Ordonez, E.; Puchelle, V.; Alhan, H.B.; et al. Linear block copolymer synthesis. Chem. Rev. 2022, 122, 14471–14553. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Hldar, U.; De, P. Block copolymer synthesis by the combination of living cationic polymerization and other polymerization methods. Front. Chem. 2021, 9, 644547. [Google Scholar] [CrossRef]
- Diaz, C.; Mehrkhodavandi, P. Strategies for the synthesis of block copolymers with biodegradable polyester segments. Polym. Chem. 2021, 12, 783–806. [Google Scholar] [CrossRef]
- Hatada, K.; Kitayama, T.; Vogl, O. (Eds.) Macromolecular Design of Polymeric Materials; Marcel Dekker Inc.: New York City, NY, USA, 1997; Chapters 3,4. [Google Scholar]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. J. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Coessens, V.; Pintauer, T.; Matyjaszewski, K. Functional polymers by atom transfer radical polymerization. Progr. Polym. Sci. 2001, 26, 337–377. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Buchmeiser, M.R. Homogeneous metathesis polymerization by well-defined group VI and group VIII transition-metal alkylidenes: Fundamentals and applications in the preparation of advanced materials. Chem. Rev. 2000, 100, 1565–1604. [Google Scholar] [CrossRef]
- Khandpur, A.K.; Förster, S.; Bates, F.S.; Hamley, I.; Ryan, A.J.; Almdal, K.; Mortensen, K. Polyisoprene-polystyrene diblock copolymer phase diagram near the order-disorder transition. Macromolecules 1995, 28, 8796–8806. [Google Scholar] [CrossRef]
- Floudas, G.; Vazaiou, B.; Schipper, F.; Ulrich, R.; Wiesner, U.; Iatrou, H.; Hadjichristidis, N. Poly(ethylene oxide-b-isoprene) diblock copolymer phase diagram. Macromolecules 2001, 34, 2947–2957. [Google Scholar] [CrossRef]
- Abetz, V.; Simon, P.F.W. Phase behaviour and morphologies of block copolymers. Adv. Polym. Sci. 2005, 189, 125–212. [Google Scholar]
- Bates, F.M.; Fredrickson, G.H. Block copolymer thermodynamics-theory and experiment. Ann. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Webber, S.E.; Munk, P.; Tuzar, Z. (Eds.) Solvents and Self-Organization of Polymers; NATO ASI Series; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; Volume 327. [Google Scholar]
- Xie, H.Q.; Xie, D. Molecular design, synthesis and properties of block and graft copolymers containing polyoxyethylene segments. Progr. Polym. Sci. 1999, 24, 275–313. [Google Scholar] [CrossRef]
- Gohy, J.-F. Block copolymer micelles. Adv. Polym. Sci. 2005, 190, 65–136. [Google Scholar]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Rodrígeuz-Hernádez, J.; Chécot, F.; Gnanou, Y.; Lecommandoux, S. Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Astafieva, I.; Zhong, X.F.; Eisenberg, A. Critical micellization phenomena in block polyelectrolyte solutions. Macromolecules 1993, 26, 7339–7352. [Google Scholar] [CrossRef]
- Prochazka, K.; Bednar, B.; Mukhtar, E.; Svoboda, P.; Trnena, J.; Almgren, M. Nonradiative energy transfer in block copolymer micelles. J. Phys. Chem. 1991, 95, 4563–4568. [Google Scholar] [CrossRef]
- Wang, Y.; Kausch, C.M.; Chun, M.; Quirk, R.P.; Mattice, W.L. Exchange of chains between micelles of labeled polystyrene-block-poly(oxyethylene) as monitored by nonradiative singlet energy transfer. Macromolecules 1995, 28, 904–911. [Google Scholar] [CrossRef]
- Sidorov, S.N.; Bronstein, L.M.; Valetsky, P.M.; Hartmann, J.; Coelfen, H.; Schnablegger, H.; Antonietti, M. Stabilization of metal nanoparticles in aqueous medium by poly(ethylene oxide)-poly(ethylene imine) block copolymers. J. Colloid Interface Sci. 1999, 212, 197–211. [Google Scholar] [CrossRef]
- Spatz, J.P.; Sheiko, S.; Möller, M. Ion stabilized block copolymer micelles film formation and inter micellar interaction. Macromolecules 1996, 29, 3220–3226. [Google Scholar] [CrossRef]
- Lazzari, M.; Scalarone, D.; Hoppe, C.E.; Vazquez-Vazquez, C.; Lòpez-Quintela, M.A. Tunable polyacrylonitrile based micellar aggregates as a potential tool for the fabrication of carbon nanofibers. Chem. Mater. 2007, 19, 5818–5820. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Bae, Y.H. Polymer Architecture and Drug Delivery. Pharm. Res. 2006, 23, 1–30. [Google Scholar] [CrossRef]
- Karanikolopoulos, N.; Pitsikalis, M.; Hadjichristidis, N.; Georgikopoulou, K.; Calogeropoulou, T.; Dunlap, J.R. pH-Responsive aggregates from double hydrophilic block copolymers carrying zwitterionic groups. Encapsulation of antiparasitic compounds for the treatment of leishmaniasis. Langmuir 2007, 23, 4214–4224. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Karanikolopoulos, N.; Zamurovic, M.; Pitsikalis, M.; Hadjichristidis, N. Poly(DL-lactide)-b-Poly(N,N-dimethylamino-2-ethyl methacrylate): Synthesis, characterization, micellization behaviour in aqueous solutions and encapsulation of the hydrophobic drug dipyridamole. Biomacromolecules 2010, 11, 430–438. [Google Scholar] [CrossRef]
- Li, J.; Barrow, D.; Howell, H.; Kalachandra, S. In vitro drug release study of methacrylate polymer blend system: Effect of polymer blend composition, drug loading and solubilizing surfactants on drug release. J. Mater. Sci. Mater. Med. 2010, 21, 583–588. [Google Scholar] [CrossRef]
- Heinzmann, C.; Salz, U.; Moszner, N.; Fiore, G.L.; Weder, C. Supramolecular Cross-Links in Poly(alkyl methacrylate) Copolymers and Their Impact on the Mechanical and Reversible Adhesive Properties. ACS Appl. Mater. Interf. 2015, 7, 13395–13404. [Google Scholar] [CrossRef] [PubMed]
- Khai, N.; Nguyen, H.; Dang, H.H.; Nguyen, L.T.; Nguyen, L.M.T.; Truong, T.T.; Nguyen, H.T.; Nguyen, T.Q.; Tran, C.D.; Nguyen, L.-T.T. Self-healing elastomers from supramolecular random copolymers of 4-vinyl pyridine. Eur. Polym. J. 2023, 199, 112474. [Google Scholar] [CrossRef]
- Trubetskoy, V.S. Polymeric micelles as carriers of diagnostic agents. Adv. Drug Deliv. Rev. 1999, 37, 81–88. [Google Scholar] [CrossRef]
- Gerst, M.; Schuch, H.; Urban, D. Amphiphilic Block Copolymers as Surfactants in Emulsion Polymerization; ACS Symposium Series; Glass, J.E., Ed.; ACS Publications: Washington, DC, USA, 2000; Volume 765, pp. 37–51. [Google Scholar]
- Yu-Su, S.Y.; Thomas, D.R.; Alford, J.E.; LaRue, I.; Pitsikalis, M.; Hadjichristidis, N.; DeSimone, J.M.; Dobrynin, A.V.; Sheiko, S.S. Molding copolymer micelles: A framework for molding of discrete objects on surfaces. Langmuir 2008, 24, 12671–12679. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, L.M.; Sidorov, S.N.; Valetsky, P.M.; Hartmann, J.; Coelfen, H.; Antonietti, M. Induced micellization by interaction of poly(2-vinylpyridine)-block-poly(ethylene oxide) with metal compounds. Micelle characteristics and metal nanoparticle formation. Langmuir 1999, 15, 6256–6262. [Google Scholar] [CrossRef]
- Munch, M.R.; Gast, A.P. Kinetics of block copolymer adsorption on dielectric surfaces from a selective solvent. Macromolecules 1990, 23, 2313–2320. [Google Scholar] [CrossRef]
- Breulmann, M.; Förster, S.; Antonietti, M. Mesoscopic surface patterns formed by block copolymer micelles. Macromol. Chem. Phys. 2000, 201, 204–211. [Google Scholar] [CrossRef]
- Roka, N.; Pitsikalis, M. Synthesis and micellization behavior of amphiphilic block copolymers of poly(N-vinyl pyrrolidone) and poly(benzyl methacrylate): Block versus statistical copolymers. Polymers 2023, 15, 2215. [Google Scholar] [CrossRef] [PubMed]
- Kontoes-Georgoudakis, P.; Plachouras, N.V.; Kokkorogianni, O.; Pitsikalis, M. Amphiphilic block copolymers of poly(N-vinyl pyrrolidone) and poly (isobornyl methacrylate). Synthesis, characterization and micellization behaviour in selective solvents. Eur. Polym. J. 2024, 208, 112873. [Google Scholar] [CrossRef]
- Roka, N.; Pitsikalis, M. Statistical copolymers of N-vinylpyrrolidone and benzyl methacrylate via RAFT: Monomer reactivity ratios, thermal properties and kinetics of thermal decomposition. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 222–230. [Google Scholar] [CrossRef]
- Karatzas, A.; Bilalis, P.; Iatrou, H.; Pitsikalis, M.; Hadjichristidis, N. Synthesis of well-defined functional macromolecular chimeras based on poly(ethylene oxide) or poly(N-vinyl pyrrolidone). React. Funct. Polym. 2009, 69, 435–440. [Google Scholar] [CrossRef]
- Bilalis, P.; Zorba, G.; Pitsikalis, M.; Hadjichristidis, N. Synthesis of poly(n-hexyl isocyanate-b-N-vinylpyrrolidone) block copolymers by the combination of anionic and nitroxide-mediated radical polymerizations: Micellization properties in aqueous solutions. J. Polym. Sci. Part A Polym. Chem. Ed. 2006, 44, 5719–5728. [Google Scholar] [CrossRef]
- Bilalis, P.; Pitsikalis, M.; Hadjichristidis, N. Controlled nitroxide-mediated and reversible addition-fragmentation chain transfer polymerization of N-vinylpyrrolidone: Synthesis of block copolymers with styrene and 2-vinylpyridine. J. Polym. Sci. Part A Polym. Chem. Ed. 2006, 44, 659–665. [Google Scholar] [CrossRef]
- Roka, N.; Kokkorogianni, O.; Pitsikalis, M. Statistical copolymers of N-vinylpyrrolidone and 2-(dimethylamino)ethyl methacrylate via RAFT: Monomer reactivity ratios, thermal properties, and kinetics of thermal decomposition. J. Polym. Sci. Polym. Chem. 2017, 55, 3776–3787. [Google Scholar] [CrossRef]
- Fokaidis-Psyllas, A.; Kokkorogianni, O.; Pitsikalis, M. Statistical copolymers of N-vinylpyrrolidone and phenoxyethyl methacrylate via RAFT polymerization: Monomer reactivity ratios, thermal properties, kinetics of thermal decomposition and self-assembly behavior in selective solvents. Polym. Bull. 2025; in press. [Google Scholar] [CrossRef]
- Plachouras, N.V.; Pitsikalis, M. Statistical copolymers of N–vinylpyrrolidone and 2–chloroethyl vinyl ether via radical RAFT polymerization: Monomer reactivity ratios, thermal properties, and kinetics of thermal decomposition of the statistical copolymers. Polymers 2023, 15, 1970. [Google Scholar] [CrossRef]
- Roka, N.; Papazoglou, T.P.; Pitsikalis, M. Block copolymers of Poly(N–vinylpyrrolidone) and Poly(vinyl esters) bearing n-alkyl side groups via radical RAFT polymerization: Synthesis, characterization and thermal properties. Polymers 2024, 16, 2447. [Google Scholar] [CrossRef]
- Rinno, H. Poly(vinyl esters). In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2000; Volume 28, pp. 469–479. [Google Scholar]
- Murray, R.E.; Lincoln, D.M. Catalytic route to vinyl esters. Catal. Today 1992, 13, 93–102. [Google Scholar] [CrossRef]
- Charmot, D.; Corpart, P.; Adam, H.; Zard, S.Z.; Biadatti, T.; Bouhadir, G. Controlled radical polymerization in dispersed media. Macromol. Symp. 2000, 150, 23–32. [Google Scholar] [CrossRef]
- Matioszek, D.; Brusylovets, O.; Wilson, D.J.; Mazières, S.; Destarac, M. Reversible addition-fragmentation chain-transfer polymerization of vinyl monomers with N,N-dimethylsdiselenocarbamates. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4361–4368. [Google Scholar] [CrossRef]
- Benard, J.; Favier, A.; Zhang, L.; Nilararoya, A.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Poly(vinyl ester) star polymers via xanthate-mediated living radical polymerization: From poly(vinyl alcohol) to glycopolymer stars. Macromolecules 2005, 38, 5475–5484. [Google Scholar] [CrossRef]
- Gu, Y.; He, J.; Li, C.; Zhou, C.; Song, S.; Yang, Y. Block copolymerization of vinyl acetate and vinyl neo-decanoate mediated by dithionosulfide. Macromolecules 2010, 43, 4500–4510. [Google Scholar] [CrossRef]
- Lipscomb, C.E.; Mahanthappa, M.K. Poly(vinyl ester) block copolymers synthesized by reversible addition-fragmentation chain transfer polymerizations. Macromolecules 2009, 42, 4571–4579. [Google Scholar] [CrossRef]
- Lipscomb, C.E.; Mahanthappa, M.K. Microphase separation mode-dependent mechanical response in poly(vinyl ester)/PEO triblock copolymers. Macromolecules 2011, 44, 4401–4409. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Yang, H.; Chen, X. Effect of non-ideal mixed solvents on dimensions of poly(N-vinylpyrrolidone) and poly(methyl methacrylate) coils. J. Macromol. Sci. Part B Polym. Phys. 2006, 45, 1125–1134. [Google Scholar]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Antonietti, M.; Heinz, S.; Schmidt, M.; Rosenauer, C. Determination of the Micelle Architecture of Polystyrene/Poly(4-vinylpyridine) Block Copolymers in Dilute Solution. Macromolecules 1994, 27, 3276–3281. [Google Scholar] [CrossRef]
- Pitsikalis, M.; Hadjichristidis, N.; Mays, J.W. Model Mono-, Di-, and Tri-ω-Functionalized Three-Arm Star Polybutadienes. Association Behavior in Dilute Solution by Dynamic Light Scattering and Viscometry. Macromolecules 1996, 29, 179–184. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Nowell, C.J.; Cipolla, D.; Rades, T.; Boyd, B.J. Direct Comparison of Standard Transmission Electron Microscopy and Cryogenic-TEM in Imaging Nanocrystals Inside Liposomes. Mol. Pharm. 2019, 16, 1775–1781. [Google Scholar] [CrossRef]

| Macro CTA (PNVP) a | Block Copolymers a | NVP | Vinyl Ester | |||
|---|---|---|---|---|---|---|
| Sample | Mn 103 (Daltons) | Ð | Mn 103 (Daltons) | Ð | % mol b | % mol b |
| PNVP-b-PVBu #1 | 8.5 | 1.30 | 16.0 | 1.90 | 22 | 78 |
| PNVP-b-PVBu #2 | 8.9 | 1.35 | 15.5 | 1.54 | 48 | 52 |
| PNVP-b-PVBu #3 | 8.9 | 1.35 | 17.5 | 1.40 | 57 | 43 |
| PNVP-b-PVBu #4 | 28.0 | 1.27 | 32.0 | 1.32 | 84 | 16 |
| PNVP-b-PVDc #1 | 5.5 | 1.47 | 12.5 | 1.60 | 38 | 62 |
| PNVP-b-PVDc #2 | 8.5 | 1.30 | 11.0 | 1.45 | 56 | 44 |
| PNVP-b-PVDc #3 | 8.5 | 1.30 | 12.5 | 1.31 | 63 | 37 |
| PNVP-b-PVDc #4 | 9.5 | 1.36 | 10.5 | 1.36 | 93 | 7 |
| PNVP-b-PVSt #1 | 7.5 | 1.30 | 10.4 | 1.51 | 61 | 39 |
| PNVP-b-PVSt #2 | 8.5 | 1.30 | 10.5 | 1.44 | 78 | 22 |
| PNVP-b-PVSt #3 | 8.1 | 1.30 | 12.5 | 1.22 | 83 | 17 |
| PNVP-b-PVSt #4 | 8.1 | 1.30 | 10.9 | 1.37 | 85 | 15 |
| Sample | Mw × 10−4 | Nw | A2 × 104 (cm3 mol/g2) |
|---|---|---|---|
| PNVP-b-PVBu #1 | 15.9 | 9.94 | 1.40 |
| PNVP-b-PVBu #2 | 3.22 | 2.08 | 4.39 |
| PNVP-b-PVBu #3 | 3.62 | 2.07 | 3.86 |
| PNVP-b-PVBu #4 | 5.69 | 1.78 | 0.69 |
| PNVP-b-PVDc #1 | 6.35 | 5.08 | 2.07 |
| PNVP-b-PVDc #2 | 3.35 | 3.05 | 2.50 |
| PNVP-b-PVDc #3 | 10.5 | 8.40 | 3.50 |
| PNVP-b-PVDc #4 | 3.59 | 3.42 | 5.16 |
| PNVP-b-PVSt #1 | 7.13 | 6.86 | 2.78 |
| PNVP-b-PVSt #2 | 3.82 | 3.64 | 1.48 |
| PNVP-b-PVSt #3 | 18.6 | 14.88 | 1.90 |
| PNVP-b-PVSt #4 | 4.44 | 4.07 | 1.30 |
| Sample | Do (cm2/s) | Kd | Rho A (nm) | Rho B (nm) |
|---|---|---|---|---|
| PNVP-b-PVBu #1 | 11.2405 | 2.04 | 4.22 | 48.45 |
| PNVP-b-PVBu #2 | 10.4839 | 4.63 | 4.53 | 32.97 |
| PNVP-b-PVBu #3 | 9.9667 | 8.58 | 4.76 | 170.06 |
| PNVP-b-PVBu #4 | 9.19953 | −2.98 | 5.16 | 96.31 |
| PNVP-b-PVDc #1 | 14.2491 | 0.96 | 3.33 | 35.82 |
| PNVP-b-PVDc #2 | 11.6172 | 5.81 | 4.08 | 76.10 |
| PNVP-b-PVDc #3 | 15.1746 | −1.11 | 3.13 | 15.58 |
| PNVP-b-PVDc #4 | 14.0547 | 0.29 | 3.38 | 85.11 |
| PNVP-b-PVSt #1 | 13.2195 | 1.37 | 3.59 | 81.37 |
| PNVP-b-PVSt #2 | 12.2076 | 3.09 | 3.89 | 71.94 |
| PNVP-b-PVSt #3 | 13.8421 | 1.40 | 3.43 | 94.93 |
| PNVP-b-PVSt #4 | 14.3697 | 0.32 | 3.30 | 84.08 |
| Sample | [η]H mL/g | [η]K mL/g | KH | Rv, nm |
|---|---|---|---|---|
| PNVP-b-PVBu #1 | 12.30 | 11.92 | 0.33 | 6.76 |
| PNVP-b-PVBu #4 | 12.82 | 12.57 | 0.43 | 4.87 |
| PNVP-b-PVDc #1 | 8.22 | 7.84 | 0.36 | 4.36 |
| PNVP-b-PVDc #2 | 5.64 | 5.64 | 0.41 | 3.10 |
| PNVP-b-PVDc #3 | 6.56 | 6.30 | 0.59 | 4.78 |
| PNVP-b-PVDc #4 | 5.01 | 5.17 | 1.07 | 3.05 |
| PNVP-b-PVSt #1 | 3.51 | 3.52 | 0.59 | 3.41 |
| PNVP-b-PVSt #2 | 1.97 | 2.11 | 1.60 | 2.82 |
| PNVP-b-PVSt #3 | 4.60 | 4.62 | 0.30 | 5.14 |
| PNVP-b-PVSt #4 | 8.00 | 7.46 | 1.20 | 3.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roka, N.; Skiadas, V.-C.; Kolovou, A.; Papazoglou, T.-P.; Pitsikalis, M. Micellization Studies of Block Copolymers of Poly(N-vinyl pyrrolidone) and n-Alkyl-Substituted Poly(vinyl esters) in Tetrahydrofuran. Polymers 2025, 17, 2842. https://doi.org/10.3390/polym17212842
Roka N, Skiadas V-C, Kolovou A, Papazoglou T-P, Pitsikalis M. Micellization Studies of Block Copolymers of Poly(N-vinyl pyrrolidone) and n-Alkyl-Substituted Poly(vinyl esters) in Tetrahydrofuran. Polymers. 2025; 17(21):2842. https://doi.org/10.3390/polym17212842
Chicago/Turabian StyleRoka, Nikoletta, Vasileios-Christos Skiadas, Areti Kolovou, Theodosia-Panagiota Papazoglou, and Marinos Pitsikalis. 2025. "Micellization Studies of Block Copolymers of Poly(N-vinyl pyrrolidone) and n-Alkyl-Substituted Poly(vinyl esters) in Tetrahydrofuran" Polymers 17, no. 21: 2842. https://doi.org/10.3390/polym17212842
APA StyleRoka, N., Skiadas, V.-C., Kolovou, A., Papazoglou, T.-P., & Pitsikalis, M. (2025). Micellization Studies of Block Copolymers of Poly(N-vinyl pyrrolidone) and n-Alkyl-Substituted Poly(vinyl esters) in Tetrahydrofuran. Polymers, 17(21), 2842. https://doi.org/10.3390/polym17212842









