Advanced Polyamidoamine Hydrogels for the Selective Cleaning of Artifacts in Heritage Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Functionalized M-GLY Oligomer
2.3. Synthesis of the H-M-GLY/MMT Hydrogel
2.4. Synthesis of the H-M-GLY Hydrogel
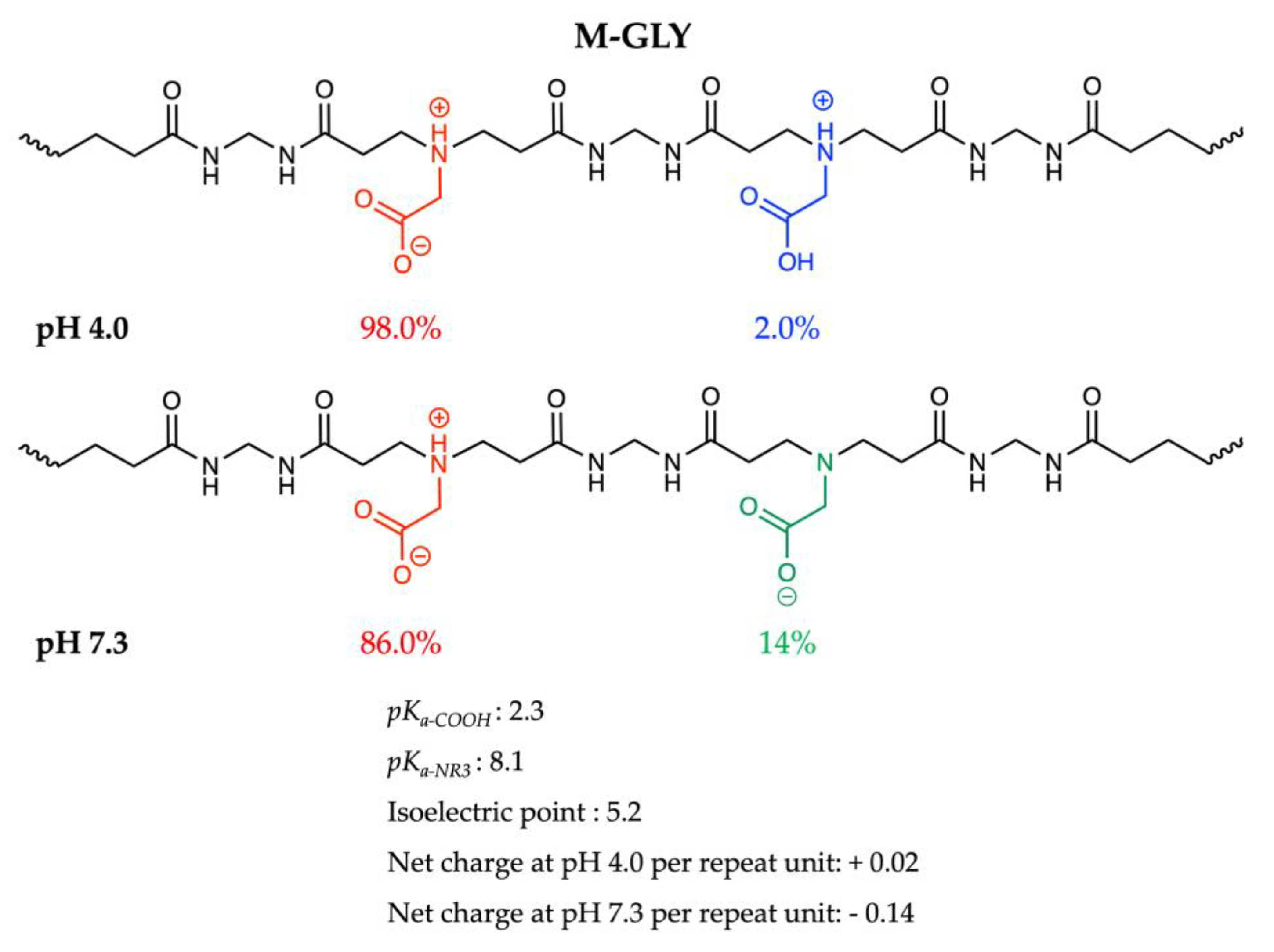
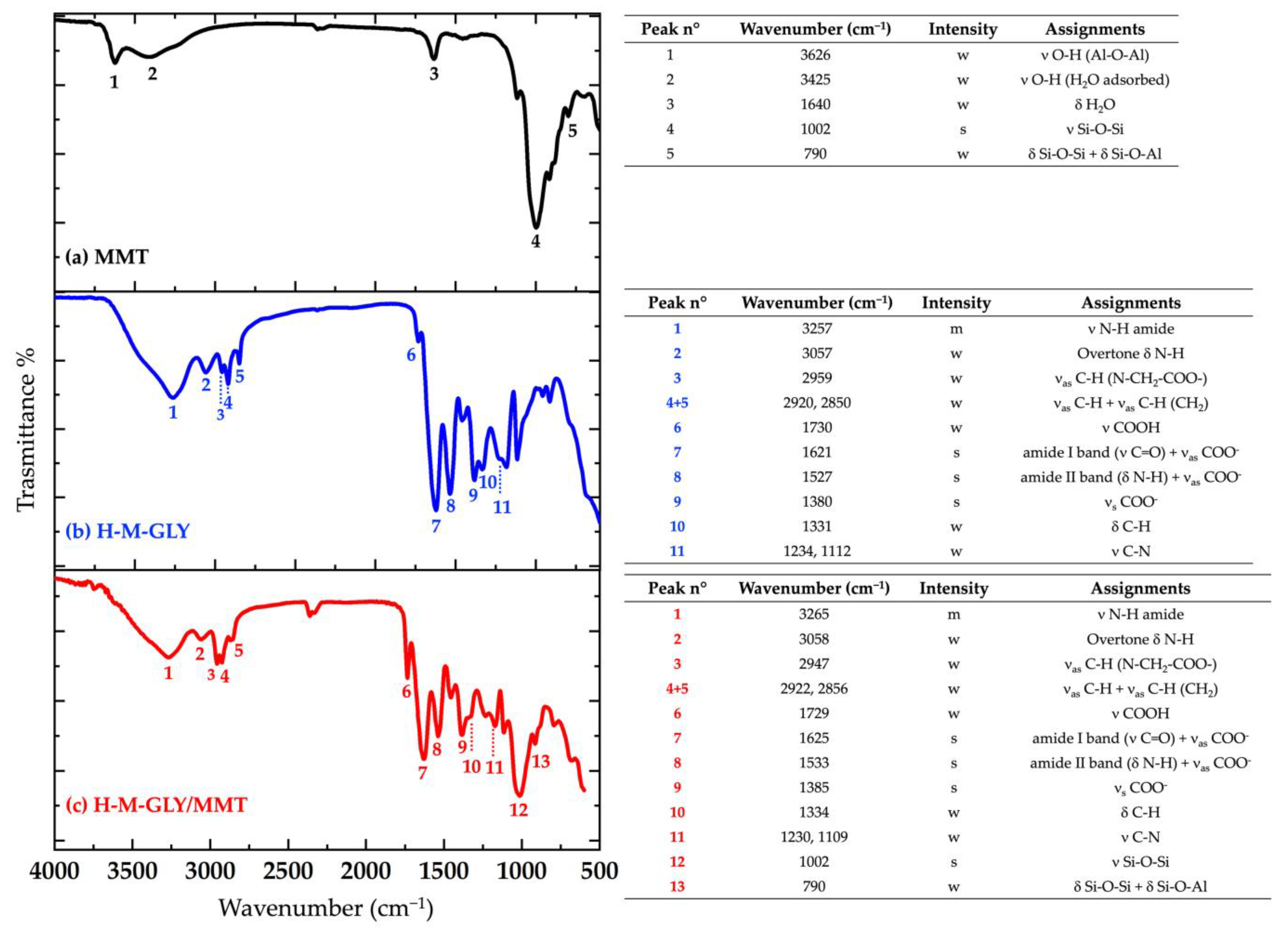
2.5. Chemical Characterization
2.6. Water Uptake Test
2.7. Morphological Analysis
2.8. X-Ray Diffraction Analysis
2.9. Compression Test
2.10. Ink Bleeding Test
2.11. Paper Deacidification Test
2.12. Wax Cleaning Test
2.13. Durability Test
3. Results
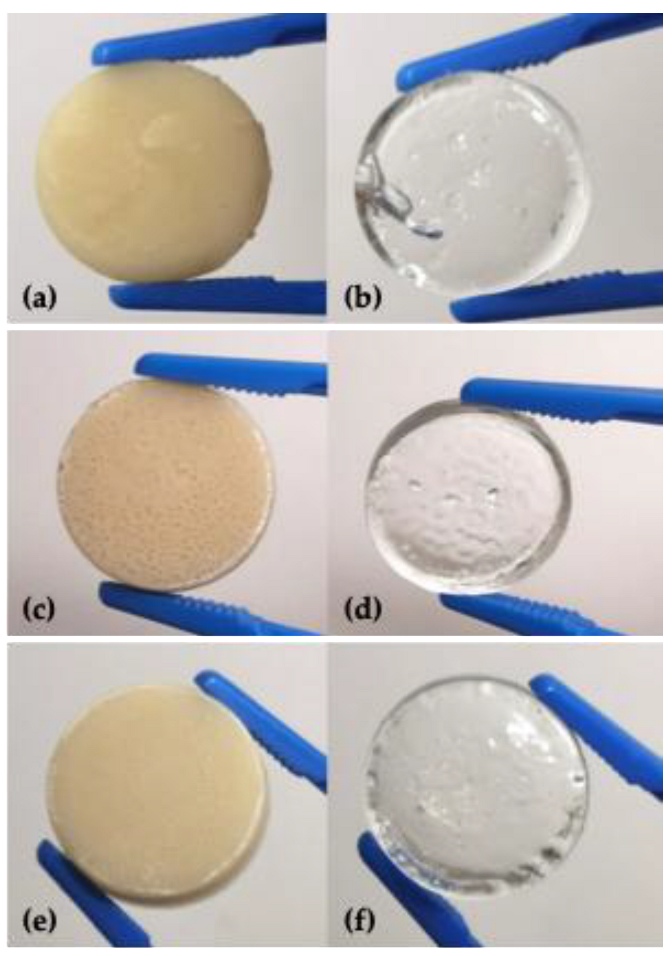
3.1. Synthesis of a,w-Acrylamide Terminated M-GLY Oligomer
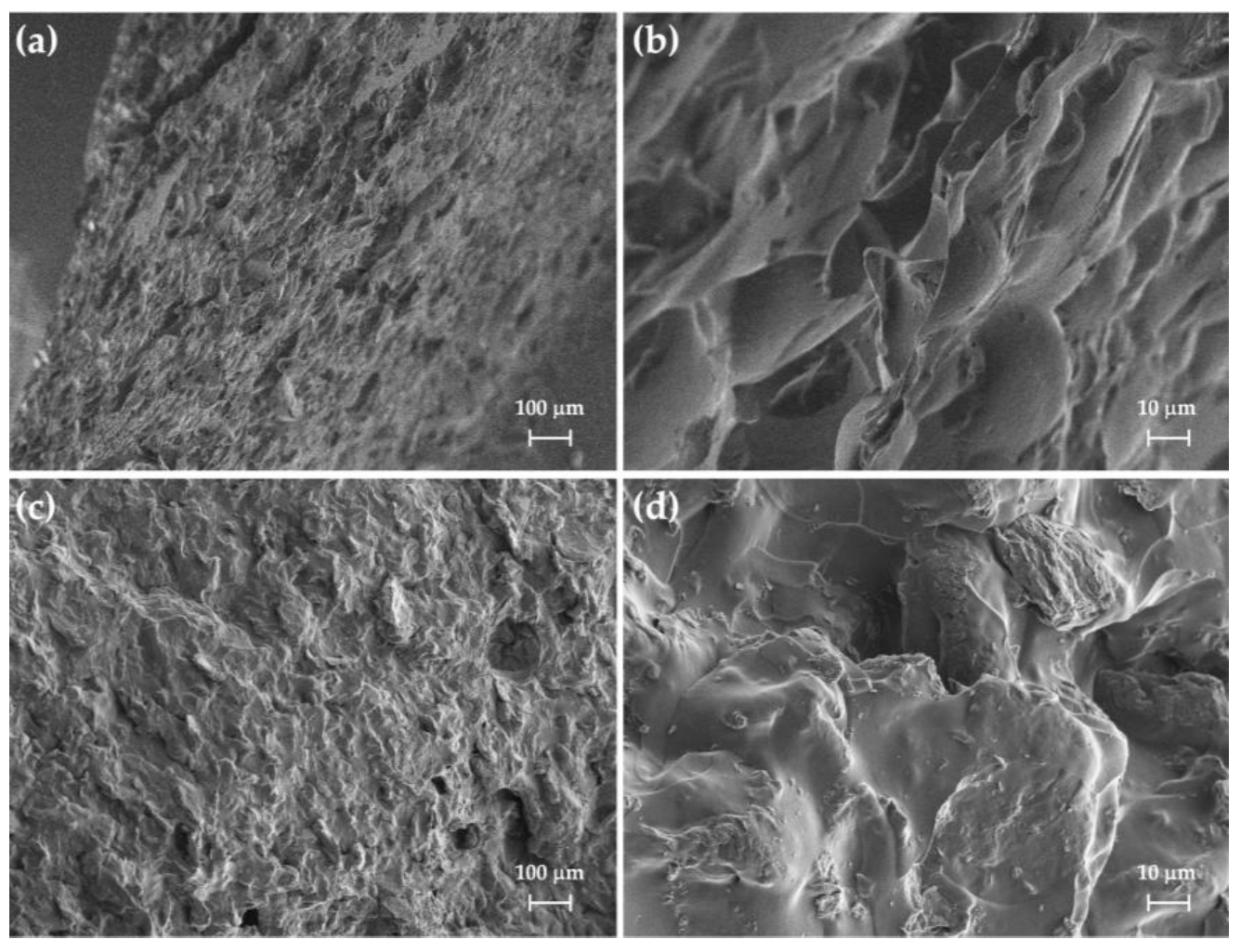
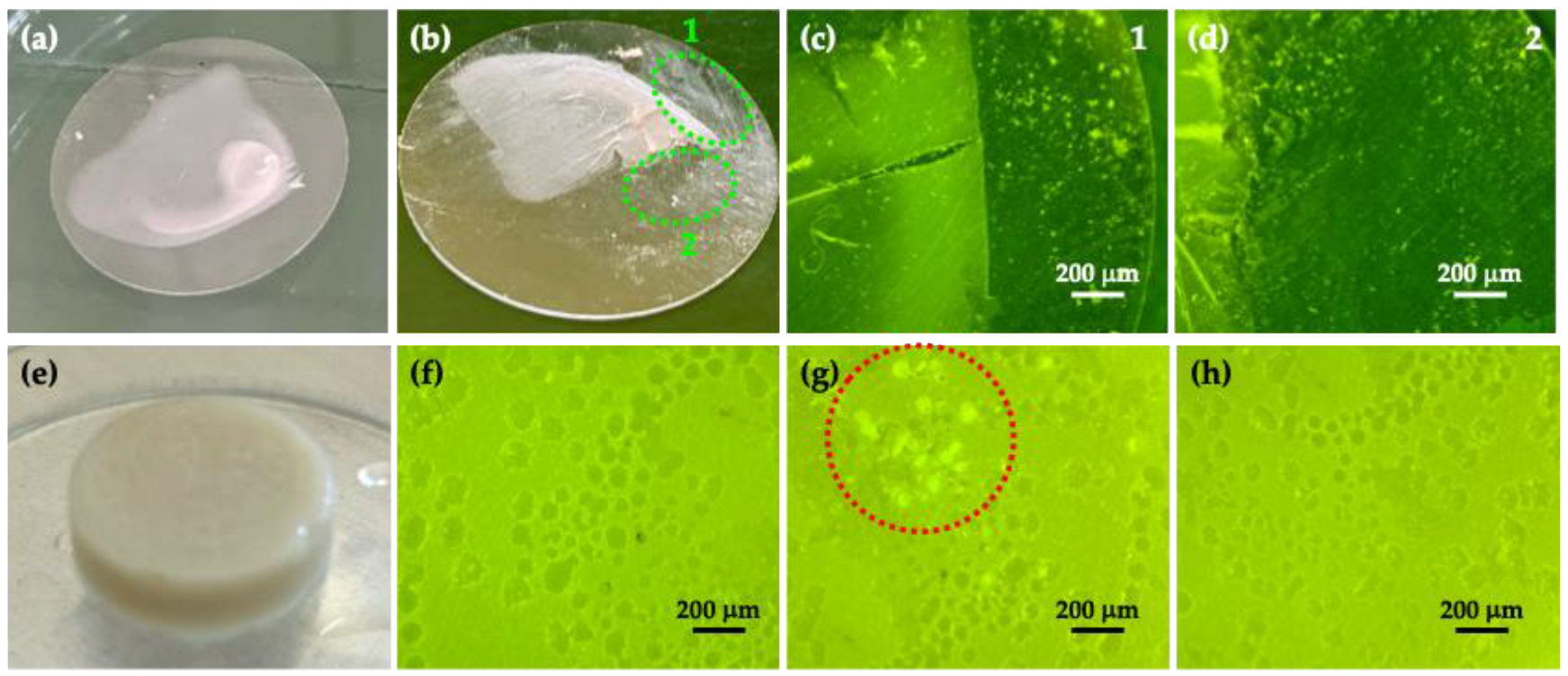
3.2. Morphological and X-Ray Diffraction Analyses
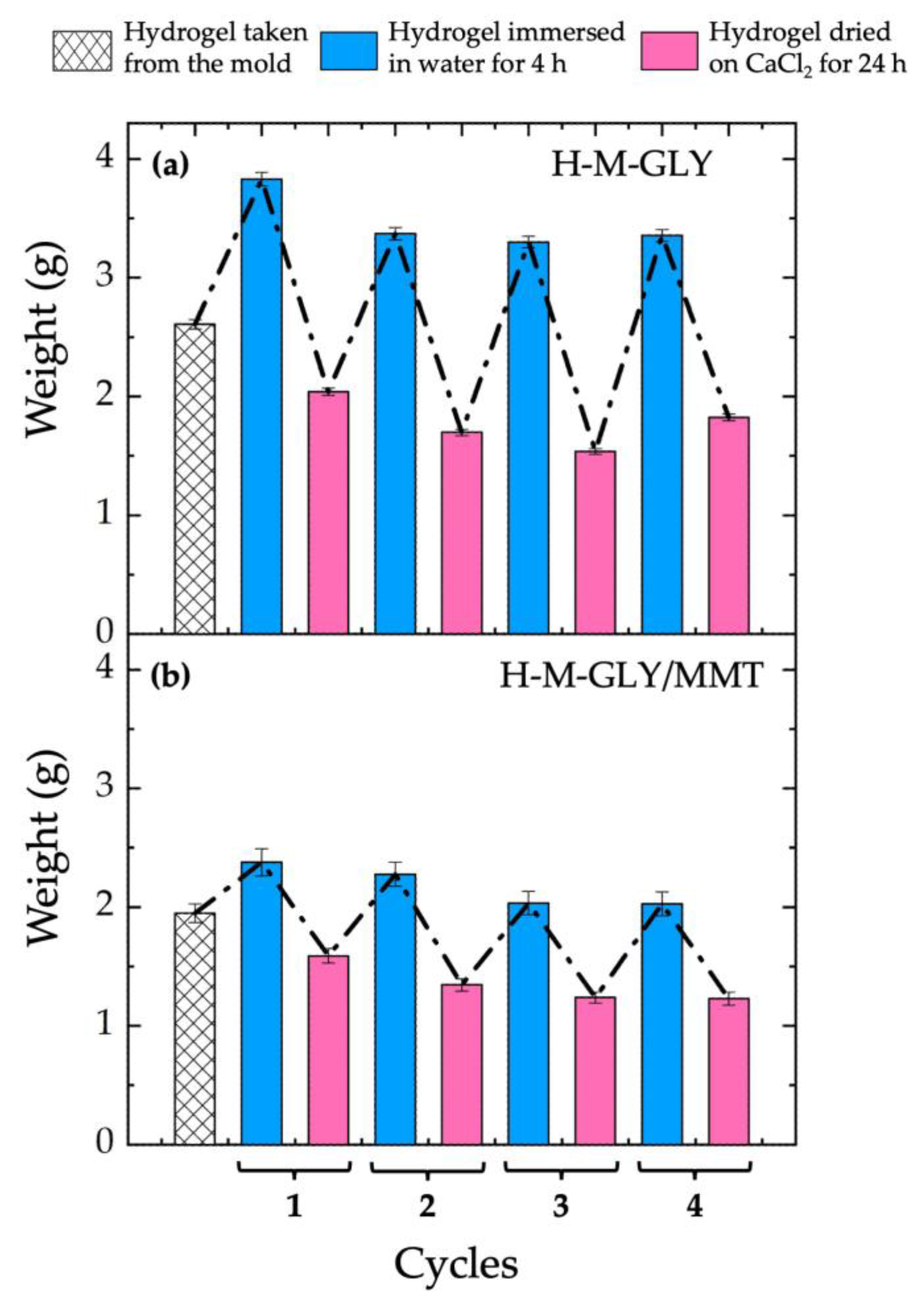
3.3. Water Uptake Tests
3.4. Compression Tests
3.5. Ink Bleeding Tests
3.6. Paper Deacidification Tests
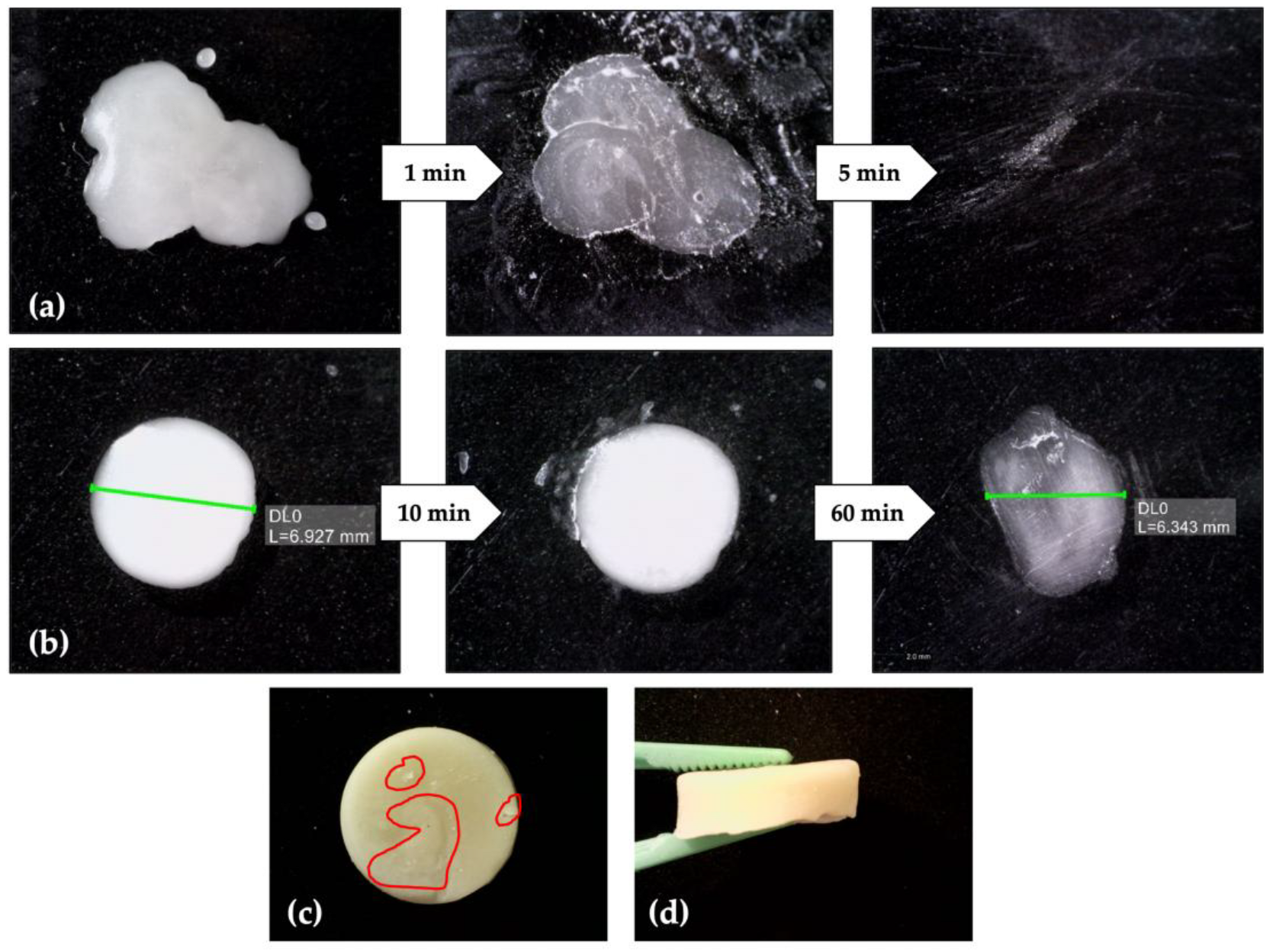
3.7. Wax Cleaning
3.8. Hydrogel Durability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Casini, A.; Chelazzi, D.; Baglioni, P. Advanced methodologies for the cleaning of works of art. Sci. China Technol. Sci. 2023, 66, 2162–2182. [Google Scholar] [CrossRef]
- Pianorsi, M.D.; Raudino, M.; Bonelli, N.; Chelazzi, D.; Giorgi, R.; Fratini, E.; Baglioni, P. Organogels for the cleaning of artifacts. Pure Appl. Chem. 2017, 89, 3–17. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Cleaning of Wall Paintings and Stones. In Nanotechnologies in the Conservation of Cultural Heritage; Baglioni, P., Chelazzi, D., Giorgi, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Chapter 3; pp. 61–82. [Google Scholar]
- Ranalli, G.; Zanardini, E. Biocleaning on Cultural Heritage: New frontiers of microbial biotechnologies. J. Appl. Microbiol. 2021, 131, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, R.; Baglioni, M.; Berti, D.; Baglioni, P. New Methodologies for the Conservation of Cultural Heritage: Micellar Solutions, Microemulsions, and Hydroxide Nanoparticles. Acc. Chem. Res. 2010, 43, 695–704. [Google Scholar] [CrossRef]
- Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle, microemulsions, and gels for the conservation of cultural heritage. Adv. Colloid Interface Sci. 2014, 205, 361–371. [Google Scholar] [CrossRef]
- Chelazzi, D.; Fratini, E.; Giorgi, R.; Mastrangelo, R.; Rossi, M.; Baglioni, P. Gels for the Cleaning of Works of Art. in Gels and Other Soft Amorphous Solids. ACS Symp. Ser. 2018, 1296, 291–314. [Google Scholar] [CrossRef]
- Baglioni, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Advanced Materials in Cultural Heritage Conservation. Molecules 2021, 26, 3967. [Google Scholar] [CrossRef]
- Carretti, E.; Giorgi, R. Cleaning IV: Applications and Case Studies. In Nanoscience for the Conservation of Works of Art; Baglioni, P., Chelazzi, D., Eds.; RSC Publishing: Cambridge, UK, 2013; Chapter 11; pp. 280–314. [Google Scholar]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. (Eds.) Cleaning of Easel Paintings. In Nanotechnologies in the Conservation of Cultural Heritage; Springer: Berlin/Heidelberg, Germany, 2015; Chapter 4; pp. 83–116. [Google Scholar]
- Lo Nostro, P. Cleaning II: Surfactants and Micellar Solutions. In Nanoscience for the Conservation of Works of Art; Baglioni, P., Chelazzi, D., Eds.; RSC Publishing: Cambridge, UK, 2013; Chapter 6; pp. 147–181. [Google Scholar]
- Saglam, R.O.; Yıldırım, S.U.; Oktar, F.N.; Genc, S.; Erdem, G.; Oner, E.T. Synthesis and characterization of interpenetrating network (IPN) based levan-polyacrylamide hydrogels and their application in conservation of cultural heritage. J. Cult. Herit. 2023, 64, 255–265. [Google Scholar] [CrossRef]
- Mazzuca, C.; Micheli, L.; Cervelli, E.; Basoli, F.; Cencetti, C.; Coviello, T.; Iannuccelli, S.; Sotgiu, S.; Palleschi, A. Cleaning of paper artworks: Development of an efficient gel-based material able to remove starch paste. ACS Appl. Mater. Interfaces 2014, 6, 16519–16528. [Google Scholar] [CrossRef]
- Bonelli, N.; Montis, C.; Antonio Mirabile, A.; Berti, D.; Baglioni, P. Restoration of paper artworks with microemulsions confined in hydrogels for safe and efficient removal of adhesive tapes. Proc. Natl. Acad. Sci. USA 2018, 115, 5932–5937. [Google Scholar] [CrossRef]
- Abdel-Maksoud, G.; Mansour, M.; Khedr, A. Fungal biodeterioration of a historical manuscript dating back to the 15th century. Herit. Sci. 2022, 10, 122. [Google Scholar]
- Pinzari, F.; Pasquariello, G.; De Mico, A. Biodeterioration of paper: A SEM study of fungal spoilage reproduced under controlled conditions. Macromol. Symp. 2006, 238, 57–66. [Google Scholar] [CrossRef]
- Blüer, A.; Vogelsanger, B. Mass Deacidification of Paper. Chimia 2001, 55, 981. [Google Scholar] [CrossRef]
- Rushdy, A.M.; Wafika, N.; Wahba, W.N.; Dacrory, S.; Kamel, S. Multi-function Gellan Gum Hydrogel for Heritage Paper Cleaning. Egypt. J. Chem. 2024, 67, 441–450. [Google Scholar] [CrossRef]
- Qingxia Meng, Q.; Liu, C.; Liu, C.; Jiao, Q.; Li, S.; Fan, H.; Ben, S. A novel nanocomposite hydrogel system for synergistic paper deacidification and reinforcement. J. Cult. Herit. 2025, 75, 31–40. [Google Scholar] [CrossRef]
- Barclay, B.; Hett, C. The Cleaning, Polishing, and Protective Waxing of Brass and Copper. Canadian Conservation Institute Notes 9/3. 2007. Available online: https://www.canada.ca/content/dam/cci-icc/documents/services/conservation-preservation-publications/canadian-conservation-institute-notes/9-3-eng.pdf?WT.contentAuthority=4.4.10 (accessed on 3 June 2025).
- Biribicchi, C.; Macchia, A.; Favero, G.; Strangis, R.; Gabriele, B.; Mancuso, R.; Mauro Francesco La Russa, M.F. Sustainable solutions for removing aged wax-based coatings from cultural heritage: Exploiting hydrophobic deep eutectic solvents (DESs). New J. Chem. 2023, 47, 5991–6000. [Google Scholar] [CrossRef]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile bioactive polymers with potential for biotechnological applications. Chem. Afr. 2019, 2, 167–193. [Google Scholar] [CrossRef]
- Dubé, M.A.; Salehpour, S. Applying the principles of green chemistry to polymer production technology. Macromol. React. Eng. 2014, 8, 7–28. [Google Scholar] [CrossRef]
- Argenziano, M.; Dianzani, C.; Ferrara, B.; Swaminathan, S.; Manfredi, A.; Ranucci, E.; Cavalli, R.; Ferruti, P. Cyclodextrin-Based Nanohydrogels Containing Polyamidoamine Units: A New Dexamethasone Delivery System for Inflammatory Diseases. Gels 2017, 3, 22. [Google Scholar] [CrossRef]
- Cavalli, R.; Primo, L.; Sessa, R.; Chiaverina, G.; di Blasio, L.; Alongi, J.; Manfredi, A.; Ranucci, E.; Ferruti, P. The AGMA1 Polyamidoamine Mediates the Efficient Delivery of SiRNA. J. Drug Target. 2017, 25, 891–898. [Google Scholar] [CrossRef]
- Alongi, J.; Costantini, A.; Ferruti, P.; Ranucci, E. Evaluation of the eco-compatibility of polyamidoamines by means of seed germination test. Polym. Degrad. Stab. 2022, 197, 109854. [Google Scholar] [CrossRef]
- Ranucci, E.; Treccani, S.; Ferruti, P.; Alongi, J. The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines. Polymers 2024, 16, 1744. [Google Scholar] [CrossRef]
- Treccani, S.; Ferruti, P.; Alongi, J.; Monti, E.; Zizioli, D.; Ranucci, E. Ecotoxicity Assessment of α-Amino Acid-Derived Polyamidoamines Using Zebrafish as a Vertebrate Model. Polymers 2024, 16, 2087. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Giammona, G.; Ranucci, E.; Ferruti, P. Synthesis of Biocompatible and Biodegradable Polyamidoamines Microgels via a Simple and Reliable Statistical Approach. Materials 2022, 15, 7280. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, C.; Bloise, N.; Mauro, N.; Ferruti, P.; Manfredi, A.; Sampaolesi, M.; Liguori, A.; Laurita, R.; Gherardi, M.; Colombo, V.; et al. Poly-L-Lactic Acid Nanofiber–Polyamidoamine Hydrogel Composites: Preparation, Properties, and Preliminary Evaluation as Scaffolds for Human Pluripotent Stem Cell Culturing. Macromol. Biosci. 2016, 16, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P.; Alongi, J.; Barabani, E.; Manfredi, A.; Ranucci, E. Silk/Polyamidoamine Membranes for Removing Chromium VI from Water. Polymers 2023, 15, 1871. [Google Scholar] [CrossRef]
- Mauro, N.; Chiellini, F.; Bartoli, C.; Gazzarri, M.; Laus, M.; Antonioli, D.; Griffiths, P.; Manfredi, A.; Ranucci, E.; Ferruti, P. RGD-mimic polyamidoamine–montmorillonite composites with tunable stiffness as scaffolds for bone tissue-engineering applications. J. Tissue Eng. Regen. Med. 2017, 11, 2164–2175. [Google Scholar] [CrossRef]
- Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Polyamidoamines derived from natural α-amino acids as effective flame retardants for cotton. Polymers 2021, 13, 3714. [Google Scholar] [CrossRef]
- Alongi, J.; Treccani, S.; Comite, V.; Fermo, P.; Ferruti, P.; Ranucci, E. Polyamidoamine-based photostabilizers for cotton fabrics. Polym. Degrad. Stab. 2024, 228, 110938. [Google Scholar] [CrossRef]
- Isca, C.; D’Avorgna, S.; Graiff, C.; Montanari, M.; Ugozzoli, F.; Predieri, G. Paper preservation with polyamidoamines: A preliminary study. Cellulose 2016, 23, 1415–1432. [Google Scholar] [CrossRef]
- Isca, C.; Di Maggio, R.; Collado, N.P.; Predieri, G.; Lottici, P.P. The use of polyamidoamines for the conservation of iron-gall inked paper. Cellulose 2019, 26, 1277–1296. [Google Scholar] [CrossRef]
- Liao, Y.; Fan, H.; Zhang, Y.; Mou, H.; Li, F.; Zhou, Z.; Liu, J. Conservation and enhancement of naturally aged paper using bi-functionalized polyamidoamine (SiPAAOH). Nord. Pulp Pap. Res. J. 2022, 37, 636–648. [Google Scholar] [CrossRef]
- Flory, P. Principles of polymer chemistry. In Molecular Size and Chemical Reactivity; Principles of Condensation Polymerization; Cornell University Press: Ithaca, NY, USA, 1953; Chapter 3; Available online: https://archive.org/details/dli.ernet.286013/page/68/mode/2up (accessed on 3 June 2025).
- Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Synergism between α-amino acid-derived polyamidoamines and sodium montmorillonite for enhancing the flame retardancy of cotton fabrics. Polym. Degrad. Stab. 2024, 225, 110764. [Google Scholar] [CrossRef]
- Treccani, S.; Alongi, J.; Ferruti, P.; Ranucci, E. α-amino acid-derived polyamidoamines as photostabilizers for cotton. Polym. Degrad. Stab. 2025, 239, 111430. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Tolmino, R.; Formoso, P.; Nicoletta, F.P. Gellan gum hybrid hydrogels for the cleaning of paper artworks contaminated with Aspergillus versicolor. Cellulose 2016, 23, 3265–3279. [Google Scholar] [CrossRef]
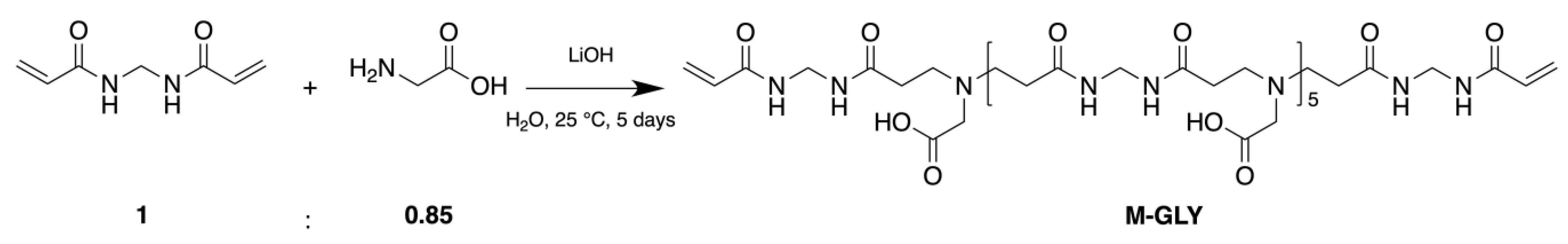
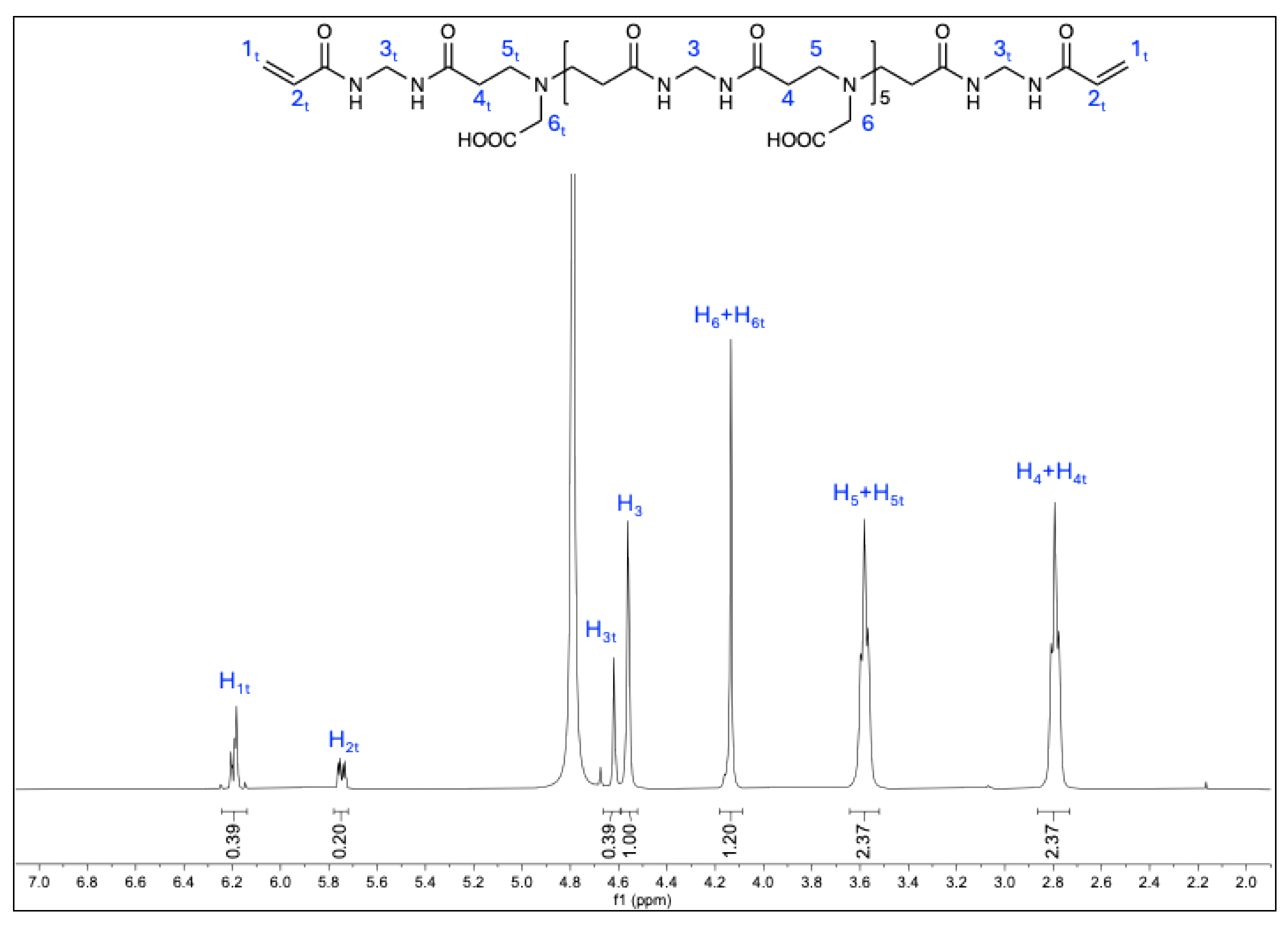



| Sample | Theoretical r b Value | Experimental r Value | n c | d | e |
|---|---|---|---|---|---|
| M-GLY | 0.85 | 0.860 | 5 | 13 | 1500 |
| Paper/Exposure Cycle to H-M-GLY Contact b | pH c |
|---|---|
| 0 | 6.22 |
| 1 | 6.22 |
| 2 | 6.25 |
| 3 | 6.58 |
| 4 | 6.60 |
| 5 | 6.57 |
| 6 | 6.65 |
| 7 | 6.70 |
| 8 | 6.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranucci, E.; Alongi, J. Advanced Polyamidoamine Hydrogels for the Selective Cleaning of Artifacts in Heritage Conservation. Polymers 2025, 17, 2680. https://doi.org/10.3390/polym17192680
Ranucci E, Alongi J. Advanced Polyamidoamine Hydrogels for the Selective Cleaning of Artifacts in Heritage Conservation. Polymers. 2025; 17(19):2680. https://doi.org/10.3390/polym17192680
Chicago/Turabian StyleRanucci, Elisabetta, and Jenny Alongi. 2025. "Advanced Polyamidoamine Hydrogels for the Selective Cleaning of Artifacts in Heritage Conservation" Polymers 17, no. 19: 2680. https://doi.org/10.3390/polym17192680
APA StyleRanucci, E., & Alongi, J. (2025). Advanced Polyamidoamine Hydrogels for the Selective Cleaning of Artifacts in Heritage Conservation. Polymers, 17(19), 2680. https://doi.org/10.3390/polym17192680







