Arabinoxylan-Based Bioplastic from Wheat Bran: A Promising Replacement for Synthetic Plastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Procedures
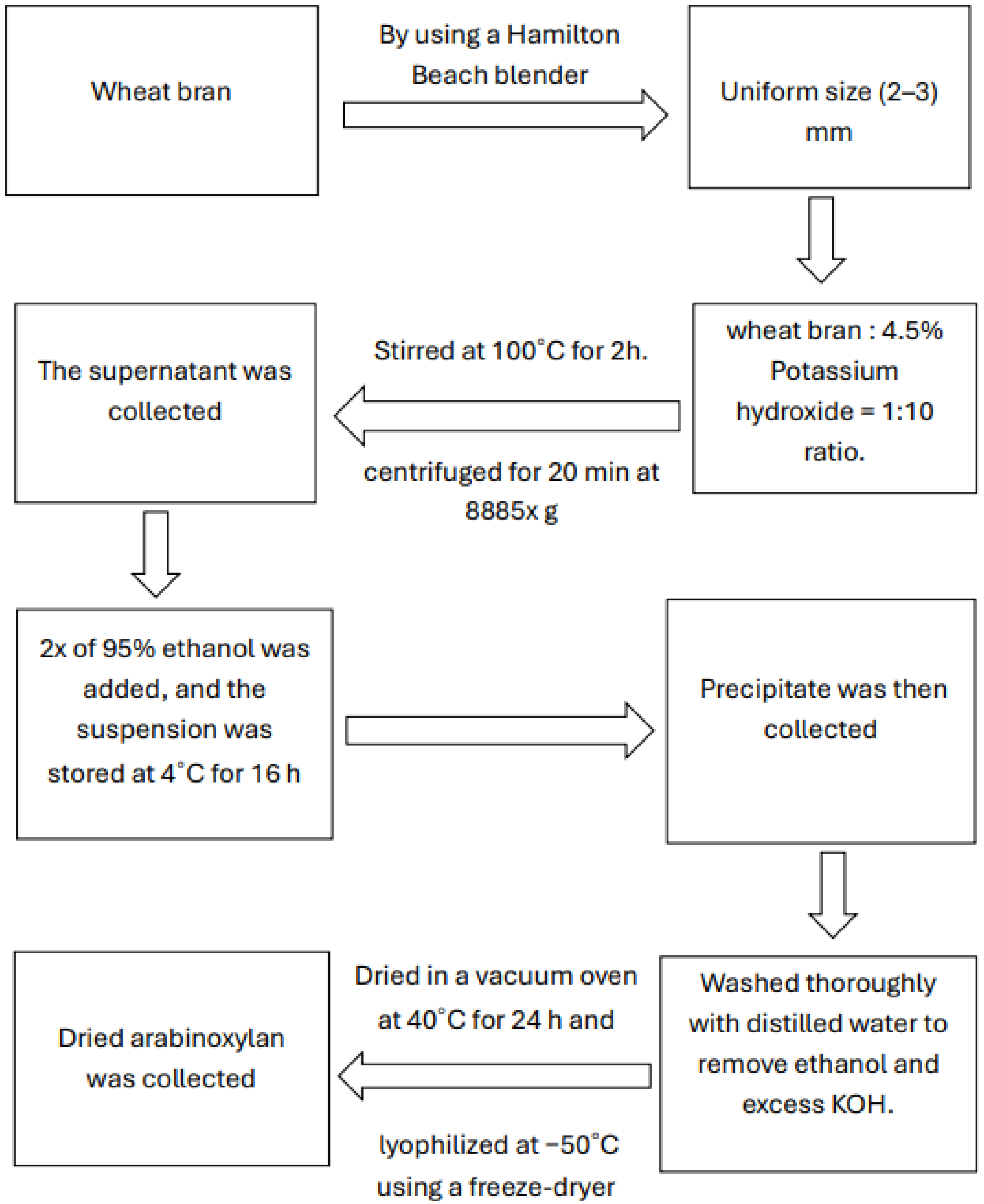
2.2.1. Wheat Bran Collection and Extraction of Arabinoxylan
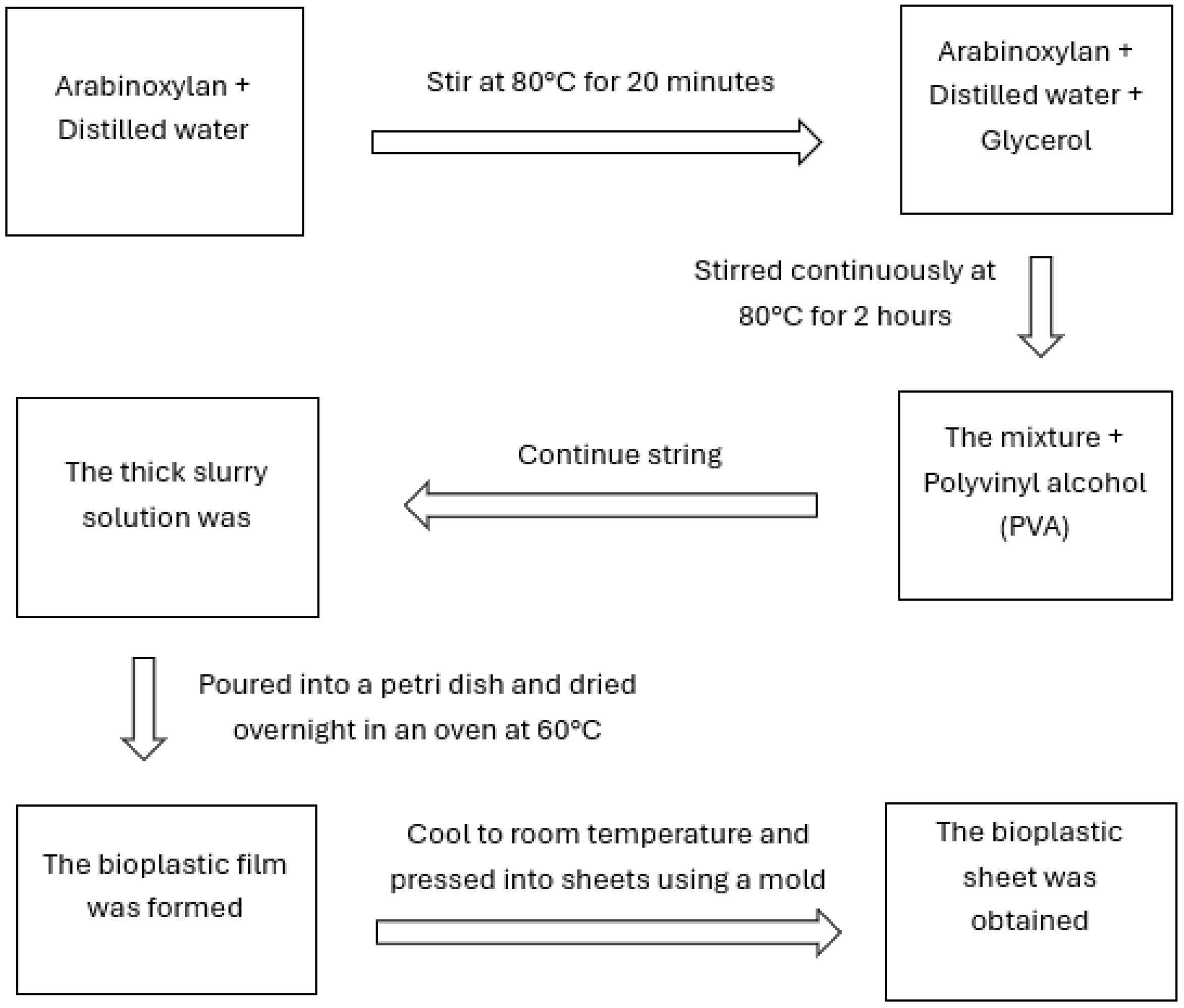
2.2.2. Bioplastic Synthesis
2.3. Characterization of the Synthesized Bioplastics
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.2. Mechanical Properties
2.3.3. Scanning Electron Microscope (SEM) Analysis
2.3.4. Film’s Thickness Measurements
2.3.5. Film’s Transparency
2.3.6. Film’s General Appearance
2.3.7. Water Contact Angle
2.3.8. Water Absorption Percentage
2.3.9. Effect of Acid
2.3.10. Effect of Alkalis
2.3.11. Biodegradability Test
3. Results
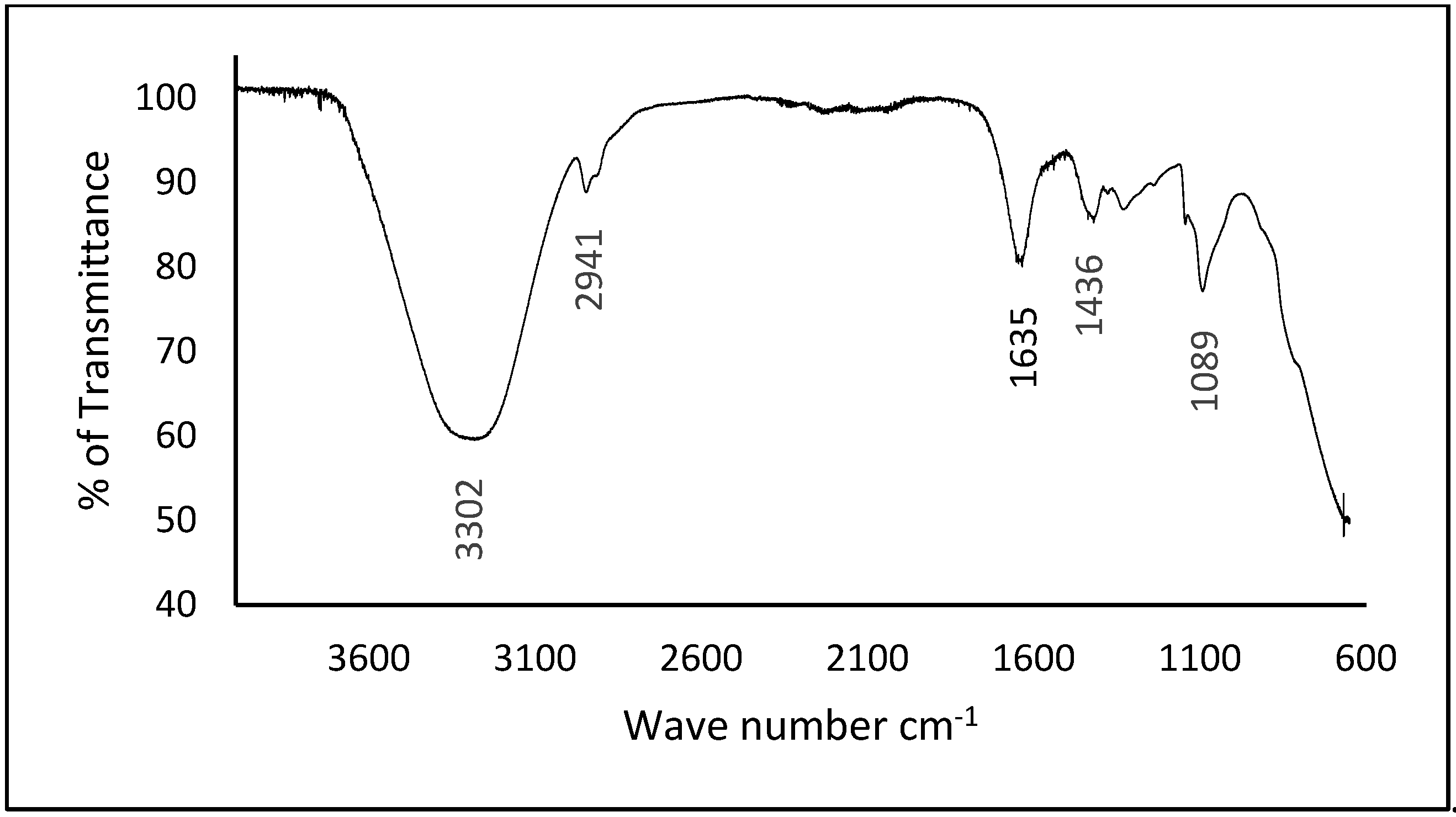
3.1. Fourier Transform Infrared (FTIR) Spectroscopy of WBAX and WBAX Bioplastic
3.2. Mechanical Properties of Synthesized Bioplastic
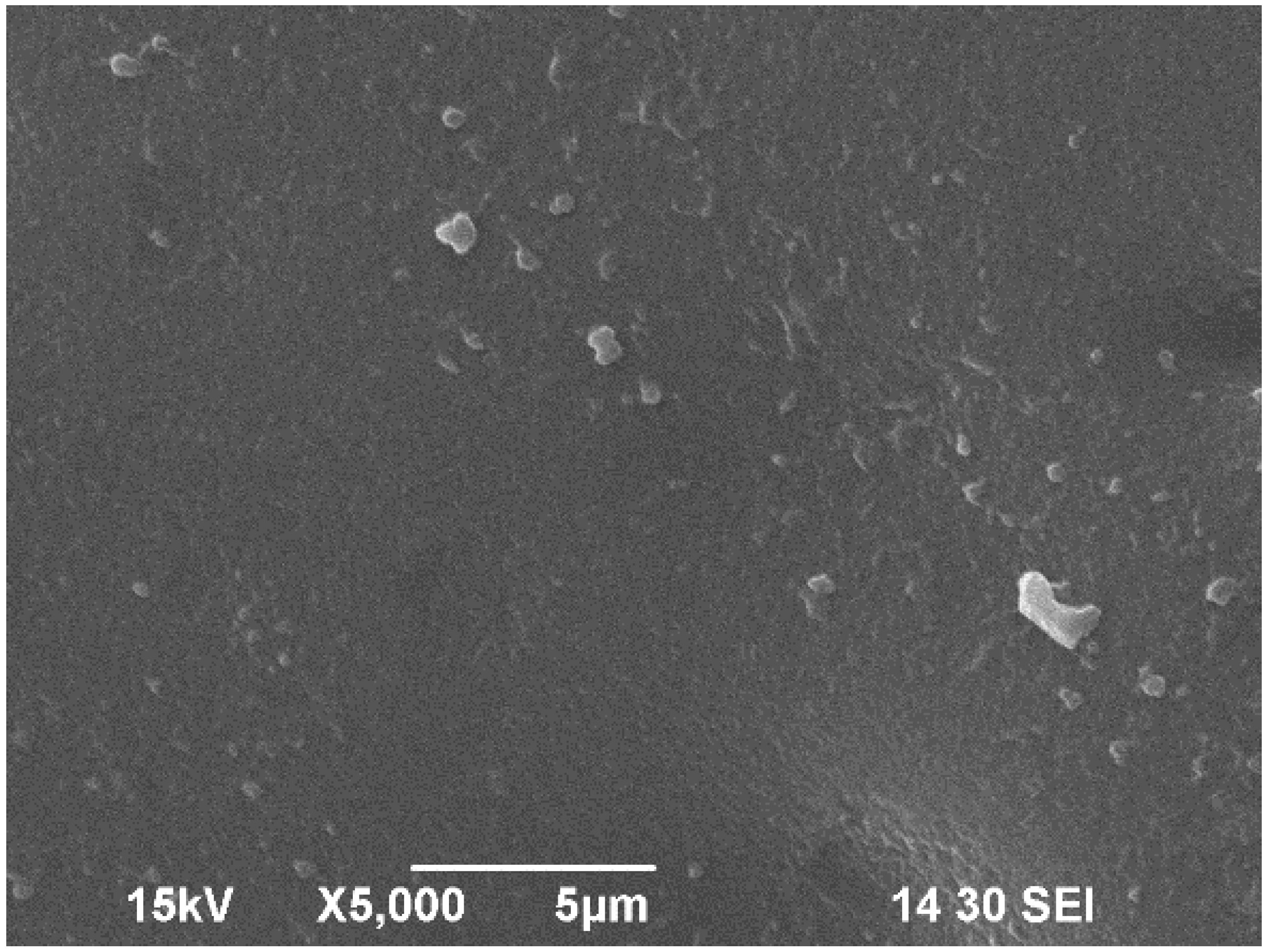
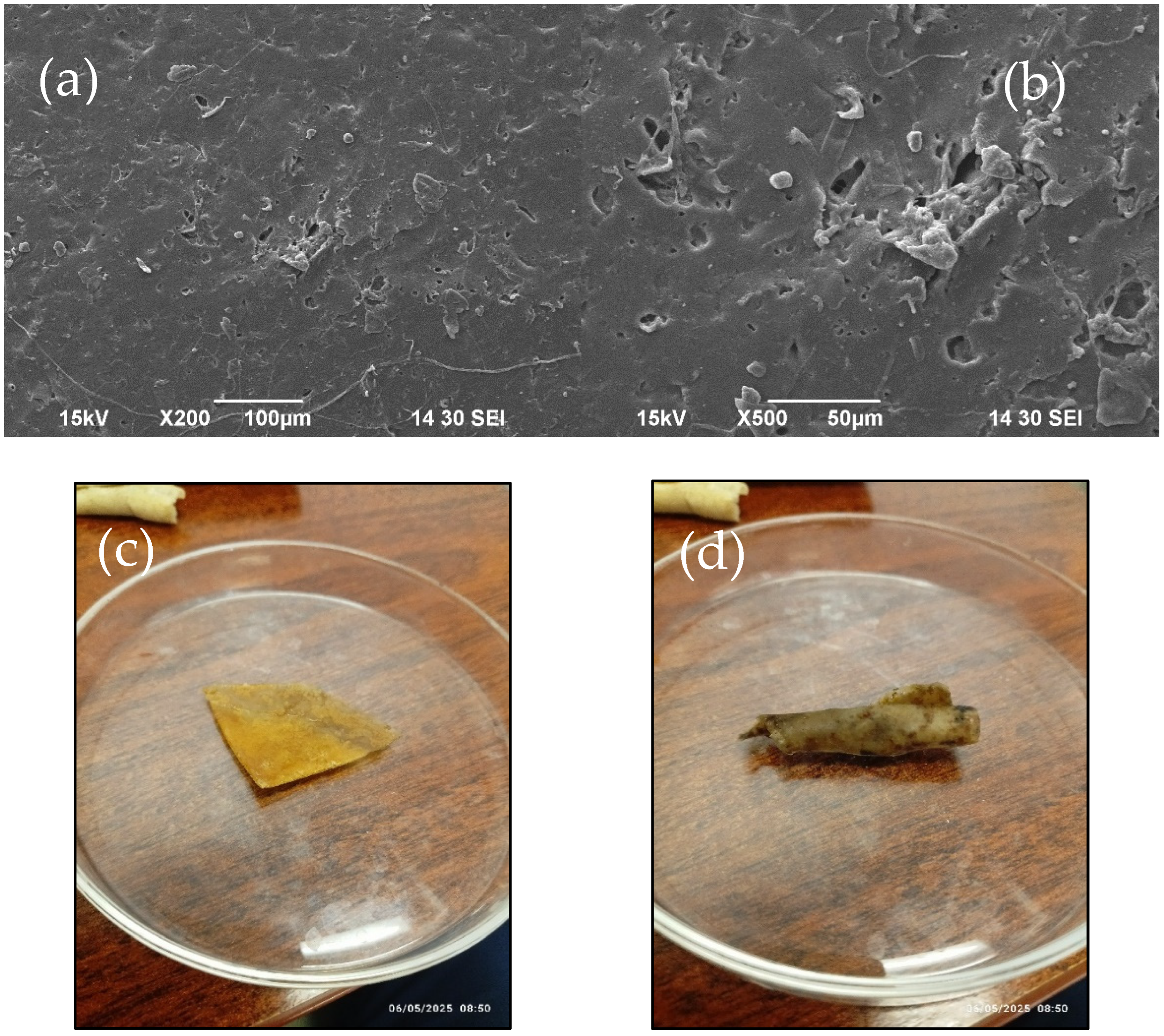
3.3. Scanning Electron Microscope (SEM) Analysis of Bioplastic
3.4. Film’s Transparency
3.5. Water Contact Angle
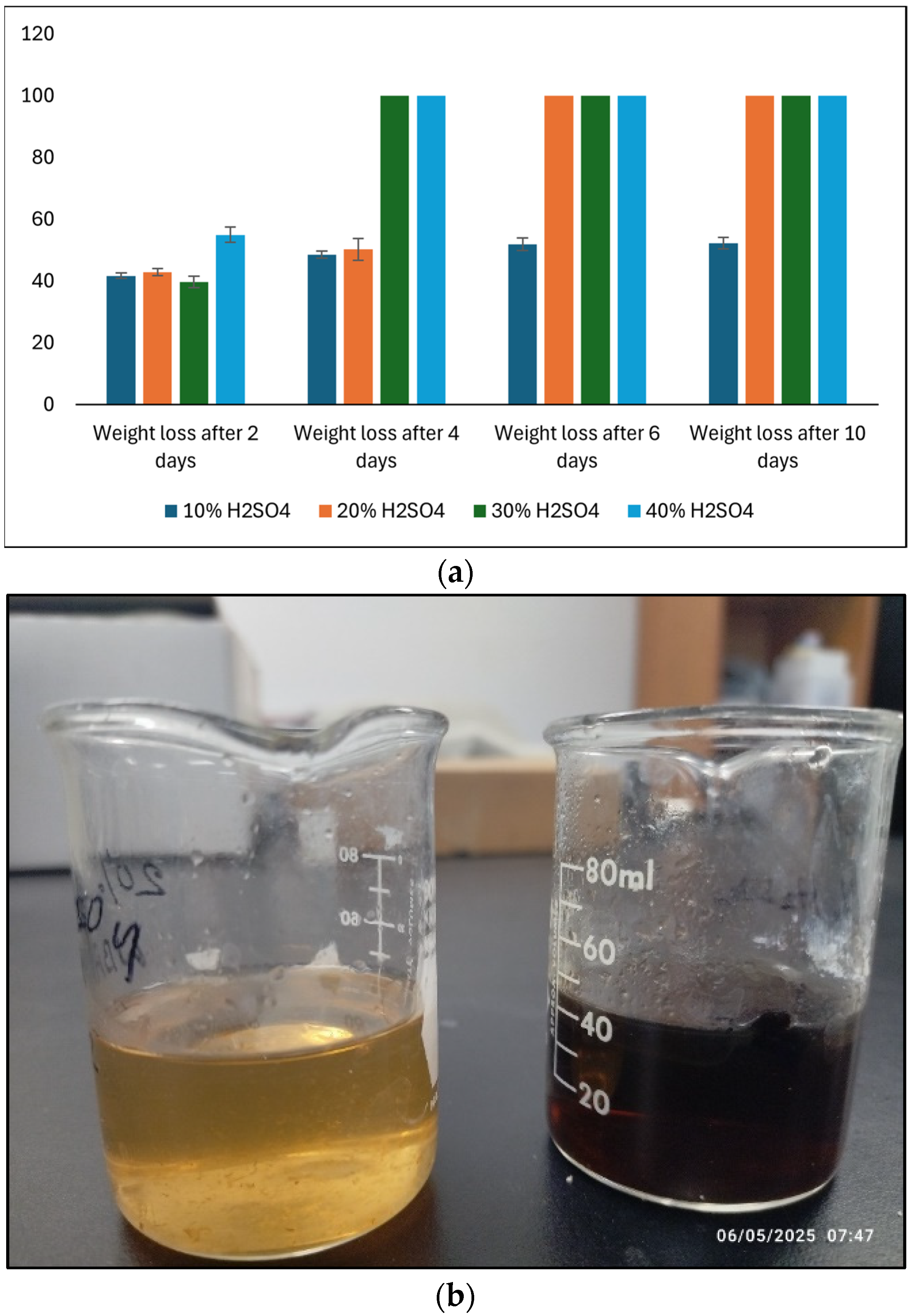
3.6. Effect of Acids on Bioplastic
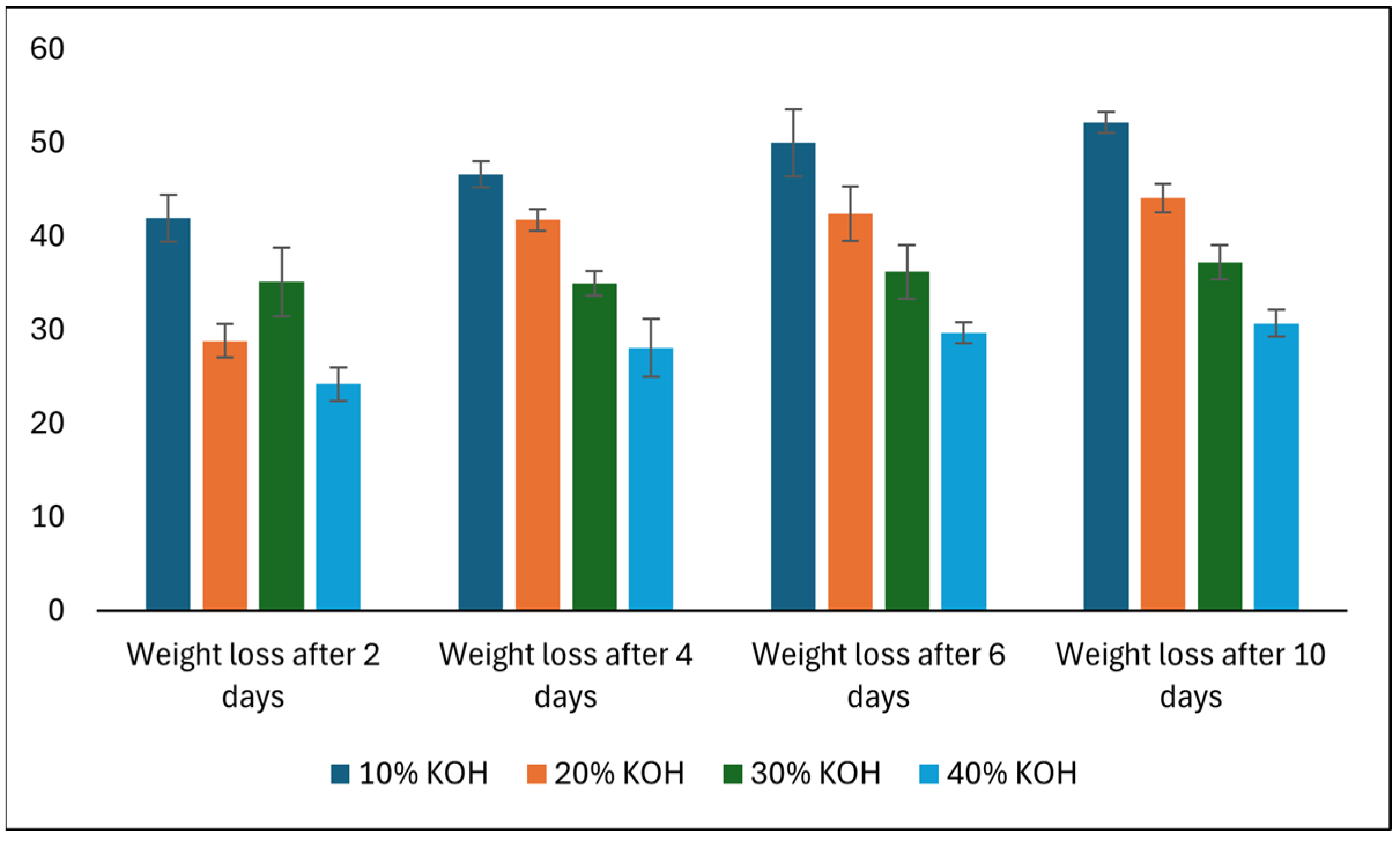
3.7. Effect of Alkalis on Bioplastic
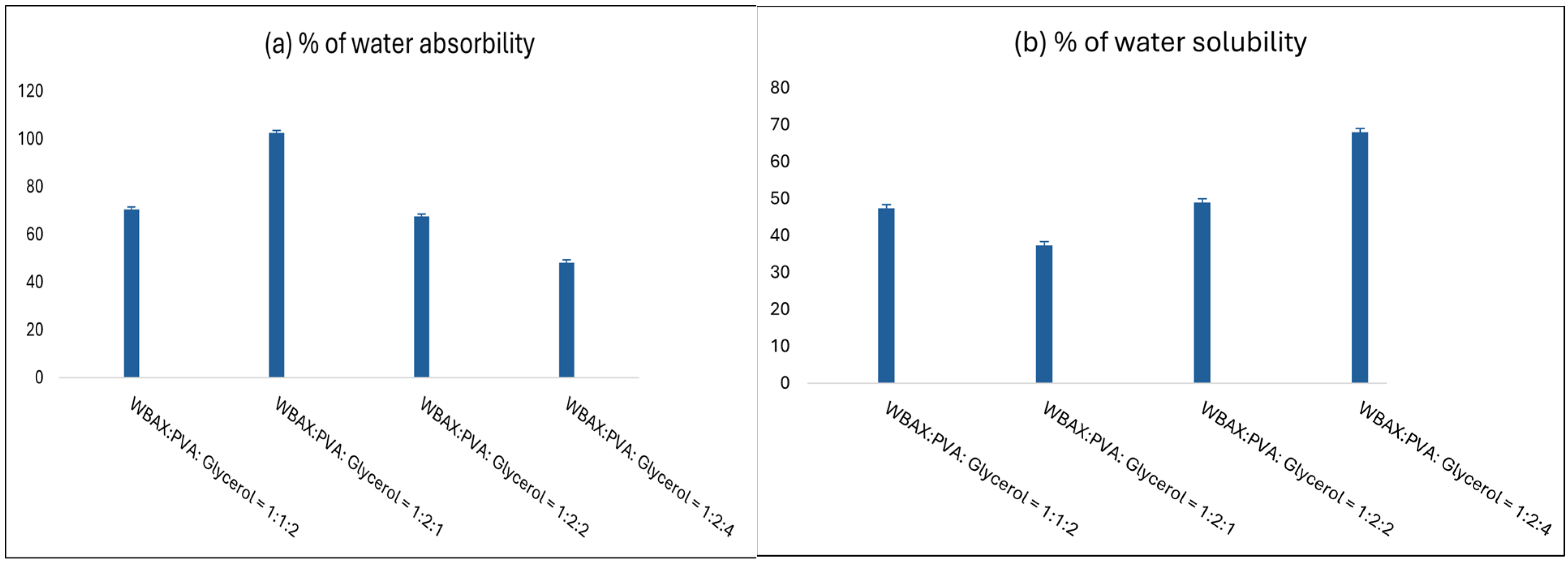
3.8. Water Absorption Percentage of Bioplastics
3.9. Biodegradability Analysis of the Bioplastic
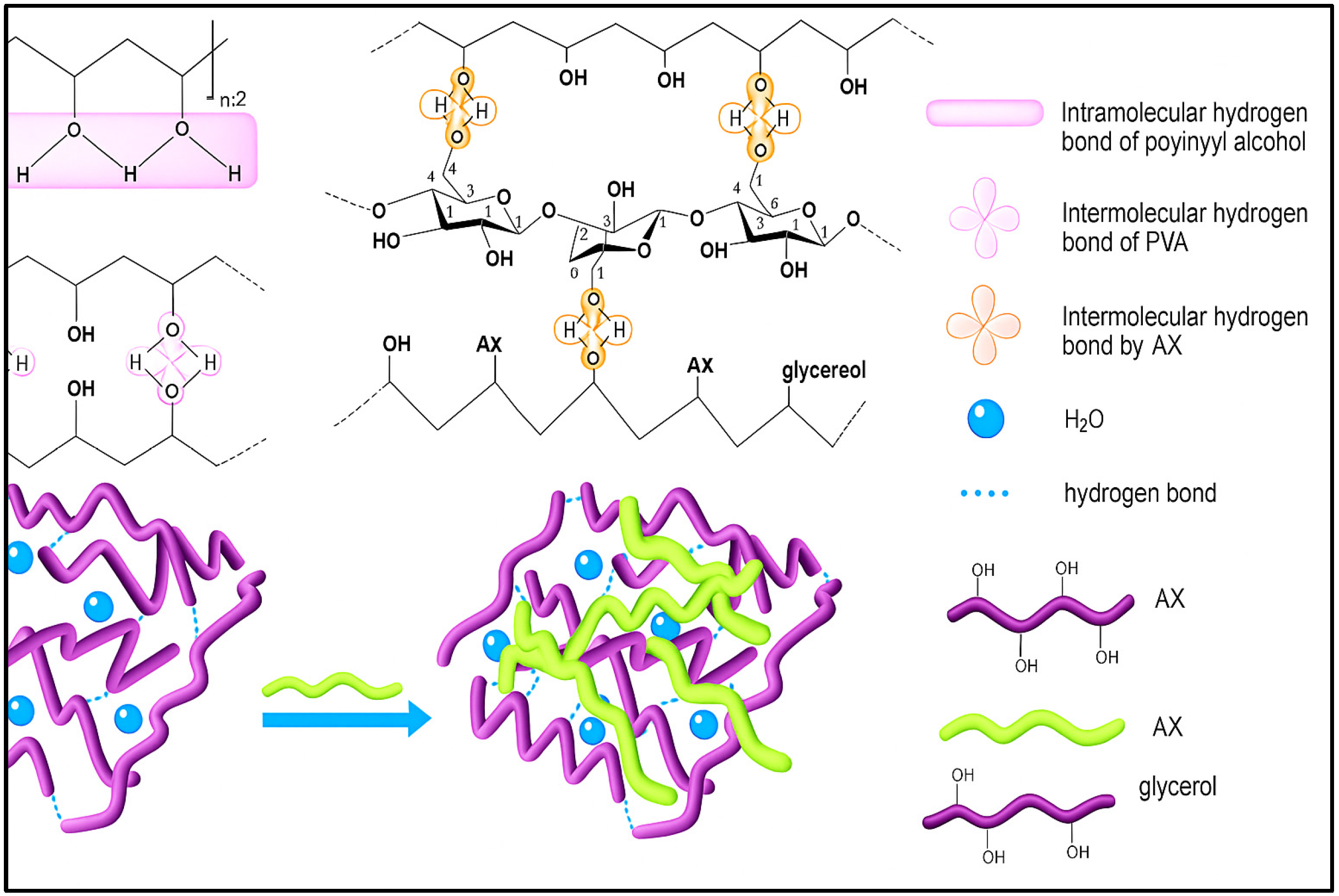
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Cellulose acetate |
| EAB | Elongation at break |
| FTIR | Fourier Transform Infrared Spectroscopy |
| LDPE | Low-density polyethylene |
| PVA | Polyvinyl alcohol |
| SEM | Scanning Electron Microscope |
| TS | Tensile strength |
| WCA | Water contact angle |
| WB | Wheat bran |
| WBAX | Wheat bran arabinoxylan |
References
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct Transformation of Edible Vegetable Waste into Bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Merino, D.; Paul, U.C.; Athanassiou, A. Bio-Based Plastic Films Prepared from Potato Peels Using Mild Acid Hydrolysis Followed by Plasticization with a Polyglycerol. Food Packag. Shelf Life 2021, 29, 100707. [Google Scholar] [CrossRef]
- Merino, D.; Simonutti, R.; Perotto, G.; Athanassiou, A. Direct Transformation of Industrial Vegetable Waste into Bioplastic Composites Intended for Agricultural Mulch Films. Green Chem. 2021, 23, 5956–5971. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Haufe, J.; Patel, M.K. Product overview and market projection of emerging bio-based plastics PRO-BIP 2009. Rep. Eur. Polysacch. Netw. Excell. (EPNOE) Eur. Bioplastics 2009, 243, 1–245. [Google Scholar]
- Kwon, E.E.; Lee, J. Polyethylene Terephthalate Production from a Carbon Neutral Resource. J. Clean. Prod. 2024, 469, 143210. [Google Scholar] [CrossRef]
- EUBIO_Admin. Materials. European Bioplastics e.V. Available online: https://www.european-bioplastics.org/bioplastics/materials/ (accessed on 20 April 2024).
- Aristei, L.; Villani, L.; Ricciardi, W. Directive 2019/904/EU. The Need to Raise Awareness on Plastic Misuse and Consequences on Health. Eur. J. Public Health 2020, 30, ckaa166.138. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Biofibers from Agricultural Byproducts for Industrial Applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef]
- Rudra, S.G.; Nishad, J.; Jakhar, N.; Kaur, C. Food industry waste: Mine of nutraceuticals. Int. J. Sci. Environ. Technol 2015, 4, 205–229. [Google Scholar]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A.; Musa, K.H. Evaluation of Fruit Leather Made from Two Cultivars of Papaya. Ital. J. Food Sci. 2016, 28, 73–82. [Google Scholar] [CrossRef]
- Chen, J.; Tang, C.; Yue, Y.; Qiao, W.; Hong, J.; Kitaoka, T.; Yang, Z. Highly Translucent All Wood Plastics via Heterogeneous Esterification in Ionic Liquid/Dimethyl Sulfoxide. Ind. Crops Prod. 2017, 108, 286–294. [Google Scholar] [CrossRef]
- Dai, H.; Chang, P.R.; Yu, J.; Geng, F.; Ma, X. Relationship of Thermoplastic Starch Crystallinity to Plasticizer Structure. Starch-Stärke 2010, 62, 86–89. [Google Scholar] [CrossRef]
- Khan, Z.A.; Butt, A.A.; Alghamdi, T.A.; Fatima, A.; Akbar, M.; Ramzan, M.; Javaid, N. Energy Management in Smart Sectors Using Fog Based Environment and Meta-Heuristic Algorithms. IEEE Access 2019, 7, 157254–157267. [Google Scholar] [CrossRef]
- Awika, J.M.; Piironen, V.; Bean, S.; American Chemical Society. Division of Agricultural and Food Chemistry. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Hensel, G.; Kastner, C.; Oleszczuk, S.; Riechen, J.; Kumlehn, J. Agrobacterium-Mediated Gene Transfer to Cereal Crop Plants: Current Protocols for Barley, Wheat, Triticale, and Maize. Int. J. Plant Genom. 2009, 2009, 835608. [Google Scholar] [CrossRef] [PubMed]
- Hemery, Y.; Rouau, X.; Lullien-Pellerin, V.; Barron, C.; Abecassis, J. Dry Processes to Develop Wheat Fractions and Products with Enhanced Nutritional Quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Onipe, O.O.; Jideani, A.I.O.; Beswa, D. Composition and Functionality of Wheat Bran and Its Application in Some Cereal Food Products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Liu, N.; Zhou, P.; Bao, Q.; Wang, X. Effect of Wheat Bran Dietary Fibre on the Rheological Properties of Dough during Fermentation and Chinese Steamed Bread Quality. Int. J. Food Sci. Technol. 2020, 56, 1623–1630. [Google Scholar] [CrossRef]
- Sarker, N.C.; Rahim, A.; Hillukka, G.; Holter, B.; Kjelland, M.; Hossain, K. Pyrolyzed Biochar from Agricultural Byproducts: Synthesis, Characterization, and Application in Water Pollutants Removal. Processes 2025, 13, 1358. [Google Scholar] [CrossRef]
- Chen, Z.; Mense, A.L.; Brewer, L.R.; Shi, Y.-C. Wheat Bran Layers: Composition, Structure, Fractionation, and Potential Uses in Foods. Crit. Rev. Food Sci. Nutr. 2023, 64, 6636–6659. [Google Scholar] [CrossRef]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A Sight to Wheat Bran: High Value-Added Products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Kaya, K.; Pehlivan, E.; Schmidt, C.; Bahadir, M. Use of Modified Wheat Bran for the Removal of Chromium(VI) from Aqueous Solutions. Food Chem. 2014, 158, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Attallah, O.A.; Mojicevic, M.; Garcia, E.L.; Azeem, M.; Chen, Y.; Asmawi, S.; Fournet, M.B. Macro and Micro Routes to High Performance Bioplastics: Bioplastic Biodegradability and Mechanical and Barrier Properties. Polymers 2021, 13, 2155. [Google Scholar] [CrossRef]
- Ave, L.; Pollet, E. Environmental Silicate Nano-Biocomposites; Springer: London, UK, 2012. [Google Scholar]
- Hossain, A.; Mahmud, M.; Koistinen, K.; Davisson, G.; Roeges, B.; Boechler, H.; Badsha, M.A.; Khan, R.; Kjelland, M.; Fereydoonpour, D.; et al. Wheat Bran Polymer Scaffolds: Supporting Triple-Negative Breast Cancer Cell Growth and Development. Bioengineering 2025, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Sarker, N.C.; Ray, P.; Pfau, C.; Kalavacharla, V.; Hossain, K.; Quadir, M. Development of functional nanomaterials from wheat bran derived arabinoxylan for nucleic acid delivery. Agric. Food Chem. 2020, 68, 4367–4373. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.F.; Norcino, L.B.; Manrich, A.; Pinheiro, A.C.M.; Oliveira, J.E.; Mattoso, L.H.C. Development, Physical-Chemical Properties, and Photodegradation of Pectin Film Reinforced with Malt Bagasse Fibers by Continuous Casting. J. Appl. Polym. Sci. 2020, 137, e49178. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Guan, F.; Liu, Y.; Yang, Q.; Zhang, X.; Xu, Y. Preparation of Electrospun Polyvinyl Alcohol/Nanocellulose Composite Film and Evaluation of Its Biomedical Performance. Gels 2021, 7, 223. [Google Scholar] [CrossRef]
- Lusiana, S.; Putri, D.; Nurazizah, I.Z.; Bahruddin. Bioplastic Properties of Sago-PVA Starch with Glycerol and Sorbitol Plasticizers. J. Phys. Conf. Ser. 2019, 1351, 012102. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, J. The Role of Hydrogen-Bonding Interaction in Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Blending Solutions and Their Films. Chin. J. Polym. Sci. 2010, 28, 903–911. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of Glycerol Plasticizer Loading on the Physical, Mechanical, Thermal, and Barrier Properties of Arrowroot (Maranta Arundinacea) Starch Biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Azmi, N.N.; Ab Patar, M.N.A.; Noor, S.N.A.M.; Mahmud, J. Testing Standards Assessment for Silicone Rubber. In Proceedings of the 2014 International Symposium on Technology Management and Emerging Technologies, Bandung, Indonesia, 27–29 May 2014. [Google Scholar] [CrossRef]
- Mulyono, N.; Suhartono, M.T.; Angelina, S. Development of Bioplastic Based on Cassava Flour and Its Starch Derrivatives for Food Packaging. J. Harmon. Res. Appl. Sci. 2015, 3, 125–133. [Google Scholar]
- Wang, B.; Duan, Y.; Xin, Z.; Yao, X.; Abliz, D.; Ziegmann, G. Fabrication of an Enriched Graphene Surface Protection of Carbon Fiber/Epoxy Composites for Lightning Strike via a Percolating-Assisted Resin Film Infusion Method. Compos. Sci. Technol. 2018, 158, 51–60. [Google Scholar] [CrossRef]
- Baba, E.M.; Cansoy, C.E.; Zayim, E.O. Optical and Wettability Properties of Polymers with Varying Surface Energies. Appl. Surf. Sci. 2015, 350, 115–120. [Google Scholar] [CrossRef]
- Deng, R.; Shen, T.; Chen, H.; Lu, J.; Yang, H.-C.; Li, W. Slippery Liquid-Infused Porous Surfaces (SLIPSs): A Perfect Solution to Both Marine Fouling and Corrosion? J. Mater. Chem. A 2020, 8, 7536–7547. [Google Scholar] [CrossRef]
- Kumar, M.; Bhardwaj, R. Wetting Characteristics of Colocasia Esculenta (Taro) Leaf and a Bioinspired Surface Thereof. Sci. Rep. 2020, 10, 935. [Google Scholar] [CrossRef]
- Liu, D.; Chen, P.; Mu, J.; Yu, Q.; Lu, C. Improvement and Mechanism of Interfacial Adhesion in PBO Fiber/Bismaleimide Composite by Oxygen Plasma Treatment. Appl. Surf. Sci. 2011, 257, 6935–6940. [Google Scholar] [CrossRef]
- ImageJ, Image Processing and Analysis in Java. Available online: https://imagej.nih.gov/ij/ (accessed on 13 June 2025).
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and Mechanical Properties of a New Edible Film Made of Pea Starch and Guar Gum as Affected by Glycols, Sugars and Polyols. Int. J. Biol. Macromol. 2017, 104, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.A.; Farag, A.A.; Abo-dief, H.M.; Tayeb, A.M. Production of Biodegradable Plastic from Agricultural Wastes. Arab. J. Chem. 2018, 11, 546–553. [Google Scholar] [CrossRef]
- Müller, R. Biodegradability of Polymers: Regulations and Methods for Testing. In Biopolymers Online; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Harunsyah; Yunus, M.; Fauzan, R. Mechanical Properties of Bioplastics Cassava Starch Film with Zinc Oxide Nanofiller as Reinforcement. IOP Conf. Ser. Mater. Sci. Eng. 2017, 210, 012015. [Google Scholar] [CrossRef]
- Rumi, S.S.; Liyanage, S.; Abidi, N. Conversion of Low-Quality Cotton to Bioplastics. Cellulose 2021, 28, 2021–2038. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of Various Plasticizers and Concentration on the Physical, Thermal, Mechanical, and Structural Properties of Cassava-Starch-Based Films. Starch-Stärke 2016, 69, 1500366. [Google Scholar] [CrossRef]
- Sena, J.; Johannissen, L.O.; Blaker, J.J.; Hay, S. A Machine Learning Model for the Prediction of Water Contact Angles on Solid Polymers. J. Phys. Chem. B 2025, 129, 2739–2745. [Google Scholar] [CrossRef]
- Burr, S.J.; Fry, S.C. Extracellular Cross-Linking of Maize Arabinoxylans by Oxidation of Feruloyl Esters to Form Oligoferuloyl Esters and Ether-like Bonds. Plant J. 2009, 58, 554–567. [Google Scholar] [CrossRef]
- Lupo, C.; Boulos, S.; Nyström, L. Influence of Partial Acid Hydrolysis on Size, Dispersity, Monosaccharide Composition, and Conformation of Linearly-Branched Water-Soluble Polysaccharides. Molecules 2020, 25, 2982. [Google Scholar] [CrossRef]
- Egüés, I.; Sanchez, C.; Mondragon, I.; Labidi, J. Effect of Alkaline and Autohydrolysis Processes on the Purity of Obtained Hemicelluloses from Corn Stalks. Bioresour. Technol. 2012, 103, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, J.; Kong, W.; Gao, C.; Liu, C.; Peng, F.; Sun, R. Influence of Urea and Glycerol on Functional Properties of Biodegradable PVA/Xylan Composite Films. Cellulose 2013, 21, 495–505. [Google Scholar] [CrossRef]
- Hanh, P.T.H.; Suwunwong, T.; Chantrapromma, S.; Choto, P.; Thanomsilp, C.; Phoungthong, K. Preparation and Characterization of Polyvinyl Alcohol (PVA)-Glycerol Composite Films Incorporating Nanosilica from Municipal Solid Waste Incinerator Bottom Ash. Heliyon 2024, 10, e25963. [Google Scholar] [CrossRef] [PubMed]
- Judawisastra, H.; Sitohang, R.D.R.; Marta, L.; Mardiyati. Water Absorption and Its Effect on the Tensile Properties of Tapioca Starch/Polyvinyl Alcohol Bioplastics. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012066. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Alahmed, A.; Simsek, S. Enhancing Mechanical Properties of Corn Bran Arabinoxylan Films for Sustainable Food Packaging. Foods 2024, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- Strand, A.; Kouko, J.; Oksanen, A.; Salminen, K.; Ketola, A.; Retulainen, E.; Sundberg, A. Enhanced Strength, Stiffness and Elongation Potential of Paper by Spray Addition of Polysaccharides. Cellulose 2019, 26, 3473–3487. [Google Scholar] [CrossRef]
- Bueno, D.; Brienzo, M. Production of Bioplastics with Chemical and Enzymatic Modificated Xylan (Lignin- and Arabinose-Free) from Sugarcane Bagasse. Biotechnol. Sustain. Mater. 2025, 2, 2. [Google Scholar] [CrossRef]
- Waluyo, J.; Purba, I.T.; Sani, K.Q.; Sayekti, N.; Ramadhani, S.S.; Pranolo, S.H.; Margono; Kaavessina, M. Bioplastic from Empty Fruit Bunch Cellulose/Chitosan/Starch: Optimization through Box-Behnken Design to Enhance the Mechanical Properties. J. Plast. Film Sheeting 2024, 40, 259–282. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Hydrophilization and Hydrophobic Recovery of PDMS by Oxygen Plasma and Chemical Treatment—An SEM Investigation. Sens. Actuators B Chem. 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics Recycling: Challenges and Opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Bangar, S.P.; Gumber, S.; Whiteside, W.S.; Phimolsiripol, Y. Arabinoxylan-Based Films and Coatings for Fresh Produce: A Review of Emerging Trends in Food Packaging. Int. J. Biol. Macromol. 2025, 310, 143097. [Google Scholar] [CrossRef]
- Coelhoso, I. Polysaccharide Films/Membranes for Food and Industrial Applications. Polysaccharides 2025, 6, 48. [Google Scholar] [CrossRef]
- Xia, G.; Ji, X.; Xu, Z.; Ji, X. Transparent Cellulose-Based Bio-Hybrid Films with Enhanced Anti-Ultraviolet, Antioxidant and Antibacterial Performance. Carbohydr. Polym. 2022, 298, 120118. [Google Scholar] [CrossRef]



| Title | Wheat Bran Arabinoxylan (WBAX) | Polyvinyl Alcohol (PVA) | Glycerol |
|---|---|---|---|
| WBAX 1:1:2 | 1 | 1 | 2 |
| WBAX 1:2:1 | 1 | 2 | 1 |
| WBAX 1:2:2 | 1 | 2 | 2 |
| WBAX 1:2:4 | 1 | 2 | 4 |
| Film Type | Absorbance | Transmission% | Thickness | Transparency |
|---|---|---|---|---|
| WBAX bioplastic film | 1.23 | 5.92 | 0.19 | 1.50 |
| CPS bioplastic film | 1.25 | 5.60 | 0.17 | 1.52 |
| Ziploc plastic bag | 0.05 | 89.23 | 0.02 | 3.57 |
| Walmart plastic bag | - | - | - | 3.78 |
| tapioca-based films | - | - | - | 3.13 |
| Film Type | Water Contact Angle (Degree) | SD |
|---|---|---|
| WBAX bioplastic film | 75.80 | 0.60 |
| Ziploc plastic bag | 124.83 | 1.11 |
| Walmart plastic bag | 76.78 | 1.10 |
| PLA/starch/lecithin film | 59.250 | 1.01 |
| Property | WBAX Bioplastic | LDPE (Synthetic Plastic) |
|---|---|---|
| Tensile Strength (MPa) | 3.34 | 10–30 |
| Elongation at Break (%) | 138 | 100–600 |
| Water Contact Angle (°) | 80 | >95 |
| Water Solubility (%) | ~40 | <1 |
| Biodegradability | Yes | No |
| Acid Resistance | Low concentration | Moderate |
| Alkali Resistance | High concentration | Moderate |
| Transparency | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badsha, M.A.R.; Kjelland, M.; Ulven, C.; Hossain, K. Arabinoxylan-Based Bioplastic from Wheat Bran: A Promising Replacement for Synthetic Plastics. Polymers 2025, 17, 2488. https://doi.org/10.3390/polym17182488
Badsha MAR, Kjelland M, Ulven C, Hossain K. Arabinoxylan-Based Bioplastic from Wheat Bran: A Promising Replacement for Synthetic Plastics. Polymers. 2025; 17(18):2488. https://doi.org/10.3390/polym17182488
Chicago/Turabian StyleBadsha, Md Abdur Rahim, Michael Kjelland, Chad Ulven, and Khwaja Hossain. 2025. "Arabinoxylan-Based Bioplastic from Wheat Bran: A Promising Replacement for Synthetic Plastics" Polymers 17, no. 18: 2488. https://doi.org/10.3390/polym17182488
APA StyleBadsha, M. A. R., Kjelland, M., Ulven, C., & Hossain, K. (2025). Arabinoxylan-Based Bioplastic from Wheat Bran: A Promising Replacement for Synthetic Plastics. Polymers, 17(18), 2488. https://doi.org/10.3390/polym17182488








