Scalable Biosynthesis and Recovery of Poly-3-Hydroxybutyrate Produced from Cotton-Derived Glucose by Cupriavidus necator
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Preparation
2.2. Media Preparation
2.3. Starter Culture Preparation
2.4. Bioreactor Procedure
2.5. Polymer Extraction
2.6. Solvent Cast Film Production
2.7. Thermal Analysis
3. Results and Discussion
3.1. Confirmation of C. necator in Culture and Film Casting
3.2. P3HB Yields
3.3. Thermal Analysis
| Test | Property | G-Derived P3HB | CCG- Derived P3HB | CWG- Derived P3HB | Purchased P3HB | Literature Source | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wang [14] | Mannina [5] | Lee [28] | Sudesh [29] | Rysbek [13] | ||||||
| TGA | Tonset | 284 °C | 285 °C | 267 °C | 283 °C | 263.4 °C | 185 °C | 200 °C | - | - |
| Tend | 302 °C | 306 °C | 283 °C | 304 °C | 269.8 °C | 267 °C | - | - | - | |
| DSC | Tg | - | - | - | - | - | - | 5 °C | 4 °C | - |
| Tc * | 37 °C | 36 °C | 26 °C | 73 °C | - | - | - | 50–60 °C | - | |
| Tcc * | 26 °C | 24 °C | 28 °C | - | - | - | - | - | - | |
| Tm * | 147 °C | 151 °C | 150 °C | 165 °C | 172.05 °C | - | 175 °C | 180 °C | 170 °C | |
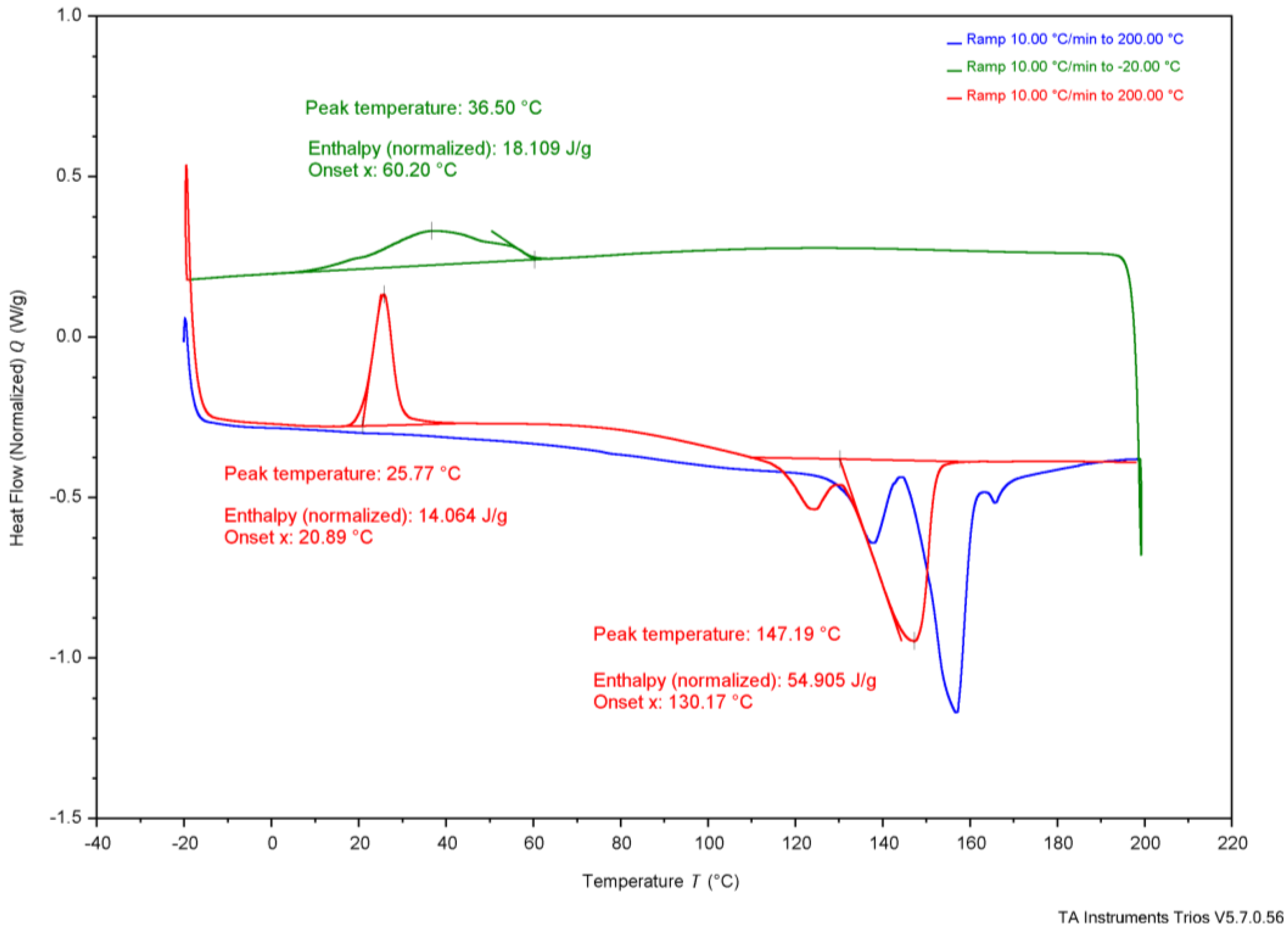

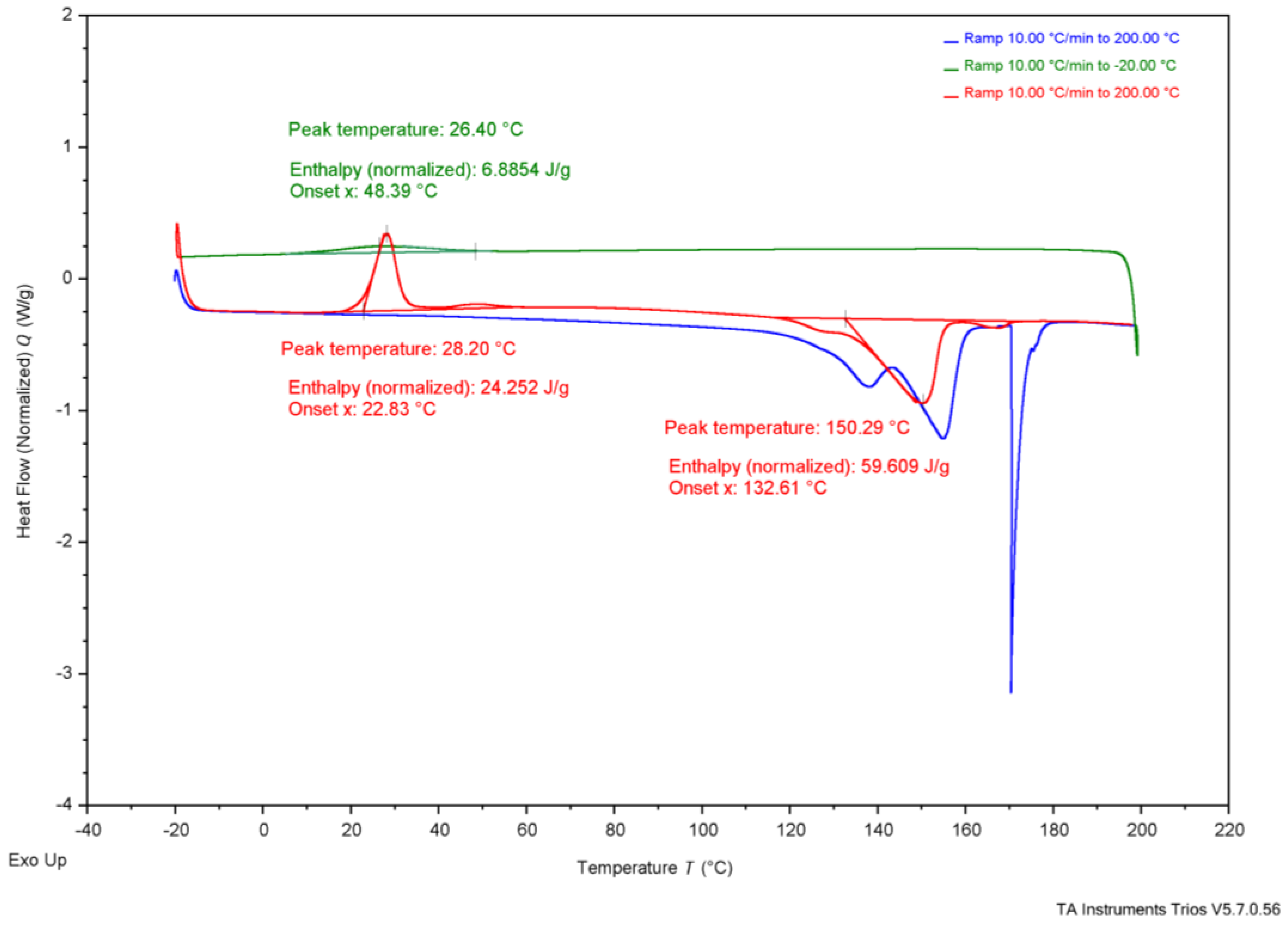

3.4. Comparing Experimental Samples
4. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P3HB | Poly-3-hydroxybutyrate |
| G | Glucose, referring to commercial glucose |
| CCG | Cotton glucose |
| CWG | Cutting waste cotton glucose |
References
- Thunman, H.; Berdugo Vilches, T.; Seemann, M.; Maric, J.; Cañete Vela, I.; Pissot, S.; Nguyen, H.N.T. Circular use of plastics-transformation of existing petrochemical clusters into thermochemical recycling plants with 100% plastics recovery. Sustain. Mater. Technol. 2019, 22, e00124. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Advancing Sustainable Materials Management: Facts and Figures Report. 2020. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 16 July 2025).
- Parashar, N.; Hait, S. Plastics in the time of COVID-19 pandemic: Protector or polluter? Sci. Total Environ. 2021, 759, 144274. [Google Scholar] [CrossRef]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Eugenia Suárez-Ojeda, M. Bioplastic recovery from wastewater: A new protocol for polyhydroxyalkanoates (PHA) extraction from mixed microbial cultures. Bioresour. Technol. 2019, 282, 361–369. [Google Scholar] [CrossRef]
- World Economic Forum; Ellen MacArthur Foundation; McKinsey & Company. The New Plastics Economy: Rethinking the Future of Plastics. 2016. Available online: https://www.ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 16 July 2025).
- Rhodes, C.J. Plastic pollution and potential solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Otari, S.V.; Jeon, J.M.; Gurav, R.; Choi, Y.K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Rajesh Banu, J.; Yoon, J.J.; et al. Biowaste-to-bioplastic (polyhydroxyalkanoates): Conversion technologies, strategies, challenges, and perspective. Bioresour. Technol. 2021, 326, 124733. [Google Scholar] [CrossRef]
- Costa, A.; Encarnação, T.; Tavares, R.; Todo Bom, T.; Mateus, A. Bioplastics: Innovation for Green Transition. Polymers 2023, 15, 517. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.M.; Dahman, Y. Production and recovery of poly-3-hydroxybutyrate bioplastics using agro-industrial residues of hemp hurd biomass. Bioprocess Biosyst. Eng. 2019, 42, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.; Rehner, R.; Kienle, A.; Grammel, H. Rapid selection of glucose-utilizing variants of the polyhydroxyalkanoate producer Ralstonia eutropha H16 by incubation with high substrate levels. Lett. Appl. Microbiol. 2012, 54, 45–51. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Grape winery waste as a promising feedstock for the production of polyhydroxyalkanoates and other value-added products. Food Bioprod. Process. 2020, 124, 1–10. [Google Scholar] [CrossRef]
- Rysbek, A.; Ramankulov, Y.; Kurmanbayev, A.; Richert, A.; Abeldenov, S. Comparative Characterization and Identification of Poly-3-hydroxybutyrate Producing Bacteria with Subsequent Optimization of Polymer Yield. Polymers 2022, 14, 335. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cai, J.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and thermal properties of PHBV produced from levulinic acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef]
- Markets and Markets. Polyhydroxyalkanoate (PHA) Market—Global Forecast to 2028. 2024. Available online: https://www.marketsandmarkets.com/Market-Reports/pha-market-395.html (accessed on 16 July 2025).
- Johnson, S.; Echeverria, D.; Venditti, R.; Jameel, H.; Yao, Y. Supply Chain of Waste Cotton Recycling and Reuse: A Review. AATCC J. Res. 2020, 7 (Suppl. S1), 19–31. [Google Scholar] [CrossRef]
- Cotton Incorporated. Monthly Economic Letter: Cotton Market Fundamentals and Price Outlook. Cotton Incorporated. 2024. Available online: https://www.cottoninc.com/wp-content/uploads/2024/11/2024-11-Monthly-Econmic-Letter.pdf (accessed on 16 July 2025).
- Vera, R.; Zambrano, F.; Suarez, A.; Pifano, A.; Marquez, R.; Farrell, M.; Ankeny, M.; Jameel, H.; Gonzalez, R. Transforming textile wastes into biobased building blocks via enzymatic hydrolysis: A review of key challenges and opportunities. Clean. Circ. Bioeconomy 2022, 3, 100026. [Google Scholar] [CrossRef]
- de Mello, A.F.M.; de Souza Vandenberghe, L.P.; Herrmann, L.W.; Letti, L.A.J.; Burgos, W.J.M.; Scapini, T.; Manzoki, M.C.; de Oliveira, P.Z.; Soccol, C.R. Strategies and engineering aspects on the scale-up of bioreactors for different bioprocesses. Syst. Microbiol. Biomanuf. 2024, 4, 365–385. [Google Scholar] [CrossRef]
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Ramsay, J.A.; Berger, E.; Voyer, R.; Chavarie, C.; Ramsay, B.A. Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnol. Tech. 1994, 8, 589–594. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- GENEWIZ. Azenta Life Sciences. 2024. Available online: https://www.genewiz.com/ (accessed on 16 July 2025).
- Jefferson Institute for Bioprocessing. 2024. Available online: https://www.linkedin.com/company/jefferson-institute-for-bioprocessing/ (accessed on 16 July 2025).
- De Sousa Junior, R.R.; Cezario, F.E.M.; Antonino, L.D.; Dos Santos, D.J.; Lackner, M. Characterization of Poly(3-hydroxybutyrate) (P3HB) from Alternative, Scalable (Waste) Feedstocks. Bioengineering 2023, 10, 1382. [Google Scholar] [CrossRef]
- García-Cerna, S.; Sánchez-Pacheco, U.; Meneses-Acosta, A.; Rojas-García, J.; Campillo-Illanes, B.; Segura-González, D.; Peña-Malacara, C. Evaluation of Poly-3-Hydroxybutyrate (P3HB) Scaffolds Used for Epidermal Cells Growth as Potential Biomatrix. Polymers 2022, 14, 4021. [Google Scholar] [CrossRef]
- Janigová, I.; Lacík, I.; Chodák, I. Thermal degradation of plasticized poly(3-hydroxybutyrate) investigated by DSC. Polym. Degrad. Stab. 2002, 77, 35–41. [Google Scholar] [CrossRef]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]

| Growth Medium | Control | G | CCG | CWG |
|---|---|---|---|---|
| Nutrient Broth | 5 mL (100% v/v) | 2.5 mL (50% v/v) | 2.5 mL (50% v/v) | 2.5 mL (50% v/v) |
| MSM 1 (5× concentration) | - | 0.25 mL (5% v/v) | 0.25 mL (5% v/v) | 0.25 mL (5% v/v) |
| MSM 2 (5× concentration) | - | 0.25 mL (5% v/v) | 0.25 mL (5% v/v) | 0.25 mL (5% v/v) |
| Commercial Glucose Solution (7.4% v/w glucose) | - | 0.625 mL (12.5% v/v) | - | - |
| Cotton Glucose Solution (8.93% v/w glucose) | - | - | 0.518 mL (10.36% v/v) | - |
| Cutting Waste Cotton Glucose Solution (9.06% v/w glucose) | - | - | - | 0.510 mL (10.2% v/v) |
| di H2O | - | 1.375 mL (27.5% v/v) | 1.482 mL (29.64% v/v) | 1.49 mL (29.8% v/v) |
| Growth Medium | G | CCG | CWG |
|---|---|---|---|
| MSM 1 (5× concentration) | 95 mL (9.5% v/v) | 95 mL (9.5% v/v) | 95 mL (9.5% v/v) |
| MSM 2 (5× concentration) | 95 mL (9.5% v/v) | 95 mL (9.5% v/v) | 95 mL (9.5% v/v) |
| Commercial Glucose Solution (7.4% v/w glucose) | 498.75 mL (49.875% v/v) | - | - |
| Cotton Glucose Solution (8.93% v/w glucose) | - | 413.3 mL (41.33% v/v) | - |
| Cutting Waste Cotton Glucose Solution (9.06% v/w glucose) | - | - | 407.368 mL (40.737% v/v) |
| di H2O | 261.25 mL (26.125% v/v) | 346.7 mL (34.67% v/v) | 352.632 mL (35.263% v/v) |
| Starter Culture Inoculum | 50 mL (5% v/v) | 50 mL (5% v/v) | 50 mL (5% v/v) |
| Glucose Source | Run | Cell Dry Weight (CDW) (g) * | P3HB Yield (g) | P3HB Yield (%) ** |
|---|---|---|---|---|
| Commercial Glucose (G) | G1 | 5.86 g | 0.10 g | 1.69% |
| G2 | 5.05 g | 0.03 g | 0.59% | |
| G3 | 6.54 g | 0.06 g | 0.92% | |
| G4 | 3.81 g | 0.10 g | 2.62% | |
| G5 | 4.50 g | 0.03 g | 0.67% | |
| Mean ± Std. Dev. | - | - | 1.30 ± 0.86% | |
| Control Cotton Glucose (CCG) | CCG1 | 3.07 g | 1.14 g | 38.5% |
| CCG2 | 6.4 g | 1.09 g | 17.0% | |
| Mean ± Std. Dev. | - | - | 27.8 ± 15.2% | |
| Cutting Waste Cotton Glucose (CWG) | CWG1 | 3.21 g | 0.9 g | 28.0% |
| CWG2 | 6.05 g | 0.82 g | 13.6% | |
| CWG3 | 9.41 g | 0.78 g | 8.29% | |
| Mean ± Std. Dev. | - | - | 16.6 ± 10.2% |
| Property | G-Derived P3HB | CCG- Derived P3HB | CWG- Derived P3HB | Purchased P3HB | Wang [14] |
|---|---|---|---|---|---|
| ΔHc J/g | 18 J/g | 38 J/g | 6.9 J/g | 65 J/g | - |
| Xc (%) | 12.3% | 26.0% | 4.7% | 44.5% | - |
| ΔHcc J/g | 14 J/g | 0.42 J/g | 24 J/g | - | - |
| Xcc (%) | 9.6% | 0.3% | 16.4% | - | - |
| ΔHm J/g | 55 J/g | 66 J/g | 60 J/g | 85 J/g | 89.7 J/g |
| Xm (%) | 37.7% | 45.2% | 41.1% | 58.2% | 61.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, A.M.; Gargano, L.E.; Fioravanti, G.M.; Schapiro, H.M.; Kander, R.G. Scalable Biosynthesis and Recovery of Poly-3-Hydroxybutyrate Produced from Cotton-Derived Glucose by Cupriavidus necator. Polymers 2025, 17, 2745. https://doi.org/10.3390/polym17202745
Clark AM, Gargano LE, Fioravanti GM, Schapiro HM, Kander RG. Scalable Biosynthesis and Recovery of Poly-3-Hydroxybutyrate Produced from Cotton-Derived Glucose by Cupriavidus necator. Polymers. 2025; 17(20):2745. https://doi.org/10.3390/polym17202745
Chicago/Turabian StyleClark, Ashley M., Lucia E. Gargano, Gabriella M. Fioravanti, Hannah M. Schapiro, and Ronald G. Kander. 2025. "Scalable Biosynthesis and Recovery of Poly-3-Hydroxybutyrate Produced from Cotton-Derived Glucose by Cupriavidus necator" Polymers 17, no. 20: 2745. https://doi.org/10.3390/polym17202745
APA StyleClark, A. M., Gargano, L. E., Fioravanti, G. M., Schapiro, H. M., & Kander, R. G. (2025). Scalable Biosynthesis and Recovery of Poly-3-Hydroxybutyrate Produced from Cotton-Derived Glucose by Cupriavidus necator. Polymers, 17(20), 2745. https://doi.org/10.3390/polym17202745






