Recycling of Polyurethanes via Covalent Adaptable Networks: The Role of Crosslink Density in Performance Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyurethane Networks
2.3. Reprocessing Conditions
2.4. Characterisation
3. Results and Discussion
3.1. Characterisation of Pristine Networks
3.2. Reprocessing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guerre, M.; Taplan, C.; Winne, J.M.; Du Prez, F.E. Vitrimers: Directing Chemical Reactivity to Control Material Properties. Chem. Sci. 2020, 11, 4855–4870. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, C.J.; Bowman, C.N. Covalent Adaptable Networks: Smart, Reconfigurable and Responsive Network Systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef]
- Nellepalli, P.; Patel, T.; Oh, J.K. Dynamic Covalent Polyurethane Network Materials: Synthesis and Self-Healability. Macromol. Rapid Commun. 2021, 42, 2100391. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef]
- Mcbride, M.K.; Worrell, B.T.; Brown, T.; Cox, L.M.; Sowan, N.; Wang, C.; Podgorski, M.; Martinez, A.M.; Bowman, C.N. Enabling Applications of Covalent Adaptable Networks. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 175–198. [Google Scholar] [CrossRef]
- Zheng, J.; Png, Z.M.; Ng, S.H.; Tham, G.X.; Ye, E.; Goh, S.S.; Loh, X.J.; Li, Z. Vitrimers: Current Research Trends and Their Emerging Applications. Mater. Today 2021, 51, 586–625. [Google Scholar] [CrossRef]
- Plastic Europe Plastics-the Fast Facts. 2024. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2024/ (accessed on 14 June 2025).
- Miravalle, E.; Bracco, P.; Brunella, V.; Barolo, C.; Zanetti, M. Improving Sustainability through Covalent Adaptable Networks in the Recycling of Polyurethane Plastics. Polymers 2023, 15, 3780. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Nevejans, S.; Reck, B.; Irusta, L.; Sardon, H.; Asua, J.M.; Ballard, N. Healable and Self-Healing Polyurethanes Using Dynamic Chemistry. Prog. Polym. Sci. 2021, 114, 101362. [Google Scholar] [CrossRef]
- Dong, J.; Yan, H.; Lv, X.; Wang, Z.; Rao, Z.; Zhu, B.; Wu, J.; Zhou, Y.; Chen, H. Reprocessable Polyurethane Elastomers Based on Reversible Ketal Exchange: Dielectric Properties and Water Resistance. J. Mater. Chem. C Mater. 2022, 11, 1369–1380. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Li, J.; Zhang, K. Catalyst-Free Room-Temperature Self-Healing Polymer Networks Based on Dynamic Covalent Quinone Methide-Secondary Amine Chemistry. Polym. Chem. 2021, 12, 6161–6166. [Google Scholar] [CrossRef]
- Li, J.; Ning, Z.; Yang, W.; Yang, B.; Zeng, Y. Hydroxyl-Terminated Polybutadiene-Based Polyurethane with Self-Healing and Reprocessing Capabilities. ACS Omega 2022, 7, 10156–10166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Yao, W.; Dong, Y.; Zhang, B.; Dong, X.; Fang, H.; Zhang, G.; Ding, Y. Dynamically Cross-Linked Waterborne Polyurethanes: Transalkylation Exchange of C-N Bonds Toward High Performance and Reprocessable Thermosets. ACS Appl. Polym. Mater. 2022, 4, 5920–5926. [Google Scholar] [CrossRef]
- Jia, Y.; Guan, Q.; Zhang, L.; Neisiany, R.E.; Yan, N.; Li, Y.; You, Z. A Fluorine-Rich Phenolic Polyurethane Elastomer with Excellent Self-Healability and Reprocessability and Its Applications for Wearable Electronics. Sci. China Mater. 2022, 65, 2553–2564. [Google Scholar] [CrossRef]
- Chen, X.; Hu, S.; Li, L.; Torkelson, J.M. Dynamic Covalent Polyurethane Networks with Excellent Property and Cross-Link Density Recovery after Recycling and Potential for Monomer Recovery. ACS Appl. Polym. Mater. 2020, 2, 2093–2101. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, T.; Guo, B.; Xu, J. Solvent-Free Thermo-Reversible and Self-Healable Crosslinked Polyurethane with Dynamic Covalent Networks Based on Phenol-Carbamate Bonds. Polymer 2019, 181, 121788. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Mechanically Robust, Self-Healable, and Highly Stretchable “Living” Crosslinked Polyurethane Based on a Reversible C–C Bond. Adv. Funct. Mater. 2018, 28, 1706050. [Google Scholar] [CrossRef]
- Miravalle, E.; Viada, G.; Bonomo, M.; Barolo, C.; Bracco, P.; Zanetti, M. Recycling of Commercially Available Biobased Thermoset Polyurethane Using Covalent Adaptable Network Mechanisms. Polymers 2024, 16, 2217. [Google Scholar] [CrossRef]
- Hao, X.Y.; Yu, B.; Li, L.; Ju, H.; Tian, M.; Cao, P.F. Semi-Interpenetrating Polyurethane Network with Fatigue Elimination and Upcycled Mechanical Performance. Macromolecules 2024, 57, 5063–5072. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, H.; Wang, Y.; Ye, J.; Guo, L.; He, L.; Li, X.; Qiu, T.; Tuo, X. Dual Dynamic Network System Constructed by Waterborne Polyurethane for Improved and Recoverable Performances. Chem. Eng. J. 2022, 442, 136204. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, T.; Zhang, Y.; Guo, B.; Xu, J. Cross-Linked Polyurethane with Dynamic Phenol-Carbamate Bonds: Properties Affected by the Chemical Structure of Isocyanate. Polym. Chem. 2021, 12, 2421–2432. [Google Scholar] [CrossRef]
- Serna, S.; Purwanto, N.S.; Fenimore, L.M.; Torkelson, J.M. Increasing the Cross-Link Density in a Dual Dissociative and Associative Polythiourethane Covalent Adaptable Network Improves Both Creep Resistance and Extrudability. Polymer 2024, 306, 127232. [Google Scholar] [CrossRef]
- Fenimore, L.M.; Suazo, M.J.; Torkelson, J.M. Covalent Adaptable Networks Made by Reactive Processing of Highly Entangled Polymer: Synthesis-Structure-Thermomechanical Property-Reprocessing Relationship in Covalent Adaptable Networks. Macromolecules 2024, 57, 2756–2772. [Google Scholar] [CrossRef]
- Koenig, A.L.; Allis, K.M.; Lehr, J.S.; Larsen, M.B. Effects of Crosslink Density and Plasticizer on Thermorheological Properties of Dissociative Guanidine-Based Covalent Adaptable Networks. Polym. Chem. 2024, 16, 52–61. [Google Scholar] [CrossRef]
- Levita, G.; De Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink Density and Fracture Toughness of Epoxy Resins. J. Mater. Sci. 1991, 26, 2348–2352. [Google Scholar] [CrossRef]
- Schroeder, J.A.; Madsen, P.A.; Foister, R.T. Structure/Property Relationships for a Series of Crosslinked Aromatic/Aliphatic Epoxy Mixtures. Polymer 1987, 28, 929–940. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Jin, K.; Torkelson, J.M. Vitrimers Designed Both to Strongly Suppress Creep and to Recover Original Cross-Link Density after Reprocessing: Quantitative Theory and Experiments. Macromolecules 2018, 51, 5537–5546. [Google Scholar] [CrossRef]
- Chen, Y.; Purwanto, N.S.; Chen, B.; Wang, T.; Kim, S.; Huang, Y.-W.; Dichtel, W.R.; Torkelson, J.M. Biobased, Catalyst-Free Non-Isocyanate Polythiourethane Foams Highly. Chem. Eng. J. 2024, 496, 154035. [Google Scholar] [CrossRef]
- ASTM D638–24; Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2024.
- Chen, W.; Zhu, C.; Gu, X. Thermosetting Polyurethanes with Water-Swollen and Shape Memory Properties. J. Appl. Polym. Sci. 2002, 84, 1504–1512. [Google Scholar] [CrossRef]
- Fridrihsone, A.; Stirna, U.; Lazdiņa, B.; Misane, M.; Vilsone, D. Characterization of Polyurethane Networks Structure and Properties Based on Rapeseed Oil Derived Polyol. Eur. Polym. J. 2013, 49, 1204–1214. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Datta, J. Novel Bio-Based Thermoplastic Poly(Ether-Urethane)s. Correlations between the Structure, Processing and Properties. Polymer 2019, 160, 1–10. [Google Scholar] [CrossRef]
- Oenema, J.; Liu, H.; De Coensel, N.; Eschenbacher, A.; Van de Vijver, R.; Weng, J.; Li, L.; Wang, C.; Van Geem, K.M. Review on the Pyrolysis Products and Thermal Decomposition Mechanisms of Polyurethanes. J. Anal. Appl. Pyrolysis 2022, 168, 105723. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal Stability and Flame Retardancy of Polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Deka, H.; Karak, N. Bio-Based Hyperbranched Polyurethanes for Surface Coating Applications. Prog. Org. Coat. 2009, 66, 192–198. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A Systematic Study Substituting Polyether Polyol with Palm Kernel Oil Based Polyester Polyol in Rigid Polyurethane Foam. Ind. Crops Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Holzworth, K.; Jia, Z.; Amirkhizi, A.V.; Qiao, J.; Nemat-Nasser, S. Effect of Isocyanate Content on Thermal and Mechanical Properties of Polyurea. Polymer 2013, 54, 3079–3085. [Google Scholar] [CrossRef]
- Trzebiatowska, P.J.; Echart, A.S.; Correas, T.C.; Eceiza, A.; Datta, J. The Changes of Crosslink Density of Polyurethanes Synthesised with Using Recycled Component. Chemical Structure and Mechanical Properties Investigations. Prog. Org. Coat. 2018, 115, 41–48. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, L.; Wu, Q.; Wu, J. A Recyclable Polyurethane with Characteristic Thermal Stiffening Behavior via B-N Coordination with Reversible B-O Bonds. Sci. China Mater. 2024, 67, 3339–3346. [Google Scholar] [CrossRef]
- Zajac, M.; Kahl, H.; Schade, B.; Rödel, T.; Dionisio, M.; Beiner, M. Relaxation Behavior of Polyurethane Networks with Different Composition and Crosslinking Density. Polymer 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Fang, Z.; Zheng, N.; Zhao, Q.; Xie, T. Healable, Reconfigurable, Reprocessable Thermoset Shape Memory Polymer with Highly Tunable Topological Rearrangement Kinetics. ACS Appl. Mater. Interfaces 2017, 9, 22077–22082. [Google Scholar] [CrossRef] [PubMed]
- Parada, G.A.; Zhao, X. Ideal Reversible Polymer Networks. Soft. Matter. 2018, 14, 5186–5196. [Google Scholar] [CrossRef] [PubMed]


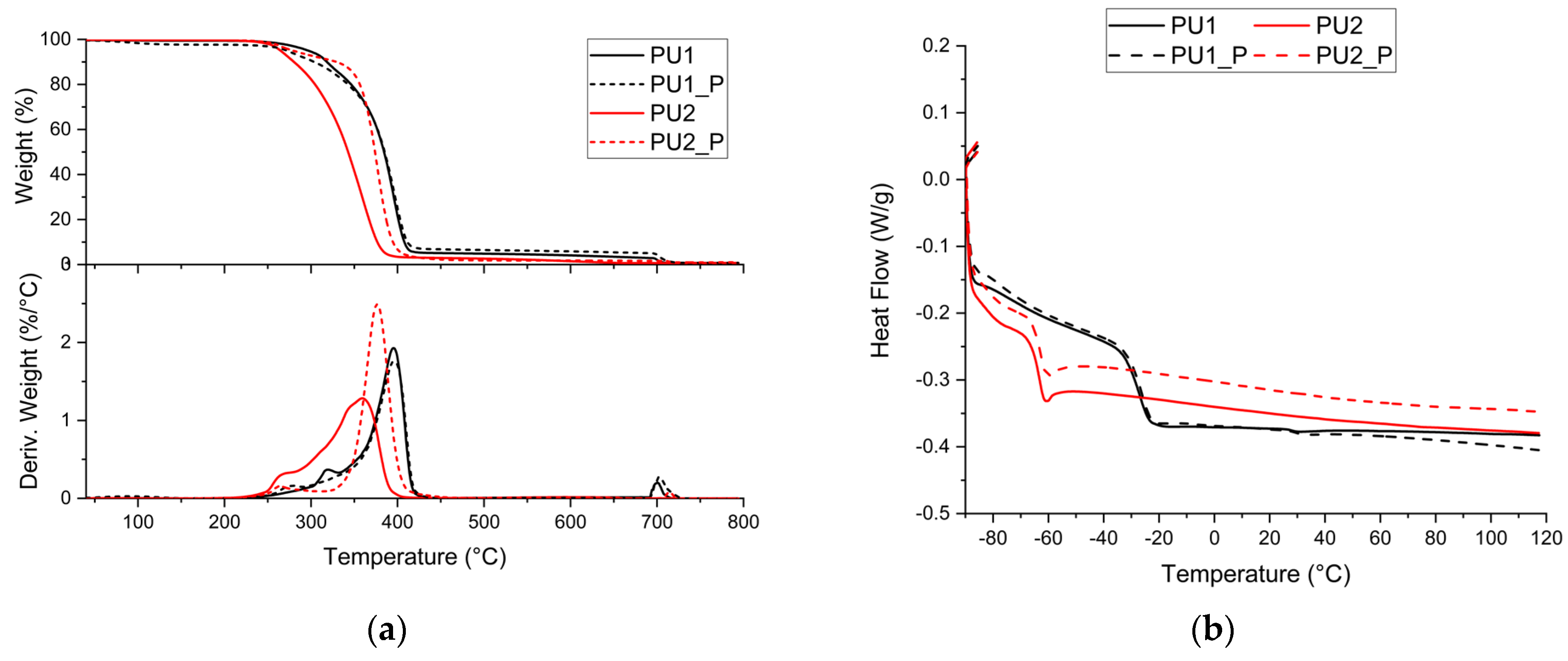
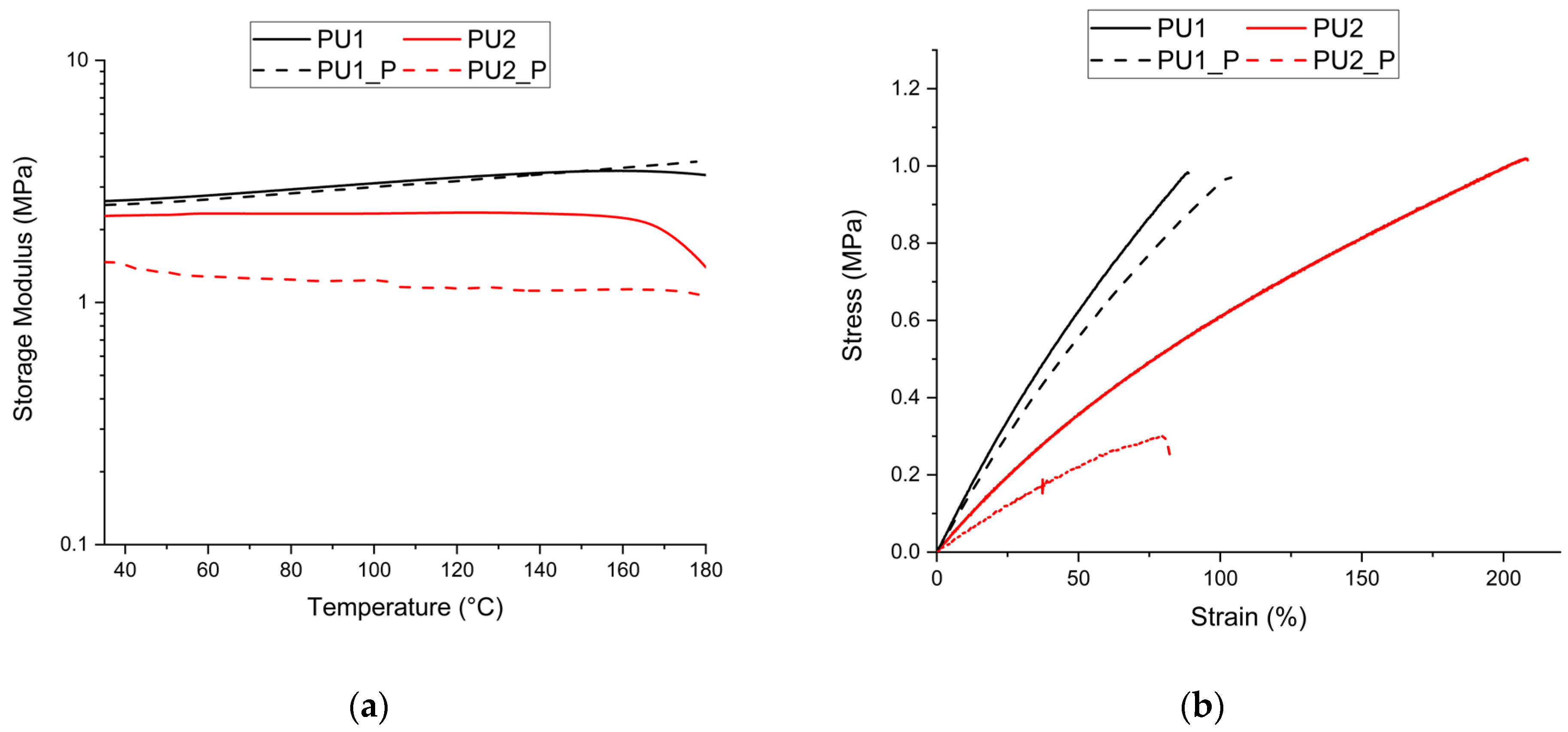


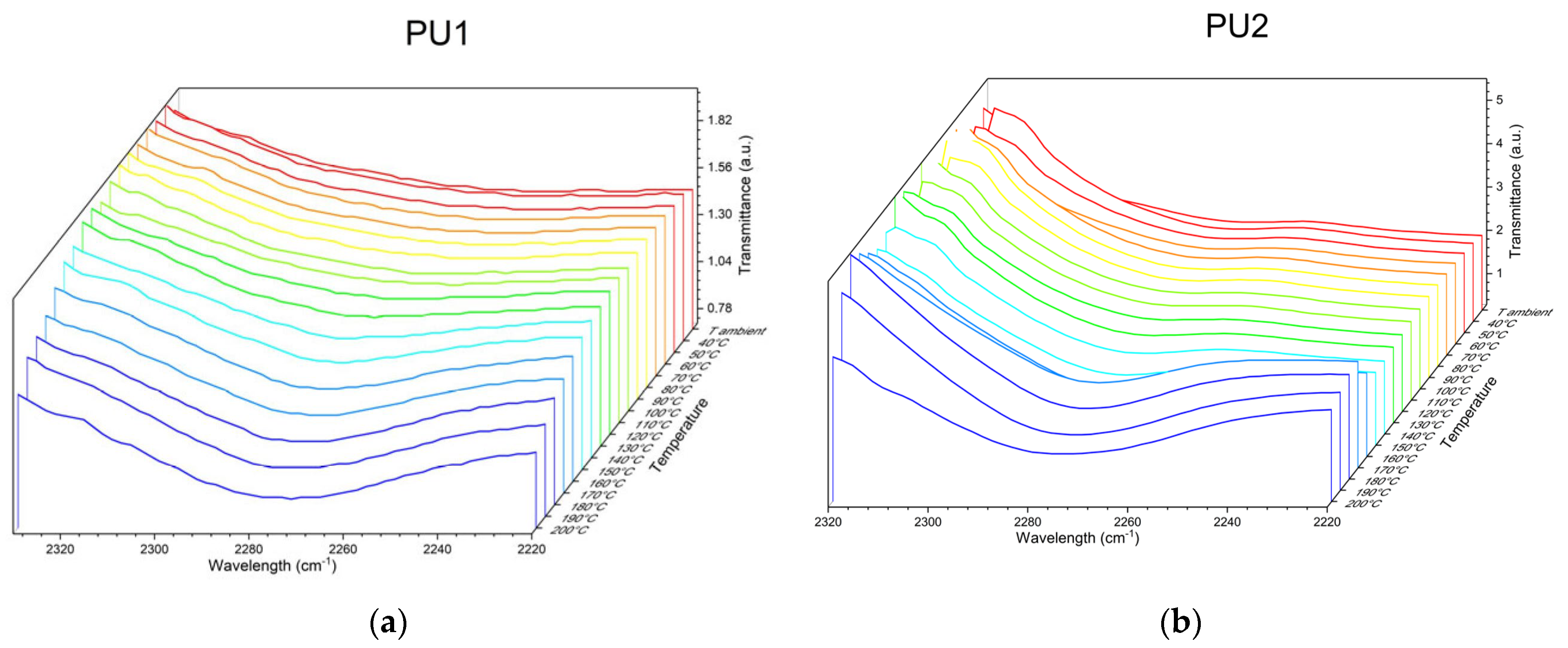
| Sample | T5% (°C) | Tmax (°C) | Tg (°C) | Swelling (%) | Mass Loss (%) |
|---|---|---|---|---|---|
| PU1 | 297 | 396 | −27 | 196 ± 4 | 8.3 ± 0.8 |
| PU1_P | 287 | 396 | −26 | 209 ± 15 | 7.7 ± 0.2 |
| PU2 | 265 | 360 | −63 | 242 ± 2 | 4.4 ± 0.5 |
| PU2_P | 280 | 377 | −63 | 287 ± 3 | 7.2 ± 0.4 |
| Sample | τKWW | β | R2 | <τ> (s) | EaKWW (KJ/mol) | τ* (s) | Ea (KJ/mol) |
|---|---|---|---|---|---|---|---|
| PU1 150 °C | 3141 | 0.91 | 0.99 | 3276 | 64.6 | 3379.2 | 88.5 |
| PU1 160 °C | 2467 | 0.87 | 0.99 | 2639 | 2565 | ||
| PU1 170 °C | 1347 | 0.89 | 0.99 | 1426 | 1080 | ||
| PU2 150 °C | 1693 | 1 | 0.97 | 1693 | 127.7 | 1510.2 | 124.8 |
| PU2 160 °C | 939 | 1 | 0.98 | 939 | 1089.6 | ||
| PU2 170 °C | 317 | 1 | 0.99 | 317 | 586.2 |
| Sample | Tensile Strength (MPa) | RIσ (%) | Elongation at Break (%) | RIε (%) | Storage Modulus (Mpa) | RIm (%) | υe (mol/m3) |
|---|---|---|---|---|---|---|---|
| PU1 | 0.93 ± 0.12 | 90 ± 16 | 3.05 ± 0.47 | 379 ± 58 | |||
| PU1_P | 1.00 ± 0.08 | 108 | 103 ± 10 | 114 | 2.88 ± 0.26 | 95 | 358 ± 32 |
| PU2 | 0.96 ± 0.14 | 197 ± 35 | 2.30 ± 0.27 | 286 ± 33 | |||
| PU2_P | 0.36 ± 0.07 | 38 | 83 ± 18 | 42 | 1.33 ± 0.15 | 58 | 165 ± 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miravalle, E.; Olariu, T.A.; Cecone, C.; Brunella, V.; Bracco, P.; Zanetti, M. Recycling of Polyurethanes via Covalent Adaptable Networks: The Role of Crosslink Density in Performance Recovery. Polymers 2025, 17, 2778. https://doi.org/10.3390/polym17202778
Miravalle E, Olariu TA, Cecone C, Brunella V, Bracco P, Zanetti M. Recycling of Polyurethanes via Covalent Adaptable Networks: The Role of Crosslink Density in Performance Recovery. Polymers. 2025; 17(20):2778. https://doi.org/10.3390/polym17202778
Chicago/Turabian StyleMiravalle, Edoardo, Teodora Andra Olariu, Claudio Cecone, Valentina Brunella, Pierangiola Bracco, and Marco Zanetti. 2025. "Recycling of Polyurethanes via Covalent Adaptable Networks: The Role of Crosslink Density in Performance Recovery" Polymers 17, no. 20: 2778. https://doi.org/10.3390/polym17202778
APA StyleMiravalle, E., Olariu, T. A., Cecone, C., Brunella, V., Bracco, P., & Zanetti, M. (2025). Recycling of Polyurethanes via Covalent Adaptable Networks: The Role of Crosslink Density in Performance Recovery. Polymers, 17(20), 2778. https://doi.org/10.3390/polym17202778









