Evaluation of Medical-Grade Polycaprolactone for 3D Printing: Mechanical, Chemical, and Biodegradation Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Medical-Grade Polycaprolactone (PCL)
2.1.2. Preparation of Specimens
2.2. Mechanical Testing
2.3. Extrusion and Printability Tests
2.3.1. Extrusion Characteristics
2.3.2. Printability
2.4. Fourier-Transform Infrared (FT-IR) Analysis
2.5. Degradation Assessment Methods
2.6. Sterilization Methods
2.7. Effect of Long-Term Thermal Exposure on Printability
3. Results and Discussion
3.1. Tensile and Compressive Properties of PCLs
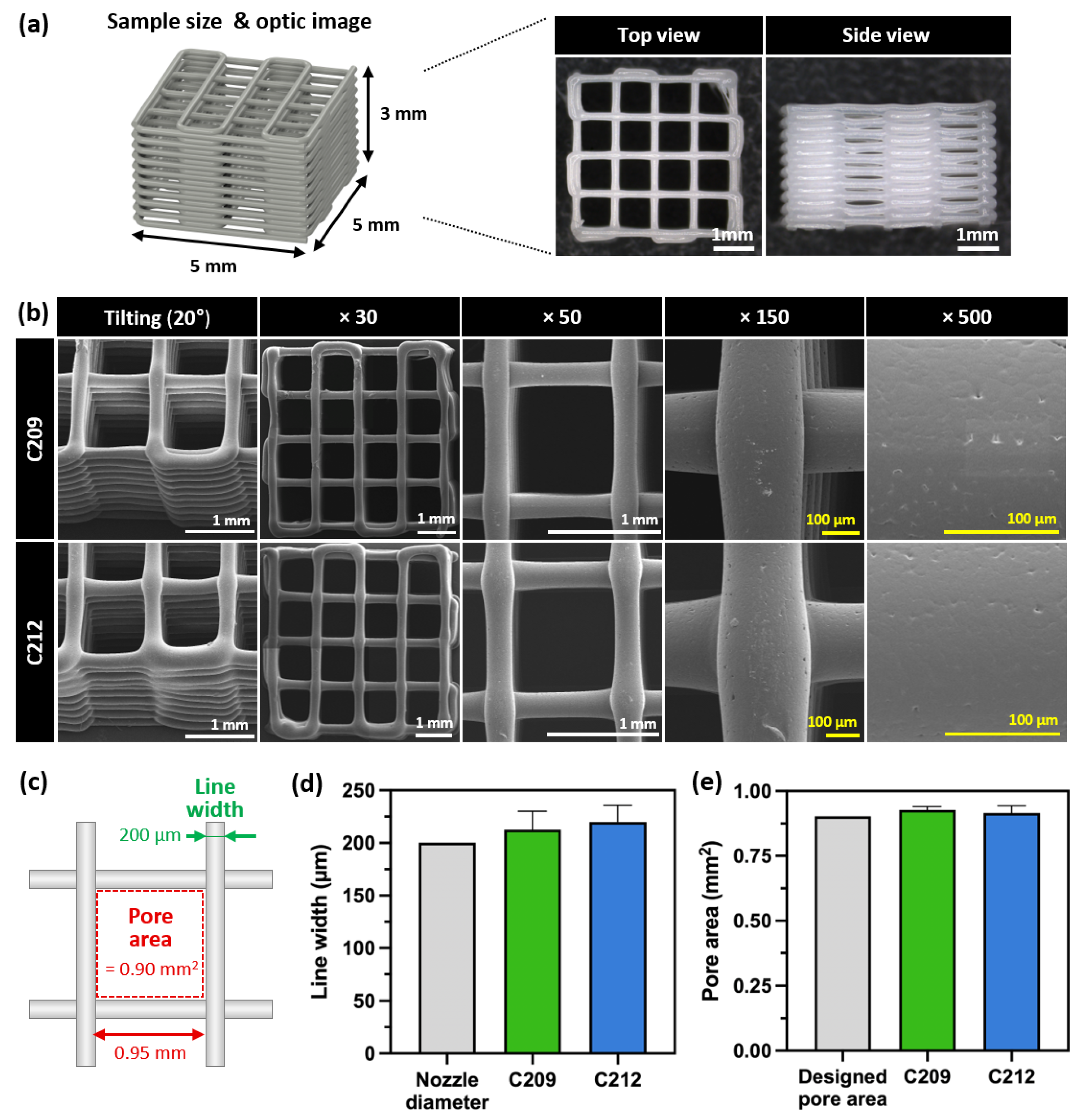
3.2. Characteristics and Morphological Analysis of 3D-Printed PCLs
3.2.1. Extrusion Properties of PCLs
3.2.2. Printability Evaluation of PCL
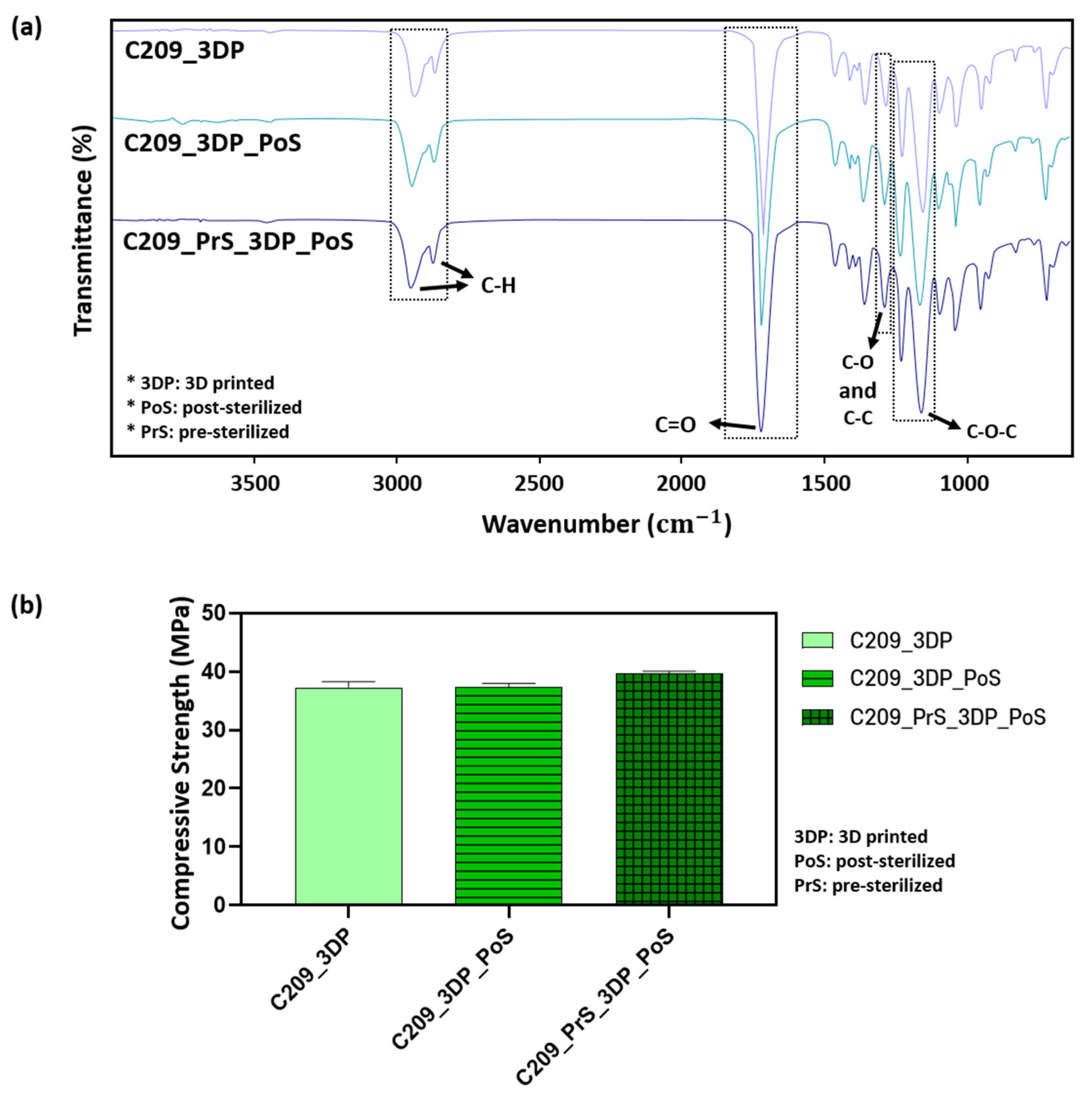
3.2.3. Evaluation of Mechanical and Chemical Properties After 3D Printing
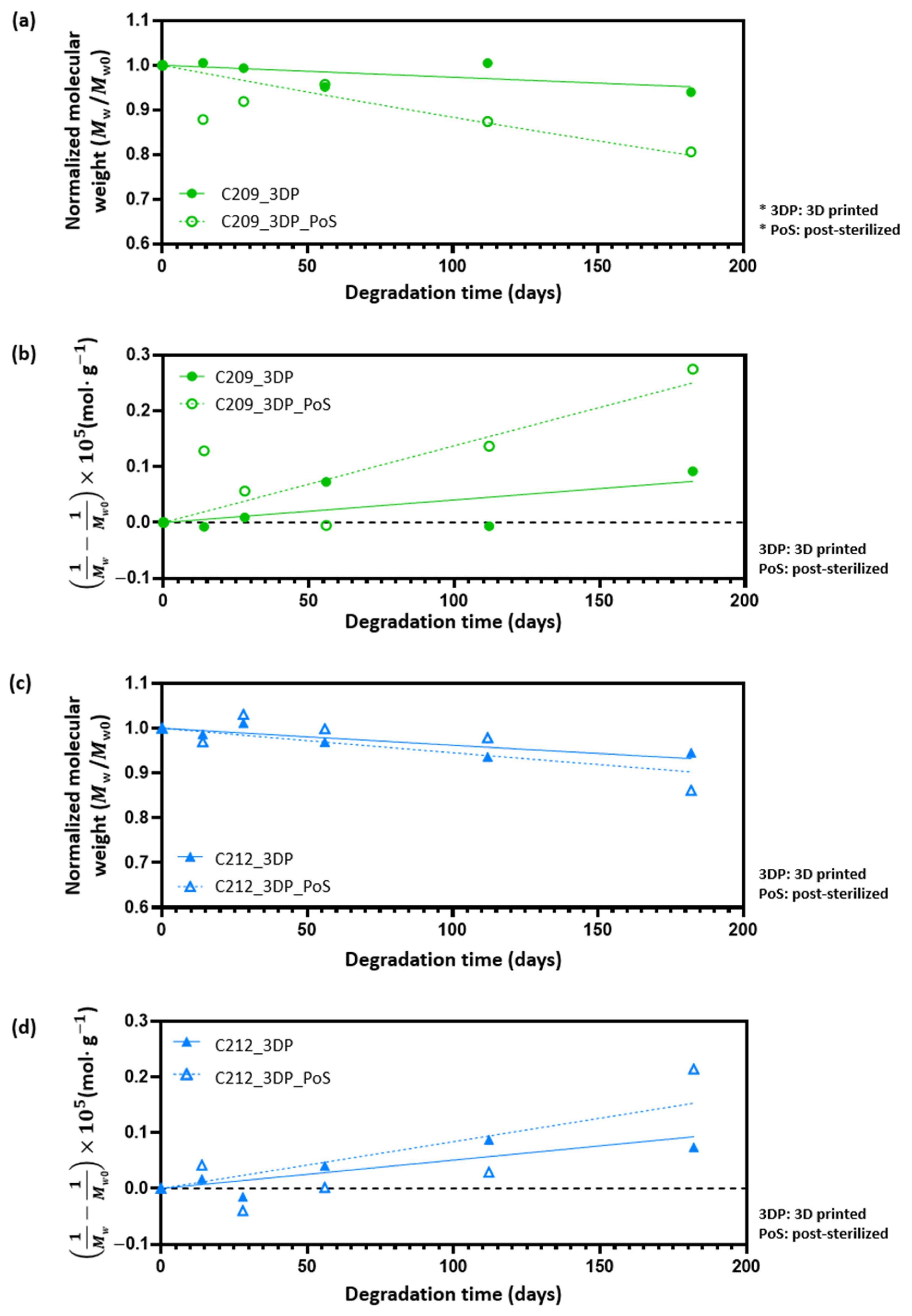
3.3. Degradation Behavior and Kinetic Modeling
3.4. Effect of Sterilization on PCL
3.5. Evaluation of Printability Under Long-Term Thermal Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gharibshahian, M.; Salehi, M.; Beheshtizadeh, N.; Kamalabadi-Farahani, M.; Atashi, A.; Nourbakhsh, M.S.; Alizadeh, M. Recent advances on 3D-printed PCL-based composite scaffolds for bone tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1168504. [Google Scholar] [CrossRef] [PubMed]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Li, P.; Gao, C.; Fu, L.; Liao, Z.; Tian, G.; Yin, H.; Li, M.; Sui, X.; Yuan, Z.; et al. Recent advances in tendon tissue engineering strategy. Front. Bioeng. Biotechnol. 2023, 11, 1115312. [Google Scholar] [CrossRef]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef]
- Wu, G.H.; Hsu, S.H. Review: Polymeric-Based 3D Printing for Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 285–292. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Rey, F.; Barzaghini, B.; Nardini, A.; Bordoni, M.; Zuccotti, G.V.; Cereda, C.; Raimondi, M.T.; Carelli, S. Advances in Tissue Engineering and Innovative Fabrication Techniques for 3-D-Structures: Translational Applications in Neurodegenerative Diseases. Cells 2020, 9, 1636. [Google Scholar] [CrossRef]
- Gleadall, A.; Visscher, D.; Yang, J.; Thomas, D.; Segal, J. Review of additive manufactured tissue engineering scaffolds: Relationship between geometry and performance. Burn. Trauma. 2018, 6, 19. [Google Scholar] [CrossRef]
- Mirasadi, K.; Yousefi, M.A.; Jin, L.; Rahmatabadi, D.; Baniassadi, M.; Liao, W.-H.; Bodaghi, M.; Baghani, M. 4D Printing of Magnetically Responsive Shape Memory Polymers: Toward Sustainable Solutions in Soft Robotics, Wearables, and Biomedical Devices. Adv. Sci. 2025, 12, e13091. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, A.M.; Cruz, R.; Rosales-Ibanez, R.; Hernandez-Sanchez, F.; Carrillo-Escalante, H.J.; Rodriguez-Martinez, J.J.; Velasquillo, C.; Talamas-Lara, D.; Ludert, J.E. In Vitro and In Vivo Evaluation of a Polycaprolactone (PCL)/Polylactic-Co-Glycolic Acid (PLGA) (80:20) Scaffold for Improved Treatment of Chondral (Cartilage) Injuries. Polymers 2023, 15, 2324. [Google Scholar] [CrossRef]
- Lizarazo-Fonseca, L.; Correa-Araujo, L.; Prieto-Abello, L.; Camacho-Rodriguez, B.; Silva-Cote, I. In vitro and in vivo evaluation of electrospun poly (epsilon-caprolactone)/collagen scaffolds and Wharton’s jelly mesenchymal stromal cells (hWJ-MSCs) constructs as potential alternative for skin tissue engineering. Regen. Ther. 2023, 24, 11–24. [Google Scholar] [CrossRef]
- Ramirez-Ruiz, F.; Nunez-Tapia, I.; Pina-Barba, M.C.; Alvarez-Perez, M.A.; Guarino, V.; Serrano-Bello, J. Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications. Bioengineering 2025, 12, 46. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Romero-Torrecilla, J.A.; Echanove-Gonzalez de Anleo, M.; Martinez-Oharriz, C.; Ripalda-Cemborain, P.; Lopez-Martinez, T.; Abizanda, G.; Valdes-Fernandez, J.; Prandota, J.; Muinos-Lopez, E.; Garbayo, E.; et al. 3D-printed polycaprolactone scaffolds functionalized with poly(lactic-co-glycolic) acid microparticles enhance bone regeneration through tunable drug release. Acta Biomater. 2025, 198, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Youssef, A.; Seher, A.; Hochleitner, G.; Dalton, P.D.; Hartmann, S.; Brands, R.C.; Muller-Richter, U.D.A.; Linz, C. Medical-grade polycaprolactone scaffolds made by melt electrospinning writing for oral bone regeneration—A pilot study in vitro. BMC Oral Health 2019, 19, 28. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Chatzinikolaidou, M.; Michalakis, K.; Tsouknidas, A. Clinical translation of polycaprolactone-based tissue engineering scaffolds, fabricated via additive manufacturing: A review of their craniofacial applications. Biomater. Adv. 2024, 162, 213902. [Google Scholar] [CrossRef]
- Russo Serafini, M.; Mowat, A.; Mustafa, S.; Saifzadeh, S.; Shabab, T.; Bas, O.; O’Rourke, N.; Hutmacher, D.W.; Medeiros Savi, F. 3D-Printed Medical-Grade Polycaprolactone (mPCL) Scaffold for the Surgical Treatment of Vaginal Prolapse and Abdominal Hernias. Bioengineering 2023, 10, 1242. [Google Scholar] [CrossRef]
- Kang, J.H.; Kaneda, J.; Jang, J.G.; Sakthiabirami, K.; Lui, E.; Kim, C.; Wang, A.; Park, S.W.; Yang, Y.P. The Influence of Electron Beam Sterilization on In Vivo Degradation of beta-TCP/PCL of Different Composite Ratios for Bone Tissue Engineering. Micromachines 2020, 11, 273. [Google Scholar] [CrossRef]
- Bruyas, A.; Moeinzadeh, S.; Kim, S.; Lowenberg, D.W.; Yang, Y.P. Effect of Electron Beam Sterilization on Three-Dimensional-Printed Polycaprolactone/Beta-Tricalcium Phosphate Scaffolds for Bone Tissue Engineering. Tissue Eng. Part A 2019, 25, 248–256. [Google Scholar] [CrossRef]
- Cubo-Mateo, N.; Rodriguez-Lorenzo, L.M. Design of Thermoplastic 3D-Printed Scaffolds for Bone Tissue Engineering: Influence of Parameters of “Hidden” Importance in the Physical Properties of Scaffolds. Polymers 2020, 12, 1546. [Google Scholar] [CrossRef]
- Yoshida, M.; Turner, P.R.; McAdam, C.J.; Ali, M.A.; Cabral, J.D. A comparison between beta-tricalcium phosphate and chitosan poly-caprolactone-based 3D melt extruded composite scaffolds. Biopolymers 2022, 113, e23482. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ghim, M.S.; Quan, M.; Kim, Y.Y.; Cho, Y.S. Experimental Verification of the Impact of the Contact Area between the Defect Site and the Scaffold on Bone Regeneration Efficacy. Polymers 2024, 16, 338. [Google Scholar] [CrossRef]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/beta-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Gwak, S.J.; Cho, Y.S. Fabrication of Polycaprolactone/Nano Hydroxyapatite (PCL/nHA) 3D Scaffold with Enhanced In Vitro Cell Response via Design for Additive Manufacturing (DfAM). Polymers 2021, 13, 1394. [Google Scholar] [CrossRef] [PubMed]
- Guedes, F.; Branquinho, M.V.; Biscaia, S.; Alvites, R.D.; Sousa, A.C.; Lopes, B.; Sousa, P.; Rema, A.; Amorim, I.; Faria, F.; et al. Gamma Irradiation Processing on 3D PCL Devices-A Preliminary Biocompatibility Assessment. Int. J. Mol. Sci. 2022, 23, 15916. [Google Scholar] [CrossRef]
- Griffin, M.; Naderi, N.; Kalaskar, D.M.; Malins, E.; Becer, R.; Thornton, C.A.; Whitaker, I.S.; Mosahebi, A.; Butler, P.E.M.; Seifalian, A.M. Evaluation of Sterilisation Techniques for Regenerative Medicine Scaffolds Fabricated with Polyurethane Nonbiodegradable and Bioabsorbable Nanocomposite Materials. Int. J. Biomater. 2018, 2018, 6565783. [Google Scholar] [CrossRef]
- Laubach, M.; Herath, B.; Bock, N.; Suresh, S.; Saifzadeh, S.; Dargaville, B.L.; McGovern, J.; Wille, M.L.; Hutmacher, D.W.; Medeiros Savi, F. In vivo characterization of 3D-printed polycaprolactone-hydroxyapatite scaffolds with Voronoi design to advance the concept of scaffold-guided bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1272348. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lo, T.S.; Lin, Y.T.; Chien, Y.H.; Lu, C.J.; Liu, S.J. Fabrication of Drug-Eluting Polycaprolactone/poly(lactic-co-glycolic Acid) Prolapse Mats Using Solution-Extrusion 3D Printing and Coaxial Electrospinning Techniques. Polymers 2021, 13, 2295. [Google Scholar] [CrossRef] [PubMed]
- KS M ISO 527-4; Plastics—Determination of Tensile Properties—Part 4: Test Conditions for Isotropic and Orthotropic Fibre-Reinforced Plastic Composites. Korea Testing & Research Institute: Gwacheon-si, Republic of Korea, 2023.
- KS M ISO 604; Plastics—Determination of Compressive Properties. Korea Testing & Research Institute: Gwacheon-si, Republic of Korea, 2002.
- KS M 0024; General Rules for Infrared Spectrophotometric Analysis. Korean Standards Service Network (KSSN): Seoul, Republic of Korea, 2022.
- ISO 11737-2; Sterilization of Health Care Products — Microbiological Methods — Part 2: Tests of Sterility Performed in the Definition, Validation and Maintenance of a Sterilization Process. International Organization for Standardization: Geneva, Switzerland, 2019.
- Nukala, S.G.; Kong, I.; Patel, V.I.; Kakarla, A.B.; Kong, W.; Buddrick, O. Development of Biodegradable Composites Using Polycaprolactone and Bamboo Powder. Polymers 2022, 14, 4169. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, H.; Mehdipour, A.; Dianat-Moghadam, H.; Salehi, R.; Khoshfetrat, A.B.; Hassani, A.; Mohammadnejad, D. PCL/Col I-based magnetic nanocomposite scaffold provides an osteoinductive environment for ADSCs in osteogenic cues-free media conditions. Stem Cell Res. Ther. 2022, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Mollaghadimi, B. Preparation and characterisation of polycaprolactone-fibroin nanofibrous scaffolds containing allicin. IET Nanobiotechnol. 2022, 16, 239–249. [Google Scholar] [CrossRef]
- Gokalp, N.; Ulker, C.; Guvenilir, Y.A. Synthesis of polycaprolactone via ring opening polymerization catalyzed by Candida antarctica lipase B immobilized onto an amorphous silica support. J. Polym. Mater. 2016, 33, 87–100. [Google Scholar]
- Rostami, M.; Jahed-Khaniki, G.; Molaee-Aghaee, E.; Shariatifar, N.; Sani, M.A.; Azami, M.; Rezvantalab, S.; Ramezani, S.; Ghorbani, M. Polycaprolactone/polyacrylic acid/graphene oxide composite nanofibers as a highly efficient sorbent to remove lead toxic metal from drinking water and apple juice. Sci. Rep. 2024, 14, 4372. [Google Scholar] [CrossRef]
- Izgordu, M.S.; Uzgur, E.I.; Ulag, S.; Sahin, A.; Karademir Yilmaz, B.; Kilic, B.; Ekren, N.; Oktar, F.N.; Gunduz, O. Investigation of 3D-Printed Polycaprolactone-/Polyvinylpyrrolidone-Based Constructs. Cartilage 2021, 13, 626S–635S. [Google Scholar] [CrossRef]
- Shojaei, S.; Nikuei, M.; Goodarzi, V.; Hakani, M.; Khonakdar, H.A.; Saeb, M.R. Disclosing the role of surface and bulk erosion on the viscoelastic behavior of biodegradable poly(ε-caprolactone)/poly(lactic acid)/hydroxyapatite nanocomposites. J. Appl. Polym. Sci. 2018, 136, 47151. [Google Scholar] [CrossRef]
- Mishra, S.; Zope, V.S.; Goje, A.S. Kinetic and thermodynamic studies of depolymerisation of poly(ethylene terephthalate) by saponification reaction. Polym. Int. 2002, 51, 1310–1315. [Google Scholar] [CrossRef]
- Zafar, S.; Khalid, N.; Daud, M.; Mirza, M.L. Kinetic Studies of the Adsorption of Thorium Ions onto Rice Husk from Aqueous Media: Linear and Nonlinear Approach. Nucleus 2015, 52, 14–19. [Google Scholar] [CrossRef]
- Gu, L.; Li, Y.; Yang, Y.; Wang, Z.; Jin, Y. Preparation and adsorption performance of cellulose-graft-polycaprolactone/polycaprolactone porous material. BioResources 2017, 12, 5539–5549. [Google Scholar] [CrossRef]
- Borovikov, P.I.; Sviridov, A.P.; Antonov, E.N.; Dunaev, A.G.; Krotova, L.I.; Fatkhudinov, T.K.; Popov, V.K. Model of aliphatic polyesters hydrolysis comprising water and oligomers diffusion. Polym. Degrad. Stab. 2019, 159, 70–78. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of photocatalytic degradation of organic compounds: A mini-review and new approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef]
- Ford Versypt, A.N.; Pack, D.W.; Braatz, R.D. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres—A review. J. Control. Release 2013, 165, 29–37. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Siparsky, G.L. Degradation Kinetics of Poly (Hydroxy) Acids: PLA and PCL; ACS Publications: Washington, DC, USA, 2000. [Google Scholar]
- Bhangare, D.; Rajput, N.; Jadav, T.; Sahu, A.K.; Tekade, R.K.; Sengupta, P. Systematic strategies for degradation kinetic study of pharmaceuticals: An issue of utmost importance concerning current stability analysis practices. J. Anal. Sci. Technol. 2022, 13, 7. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Polycaprolactone Composites/Blends and Their Applications Especially in Water Treatment. ChemEngineering 2023, 7, 104. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [PubMed]
- Carranza, T.; Zalba-Balda, M.; Baraibar, M.J.B.; de la Caba, K.; Guerrero, P. Effect of sterilization processes on alginate/gelatin inks for three-dimensional printing. Int. J. Bioprint. 2023, 9, 645. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Lykins, W.R.; Bernards, D.A.; Schlesinger, E.B.; Wisniewski, K.; Desai, T.A. Tuning polycaprolactone degradation for long acting implantables. Polymer 2022, 262, 125473. [Google Scholar] [CrossRef]
- Arraiza, A.L.; Sarasua, J.; Verdu, J.; Colin, X. Rheological behavior and modeling of thermal degradation of poly (∊-caprolactone) and poly (L-lactide). Int. Polym. Process. 2007, 22, 389–394. [Google Scholar] [CrossRef]






| Polymer Name | Inherent Viscosity (dL/g) | Composition | End Group | Company (Country) |
|---|---|---|---|---|
| RESOMER® C203 | 0.35–0.43 | Poly (ɛ-caprolactone) | Ester | Evonik (Germany) |
| RESOMER® C209 | 0.8–1.0 | |||
| RESOMER® C212 | 1.13–1.38 | |||
| RESOMER® C217 | 1.53–1.87 |
| Specimen Name | Pre-Sterilization | Post-Sterilization |
|---|---|---|
| PCL-3DP 1 | No | No |
| PCL-PrS 2 -3DP | Yes | No |
| PCL-3DP-PoS 3 | No | Yes |
| PCL-PrS-3DP-PoS | Yes | Yes |
| Kinetic Model | Material | (day−1) | |
|---|---|---|---|
| Pseudo-First-Order | C209 | 0.298 | |
| C212 | 8.294 × 10−7 | 0.770 | |
| Kinetic Model | Material | (day−1) | |
| Pseudo-Second-Order | C209 | 5.236 × 10−4 | 0.326 |
| C212 | 1.156 × 10−3 | 0.695 |
| Kinetic Model | Material | Post-Sterilization | (day−1) | |
|---|---|---|---|---|
| Pseudo-First-Order | C209 | No | 0.399 | |
| Yes | 0.413 | |||
| C212 | No | 0.715 | ||
| Yes | 0.604 | |||
| Kinetic Model | Material | Post-Sterilization | (day−1) | |
| Pseudo-Second-Order | C209 | No | 0.408 | |
| Yes | 0.642 | |||
| C212 | No | 0.715 | ||
| Yes | 0.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.C.; Kim, J.-S.; Yu, Y.J.; Yu, S.-G.; Lee, D.Y.; Lee, D.-M.; Gwak, S.-J.; Seo, K.D.; Lee, S.-J. Evaluation of Medical-Grade Polycaprolactone for 3D Printing: Mechanical, Chemical, and Biodegradation Characteristics. Polymers 2025, 17, 2730. https://doi.org/10.3390/polym17202730
Kim EC, Kim J-S, Yu YJ, Yu S-G, Lee DY, Lee D-M, Gwak S-J, Seo KD, Lee S-J. Evaluation of Medical-Grade Polycaprolactone for 3D Printing: Mechanical, Chemical, and Biodegradation Characteristics. Polymers. 2025; 17(20):2730. https://doi.org/10.3390/polym17202730
Chicago/Turabian StyleKim, Eun Chae, Jae-Seok Kim, Yun Jin Yu, Sang-Gi Yu, Dong Yeop Lee, Dong-Mok Lee, So-Jung Gwak, Kyoung Duck Seo, and Seung-Jae Lee. 2025. "Evaluation of Medical-Grade Polycaprolactone for 3D Printing: Mechanical, Chemical, and Biodegradation Characteristics" Polymers 17, no. 20: 2730. https://doi.org/10.3390/polym17202730
APA StyleKim, E. C., Kim, J.-S., Yu, Y. J., Yu, S.-G., Lee, D. Y., Lee, D.-M., Gwak, S.-J., Seo, K. D., & Lee, S.-J. (2025). Evaluation of Medical-Grade Polycaprolactone for 3D Printing: Mechanical, Chemical, and Biodegradation Characteristics. Polymers, 17(20), 2730. https://doi.org/10.3390/polym17202730







