Impact of Thermal Treatment and Aging on Lignin Properties in Spruce Wood: Pathways to Value-Added Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Thermal Modification
- Heating and drying. In this phase, the temperature in the oven increased to approximately 100 °C. Throughout this stage, the wood is dried to approximately zero moisture content.
- Thermal modification. In the second phase, the temperature is increased to the desired level (160 °C, 180 °C, and 210 °C, respectively). After reaching the desired temperature, the oven is kept in a steady state and the actual treatment takes place for 3 h. During the thermal modification, the timber is protected using steam.
- Cooling and climatization. In the third phase, the wood is gradually cooled to 80–90 °C, and the moisture content of the final product is 4–7%.
2.3. Accelerated Aging
2.4. Chemical Analyses
2.5. Nitrobenzene Oxidation
2.6. Size-Exclusion Chromatography
2.7. Thermal Analysis
2.8. ATR–FTIR Analysis
2.9. Statistical Evaluation
3. Results and Discussion
3.1. Extractives and Lignin Yields
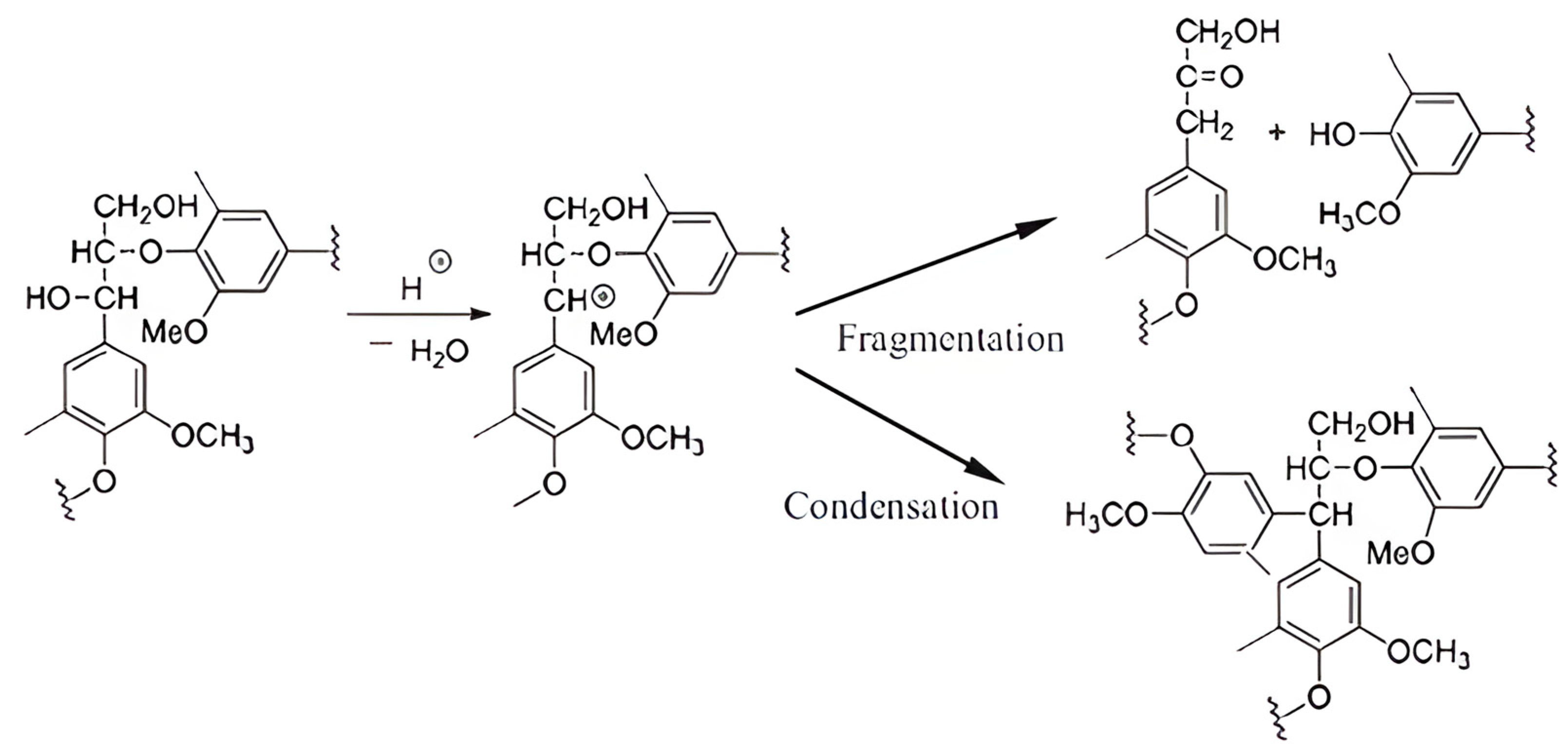
3.2. Nitrobenzene Oxidation Products
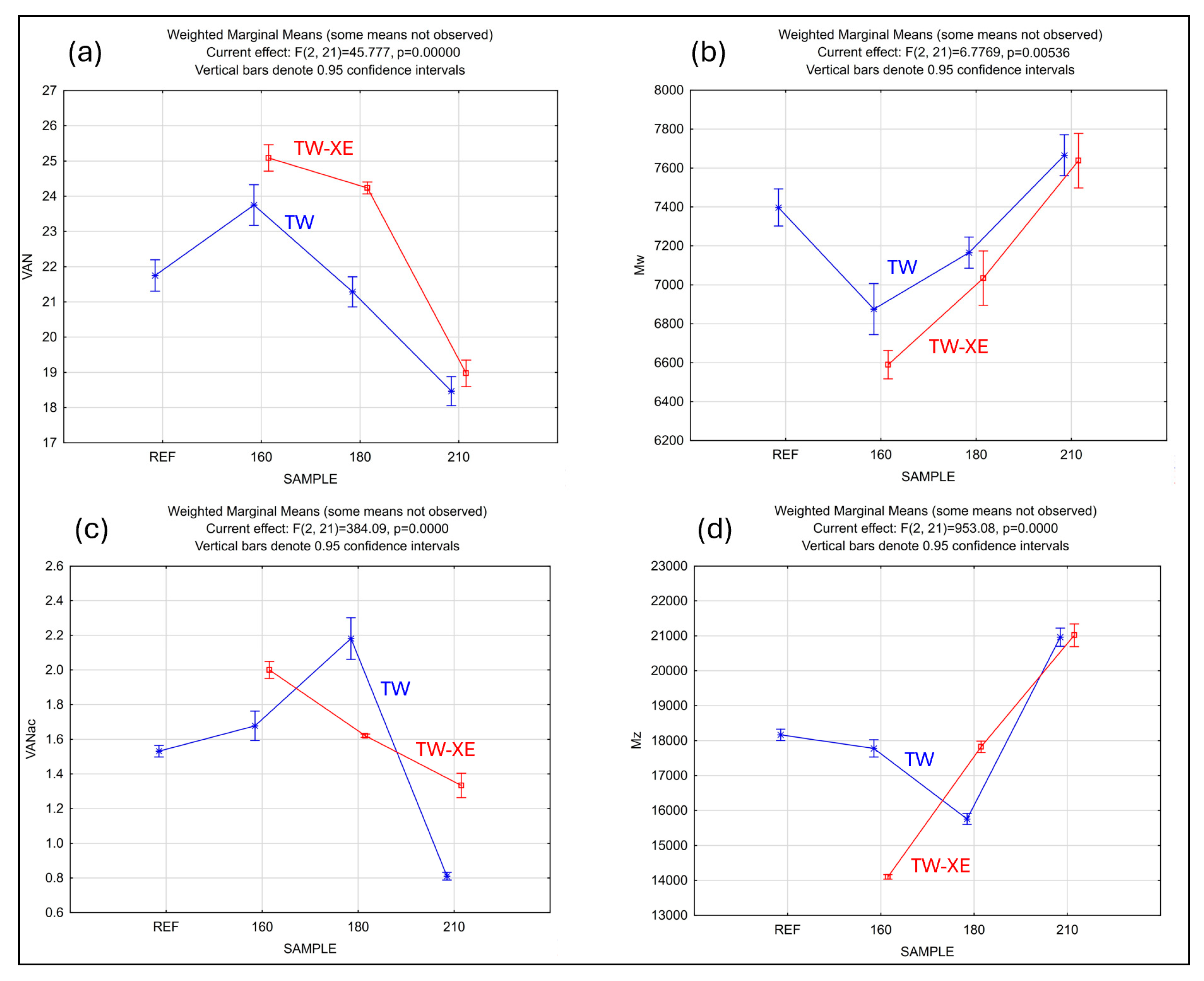
3.3. Molecular Weight and Molecular Weight Distribution of Lignins
| T (°C) | Mn (Da) | Mw (Da) | Mz (Da) | PDI |
|---|---|---|---|---|
| REF | 2689 (29) | 7397 (60) | 18,164 (102) | 2.75 (0.01) |
| 160-TW | 2983 (65) | 6875 (82) | 17,779 (156) | 2.31 (0.02) |
| 180-TW | 2670 (33) | 7165 (50) | 15,758 (98) | 2.68 (0.02) |
| 210-TW | 2744 (19) | 7666 (66) | 20,961 (164) | 2.79 (0.04) |
| 160-XE | 2506 (31) | 6590 (45) | 14,101 (40) | 2.63 (0.02) |
| 180-XE | 3116 (66) | 7034 (88) | 17,825 (102) | 2.26 (0.02) |
| 210-XE | 2904 (46) | 7638 (88) | 21,015 (205) | 2.63 (0.01) |
3.4. Thermal Analysis of Lignins
3.5. FTIR Analyses
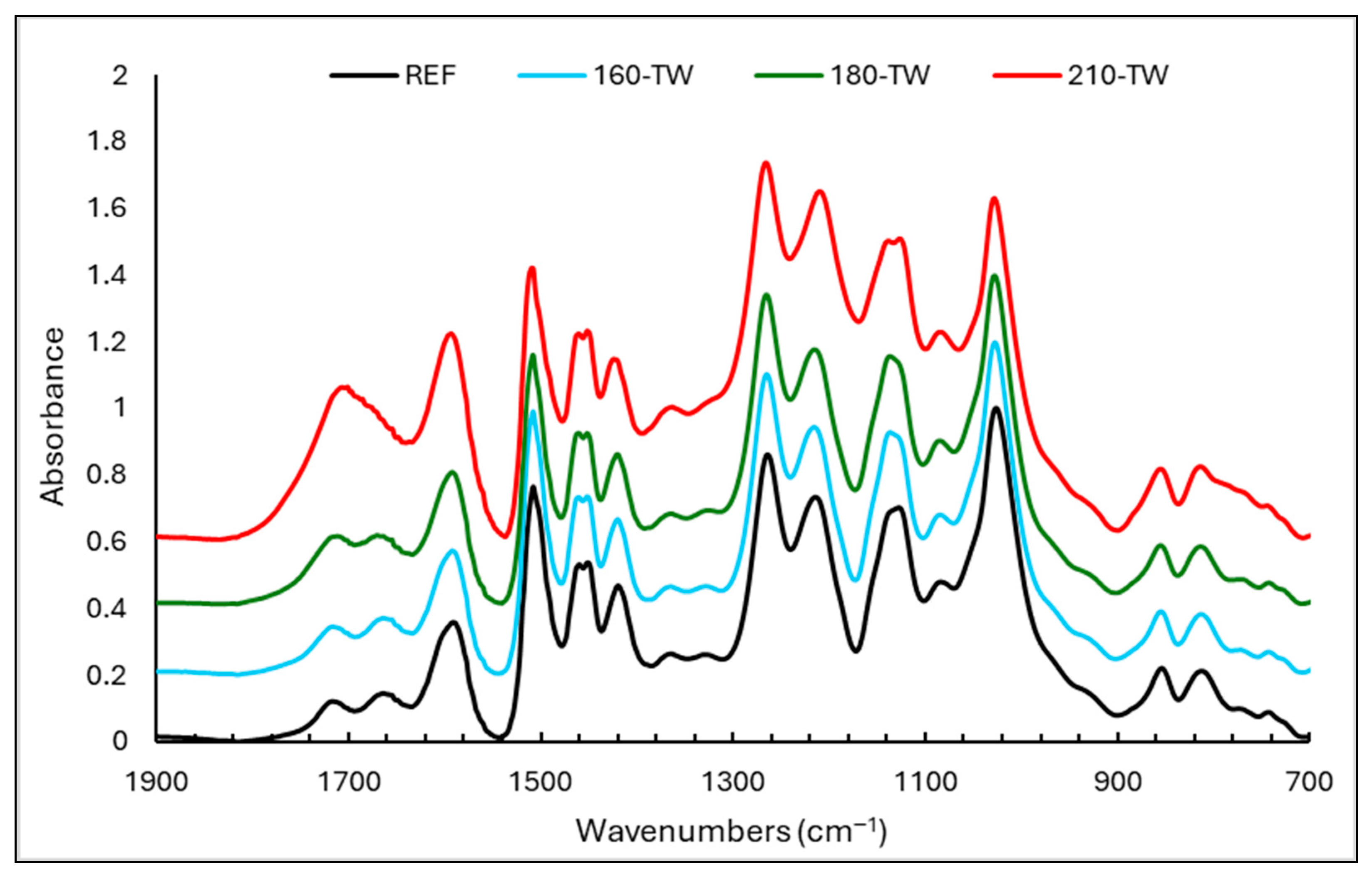

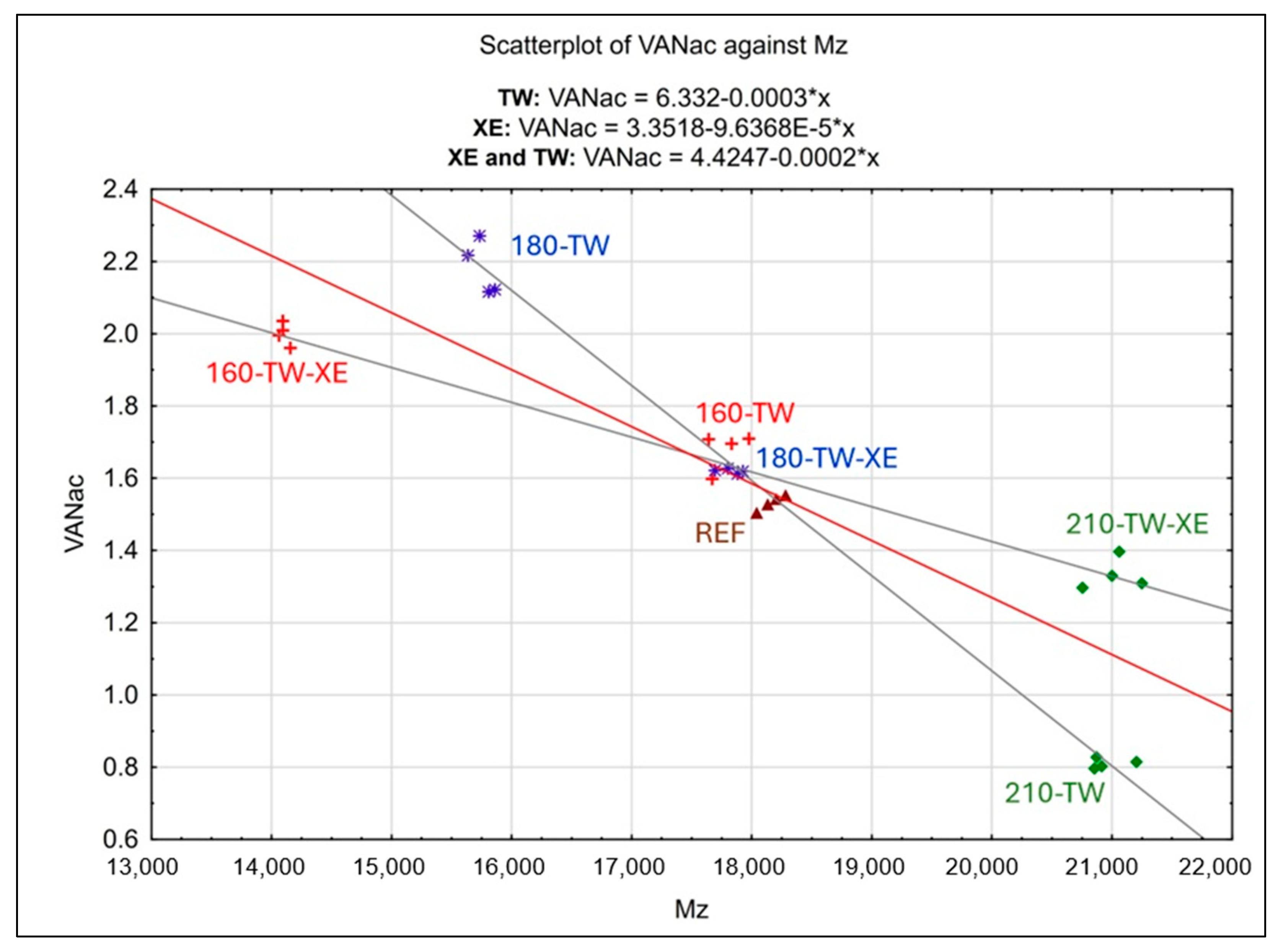
3.6. Statistical Analysis
4. Conclusions
- Depolymerization at lower temperatures is explained by reactions involving the cleavage of β-O-4 bonds, resulting in a decreased molecular weight and an increased yield of degradation products, such as vanillin and vanillic acid.
- Condensation at higher temperatures (i.e., 210 °C) leads to an increased molecular weight and structural stability and, conversely, a decreased yield of NBO products due to the reduced cleavage of lignin inter-unit bonds.
- Accelerated aging results in a relative increase in the lignin content due to the leaching of water-soluble extractives; lignin in aged samples has a reduced molecular weight (Mw) compared with thermally treated samples, indicating further degradation and yielding higher amounts of aromatic monomers; aging also results in lower decomposition temperatures, which improves its suitability for further processing.
- Vanillin yield is highest at lower modification temperatures (160 °C) and decreases with increasing temperature; a strong negative correlation (–0.9255) shows that lower molecular weight lignin produces more vanillin.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood modification technologies—A review. iFor.-Biogeosci. For. 2017, 10, 895–908. [Google Scholar] [CrossRef]
- Jones, D.; Sandberg, D.; Goli, G.; Todaro, L. Wood Modification in Europe: A State-of-the-Art About Processes, Products, and Applications; Firenze University Press: Firenze, Italy, 2019. [Google Scholar]
- Robles, E.; Herrera, R.; De Hoyos Martínez, P.L.; Fernández Rodríguez, J.; Labidi, J. Valorization of Heat-Treated Wood after Service Life through a Cascading Process for the Production of Lignocellulosic Derivatives. Resour. Conserv. Recycl. 2021, 170, 105602. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Curling, S.F.; Hale, M.D. On the effect of heat on the chemical composition and dimensions of thermally-modified wood. Polym. Degrad. Stabil. 2009, 94, 2184–2193. [Google Scholar] [CrossRef]
- Candelier, K.; Dumarc, S.; Pétrissans, A.; Pétrissans, M.; Kamdem, P.; Gérardin, P. Thermodesorption coupled to GC–MS to characterize volatiles formation kinetic during wood thermodegradation. J. Anal. Appl. Pyrolysis 2013, 101, 96–102. [Google Scholar] [CrossRef]
- Hill, C.; Altgen, M.; Rautkari, L. Thermal modification of wood—A review: Chemical changes and hygroscopicity. J. Mater. Sci. 2021, 56, 6581–6614. [Google Scholar] [CrossRef]
- Tanis, M.H.; Wallberg, O.; Galbe, M.; Al-Rudainy, B. Lignin Extraction by Using Two-Step Fractionation: A Review. Molecules 2024, 29, 98. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.A.; Zhu, X.H.; Sulaeva, I.; Potthast, A.; Rosenau, T.; Rojas, O.J. Spruce milled wood lignin: Linear, branched or cross-linked? Green Chem. 2020, 22, 3985–4001. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Li, F.; Cao, X.; Sun, R. Production of vanillin from lignin: The relationship between β-O-4 linkages and vanillin yield. Ind. Crop. Prod. 2018, 116, 116–121. [Google Scholar] [CrossRef]
- Hosoya, T.; Yamamoto, K.; Miyafuji, H.; Yamada, T. Selective Production of Bio-Based Aromatics by Aerobic Oxidation of Native Soft Wood Lignin in Tetrabutylammonium Hydroxide. RSC Adv. 2020, 10, 19199–19210. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Wikberg, H.; Ohra-aho, T.; Pileidis, F.; Titirici, M.M. Structural and morphological changes in kraft lignin during hydrothermal carbonization. ACS Sustain. Chem. Eng. 2015, 3, 2737–2745. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sampedro, R.; Capanema, E.A.; Hoeger, I.; Villar, J.C.; Rojas, O.J. Lignin changes after steam explosion and laccase-mediator treatment of eucalyptus wood chips. J. Agric. Food. Chem. 2011, 59, 8761–8769. [Google Scholar] [CrossRef] [PubMed]
- Hartati, I.; Sulistyo, H.; Sediawan, W.B.; Azis, M.M.; Fahrurrozi, M. Microwave-assisted urea-based- hydrotropic pretreatment of rice straw: Experimental data and mechanistic kinetic models. ACS Omega 2021, 6, 13225–13239. [Google Scholar] [CrossRef]
- Steinmetz, V.; Villain-Gambier, M.; Klem, A.; Ziegler, I.; Dumarcay, S.; Trebouet, D. Lignin Carbohydrate Complexes structure preserved throughout downstream processes for their valorization after recovery from industrial process water. Int. J. Biol. Macromol. 2020, 157, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Ru, B.; Wang, S.; Dai, G.; Zhang, L. Effect of Torrefaction on Biomass Physicochemical Characteristics and the Resulting Pyrolysis Behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, H.; Oh, S.; Kim, Y.S.; Kim, U.J.; Choi, J.W. Investigation of structural modification and thermal characteristics of lignin after heat treatment. Int. J. Biol. Macromol. 2014, 66, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FT-IR and PY-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Sette, M.; Wechselberger, R.; Crestini, C. Elucidation of lignin structure by quantitative 2D NMR. Chem. Eur. J. 2011, 17, 9529–9535. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.W.C.; Moutinho, V.C.P.; Severo, E.T.D.; Ferreira, J.S.S.; Ballarin, A.W. Impact of thermal modification on swelling and mechanical behavior of Couratari spp. BioResources 2023, 18, 5242. [Google Scholar] [CrossRef]
- Das, L.; Li, M.; Stevens, J.; Li, W.Q.; Pu, Y.Q.; Ragauskas, A.J.; Shi, J. Characterization and Catalytic Transfer Hydrogenolysis of Deep Eutectic Solvent Extracted Sorghum Lignin to Phenolic Compounds. ACS Sustain. Chem. Eng. 2018, 6, 10408–10420. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Sikora, A.; Kačík, F.; Gaff, M.; Vondrová, V.; Bubeníková, T.; Kubovský, I. Impact of thermal modification on color and chemical changes of spruce and oak wood. J. Wood Sci. 2018, 64, 406–416. [Google Scholar] [CrossRef]
- Kačíková, D.; Kubovský, I.; Gaff, M.; Kačík, F. Changes of Meranti, Padauk, and Merbau Wood Lignin during the ThermoWood Process. Polymers 2021, 13, 993. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Ragauskas, A.J. Lignin Structural Alterations in Thermochemical Pretreatments with Limited Delignification. Bioenergy Res. 2015, 8, 992–1003. [Google Scholar] [CrossRef]
- Godinho, D.; Ferreira, C.; Lourenço, A.; de Oliveira Araújo, S.; Quilhó, T.; Diamantino, T.C.; Gominho, J. The behavior of thermally modified wood after exposure in maritime/industrial and urban environments. Heliyon 2024, 10, e25020. [Google Scholar] [CrossRef]
- Kúdela, J.; Ihracký, P.; Kačík, F. Discoloration and Surface Changes in Spruce Wood after Accelerated Aging. Polymers 2024, 16, 1191. [Google Scholar] [CrossRef] [PubMed]
- Daou, M.; Farfan Soto, C.; Majira, A.; Cézard, L.; Cottyn, B.; Pion, F.; Navarro, D.; Oliveira Correia, L.; Drula, E.; Record, E.; et al. Fungal Treatment for the Valorization of Technical Soda Lignin. J. Fungi 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Kim, J.H.; Kwon, O.H.; Park, W.H.; Cho, D.H. Effects of Thermal Stabilization and Electron Beam Irradiation on the Characteristics of Lignin Extracted from Black Liquor. Defect Diffus. 2017, 381, 64–68. [Google Scholar] [CrossRef]
- Kačíková, D.; Kubovský, I.; Ulbriková, N.; Kačík, F. The Impact of Thermal Treatment on Structural Changes of Teak and Iroko Wood Lignins. Appl. Sci. 2020, 10, 5021. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Y.; Liu, Y.; Gao, J.; Chen, Y.; Fan, Y. Heat-Induced Discoloration of Chromophore Structures in Eucalyptus Lignin. Materials 2018, 11, 1686. [Google Scholar] [CrossRef]
- Argyropoulos, D.S.; Jurasek, L.; Kristofova, L.; Xia, Z.C.; Sun, Y.J.; Palus, E. Abundance and reactivity of dibenzodioxocins in softwood lignin. J. Agric. Food Chem. 2002, 50, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Košíková, B.; Lábaj, J.; Gregorová, A.; Slameňová, D. Lignin antioxidants for preventing oxidation damage of DNA and for stabilizing polymeric composites. Holzforschung 2006, 60, 166–170. [Google Scholar] [CrossRef]
- Mahmood, Z.; Yameen, M.; Jahangeer, M.; Riaz, M.; Ghaffar, A.; Javid, I. Lignin as Natural Antioxidant Capacity. In Lignin—Trends and Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- ASTM G155; Standard Practice for Operating Xenon Arc Light Apparatus for Exposure of Non-Metallic Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013.
- ASTM D1107-21; Standard Test Method for Ethanol-Toluene Solubility of Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Zinovyev, G.; Sulaeva, I.; Podzimek, S.; Rçssner, D. Getting Closer to Absolute Molar Masses of Technical Lignins. ChemSusChem 2018, 11, 3259–3326. [Google Scholar] [CrossRef]
- Pittman, Z.A.; Lynn, B.; Thies, M.C.; Kitchens, C.L. Improved Multiangle Light Scattering for Molar Mass Determination of Fluoresecent Lignins. ACS Sustain. Chem. Eng. 2023, 11, 16739–16748. [Google Scholar] [CrossRef]
- Gupta, A.; Dutt, B.; Sharma, S. Analysis of Chemical Properties of Thermally Treated Pinus Roxburghii Sargent Wood. Bioresources 2023, 18, 4598–4609. [Google Scholar] [CrossRef]
- Kačík, F.; Kubovský, I.; Bouček, J.; Hrčka, R.; Gaff, M.; Kačíková, D. Colour and Chemical Changes of Black Locust Wood during Heat Treatment. Forests 2023, 14, 73. [Google Scholar] [CrossRef]
- Torniainen, P.; Jones, D.; Sandberg, D. Colour as a quality indicator for industrially manufactured ThermoWood®. Wood Mater. Sci. Eng. 2021, 16, 287–289. [Google Scholar] [CrossRef]
- Torniainen, P.; Popescu, C.-M.; Jones, D.; Scharf, A.; Sandberg, D. Correlation of Studies between Colour, Structure and Mechanical Properties of Commercially Produced ThermoWood® Treated Norway Spruce and Scots Pine. Forests 2021, 12, 1165. [Google Scholar] [CrossRef]
- Mastouri, A.; Azadfallah, M.; Kamboj, G.; Rezaei, F.; Tarmian, A.; Efhamisisi, D.; Mahmoudkia, M.; Corcione, C.E. Kinetic Studies on Photo-Degradation of Thermally-Treated Spruce Wood during Natural Weathering: Surface Performance, Lignin and Cellulose Crystallinity. Constr. Build. Mater. 2023, 392, 131923. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, Y.; Ran, Y.; Cao, J. Pseudo lignin formed from hygrothermally treated holocellulose and its effect on fungal degradation. Ind. Crops Prod. 2022, 184, 115004. [Google Scholar] [CrossRef]
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour. Technol. 2012, 117, 7–12. [Google Scholar] [CrossRef]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocavailableellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Rawat, S.; Kumar, A.; Narani, A.; Bhaskar, T. Isolation of lignin from spruce and pine wood: Role of structural difference for potential value addition. Biomass Biofuels Biochem. 2021, 173–191. [Google Scholar] [CrossRef]
- Li, J.; Gellerstedt, G.; Toven, K. Steam explosion lignins; their extraction, structure and potential as feedstock for biodiesel and chemicals. Bioresour. Technol. 2009, 100, 2556–2561. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Vega-Aguilar, C.A.; Rodrigues, A.E. Added-Value Chemicals from Lignin Oxidation. Molecules 2021, 26, 4602. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L. Nitrobenzene and Cupric Oxide Oxidations. In Methods in Lignin Chemistry; Lin, S., Dence, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar] [CrossRef]
- Lappiere, C. Determining structure by chemical degradation. In Lignin and Lignans: Advances in Chemistry; CRC Press: Boca Raton, FL, USA, 2010; pp. 11–48. [Google Scholar]
- Fang, L.; Yazdi, S.; Frazier, C.E. Catalytic Acidolysis: Impact on In Situ Pine-Lignin Repolymerization. ACS Sustain. Chem. Eng. 2023, 11, 10709–10716. [Google Scholar] [CrossRef]
- Rousset, P.; Lapierre, C.; Pollet, B.; Quirino, W.; Perre, P. Effect of severe thermal treatment on spruce and beech wood lignins. Ann. For. Sci. 2009, 66, 1. [Google Scholar] [CrossRef]
- Baumberger, S.; Abaecherli, A.; Fasching, M.; Gellerstedt, G.; Gosselink, R.; Hortling, B.; Li, J.; Saake, B.; de Jong, E. Molar mass determination of lignins by size-exclusion chromatography: Towards standardisation of the method. Holzforschung 2007, 61, 459–468. [Google Scholar] [CrossRef]
- Alén, R.; Kotilainen, R.; Zaman, A. Thermochemical behavior of Norway spruce (Picea abies) at 180–225 °C. Wood Sci. Technol. 2002, 36, 163–171. [Google Scholar] [CrossRef]
- Esteves, B.; Domingos, I.; Pereira, H. Pine wood modification by heat treatment in air. BioResources 2008, 3, 142–154. [Google Scholar] [CrossRef]
- Gerardin, P. New alternatives for wood preservation based on thermal and chemical modification of wood-a review. Ann. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef]
- Weiland, J.J.; Guyonnet, R. Study of chemical modifications and fungi degradation of thermally modified wood using DRIFT spectroscopy. Holz Roh Werkst. 2003, 61, 216–220. [Google Scholar] [CrossRef]
- Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Ranzi, E. Detailed kinetic modeling of the thermal degradation of lignins. Biomass Bioenerg. 2010, 34, 290–301. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Zhang, Q.; Wang, J.; Ma, Q.; Yang, Y.; Luo, X.; Zhang, W. Comparison of the physicochemical characteristics of bio-char pyrolyzed from moso bamboo and rice husk with different pyrolysis temperatures. BioResources 2017, 12, 4652–4669. [Google Scholar] [CrossRef]
- Chu, S.; Subrahmanyam, A.V.; Huber, G.W. The pyrolysis chemistry of a β-O-4 type oligomeric lignin model compound. Green Chem. 2013, 15, 125–136. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Domínguez, J.C.; Santos, T.M.; Rigual, V.; Oliet, M.; Alonso, M.V.; Rodriguez, F. Thermal stability, degradation kinetics, and molecular weight of organosolv lignins from Pinus radiata. Ind. Crops Prod. 2018, 111, 889–898. [Google Scholar] [CrossRef]
- Araújo, L.C.P.; Yamaji, F.M.; Lima, V.H.; Botaro, V.R. Kraft Lignin Fractionation by Organic Solvents: Correlation between Molar Mass and Higher Heating Value. Bioresour. Technol. 2020, 314, 123757. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, D.S.; Sun, Y. Photochemically induced solid-state degradation, condensation and rearrangement reactions in lignin model compounds and milled wood lignin. Photochem. Photobiol. 1996, 64, 510–517. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FTIR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Blindheim, F.H.; Ruwoldt, J. The Effect of Sample Preparation Techniques on Lignin Fourier Transform Infrared Spectroscopy. Polymers 2023, 15, 2901. [Google Scholar] [CrossRef] [PubMed]
- Pylypchuk, I.V.; Riazanova, A.; Lindström, M.E.; Sevastyanova, O. Structural and Molecular-Weight-Dependency in the Formation of Lignin Nanoparticles from Fractionated Soft- and Hardwood Lignins. Green Chem. 2021, 23, 3061–3072. [Google Scholar] [CrossRef]
- Awan, I.Z.; Tanchoux, N.; Quignard, F.; Albonetti, S.; Cavani, F.; Di Renzo, F. Heterogeneous Catalysis as a Tool for Production of Aromatic Compounds From Lignin. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–275. [Google Scholar]
- Dos Santos, R.J.; Vallejos, M.E.; Area, M.C.; Felissia, F.E. Kinetics of Vanillin and Vanillic Acid Production from Pine Kraft Lignin. Processes 2024, 12, 1472. [Google Scholar] [CrossRef]


| T (°C) | Extractives (%) | Lignin (%) | Dioxane Lignin Yields | |||||
|---|---|---|---|---|---|---|---|---|
| TW | TW-XE | TW | TW-XE | (% Wood) | (% Klason Lignin) | |||
| TW | TW-XE | TW | TW-XE | |||||
| REF | 0.98 (0.05) | 0.98 (0.05) | 26.24 (0.04) | 26.24 (0.04) | 4.75 (0.21) | 4.75 (0.21) | 18.11 (0.78) | 18.11 (0.78) |
| 160 | 2.51 (0.20) | 1.63 (0.10) | 26.45 (0.07) | 27.68 (0.40) | 5.38 (0.15) | 5.15 (0.21) | 20.32 (0.57) | 18.59 (0.88) |
| 180 | 2.71 (0.26) | 2.25 (0.14) | 28.65 (0.09) | 32.14 (0.18) | 7.68 (0.29) | 8.40 (0.41) | 26.81 (1.03) | 26.16 (1.28) |
| 210 | 3.49 (0.25) | 3.07 (0.23) | 33.08 (0.05) | 35.26 (0.43) | 9.13 (0.22) | 9.92 (0.18) | 27.58 (0.63) | 28.13 (0.18) |
| T (°) | TW | TW-XE | ||||
|---|---|---|---|---|---|---|
| Vanillin | Vanillic Acid | SUM | Vanillin | Vanillic Acid | SUM | |
| REF | 21.75 (0.28) | 1.53 (0.02) | 23.28 (0.29) | 21.75 (0.28) | 1.53 (0.02) | 23.28 (0.29) |
| 160 | 23.75 (0.36) | 1.68 (0.05) | 25.43 (0.60) | 25.09 (0.23) | 2.00 (0.03) | 27.09 (0.26) |
| 180 | 21.29 (0.27) | 2.18 (0.08) | 23.47 (0.34) | 24.23 (0.11) | 1.62 (0.01) | 25.85 (0.10) |
| 210 | 18.47 (0.26) | 0.81 (0.01) | 19.28 (0.25) | 18.97 (0.24) | 1.33 (0.04) | 20.31 (0.28) |
| Sample | Peak Temperature (°C)/Mass Loss (%) | Residual Mass (%) | ||
|---|---|---|---|---|
| First Region | Second Region | Third Region | ||
| REF | 83/1.89 | 371/30.76 | 395–600/19.45 | 47.95 |
| 160-TW | 60/2.11 | 369/31.94 | 395–600/19.57 | 46.39 |
| 180-TW | 64/2.31 | 366/31.22 | 395–600/19.27 | 47.18 |
| 210-TW | 64/2.31 | 374/24.36 | 395–600/19.27 | 52.90 |
| 160-TW-XE | 62/2.08 | 344/27.39 | 392–600/22.36 | 48.11 |
| 180-TW-XE | 64/2.22 | 346/24.47 | 393–600/24.42 | 48.86 |
| 210-TW-XE | 66/2.50 | 360/20.26 | 392–600/27.25 | 49.97 |
| Variable | VAN | VANac | Mn | Mw | Mz | PDI |
|---|---|---|---|---|---|---|
| VAN | 1.000000 | 0.664343 | 0.039300 | −0.925533 | −0.787225 | −0.626182 |
| VANac | 0.664343 | 1.000000 | −0.259399 | −0.727719 | −0.886412 | −0.246322 |
| Mn | 0.039300 | −0.259399 | 1.000000 | 0.161560 | 0.459996 | −0.769325 |
| Mw | −0.925533 | −0.727719 | 0.161560 | 1.000000 | 0.869334 | 0.505147 |
| Mz | −0.787225 | −0.886412 | 0.459996 | 0.869334 | 1.000000 | 0.154899 |
| PDI | −0.626182 | −0.246322 | −0.769325 | 0.505147 | 0.154899 | 1.000000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kačík, F.; Výbohová, E.; Jurczyková, T.; Eštoková, A.; Kmeťová, E.; Kačíková, D. Impact of Thermal Treatment and Aging on Lignin Properties in Spruce Wood: Pathways to Value-Added Applications. Polymers 2025, 17, 238. https://doi.org/10.3390/polym17020238
Kačík F, Výbohová E, Jurczyková T, Eštoková A, Kmeťová E, Kačíková D. Impact of Thermal Treatment and Aging on Lignin Properties in Spruce Wood: Pathways to Value-Added Applications. Polymers. 2025; 17(2):238. https://doi.org/10.3390/polym17020238
Chicago/Turabian StyleKačík, František, Eva Výbohová, Tereza Jurczyková, Adriana Eštoková, Elena Kmeťová, and Danica Kačíková. 2025. "Impact of Thermal Treatment and Aging on Lignin Properties in Spruce Wood: Pathways to Value-Added Applications" Polymers 17, no. 2: 238. https://doi.org/10.3390/polym17020238
APA StyleKačík, F., Výbohová, E., Jurczyková, T., Eštoková, A., Kmeťová, E., & Kačíková, D. (2025). Impact of Thermal Treatment and Aging on Lignin Properties in Spruce Wood: Pathways to Value-Added Applications. Polymers, 17(2), 238. https://doi.org/10.3390/polym17020238









