Recent Progress in Cellulose-Based Aerogels for Sustainable Oil–Water Separation Technologies
Abstract
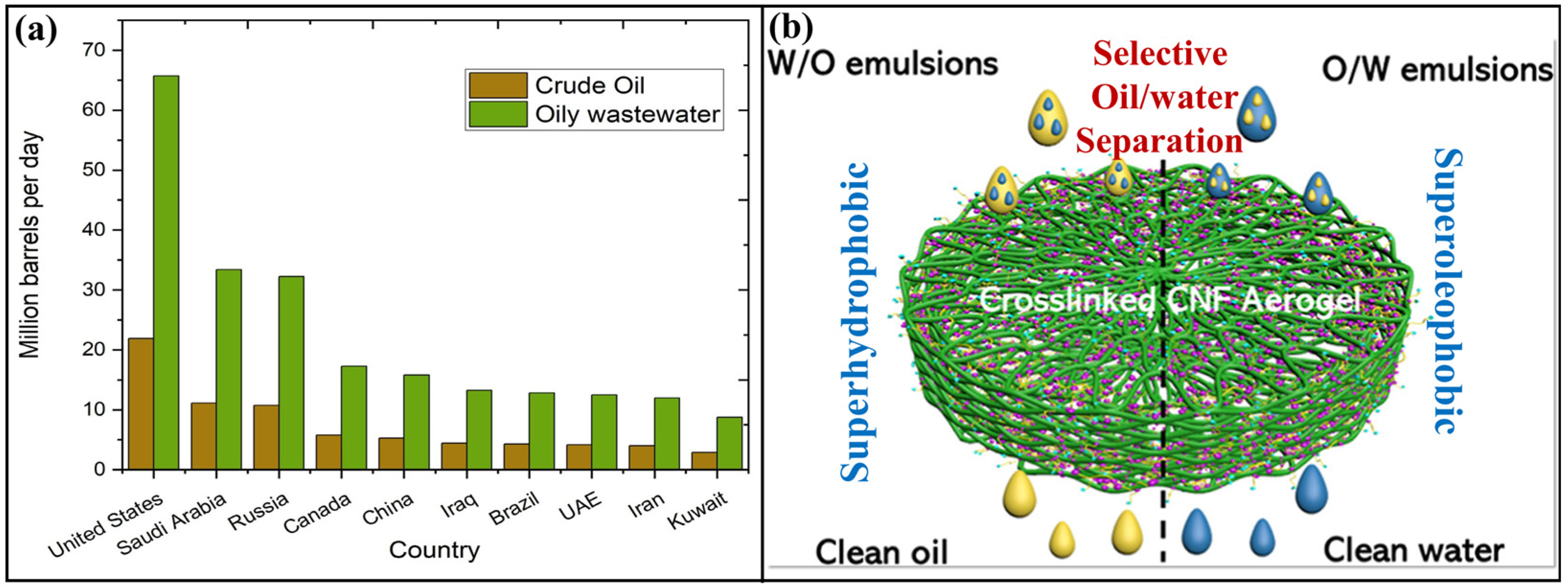
1. Introduction
1.1. Aerogels for Oil–Water Separation
1.2. Importance of Cellulose-Based Aerogels
2. Cellulose-Based Aerogels
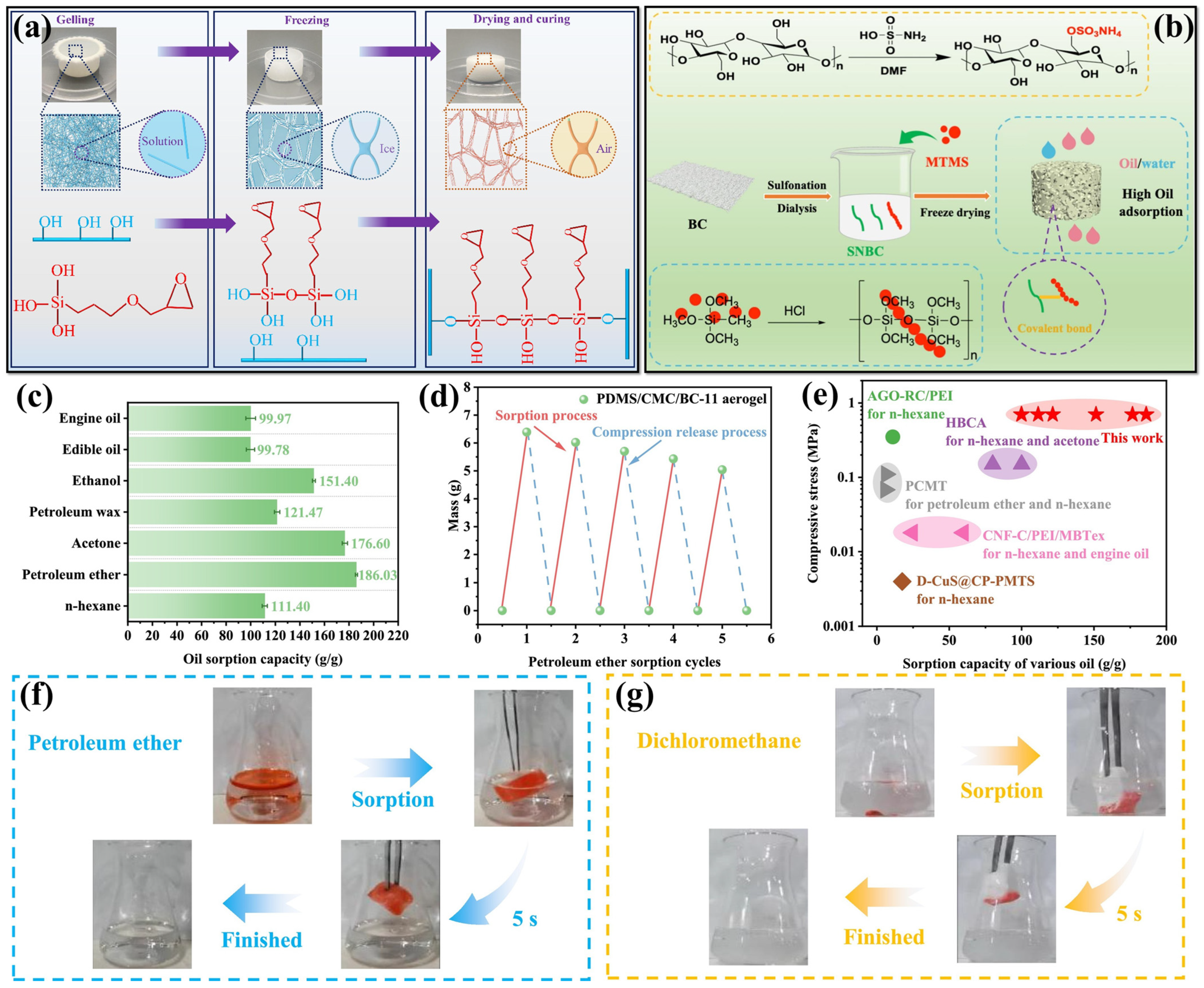
2.1. Regenerated Cellulose-Based Aerogels
2.2. Bacterial Cellulose-Based Aerogel
3. Cellulose with Different Kinds of Biopolymer Aerogels
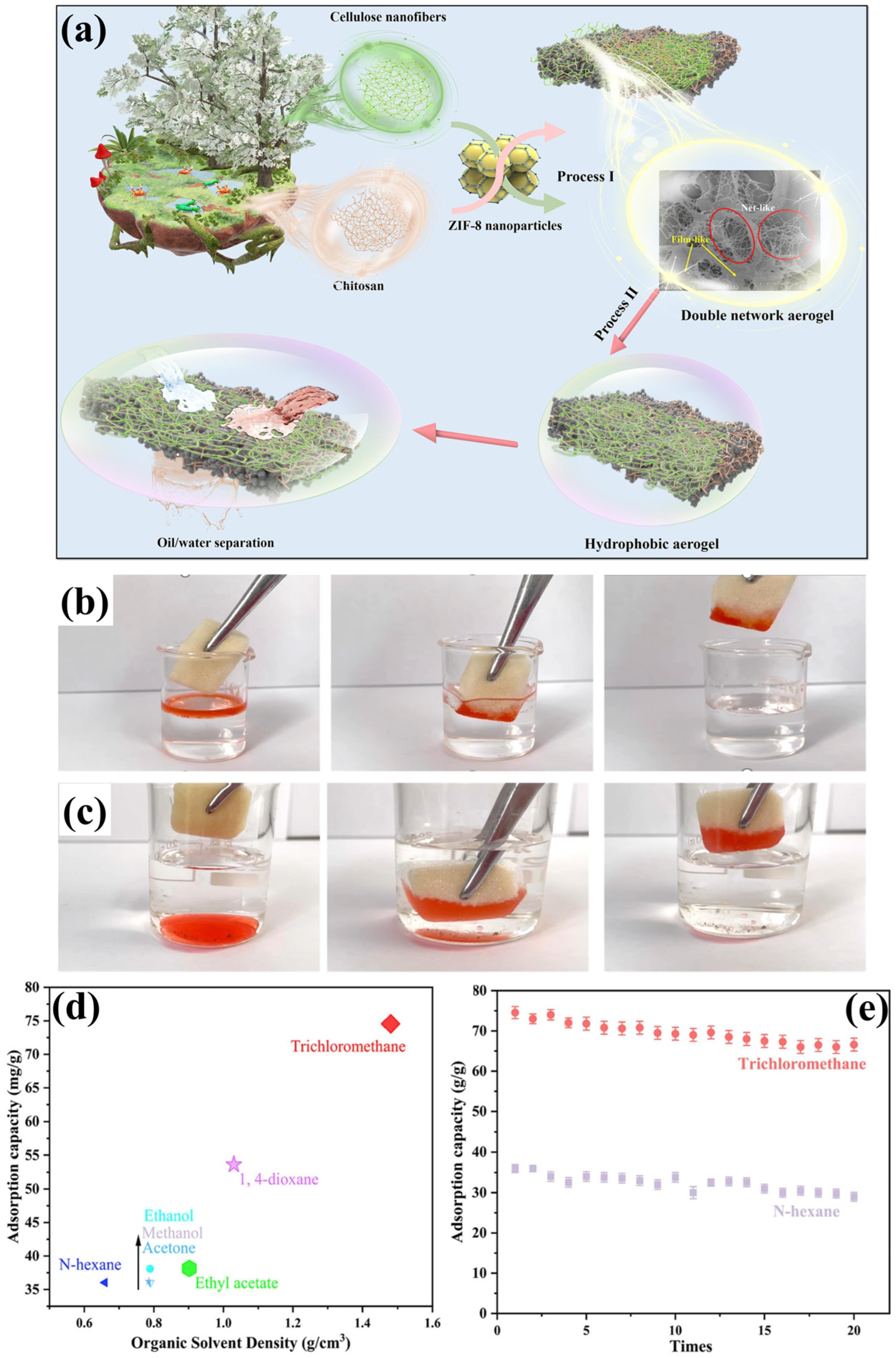
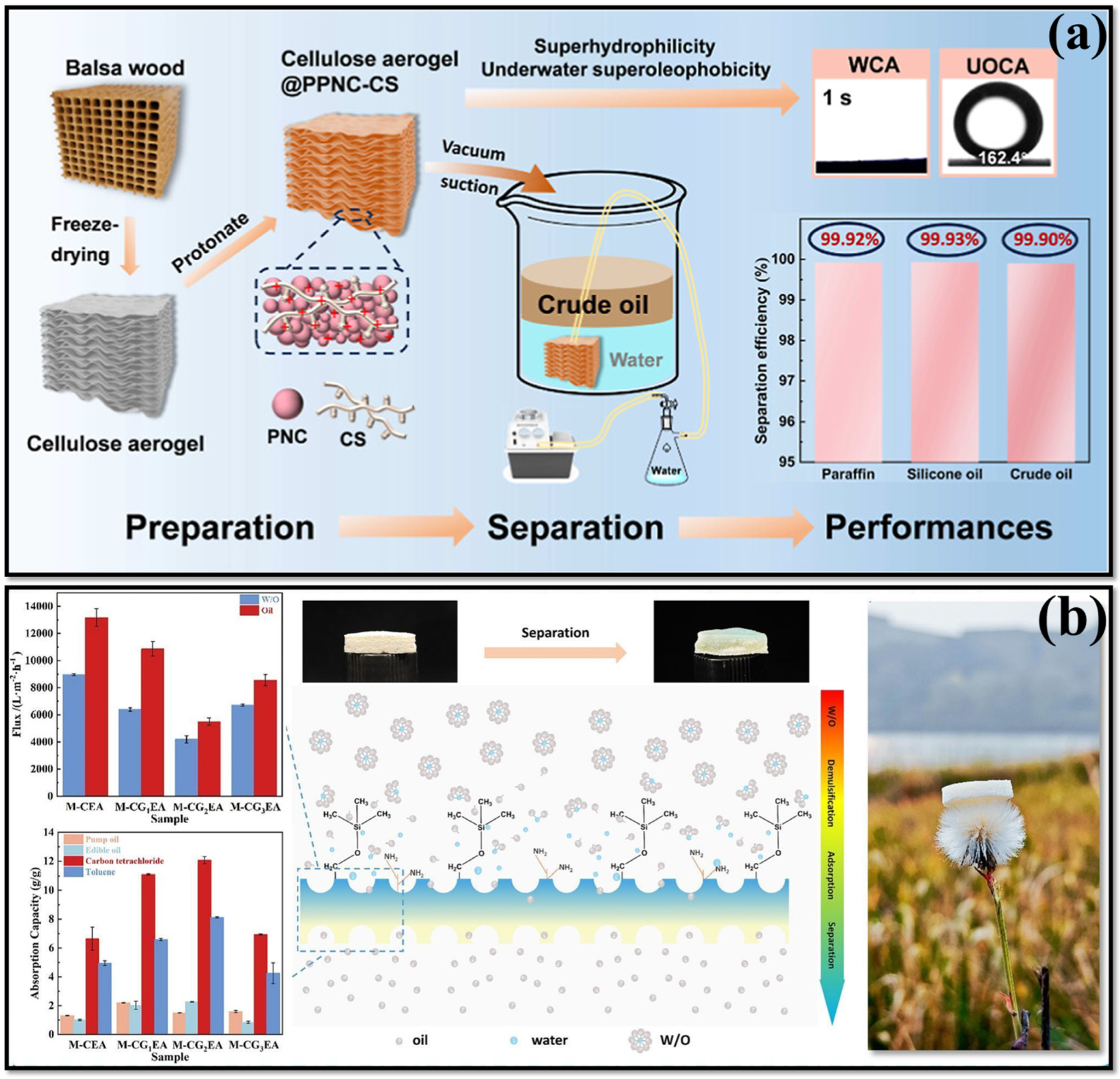
3.1. Chitosan-Cellulose Based Aerogel
3.2. Lignin-Cellulose Based Aerogels
3.3. Alginate-Cellulose Based Aerogels
4. Cellulose with Various Synthetic Polymer Aerogels
4.1. PVA-Cellulose Based Aerogels
4.2. PEI-Cellulose Based Aerogels
4.3. PDA-Cellulose Based Aerogel
4.4. Cellulose with Other Polymers as Aerogels
5. Cellulose and Organic/Inorganic Material Composite Aerogels
6. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ADP | Ammonium dihydrogen phosphate |
| APDMH | 3-(3′-acrylicacidpropylester)-5,5-dimethyl hydantoin |
| BC | Bacterial cellulose |
| bPEI | Branched polyethyleneimine |
| BTCA | 1,2,3,4-butanetetracarboxylic acid |
| CA | Contact angle |
| CC | Cyanuric chloride |
| CMC | Carboxymethyl cellulose |
| CNC | Cellulose nanocrystals |
| CS | Chitosan |
| CVD | Chemical vapor deposition |
| DES | Deep eutectic solvent |
| DNS | Deep eutectic solvent—N-methyl morpholine-N-oxide monohydrate |
| DPPA | Dipentaerythritol pentaacrylate |
| DTOS | Dodecyltrimethoxysilane |
| ECH | Epichlorhydrin |
| ETOS | Ethyl trimethoxysilane |
| GA | Glutaraldehyde |
| GL | Glycerol |
| GO | Graphene oxide |
| GPTMS | 3-glycidoxypropyltrimethoxysilane |
| HBCA | Hydrophobic BC-based aerogel |
| HDTMS | Hexadecyltrimethoxysilane |
| ICO | Silylated castor oil |
| IPTES | 3-isocyanatopropyltriethoxysilane |
| JACA | Janus all -cellulose aerogel |
| LCMA | Lignin containing cellulose aerogel |
| MOF | Metal–Organic Framework |
| MPa | Megapascal |
| mPEI | modified polyethyleneimine |
| MTCS | Methyltrichlorosilane |
| MTMS | Methyltrimethoxylsilane |
| MWCNT | Multi-Walled Carbon Nanotube |
| NFC | Nanofibrillated cellulose |
| NM | Natural microfibrils |
| NMMO⋅H2O | N-methyl morpholine-N-oxide monohydrate |
| NP | Nanoparticle |
| OCA | Oil contact angle |
| OTMS | Octadecyltrichlorosilane |
| PDA | Polydopamine |
| PDMS | Polydimethylsiloxane |
| PDSO | Poly(dodecylsiloxane) |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PLA | Polylactic acid |
| PMTS | Polymethyltrimethoxysilane |
| PNC-CS | Polymeric nanocross-linked chitosan |
| PU | Polyurethane |
| PUF | Polyurethane foam |
| PVA | Polyvinyl alcohol |
| PVTMS | Polyvinyltrimethoxysilane |
| QHS | Quaternarized N-halamine siloxane |
| RCA | Regenerated cellulose |
| SA | Sodium alginate |
| SBC | Silanized bacterial cellulose |
| SBCA | Silylated bacterial cellulose aerogels |
| SHI–OP | Superhydrophilic–oleophobic |
| SNBC | Sulfonated nanofibrillated bacterial cellulose |
| SiO2 | Silica |
| TA | Tannic acid |
| TEOS | Tetraethyl orthosilicate |
| TiO2 | Titanium dioxide |
| VNFC | Vinylated nanofibrillated cellulose |
| WCA | Water contact angle |
References
- Saxena, V. Water quality, air pollution, and climate change: Investigating the environmental impacts of industrialization and urbanization. Water Air Soil Pollut. 2025, 236, 73. [Google Scholar] [CrossRef]
- Swain, C.K. Environmental pollution indices: A review on concentration of heavy metals in air, water, and soil near industrialization and urbanisation. Discov. Environ. 2024, 2, 5. [Google Scholar] [CrossRef]
- Singha, W.J.; Deka, H. Ecological and human health risk associated with heavy metals (HMs) contaminant sourced from petroleum refinery oily sludge. J. Hazard. Mater. 2024, 476, 135077. [Google Scholar] [CrossRef]
- Sharma, K.; Rajan, S.; Nayak, S.K. Water pollution: Primary sources and associated human health hazards with special emphasis on rural areas. In Water Resources Management for Rural Development; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–14. [Google Scholar]
- Singh, V. Water pollution. In Textbook of Environment and Ecology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 253–266. [Google Scholar]
- Erfani, H.; Madhu, N.R.; Khodayari, S.; Qureshi, M.A.; Swetanshu; Singh, P.; Jadoun, S. Separation and removal of oil from water/wastewater in the oil industry: A review. Environ. Technol. Rev. 2024, 13, 325–343. [Google Scholar] [CrossRef]
- Odoom, J.; Iorhemen, O.T.; Li, J. Advances in adsorption for oily wastewater treatment: Eco-friendly adsorbents and analytical insights. Energy Ecol. Environ. 2025, 10, 15–44. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Liu, X.; He, C.; Luo, S.; Tian, Q. Facile Preparation of iPP Fibrous Membranes from In Situ Microfibrillar Composites for Oil/Water Separation. Polymers 2025, 17, 2114. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Li, Y.; Zheng, Y.; Chen, J.; Zhang, X.; Li, K.; Shen, J.; Guo, X. Study on the Electrospinning Fabrication of PCL/CNTs Fiber Membranes and Their Oil–Water Separation Performance. Polymers 2025, 17, 1705. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liang, L.; Tu, F.; Li, Z.; Tang, X.; Dai, L.; Li, L. Research progress on membrane separation technology for oily wastewater treatment. Toxics 2024, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Kovrov, O.; Kulikova, D. Development of the oil-contaminated wastewater treatment technology for trucking companies. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2024; Volume 1348, p. 012023. [Google Scholar] [CrossRef]
- Chaouch, N.; Bouziane, K. Treatment of Oily Wastewater from Oil Fields in Southeast Algeria; Springer: Cham, Switzerland, 2024; pp. 347–353. [Google Scholar]
- Jiang, J.; Wan, S.; Wen, C.; Tang, L.; Xu, N. Frontiers in Innovative Materials and Technologies for Oil–Water Separation. Polymers 2025, 17, 1635. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Abousnina, R.; Ghaffour, N.; Nghiem, L.D. Mitigating offshore oily wastewater pollution: Sustainable strategies for treatment, disposal, and reuse. Mar. Pollut. Bull. 2025, 219, 118240. [Google Scholar] [CrossRef] [PubMed]
- Nath, F.; Chowdhury, M.O.; Rhaman, M.M. Navigating Produced Water Sustainability in the Oil and Gas Sector: A Critical Review of Reuse Challenges, Treatment Technologies, and Prospects Ahead. Water 2023, 15, 4088. [Google Scholar] [CrossRef]
- Al-Rajabi, M.M.; Almanassra, I.W.; Khalil, A.K.A.; Atieh, M.A.; Laoui, T.; Khalil, K.A. Facile Coaxial Electrospinning Synthesis of Polyacrylonitrile/Cellulose Acetate Nanofiber Membrane for Oil–Water Separations. Polymers 2023, 15, 4594. [Google Scholar] [CrossRef]
- Shahbaz, M.; Rashid, N.; Saleem, J.; Mackey, H.; McKay, G.; Al-Ansari, T. A review of waste management approaches to maximise sustainable value of waste from the oil and gas industry and potential for the State of Qatar. Fuel 2023, 332, 126220. [Google Scholar] [CrossRef]
- Abousnina, R.M.; Nghiem, L.D.; Bundschuh, J. Comparison between oily and coal seam gas produced water with respect to quantity, characteristics and treatment technologies: A review. Desalination Water Treat. 2015, 54, 1793–1808. [Google Scholar] [CrossRef]
- Scurtu, C.T. Treatment of Produced Water: Targeting Dissolved Compounds to Meet a Zero Harmful Discharge in Oil and Gas Production. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2009. [Google Scholar]
- Mamozai, W.; Hesam, A.M.; Hemma, W.H. Impacts of crude oils on water quality: A comprehensive review. Eur. J. Theor. Appl. Sci. 2024, 2, 126–138. [Google Scholar] [CrossRef]
- Muvel, H.; Jindal, M.K.; Tewari, P.K.; Anand, V. Minimizing oil pollution: A review of current status and its treatment options. RSC Sustain. 2025, 3, 3681–3723. [Google Scholar] [CrossRef]
- Pal, D.; Sen, S. In-depth coverage of petroleum waste sources, characteristics, environmental impact, and sustainable remediation process. In Impact of Petroleum Waste on Environmental Pollution and Its Sustainable Management Through Circular Economy; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–38. [Google Scholar]
- Yang, K.; Wang, S.; Du, B.; Zhou, S. An Environmentally Friendly Superhydrophobic Wood Sponge with Photo/Electrothermal Effects Prepared from Natural Wood for All-Weather High-Viscosity Oil–Water Separation. Polymers 2024, 16, 3256. [Google Scholar] [CrossRef]
- Bi, Q.; Zhao, Q.; Qin, Z.; Gao, W.; Li, Z.; Deng, S.; Mo, L. Lightweight, robust, and antimicrobial cellulose nanofibril aerogels decorated with TiO2@PDA core–shell nanoparticles for highly efficient oil–water separation. Chem. Eng. J. 2025, 507, 159989. [Google Scholar] [CrossRef]
- Pi, P.; Ren, Z.; Yang, Y.; Chen, W.; Lin, Y. A review of various dimensional superwetting materials for oil–water separation. Nanoscale 2024, 16, 17248–17275. [Google Scholar] [CrossRef]
- Huang, J.; Ran, X.; Sun, L.; Bi, H.; Wu, X. Recent advances in membrane technologies applied in oil–water separation. Discov. Nano 2024, 19, 66. [Google Scholar] [CrossRef]
- Wang, X.; An, J.; Hassan, A.; Gao, Q.; Liu, X.; Boudaoud, H. Innovative 3D-Printed Superhydrophobic Porous Architectures for Continuous Oil–Water Separation. Polymers 2025, 17, 1465. [Google Scholar] [CrossRef]
- Jiang, Q.; Mo, J.; Han, S.; Liu, X.; Qu, B.; Xie, J.; Wang, X.; Zhao, J. Balsam-Pear-Skin-Like-Structure Polyvinylidene Fluoride/Ethylene–Vinyl Alcohol Fibrous Membrane for Highly Efficient Oil/Water Separation Through One-Step Electrospinning. Polymers 2025, 17, 1389. [Google Scholar] [CrossRef]
- Amran, N.A.; Mustapha, S.N.A. Oil–water separation techniques for bilge water treatment. In Resources of Water; BoD: Delhi, India, 2021; Volume 147. [Google Scholar]
- Zulfiqar, I.; Shehzadi, I.; Hussain, N. Principles of oil-water separation strategies. In Nanotechnology for Oil-Water Separation; Elsevier: Amsterdam, The Netherlands, 2024; pp. 49–81. [Google Scholar]
- Sadatshojaie, A.; Wood, D.A.; Jokar, S.M.; Rahimpour, M.R. Applying ultrasonic fields to separate water contained in medium-gravity crude oil emulsions and determining crude oil adhesion coefficients. Ultrason. Sonochem. 2021, 70, 105303. [Google Scholar] [PubMed]
- Yu, J.; Cao, C.; Pan, Y. Advances of adsorption and filtration techniques in separating highly viscous crude oil/water mixtures. Adv. Mater. Interfaces 2021, 8, 2100061. [Google Scholar] [CrossRef]
- Saini, H.; Otyepková, E.; Schneemann, A.; Zbořil, R.; Otyepka, M.; Fischer, R.A.; Jayaramulu, K. Hierarchical porous metal–organic framework materials for efficient oil–water separation. J. Mater. Chem. A 2022, 10, 2751–2785. [Google Scholar]
- Maggay, I.V.; Chang, Y.; Venault, A.; Dizon, G.V.; Wu, C.-J. Functionalized porous filtration media for gravity-driven filtration: Reviewing a new emerging approach for oil and water emulsions separation. Sep. Purif. Technol. 2021, 259, 117983. [Google Scholar] [CrossRef]
- Fouladi, M.; Kavousi Heidari, M.; Tavakoli, O. Development of porous biodegradable sorbents for oil/water separation: A critical review. J. Porous Mater. 2023, 30, 1037–1053. [Google Scholar] [CrossRef]
- Feng, T.; Fu, L.; Mu, Z.; Wei, W.; Li, W.; Liang, X.; Ma, L.; Wu, Y.; Wang, X.; Wu, T.; et al. Bicomponent Electrospinning of PVDF-Based Nanofiber Membranes for Air Filtration and Oil–Water Separation. Polymers 2025, 17, 703. [Google Scholar] [CrossRef]
- Tang, C.; Yan, X.; Tam, K.C. Cross-Linked Cellulose Nanofibril Aerogel with Multiscaled Pores and Dual Wettability for Emulsion Separation. ACS Sustain. Chem. Eng. 2024, 12, 656–664. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Liu, Y.; Craig, V.S.J.; Lai, Z. Selective separation of oil and water with mesh membranes by capillarity. Adv. Colloid Interface Sci. 2016, 235, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Patel, R. Review on oil/water separation membrane technology. Membr. J. 2020, 30, 359–372. [Google Scholar] [CrossRef]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef]
- Liu, P.; Chen, X.; Li, Y.; Cheng, P.; Tang, Z.; Lv, J.; Aftab, W.; Wang, G. Aerogels meet phase change materials: Fundamentals, advances, and beyond. ACS Nano 2022, 16, 15586–15626. [Google Scholar] [CrossRef]
- Liebner, F.; Plappert, S. Nanocellulose-Based Aerogels. In The Handbook of Paper-Based Sensors and Devices: Volume 1: Materials and Technologies; Springer: Berlin/Heidelberg, Germany, 2025; pp. 519–546. [Google Scholar]
- Segneanu, A.-E.; Herea, D.-D.; Buema, G.; Bradu, I.A.; Cepan, M.; Grozescu, I. Advanced Aerogels for Water Remediation: Unraveling Their Potential in Fats, Oils, and Grease Sorption—A Comprehensive Review. Gels 2025, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hao, M.; Chen, Z.; Ramakrishna, S.; Liu, Y.; Wang, X.; Hu, X.; Wei, Y. Recent advances in the development of nanofiber-based aerogel for oil-water separation: A review. Fuel 2023, 354, 129338. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; He, J.; Zhao, Y.; Mu, L.; Liu, X.; Zhang, Y.; Sun, C.-L.; Zhang, N.; Qu, M. A review of superwetting aerogel-based oil-water separation materials. Mater. Today Sustain. 2024, 26, 100741. [Google Scholar] [CrossRef]
- Fu, Y.; Guo, Z. Natural polysaccharide-based aerogels and their applications in oil–water separations: A review. J. Mater. Chem. A 2022, 10, 8129–8158. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Wang, X.; Wang, Z.; Yu, D.; Ji, D. High-hydrophobic ZIF-67@PLA honeycomb aerogel for efficient oil–water separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130768. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Liu, X.; Su, Y.; Wang, J.; Fan, T.; Ning, X.; Ramakrishn, S.; Long, Y.-Z. Ultralight and multifunctional PVDF/SiO2@GO nanofibrous aerogel for efficient harsh environmental oil-water separation and crude oil absorption. Carbon 2022, 193, 77–87. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, S.; Huang, C.; Deng, F.; Liu, J.; Liu, C.; Li, Y. Superhydrophilic and Underwater Superoleophobic Copper Mesh Coated with Bamboo Cellulose Hydrogel for Efficient Oil/Water Separation. Polymers 2024, 16, 14. [Google Scholar] [CrossRef]
- Romero-Montero, A.; Valencia-Bermúdez, J.L.; Rosas-Meléndez, S.A.; Núñez-Tapia, I.; Piña-Barba, M.C.; Leyva-Gómez, G.; Del Prado-Audelo, M.L. Biopolymeric Fibrous Aerogels: The Sustainable Alternative for Water Remediation. Polymers 2023, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Yagoub, H.; Zhu, L.; Shibraen, M.H.M.A.; Altam, A.A.; Babiker, D.M.D.; Liang, S.; Jin, Y.; Yang, S. Complex Aerogels Generated from Nano-Polysaccharides and Its Derivatives for Oil–Water Separation. Polymers 2019, 11, 1593. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, T.; Ge-Zhang, S.; Mu, P.; Liu, Y.; Cui, J. Wood Sponge for Oil–Water Separation. Polymers 2024, 16, 2362. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Y.; Tao, H.; Yuan, B. Bio-Based Porous Aerogel with Bionic Structure and Hydrophobic Polymer Coating for Efficient Absorption of Oil/Organic Liquids. Polymers 2022, 14, 4579. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.A.; HPS, A.K.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; A.K., A.S.; Adnan, A.S. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Zhang, Y.-Q.; Gao, C.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R. Superhydrophobic aerogel membrane with integrated functions of biopolymers for efficient oil/water separation. Sep. Purif. Technol. 2022, 282, 120138. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef]
- Sozcu, S.; Frajova, J.; Wiener, J.; Venkataraman, M.; Tomkova, B.; Militky, J. Effect of Drying Methods on the Thermal and Mechanical Behavior of Bacterial Cellulose Aerogel. Gels 2024, 10, 474. [Google Scholar] [CrossRef]
- Amini, M.; Isari, A.A.; Ghasemi, S.; Banvillet, G.; Rojas, O.J.; Kamkar, M.; Arjmand, M. Tailoring pore structure in nanocellulose cryogels: Enhancing thermal and electromagnetic interference shielding properties. Carbohydr. Polym. 2025, 357, 123435. [Google Scholar] [CrossRef]
- Shang, Q.; Chen, J.; Hu, Y.; Yang, X.; Hu, L.; Liu, C.; Ren, X.; Zhou, Y. Facile Fabrication of Superhydrophobic Cross-Linked Nanocellulose Aerogels for Oil–Water Separation. Polymers 2021, 13, 625. [Google Scholar] [CrossRef]
- Romero-Montero, A.; Rosas-Melendez, S.A.; Valencia-Bermúdez, J.L.; Nuñez-Tapia, I.; Piña-Barba, M.C.; Melgoza-Ramírez, L.J.; Leyva-Gómez, G.; Del Prado-Audelo, M.L. Oil/water separation by super-hydrophobic wastepaper cellulose-candelilla wax cryogel: A circular material-based alternative. Front. Mater. 2023, 10, 1308094. [Google Scholar]
- He, Z.; Zhang, X.; Batchelor, W. Cellulose nanofibre aerogel filter with tuneable pore structure for oil/water separation and recovery. RSC Adv. 2016, 6, 21435–21438. [Google Scholar] [CrossRef]
- Tan, Z.; Hu, L.; Yang, D.; Zheng, D.; Qiu, X. Lignin: Excellent hydrogel swelling promoter used in cellulose aerogel for efficient oil/water separation. J. Colloid Interface Sci. 2023, 629, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Nikiforov, A.; Gromov, M.; Ostrikov, K.; De Geyter, N.; Morent, R. Plasma-aerosol-assisted surface engineering for scalable oil/water membrane separation. Appl. Surf. Sci. 2022, 606, 154807. [Google Scholar] [CrossRef]
- Tang, R.; Xu, S.; Hu, Y.; Wang, J.; Lu, C.; Wang, L.; Zhou, Z.; Liao, D.; Zhang, H.; Tong, Z. Multifunctional nano-cellulose aerogel for efficient oil–water separation: Vital roles of magnetic exfoliated bentonite and polyethyleneimine. Sep. Purif. Technol. 2023, 314, 123557. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Liao, Q.; Su, X.; Zhu, W.; Hua, W.; Qian, Z.; Liu, L.; Yao, J. Flexible and durable cellulose aerogels for highly effective oil/water separation. RSC Adv. 2016, 6, 63773–63781. [Google Scholar] [CrossRef]
- Abdullah; Zou, Y.; Farooq, S.; Walayat, N.; Zhang, H.; Faieta, M.; Pittia, P.; Huang, Q. Bio-aerogels: Fabrication, properties and food applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 6687–6709. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Pour, G.; Beauger, C.; Rigacci, A.; Budtova, T. Xerocellulose: Lightweight, porous and hydrophobic cellulose prepared via ambient drying. J. Mater. Sci. 2015, 50, 4526–4535. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Yu, Y.; Chen, W.; Hu, H.; Li, H. Naturally Dried Graphene Aerogels with Superelasticity and Tunable Poisson’s Ratio. Adv. Mater. (Deerfield Beach Fla.) 2016, 28, 9223–9230. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Azimi, B.; Sepahvand, S.; Ismaeilimoghadam, S.; Kargarzadeh, H.; Ashori, A.; Jonoobi, M.; Danti, S. Application of cellulose-based materials as water purification filters; a state-of-the-art review. J. Polym. Environ. 2024, 32, 345–366. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, X.; Weber, C.C.; Varley, R.J.; Henderson, L.C. Review of plant cellulose-based aerogel materials for oil/water mixture separation. J. Environ. Chem. Eng. 2024, 12, 113716. [Google Scholar] [CrossRef]
- Chhajed, M.; Verma, C.; Maji, P.K. Recent advances in hydrophobic nanocellulose aerogels for oil spill applications: A review. Mar. Pollut. Bull. 2024, 199, 116024. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, J.; Cai, M.; Xu, Q.; Zhang, J.; Cao, X.; Zhang, J.; Chen, Y. Advanced superhydrophobic and multifunctional nanocellulose aerogels for oil/water separation: A review. Carbohydr. Polym. 2023, 300, 120242. [Google Scholar] [CrossRef]
- Liao, W.; Wang, Y.-Z. Cellulose-Based Absorbents for Oil Contaminant Removal. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 951–977. [Google Scholar]
- Hammouda, S.b.; Chen, Z.; An, C.; Lee, K. Recent advances in developing cellulosic sorbent materials for oil spill cleanup: A state-of-the-art review. J. Clean. Prod. 2021, 311, 127630. [Google Scholar] [CrossRef]
- Rafieian, F.; Hosseini, M.; Jonoobi, M.; Yu, Q. Development of hydrophobic nanocellulose-based aerogel via chemical vapor deposition for oil separation for water treatment. Cellulose 2018, 25, 4695–4710. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Cellulose nanofibril aerogels: Synergistic improvement of hydrophobicity, strength, and thermal stability via cross-linking with diisocyanate. ACS Appl. Mater. Interfaces 2017, 9, 2825–2834. [Google Scholar] [CrossRef]
- Fumagalli, M.; Ouhab, D.; Boisseau, S.M.; Heux, L. Versatile gas-phase reactions for surface to bulk esterification of cellulose microfibrils aerogels. Biomacromolecules 2013, 14, 3246–3255. [Google Scholar] [CrossRef]
- Russler, A.; Wieland, M.; Bacher, M.; Henniges, U.; Miethe, P.; Liebner, F.; Potthast, A.; Rosenau, T. AKD-Modification of bacterial cellulose aerogels in supercritical CO2. Cellulose 2012, 19, 1337–1349. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, G.; Xu, B.; Guo, J. Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw. Green Process. Synth. 2024, 13, 20240063. [Google Scholar] [CrossRef]
- Buchtová, N.; Pradille, C.; Bouvard, J.-L.; Budtova, T. Mechanical properties of cellulose aerogels and cryogels. Soft Matter 2019, 15, 7901–7908. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Xing, T.; He, A.; Luo, Y.; Wang, M.; Qiao, S.; Tong, A.; Shi, Z.; Liao, X.; et al. Advances in regenerated cellulosic aerogel from waste cotton textile for emerging multidimensional applications. Int. J. Biol. Macromol. 2024, 270, 132462. [Google Scholar] [CrossRef] [PubMed]
- Suriyachai, N.; Khongchamnan, P.; Laosiripojana, N.; Kreetachat, T.; Wongcharee, S.; Sakulthaew, C.; Chokejaroenrat, C.; Imman, S. Optimization of Cellulose Recovery Using Deep Eutectic Solvent Fractionation: A Response Surface Method Approach. Energies 2024, 17, 4257. [Google Scholar] [CrossRef]
- Orozco, S.E.; Hoheneder, R.; Steiner, K.; Frécaut, S.; Fitz, E.; Bischof, R.H. Coagulation temperature during cellulose regeneration from N-methylmorpholine-N-oxide (NMMO) influences the structure and ease of saccharification of lyocell byproducts. Carbohydr. Polym. 2025, 363, 123618. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Xu, Y.; Zhang, H.; Cai, R.; Guo, Z.; Zhou, L.; Zhang, J.; Yuan, Y. Mechanism of cellulose regeneration from its ionic liquid solution as revealed by infrared spectroscopy. Polymer 2022, 257, 125280. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Li, J.; Xie, F.; Yang, H.; Wang, C.; Fahlman, B.D.; Li, W. Natural microfibrils/regenerated cellulose-based carbon aerogel for highly efficient oil/water separation. J. Hazard. Mater. 2023, 454, 131397. [Google Scholar] [CrossRef]
- Dong, B.; Yao, P.; Xie, F.; Yang, H.; Arenal, R.; Li, W. Kapok/regenerated cellulose based carbon aerogel prepared at a low-temperature carbonization for oil/water separation. Ind. Crops Prod. 2024, 222, 119743. [Google Scholar] [CrossRef]
- Ma, X.; Dong, B.; Xie, F.; Yang, H.; Wang, C.; Bittencourt, C.; Snyders, R.; Li, W. A novel cost-effective kapok fibers and regenerated cellulose-based carbon aerogel for continuous oil/water separation. Sep. Purif. Technol. 2025, 353, 128435. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Yu, Y.; Li, H.; Li, H.; Bai, J.; Shi, F.; Liu, J. Bacterial cellulose biomass aerogels for oil-water separation and thermal insulation. J. Environ. Chem. Eng. 2023, 11, 110403. [Google Scholar] [CrossRef]
- Hu, X.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Ramakrishna, S.; Wang, X.; Yao, J. Preparation of high elastic bacterial cellulose aerogel through thermochemical vapor deposition catalyzed by solid acid for oil-water separation. Carbohydr. Polym. 2023, 305, 120538. [Google Scholar] [CrossRef]
- Ke, W.; Ge, F.; Shi, X.; Zhang, Y.; Wu, T.; Zhu, X.; Cheng, Y.; Shi, Y.; Wang, Z.; Yuan, L. Superelastic and superflexible cellulose aerogels for thermal insulation and oil/water separation. Int. J. Biol. Macromol. 2024, 260, 129245. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, S.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Ramakrishna, S.; Wang, X.; Yao, J. Bacterial cellulose composite aerogel with high elasticity and adjustable wettability for dye absorption and oil–water separation. Appl. Surf. Sci. 2023, 640, 158299. [Google Scholar] [CrossRef]
- Hu, C.; Zhuang, J.; Xia, Y.; Zhang, J.; Zhang, X. Facile fabrication of sulfonated bacterial cellulose-silane composite aerogels via in suit polymerization for enhanced oil/water separation performance. Int. J. Biol. Macromol. 2024, 283, 137650. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Qi, J.; Liu, T.; Feng, Y.; Liu, C.; Shen, C. Eco-friendly bacterial cellulose/MXene aerogel with excellent photothermal and electrothermal conversion capabilities for efficient separation of crude oil/seawater mixture. Carbohydr. Polym. 2024, 336, 122140. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Zheng, L.; Zheng, H. Cellulose-based aerogels for efficient dye sorption and oil-water separation in textile wastewater treatment. Int. J. Biol. Macromol. 2025, 310, 143612. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Shi, R.; Chen, L.; Fan, M. A robust salt-tolerant superoleophobic chitosan/nanofibrillated cellulose aerogel for highly efficient oil/water separation. Carbohydr. Polym. 2018, 200, 611–615. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.; Li, L.; Fan, B.; Liu, Y.; Li, R.; Ren, X.; Huang, T.-S.; Kim, I.S. Construction of aerogels based on nanocrystalline cellulose and chitosan for high efficient oil/water separation and water disinfection. Carbohydr. Polym. 2020, 243, 116461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pan, Y.; Wang, W.; Lin, R.; Liu, X. Preparation of cellulose/chitosan superoleophobic aerogel with cellular pores for oil/water separation. Ind. Crops Prod. 2023, 194, 116303. [Google Scholar] [CrossRef]
- Si, R.; Luo, H.; Zhang, T.; Pu, J. High hydrophobic ZIF-8@ cellulose nanofibers/chitosan double network aerogel for oil adsorbent and oil/water separation. Int. J. Biol. Macromol. 2023, 238, 124008. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, J.; Wu, J.; Sun, D. Highly efficient separation for aqueous viscous oils enabled by a wood-based cellulose aerogel with a superhydrophilic protonated coating. ACS Sustain. Chem. Eng. 2024, 12, 7457–7465. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, J.; Ju, X.; Liu, Y.; Wang, G. Fabrication of glycerol-modulated cellulose/chitosan superhydrophobic aerogel for excellent mechanical performance and high oil-water separation efficiency. J. Environ. Chem. Eng. 2025, 13, 116641. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, F.; Wang, K.; Gao, Y.; Lu, Y.; Han, J.; Chen, L.; Wang, H.; Zhao, Y. Preparation and oil–water separation properties of PAMAM-modified chitosan/cellulose sequential interpenetrating polymer network aerogels. Int. J. Biol. Macromol. 2025, 304, 140704. [Google Scholar] [CrossRef]
- Chen, S.; Shao, Q.; Hu, L.; Tan, Z.; Zheng, D. Hydrophobic and magnetic fabrication of hydroxyethyl cellulose-lignin aerogel through ultrasound enhancement for efficient oil/water separation. J. Water Process Eng. 2023, 52, 103503. [Google Scholar] [CrossRef]
- Tan, Z.; Yoo, C.G.; Yang, D.; Liu, W.; Qiu, X.; Zheng, D. “Rigid-flexible” anisotropic biomass-derived aerogels with superior mechanical properties for oil recovery and thermal insulation. ACS Appl. Mater. Interfaces 2023, 15, 42080–42093. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Li, Y.; Yin, Z.; Bao, M. Construction of a superhydrophobic sodium alginate aerogel for efficient oil absorption and emulsion separation. Langmuir 2021, 37, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Y. Sodium alginate-based functional materials toward sustainable applications: Water treatment and energy storage. Ind. Eng. Chem. Res. 2023, 62, 11279–11304. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Hu, C.; Zheng, J.; Zhu, Z.; Liu, B. Design of carboxymethyl cellulose/alginate aerogels with anti-fouling and light-driven self-cleaning for enhanced oily wastewater remediation. Carbohydr. Polym. 2024, 342, 122358. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Feng, Q.; Chen, C.; Xu, Z. Preparation of antifouling and highly hydrophobic cellulose nanofibers/alginate aerogels by bidirectional freeze-drying for water-oil separation in the ocean environment. J. Hazard. Mater. 2023, 441, 129965. [Google Scholar] [CrossRef]
- Zhou, L.; Zhai, S.; Chen, Y.; Xu, Z. Anisotropic Cellulose Nanofibers/Polyvinyl Alcohol/Graphene Aerogels Fabricated by Directional Freeze-drying as Effective Oil Adsorbents. Polymers 2019, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cai, Z.; Gong, S. Green synthesis of polyvinyl alcohol (PVA)–cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Boroujeni, F.M.; Fioravanti, G.; Kander, R. Synthesis and characterization of cellulose microfibril-reinforced polyvinyl alcohol biodegradable composites. Materials 2024, 17, 526. [Google Scholar] [CrossRef]
- Akhlamadi, G.; Goharshadi, E.K. Sustainable and superhydrophobic cellulose nanocrystal-based aerogel derived from waste tissue paper as a sorbent for efficient oil/water separation. Process Saf. Environ. Prot. 2021, 154, 155–167. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, X.; Liu, L.; Yao, J. Highly compressible and durable superhydrophobic cellulose aerogels for oil/water emulsion separation with high flux. J. Mater. Sci. 2021, 56, 2763–2776. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, H.; Li, L.; Hu, Y. Preparation of a PVA/CNF/ETOS elastic aerogel by directional freezing and its application in oil–water separation. ACS Appl. Polym. Mater. 2023, 5, 3554–3563. [Google Scholar] [CrossRef]
- Chen, B.; Hu, Y. Hierarchical aerogels based on cellulose nanofibers and long-chain polymers for enhancing oil–water separation efficiency. Mater. Today Nano 2024, 26, 100469. [Google Scholar] [CrossRef]
- Zhan, B.; Chen, Z.; Zhou, W.; Li, X.; Wang, G.; Liu, Y. Superwetting PVA/cellulose aerogel with asymmetric structure for oil/water separation and solar-driven seawater desalination. J. Hazard. Mater. 2024, 476, 135131. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Qin, M.; Yang, X.; Zhang, F.; Wang, Z.; Ji, D.; Yu, D. Synthesis and characterization of UiO-66-NH2 incorporated PVA/cellulose nanofibers composite aerogel for enhanced oil–water separation and formaldehyde adsorption. Sep. Purif. Technol. 2023, 325, 124673. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, S.; Zhao, X.; Liu, B.; Zhang, Z.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Preparation of hydrophobic and lipophilic carboxymethyl cellulose composite aerogel using ferrous ion/persulfate and its directed oxidation for oil–water emulsion separation. Carbohydr. Polym. 2025, 348, 122814. [Google Scholar] [CrossRef]
- Fan, B.; Qi, B.; Wang, P.; Liu, Y.; Yu, Y.; Wang, Q.; Ren, X. Mechanically tough and regenerable antibacterial nanofibrillated cellulose-based aerogels for oil/water separation. Langmuir 2022, 38, 10716–10727. [Google Scholar] [CrossRef]
- Fan, B.; Lin, C.; Li, Z.; Cui, L.; Xu, B.; Yu, Y.; Wang, Q.; Wang, P. Peroxidase-catalyzed fabrication of a mechanically robust nanofibrillated cellulose-based aerogel with antibacterial activity for efficient oil–water separation. ACS Appl. Polym. Mater. 2023, 5, 10494–10505. [Google Scholar] [CrossRef]
- Fan, B.; Wu, L.; Ming, A.; Liu, Y.; Yu, Y.; Cui, L.; Zhou, M.; Wang, Q.; Wang, P. Highly compressible and hydrophobic nanofibrillated cellulose aerogels for cyclic oil/water separation. Int. J. Biol. Macromol. 2023, 242, 125066. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Jiang, W.; Fang, X.; Xia, Z.; Niu, H.; Zhou, H. Multifunctional amphibious superhydrophilic-oleophobic cellulose nanofiber aerogels for oil and water purification. Carbohydr. Polym. 2024, 330, 121774. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shu, S.; Hu, X.; Luo, Z.; Gong, H.; Fang, D.; Kou, Y.; Zhang, X.; Li, X.; Deng, Z. Hydrophobic cellulose nanofiber based aerogel for high-efficiency oil-water separation. Int. J. Biol. Macromol. 2025, 305, 141173. [Google Scholar] [CrossRef]
- Fei, Y.; Tan, Y.; Deng, Y.; Xia, P.; Cheng, J.; Wang, C.; Zhang, J.; Niu, C.; Fu, Q.; Lu, L. In situ construction strategy for three-dimensional Janus cellulose aerogel with highly efficient oil–water separation performance: From hydrophobicity to asymmetric wettability. Green Chem. 2022, 24, 7074–7081. [Google Scholar] [CrossRef]
- Mari, T.; Zimmermann, M.V.G.; Fenner, B.R.; Monticeli, F.M.; Ornaghi Júnior, H.L.; Baldasso, C.; Zattera, A.J. Use of a Sorption Column with Polyurethane/Graphene Core Combined with an Electroflotation Reactor for Oily Wastewater Treatment. Polymers 2025, 17, 1127. [Google Scholar] [CrossRef]
- Alazab, A.A.; Saleh, T.A. Magnetic hydrophobic cellulose-modified polyurethane filter for efficient oil-water separation in a complex water environment. J. Water Process Eng. 2022, 50, 103125. [Google Scholar] [CrossRef]
- Zhou, S.; You, T.; Zhang, X.; Xu, F. Superhydrophobic cellulose nanofiber-assembled aerogels for highly efficient water-in-oil emulsions separation. ACS Appl. Nano Mater. 2018, 1, 2095–2103. [Google Scholar] [CrossRef]
- Qiao, A.; Huang, R.; Penkova, A.; Qi, W.; He, Z.; Su, R. Superhydrophobic, elastic and anisotropic cellulose nanofiber aerogels for highly effective oil/water separation. Sep. Purif. Technol. 2022, 295, 121266. [Google Scholar] [CrossRef]
- Shang, Q.; Cheng, J.; Hu, L.; Bo, C.; Yang, X.; Hu, Y.; Liu, C.; Zhou, Y. Bio-inspired castor oil modified cellulose aerogels for oil recovery and emulsion separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128043. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, J.; Li, P.; Wang, X.; Cheng, Z.; Li, J.; Wang, B.; Gao, W.; Xu, J. Rechargeable nanofibrillated cellulose aerogel with excellent biocidal properties for efficient oil/water separation. Sep. Purif. Technol. 2022, 301, 121955. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Lin, Z.; Fu, Q.; Niu, C.; Zhao, Z.; Lu, L. Asymmetric wettable three-dimensional Janus all-cellulose aerogel: Controllable construction with a similar phase self-assembling strategy and oil-water separation performance. J. Environ. Chem. Eng. 2023, 11, 110776. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, C.; Dong, H. Oil/water separation with hydrophobic/oleophilic silica-shelled cellulose aerogels. Geoenergy Sci. Eng. 2024, 240, 213043. [Google Scholar] [CrossRef]
- Zeng, M.; Xu, Q.; Yu, J. Polyvinyltrimethoxysilane-enhanced TEMPO-oxidized cellulose nanofiber aerogels for exceptional anisotropic thermal insulation, flame retardancy and oil/water separation. Ind. Crops Prod. 2025, 228, 120862. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Wang, S.; Li, X.; Miao, Y.; Zhou, J.; Liu, Z. Hydrophobic Ti3C2Tx/TEMPO Oxidized Cellulose Nanofibers Composite Aerogel for Efficient Oil-Water Separation. Polymers 2025, 17, 273. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, D.; Wang, L.; Wang, J.; Ye, C.; Liang, Y.; Li, Y.; Tang, Y. Highly compressible and hydrophobic aerogel from microfibrillated cellulose for efficient oil/water separation. Ind. Crops Prod. 2025, 230, 121063. [Google Scholar] [CrossRef]


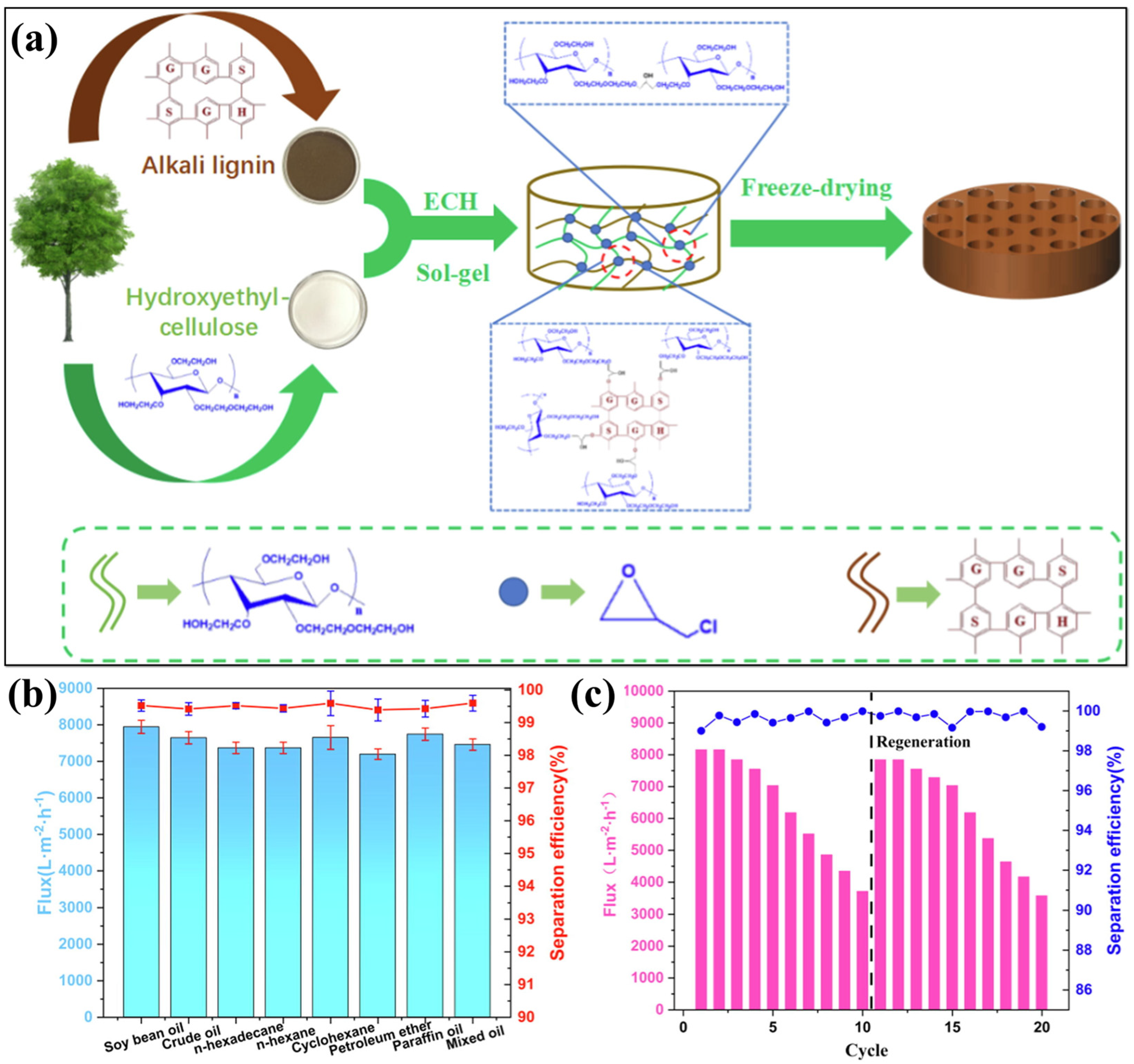



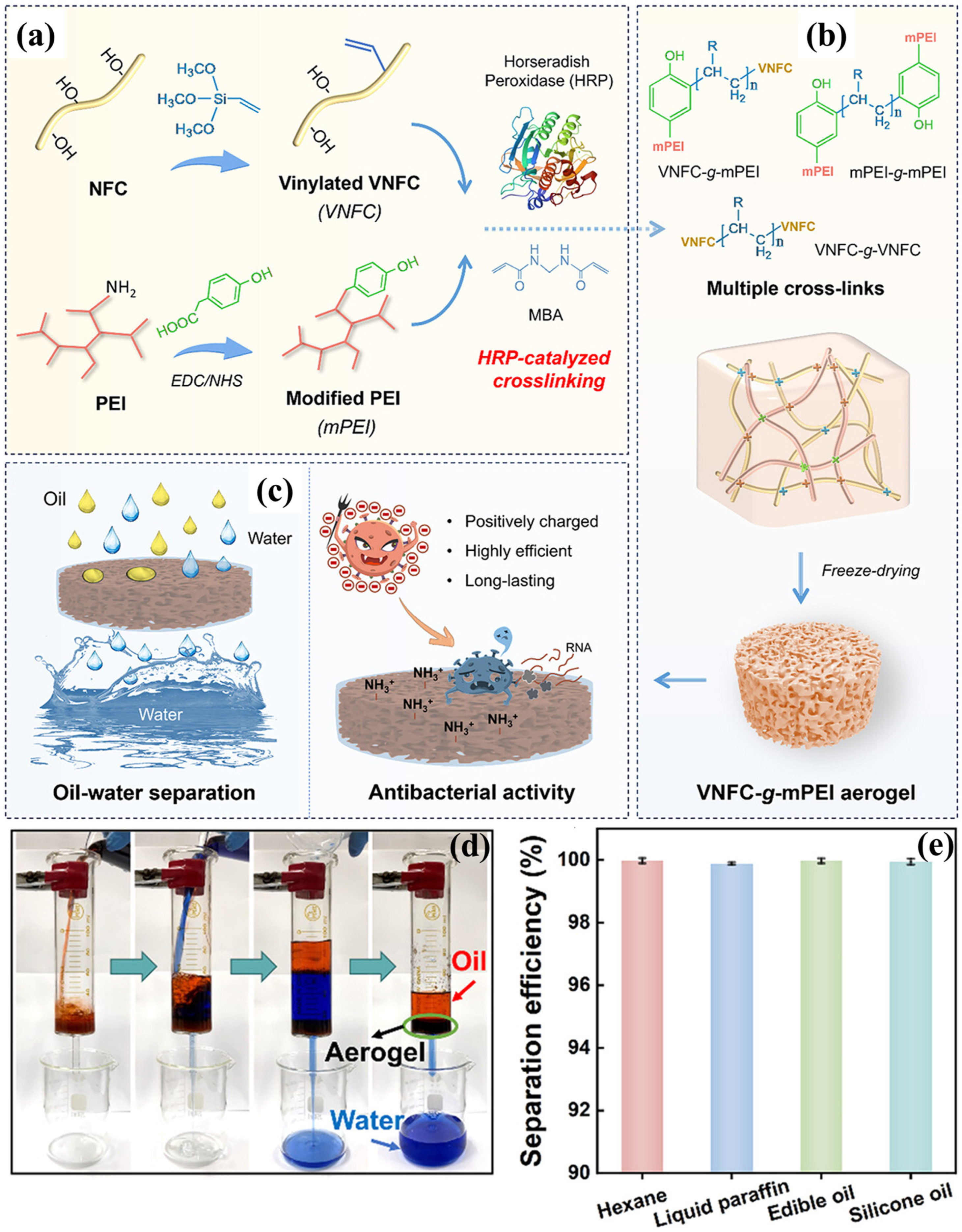


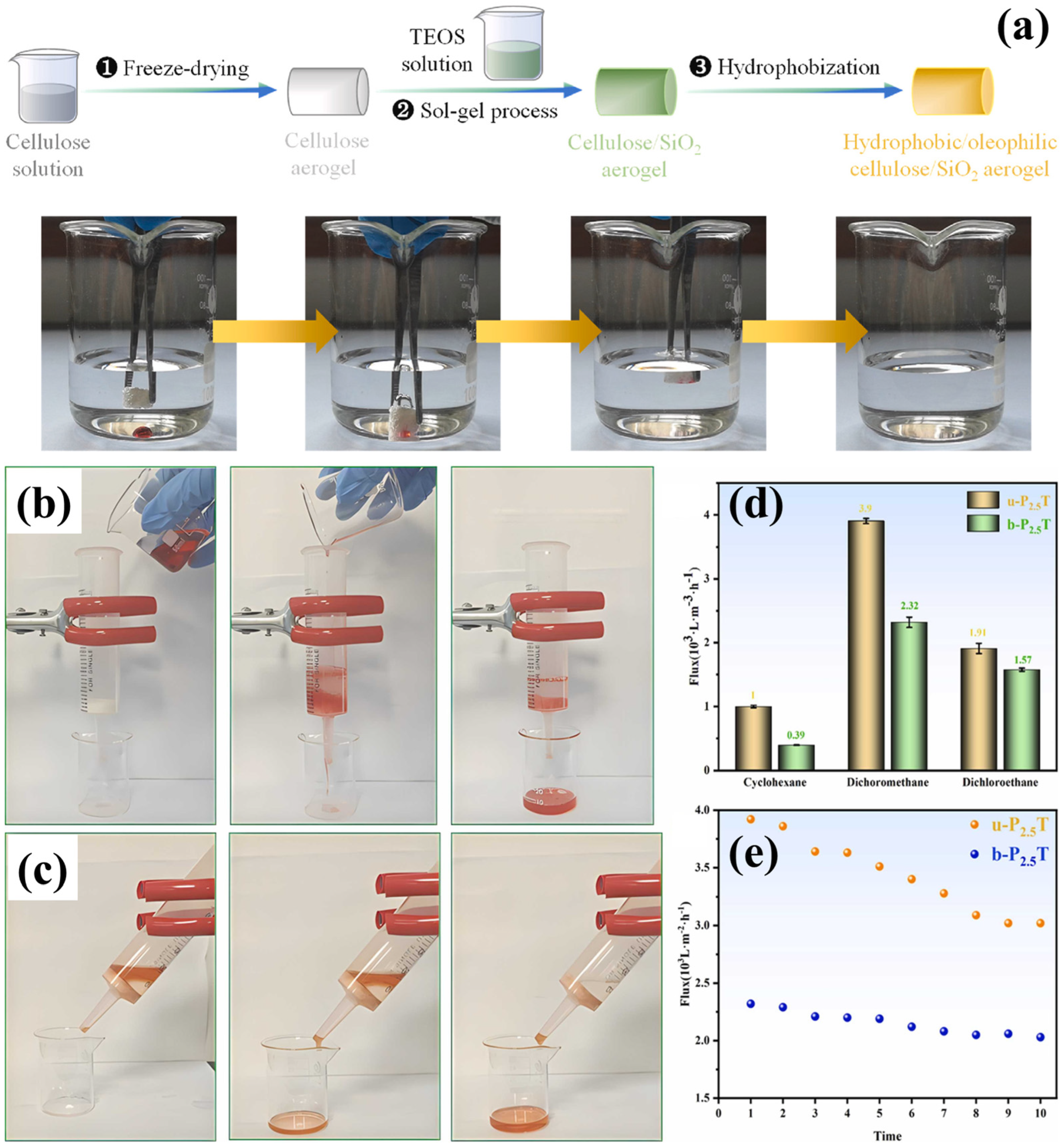
| Materials | Density | Specific Surface Area | Porosity | WCA | Mechanical | Flux | Efficiency | Sorption | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CNF/PDA/TiO2/OTMS | 0.1171 g/mL | − | 87.6723% | 113.9° | 41.83 kPa | 23.8095 L/hg | 96.15% | 59.9 (g/g) | [25] |
| CNC, PEG, Maleic anhydride, DMAP | 31.5 mg/cm3 | − | 96.7% | − | − | − | >97% | − | [38] |
| Hydroxyethyl cellulose, alkali lignin, epichlorhydrin | 0.0198–0.0838 g/cm3 | − | 91.87–98.13% | − | − | 7646 ± 167 L/m2h | >99% | − | [63] |
| Natural cellulose fibers, N-methyl morpholine-N-oxide monohydrate (NMMO·H2O) | 8–10 mg/cm3 | 768.89 m2/g | − | 151.5° | − | 11,718.8 L/m2h (carbon tetrachloride (CCl4)) | − | − | [91] |
| Kapok fiber/regenerated cellulose (carbon aerogel) | − | − | − | − | − | − | − | 98–232.1 (g/g) | [92] |
| Cellulose (kapok fibers), N-methyl morpholine-N-oxide monohydrate, deep eutectic solvent | 3.78 mg/cm3 | − | 98.9% | 144.7° | 50% strain at 100 cycles | 21,972.7 L/m2h | − | 137.5–371.7 (g/g) | [93] |
| Bacterial cellulose | 5.694 mg/cm3 | − | 98–99% | − | − | − | − | − | [94] |
| Bacterial cellulose, 1,2,3,4-butanetetracarboxylic acid, MTMS | 5.2 mg/cm3 | 63.4 m2/g to 49.7 m2/g | 99.7% | 142° | − | − | − | 74–165 g/g | [95] |
| Bacterial cellulose/γ-(2,3-epoxypropoxy) propytrimethoxysilane trimethoxysilane | 4.5 and 14.7 mg/cm3 | 6.5 m2/g | 98.9–99.7% | 146.8° | − | − | − | 66 mg/g | [97] |
| Bacterial cellulose | 10.25 mg/cm3 | 85.25 m2/g | 99.4% | 0° | [96] | ||||
| Bacterial cellulose/MTMS | 10–15 mg/cm3 | 88.07 m2/g | 99.1% | >120° | 162 L/hg | − | 65–156 (g/g) | ||
| sulfonated nano-fibrillated Bacterial cellulose/MTMS | − | 6.039 m2/g | − | 152.4° | − | − | 98.48% | 42.14–85.37 (g/g) | [98] |
| BC, Ti3C2Tx, MTMS | 0.06 g/cm3 | − | 96.5% | 136° | − | 630 kg/m2h1 | − | − | [99] |
| Bacterial cellulose/CMC/PDMS | 0.097 g/cm3 | − | 96% | 145.8° | 0.70 MPa at 80% strain | − | − | 484 mg/g | [100] |
| NFC, chitosan | 18.6 mg/cm3 | − | 98.7% | − | 42.8 kPa | − | >99% | − | [102] |
| Nanocrystalline cellulose, chitosan, | 22.22 kg/m3 to 40.82 kg/m3 | − | ≥97.66% | − | − | − | >99.9% | − | [103] |
| Nanofibrillated cellulose, chitosan, | 0.008 g/cm3 | 58.095 m2/g | 99.46% | − | 87.16 kPa at 80% strain | 0.826 L/m2s1 | >99.96% | − | [104] |
| Cellulose nanofibers/chitosan/methyltrimethoxysilane (MTMS), Zinc acetate (Zn (CH3COO))2 | 15.87 mg/cm3 | 5.51 m2/g | 99.01% | 132.6° | − | 13,167.5 L/m2h (CHCl3) | 98.5% | 35–75 g/g | [105] |
| Wood based cellulose, chitosan | − | − | − | − | − | 139,100 L/m2h | 99.90% | − | [106] |
| Cellulose nanofibers (CNF)/chitosan/trimethylchlorosilane (PMTS) | − | − | − | 141° | − | 18,000 to 28,000 L/m2h | 99.3% | 65 (g/g) | [107] |
| Polyamidoamine (PAMAM)-modified chitosan/cellulose/methyltrimethoxy-silane (MTMS) | 0.06–0.11 g/cm3 | 1.93–12.56 m2/g | − | 139.5° | 2.28 MPa | 5501.85 L/m2h (carbon tetrachloride/water), 4198.60 L/m2h, 96.67% (water-in-oil emulsions) | 96.67% (water/oil emulsion) | 1.49–12.07 (g/g) (CCl4) | [108] |
| Hydroxyethyl cellulose, alkali lignin, epichlorohydrin, n-dodecyl mercaptan (NDM), Fe3O4/PDA | 0.0443 g/cm3 to 0.0718 g/cm3 | − | − | 111.18° | − | 2986 L/m2h | >99% | − | [109] |
| Cellulose/lignin, Methyltrichlorosilane (MTCS) | 15.3 ± 0.7 mg/cm3 | 3.2 m2/g | 98.87% | 168° | 11.6% (plastic deformation) | >1000 L/m2h | − | 38.6–87.9 (g/g) | [110] |
| Sodium CMC, SA, TiO2, CaCl2 | − | − | − | − | − | 7650 L/m2h | 99.9% | − | [113] |
| Cellulose nano fibers/Sodium alginate, Methyltrimethoxysilane (MTMS) | 24.2 mg/cm3 | − | 97.85% | 144.5° | 340 kPa at 90% strain | − | − | 88.91 (g/g) | [114] |
| CNC/PVA/TEOS | 0.017 g/cm3 | 76 m2/g | 98.42% | − | − | − | − | 69–168 g/g | [118] |
| Cellulose, PVA, N,N’- methylenebisacrylamide (MBA) and methyltrichlorosilane (MTCS) | 50.4 mg/cm3 | − | 96.6% | 156.6° | 490.7 kPa at 90% strain | 7176.3 L/m2h | 98.5% | − | [119] |
| CNF/PVA/ethyltrimethoxysilane | 10.8 kg/cm3 | 27.9 m2/g | 98.4% | 148° | − | − | − | − | [120] |
| CNF/PVA/TEMPO/DTOS | 11.4 kg/m3 | 99.2% | 152° | − | − | − | − | [121] | |
| PVA/cellulose/methyltrichlorosilane | − | − | − | − | − | 631.9–2368.7 L/m2h | − | − | [122] |
| Carboxymethyl cellulose/PVA/SiO2/Fe2+ | 0.0211 g/cm3 | 132.13 m2/g | 98.62% | 139° | − | 19,130 L/m2h | − | − | [124] |
| Nanofibrillated cellulose, 3-(3′-acrylicacidpropylester)-5,5-dimethyl hydantoin (APDMH), poly(ethyleneimine) (PEI), 3-glycidoxypropyltrimethox (GPTMS) | 67 mg/cm3 | 94% | − | Recover to 96.76% after 5 compression-release cycles | 9500 L/m2h | 99% | − | [125] | |
| Vinylated nanofibrinogen cellulose/Horseradish peroxidase/modified polyethyleneamine, Vinyltrimethoxysilane (VTMS) | 55.1 mg/cm3 | − | 95.5% | 0° | 42.0 kPa | 5000 L/m2h | 99% | − | [126] |
| NFC, Polyethyleneimine, methyl trichlorosilane (MTS), Ethylene glycol diglycidyl ether (EGDE) | 53.80 mg/cm3 | − | 95.73% | 130.0° | Elasticity (95.86%) | 5000 L/m2h | 99% | − | [127] |
| CNF, GPTMS, PEI, fluorine-contained compound (FS-60) | 0.0256 g/cm3 | 127.87 m2/g | 98.30% | − | − | 9060 L/m2h | 99% | − | [128] |
| Cellulose nanofibers/TEMPO/PEI/Tannic acid/MTMS | 2.96 ± 0.31 kg/m3 | 10–284 m2/g | 46.45 ± 0.18% | − | − | − | − | 102.8 (g/g) | [129] |
| Cellulose, Dopamine hydrochloride (DA), trimethylchlorosilane (TMCS), Methyltrimethoxysilane (MTMS) | 0.0405 g/cm3 | − | − | 142° | 1804.5 Pa and 59.0% resilience | 3121 L/m2h | 99.5% | − | [130] |
| Polyurethane, Fe3O4, Cellulose | − | − | − | − | − | 48,750 L/m2h | >97.68% | − | [132] |
| CNF, SiO2,MTMS | 6.43 mg/cm3 | − | 99.6% | 168.4° | − | 1910 ± 60 L/m2h | 99.5% | − | [133] |
| CNF, PDMS | 22.7 mg/cm3 | − | − | 163.5° | − | 2800 L/m2h | 99.9% | 24 to 48 (g/g) | [134] |
| CNF, tannic acid, silylated castor oil, 3-isocyanatopropyltriethoxysilane (IPTES) | 24.0 mg/cm3 | − | 98.32% | 135.6° ± 0.8° | − | 123.3 to 473.8 L/m2 h | 94.4% to 97.1% | 53.2 to 113.8 g/g | [135] |
| Cellulose nanofiber/SiO2/MTMS | 34.83 mg/cm3 | − | 84.48% | 143° | − | − | − | − | [138] |
| CNF/TEMPO/PVTMS | 23.5 (b.a) 18.6 (u.a) | 16.0 (b.a) 12.6 (u.a) | 98.1% (b.a) 98.5 (u.a) | 115.6° (b.a) 112.4 (u.a) | − | 3900 L/m2h (unidirectional aerogel) | − | 34–66 (g/g) (bidirectional aerogel) | [139] |
| NFC, cyanuric chloride, hexadecyltrimethoxysilane (HDTMS) | − | − | − | − | − | − | − | 50 g/g | [136] |
| Janus all-cellulose/methyltrimethoxysilane (MTMS) and tetraethylsilicate (TEOS) | 0.041 g/cm3 | − | 97.42% | − | − | 3111 L/m2h | 99.51% | − | [137] |
| Microfibrillated cellulose/MTMS | 6.06–12.21 mg/cm3 | 10.87 m2/g | 99.33–99.58% | 133.15° | 27.84 kPa | − | − | 66–122 g/g | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanisamy, K.; Palanisamy, G.; Im, Y.M.; Thangarasu, S.; Phutela, U.G.; Oh, T.H. Recent Progress in Cellulose-Based Aerogels for Sustainable Oil–Water Separation Technologies. Polymers 2025, 17, 2723. https://doi.org/10.3390/polym17202723
Palanisamy K, Palanisamy G, Im YM, Thangarasu S, Phutela UG, Oh TH. Recent Progress in Cellulose-Based Aerogels for Sustainable Oil–Water Separation Technologies. Polymers. 2025; 17(20):2723. https://doi.org/10.3390/polym17202723
Chicago/Turabian StylePalanisamy, Karvembu, Gowthami Palanisamy, Yeong Min Im, Sadhasivam Thangarasu, Urmila Gupta Phutela, and Tae Hwan Oh. 2025. "Recent Progress in Cellulose-Based Aerogels for Sustainable Oil–Water Separation Technologies" Polymers 17, no. 20: 2723. https://doi.org/10.3390/polym17202723
APA StylePalanisamy, K., Palanisamy, G., Im, Y. M., Thangarasu, S., Phutela, U. G., & Oh, T. H. (2025). Recent Progress in Cellulose-Based Aerogels for Sustainable Oil–Water Separation Technologies. Polymers, 17(20), 2723. https://doi.org/10.3390/polym17202723






