Influence of Post-Washing Time and Build Orientation on Mechanical Properties and Biocompatibility of Additively Manufactured Permanent Dental Resin Material
Abstract
1. Introduction
2. Materials and Methods
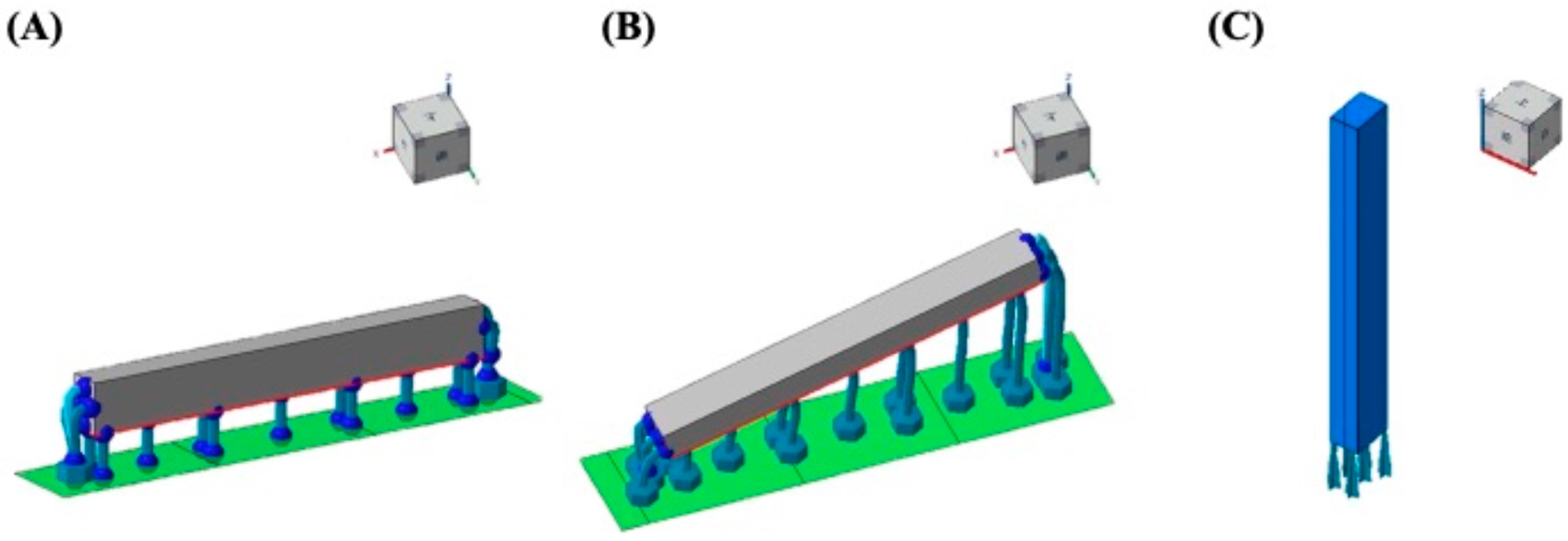
2.1. 3D CAD and Specimen Preparation
2.2. Accuracy of DLP-Fabricated Specimens
2.3. Mechanical Properties
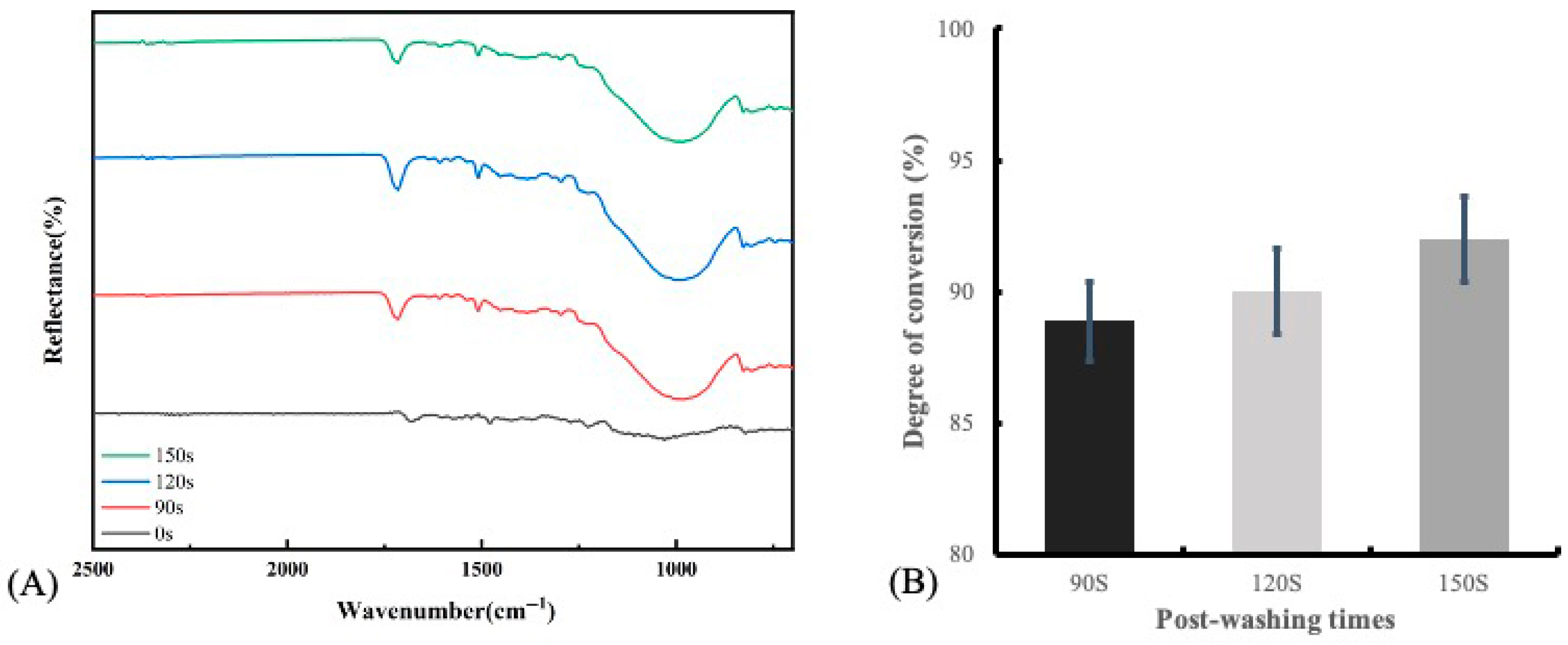
2.4. Degree of Conversion (DC)
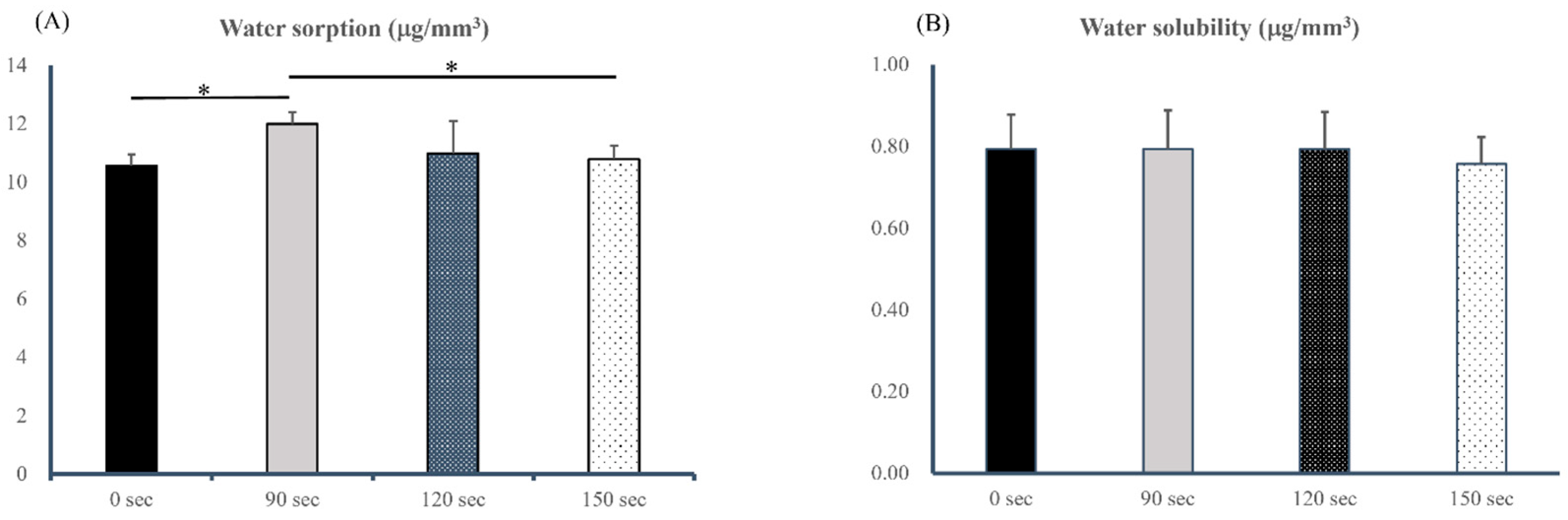
2.5. Water Sorption and Solubility
2.6. Cytotoxicity Assay
2.7. Statistical Analysis
3. Results
3.1. Dimensional Accuracy
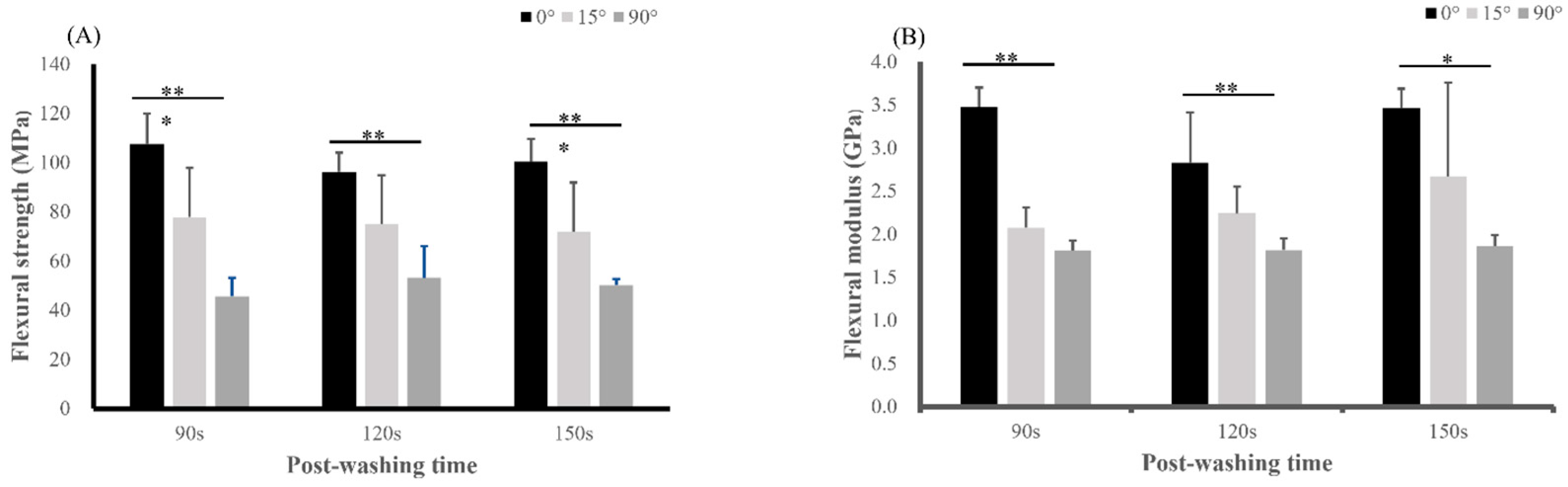
3.2. Mechanical Properties
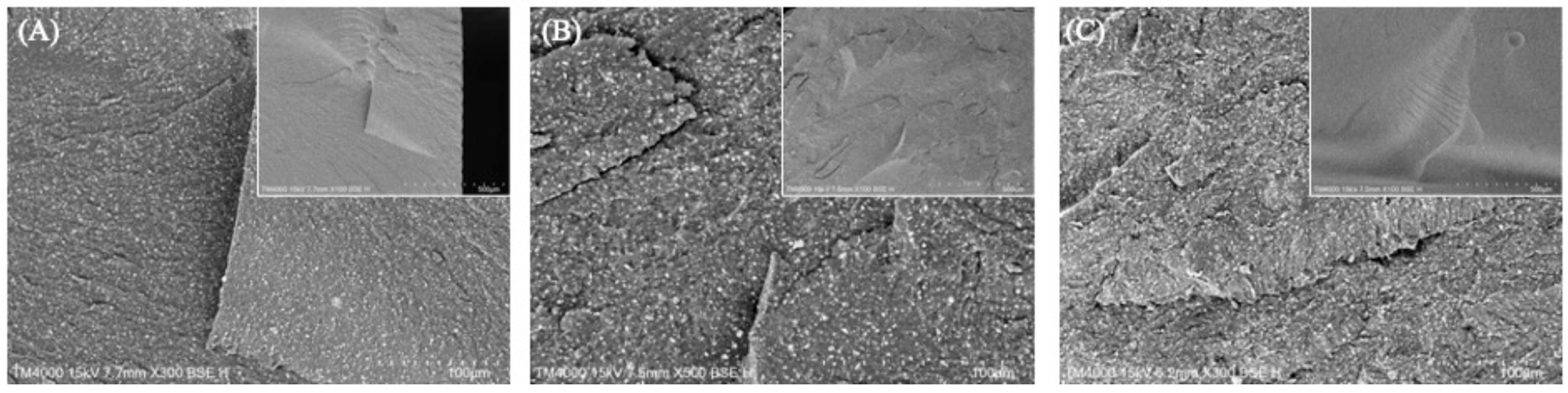
3.3. Degree of Conversion (DC)
3.4. Water Sorption and Solubility
3.5. Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLP | Digital light processing |
| CAD/CAM | Computer-aided design/computer-aided manufacturing |
| SLA | Stereolithography apparatus |
References
- Methani, M.M.; Revilla-León, M.; Zandinejad, A. The potential of additive manufacturing technologies and their processing parameters for the fabrication of all-ceramic crowns: A review. J. Esthet. Restor. Dent. 2020, 32, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.W.; Hsu, C.Y.; Huang, H.Y.; Chao, J.C.; Lee, W.F. Trueness of removable partial denture frameworks additively manufactured with selective laser melting. J. Prosthet. Dent. 2022, 127, 122–127. [Google Scholar] [CrossRef]
- Peng, M.; Li, C.; Huang, C.; Liang, S. Digital technologies to facilitate minimally invasive rehabilitation of a severely worn dentition: A dental technique. J. Prosthet. Dent. 2021, 126, 167–172. [Google Scholar] [CrossRef]
- Ramezani, M.; Dommati, H.; Wang, J.C.; Pasang, T.; Lee, C. Tribological Characterization of Alumina Ceramic Manufactured by Solvent-Based Slurry Stereolithography. J. Mater. Eng. Perform. 2023, 32, 8325–8336. [Google Scholar] [CrossRef]
- Alshamrani, A.A.; Raju, R.; Ellakwa, A. Effect of printing layer thickness and postprinting conditions on the flexural strength and hardness of a 3D-printed resin. BioMed Res. Int. 2022, 2022, 8353137. [Google Scholar] [CrossRef]
- Yang, M.S.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Park, J.M. Investigation of the marginal fit of a 3D-printed three-unit resin prosthesis with different build orientations and layer thicknesses. J. Adv. Prosthodont. 2022, 14, 250–261. [Google Scholar] [CrossRef]
- Waltimo, A.; Könönen, M. Maximal bite force and its association with signs and symptoms of craniomandibular disorders in young Finnish non-patients. Acta Odontol. Scand. 1995, 53, 254–258. [Google Scholar] [CrossRef]
- Reymus, M.; Fabritius, R.; Kebler, A.; Hickel, R.; Edelhoff, D.; Stawarczyk, B. Fracture load of 3D-printed fixed dental prostheses compared with milled and conventionally fabricated ones: The impact of resin material, build direction, post-curing, and artificial aging-an in vitro study. Clin. Oral Investig. 2020, 24, 701–710. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Okamoto, A.; Fukushima, M.; Okiji, T. Evaluation of flowable resin composite surfaces eroded by acidic and alcoholic drinks. Dent. Mater. J. 2008, 27, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Stawarczyk, B.; Vogt, K.; Hickel, R.; Edelhoff, D.; Reymus, M. Influence of cleaning methods after 3D printing on two-body wear and fracture load of resin-based temporary crown and bridge material. Clin. Oral Investig. 2021, 25, 5987–5996. [Google Scholar] [CrossRef]
- Alharbi, N.; Wismeijer, D.; Osman, R.B. Additive Manufacturing Techniques in Prosthodontics: Where Do We Currently Stand? A Critical Review. Int. J. Prosthodont. 2017, 30, 474–484. [Google Scholar] [CrossRef]
- Park, G.S.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Seo, D.G. Effects of printing parameters on the fit of implant-supported 3D printing resin prosthetics. Materials 2019, 12, 2533. [Google Scholar] [CrossRef] [PubMed]
- Favero, C.S.; English, J.D.; Cozad, B.E.; Wirthlin, J.O.; Short, M.M.; Kasper, F.K. Effect of print layer height and printer type on the accuracy of 3-dimensional printed orthodontic models. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 557–565. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Bui, P.H.B.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef]
- Di Fiore, A.; Stellini, E.; Alsayed, H.D.; Alhotan, A. Influence of different post-washing solutions on the mechanical and surface properties of 3D-printed material for definitive restorations: An in vitro study. J. Dent. 2025, 161, 105980. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Gu, H.; Jang, M.; Bayarsaikhan, E.; Lim, J.H.; Shim, J.S.; Lee, K.W.; Kim, J.E. Influence of postwashing process on the elution of residual monomers, degree of conversion, and mechanical properties of a 3D printed crown and bridge materials. Dent. Mater. 2022, 38, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Derban, P.; Negrea, R.; Rominu, M.; Marsavina, L. Influence of the printing angle and load direction on flexure strength in 3D printed materials for provisional dental restorations. Materials 2021, 14, 3376. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, G.; Lim, J.H.; Kim, J.E. Effects of washing agents on the mechanical and biocompatibility properties of water-washable 3D printing crown and bridge resin. Sci. Rep. 2024, 14, 9909. [Google Scholar] [CrossRef]
- DETAX. Highend Medical Matetials. Available online: https://www.detax.de/en/shop/produkte/Freeprint-temp.php (accessed on 14 April 2021).
- ISO-10477; Dentistry—Polymer-Based Crown and Veneering Materials. International Standards Organization: Geneva, Switzerland, 2020.
- Nam, N.E.; Hwangbo, N.K.; Kim, J.E. Effects of surface glazing on the mechanical and biological properties of 3D printed permanent dental resin materials. J. Prosthodont. Res. 2024, 68, 273–282. [Google Scholar] [CrossRef]
- Altarazi, A.; Haider, J.; Alhotan, A.; Silikas, N.; Devlin, H. Assessing the physical and mechanical properties of 3D printed acrylic material for denture base application. Dent. Mater. 2022, 38, 1841–1854. [Google Scholar] [CrossRef]
- Akin, H.; Tugut, F.; Polat, Z.A. In vitro comparison of the cytotoxicity and water sorption of two different denture base systems. J. Prosthodont. 2015, 24, 152–155. [Google Scholar] [CrossRef] [PubMed]
- ISO-10993-5:2009; Biological Evaluation of Medical Devices: Part 5: Tests for In Vitro Cytotoxicity. International Standards Organization: Geneva, Switzerland, 2009.
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef]
- ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials. ISO: Geneva, Switzerland, 2019.
- Ciccone-Nogueira, J.C.; Borsatto, M.C.; Souza-Zaron, W.C.; Ramos, R.P.; Palma-Dibb, R.G. Microhardness of composite resins at different depths varying the post-irradiation time. J. Appl. Oral Sci. 2007, 15, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hashimoto, M.; Wadgaonkar, B.; Svizero, N.; Carvalho, R.M.; Yiu, C.; Rueggeberg, F.A.; Foulger, S.; Saito, T.; Nishitani, Y. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 2005, 26, 6449–6459. [Google Scholar] [CrossRef]
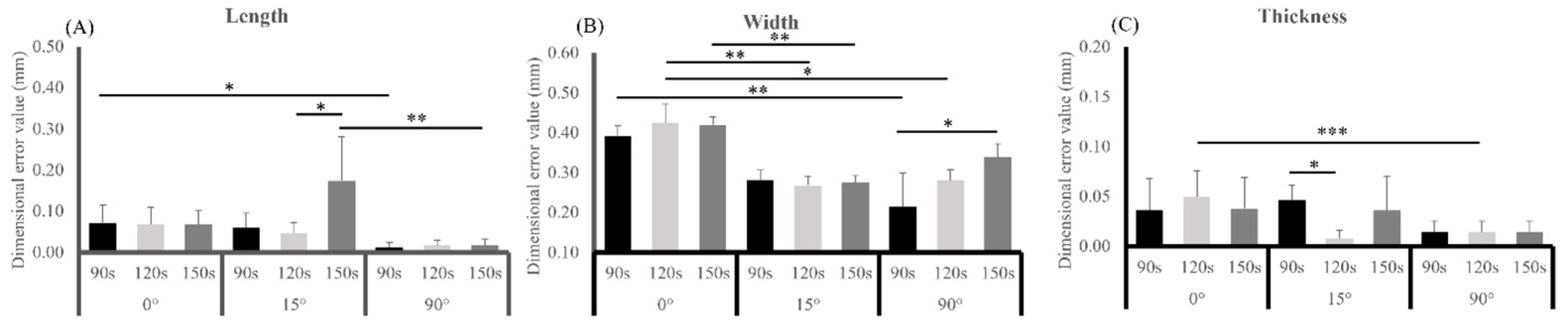


| Dimensional Direction | Source | Type III Sum Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|---|
| Length | Build orientation | 0.049 | 2 | 0.025 | 11.641 | <0.001 *** |
| Post-washing time | 0.016 | 2 | 0.008 | 3.923 | 0.029 * | |
| Build orientation × Post-washing time | 0.033 | 4 | 0.008 | 3.935 | 0.009 ** | |
| Width | Build orientation | 0.186 | 2 | 0.093 | 60.417 | <0.001 *** |
| Post-washing time | 0.018 | 2 | 0.009 | 5.832 | 0.006 ** | |
| Build orientation × Post-washing time | 0.024 | 4 | 0.006 | 3.890 | 0.010 ** | |
| Thickness | Build orientation | 0.006 | 2 | 0.003 | 5.696 | 0.007 ** |
| Post-washing time | 0.000 | 2 | 0.000 | 0.501 | 0.610 | |
| Build orientation × Post-washing time | 0.004 | 4 | 0.001 | 1.991 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, P.-W.; Chou, J.-S.; Chen, L.-X.; Chuang, P.-E.; Takahashi, H.; Hsieh, M.-C.; Lee, W.-F. Influence of Post-Washing Time and Build Orientation on Mechanical Properties and Biocompatibility of Additively Manufactured Permanent Dental Resin Material. Polymers 2025, 17, 2694. https://doi.org/10.3390/polym17192694
Peng P-W, Chou J-S, Chen L-X, Chuang P-E, Takahashi H, Hsieh M-C, Lee W-F. Influence of Post-Washing Time and Build Orientation on Mechanical Properties and Biocompatibility of Additively Manufactured Permanent Dental Resin Material. Polymers. 2025; 17(19):2694. https://doi.org/10.3390/polym17192694
Chicago/Turabian StylePeng, Pei-Wen, Jia-Syuan Chou, Le-Xin Chen, Po-En Chuang, Hidekazu Takahashi, Min-Chieh Hsieh, and Wei-Fang Lee. 2025. "Influence of Post-Washing Time and Build Orientation on Mechanical Properties and Biocompatibility of Additively Manufactured Permanent Dental Resin Material" Polymers 17, no. 19: 2694. https://doi.org/10.3390/polym17192694
APA StylePeng, P.-W., Chou, J.-S., Chen, L.-X., Chuang, P.-E., Takahashi, H., Hsieh, M.-C., & Lee, W.-F. (2025). Influence of Post-Washing Time and Build Orientation on Mechanical Properties and Biocompatibility of Additively Manufactured Permanent Dental Resin Material. Polymers, 17(19), 2694. https://doi.org/10.3390/polym17192694





