Preparation of Crosslinked Gelatin Microparticles and Study on Their Loading Capacity for Folic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microparticlees
2.2.1. Preparation of GMPs
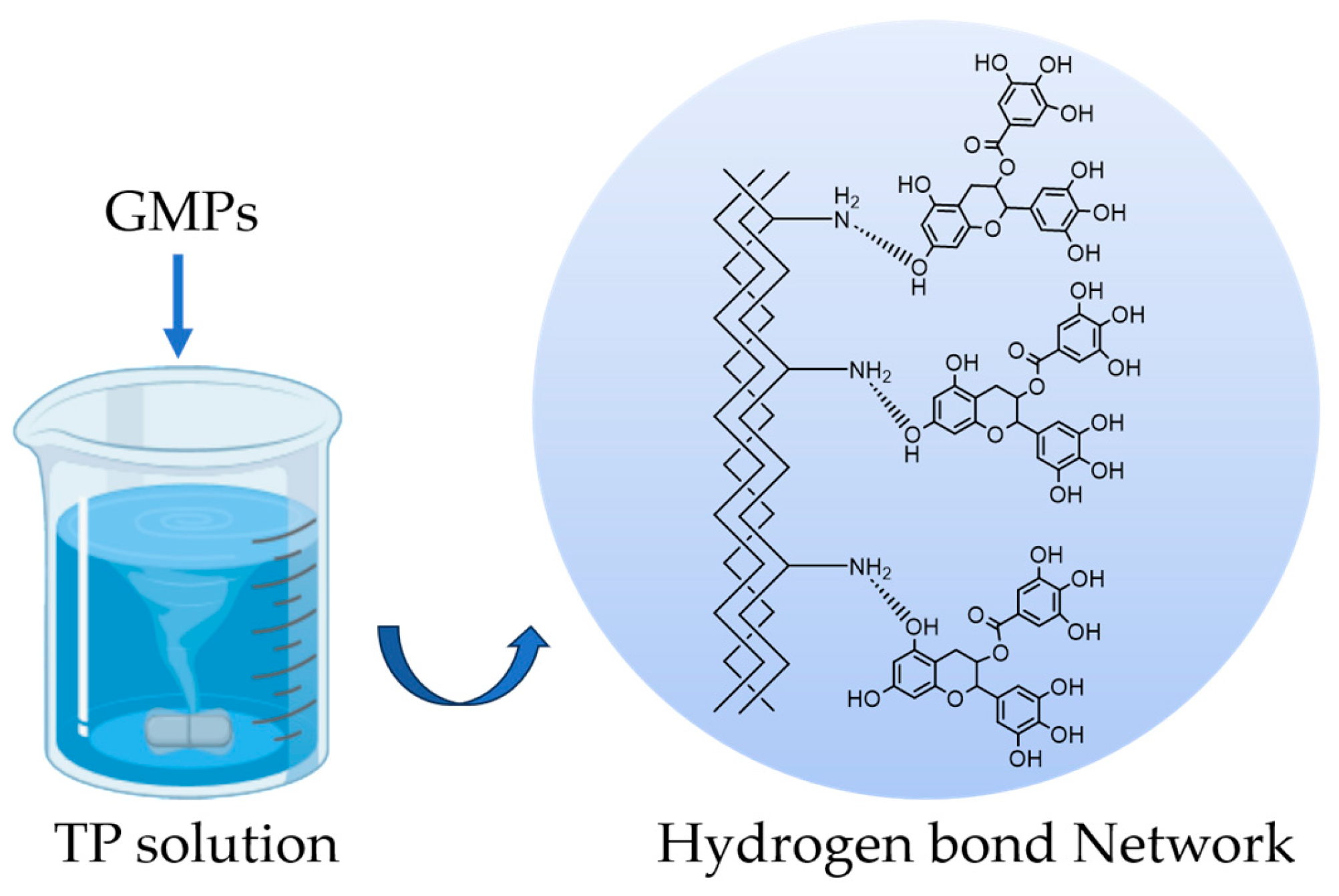
2.2.2. Preparation of cGMPs
2.3. Preparation of Substrate-Loaded cGMPs
2.3.1. Preparation of MB-Loaded cGMPs (MB-cGMPs)
2.3.2. Preparation of FA-Loaded cGMPs (FA-cGMPs)
2.4. Determination of the MB Retention Rate
2.5. Determination of FA Concentration
2.6. Effect of Crosslinking Conditions on MB Retention Rate
2.7. Characterization Methods
2.7.1. Scanning Electron Microscopy (SEM)
2.7.2. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.7.3. X-Ray Diffraction (XRD)
2.7.4. Differential Scanning Calorimetry (DSC)
2.8. Stability Testing of FA-cGMPs in High-Temperature Aqueous Solutions
2.9. In Vitro Digestion Protocol of FA-cGMPs
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optimisation of the cGMPs Preparation Process
3.2. Characterization Results
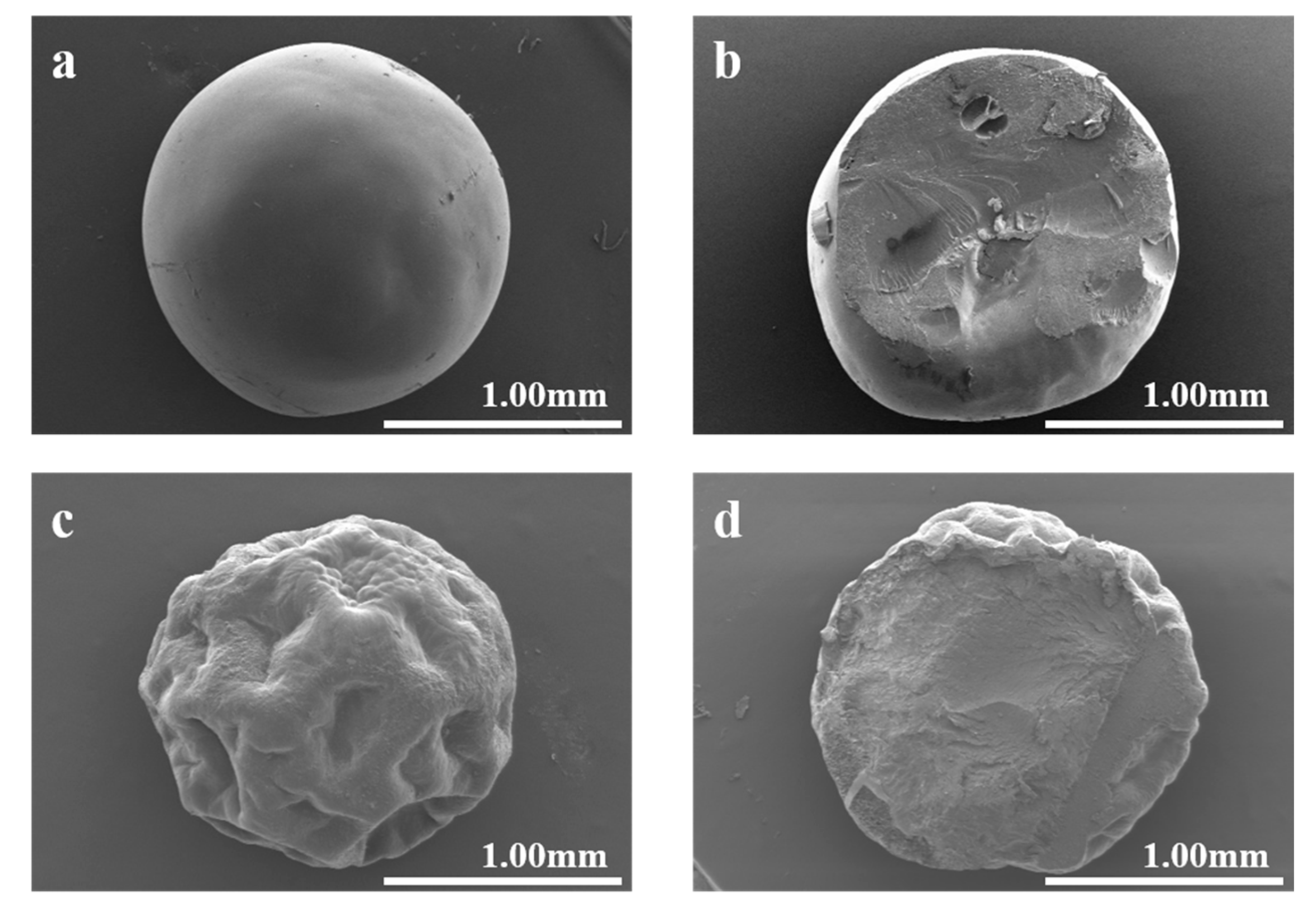
3.2.1. SEM
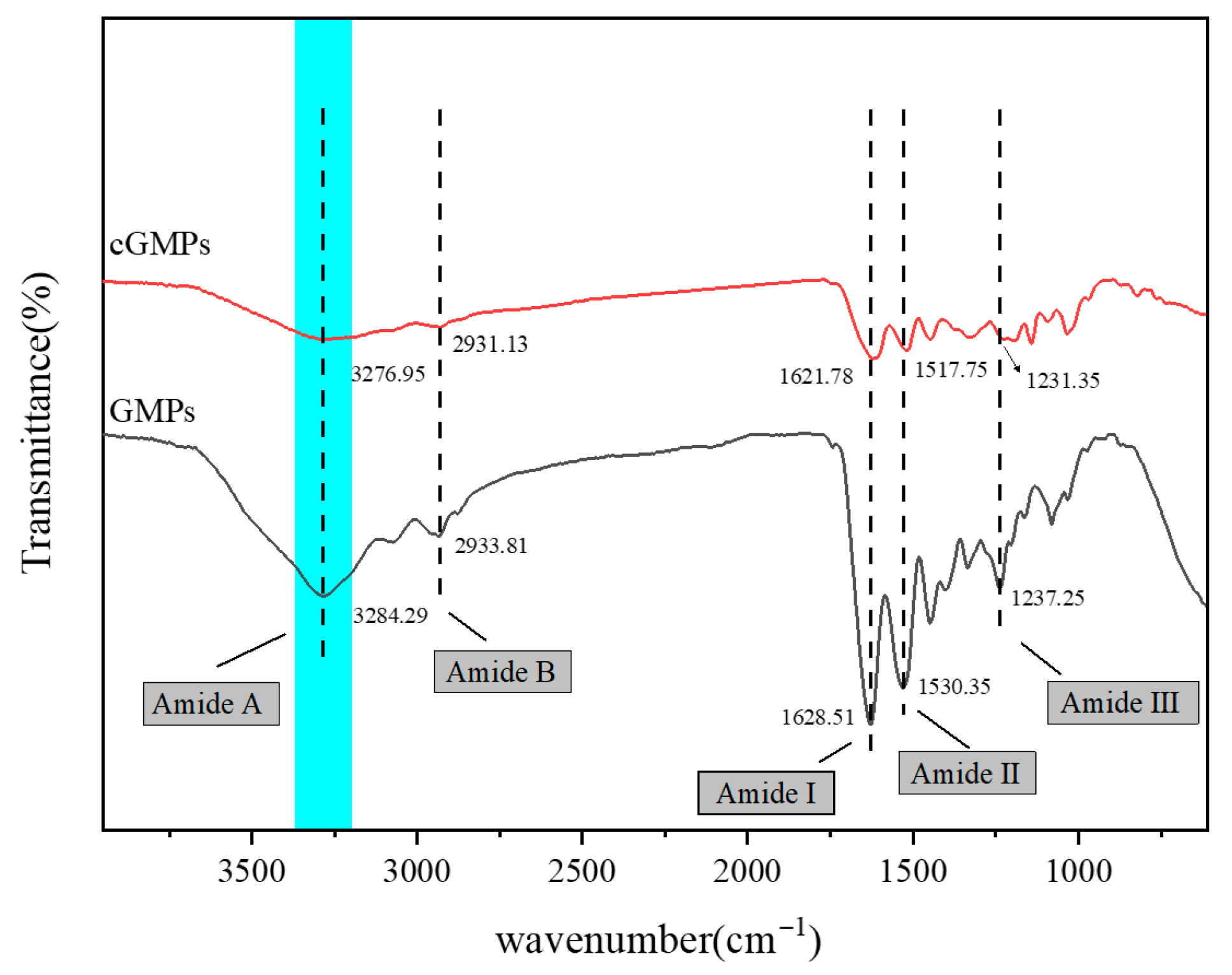
3.2.2. ATR-FTIR Spectroscopy
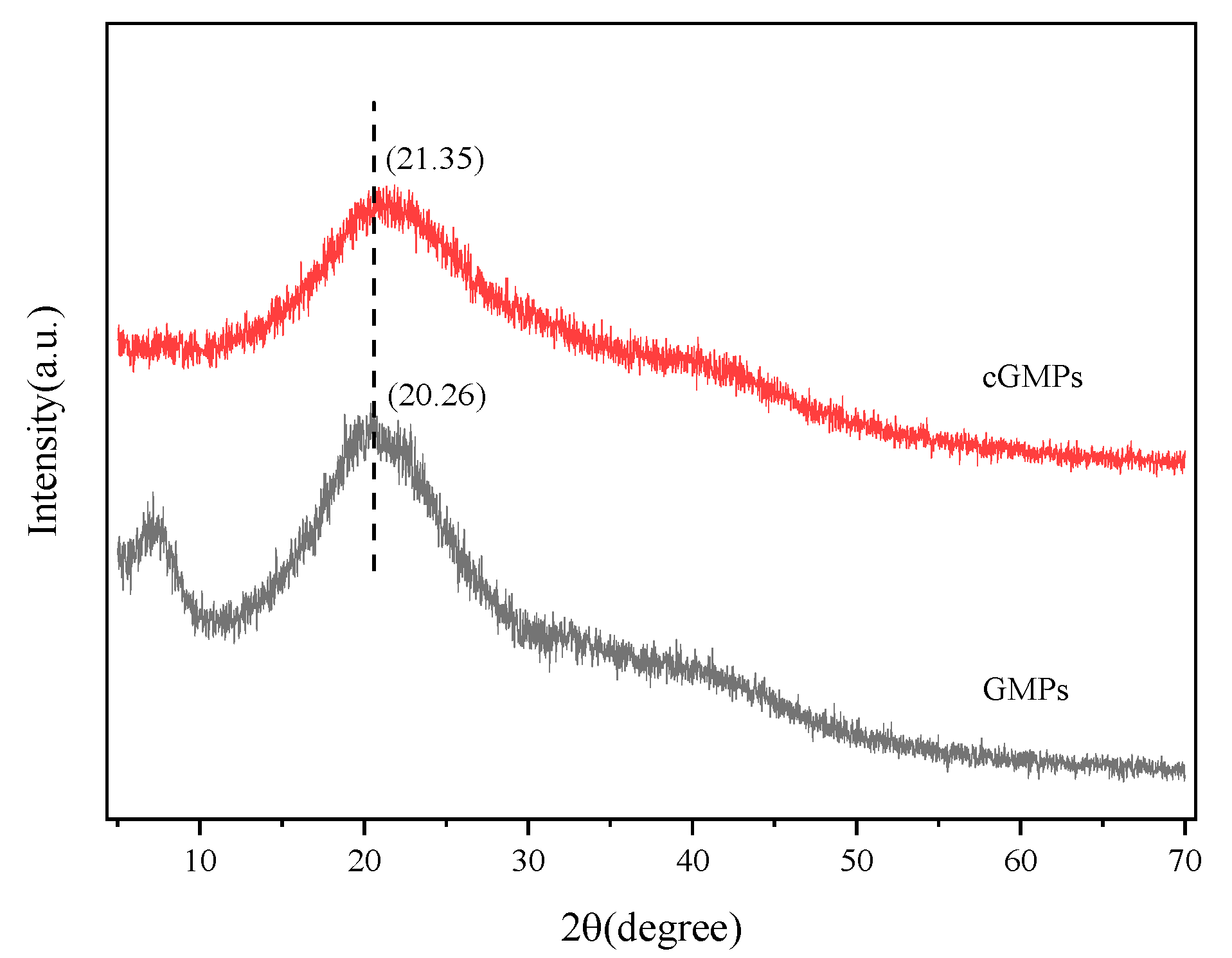
3.2.3. XRD
3.2.4. DSC
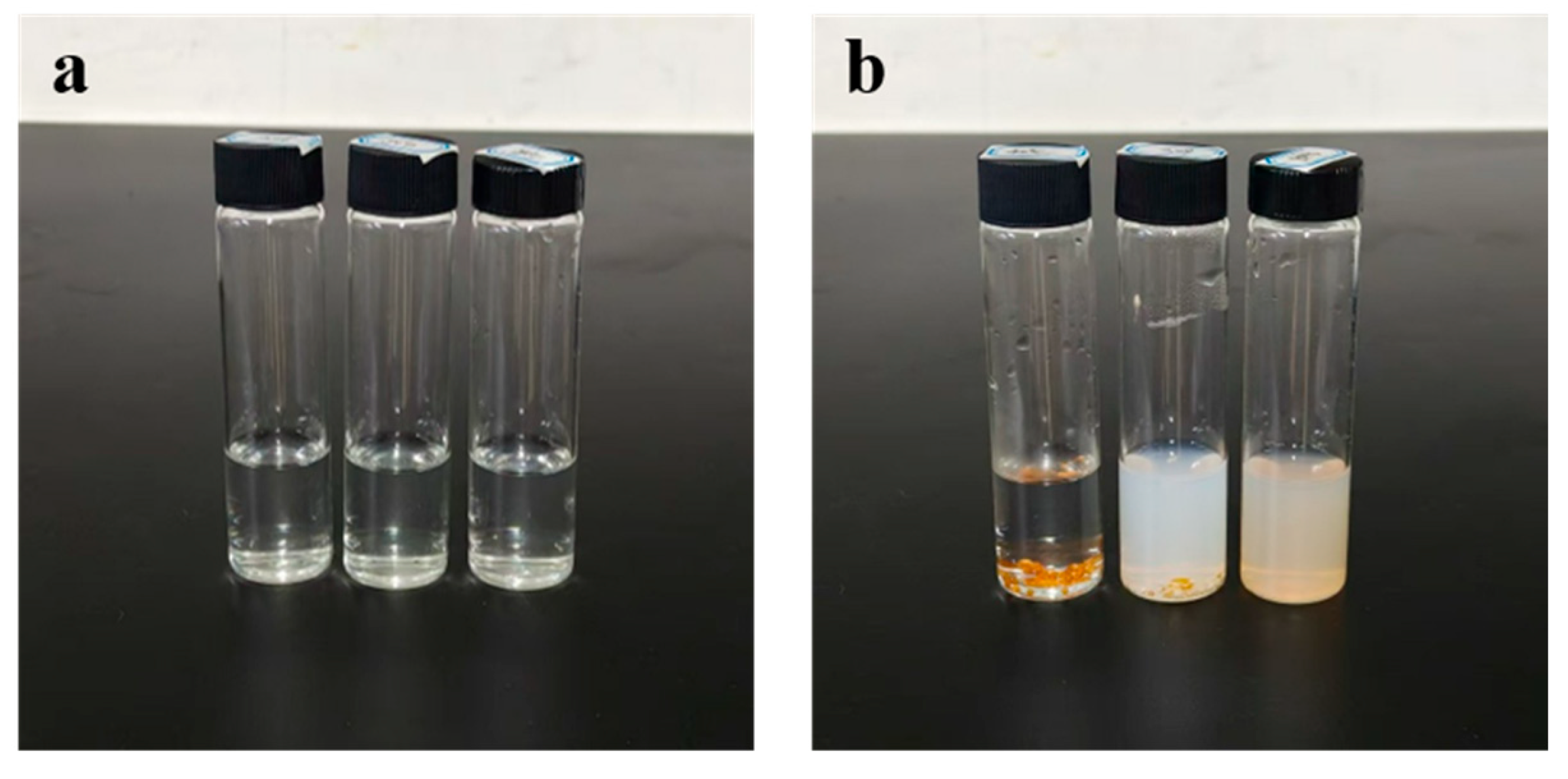
3.3. Stability Analysis of FA-cGMPs in High-Temperature Aqueous Solutions
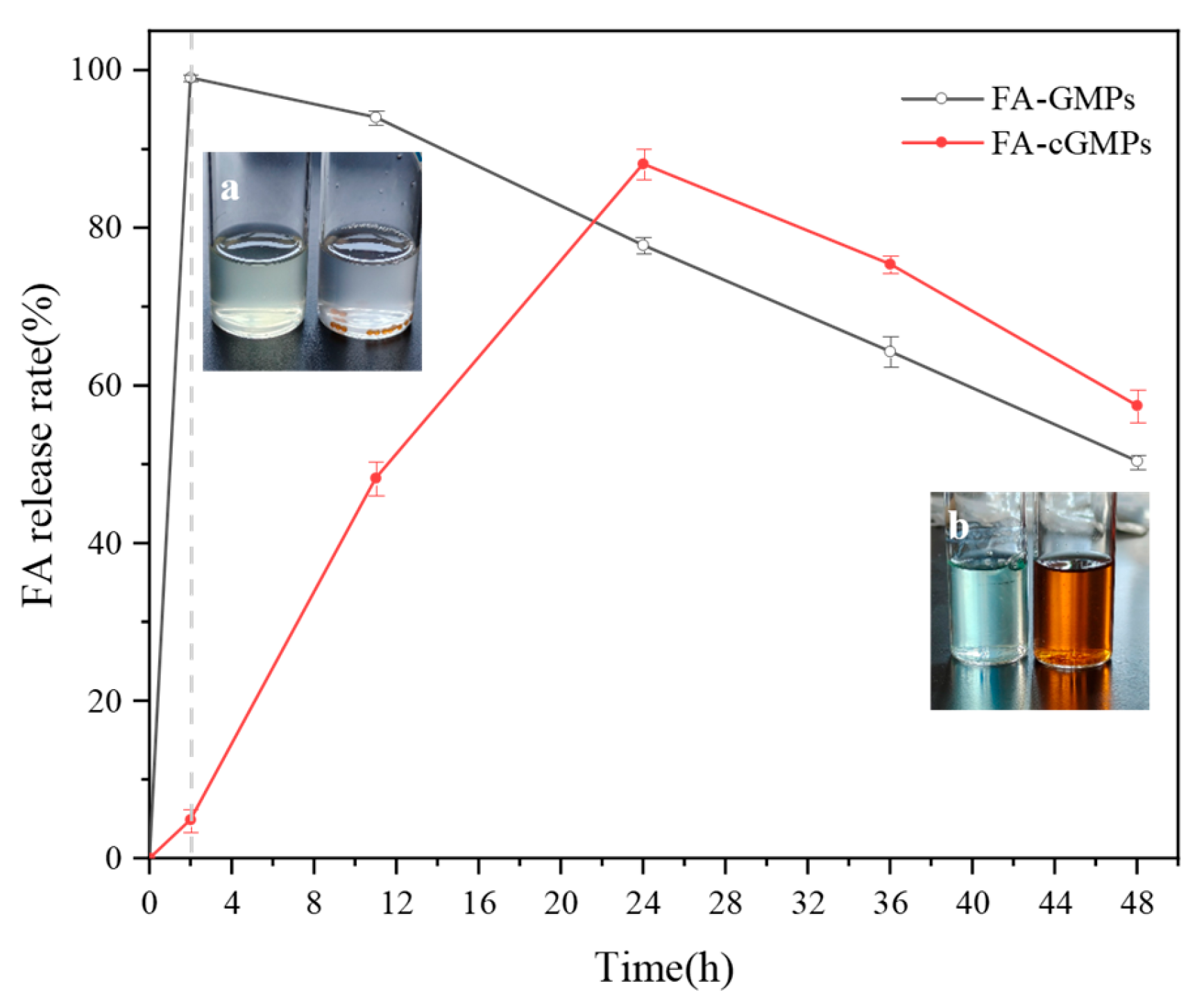
3.4. In Vitro Digestion of FA-cGMPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GMPs | Gelatin microparticles |
| cGMPs | Crosslinked gelatin microparticles |
| PVA | Poly(vinyl alcohol) |
| FA | Folic acid |
| TP | Tea polyphenols |
| FA-GMPs | FA-loaded GMPs |
| FA-cGMPs | FA-loaded cGMPs |
| MCT | Medium-chain triglycerides |
| MB | Methylene blue |
| MB-GMS | MB-loaded GMPs |
| MB-cGMS | MB-loaded cGMPs |
| SEM | Scanning electron microscopy |
| ATR-FTIR | Attenuated total reflection Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
| DSC | Differential scanning calorimetry |
References
- Lee, D.H.; Tamura, A.; Arisaka, Y.; Seo, J.H.; Yui, N. Mechanically Reinforced Gelatin Hydrogels by Introducing Slidable Supramolecular Cross-Linkers. Polymers 2019, 11, 1787. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Li, Y.H.; Pan, J.F.; Liu, F.; Dai, H.J.; Fu, Y.; Huang, T.; Farooq, S.; Zhang, H. Collagen and Gelatin: Structure, Properties, and Applications in Food Industry. Int. J. Biol. Macromol. 2024, 254, 128037. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Hernández-Muñoz, P. Encapsulation of Curcumin in Electrosprayed Gelatin Microspheres Enhances its Bioaccessibility and Widens its Uses in Food Applications. Innov. Food Sci. Emergine Technol. 2015, 29, 302–307. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Wang, Y.X.; Huo, L.; Wang, Y.; Jin, L.’E. Preparation and Properties of Gelatin-Tannic Acid Histidine Metal Complex Microspheres. Eur. Polym. J. 2023, 196, 112318. [Google Scholar] [CrossRef]
- Mizukami, Y.; Moriya, A.; Takahashi, Y.; Shimizu, K.; Konishi, S.; Takakura, Y.; Nishikawa, M. Incorporation of Gelatin Microspheres into HepG2 Human Hepatocyte Spheroids for Functional Improvement through Improved Oxygen Supply to Spheroid Core. Biol. Pharm. Bull. 2020, 43, 1220–1225. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Du, Y.; Yu, M.L.; Ren, L.R.; Guo, Y.F.; Li, Q.H.; Yin, M.M.; Li, X.L.; Chen, F.L. Controlled Release of Dinotefuran with Temperature/pH-Responsive Chitosan-Gelatin Microspheres to Reduce Leaching Risk During Application. Carbohydr. Polym. 2022, 277, 118880. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef]
- Chang, K.C.; Chang, P.J.; Chen, J.C.; Huang, S.M.; Liu, S.M.; Shih, C.J.; Chen, W.C. In Vitro Characterizations of Post-Crosslinked Gelatin-Based Microspheres Modified by Phosphatidylcholine or Diammonium Phosphate as Antibiotic Delivery Vehicles. Polymers 2023, 15, 1504. [Google Scholar] [CrossRef]
- De Clercq, K.; Schelfhout, C.; Bracke, M.; De Wever, O.; Van Bockstal, M.; Ceelen, W.; Remon, J.P.; Vervaet, C. Genipin-Crosslinked Gelatin Microspheres as a Strategy to Prevent Postsurgical Peritoneal Adhesions: In Vitro and in Vivo Characterization. Biomaterials 2016, 96, 33–46. [Google Scholar] [CrossRef]
- Sabbagh, F.; Deshmukh, A.R.; Choi, Y.; Kim, B.S. Effect of Microsphere Concentration on Catechin Release from Microneedle Arrays. ACS Appl. Mater. Interfaces 2024, 16, 28276–28289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ng, S.C.; Yu, J.S.; Tsai, W.B. Modification and Crosslinking of Gelatin-Based Biomaterials as Tissue Adhesives. Colloids Surf. B-Biointerfaces 2019, 174, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Muvaffak, A.; Gürhan, I.; Hasirci, N. Cytotoxicity of 5-Fluorouracil Entrapped in Gelatin Microspheres. J. Microencapsul. 2004, 21, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, X.J.; Chen, Q.Q.; Ye, X.Y.; Zeng, Q.Z.; Yuan, Y.; Dong, L.H.; Huang, F.; Su, D.X. Hydrogel with the Network Structure Fabricated by Anthocyanin-Gelatin Crosslinking and Improved Mineral Encapsulation Ability. Int. J. Food Sci. Technol. 2022, 57, 7143–7155. [Google Scholar] [CrossRef]
- Delchier, N.; Herbig, A.L.; Rychlik, M.; Renard, C.M.G.C. Folates in Fruits and Vegetables: Contents, Processing, and Stability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 506–528. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Maria Lagaron, J.; López-Rubio, A. Photoprotection of Folic Acid Upon Encapsulation in Food-Grade Amaranth (Amaranthus hypochondriacus L.) Protein Isolate—Pullulan Electrospun Fibers. LWT Food Sci. Technol. 2015, 62, 970–975. [Google Scholar] [CrossRef]
- Prasertmanakit, S.; Praphairaksit, N.; Chiangthong, W.; Muangsin, N. Ethyl Cellulose Microcapsules for Protecting and Controlled Release of Folic Acid. AAPS PharmSciTech 2009, 10, 1104–1112. [Google Scholar] [CrossRef]
- Fathima, E.; Nallamuthu, I.; Anand, T.; Naika, M.; Khanum, F. Enhanced Cellular Uptake, Transport and Oral Bioavailability of Optimized Folic Acid-Loaded Chitosan Nanoparticles. Int. J. Biol. Macromol. 2022, 208, 596–610. [Google Scholar] [CrossRef]
- Fang, C.H.; Kanemaru, K.; Carvalho, W.S.P.; Fruehauf, K.R.; Zhang, S.S.E.; Das, P.P.; Xu, C.S.; Lu, Y.P.; Rajagopalan, N.; Kulka, M.; et al. Self-Assembled Poloxamer-Legumin/Vicilin Nanoparticles for the Nanoencapsulation and Controlled Release of Folic Acid. Int. J. Biol. Macromol. 2024, 268, 131646. [Google Scholar] [CrossRef]
- Salevic-Jelic, A.; Rakic, V.; Balanc, B.; Levic, S.; Radovanovic, Z.; Dordevic, V.; Knezevic-Jugovic, Z.; Nedovic, V. Application of Pumpkin-Leaf Protein Concentrate as A Matrix Component for the Encapsulation of Folic Acid. Food Chem. X 2025, 26, 102310. [Google Scholar] [CrossRef]
- Wang, L.; You, D.S.; Guo, D.Y.; Zhuang, X.C.; Yuan, T.; Qiu, D. Preparation and Properties of Sodium Carboxymethyl Cellulose Microspheres by Dropping Method. ACS Omega 2025, 10, 4754–4762. [Google Scholar] [CrossRef]
- Zuo, R.Z.; Qiu, D.; Yang, Q.Q.; Deng, S.G.; Wang, Y.J. Preparation and Properties of Starch-Based Microspheres by Dropping Method. Fine Chem. 2024, 41, 2290–2299. [Google Scholar]
- Zhu, Y.C.; Zhang, D.D.; He, S.D.; Huang, Z.H.; Zhang, Z.Y.; Zhu, J.J.; Cao, Y.P. Controlled Release of Methylene Blue from Glutaraldehyde-Modified Gelatin. J. Food Biochem. 2019, 43, 12977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Gan, C.S.; Xu, K.; Wang, H.L.; Li, H.Y.; Yang, L.; Sun, S.J. Fabrication and Characterization of Zein/Gelatin/Carboxymethyl Starch Nanoparticles as An Efficient Delivery Vehicle for Quercetin with Improved Stability and Bioaccessibility. Int. J. Biol. Macromol. 2025, 308, 142409. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.Y.; Shen, Y.Q.; Li, Z.P. Control of Size and Morphology of Gelatin Microspheres. J. Macromol. Sci. Part B-Phys. 2012, 51, 12–21. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Awad, E.A.; Leo, T.K.; Imlan, J.C.; Sazili, A.Q. Characterization of Gelatin From Bovine Skin Extracted Using Ultrasound Subsequent to Bromelain Pretreatment. Food Hydrocoll. 2018, 80, 264–273. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Brachais, C.H.; Debeaufort, F. Coupling Tyrosol, Quercetin or Ferulic Acid and Electron Beam Irradiation to Cross-Link Chitosan-Gelatin Films: A Structure-Function Approach. Eur. Polym. J. 2015, 67, 113–127. [Google Scholar] [CrossRef]
- Lin, J.J.; Pan, D.D.; Sun, Y.Y.; Ou, C.R.; Wang, Y.; Cao, J.X. The Modification of Gelatin Films: Based on Various Cross-Linking Mechanism of Glutaraldehyde at Acidic and Alkaline Conditions. Food Sci. Nutr. 2019, 7, 4140–4146. [Google Scholar] [CrossRef]
- Yan, J.N.; Dai, M.Q.; Zhang, Z.J.; Wang, C.; Lai, B.; Wu, H.T. Comparison of Gel and Functional Properties of Gelatin Derived from Two Jellyfish Stomolophus Meleagris and Rhopilema Esculentum Kishinouye. Food Hydrocoll. 2025, 159, 110658. [Google Scholar] [CrossRef]
- Liu, F.Y.; Edelmann, M.; Piironen, V.; Kariluoto, S. The Bioaccessibility of Folate in Breads and the Stability of Folate Vitamers During in Vitro Digestion. Food Funct. 2022, 13, 3220–3233. [Google Scholar] [CrossRef]
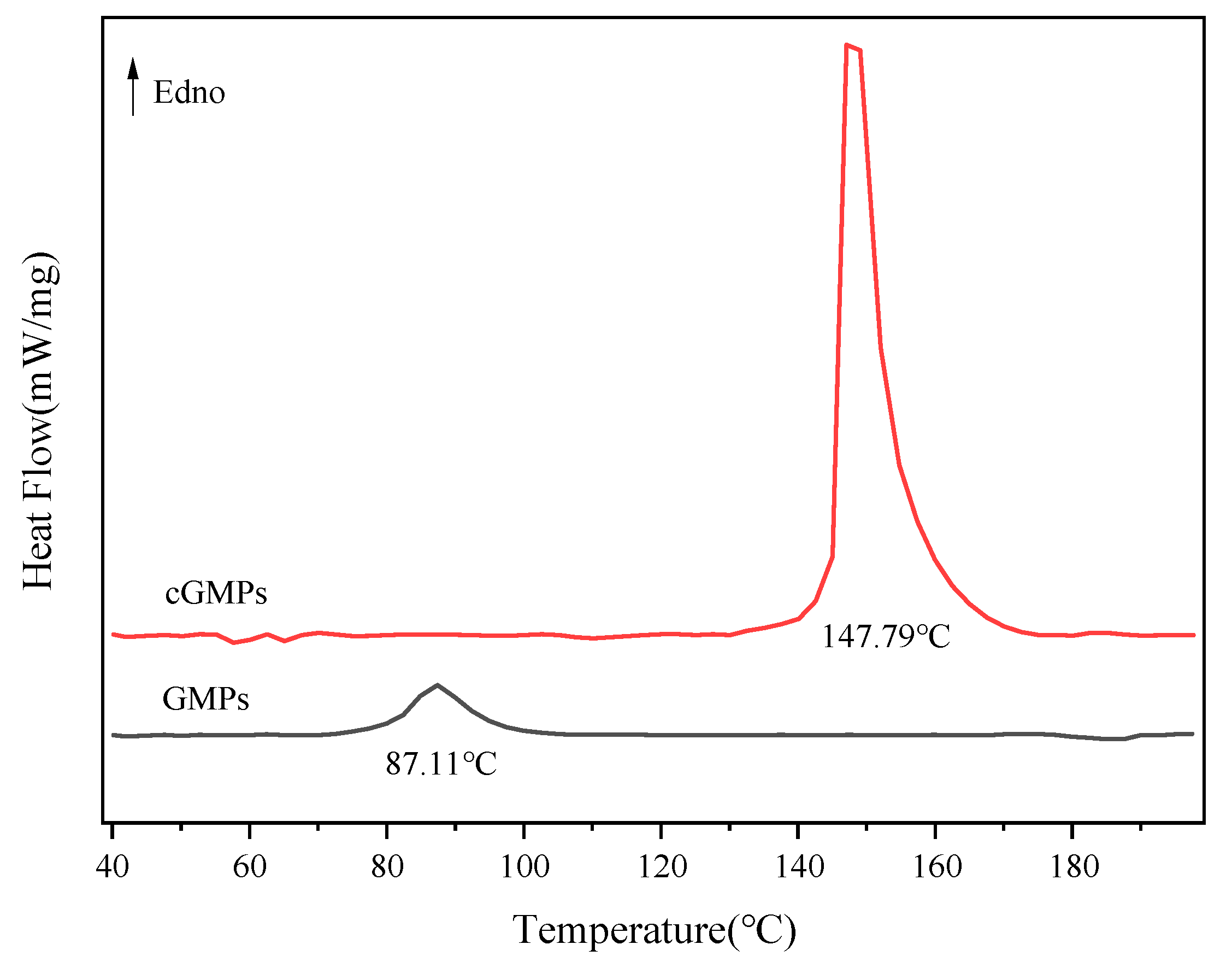

| Samples | (°C) | (J/g) |
|---|---|---|
| GMPs | 87.11 | 15.74 |
| cGMPs | 147.79 | 100.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.-Y.; Hu, X.-F.; Qiu, D.; Wang, Y.-J.; Tong, Z.-F. Preparation of Crosslinked Gelatin Microparticles and Study on Their Loading Capacity for Folic Acid. Polymers 2025, 17, 2815. https://doi.org/10.3390/polym17212815
Qi J-Y, Hu X-F, Qiu D, Wang Y-J, Tong Z-F. Preparation of Crosslinked Gelatin Microparticles and Study on Their Loading Capacity for Folic Acid. Polymers. 2025; 17(21):2815. https://doi.org/10.3390/polym17212815
Chicago/Turabian StyleQi, Jia-Yi, Xiao-Feng Hu, Dan Qiu, Ya-Juan Wang, and Zhang-Fa Tong. 2025. "Preparation of Crosslinked Gelatin Microparticles and Study on Their Loading Capacity for Folic Acid" Polymers 17, no. 21: 2815. https://doi.org/10.3390/polym17212815
APA StyleQi, J.-Y., Hu, X.-F., Qiu, D., Wang, Y.-J., & Tong, Z.-F. (2025). Preparation of Crosslinked Gelatin Microparticles and Study on Their Loading Capacity for Folic Acid. Polymers, 17(21), 2815. https://doi.org/10.3390/polym17212815






