Electrospinning PLLA/PCL Blend Fibre-Based Materials and Their Biomedical Application: A Mini Review
Abstract
1. Introduction
2. Electrospinning
3. Poly (L-Lactic Acid)
4. Polycaprolactone
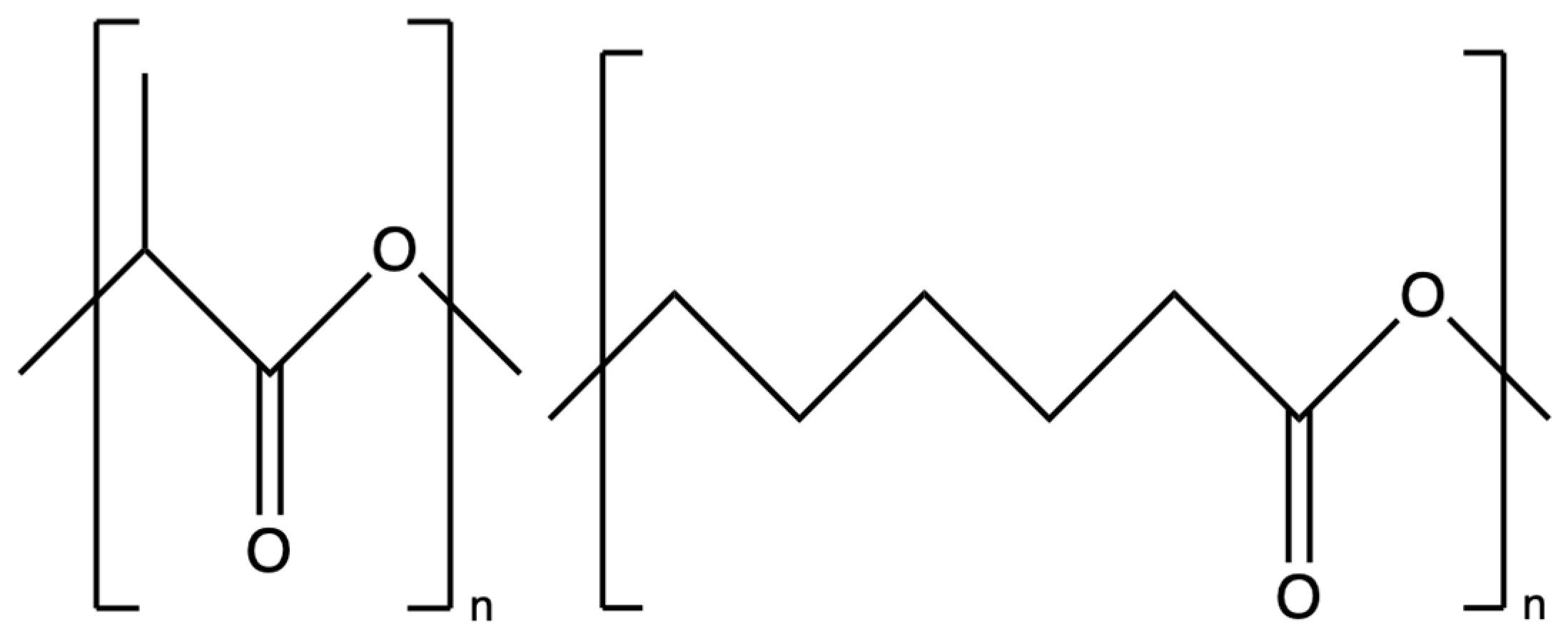
5. Electrospun PLLA/PCL
6. Electrospun PLLA/PCL Hybrids
7. Electrospun PLLA/PCL-Based 3D Structure
| Author | Year | Materials | Fabrication Method | Application | Reference |
|---|---|---|---|---|---|
| Hassanajili et al. | 2019 | PLLA, PCL and HA | 3D printing | Bone tissue engineering, MG63 osteocarcinoma cells attachment, proliferation and ALP activity | [151] |
| Wang et al. | 2021 | PLLA and PCL | Electrospinning/hot pressing/supercritical CO2 batch foaming method. | Vascular patch, human umbilical endothelial cell adhesion and migration | [162] |
| Shahverdi et al. | 2022 | PLLA and PCL | Melt electrowriting | Mouse murine fibroblast and human umbilical vein endothelial cells attachment and proliferation | [163] |
| Guerra et al. | 2018 | PLLA and PCL | 3D printing | Biodegradable stents, murine 3T3 fibroblast cells attachment and proliferation | [164] |
| Yao et al. | 2017 | PLLA and PCL | Electrospinning/thermally induced self-agglomeration | Bone tissue engineering, human mesenchymal stem cells, osteogenic differentiation, and bone formation in a mouse model | [159] |
| Sadiasa et al. | 2013 | PLLA and PCL | Salt leaching | Bone tissue engineering, MG63 osteoblast-like cells proliferation, bone formation in rabbit model | [150] |
| Samadian et al. | 2020 | PLLA, PCL, gelatin and taurine | Electrospinning/thermally induced phase separation | Bone tissue engineering, MG63 osteoblast-like cells proliferation, bone formation in mouse model | [165] |
| Meng et al. | 2024 | PLLA, PCL and bioactive glass | Electrospinning/progen leaching | Bone tissue engineering, human osteogenic sarcoma cells proliferation and infiltration | [161] |
| Qiu et al. | 2016 | PLLA, PCL, silica and dexamethasone | Thermally induced phase separation/electrophoretic deposition | Bone tissue engineering, primary rat bone marrow mesenchymal stem cells proliferation and differentiation, bone formation in mouse model | [152] |
| Dong et al. | 2019 | PLLA and PCL | Electrospinning with water bath collection | MG63 osteoblast-like cells proliferation and infiltration | [166] |
| Peiravi et al. | 2025 | PLLA, PCL and ZnO | 3D printing | Osteoarthritis treatment, MG63 osteoblast-like cells proliferation and mineralization, cartilage tissue repair in rabbit model | [167] |
| Dhayer et al. | 2025 | PLLA and PCL | Melt-spinning/knitting | Adipose tissue reconstruction, murine bone marrow mesenchymal stem cells and preadipocytes cells differentiation, in vivo study with mouse model | [168] |
8. Perspectives and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Toncheva, A.; Spasova, M.; Paneva, D.; Manolova, N.; Rashkov, I. Polylactide (PLA)-based electrospun fibrous materials containing ionic drugs as wound dressing materials: A review. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 657–671. [Google Scholar] [CrossRef]
- Ziabicki, A. Fundamentals of Fibre Information: The Science of Fibre Spinning and Drawing; Wiley: London, UK, 1976. [Google Scholar]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2014, 45, 659–670. [Google Scholar] [CrossRef]
- Zupančič, Š. Core-shell nanofibers as drug-delivery systems. Acta Pharm. 2019, 69, 131–153. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Zhao, Y.; Jiang, L. Electrospinning of multilevel structured functional micro-/nanofibers and their applications. J. Mater. Chem. A 2013, 1, 7290–7305. [Google Scholar] [CrossRef]
- Tian, D.; Lu, X.; Nie, G.; Gao, M.; Wang, C. Direct growth of Ni–Mn–O nanosheets on flexible electrospun carbon nanofibers for high performance supercapacitor applications. Inorg. Chem. Front. 2018, 5, 635–642. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Pereira-Lobato, C.; Echeverry-Rendón, M.; Fernández-Blázquez, J.; Llorca, J.; González, C. PLA/PCL composites manufactured from commingled yarns for biomedical applications. J. Mech. Behav. Biomed. Mater. 2025, 163, 106819. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Azimi, B.; Ismaeilimoghadam, S.; Danti, S. Poly (lactic acid)-based electrospun fibrous structures for biomedical applications. Appl. Sci. 2022, 12, 3192. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun core–shell fibrous 2D scaffold with biocompatible poly (glycerol sebacate) and poly-L-lactic acid for wound healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef]
- Alves, P.E.; Soares, B.G.; Lins, L.C.; Livi, S.; Santos, E.P. Controlled delivery of dexamethasone and betamethasone from PLA electrospun fibers: A comparative study. Eur. Polym. J. 2019, 117, 1–9. [Google Scholar] [CrossRef]
- Fattahi, F.-S.; Khoddami, A.; Avinc, O. Poly (lactic acid)(PLA) nanofibers for bone tissue engineering. J. Text. Polym. 2019, 7, 47–64. [Google Scholar]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Schoenmaker, B.; Kalaoglu, Ö.I.; De Clerck, K. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur. Polym. J. 2011, 47, 1256–1263. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.; Dalton, P.; Hutmacher, D. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Mishra, N.; Goyal, A.K.; Khatri, K.; Vaidya, B.; Paliwal, R.; Rai, S.; Mehta, A.; Tiwari, S.; Vyas, S.; Vyas, S.P. Biodegradable polymer based particulate carrier(s) for the delivery of proteins and peptides. Anti-Inflamm. Anti-Allergy Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Inflamm. Anti-Allergy Agents) 2008, 7, 240–251. [Google Scholar] [CrossRef]
- Bezwada, R.S.; Jamiolkowski, D.D.; Lee, I.-Y.; Agarwal, V.; Persivale, J.; Trenka-Benthin, S.; Erneta, M.; Suryadevara, J.; Yang, A.; Liu, S. Monocryl® suture, a new ultra-pliable absorbable monofilament suture. Biomaterials 1995, 16, 1141–1148. [Google Scholar] [CrossRef]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Xiong, M.; Liu, X.; Luo, S.; Luo, J.; Wang, Y. Electrospun silk fibroin/fibrin vascular scaffold with superior mechanical properties and biocompatibility for applications in tissue engineering. Sci. Rep. 2024, 14, 3942. [Google Scholar] [CrossRef]
- Dieterle, M.P.; Steinberg, T.; Tomakidi, P.; Nohava, J.; Vach, K.; Schulz, S.D.; Hellwig, E.; Proksch, S. Novel in Situ-Cross-Linked electrospun gelatin/hydroxyapatite nonwoven scaffolds prove suitable for periodontal tissue engineering. Pharmaceutics 2022, 14, 1286. [Google Scholar] [CrossRef]
- Puigmal, A.C.; Ayran, M.; Ulag, S.; Altan, E.; Guncu, M.M.; Aksu, B.; Durukan, B.K.; Sasmazel, H.T.; Perez, R.A.; Koc, E. Fucoidan-loaded electrospun Polyvinyl-alcohol/Chitosan nanofibers with enhanced antibacterial activity for skin tissue engineering. J. Mech. Behav. Biomed. Mater. 2023, 148, 106163. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.-R.; Ghasemi-Mobarakeh, L.; Itel, F.; Schoeller, J.; Fashandi, H.; Borzi, A.; Neels, A.; Fortunato, G.; Rossi, R.M. Emulsion electrospinning of sodium alginate/poly (ε-caprolactone) core/shell nanofibers for biomedical applications. Nanoscale Adv. 2022, 4, 2929–2941. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.S.; Kamoun, E.A.; Eldin, M.S.; Soliman, H.M.; EL-Moslamy, S.H.; El-Fakharany, E.M.; Shokr, A.-b.M. Electrospun PVA–dextran nanofibrous scaffolds for acceleration of topical wound healing: Nanofiber optimization, characterization and in vitro assessment. Arab. J. Sci. Eng. 2023, 48, 205–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, Y.; Jiang, X.; Li, X.; Wu, X.; Zhong, J.; Jia, X.; Li, Q.; Wang, X.; Zhao, K. Stepwise degradable PGA-SF core-shell electrospinning scaffold with superior tenacity in wetting regime for promoting bone regeneration. Mater. Today Bio 2024, 26, 101023. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soria, A.; Moreno-Serna, V.; Canales, D.A.; García-Herrera, C.; Zapata, P.A.; Orihuela, P.A. Effect of electrospun PLGA/collagen scaffolds on cell adhesion, viability, and collagen release: Potential applications in tissue engineering. Polymers 2023, 15, 1079. [Google Scholar] [CrossRef]
- Virijević, K.; Zivanovic, M.N.; Nikolic, D.; Milivojevic, N.; Pavic, J.; Moric, I.; Šenerović, L.; Dragacevic, L.; Thurner, P.J.; Rufin, M. AI-driven optimization of PCL/PEG electrospun scaffolds for enhanced in vivo wound healing. ACS Appl. Mater. Interfaces 2024, 16, 22989–23002. [Google Scholar]
- Bani Mustafa, D.; Sakai, T.; Sato, O.; Ikebe, M.; Chou, S.-F. Electrospun ibuprofen-loaded blend PCL/PEO fibers for topical drug delivery applications. Polymers 2024, 16, 1934. [Google Scholar] [CrossRef]
- Mashhadikhan, M.; Soleimani, M.; Parivar, K.; Yaghmaei, P. ADSCs on PLLA/PCL hybrid nanoscaffold and gelatin modification: Cytocompatibility and mechanical properties. Avicenna J. Med. Biotechnol. 2015, 7, 32. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-L-lactic acid (PLLA)-based biomaterials for regenerative medicine: A review on processing and applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Vilay, V.; Mariatti, M.; Ahmad, Z.; Pasomsouk, K.; Todo, M. Characterization of the mechanical and thermal properties and morphological behavior of biodegradable poly (L-lactide)/poly (ε-caprolactone) and poly (L-lactide)/poly (butylene succinate-co-L-lactate) polymeric blends. J. Appl. Polym. Sci. 2009, 114, 1784–1792. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Jhurry, D. Biodegradable polymer blends: Miscibility, physicochemical properties and biological response of scaffolds. Polym. Int. 2015, 64, 1289–1302. [Google Scholar] [CrossRef]
- López-Rodríguez, N.; López-Arraiza, A.; Meaurio, E.; Sarasua, J. Crystallization, morphology, and mechanical behavior of polylactide/poly (ε-caprolactone) blends. Polym. Eng. Sci. 2006, 46, 1299–1308. [Google Scholar] [CrossRef]
- Bai, H.; Xiu, H.; Gao, J.; Deng, H.; Zhang, Q.; Yang, M.; Fu, Q. Tailoring impact toughness of poly (L-lactide)/poly (ε-caprolactone)(PLLA/PCL) blends by controlling crystallization of PLLA matrix. ACS Appl. Mater. Interfaces 2012, 4, 897–905. [Google Scholar] [CrossRef]
- Wei, M.; Tonelli, A.E. Compatiblization of polymers via coalescence from their common cyclodextrin inclusion compounds. Macromolecules 2001, 34, 4061–4065. [Google Scholar] [CrossRef]
- Wojasiński, M.; Faliszewski, K.; Ciach, T. Electrospinning production of PLLA fibrous scaffolds for tissue engineering. Chall. Mod. Technol. 2013, 4. [Google Scholar]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nourbakhsh, M. Electrospun polycaprolactone scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 527–539. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Del Bakhshayesh, A.R.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The efficiency of PCL/HAp electrospun nanofibers in bone regeneration: A review. J. Med. Eng. Technol. 2021, 45, 511–531. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I. Disintegration of water drops in an electric field. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1964, 280, 383–397. [Google Scholar]
- Taylor, G.I. The force exerted by an electric field on a long cylindrical conductor. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1966, 291, 145–158. [Google Scholar]
- Taylor, G.I. Electrically driven jets. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1969, 313, 453–475. [Google Scholar]
- Nalbandian, M.J. Development and Optimization of Chemically-Active Electrospun Nanofibers for Treatment of Impaired Water Sources. Ph.D. Thesis, University of California, Riverside, CA, USA, 2014. [Google Scholar]
- Natarajan, L.; New, J.; Dasari, A.; Yu, S.; Manan, M.A. Surface morphology of electrospun PLA fibers: Mechanisms of pore formation. RSC Adv. 2014, 4, 44082–44088. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Huang, L.; Nagapudi, K.; Apkarian, P.R.; Chaikof, E.L. Engineered collagen–PEO nanofibers and fabrics. J. Biomater. Sci. Polym. Ed. 2001, 12, 979–993. [Google Scholar] [CrossRef]
- Sajkiewicz, P.; Kołbuk, D. Electrospinning of gelatin for tissue engineering–molecular conformation as one of the overlooked problems. J. Biomater. Sci. Polym. Ed. 2014, 25, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cao, C.; Ma, X. A novel three-dimensional tubular scaffold prepared from silk fibroin by electrospinning. Int. J. Biol. Macromol. 2009, 45, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospun Antibacterial Chitosan-B ased Fibers. Macromol. Biosci. 2013, 13, 860–872. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Neumann, I.A.; Flores-Sahagun, T.H.S.; Ribeiro, A.M. Biodegradable poly (L-lactic acid)(PLLA) and PLLA-3-arm blend membranes: The use of PLLA-3-arm as a plasticizer. Polym. Test. 2017, 60, 84–93. [Google Scholar] [CrossRef]
- Mariano, M.; Pilate, F.; de Oliveira, F.B.; Khelifa, F.; Dubois, P.; Raquez, J.-M.; Dufresne, A. Preparation of cellulose nanocrystal-reinforced poly (lactic acid) nanocomposites through noncovalent modification with PLLA-based surfactants. Acs Omega 2017, 2, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Basu, B. Three dimensional porous scaffolds: Mechanical and biocompatibility properties. In Biomaterials for Musculoskeletal Regeneration: Concepts; Springer: Singapore, 2017; pp. 353–384. [Google Scholar]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.Y.; Huang, Y.Y.; Lim, S.T.; Wong, P.; Joner, M.; Foin, N. Mechanical behavior of polymer-based vs. metallic-based bioresorbable stents. J. Thorac. Dis. 2017, 9 (Suppl. S9), S923. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, L. Research into biodegradable polymeric stents: A review of experimental and modelling work. Vessel. Plus 2018, 2, 12. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic degradation and erosion of polyester biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, S.; Liu, K.; Wen, W.; Lu, L.; Ding, S.; Zhou, C.; Luo, B. 3D poly (L-lactide)/chitosan micro/nano fibrous scaffolds functionalized with quercetin-polydopamine for enhanced osteogenic and anti-inflammatory activities. Chem. Eng. J. 2020, 391, 123524. [Google Scholar] [CrossRef]
- Zhu, M.; Gu, J.; He, L.; Mahar, F.K.; Kim, I.; Wei, K. Fabrication and osteoblastic adhesion behavior of regenerated silk fibroin/PLLA Nanofibrous scaffold by double syringe electrospinning. Fibers Polym. 2019, 20, 1850–1856. [Google Scholar] [CrossRef]
- Ghersi, G.; Carfi’Pavia, F.; Conoscenti, G.; Mannella, G.; Greco, S.; Rigogliuso, S.; La Carrubba, V.; Brucato, V. PLLA scaffold via TIPS for bone tissue engineering. Chem. Eng. Trans. 2016, 49, 301–306. [Google Scholar]
- Puglia, D.; Ceccolini, R.; Fortunati, E.; Armentano, I.; Morena, F.; Martino, S.; Aluigi, A.; Torre, L.; Kenny, J.M. Effect of processing techniques on the 3D microstructure of poly (L-lactic acid) scaffolds reinforced with wool keratin from different sources. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Elektrospinnen: Eine faszinierende Methode zur Präparation ultradünner Fasern. Angew. Chem. 2007, 119, 5770–5805. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Bhattarai, N.; Yi, H.K.; Hwang, P.H.; Cha, D.I.; Kim, H.Y. Novel biodegradable electrospun membrane: Scaffold for tissue engineering. Biomaterials 2004, 25, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Derakhshan, M.A.; Pourmand, G.; Ai, J.; Ghanbari, H.; Dinarvand, R.; Naji, M.; Faridi-Majidi, R. Electrospun PLLA nanofiber scaffolds for bladder smooth muscle reconstruction. Int. Urol. Nephrol. 2016, 48, 1097–1104. [Google Scholar] [CrossRef]
- Karanth, D.; Puleo, D.; Dawson, D.; Holliday, L.; Sharab, L. Characterization of 3D printed biodegradable piezoelectric scaffolds for bone regeneration. Clin. Exp. Dent. Res. 2023, 9, 398–408. [Google Scholar] [CrossRef]
- Das, R.; Le, T.T.; Schiff, B.; Chorsi, M.T.; Park, J.; Lam, P.; Kemerley, A.; Supran, A.M.; Eshed, A.; Luu, N. Biodegradable piezoelectric skin-wound scaffold. Biomaterials 2023, 301, 122270. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Li, D.; Wu, T.; He, N.; Wang, J.; Chen, W.; He, L.; Huang, C.; Ei-Hamshary, H.A.; Al-Deyab, S.S.; Ke, Q. Three-dimensional polycaprolactone scaffold via needleless electrospinning promotes cell proliferation and infiltration. Colloids Surf. B Biointerfaces 2014, 121, 432–443. [Google Scholar] [CrossRef]
- Safaeijavan, R.; Soleimani, M.; Divsalar, A.; Eidi, A.; Ardeshirylajimi, A. Comparison of random and aligned PCL nanofibrous electrospun scaffolds on cardiomyocyte differentiation of human adipose-derived stem cells. Iran. J. Basic Med. Sci. 2014, 17, 903. [Google Scholar]
- Meng, Q.; Xu, H.; Li, Y.; Liu, F.; Shao, H.; Ling, P.; Wu, S. Conjugated electrospinning toward a polycaprolactone scaffold simultaneously containing micro-/nano-fibers for potential biomedical application. J. Polym. Res. 2024, 31, 301. [Google Scholar] [CrossRef]
- Singhvi, M.; Zinjarde, S.; Gokhale, D. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Sun, Q.S.; Dong, J.; Lin, Z.X.; Yang, B.; Wang, J.Y. Comparison of cytocompatibility of zein film with other biomaterials and its degradability in vitro. Biopolymers 2005, 78, 268–274. [Google Scholar] [CrossRef]
- Oztemur, J.; Ozdemir, S.; Tezcan-Unlu, H.; Cecener, G.; Sezgin, H.; Yalcin-Enis, I. Investigation of biodegradability and cellular activity of PCL/PLA and PCL/PLLA electrospun webs for tissue engineering applications. Biopolymers 2023, 114, e23564. [Google Scholar] [CrossRef]
- La Carrubba, V.; Pavia, F.C.; Brucato, V.; Piccarolo, S. PLLA/PLA scaffolds prepared via Thermally Induced Phase Separation (TIPS): Tuning of properties and biodegradability. Int. J. Mater. Form. 2008, 1, 619–622. [Google Scholar] [CrossRef]
- Bolbasov, E.; Goreninskii, S.; Tverdokhlebov, S.; Mishanin, A.; Viknianshchuk, A.; Bezuidenhout, D.; Golovkin, A. Comparative study of the physical, topographical and biological properties of electrospinning PCL, PLLA, their blend and copolymer scaffolds. IOP Conf. Ser. Mater. Sci. Eng. 2018, 350, 012012. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, E.J.; Park, J.K. Effect of PEG molecular weight on the tensile toughness of starch/PCL/PEG blends. J. Appl. Polym. Sci. 2000, 77, 2049–2056. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chueh, J.-Y.; Tseng, H.; Huang, H.-M.; Lee, S.-Y. Preparation and characterisation of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Patricio, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia Cirp 2013, 5, 110–114. [Google Scholar] [CrossRef]
- Behtaj, S.; Karamali, F.; Masaeli, E.; Anissimov, Y.G.; Rybachuk, M. Electrospun PGS/PCL, PLLA/PCL, PLGA/PCL and pure PCL scaffolds for retinal progenitor cell cultivation. Biochem. Eng. J. 2021, 166, 107846. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly (lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- De Witte, T.; Fratila-Apachitei, L.; Zadpoor, A.; Peppas, N. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Wu, J.; Hu, C.; Tang, Z.; Yu, Q.; Liu, X.; Chen, H. Tissue-engineered vascular grafts: Balance of the four major requirements. Colloid Interface Sci. Commun. 2018, 23, 34–44. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, C.; Deng, X. Biodegradable polyester blends for biomedical application. J. Appl. Polym. Sci. 1995, 56, 103–112. [Google Scholar] [CrossRef]
- Hasan, A.; Soliman, S.; El Hajj, F.; Tseng, Y.-T.; Yalcin, H.C.; Marei, H.E. Fabrication and in vitro characterization of a tissue engineered PCL-PLLA heart valve. Sci. Rep. 2018, 8, 8187. [Google Scholar] [CrossRef]
- Vaz, C.M.; Van Tuijl, S.; Bouten, C.; Baaijens, F. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005, 1, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Can, E.; Udenir, G.; Kanneci, A.I.; Kose, G.; Bucak, S. Investigation of PLLA/PCL blends and paclitaxel release profiles. Aaps Pharmscitech 2011, 12, 1442–1453. [Google Scholar] [CrossRef]
- Cardoso, G.B.; Perea, G.N.; D’Avila, M.A.; Dias, C.G.; Zavaglia, C.A.; Arruda, A.C. Initial study of electrospinning PCL/PLLA blends. Adv. Mater. Phys. Chem. 2011, 1, 94. [Google Scholar] [CrossRef]
- Lu, L.; Wu, D.; Zhang, M.; Zhou, W. Fabrication of polylactide/poly (ε-caprolactone) blend fibers by electrospinning: Morphology and orientation. Ind. Eng. Chem. Res. 2012, 51, 3682–3691. [Google Scholar] [CrossRef]
- Madheswaran, D.; Sivan, M.; Hauzerova, S.; Kostakova, E.K.; Jencova, V.; Valtera, J.; Behalek, L.; Mullerova, J.; Nguyen, N.H.; Capek, L. Continuous fabrication of braided composite nanofibrous surgical yarns using advanced AC electrospinning and braiding technology. Compos. Commun. 2024, 48, 101932. [Google Scholar] [CrossRef]
- Chen, L.; Bai, Y.; Liao, G.; Peng, E.; Wu, B.; Wang, Y.; Zeng, X.; Xie, X. Electrospun poly (L-lactide)/poly (ε-caprolactone) blend nanofibrous scaffold: Characterization and biocompatibility with human adipose-derived stem cells. PLoS ONE 2013, 8, e71265. [Google Scholar] [CrossRef]
- Meng, C.; Tang, D.; Liu, X.; Meng, J.; Wei, W.; Gong, R.H.; Li, J. Heterogeneous porous PLLA/PCL fibrous scaffold for bone tissue regeneration. Int. J. Biol. Macromol. 2023, 235, 123781. [Google Scholar] [CrossRef]
- Oztemur, J.; Ozdemir, S.; Yalcin-Enis, I. Effect of blending ratio on morphological, chemical, and thermal characteristics of PLA/PCL and PLLA/PCL electrospun fibrous webs. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 793–803. [Google Scholar] [CrossRef]
- Vida, T.A.; Motta, A.C.; Santos, A.R., Jr.; Cardoso, G.B.C.; Brito, C.C.d.; Zavaglia, C.A.d.C. Fibrous PCL/PLLA scaffolds obtained by rotary jet spinning and electrospinning. Mater. Res. 2018, 20 (Suppl. S2), 910–916. [Google Scholar] [CrossRef]
- Li, H.; Qiao, T.; Song, P.; Guo, H.; Song, X.; Zhang, B.; Chen, X. Star-shaped PCL/PLLA blended fiber membrane via electrospinning. J. Biomater. Sci. Polym. Ed. 2015, 26, 420–432. [Google Scholar] [CrossRef]
- Lui, Y.S.; Lewis, M.P.; Loo, S.C.J. Sustained-release of naproxen sodium from electrospun-aligned PLLA–PCL scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 1011–1021. [Google Scholar] [CrossRef]
- Shakhssalim, N.; Rasouli, J.; Moghadasali, R.; Aghdas, F.S.; Naji, M.; Soleimani, M. Bladder smooth muscle cells interaction and proliferation on PCL/PLLA electrospun nanofibrous scaffold. Int. J. Artif. Organs 2013, 36, 113–120. [Google Scholar] [CrossRef]
- Liao, G.Y.; Chen, L.; Zeng, X.Y.; Zhou, X.P.; Xie, X.L.; Peng, E.J.; Ye, Z.Q.; Mai, Y.W. Electrospun poly (L-lactide)/poly (ε-caprolactone) blend fibers and their cellular response to adipose-derived stem cells. J. Appl. Polym. Sci. 2011, 120, 2154–2165. [Google Scholar] [CrossRef]
- Mobarra, N.; Soleimani, M.; Pakzad, R.; Enderami, S.E.; Pasalar, P. Three-dimensional nanofiberous PLLA/PCL scaffold improved biochemical and molecular markers hiPS cell-derived insulin-producing islet-like cells. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), 685–692. [Google Scholar] [CrossRef]
- Liao, G.-Y.; Jiang, S.; Xia, H.; Jiang, K. Preparation and characterization of aligned PLLA/PCL/HA composite fibrous membranes. J. Macromol. Sci. Part A 2012, 49, 946–951. [Google Scholar] [CrossRef]
- Griffith, L. Polymeric biomaterials. Acta Mater. 2000, 48, 263–277. [Google Scholar] [CrossRef]
- Jiang, S.; Song, P.; Guo, H.; Zhang, X.; Ren, Y.; Liu, H.; Song, X.; Kong, M. Blending PLLA/tannin-grafted PCL fiber membrane for skin tissue engineering. J. Mater. Sci. 2017, 52, 1617–1624. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Du, J.; Tan, E.; Kim, H.J.; Zhang, A.; Bhattacharya, R.; Yarema, K.J. Comparative evaluation of chitosan, cellulose acetate, and polyethersulfone nanofiber scaffolds for neural differentiation. Carbohydr. Polym. 2014, 99, 483–490. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Z.; Pu, X.; Yin, G.; Zhang, J. Fabrication of Chitosan/Polypyrrole-coated poly (L-lactic acid)/Polycaprolactone aligned fibre films for enhancement of neural cell compatibility and neurite growth. Cell Prolif. 2019, 52, e12588. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, W.; Cao, Y.; Yao, J.; Wu, J.; Gu, X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 2005, 128, 1897–1910. [Google Scholar] [CrossRef]
- Matus-Munoz, M.R.; Ruiz-Ramos, R.; Altuzar, V.; Beltrán, H.I.; Palomino-Ovando, M.A.; Mendoza-Barrera, C. Fabrication and characterization of PCL/PLLA/CS composite fibers as extracellular matrix (ECM) mimetics. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 728–739. [Google Scholar] [CrossRef]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef]
- Doosti-Telgerd, M.; Mahdavi, F.S.; Moradikhah, F.; Daryasari, M.P.; Atashgah, R.B.; Dolatyar, B.; Javar, H.A.; Seyedjafari, E.; Shabani, I.; Arefian, E. Nanofibrous scaffolds containing hydroxyapatite and microfluidic-prepared polyamidoamin/BMP-2 plasmid dendriplexes for bone tissue engineering applications. Int. J. Nanomed. 2020, 15, 2633–2646. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dai, T.; Wang, W.; Ouyang, J. BMP-2 loaded Bioactive PLLA/PCL Blended nanofibers for synergistic influences on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells via TGF-β/Smad2/3 signaling pathway. J. Drug Deliv. Sci. Technol. 2025, 105, 106639. [Google Scholar] [CrossRef]
- Doyle, C.; Tanner, E.; Bonfield, W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zheng, D.; Yao, H.; Fu, S.; Wei, Z.; Wang, Z.; Xie, M. An extracellular matrix-mimicking, bilayered, heterogeneous, porous, nanofibrous scaffold for anterior urethroplasty in a rabbit model. Biomed. Mater. 2020, 15, 065008. [Google Scholar] [CrossRef]
- Kontogianni, G.-I.; Bonatti, A.F.; De Maria, C.; Naseem, R.; Melo, P.; Coelho, C.; Vozzi, G.; Dalgarno, K.; Quadros, P.; Vitale-Brovarone, C. Promotion of in vitro osteogenic activity by melt extrusion-based plla/pcl/phbv scaffolds enriched with nano-hydroxyapatite and strontium substituted nano-hydroxyapatite. Polymers 2023, 15, 1052. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, M.; Zhao, W.; Liu, M.; Fang, W.; Wang, Y.; Gao, G.; Wang, Y.; Fu, Q. Scaffold-based tissue engineering strategies for urethral repair and reconstruction. Biofabrication 2024, 17, 012003. [Google Scholar] [CrossRef]
- Wan, X.; Xie, M.-K.; Xu, H.; Wei, Z.-W.; Yao, H.-J.; Wang, Z.; Zheng, D.-C. Hypoxia-preconditioned adipose-derived stem cells combined with scaffold promote urethral reconstruction by upregulation of angiogenesis and glycolysis. Stem Cell Res. Ther. 2020, 11, 535. [Google Scholar] [CrossRef]
- Sharifisamani, E.; Mousazadegan, F.; Bagherzadeh, R.; Latifi, M. PEG-PLA-PCL based electrospun yarns with curcumin control release property as suture. Polym. Eng. Sci. 2020, 60, 1520–1529. [Google Scholar] [CrossRef]
- Mobarakeh, A.I.; Ramsheh, A.S.; Khanshan, A.; Aghaei, S.; Mirbagheri, M.S.; Esmaeili, J. Fabrication and evaluation of a bi-layered electrospun PCL/PVA patch for wound healing: Release of vitamins and silver nanoparticle. Heliyon 2024, 10, e33178. [Google Scholar] [CrossRef]
- Haroosh, H.J.; Chaudhary, D.S.; Dong, Y. Electrospun PLA/PCL fibers with tubular nanoclay: Morphological and structural analysis. J. Appl. Polym. Sci. 2012, 124, 3930–3939. [Google Scholar] [CrossRef]
- Touny, A.H.; Lawrence, J.G.; Jones, A.D.; Bhaduri, S.B. Effect of electrospinning parameters on the characterization of PLA/HNT nanocomposite fibers. J. Mater. Res. 2010, 25, 857–865. [Google Scholar] [CrossRef]
- Lv, G.; He, F.; Wang, X.; Gao, F.; Zhang, G.; Wang, T.; Jiang, H.; Wu, C.; Guo, D.; Li, X. Novel nanocomposite of nano Fe3O4 and polylactide nanofibers for application in drug uptake and induction of cell death of leukemia cancer cells. Langmuir 2008, 24, 2151–2156. [Google Scholar] [CrossRef]
- Haroosh, H.J.; Dong, Y.; Ingram, G.D. Synthesis, morphological structures, and material characterization of electrospun PLA: PCL/magnetic nanoparticle composites for drug delivery. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1607–1617. [Google Scholar] [CrossRef]
- Zeinali, R.; Del Valle, L.J.; Torras, J.; Puiggali, J. Recent progress on biodegradable tissue engineering scaffolds prepared by thermally-induced phase separation (Tips). Int. J. Mol. Sci. 2021, 22, 3504. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Ciapetti, G.; Granchi, D.; Devescovi, V.; Baglio, S.R.; Leonardi, E.; Martini, D.; Jurado, M.J.; Olalde, B.; Armentano, I.; Kenny, J.M. Enhancing osteoconduction of PLLA-based nanocomposite scaffolds for bone regeneration using different biomimetic signals to MSCs. Int. J. Mol. Sci. 2012, 13, 2439–2458. [Google Scholar] [CrossRef]
- Jaiswal, A.; Chhabra, H.; Soni, V.; Bellare, J. Enhanced mechanical strength and biocompatibility of electrospun polycaprolactone-gelatin scaffold with surface deposited nano-hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Yang, F.; Seyednejad, H.; Chen, Z.; Hennink, W.E.; Anderson, J.M.; van den Beucken, J.J.; Jansen, J.A. Biocompatibility and degradation characteristics of PLGA-based electrospun nanofibrous scaffolds with nanoapatite incorporation. Biomaterials 2012, 33, 6604–6614. [Google Scholar] [CrossRef] [PubMed]
- Chuenjitkuntaworn, B.; Supaphol, P.; Pavasant, P.; Damrongsri, D. Electrospun poly (L-lactic acid)/hydroxyapatite composite fibrous scaffolds for bone tissue engineering. Polym. Int. 2010, 59, 227–235. [Google Scholar] [CrossRef]
- Qi, H.; Ye, Z.; Ren, H.; Chen, N.; Zeng, Q.; Wu, X.; Lu, T. Bioactivity assessment of PLLA/PCL/HAP electrospun nanofibrous scaffolds for bone tissue engineering. Life Sci. 2016, 148, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Z.; Tian, F.; Chen, S.; Lei, L.; Jiang, T.; Feng, Q.; Fan, Y. Sustained delivery of calcium and orthophosphate ions from amorphous calcium phosphate and poly (L-lactic acid)-based electrospinning nanofibrous scaffold. Sci. Rep. 2017, 7, 45655. [Google Scholar] [CrossRef]
- Meng, C.; Liu, X.; Li, J. Hierarchical porous PLLA/ACP fibrous membrane towards bone tissue scaffold. J. Mech. Behav. Biomed. Mater. 2024, 152, 106455. [Google Scholar] [CrossRef]
- Canales, D.A.; Piñones, N.; Saavedra, M.; Loyo, C.; Palza, H.; Peponi, L.; Leonés, A.; Baier, R.V.; Boccaccini, A.R.; Grünelwald, A. Fabrication and assessment of bifunctional electrospun poly (L-lactic acid) scaffolds with bioglass and zinc oxide nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2023, 228, 78–88. [Google Scholar] [CrossRef]
- Piatti, E.; Miola, M.; Liverani, L.; Verné, E.; Boccaccini, A.R. Poly (ε-caprolactone)/bioactive glass composite electrospun fibers for tissue engineering applications. J. Biomed. Mater. Res. Part A 2023, 111, 1692–1709. [Google Scholar] [CrossRef]
- Liao, G.; Jiang, S.; Xu, X.; Ke, Y. Electrospun aligned PLLA/PCL/HA composite fibrous membranes and their in vitro degradation behaviors. Mater. Lett. 2012, 82, 159–162. [Google Scholar] [CrossRef]
- de Siqueira, L.; Ribeiro, N.; Paredes, M.B.; Grenho, L.; Cunha-Reis, C.; Trichês, E.S.; Fernandes, M.H.; Sousa, S.R.; Monteiro, F.J. Influence of PLLA/PCL/HA scaffold fiber orientation on mechanical properties and osteoblast behavior. Materials 2019, 12, 3879. [Google Scholar] [CrossRef] [PubMed]
- Kalvand, E.; Bakhshandeh, H.; Nadri, S.; Habibizadeh, M.; Rostamizadeh, K. Poly-ε-caprolactone (PCL)/poly-l-lactic acid (PLLA) nanofibers loaded by nanoparticles-containing TGF-β1 with linearly arranged transforming structure as a scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2023, 111, 1838–1849. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Yang, H.; He, W.; Wang, B.; Liu, S.; Fan, Y. Construction of a Curcumin-Loaded PLLA/PCL Micro-Nano Conjugated Fibrous Membrane to Synergistically Prevent Postoperative Adhesion from Multiple Perspectives. Adv. Funct. Mater. 2024, 34, 2407983. [Google Scholar] [CrossRef]
- Liao, G.-Y.; Zhou, X.-P.; Chen, L.; Zeng, X.-Y.; Xie, X.-L.; Mai, Y.-W. Electrospun aligned PLLA/PCL/functionalised multiwalled carbon nanotube composite fibrous membranes and their bio/mechanical properties. Compos. Sci. Technol. 2012, 72, 248–255. [Google Scholar] [CrossRef]
- Liao, G.-Y.; Peng, X.; Jiang, S. Electrospun aligned poly (L-lactide)/poly (ϵ-caprolactone)/poly (ethylene glycol) blend fibrous membranes. J. Macromol. Sci. Part A 2012, 49, 466–472. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P.; Ayyar, M.; Krishnasamy, N.P.; Nageswaran, G. Blood compatibility and physicochemical assessment of novel nanocomposite comprising polyurethane and dietary carotino oil for cardiac tissue engineering applications. J. Appl. Polym. Sci. 2018, 135, 45691. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Jiang, Y.-C.; Jiang, L.; Huang, A.; Wang, X.-F.; Li, Q.; Turng, L.-S. Electrospun polycaprolactone/gelatin composites with enhanced cell–matrix interactions as blood vessel endothelial layer scaffolds. Mater. Sci. Eng. C 2017, 71, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Miszuk, J.M.; Zhao, Y.; Sun, H.; Fong, H. Electrospun polycaprolactone 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv. Healthc. Mater. 2015, 4, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Sadiasa, A.; Nguyen, T.H.; Lee, B.-T. In vitro and in vivo evaluation of porous PCL-PLLA 3D polymer scaffolds fabricated via salt leaching method for bone tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2014, 25, 150–167. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Qiu, K.; Chen, B.; Nie, W.; Zhou, X.; Feng, W.; Wang, W.; Chen, L.; Mo, X.; Wei, Y.; He, C. Electrophoretic deposition of dexamethasone-loaded mesoporous silica nanoparticles onto poly (L-lactic acid)/poly (ε-caprolactone) composite scaffold for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 4137–4148. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Morsi, Y.; El-Hamshary, H.; El-Newhy, M.; Fan, C.; Mo, X. Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 24415–24425. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, Y.; Shi, X. Construction of electrospun organic/inorganic hybrid nanofibers for drug delivery and tissue engineering applications. Adv. Fiber Mater. 2019, 1, 32–45. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.-J.; Dean, D.R.; Jun, H.-W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, X.-J.; Zhang, S.; Zhang, J.-C.; Li, Y.-F.; You, Q.-Z.; Long, Y.-Z. Electrospun anisotropic architectures and porous structures for tissue engineering. J. Mater. Chem. B 2015, 3, 5389–5410. [Google Scholar] [CrossRef]
- Yao, Q.; Cosme, J.G.; Xu, T.; Miszuk, J.M.; Picciani, P.H.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Mader, M.; Jérôme, V.; Freitag, R.; Agarwal, S.; Greiner, A. Ultraporous, compressible, wettable polylactide/polycaprolactone sponges for tissue engineering. Biomacromolecules 2018, 19, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Liu, X.; Li, R.; Malekmohammadi, S.; Feng, Y.; Song, J.; Gong, R.H.; Li, J. 3D Poly (L-lactic acid) fibrous sponge with interconnected porous structure for bone tissue scaffold. Int. J. Biol. Macromol. 2024, 268, 131688. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Zhou, L.; Bi, Z.; Shi, M.; Wang, D.; Li, Q. Fabrication of a novel Three-Dimensional porous PCL/PLA tissue engineering scaffold with high connectivity for endothelial cell migration. Eur. Polym. J. 2021, 161, 110834. [Google Scholar] [CrossRef]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt electrowriting of PLA, PCL, and composite PLA/PCL scaffolds for tissue engineering application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-printed PCL/PLA composite stents: Towards a new solution to cardiovascular problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef]
- Samadian, H.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Bit, A.; Alam, M.; Goodarzi, A.; Darya, G.; Salehi, M. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and Taurine for bone regeneration. Sci. Rep. 2020, 10, 13366. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, J.; Pang, L.; Chen, J.; Qi, M.; You, S.; Ren, N. An anisotropic three-dimensional electrospun micro/nanofibrous hybrid PLA/PCL scaffold. RSC Adv. 2019, 9, 9838–9844. [Google Scholar] [CrossRef] [PubMed]
- Peiravi, M.; Sherafat, Z.; Sani, M.; Azarpira, N. In vitro and in vivo assessment of 3D-printed PCL/PLA/ZnO nanocomposite scaffolds for osteoarthritis treatment. Compos. Commun. 2025, 57, 102432. [Google Scholar] [CrossRef]
- Dhayer, M.; Barral, V.; Cleret, D.; Jordao, A.; Drucbert, A.-S.; Germain, N.; Dropsit, S.; Maboudou, P.; Dekiouk, S.; Brun, S. Material and biological characterization of 3D knitted bioresorbable poly (D,L-lactide)(PLA) and polycaprolactone (PCL) scaffolds for soft tissue regeneration: From fabrication to in vivo performance. J. Biol. Eng. 2025, 19, 53. [Google Scholar] [CrossRef]

| Author | Year | Materials | Application | Reference |
|---|---|---|---|---|
| Yang et al. | 2024 | Silk fibroin/fibrin | Artificial blood vessels | [22] |
| Dieterle et al. | 2022 | Gelatin/Hydroxyapatite | Periodontal tissue engineering | [23] |
| Puigmal et al. | 2023 | Polyvinyl alcohol/Chitosan | Skin tissue engineering | [24] |
| Norouzi et al. | 2022 | Sodium alginate/Polycaprolactone | Novel nanofibrous biomaterial | [25] |
| Kenawy et al. | 2022 | Polyvinyl alcohol/Dextran | Wound healing | [26] |
| Zhang et al. | 2024 | Silk fibroin/Polyglycolic acid | Bone regeneration | [27] |
| Guzmán-Soria et al. | 2023 | Poly lactic-co-glycolic acid/Collagen | Tissue engineering | [28] |
| Virijević et al. | 2024 | Polycaprolactone/Polyethylene glycol | Wound Healing | [29] |
| Mustafa et al. | 2024 | Polycaprolactone/Polyethene oxide | Drug delivery | [30] |
| Author | Year | Solvent System | Application | Reference |
|---|---|---|---|---|
| Behtaj et al. | 2021 | Chloroform/Ethanol (9/1) | Retinal progenitor cell proliferation | [86] |
| Oztemur et al. | 2023 | Chloroform/Ethanol/Acetic acid (8/1/1) | Analysis of morphological, chemical and thermal properties | [99] |
| Bolbasov et al. | 2018 | Hexafluoro-2-propanol | Fat-derived multipotent mesenchyme stem cells cell proliferation | [82] |
| Oztemur et al. | 2023 | Chloroform/Ethanol/Acetic acid (8/1/1) | Fibroblast cells and human umbilical endothelial cells proliferation | [80] |
| Vida et al. | 2017 | Chloroform and Chloroform/Acetone (4/1) | Vero cells and fibroblastic cells proliferation | [100] |
| Li et al. | 2015 | Chloroform | MC3T3-E1 cells proliferation | [101] |
| Meng et al. | 2023 | Dichloromethane/ Dimethylformamide (19/1) | Bone tissue scaffold, human osteogenic sarcoma cells proliferation | [98] |
| Lui et al. | 2015 | 1,1,1,3,3,3-hexafluoro-2-propanol | Tendon regeneration, naproxen sodium release, L929 murine fibroblast cells proliferation | [102] |
| Shakhssalim et al. | 2013 | Chloroform/N, N-dimethylformamide (10/1) | Bladder reconstruction, human bladder smooth muscle cells proliferation | [103] |
| Liao et al. | 2010 | Chloroform/Methanol (3/1) | Adipose-derived stem cells proliferation and differentiation | [104] |
| Mobarra et al. | 2018 | Chloroform/N, N-dimethylformamide (8/2) | Diabetes mellitus therapy, human-induced pluripotent stem cells differentiation to beta islet-like cluster cells | [105] |
| Chen et al. | 2013 | Chloroform/Methanol (3/1) | Human adipose-derived stem cells proliferation and differentiation | [97] |
| Lu et al. | 2012 | Chloroform/ Dimethylformamide (4/1) | Analysis of surface morphology, phase structure, and hierarchical structures within the fibres | [95] |
| Author | Year | Type of Addition | Addition Constituents | Application | Reference |
|---|---|---|---|---|---|
| Qi et al. | 2016 | Bioceramic | Hydroxyapatite particles | Bone tissue engineering, mouse calvaria-derived pre-osteoblastic cells proliferation, differentiation and mineralization of osteoblasts | [134] |
| Liao et al. | 2012 | Bioceramic | Hydroxyapatite particles | Higher porosity, higher hydrophilic properties and higher biodegradation properties | [140] |
| De Siqueira et al. | 2019 | Bioceramic | Hydroxyapatite particles | Osteoblast cells adhesion and proliferation | [141] |
| Mashhadikhan et al. | 2015 | Natural Molecule | Gelatin | Adipose Derived Stem Cells attachment, viability and proliferation | [31] |
| Jiang et al. | 2017 | Natural Molecule | Tannin | Skin tissue engineering, Neonatal human dermal fibroblast cells viability and proliferation | [108] |
| Matus-Munoz et al. | 2022 | Natural Molecule | Chitosan | Human keratinocyte cells proliferation and differentiation | [113] |
| Kalvand et al. | 2023 | Natural Molecule | Chitosan/Dextran/TGF-β1 | Cartilage tissue engineering, mesenchymal stem cells differentiation | [142] |
| Wang et al. | 2025 | Natural Molecule | BMP-2 | Bone tissue engineering, rat bone marrow-derived mesenchymal stem cells proliferation and osteogenic differentiation | [116] |
| Liao et al. | 2024 | Natural Molecule | Curcumin | Prevent postoperative adhesion, inhibit fibroblast adhesion, proliferation, and differentiation | [143] |
| Xu et al. | 2019 | Natural Molecule/Synthetic Material | Chitosan/Polypyrrole | Nerve repair and regeneration, PC12 cells differentiation, neurite growth and alignment | [111] |
| Liao et al. | 2012 | Synthetic Material | Multiwalled carbon nanotube | Adipose-derived stem cells proliferation and reorientation | [144] |
| Liao et al. | 2012 | Synthetic Material | Poly (ethylene glycol) | Adipose Derived Stem Cells attachment, viability and proliferation | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C. Electrospinning PLLA/PCL Blend Fibre-Based Materials and Their Biomedical Application: A Mini Review. Polymers 2025, 17, 2802. https://doi.org/10.3390/polym17202802
Meng C. Electrospinning PLLA/PCL Blend Fibre-Based Materials and Their Biomedical Application: A Mini Review. Polymers. 2025; 17(20):2802. https://doi.org/10.3390/polym17202802
Chicago/Turabian StyleMeng, Chen. 2025. "Electrospinning PLLA/PCL Blend Fibre-Based Materials and Their Biomedical Application: A Mini Review" Polymers 17, no. 20: 2802. https://doi.org/10.3390/polym17202802
APA StyleMeng, C. (2025). Electrospinning PLLA/PCL Blend Fibre-Based Materials and Their Biomedical Application: A Mini Review. Polymers, 17(20), 2802. https://doi.org/10.3390/polym17202802







