Active Polylactide-poly(ethylene glycol) Films Loaded with Olive Leaf Extract for Food Packaging—Antibacterial Activity, Surface, Thermal and Mechanical Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Olive Leaf Extract—Obtainment and Characterization
2.3. Polylactide Films Formation
2.4. Contact Angle Measurements and Surface Free Energy Calculation
2.5. Atomic Force Microscopy (AFM)
2.6. Thermogravimetric Analysis (TGA)
2.7. Mechanical Properties
2.8. Antibacterial Properties
2.8.1. Microorganisms and Cultivation Conditions
2.8.2. Bacteriostatic Activity
2.8.3. Antibacterial Activity
2.8.4. Anti-Adhesive Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Olive Leaf Extract—Obtainment and Characterization
3.2. Contact Angle Measurements
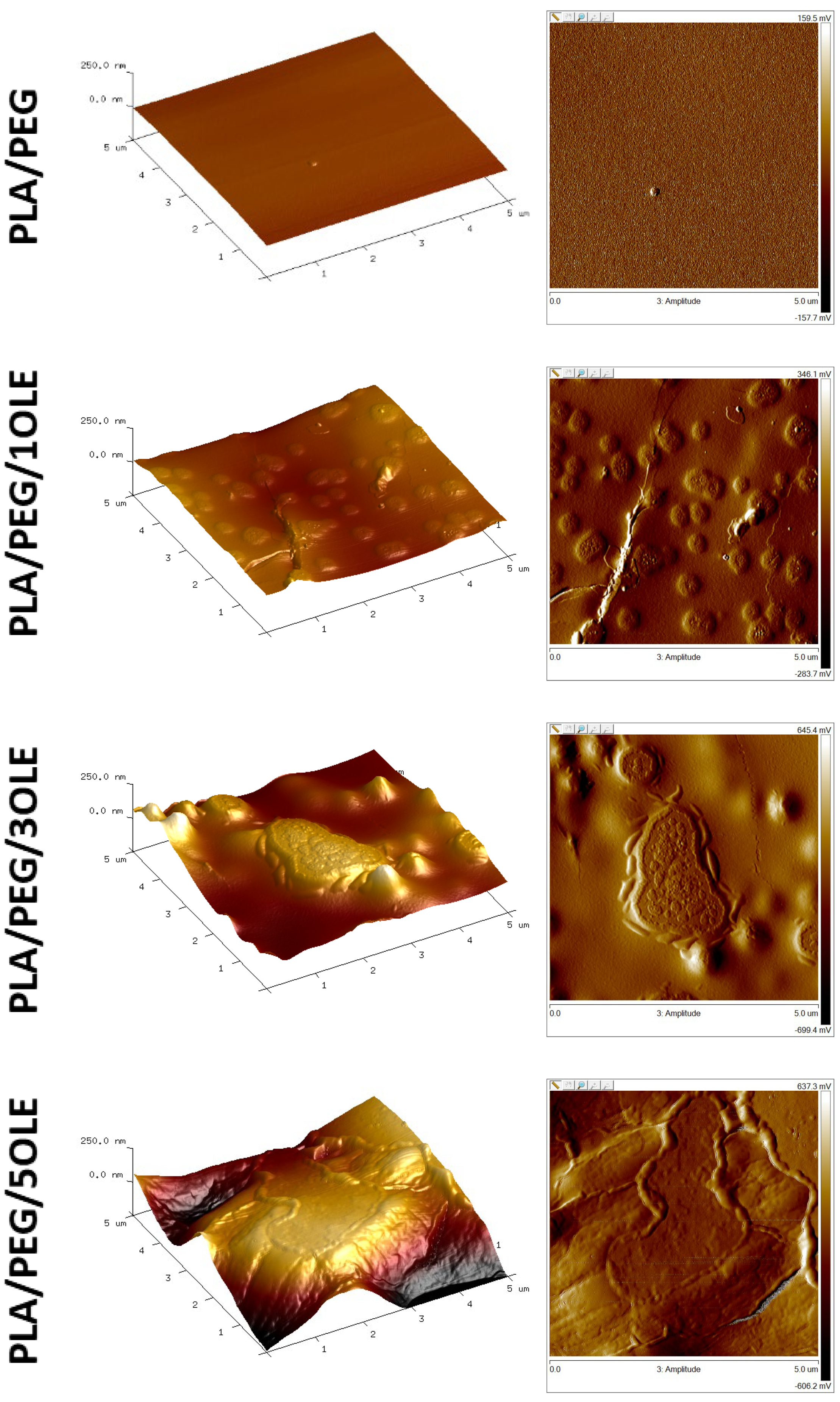
3.3. Atomic Force Microscopy (AFM)
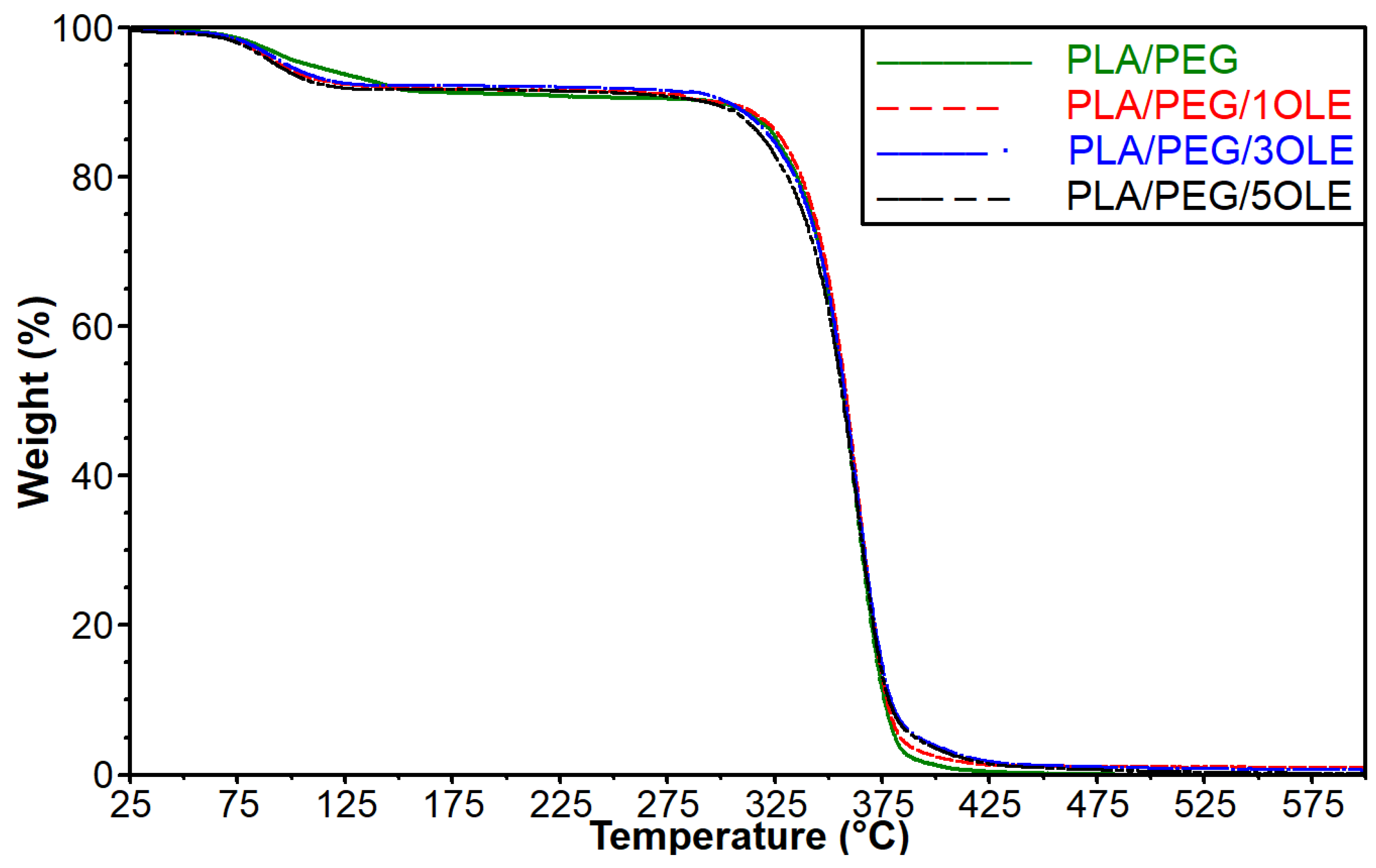
3.4. Thermogravimetric Analysis (TGA)
3.5. Mechanical Properties
3.6. Bacteriostatic, Bactericidal and Antiadhesive Activity
3.6.1. Antibacterial Activity
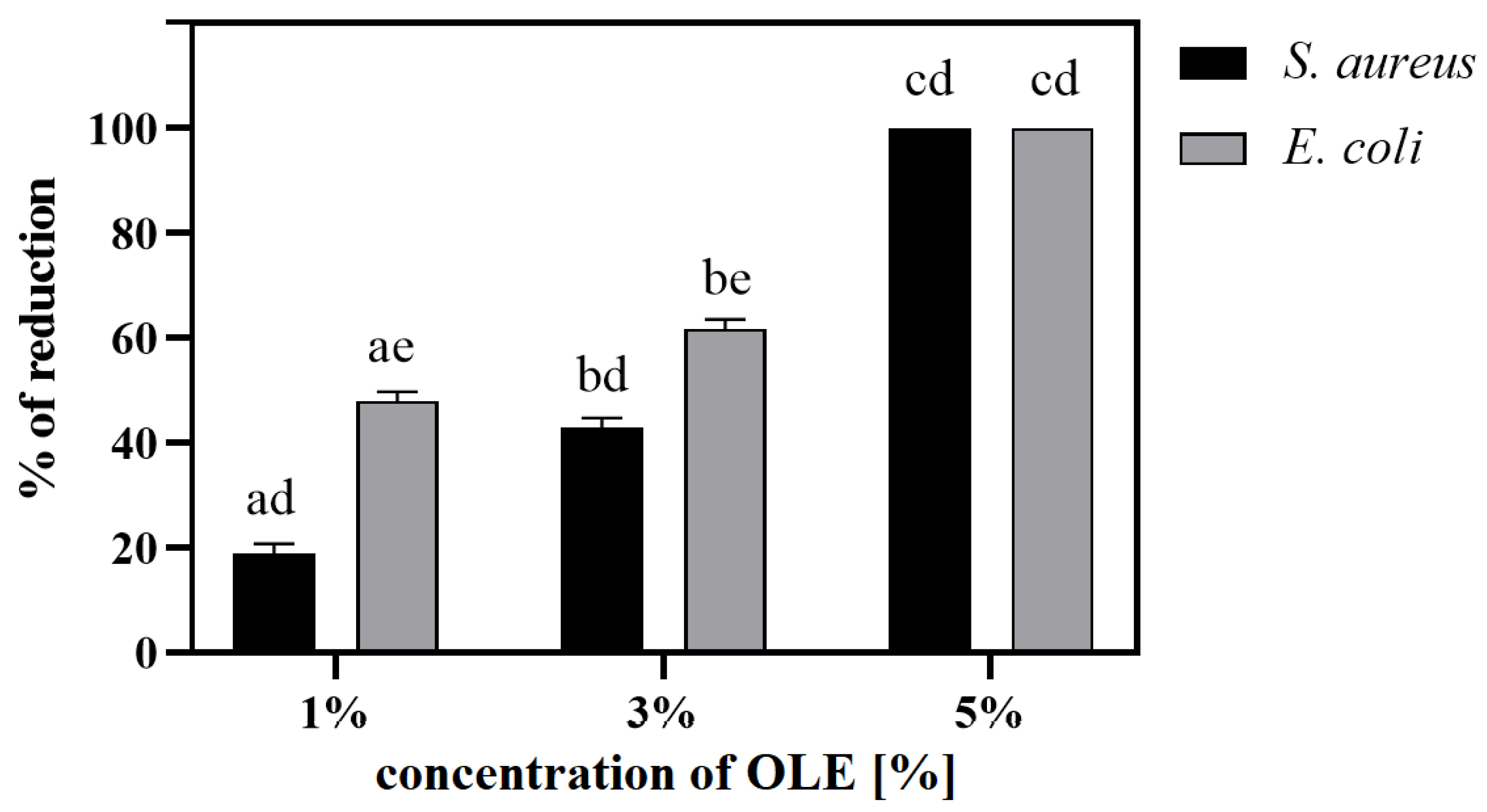
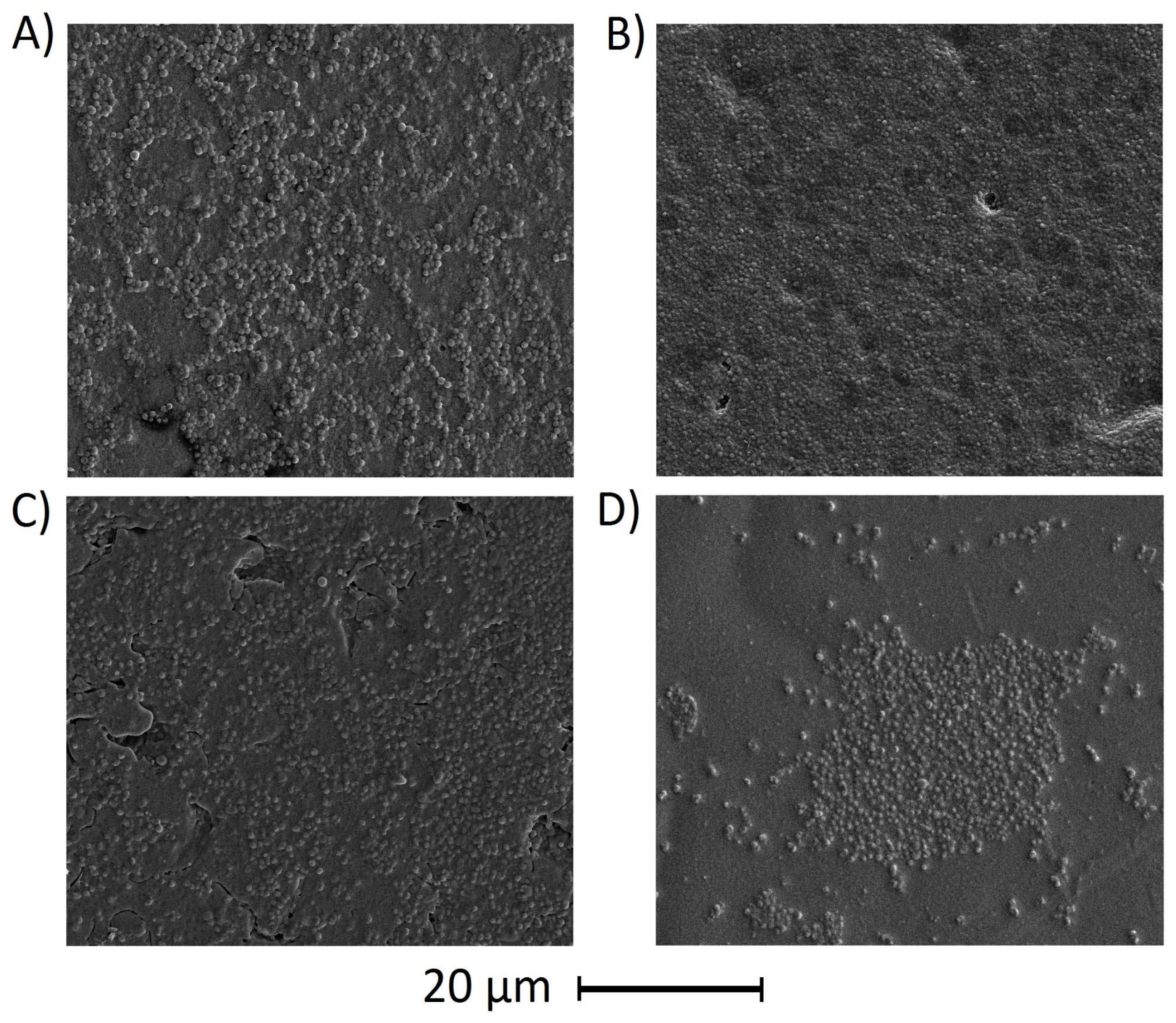
3.6.2. Antiadhesive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and Intelligent Packaging in Meat Industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.; Goddard, J.M. Active Packaging Coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef]
- Roopa, H.; Panghal, A.; Kumari, A.; Chhikara, N.; Sehgal, E.; Rawat, K. Active Packaging in Food Industry. In Novel Technologies in Food Science; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A Review of Active Packaging in Bakery Products: Applications and Future Trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Singh, A.K.; Ramakanth, D.; Kumar, A.; Lee, Y.S.; Gaikwad, K.K. Active Packaging Technologies for Clean Label Food Products: A Review. J. Food Meas. Charact. 2021, 15, 4314–4324. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)–Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- Malek, N.S.A.; Faizuwan, M.; Khusaimi, Z.; Bonnia, N.N.; Rusop, M.; Asli, N.A. Preparation and Characterization of Biodegradable Polylactic Acid (PLA) Film for Food Packaging Application: A Review. J. Phys. Conf. Ser. 2021, 1892, 012037. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Tarach, I.; Olewnik-Kruszkowska, E.; Richert, A.; Gierszewska, M.; Rudawska, A. Influence of Tea Tree Essential Oil and Poly(Ethylene Glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films. Materials 2020, 13, 4953. [Google Scholar] [CrossRef] [PubMed]
- Richert, A.; Olewnik-Kruszkowska, E.; Dąbrowska, G.B.; Dąbrowski, H.P. The Role of Birch Tar in Changing the Physicochemical and Biocidal Properties of Polylactide-Based Films. Int. J. Mol. Sci. 2021, 23, 268. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Ferri, M.; Cardeira, M.C.; Gierszewska, M.; Rudawska, A. Comparison of Polylactide-Based Active Films Containing Berberine and Quercetin as Systems for Maintaining the Quality and Safety of Blueberries. Polymers 2024, 16, 1577. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Wrona, M.; Nerin, C.; Grabska-Zielińska, S. Polylactide-Based Films with the Addition of Poly(Ethylene Glycol) and Extract of Propolis—Physico-Chemical and Storage Properties. Foods 2022, 11, 1488. [Google Scholar] [CrossRef] [PubMed]
- Ozkoc, G.; Kemaloglu, S. Morphology, Biodegradability, Mechanical, and Thermal Properties of Nanocomposite Films Based on PLA and Plasticized PLA. J. Appl. Polym. Sci. 2009, 114, 2481–2487. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Richert, A.; Grabska-Zielińska, S.; Rudawska, A.; Bouaziz, M. Antibacterial Films Based on Polylactide with the Addition of Quercetin and Poly(Ethylene Glycol). Materials 2021, 14, 1643. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa-Fernández, E.J. Characterization of Olive Leaf Extract Polyphenols Loaded by Supercritical Solvent Impregnation into PET/PP Food Packaging Films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of Active/Intelligent Food Packaging Film Containing Amaranthus Leaf Extract for Shelf Life Extension of Chicken/Fish during Chilled Storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Liao, G.; Sun, E.; Kana, E.B.G.; Huang, H.; Sanusi, I.A.; Qu, P.; Jin, H.; Liu, J.; Shuai, L. Renewable Hemicellulose-Based Materials for Value-Added Applications. Carbohydr. Polym. 2024, 341, 122351. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of Active Packaging Film Made from Poly (Lactic Acid) Incorporated Essential Oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of Polylactic Acid Fibres Containing Tea Tree and Manuka Oil. React. Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Natural Polymeric Compound Based on High Thermal Stability Catechin from Green Tea. Biomolecules 2020, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Olejnik, O. Aging Resistance of Biocomposites Crosslinked with Silica and Quercetin. Int. J. Mol. Sci. 2021, 22, 10894. [Google Scholar] [CrossRef]
- Pawłowska, A.; Stepczyńska, M. Natural Biocidal Compounds of Plant Origin as Biodegradable Materials Modifiers. J. Polym. Environ. 2022, 30, 1683–1708. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, D.U.; Eun, J.B.; Moon, S.H. Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat. Antioxidants 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and Antimicrobial Activity of Rosemary, Pomegranate and Olive Extracts in Fish Patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef]
- Martiny, T.R.; Pacheco, B.S.; Pereira, C.M.P.; Mansilla, A.; Astorga–España, M.S.; Dotto, G.L.; Moraes, C.C.; Rosa, G.S. A Novel Biodegradable Film Based on κ-Carrageenan Activated with Olive Leaves Extract. Food Sci. Nutr. 2020, 8, 3147–3156. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, R.; Gupta, V.; Kumar, P.; Kumar, A.; Singh, S.; Gaikwad, K.K. Development and Characterization of PVA-Starch Incorporated with Coconut Shell Extract and Sepiolite Clay as an Antioxidant Film for Active Food Packaging Applications. Int. J. Biol. Macromol. 2021, 185, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Martiny, T.R.; Raghavan, V.; de Moraes, C.C.; da Rosa, G.S.; Dotto, G.L. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef]
- Yahyaoui, M.; Gordobil, O.; Herrera Díaz, R.; Abderrabba, M.; Labidi, J. Development of Novel Antimicrobial Films Based on Poly(Lactic Acid) and Essential Oils. React. Funct. Polym. 2016, 109, 1–8. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial, Rheological, and Thermal Properties of Plasticized Polylactide Films Incorporated with Essential Oils to Inhibit Staphylococcus Aureus and Campylobacter Jejuni. J. Food Sci. 2016, 81, E419–E429. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Szydłowska-Czerniak, A.; Nowaczyk, J.; Olewnik-Kruszkowska, E. Development and Characterization of Active Packaging Films Based on Chitosan, Plasticizer, and Quercetin for Repassed Oil Storage. Food Chem. 2023, 399, 133934. [Google Scholar] [CrossRef] [PubMed]
- Masek, A. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Korukluoglu, M.; Sahan, Y.; Yigit, A. Antifungal Properties of Olive Leaf Extracts and Their Phenolic Compounds. J. Food Saf. 2008, 28, 76–87. [Google Scholar] [CrossRef]
- Talhaoui, N.; Vezza, T.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Gálvez, J.; Segura-Carretero, A. Phenolic Compounds and in Vitro Immunomodulatory Properties of Three Andalusian Olive Leaf Extracts. J. Funct. Foods 2016, 22, 270–277. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial Effect of Olive (Olea europaea L.) Leaves Extract in Raw Peeled Undeveined Shrimp (Penaeus Semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- Altiok, E.; Bayçin, D.; Bayraktar, O.; Ülkü, S. Isolation of Polyphenols from the Extracts of Olive Leaves (Olea europaea L.) by Adsorption on Silk Fibroin. Sep. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef]
- Amaro-Blanco, G.; Delgado-Adámez, J.; Martín, M.J.; Ramírez, R. Active Packaging Using an Olive Leaf Extract and High Pressure Processing for the Preservation of Sliced Dry-Cured Shoulders from Iberian Pigs. Innov. Food Sci. Emerg. Technol. 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Bouaziz, M.; Sayadi, S. Isolation and Evaluation of Antioxidants from Leaves of a Tunisian Cultivar Olive Tree. Eur. J. Lipid Sci. Technol. 2005, 107, 497–504. [Google Scholar] [CrossRef]
- Erdohan, Z.Ö.; Çam, B.; Turhan, K.N. Characterization of Antimicrobial Polylactic Acid Based Films. J. Food Eng. 2013, 119, 308–315. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and Antioxidant Activity of Olive Leaf Extracts from Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Macciola, E.; Succi, M.; Tremonte, P.; Iorizzo, M. Efficacy of Olive Leaf Extract (Olea Europaea L. Cv Gentile Di Larino) in Marinated Anchovies (Engraulis Encrasicolus, L.) Process. Heliyon 2019, 5, e01727. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.X.; Rico, D.; McHugh, T.H. Antimicrobial Olive Leaf Gelatin Films for Enhancing the Quality of Cold-Smoked Salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Development of Biodegradable Films with Improved Antioxidant Properties Based on the Addition of Carrageenan Containing Olive Leaf Extract for Food Packaging Applications. J. Polym. Environ. 2020, 28, 123–130. [Google Scholar] [CrossRef]
- Musella, E.; El Ouazzani, I.C.; Mendes, A.R.; Rovera, C.; Farris, S.; Mena, C.; Teixeira, P.; Poças, F. Preparation and Characterization of Bioactive Chitosan-Based Films Incorporated with Olive Leaves Extract for Food Packaging Applications. Coatings 2021, 11, 1339. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Bouaziz, M.M.A. Polylactide Films with the Addition of Olive Leaf Extract— Physico-Chemical Characterization. Materials 2021, 14, 7623. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves-A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of Bioactive Compounds in Commercial Olive Leaf Extracts, and Olive Leaves and Their Infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef]
- Frikha, N.; Bouguerra, S.; Kit, G.; Abdelhedi, R.; Bouaziz, M. Smectite Clay KSF as Effective Catalyst for Oxidation of M-Tyrosol with H2O2 to Hydroxytyrosol. React. Kinet. Mech. Catal. 2019, 127, 505–521. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Zapata, A.; Ramirez-Arcos, S. A Comparative Study of McFarland Turbidity Standards and the Densimat Photometer to Determine Bacterial Cell Density. Curr. Microbiol. 2015, 70, 907–909. [Google Scholar] [CrossRef] [PubMed]
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2011.
- Dingreville, R.; Qu, J.; Cherkaoui, M. Surface Free Energy and Its Effect on the Elastic Behavior of Nano-Sized Particles, Wires and Films. J. Mech. Phys. Solids 2005, 53, 1827–1854. [Google Scholar] [CrossRef]
- Novoselić, A.; Klisović, D.; Lukić, I.; Lukić, M.; Brkić Bubola, K. The Use of Olive Leaves in Buža Olive Cultivar Oil Production: Exploring the Impact on Oil Yield and Chemical Composition. Agriculture 2021, 11, 917. [Google Scholar] [CrossRef]
- Kong, I.; Lamudji, I.G.; Angkow, K.J.; Insani, R.M.S.; Mas, M.A.; Pui, L.P. Application of Edible Film with Asian Plant Extracts as an Innovative Food Packaging: A Review. Coatings 2023, 13, 245. [Google Scholar] [CrossRef]
- Köse, M.D.; Gümüş Işık, Ş.; Bayraktar, O. Olive Leaf Polyphenols Loaded Mucoadhesive Oral Films. J. Eng. Sci. Des. 2021, 9, 366–380. [Google Scholar] [CrossRef]
- Moraczewski, K.; Pawłowska, A.; Stepczyńska, M.; Malinowski, R.; Kaczor, D.; Budner, B.; Gocman, K.; Rytlewski, P. Plant Extracts as Natural Additives for Environmentally Friendly Polylactide Films. Food Packag. Shelf Life 2020, 26, 100593. [Google Scholar] [CrossRef]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of Flexible Bactericidal Films Based on Poly(Lactic Acid) and Essential Oil and Its Effectiveness to Reduce Microbial Growth of Refrigerated Rainbow Trout. LWT 2016, 72, 251–260. [Google Scholar] [CrossRef]
- Erdohan, Z.Ö.; Turhan, K.N. Olive Leaf Extract and Usage for Development of Antimicrobial Food Packaging. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2012; Volume 1, pp. 1094–1101. [Google Scholar]
- Azman, N.H.; Khairul, W.M.; Sarbon, N.M. A Comprehensive Review on Biocompatible Film Sensor Containing Natural Extract: Active/Intelligent Food Packaging. Food Control 2022, 141, 109189. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Bustos, F.; Guarda, A.; Galotto, M.J. Cross-Linked Methyl Cellulose Films with Murta Fruit Extract for Antioxidant and Antimicrobial Active Food Packaging. Food Hydrocoll. 2016, 60, 335–344. [Google Scholar] [CrossRef]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple Peel Extract Incorporated Poly(Vinyl Alcohol)-Corn Starch Film for Active Food Packaging: Preparation, Characterization and Antioxidant Activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ayana, B.; Turhan, K.N. Use of Antimicrobial Methylcellulose Films to Control Staphylococcus Aureus during Storage of Kasar Cheese. Packag. Technol. Sci. 2009, 22, 461–469. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and Safety of PLA and Its Copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Aliaa, N.S.N.S.; Fazliah, M.N.S.N.; Fatimah, S.S.; Syazana, A.N. Synthesis and Characterization of PLA-PEG Biocomposite Incorporated with Sol-Gel Derived 45S5 Bioactive Glass. Mater. Today Proc. 2019, 17, 982–988. [Google Scholar] [CrossRef]
- Ferreira, K.N.; Oliveira, R.R.; Castellano, L.R.C.; Bonan, P.R.F.; Carvalho, O.V.; Pena, L.; Souza, J.R.; Oliveira, J.E.; Medeiros, E.S. Controlled Release and Antiviral Activity of Acyclovir-Loaded PLA/PEG Nanofibers Produced by Solution Blow Spinning. Biomater. Adv. 2022, 136, 212785. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Olive Leaf Extract to Obtain Bioactive Films Effective in Cherry Tomato Preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial Activity of Commercial Olea Europaea (Olive) Leaf Extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef]
- Lim, A.; Subhan, N.; Jazayeri, J.A.; John, G.; Vanniasinkam, T.; Obied, H.K. Plant Phenols as Antibiotic Boosters: In Vitro Interaction of Olive Leaf Phenols with Ampicillin. Phytother. Res. 2016, 30, 503–509. [Google Scholar] [CrossRef]
| Sample | Contact Angle [°] | SFE [mJ/m2] | [mJ/m2] | [mJ/m2] | |
|---|---|---|---|---|---|
| G | D | ||||
| PLA/PEG | 74.62 ± 0.62 | 48.55 ± 0.23 | 35.31 ± 0.09 | 30.39 ± 0.05 | 35.31 ± 0.09 |
| PLA/PEG/1OLE | 85.72 ± 0.91 * | 62.20 ± 1.65 * | 27.17 ± 0.45 * | 24.24 ± 0.35 * | 27.17 ± 0.45 * |
| PLA/PEG/3OLE | 99.27 ± 1.06 * | 64.34 ± 0.88 * | 26.25 ± 0.21 * | 26.06 ± 0.20 * | 26.25 ± 0.21 * |
| PLA/PEG/5OLE | 96.23 ± 0.70 * | 61.54 ± 2.27 * | 27.63 ± 0.55 * | 27.26 ± 0.50 * | 27.63 ± 0.55 * |
| Specimen | Ra [nm] | Rq [nm] | Rmax [nm] |
|---|---|---|---|
| PLA/PEG | 4.79 ± 0.08 | 5.87 ± 0.18 | 35.47 ± 0.46 |
| PLA/PEG/1OLE | 21.90 ± 1.82 | 25.40 ± 1.29 | 138.93 ± 3.90 |
| PLA/PEG/3OLE | 45.10 ± 1.56 | 54.60 ± 3.09 | 280.67 ± 8.02 |
| PLA/PEG/5OLE | 77.20 ± 3.22 | 94.60 ± 4.74 | 461.67 ± 7.02 |
| Sample | Temperature (°C) at Mass Loss | ΔT (T10%-T5%) | ||
|---|---|---|---|---|
| T5% | T10% | T50% | ||
| PLA/PEG | 108.82 | 297.75 | 357.43 | 188.93 |
| PLA/PEG/1OLE | 95.52 | 299.74 | 358.55 | 204.22 |
| PLA/PEG/3OLE | 98.56 | 303.05 | 358.07 | 204.49 |
| PLA/PEG/5OLE | 93.53 | 293.47 | 356.84 | 199.94 |
| Sample | Hardness (H; MPa) | Reduced Young’s Modulus (Er; MPa) | H/Er | H3/Er2 (MPa) |
|---|---|---|---|---|
| PLA/PEG | 21.3 ± 1.5 | 218.9 ± 17.0 | 0.097 ± 0.003 | 0.201 ± 0.030 |
| PLA/PEG/1OLE | 20.2 ± 0.8 | 204.6 ± 10.3 | 0.091 ± 0.005 | 0.196 ± 0.015 |
| PLA/PEG/3OLE | 19.8 ± 0.6 | 208.2 ± 14.7 | 0.095 ± 0.016 | 0.179 ± 0.028 |
| PLA/PEG/5OLE | 18.3 ± 1.2 * | 172.1 ± 7.4 * | 0.106 ± 0.011 | 0.207 ± 0.017 |
| McFarland Standard Values Specifying the Number of Staphylococcus aureus Bacteria | ||||
|---|---|---|---|---|
| Time [h] | PLA/PEG | PLA/PEG/1OLE | PLA/PEG/3OLE | PLA/PEG/5OLE |
| 0 | 0.3 | |||
| 0.5 | 0.53 + 0.02 | 0.53 + 0.02 | 0.52 + 0.01 | 0.50 + 0.02 |
| 1.0 | 0.86 + 0.01 | 0.84 + 0.01 | 0.75 + 0.01 * | 0.73 + 0.01 * |
| 2.0 | 1.74 + 0.01 | 1.42 + 0.02 * | 1.12 + 0.02 * | 1.07 + 0.04 * |
| 3.0 | 2.10 + 0.01 | 1.72 + 0.02 * | 1.53 + 0.02 * | 1.46 + 0.01 * |
| 4.0 | 3.22 + 0.02 | 2.65 + 0.02 * | 2.38 + 0.04 * | 2.29 + 0.03 * |
| 4.5 | 3.49 + 0.02 | 3.09 + 0.02 * | 2.61 + 0.02 * | 2.42 + 0.02 * |
| 5.0 | >4 | 3.66 + 0.02 * | 3.35 + 0.03 * | 3.11 + 0.02 * |
| 5.5 | >4 | 3.72 + 0.05 * | 3.49 + 0.03 * | |
| 6.0 | >4 | >4 | ||
| McFarland Standard Values Specifying the Number of Escherichia coli Bacteria | ||||
|---|---|---|---|---|
| Time [h] | PLA/PEG | PLA/PEG/1OLE | PLA/PEG/3OLE | PLA/PEG/5OLE |
| 0 | 0.3 | |||
| 0.5 | 0.57 + 0.02 | 0.57 + 0.02 | 0.52 + 0.01 * | 0.52 + 0.01 * |
| 1.0 | 1.42 + 0.02 | 1.41 + 0.01 | 1.16 + 0.02 * | 1.12 + 0.01 * |
| 2.0 | 2.61 + 0.01 | 2.60 + 0.02 | 2.14 + 0.04 * | 2.01 + 0.04 * |
| 3.0 | >4 | >4 | 3.36 + 0.02 * | 3.34 + 0.01 * |
| 4.0 | >4 | >4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabska-Zielińska, S.; Olewnik-Kruszkowska, E.; Gierszewska, M.; Bouaziz, M.; Wekwejt, M.; Pałubicka, A.; Żywicka, A.; Kaczmarek-Szczepańska, B. Active Polylactide-poly(ethylene glycol) Films Loaded with Olive Leaf Extract for Food Packaging—Antibacterial Activity, Surface, Thermal and Mechanical Evaluation. Polymers 2025, 17, 205. https://doi.org/10.3390/polym17020205
Grabska-Zielińska S, Olewnik-Kruszkowska E, Gierszewska M, Bouaziz M, Wekwejt M, Pałubicka A, Żywicka A, Kaczmarek-Szczepańska B. Active Polylactide-poly(ethylene glycol) Films Loaded with Olive Leaf Extract for Food Packaging—Antibacterial Activity, Surface, Thermal and Mechanical Evaluation. Polymers. 2025; 17(2):205. https://doi.org/10.3390/polym17020205
Chicago/Turabian StyleGrabska-Zielińska, Sylwia, Ewa Olewnik-Kruszkowska, Magdalena Gierszewska, Mohamed Bouaziz, Marcin Wekwejt, Anna Pałubicka, Anna Żywicka, and Beata Kaczmarek-Szczepańska. 2025. "Active Polylactide-poly(ethylene glycol) Films Loaded with Olive Leaf Extract for Food Packaging—Antibacterial Activity, Surface, Thermal and Mechanical Evaluation" Polymers 17, no. 2: 205. https://doi.org/10.3390/polym17020205
APA StyleGrabska-Zielińska, S., Olewnik-Kruszkowska, E., Gierszewska, M., Bouaziz, M., Wekwejt, M., Pałubicka, A., Żywicka, A., & Kaczmarek-Szczepańska, B. (2025). Active Polylactide-poly(ethylene glycol) Films Loaded with Olive Leaf Extract for Food Packaging—Antibacterial Activity, Surface, Thermal and Mechanical Evaluation. Polymers, 17(2), 205. https://doi.org/10.3390/polym17020205










