Effects of Chitosan on Drug Load and Release for Cisplatin–Hydroxyapatite–Gelatin Composite Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydroxyapatite Microspheres (HAp)
2.1.1. Synthesis of HAp
2.1.2. Characterization of Synthesized HAp
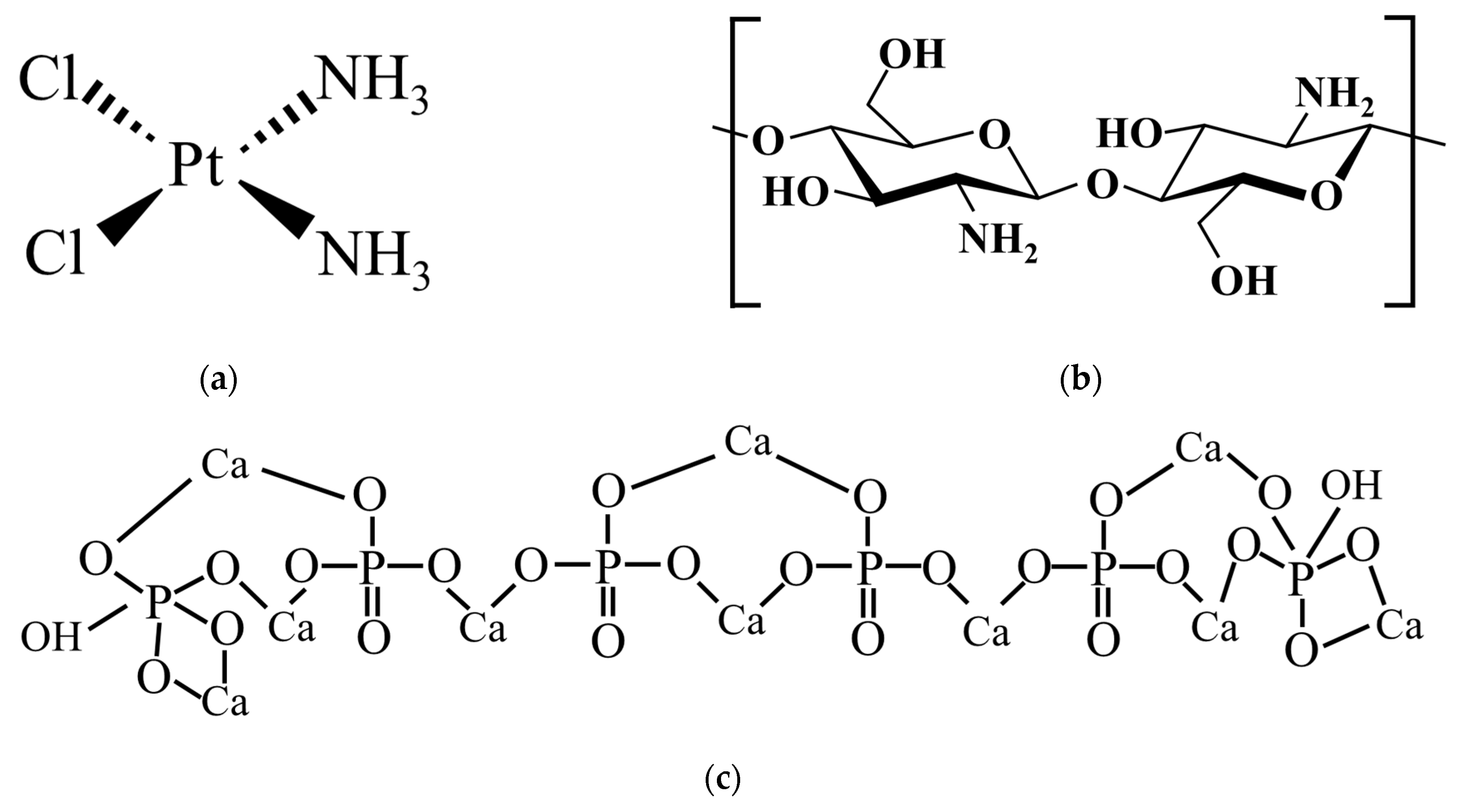
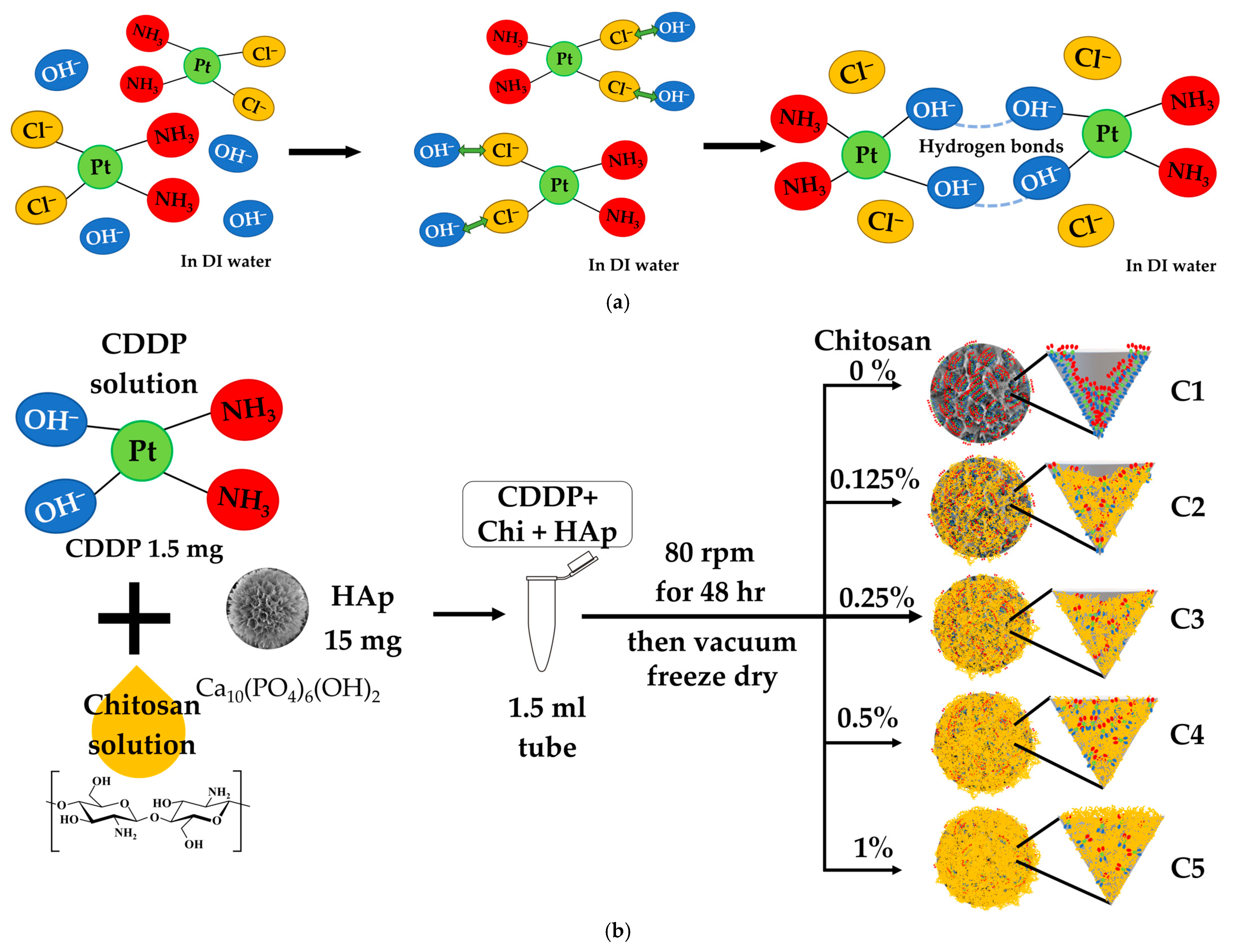
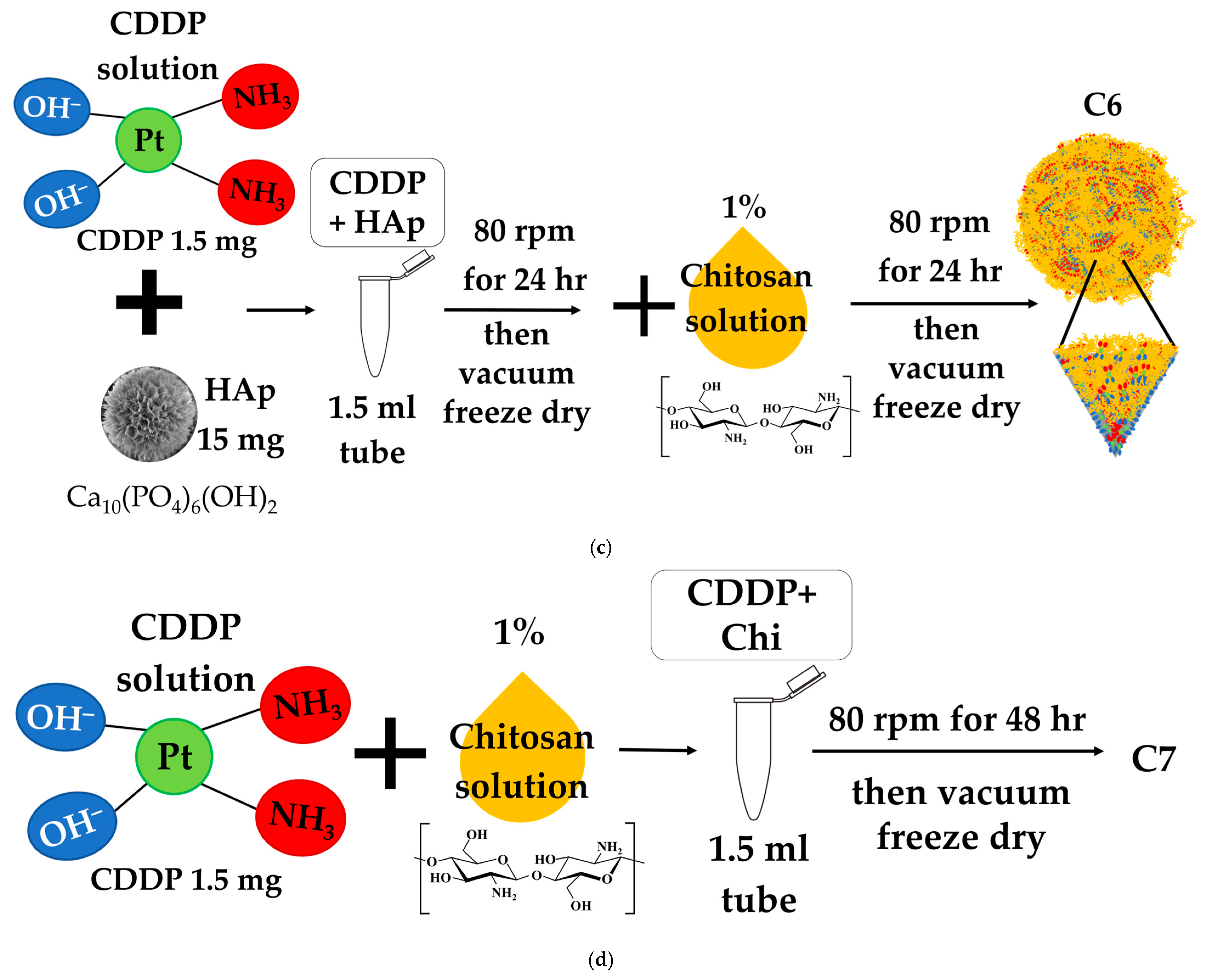
2.2. Drug-Loading Protocols
2.3. Drug-Loading Content (DLC) and Drug Encapsulation Efficiency (DEE) Analysis
2.3.1. UV-Visible Calibration Curve Establishment for CDDP Quantification
2.3.2. Assessment of Cisplatin Loading and Entrapment Performance
2.4. In Vitro Drug Release Study
2.5. Cell Cytotoxicity Assay
3. Results and Discussion
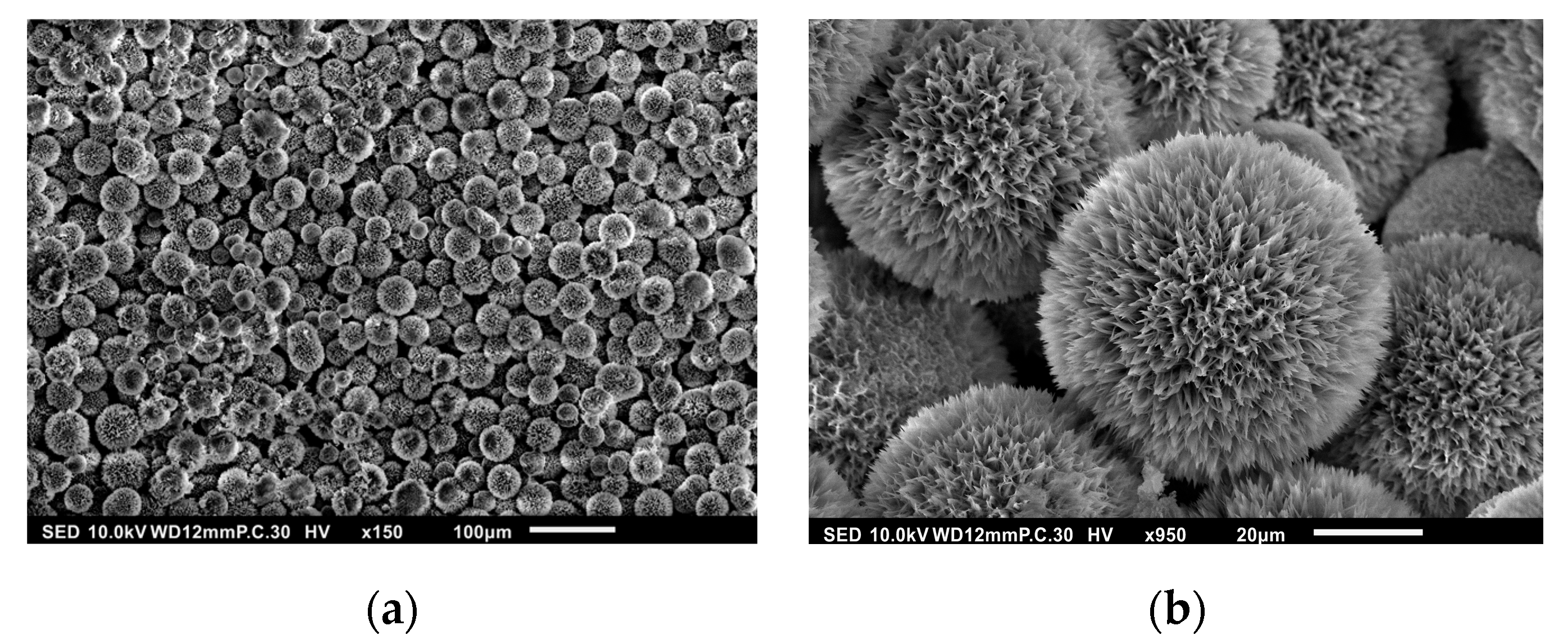
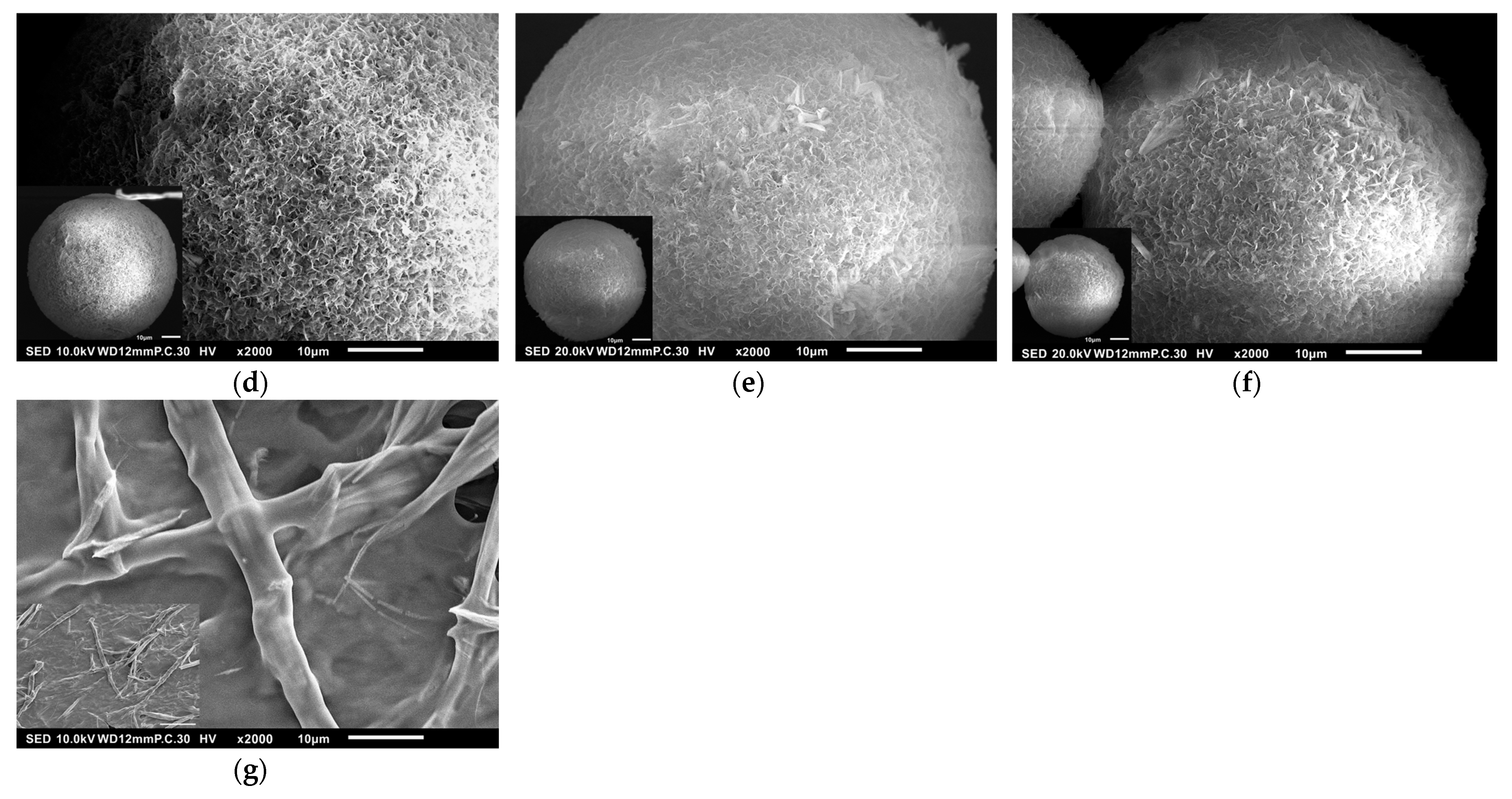
3.1. Surface Morphology of HAp
3.2. Drug Entrapment Efficiency (DEE) and Drug-Loading Content (DLC)
3.2.1. Calibration Curve for CDDP Analysis
3.2.2. DLC and DEE Analysis
3.3. CDDP Release Profiles
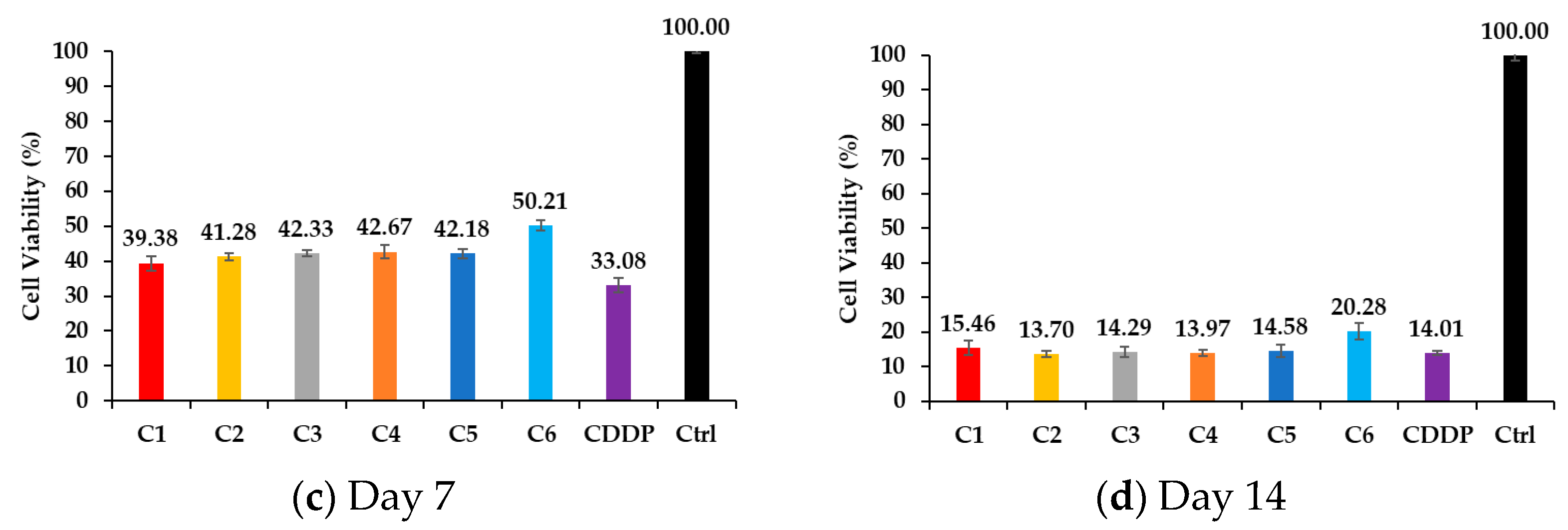
3.4. Cell Viability Through MTT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourmadadi, M.; Eshaghi, M.M.; Rahmani, E.; Ajalli, N.; Bakhshi, S.; Mirkhaef, H.; Lasemi, M.V.; Rahdar, A.; Behzadmehr, R.; Díez-Pascual, A.M. Cisplatin-loaded nanoformulations for cancer therapy: A comprehensive review. J. Drug Deliv. Sci. Technol. 2022, 77, 103928. [Google Scholar] [CrossRef]
- Gorgzadeh, A.; Hheidari, A.; Ghanbarikondori, P.; Arastonejad, M.; Goki, T.; Aria, M. Investigating the properties and cytotoxicity of cisplatin-loaded nano-polybutylcyanoacrylate on breast cancer cells. Asian Pac. J. Cancer Biol. 2023, 8, 345–350. [Google Scholar] [CrossRef]
- Sultan, M.H.; Moni, S.S.; Madkhali, O.A.; Bakkari, M.A.; Alshahrani, S.; Alqahtani, S.S.; Alhakamy, N.A.; Mohan, S.; Ghazwani, M.; Bukhary, H.A.; et al. Characterization of cisplatin-loaded chitosan nanoparticles and rituximab-linked surfaces as target-specific injectable nano-formulations for combating cancer. Sci. Rep. 2022, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Meng, F.; Yang, Z.; Lafuente-Merchan, M.; Fernández, L.M.; Cao, Y.; Kusamori, K.; Nishikawa, M.; Itakura, S.; Chen, J.; et al. Nano-drug delivery system for the treatment of multidrug-resistant breast cancer: Current status and future perspectives. Biomed. Pharmacother. 2024, 179, 117327. [Google Scholar] [CrossRef]
- Heydari, S.R.; Samadi, M.; Shirangi, A.; Farokhi, M.; Moradi, A.; Bafkary, R.; Atyabi, F.; Mottaghitalab, F.; Dinarvand, R. Dual responsive hydroxyapatite capped mesoporous silica nanoparticles for controlled delivery of Palbociclib to treat osteosarcoma. J. Drug Deliv. Sci. Technol. 2023, 82, 104356. [Google Scholar] [CrossRef]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent Progress in Nanostructured Smart Drug Delivery Systems for Cancer Therapy: A Review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef]
- Wang, Z.; Nogueira, L.P.; Haugen, H.J.; Van Der Geest, I.C.M.; de Almeida Rodrigues, P.C.; Janssen, D.; Bitter, T.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G. Dual-functional porous and cisplatin-loaded polymethylmethacrylate cement for reconstruction of load-bearing bone defect kills bone tumor cells. Bioact. Mater. 2022, 15, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Effendy, W.; Mydin, R.; Sreekantan, S.; Gazzali, A.M.; Musa, M.Y. Cisplatin encapsulation efficiency profiles using titania nanotube arrays platform in targeted cancer therapy. Biointerface Res. Appl. Chem. 2023, 13, 255–266. [Google Scholar]
- Romani, A.M.P. Cisplatin in cancer treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef]
- Han, Y.; Wen, P.; Li, J.; Kataoka, K. Targeted nanomedicine in cisplatin-based cancer therapeutics. J. Control. Release 2022, 345, 709–720. [Google Scholar] [CrossRef]
- Eslami Moghadam, M.; Sadeghi, M.; Mansouri-Torshizi, H.; Saidifar, M. High cancer selectivity and improving drug release from mesoporous silica nanoparticles in the presence of human serum albumin in cisplatin, carboplatin, oxaliplatin, and oxalipalladium treatment. Eur. J. Pharm. Sci. 2023, 187, 106477. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-A.; Lu, Y.-J.; Dash, B.S.; Chao, Y.-K.; Chen, J.-P. Hyaluronic Acid-Modified Cisplatin-Encapsulated Poly(Lactic-co-Glycolic Acid) Magnetic Nanoparticles for Dual-Targeted NIR-Responsive Chemo-Photothermal Combination Cancer Therapy. Pharmaceutics 2023, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Bao, Z.; Wang, P.; Deng, Y.; Fan, J.; Zhu, X.; Xia, X.; Song, Y.; Yao, H.; Li, D. Gelatin-Functionalized Carbon Nanotubes Loaded with Cisplatin for Anti-Cancer Therapy. Polymers 2023, 15, 3333. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, N.; Rezaie, H.R.; Javadpour, J.; Kharaziha, M. Cisplatin loaded polycaprolactone—Zeolite nanocomposite scaffolds for bone cancer treatment. J. Sci. Adv. Mater. Devices 2022, 7, 100377. [Google Scholar] [CrossRef]
- Ahmari, A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Khanbeigi, K.A. A green approach for preparation of chitosan/hydroxyapatite/graphitic carbon nitride hydrogel nanocomposite for improved 5-FU delivery. Int. J. Biol. Macromol. 2024, 258, 128736. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, M.; Rajeev, M.R. Modified chitosan-hyaluronic acid based hydrogel for the pH-responsive Co-delivery of cisplatin and doxorubicin. Int. J. Biol. Macromol. 2022, 201, 378–388. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- De Lama-Odría, M.D.; del Valle, L.J.; Puiggalí, J. Hydroxyapatite Biobased Materials for Treatment and Diagnosis of Cancer. Int. J. Mol. Sci. 2022, 23, 11352. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Aghajani, H.; Farrokhi-Rad, M. Vancomycin loaded-mesoporous bioglass/hydroxyapatite/chitosan coatings by electrophoretic deposition. Ceram. Int. 2022, 48, 20176–20186. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Wang, C.; Sun, R.; Tang, Y. Effects of physico-chemical properties of ions-doped hydroxyapatite on adsorption and release performance of doxorubicin as a model anticancer drug. Mater. Chem. Phys. 2022, 276, 125440. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Dang, J.; Wang, S.; Cheng, W.; Ran, Z.; Zhu, H.; Deng, H.; Xiong, C.; Xu, W.; et al. Anticancer and bone-enhanced nano-hydroxyapatite/gelatin/polylactic acid fibrous membrane with dual drug delivery and sequential release for osteosarcoma. Int. J. Biol. Macromol. 2023, 240, 124406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nadeem, A.; Sebastian, S.; Olsson, M.A.; Wai, S.N.; Styring, E.; Engellau, J.; Isaksson, H.; Tägil, M.; Lidgren, L.; et al. Bone mineral: A trojan horse for bone cancers. Efficient mitochondria targeted delivery and tumor eradication with nano hydroxyapatite containing doxorubicin. Mater. Today Bio. 2022, 14, 100227. [Google Scholar] [CrossRef]
- Deng, T.; Luo, D.; Zhang, R.; Zhao, R.; Hu, Y.; Zhao, Q.; Wang, S.; Iqbal, M.Z.; Kong, X. DOX-loaded hydroxyapatite nanoclusters for colorectal cancer (CRC) chemotherapy: Evaluation based on the cancer cells and organoids. SLAS Technol. 2023, 28, 22–31. [Google Scholar] [CrossRef]
- Kargozar, S.; Mollazadeh, S.; Kermani, F.; Webster, T.J.; Nazarnezhad, S.; Hamzehlou, S.; Baino, F. Hydroxyapatite Nanoparticles for Improved Cancer Theranostics. J. Funct. Biomater. 2022, 13, 100. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Pang, Z. Chapter 4—Chitosan in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 101–119. [Google Scholar]
- Giri, T.K. Chitosan based nanoparticulate system for controlled delivery of biological macromolecules. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sarwar, M.S.; Huang, Q.; Ghaffar, A.; Abid, M.A.; Zafar, M.S.; Khurshid, Z.; Latif, M. A Smart Drug Delivery System Based on Biodegradable Chitosan/Poly(allylamine hydrochloride) Blend Films. Pharmaceutics 2020, 12, 131. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Kolawole, O.M. Recent advances in modified chitosan-based drug delivery systems for transmucosal applications: A comprehensive review. Int. J. Biol. Macromol. 2024, 277, 134531. [Google Scholar] [CrossRef] [PubMed]
- Tousian, B.; Ghasemi, M.H.; Khosravi, A.R. Targeted chitosan nanoparticles embedded into graphene oxide functionalized with caffeic acid as a potential drug delivery system: New insight into cancer therapy. Int. J. Biol. Macromol. 2022, 222, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release Off. J. Control. Release Society 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Kao, I.F.; Fu, C.-Y.; Yen, S.-K. Effects of Adding Chitosan on Drug Entrapment Efficiency and Release Duration for Paclitaxel-Loaded Hydroxyapatite—Gelatin Composite Microspheres. Pharmaceutics 2023, 15, 2025. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Liang, Y.-H.; Yen, S.-K. Effects of Chitosan on Loading and Releasing for Doxorubicin Loaded Porous Hydroxyapatite–Gelatin Composite Microspheres. Polymers 2022, 14, 4276. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Kuo, Y.-T.; Kao, I.F.; Yen, S.-K. Porous Chitosan/Hydroxyapatite Composite Microspheres for Vancomycin Loading and Releasing. Pharmaceutics 2024, 16, 730. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Huang, S.-W.; Kao, I.F.; Yen, S.-K. The Preparation and Characterization of Chitosan/Calcium Phosphate Composite Microspheres for Biomedical Applications. Polymers 2024, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Lai, Y.-L.; Lin, C.-C.; Hsu, S.-R.; Yen, S.-K. Electrochemical Deposition of Cisplatin on Pure Magnesium. J. Electrochem. Soc. 2018, 165, D196. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Zhou, W.; Liu, C.; Zhang, H. Reregulated mitochondrial dysfunction reverses cisplatin resistance microenvironment in colorectal cancer. Smart Med. 2022, 1, e20220013. [Google Scholar] [CrossRef]
- Xiang, J.; Li, Y.; Zhang, Y.; Wang, G.; Xu, H.; Zhou, Z.; Tang, J.; Shen, Y. Polyphenol-cisplatin complexation forming core-shell nanoparticles with improved tumor accumulation and dual-responsive drug release for enhanced cancer chemotherapy. J. Control. Release 2021, 330, 992–1003. [Google Scholar] [CrossRef]



| DEE (%) | DLC (%) | |
|---|---|---|
| C1 | 58.33 ± 0.88 | 5.83 ± 0.09 |
| C2 | 50.24 ± 1.47 | 5.02 ± 0.15 |
| C3 | 48.36 ± 1.47 | 4.84 ± 0.15 |
| C4 | 40.17 ± 1.11 | 4.02 ± 0.11 |
| C5 | 32.91 ± 2.05 | 3.29 ± 0.21 |
| C6 | 99.43 ± 0.21 | 9.94 ± 0.02 |
| C7 | 75.07 ± 3.57 | 34.17 ± 7.89 |
| C8 | 84.63 ± 0.69 | 18.51 ± 0.15 |
| C9 | 74.34 ± 0.99 | 29.97 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-Y.; Kao, I.-F.; Yen, S.-K. Effects of Chitosan on Drug Load and Release for Cisplatin–Hydroxyapatite–Gelatin Composite Microspheres. Polymers 2025, 17, 1485. https://doi.org/10.3390/polym17111485
Wu M-Y, Kao I-F, Yen S-K. Effects of Chitosan on Drug Load and Release for Cisplatin–Hydroxyapatite–Gelatin Composite Microspheres. Polymers. 2025; 17(11):1485. https://doi.org/10.3390/polym17111485
Chicago/Turabian StyleWu, Meng-Ying, I-Fang Kao, and Shiow-Kang Yen. 2025. "Effects of Chitosan on Drug Load and Release for Cisplatin–Hydroxyapatite–Gelatin Composite Microspheres" Polymers 17, no. 11: 1485. https://doi.org/10.3390/polym17111485
APA StyleWu, M.-Y., Kao, I.-F., & Yen, S.-K. (2025). Effects of Chitosan on Drug Load and Release for Cisplatin–Hydroxyapatite–Gelatin Composite Microspheres. Polymers, 17(11), 1485. https://doi.org/10.3390/polym17111485







