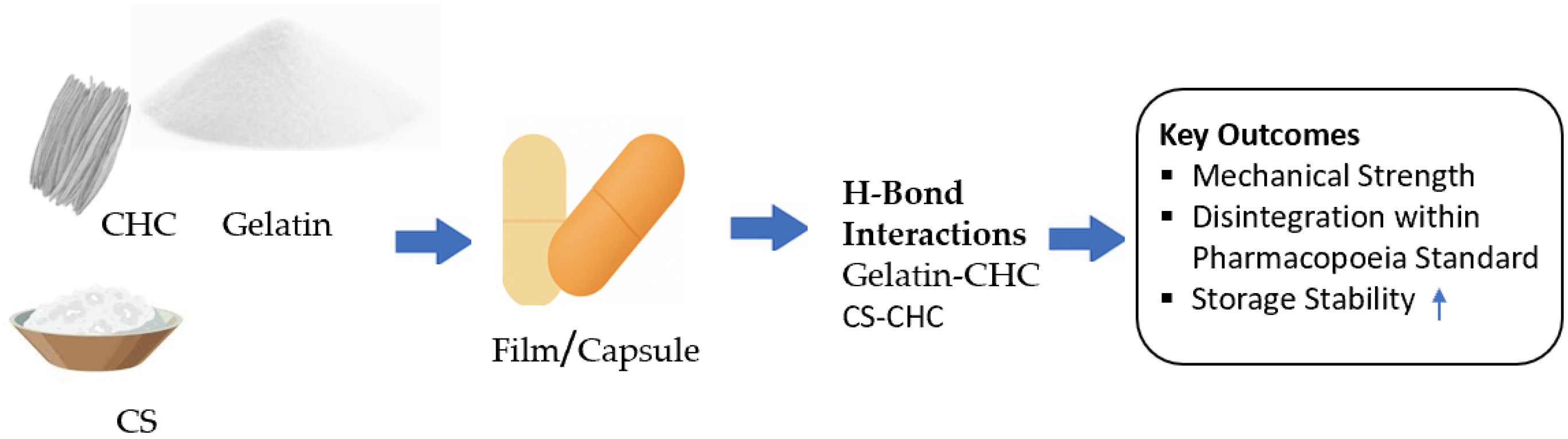
Structural and Functional Enhancement of Halal Gelatin Capsules Reinforced with Corn Husk Cellulose
Abstract
1. Introduction
2. Materials and Methods
- Pretreatment of Corn Husks
- Film preparation
2.1. Mechanical Properties
Tensile Strength (MPa) and Elongation at Break (%)
2.2. Storage and Morphological Appearance
2.2.1. Capsule Surface by Optical Microscopy
2.2.2. Temperature Humidity Stability Test
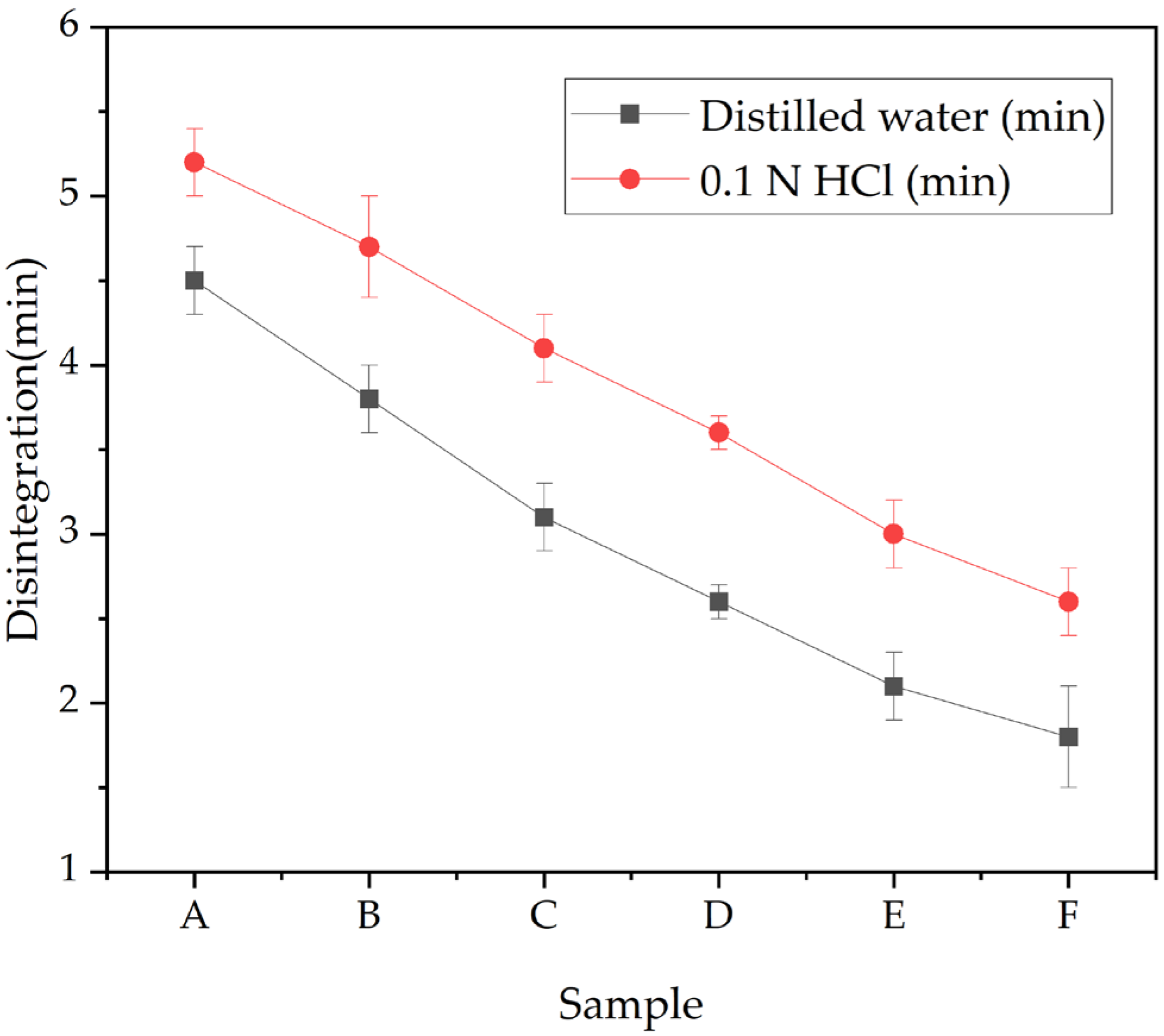
2.3. Disintegration Test
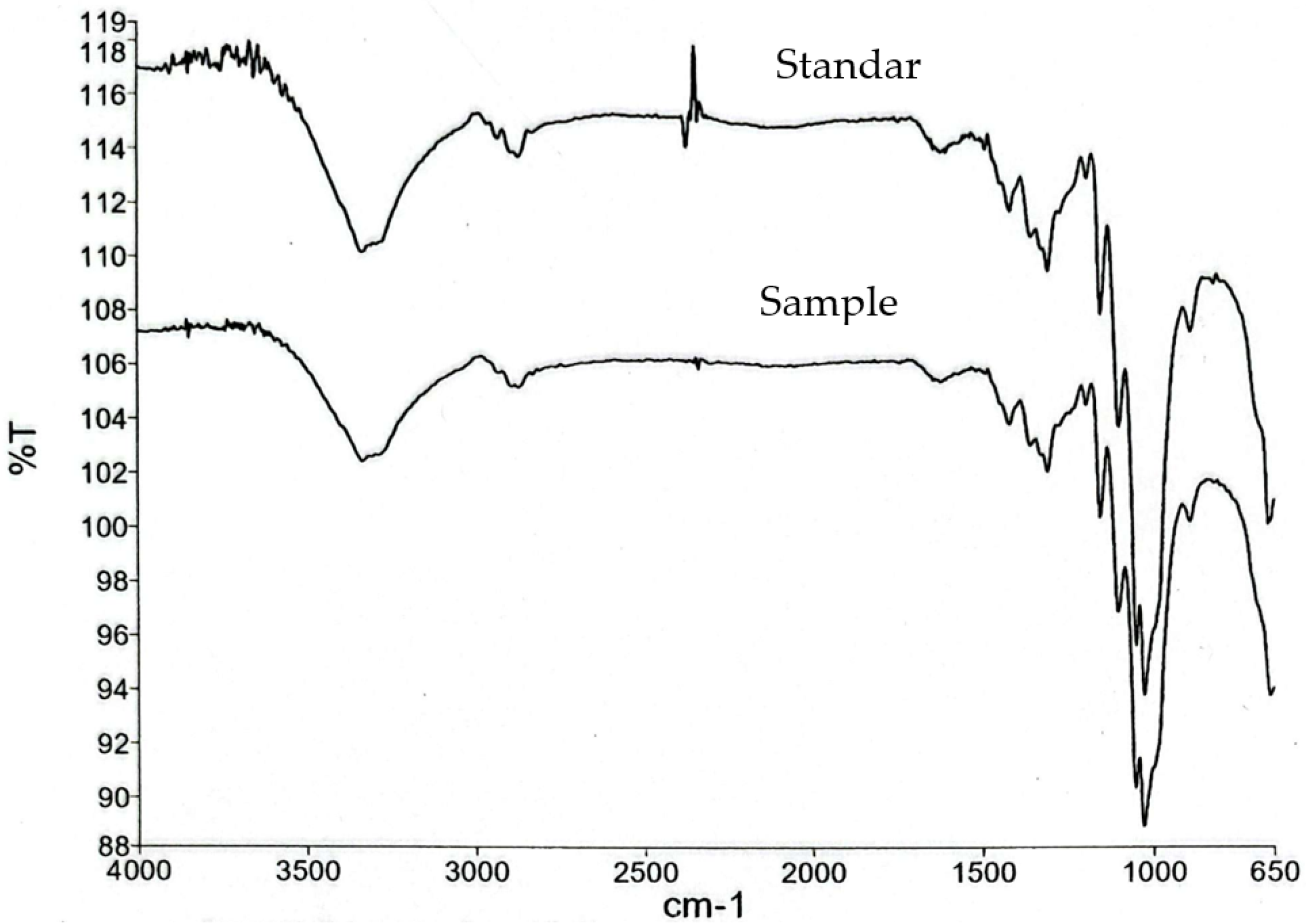
2.4. Fourier-Transform Infrared Spectroscopy
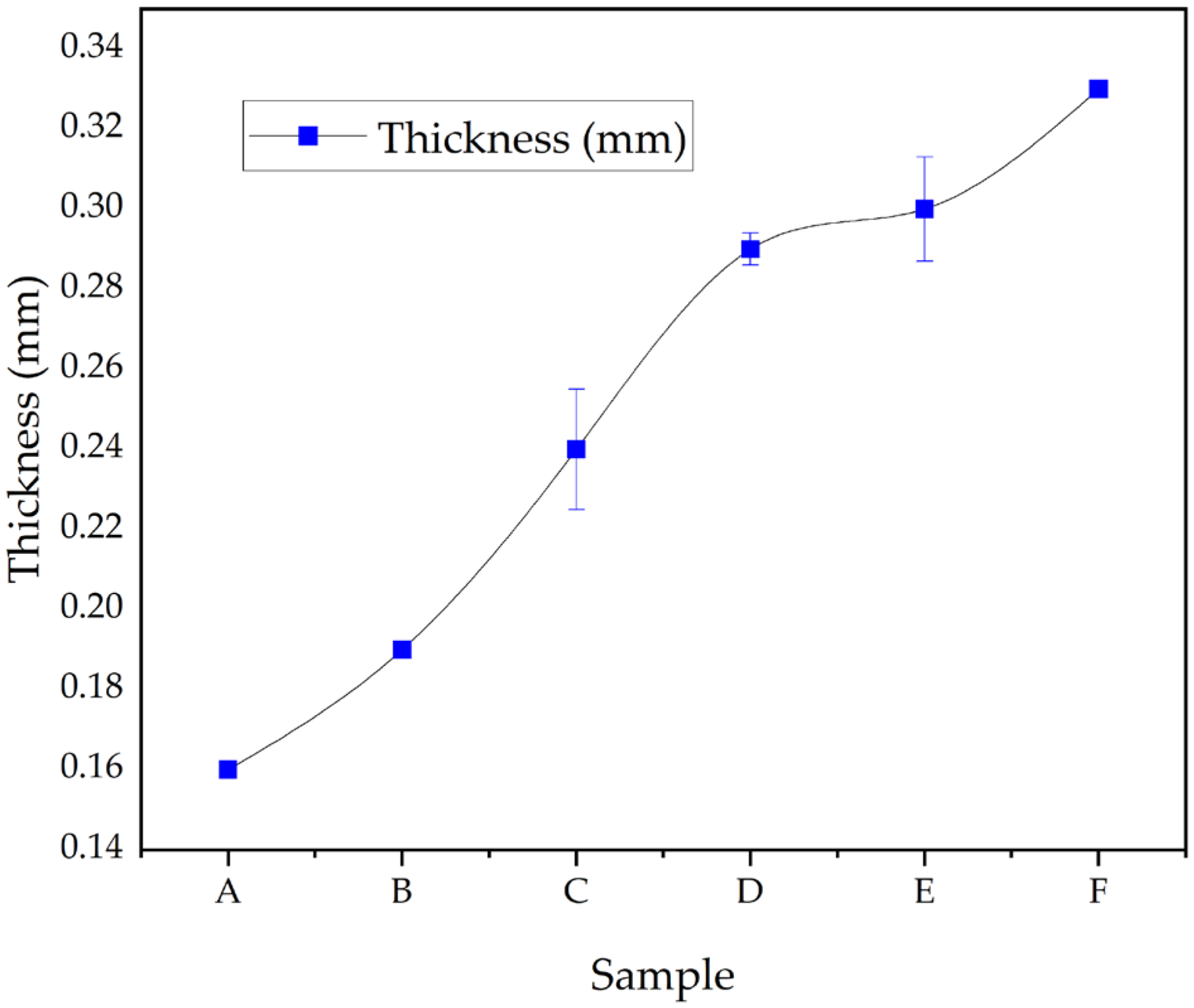
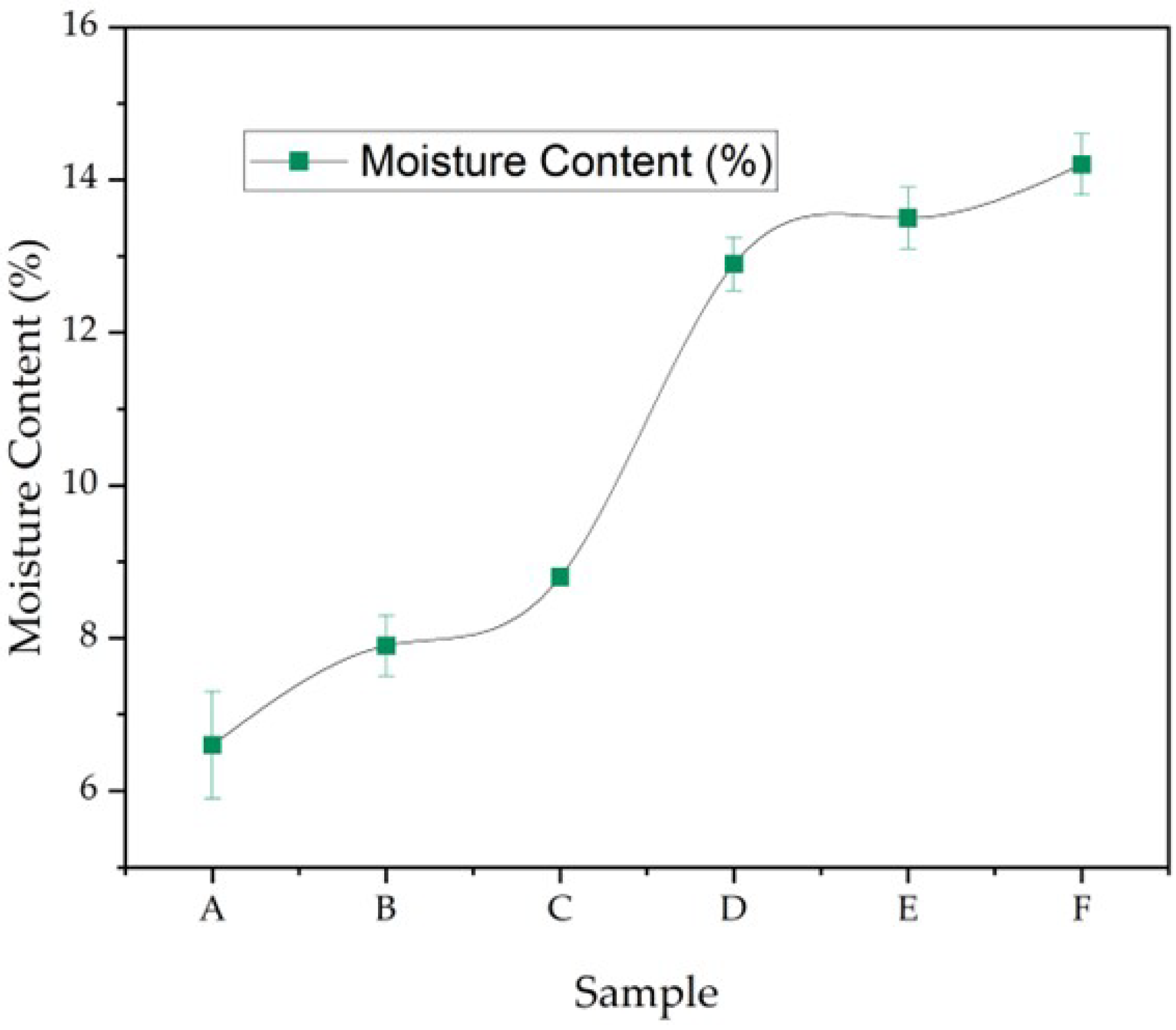
2.5. Film Thickness and Moisture Content (Basic Physicals)
2.6. Swelling Index (Water Uptake Behavior)
2.7. Ash Content (Inorganic Residue/Purity)
3. Result and Discussion
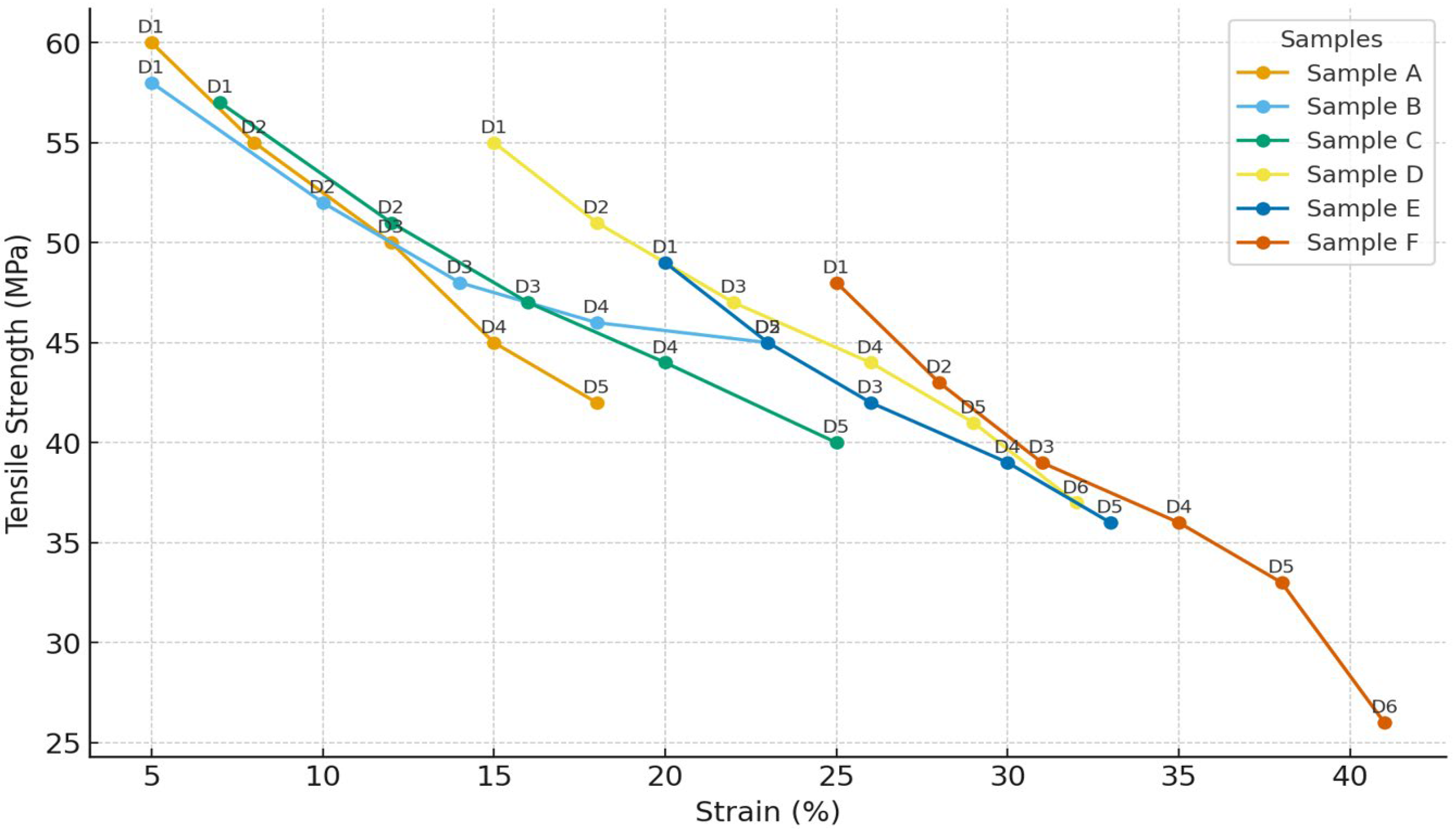
3.1. Mechanical Properties and Storage Appearance
Tensile Strength and Elongation at Break
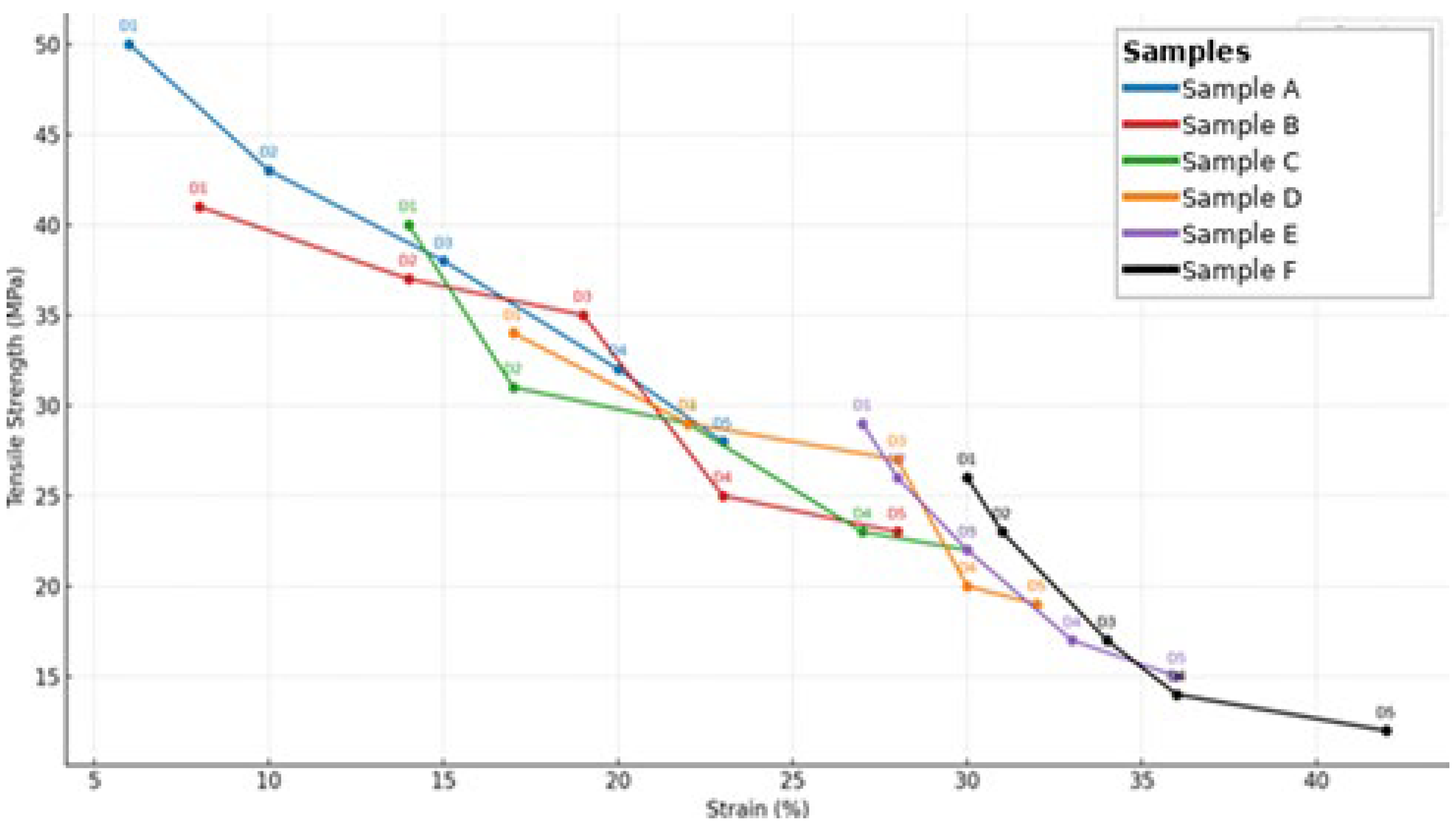
- Mechanical Stability of Capsule Films under Varying Storage Conditions
3.2. Storage Appearance
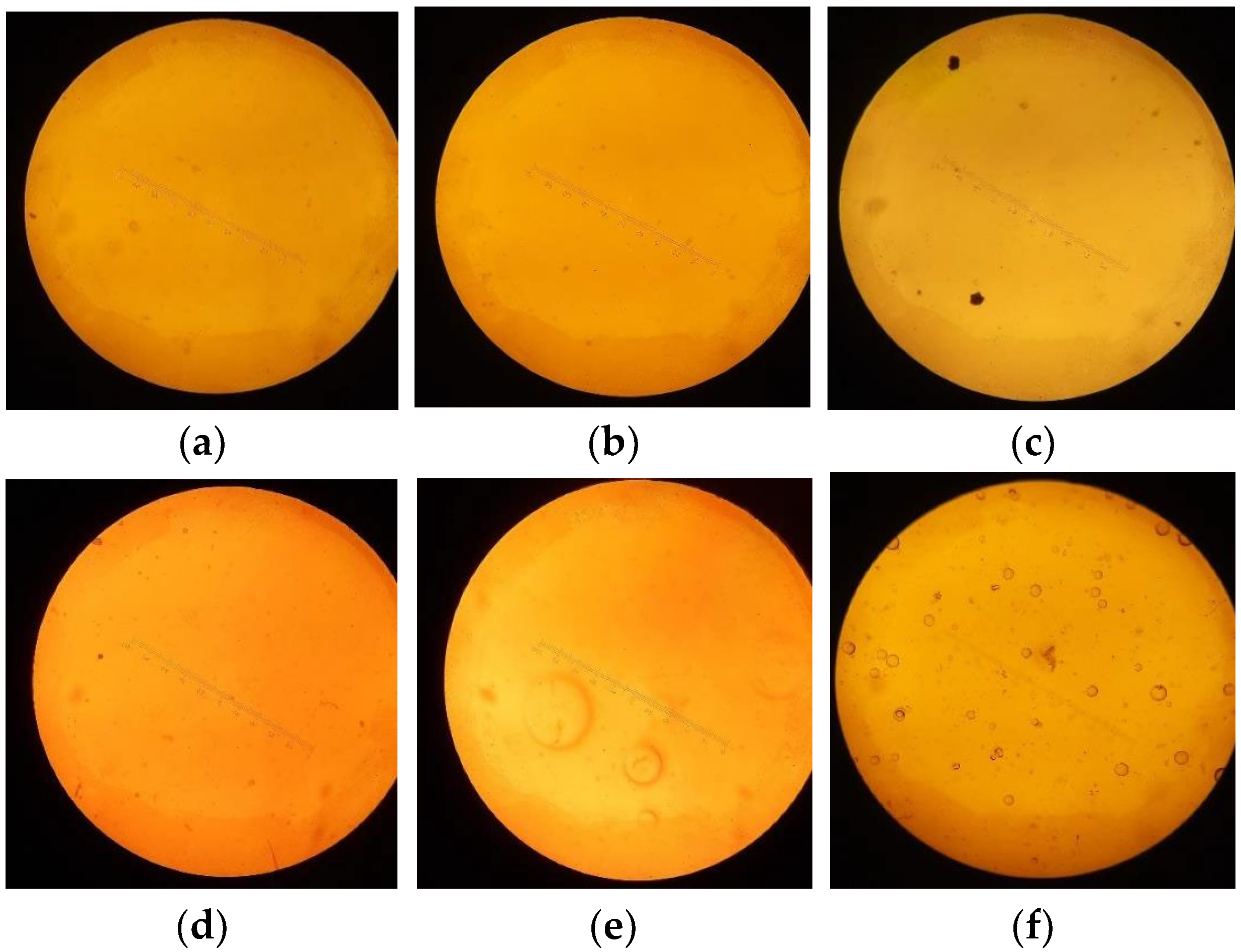
3.2.1. Capsule Shell Surface by Optical Microscopy
3.2.2. Capsule Clarity and Structural Assessment by UV–Vis and FTIR
3.2.3. Temperature and Humidity Stability
3.3. Disintegration of Capsule Film
3.4. FTIR Analysis of Gelatin–CHC Composite Films
3.5. Capsule Film Thickness and Moisture Content
3.5.1. Capsule Film Thickness
3.5.2. Moisture Content
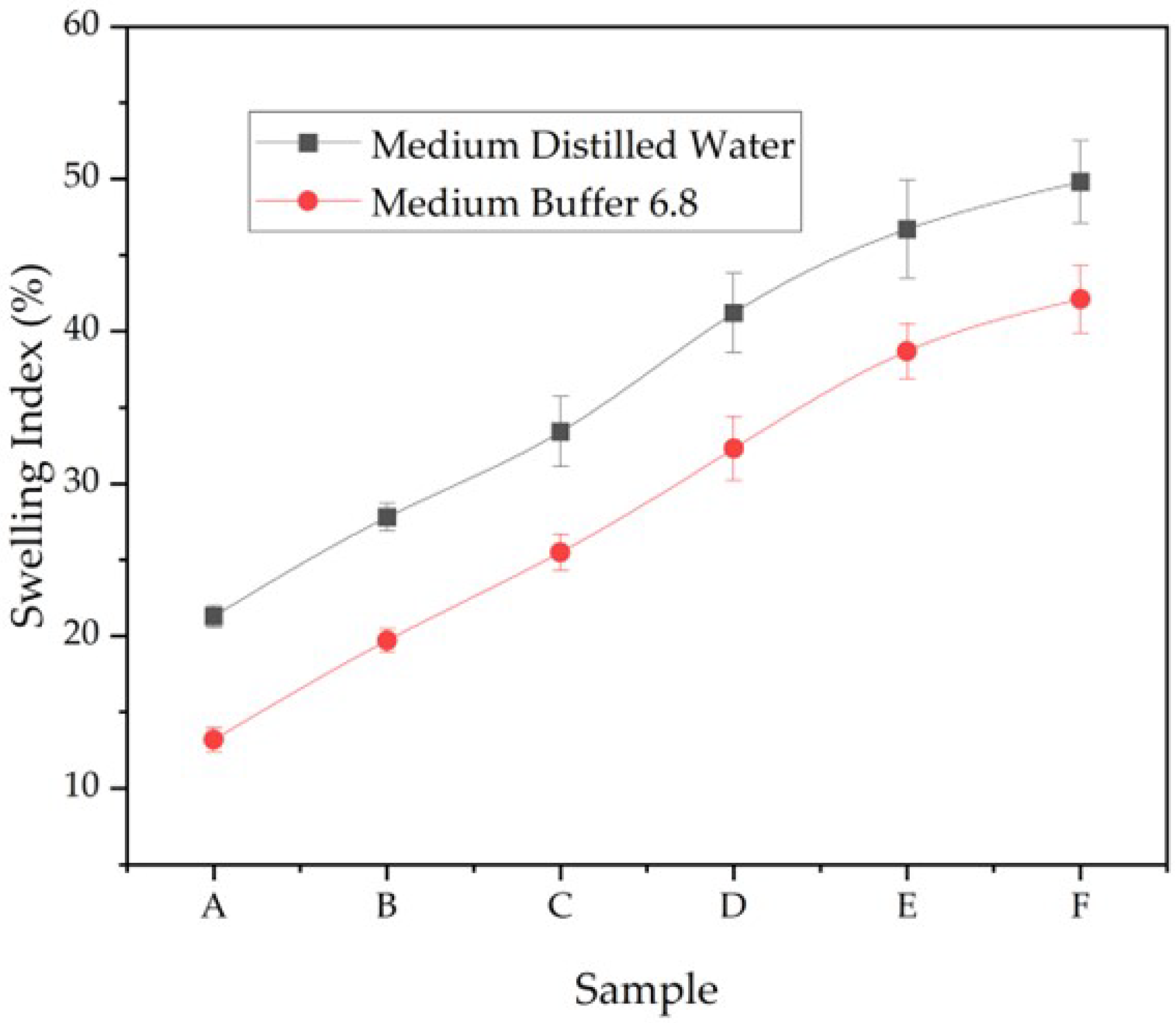
3.6. Swelling Index
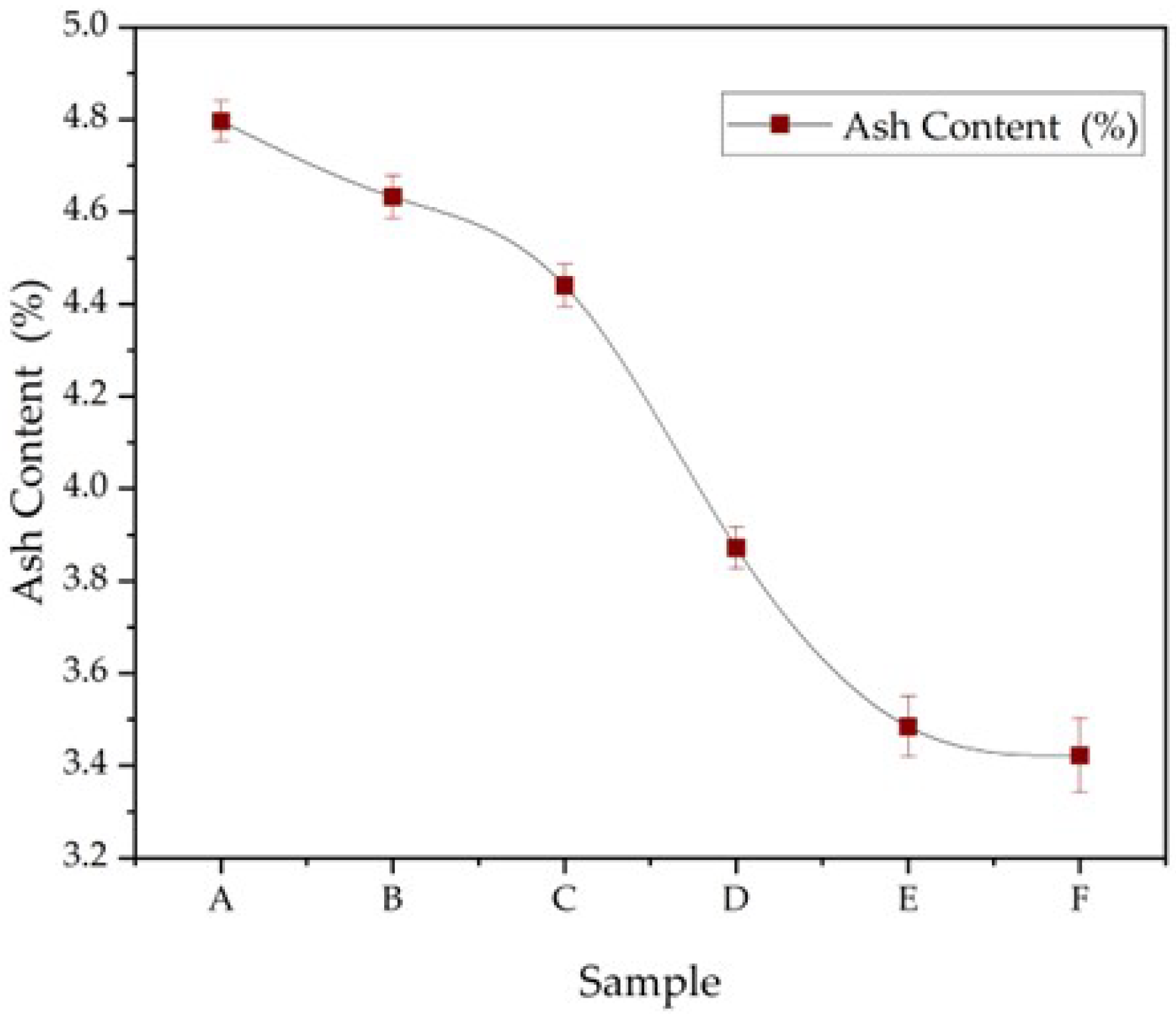
3.7. Ash Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Zea mays starch content data. In FAOSTAT; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023. [Google Scholar]
- Krstic, M.; Maksimovic, Z.; Ibric, S.; Bakic, T.; Prodanovic, J.; Razic, S. Lignocellulosic biomass as a source of microcrystalline cellulose—Chemical and technological characterization and future perspectives. Cellulose Chem. Technol. 2018, 52, 577–588. [Google Scholar]
- Liu, Y.; Liu, S.; Liu, J.; Zheng, X.; Tang, K. Effect of gelatin type on the structure and properties of microfibrillated cellulose-reinforced gelatin edible films. J. Appl. Polym. Sci. 2022, 139, 52119. [Google Scholar] [CrossRef]
- Cadé, D.; Cole, E.T.; Mayer, J.P.; Wittwer, F. Liquid-filled gelatin capsules. Pharm. Sci. Technol. Today 2002, 5, 411–420. [Google Scholar]
- Abdalbasit Adam, M.; Hadia Fadol, A. Review: Gelatin, source, extraction, and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Noh, S.W.; Kang, C.-S.; Park, J.-H.; Park, J.-W. Interaction of Porcine Myofibrillar Proteins and Various Gelatins (Bovine Hide, Porcine Skin, Fish). Foods 2019, 8, 188. [Google Scholar]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Latorre, M.E.; Ravazzoli, P.D.; Velazquez, D.E. Mechanical, surface, and thermal properties of gelatin-collagen blend films with potential application to biodegradable packaging. Int. J. Food Prop. 2025, 28, 2540851. [Google Scholar] [CrossRef]
- Luo, W.; Chen, W.; Liu, D.; Huang, X.; Ma, B. Effects of temperature and humidity on mechanical properties of gelatin-based materials. Int. J. Biol. Macromol. 2020, 164, 2990–2999. [Google Scholar]
- United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary (USP 43–NF 38). General Chapter <1241> Capsules—Moisture Permeation and Content; United States Pharmacopeial Convention: Rockville, MD, USA, 2020. [Google Scholar]
- Chavarría-Rojas, M.; Acuña-Amador, D.; Madrigal-Redondo, G.L. Gelatin and non-gelatin soft gel capsules: A review. J. Excip. Food Chem. 2021, 12, 19–29. [Google Scholar]
- United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary (USP 43–NF 38). General Chapter <1151> Pharmaceutical Dosage Forms—Capsules; United States Pharmacopeial Convention: Rockville, MD, USA, 2020. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). European Pharmacopoeia 10.0. Monographs on Capsules; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2020. [Google Scholar]
- United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary (USP 43–NF 38). General Chapter <701> Disintegration; United States Pharmacopeial Convention: Rockville, MD, USA, 2020. [Google Scholar]
- Firdaus, F.E.; Calluna, B.F.; Nasution, S.K. Antibacterial activity of lemongrass (Cymbopogon citratus) against Staphylococcus aureus in cellulose nanofibers from lignocellulosic biomass and glycerol. In BIO Web Conference, Proceedings of the 2024 6th International Conference on Biotechnology and Agriculture Engineering (ICBAE 2024), Tokyo, Japan, 9–11 October 2024; EDP Sciences: Les Ulis, France, 2025; Volume 150, p. 002003. [Google Scholar]
- Ministry of Health of the Republic of Indonesia. Pharmaceutical Raw Material Standards for Capsules. In Regulation No. 660/Menkes/Per/VII/1995; Ministry of Health: Jakarta, Indonesia, 1995. [Google Scholar]

| Sample | Gelatin (g) | CS (g) | CHC (g) | Glycerol (g) |
|---|---|---|---|---|
| A | 30 | 10 | 55 | 4.75 |
| B | 25 | 15 | 45 | 12.75 |
| C | 35 | 10 | 35 | 16 |
| D | 25 | 20 | 30 | 18.75 |
| E | 20 | 25 | 20 | 22.75 |
| F | 15 | 30 | 10 | 24.75 |
| Sample | Tensile Strength @25 °C (MPa) | Elongation @25 °C (%) | Tensile Strength @30 °C (MPa) | Elongation @30 °C (%) | Mechanical Classification |
|---|---|---|---|---|---|
| A | 50.2 ± 1.4 | 66.7 ± 3.2 | 45.1 ± 1.1 | 183.3± 3.1 | Excellent (rigid, elastic) |
| B | 48.7 ± 1.6 | 83.3 ± 3.9 | 42.3 ± 1.3 | 250.1 ± 3.5 | Flexible, stable |
| C | 47.3 ± 1.8 | 95.2 ± 4.1 | 39.3 ± 1.2 | 171.4 ± 3.9 | Moderate |
| D | 45.9 ± 1.5 | 120.8 ± 5.0 | 35.3± 1.7 | 160.9± 4.8 | Ductile |
| E | 44.5 ± 1.7 | 137.2 ± 4.3 | 28.2 ± 1.5 | 127.3± 3.7 | Soft, reduced integrity |
| F | 43.1 ± 1.7 | 153.8 ± 4.6 | 26.7± 1.7 | 140 ± 4.2 | Poor mechanical retention |
| Sample | Visual/Microscopic Surface Observation | Interpretation |
|---|---|---|
| A–B | Smooth, dense surface; no visible pores or defects under 100× microscopy | Well-formed matrix with good film integrity correlates with high tensile strength and low ductility |
| C–D | Slightly rough surface; occasional shallow voids or minor irregularities | Moderate matrix disruption; corresponds with balanced strength and flexibility |
| E–F | Irregular surface with visible bubbles and microvoids under light microscopy and SEM | Poor film formation with trapped air or moisture explains low strength and high elongation |
| Sample | Absorbance (cm−1) | Interpretation |
|---|---|---|
| A | 0.7109 | Clearer (hard capsule) |
| B | 0.7499 | – |
| C | 0.8005 | – |
| D | 0.8498 | – |
| E | 0.9724 | – |
| F | 1.0023 | Less clear (soft capsule) |
| Wavenumber (cm−1) | Assignment | Interpretation |
|---|---|---|
| ~3400 | O–H stretching | Hydrogen bonding (gelatin–cellulose) |
| ~2900 | C–H stretching | Aliphatic chains |
| ~1650 | Amide I (C=O stretch) | Peptide backbone of gelatin |
| ~1540 | Amide II (N–H bend) | Gelatin–cellulose interaction |
| ~1030 | C–O stretching | Polysaccharide (cellulose/starch) units |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firdaus, F.E.; Kinanti, A. Structural and Functional Enhancement of Halal Gelatin Capsules Reinforced with Corn Husk Cellulose. Polymers 2025, 17, 2803. https://doi.org/10.3390/polym17202803
Firdaus FE, Kinanti A. Structural and Functional Enhancement of Halal Gelatin Capsules Reinforced with Corn Husk Cellulose. Polymers. 2025; 17(20):2803. https://doi.org/10.3390/polym17202803
Chicago/Turabian StyleFirdaus, Flora Elvistia, and Aurelia Kinanti. 2025. "Structural and Functional Enhancement of Halal Gelatin Capsules Reinforced with Corn Husk Cellulose" Polymers 17, no. 20: 2803. https://doi.org/10.3390/polym17202803
APA StyleFirdaus, F. E., & Kinanti, A. (2025). Structural and Functional Enhancement of Halal Gelatin Capsules Reinforced with Corn Husk Cellulose. Polymers, 17(20), 2803. https://doi.org/10.3390/polym17202803







