The Effects of Soft-Segment Molecular Weight on the Structure and Properties of Poly(trimethylene terephthalate)-block-poly(tetramethylene glycol) Copolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PTT-b-PTMG Copolymers
2.3. Characterizations
3. Results and Discussion
3.1. Structure and Composition of PTT-b-PTMG Copolymers
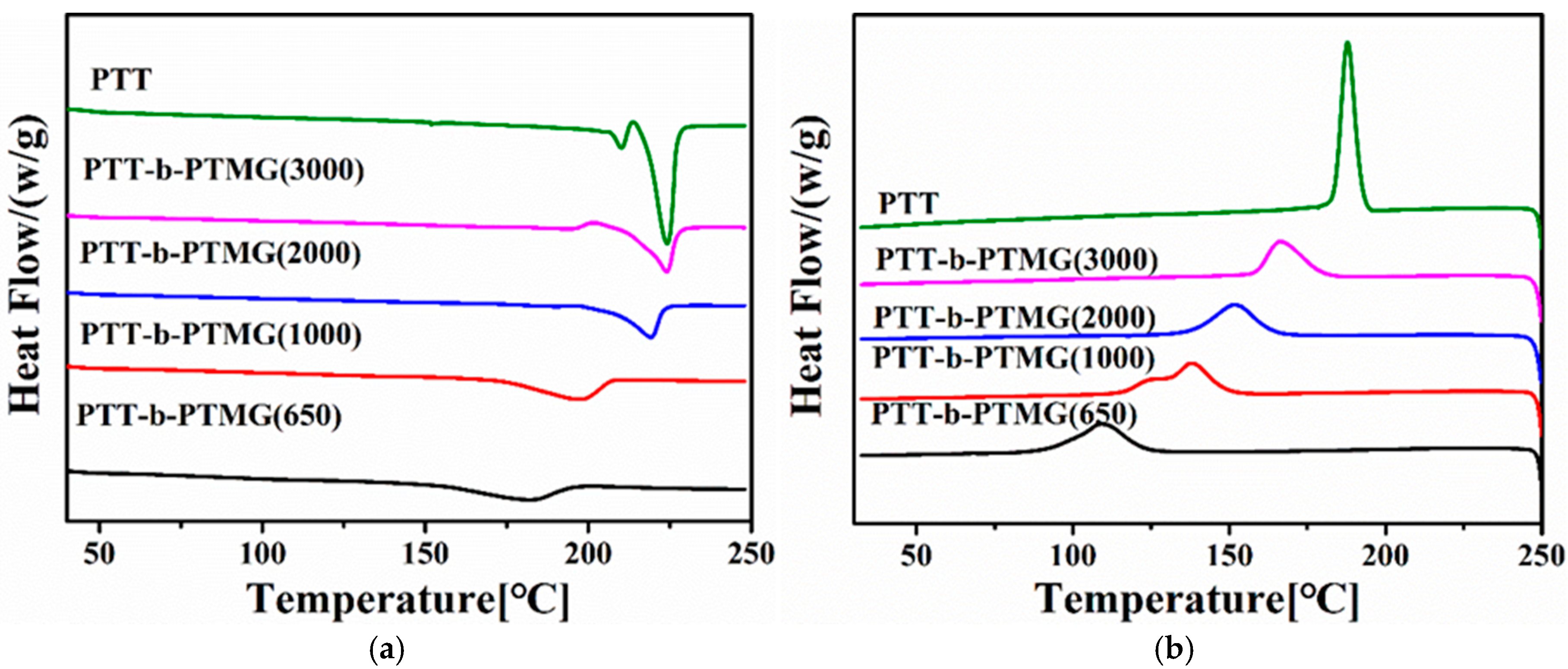
3.2. Melt and Crystallization Behavior PTT-b-PTMG Copolymers
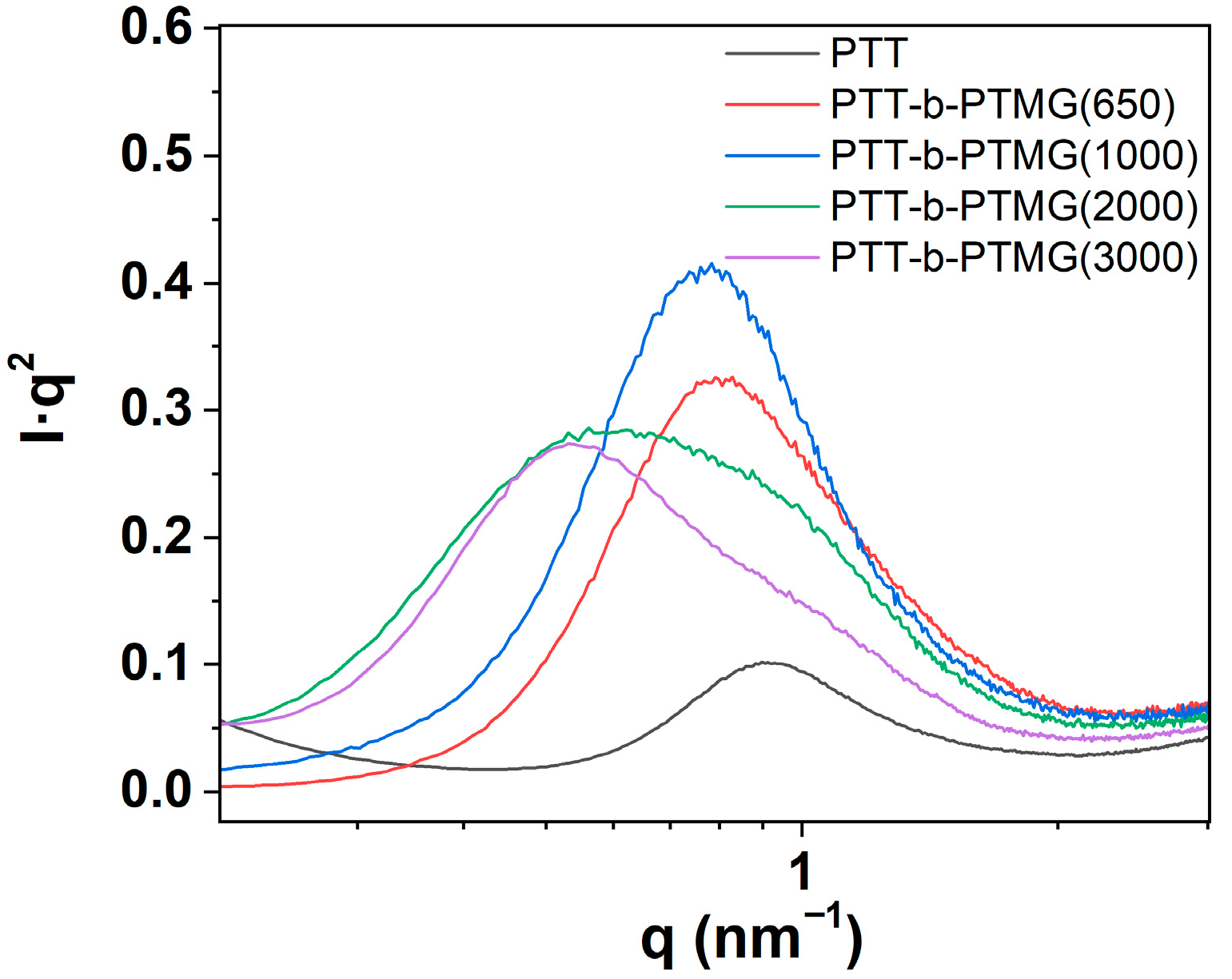
3.3. Phase Structure of PTT-b-PTMG Copolymers
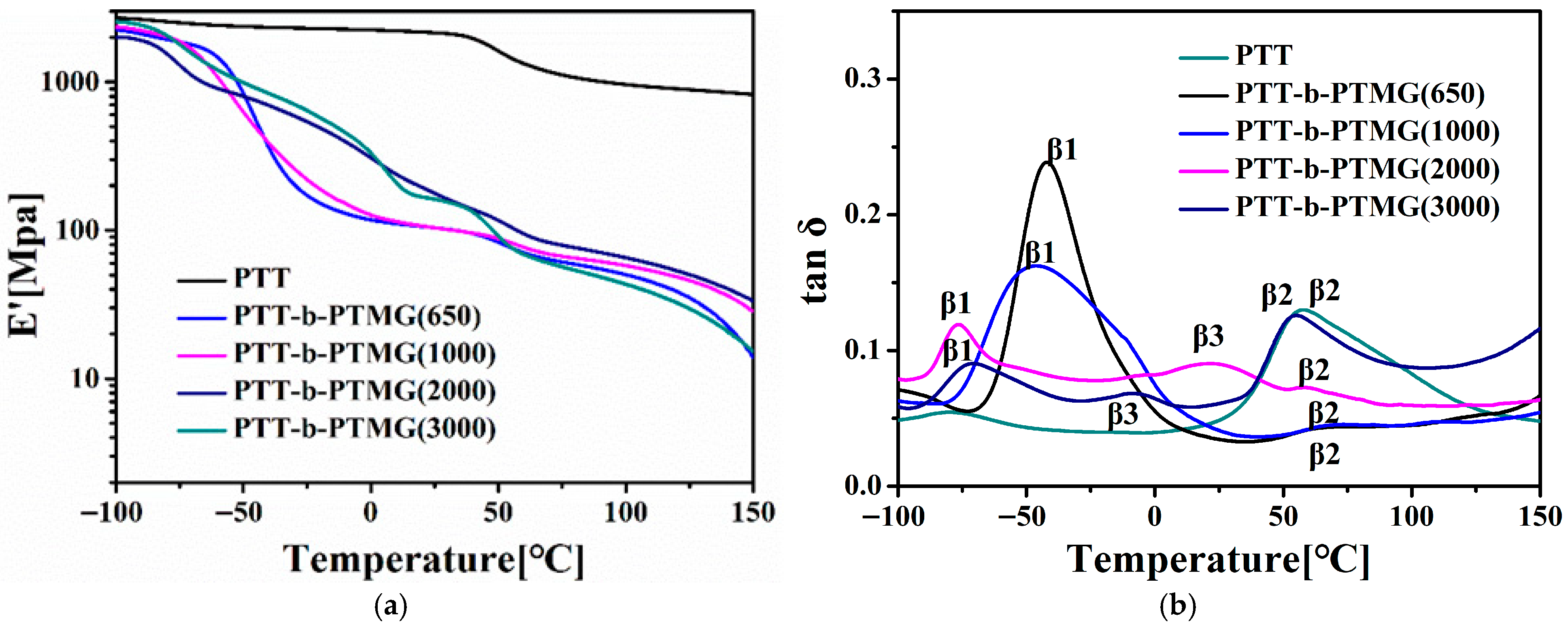
3.4. Thermal Stability of PTT-b-PTMG Copolymers
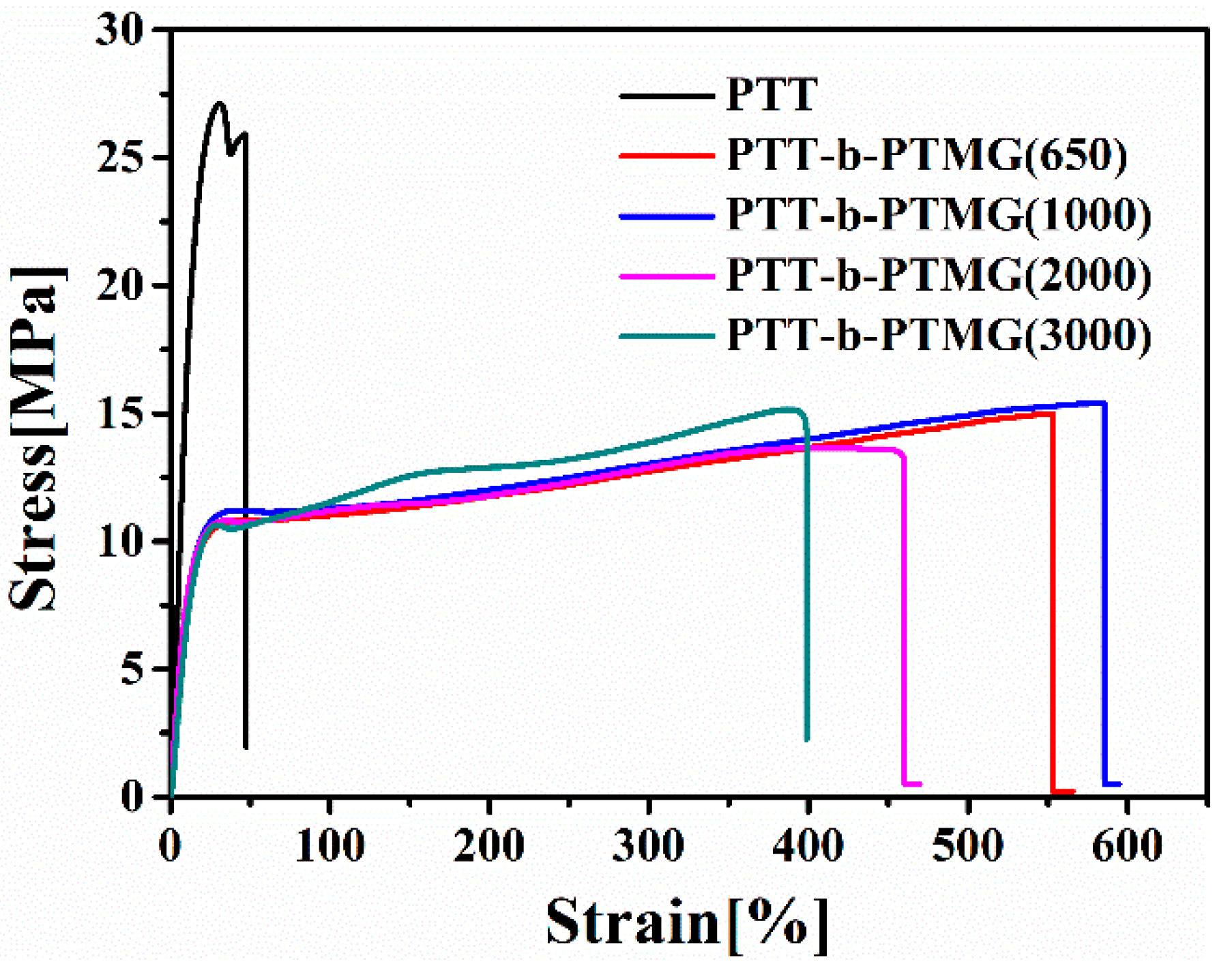
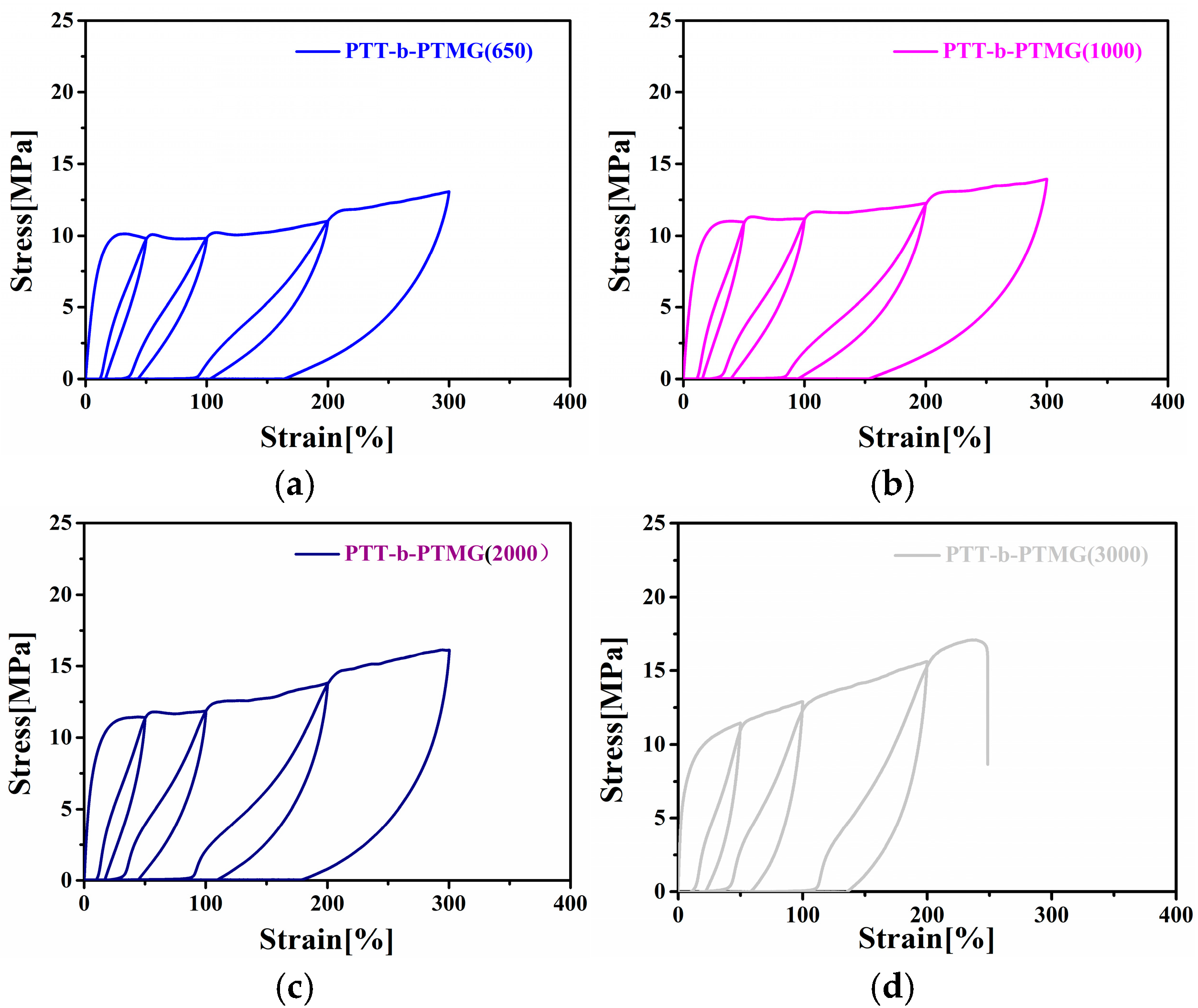
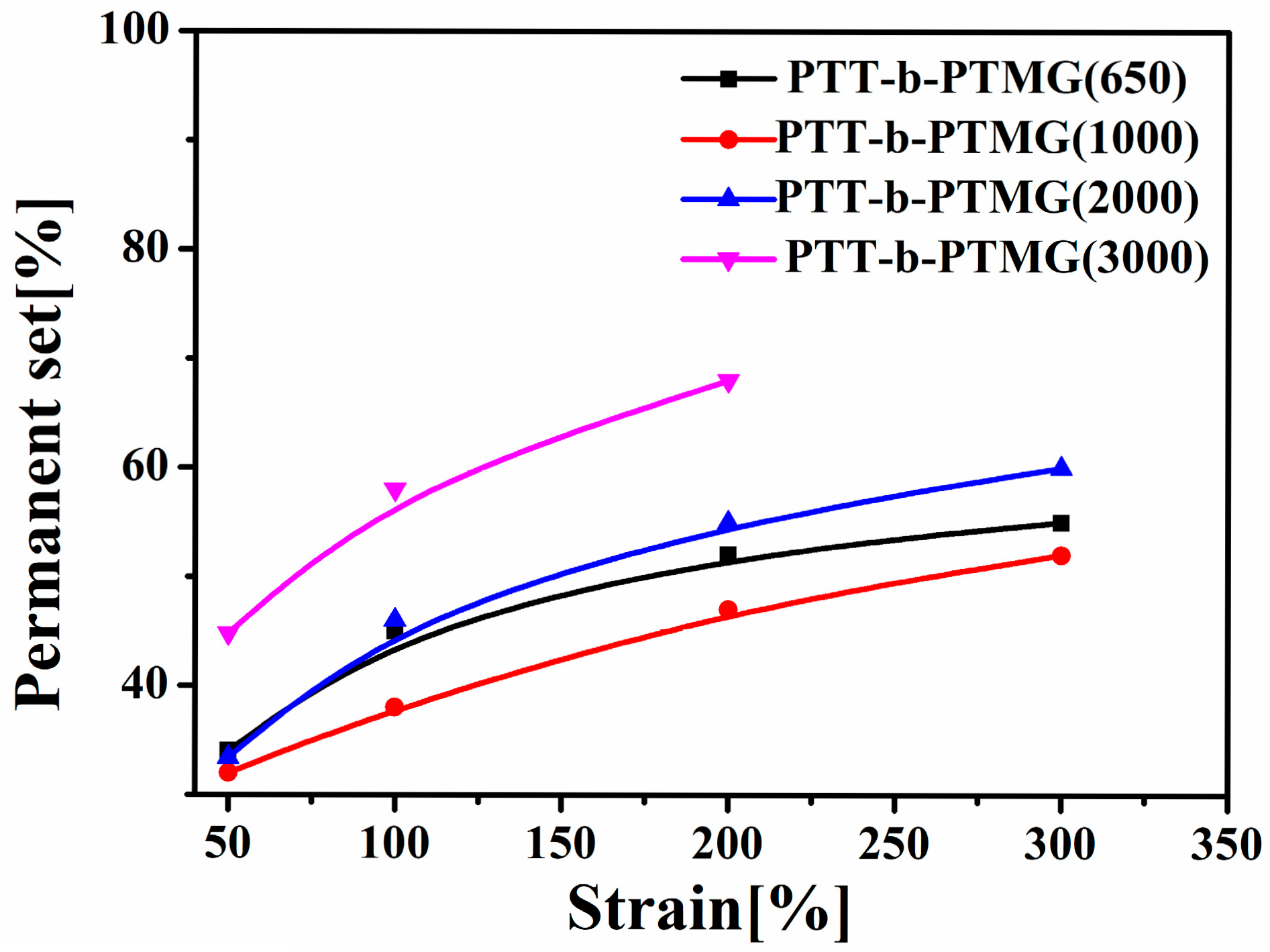
3.5. Mechanical Properties of PTT-b-PTMG Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Xiao, Y.; Wang, W.; Liu, P.W.; Wang, Q.Y.; Jie, S.Y.; Hu, J.; Yao, Z.; Li, B. State-of-the-art and research progress of block-type thermoplastic elastomers. Acta Polym. Sin. 2025, 56, 1463–1479. [Google Scholar] [CrossRef]
- Miah, M.R.; Mahmud, S.; Khan, A.N.; Jalil, M.A.; Wang, J.; Zhu, J. Recent advances in sustainable thermoplastic polyester Elastomers: Synthesis, properties and applications. Polymer 2025, 336, 128878. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Wang, Z.; Zhang, L. Synthesis of novel thermoplastic polyester elastomers with biobased amorphous polyester as the soft segment. Polym. Test. 2023, 124, 108088. [Google Scholar] [CrossRef]
- Stribeck, A.; Eling, B.; Pöselt, E.; Malfois, M.; Schander, E. Melting, Solidification, and Crystallization of a Thermoplastic Polyurethane as a Function of Hard Segment Content. Macromol. Chem. Phys. 2019, 220, 1900074. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Wang, R.; Qu, C.; Ren, C.; Chen, H.; Yin, P. Bio-based functional monomer-based high thermal and mechanical performance TPEE. Eur. Polym. J. 2025, 236, 114105. [Google Scholar] [CrossRef]
- Bonart, R. Thermoplastic elastomers. Polymer 1979, 20, 1389–1403. [Google Scholar] [CrossRef]
- Xiang, F.; Givens, T.M.; Ward, S.M.; Grunlan, J.C. Elastomeric Polymer Multilayer Thin Film with Sustainable Gas Barrier at High Strain. ACS Appl. Mater. Interfaces 2015, 7, 16148–16151. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Rodriguez, I.A.; Wesner, D.; Schönherr, H.; Bowlin, G.L.; Jhurry, D. Poly(ester-ether)s: III. assessment of cell behaviour on nanofibrous scaffolds of PCL, PLLA and PDX blended with amorphous PMeDX. J. Mater. Chem. B 2015, 3, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Masser, H.; Shiau, H.-S.; Liang, S.; Runt, J.; Painter, P.C.; Colby, R.H. Linear Viscoelasticity and Fourier Transform Infrared Spectroscopy of Polyether–Ester–Sulfonate Copolymer Ionomers. Macromolecules 2014, 47, 3635–3644. [Google Scholar] [CrossRef]
- Szymczyk, A.; Roslaniec, Z. Sulfonated poly(ether–block–ester) ionomers with anions in the polyester hard segments. Polym. Adv. Technol. 1999, 10, 579–587. [Google Scholar] [CrossRef]
- Gan, Z.; Bing, D.; Qu, S.; Li, S.; Tan, T.; Yang, J. In situ synthesis of poly(ether ester) via direct polycondensation of terephthalic acid and 1,3-propanediol with sulfonic acids as catalysts. Polym. Chem. 2019, 10, 3629–3638. [Google Scholar] [CrossRef]
- Coleman, D. Block copolymers: Copolymerization of ethylene terephthalate and polyoxyethylene glycols. J. Polym. Sci. 1954, 14, 15–28. [Google Scholar] [CrossRef]
- Tang, S.; Li, J.; Wang, R.; Zhang, J.; Lu, Y.; Hu, G.H.; Wang, Z.; Zhang, L. Current trends in bio-based elastomer materials. SusMat 2022, 2, 2–33. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Fei, X.; Gao, R.; Liu, F.; Fan, L.; Zhu, J.; Liu, X. Synthesis of High Thermal-Resistant Poly(ester-ether) Elastomers from Bio-Based 2,5-Furandicarboxylic Acid. ACS Sustain. Chem. Eng. 2022, 10, 13595–13606. [Google Scholar] [CrossRef]
- Zeilstra, J.J. Influencing the crystallization behavior of PET-based segmented copoly(ether-ester). J. Appl. Polym. Sci. 1986, 31, 1977–1997. [Google Scholar] [CrossRef]
- Stevenson, J.C.; Cooper, S.L. Multiple endothermic melting behavior in poly(tetramethylene terephthalate)-containing polyesters and block copolyether-esters. J. Polym. Sci. Part B Polym. Phys. 1988, 26, 953–966. [Google Scholar] [CrossRef]
- Nagai, Y.; Nakamura, D.; Miyake, T.; Ueno, H.; Matsumoto, N.; Kaji, A.; Ohishi, F. Photodegradation mechanisms in poly(2,6-butylenenaphthalate-co-tetramethyleneglycol) (PBN–PTMG). I: Influence of the PTMG content. Polym. Degrad. Stab. 2005, 88, 251–255. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, M.; Xu, Y.; Wang, W.; Wang, Z.; Zhang, L. Bio-based polyesters: Recent progress and future prospects. Prog. Polym. Sci. 2021, 120, 101430. [Google Scholar] [CrossRef]
- Basak, S. Thermoplastic elastomers in biomedical industry—Evolution and current trends. J. Macromol. Sci. Part A 2021, 58, 579–593. [Google Scholar] [CrossRef]
- Yang, T.; Liu, F.; Gao, R.; Zhang, X.; Li, J.; Wang, J.; Zhu, J. Fabrication of Thermoplastic Poly(ether-ester) Elastomers with High Melting Temperature and Elasticity from Bio-based 2,5-Furandicarboxylic Acid. ACS Sustain. Resour. Manag. 2024, 1, 1520–1533. [Google Scholar] [CrossRef]
- Kurian, J.V. A New Polymer Platform for the Future—Sorona® from Corn Derived 1,3-Propanediol. J. Polym. Environ. 2005, 13, 159–167. [Google Scholar] [CrossRef]
- Xie, Q.; Hu, X.; Hu, T.; Xiao, P.; Xu, Y.; Leffew, K.W. Polytrimethylene Terephthalate: An Example of an Industrial Polymer Platform Development in China. Macromol. React. Eng. 2015, 9, 401–408. [Google Scholar] [CrossRef]
- Szymczyk, A.; Senderek, E.; Nastalczyk, J.; Roslaniec, Z. New multiblock poly(ether-ester)s based on poly(trimethylene terephthalate) as rigid segments. Eur. Polym. J. 2008, 44, 436–443. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Zheng, Z.; Liu, D. 1,3-Propanediol and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk, A.; Nastalczyk, J.; Sablong, R.J.; Roslaniec, Z. The influence of soft segment length on structure and properties of poly(trimethylene terephthalate)-block-poly(tetramethylene oxide) segmented random copolymers. Polym. Adv. Technol. 2010, 22, 72–83. [Google Scholar] [CrossRef]
- Szymczyk, A. Structure and properties of new polyester elastomers composed of poly(trimethylene terephthalate) and poly(ethylene oxide). Eur. Polym. J. 2009, 45, 2653–2664. [Google Scholar] [CrossRef]
- Szymczyk, A. Poly(trimethylene terephthalate-block-tetramethylene oxide) elastomer/single-walled carbon nanotubes nanocomposites: Synthesis, structure, and properties. J. Appl. Polym. Sci. 2012, 126, 796–807. [Google Scholar] [CrossRef]
- Piesowicz, E.; Paszkiewicz, S.; Szymczyk, A. Phase Separation and Elastic Properties of Poly(Trimethylene Terephthalate)-block-poly(Ethylene Oxide) Copolymers. Polymers 2016, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, S.; Szymczyk, A.; Pilawka, R.; Przybyszewski, B.; Czulak, A.; RosŁaniec, Z. Improved Thermal Conductivity of Poly(trimethylene terephthalate-block-poly(tetramethylene oxide) Based Nanocomposites Containing Hybrid Single-Walled Carbon Nanotubes/Graphene Nanoplatelets Fillers. Adv. Polym. Technol. 2015, 36, 236–242. [Google Scholar] [CrossRef]
- Yao, C.; Yang, G. Crystallization-induced aging in poly(trimethylene terephthalate)/poly(ethylene oxide terephthalate) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 411–416. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, J.; Huang, W.; Qu, T.; Li, X.; Li, Y.; Yang, X.; Tu, Y. One Pot, One Feeding Step, Two-Stage Polymerization Synthesis and Characterization of (PTT-b-PTMO-b-PTT)n Multiblock Copolymers. Macromolecules 2013, 46, 7274–7281. [Google Scholar] [CrossRef]
- Wu, M.Y.; Shi, M.L.; Cheng, Y.Q.; Chen, C.F.; Yu, S.H. The study on poly (tetramethylene terephthalate)-poly (tetramethylene ether) multi-block copolymer. Polym. Commun. 1980, 2, 77–83. [Google Scholar]
- Li, Y.; Liu, Y.; Liu, A.; Xu, C.; Zhang, C.; Yu, J.; Yuan, R.; Li, F. Poly(trimethylene terephthalate-b-poly(trimethylene ether) glycol) copolymers: From bio-based thermoplastic elastomers to elastic fibers for apparel. Eur. Polym. J. 2025, 225, 113706. [Google Scholar] [CrossRef]
- Kong, W.; Yang, Y.; Liu, Z.; Jiang, L.; Zhou, C.; Lei, J. Structure–property relations of nylon-6 and polytetramethylene glycol based multiblock copolymers with microphase separation prepared through reactive processing. Polym. Int. 2016, 66, 436–442. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Irska, I.; Pawlikowska, D.; Piesowicz, E. Synthesis, structure, and physical properties of poly(tri-methylene terephthalate)-block-poly(caprolactone) copolymers. J. Appl. Polym. Sci. 2018, 136, 47341. [Google Scholar] [CrossRef]
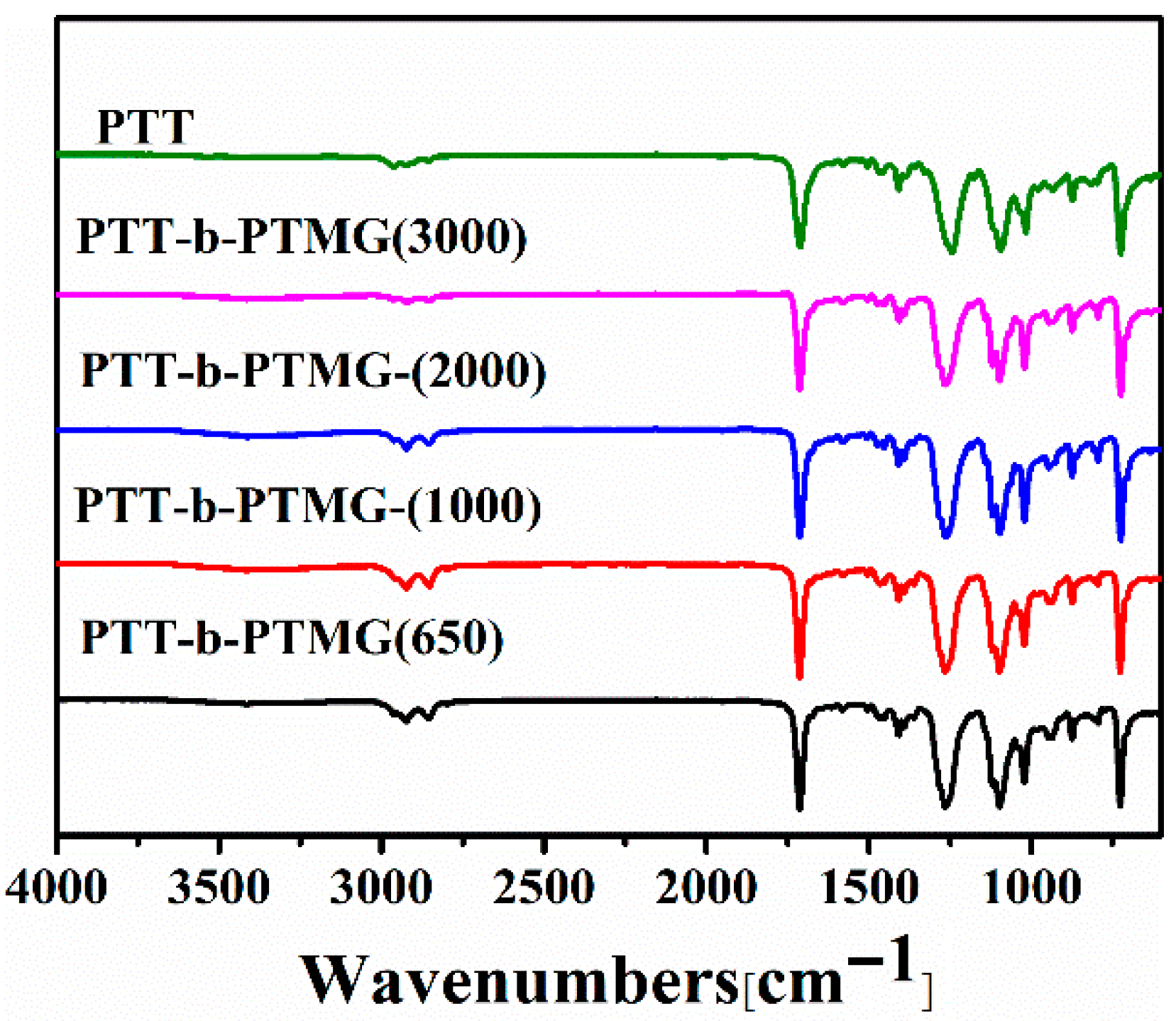

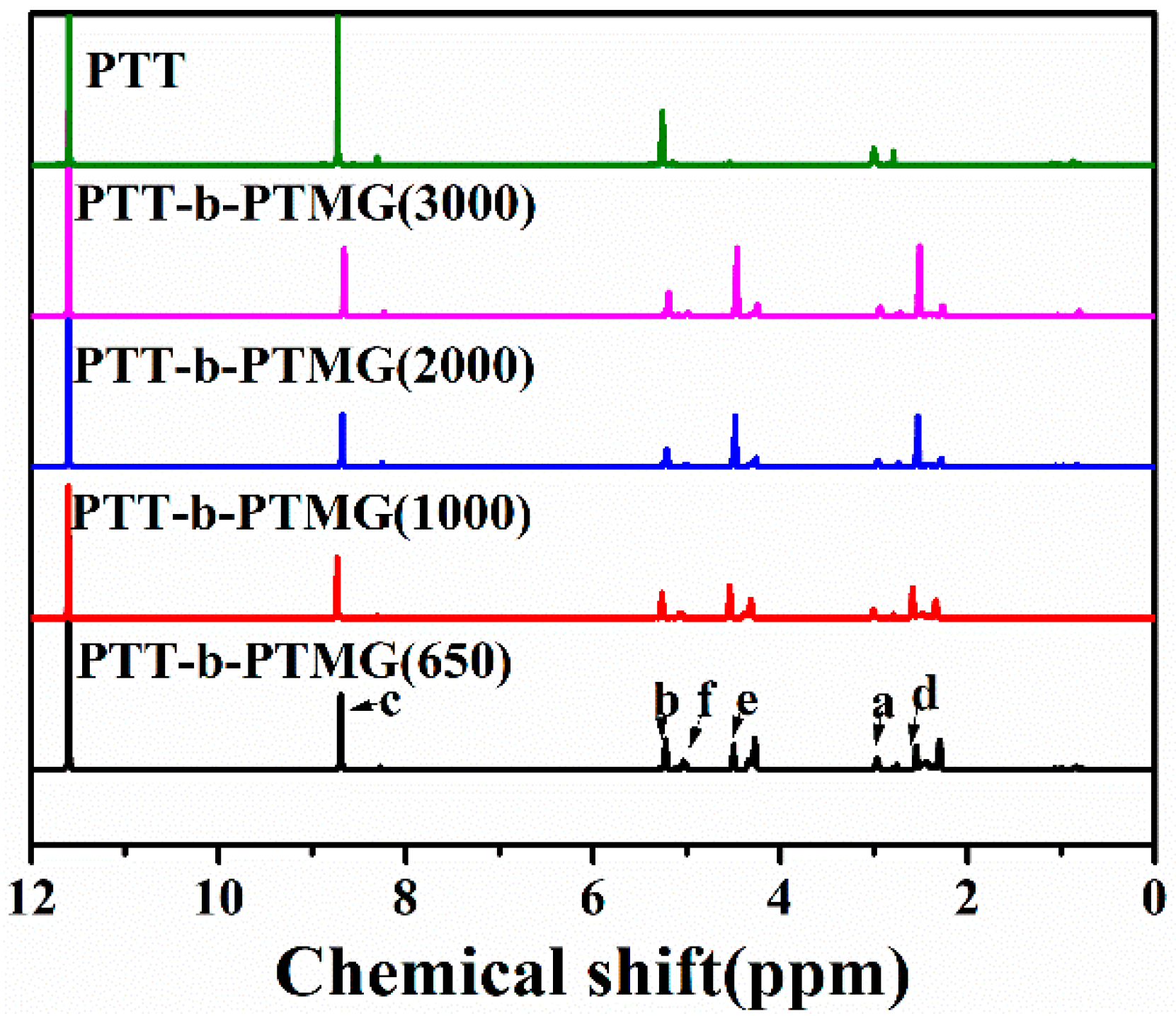
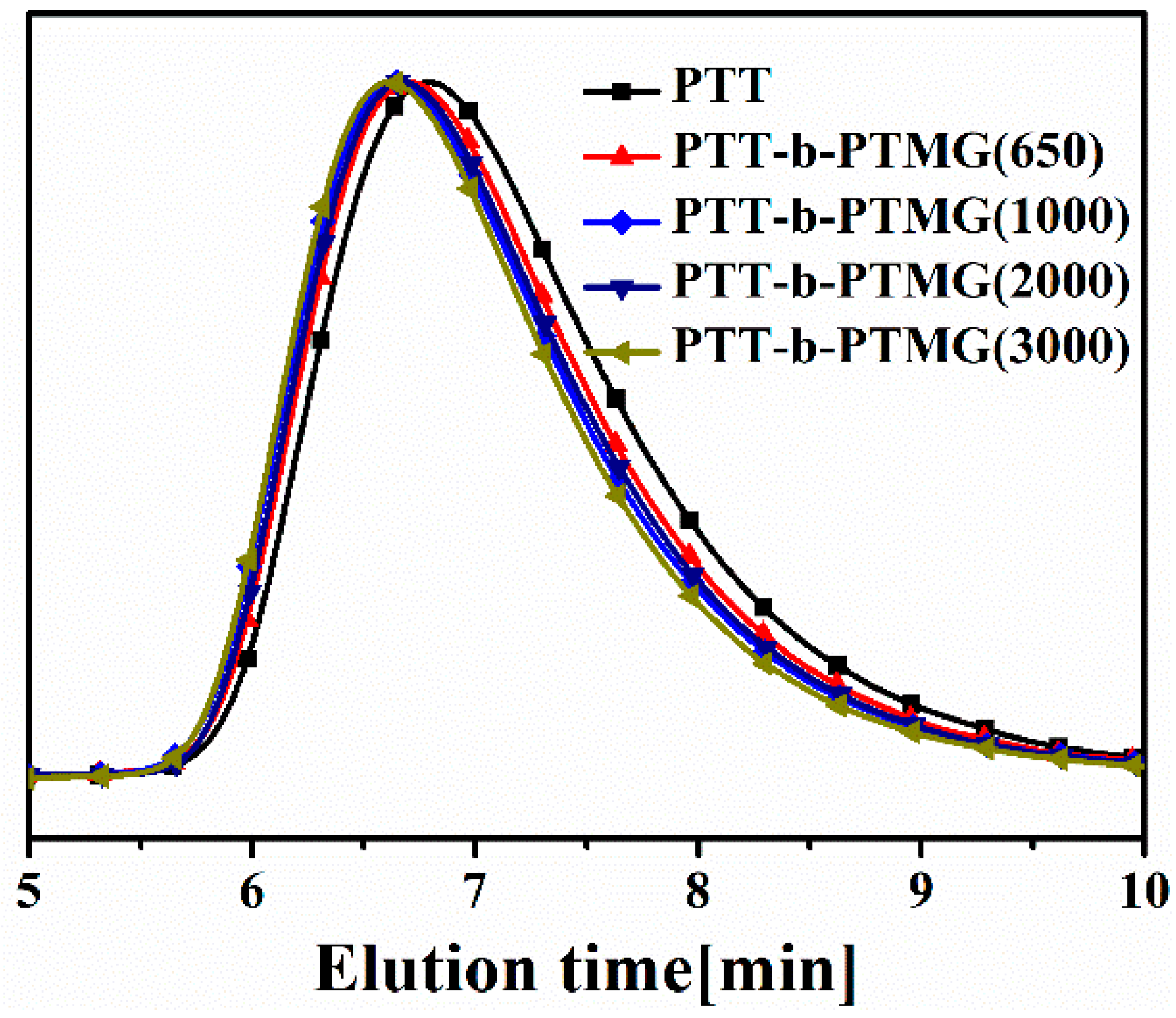


| Sample | Ws (wt%) | Wh (wt%) | IV | Mn × 103 (g/mol) | Mw × 103 (g/mol) | Mw/Mn | Ln,T | RC |
|---|---|---|---|---|---|---|---|---|
| PTT | - | - | 0.92 | 38.6 | 71.3 | 1.84 | - | - |
| PTT-b-PTMG(650) | 54.8 | 45.2 | 1.22 | 41.2 | 76.6 | 1.86 | 4 | 0.44 |
| PTT-b-PTMG(1000) | 49.6 | 50.4 | 1.27 | 43.9 | 81.3 | 1.85 | 6.5 | 0.43 |
| PTT-b-PTMG(2000) | 46.5 | 53.5 | 1.23 | 42.5 | 79.9 | 1.88 | 12.5 | 0.41 |
| PTT-b-PTMG(3000) | 45.7 | 54.3 | 1.25 | 43.6 | 82.8 | 1.90 | 20.0 | 0.36 |
| Sample | Tm/°C | ΔHm (J·g−1) | Tp/°C | ΔHc (J·g−1) |
|---|---|---|---|---|
| PTT | 224.0 | 43.5 | 187.8 | −46.7 |
| PTT-b-PTMG(650) | 181.0 | 21.4 | 108.9 | −28.5 |
| PTT-b-PTMG(1000) | 197.0 | 20.6 | 137.7 | −29.1 |
| PTT-b-PTMG(2000) | 219.3 | 17.9 | 151.6 | −26.6 |
| PTT-b-PTMG(3000) | 224.0 | 17.5 | 166.3 | −23.6 |
| Sample | T5%/°C | T25%/°C | T50%/°C | Tmax/°C | Wf/% | Ea (R) (kJ/mol) |
|---|---|---|---|---|---|---|
| PTT | 367.5 | 388.0 | 399.3 | 399.7 | 6.8 | 382 (0.996) |
| PTT-b-PTMG(650) | 361.3 | 387.0 | 398.5 | 400.5 | 2.8 | 374 (0.997) |
| PTT-b-PTMG(1000) | 360.2 | 386.6 | 399.6 | 400.3 | 2.5 | 380 (0.995) |
| PTT-b-PTMG(2000) | 360.8 | 387.7 | 399.5 | 400.5 | 3.0 | 376 (0.996) |
| PTT-b-PTMG(3000) | 366.0 | 389.8 | 400.6 | 401.2 | 2.5 | 390 (0.997) |
| Sample | E/MPa | σ/Mpa | ε/% |
|---|---|---|---|
| PTT | 2012 ± 3.2 | 26 ± 0.3 | 49 ± 10 |
| PTT-b-PTMG(650) | 105 ± 3.5 | 15 ± 0.2 | 551 ± 16 |
| PTT-b-PTMG(1000) | 107 ± 2.6 | 14 ± 0.3 | 585 ± 14 |
| PTT-b-PTMG(2000) | 184 ± 2.1 | 15 ± 0.2 | 459 ± 14 |
| PTT-b-PTMG(3000) | 215 ± 2.3 | 15 ± 0.5 | 393 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Tian, Y.; Li, J.; Shi, J.; Kuang, J.; Zhou, W.; Chen, Y. The Effects of Soft-Segment Molecular Weight on the Structure and Properties of Poly(trimethylene terephthalate)-block-poly(tetramethylene glycol) Copolymers. Polymers 2025, 17, 2781. https://doi.org/10.3390/polym17202781
Dong H, Tian Y, Li J, Shi J, Kuang J, Zhou W, Chen Y. The Effects of Soft-Segment Molecular Weight on the Structure and Properties of Poly(trimethylene terephthalate)-block-poly(tetramethylene glycol) Copolymers. Polymers. 2025; 17(20):2781. https://doi.org/10.3390/polym17202781
Chicago/Turabian StyleDong, Hailiang, Yuchuang Tian, Junyu Li, Jiyou Shi, Jun Kuang, Wenle Zhou, and Ye Chen. 2025. "The Effects of Soft-Segment Molecular Weight on the Structure and Properties of Poly(trimethylene terephthalate)-block-poly(tetramethylene glycol) Copolymers" Polymers 17, no. 20: 2781. https://doi.org/10.3390/polym17202781
APA StyleDong, H., Tian, Y., Li, J., Shi, J., Kuang, J., Zhou, W., & Chen, Y. (2025). The Effects of Soft-Segment Molecular Weight on the Structure and Properties of Poly(trimethylene terephthalate)-block-poly(tetramethylene glycol) Copolymers. Polymers, 17(20), 2781. https://doi.org/10.3390/polym17202781






