Electrospun Polymeric Film in Red BF-4B Dye Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
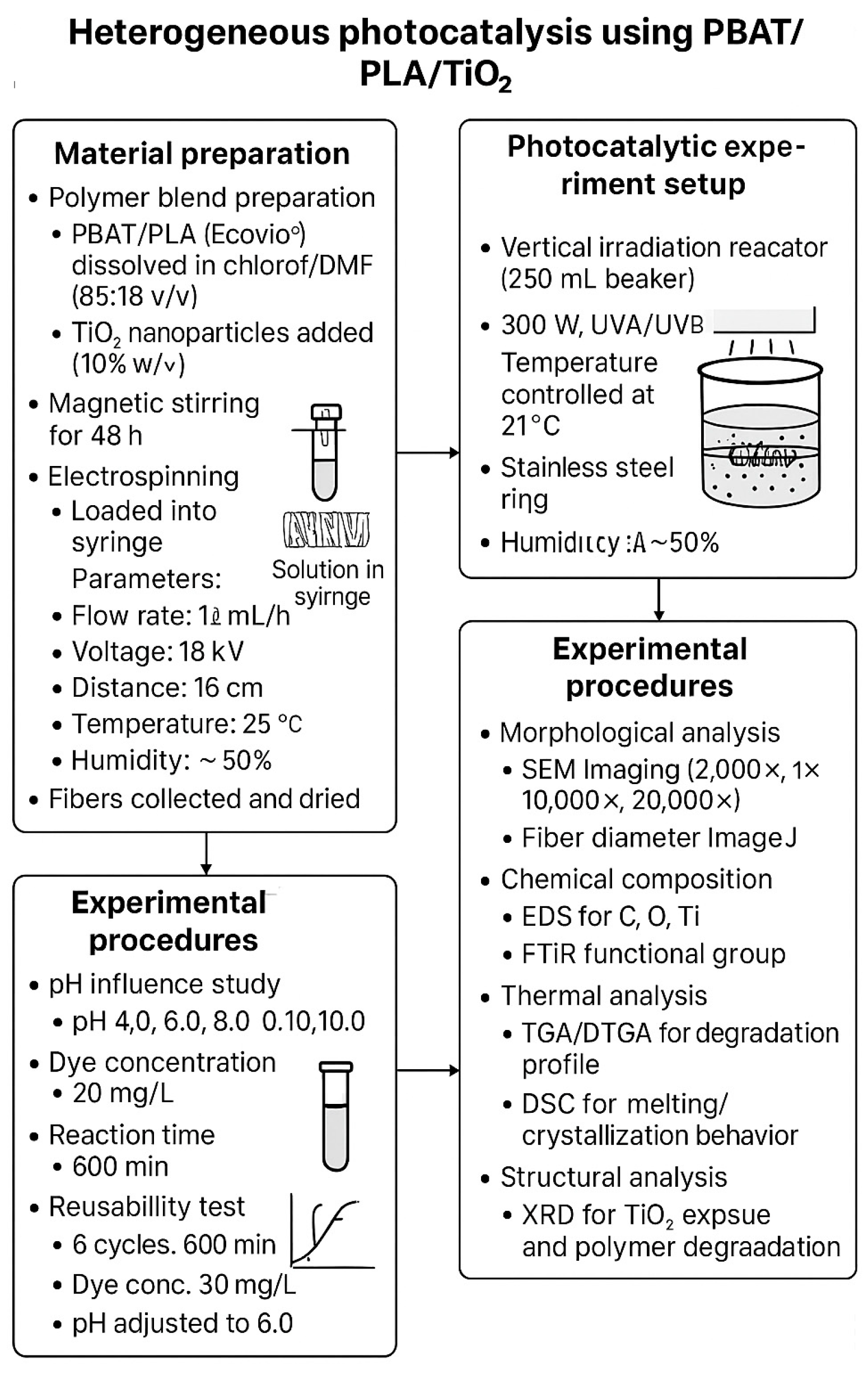
2.2.1. Preparation of Electrospun Fibers and Electrospinning Conditions
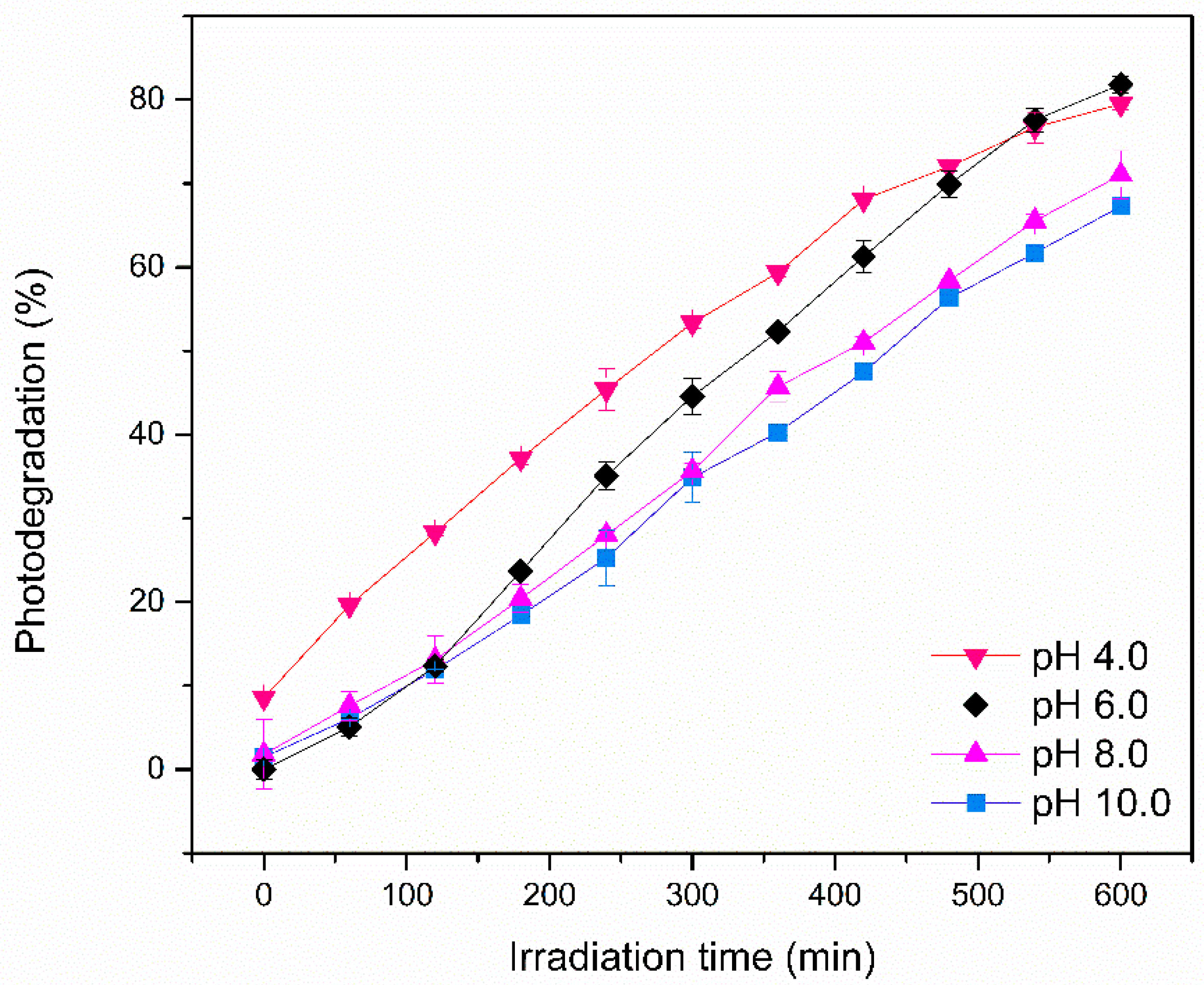
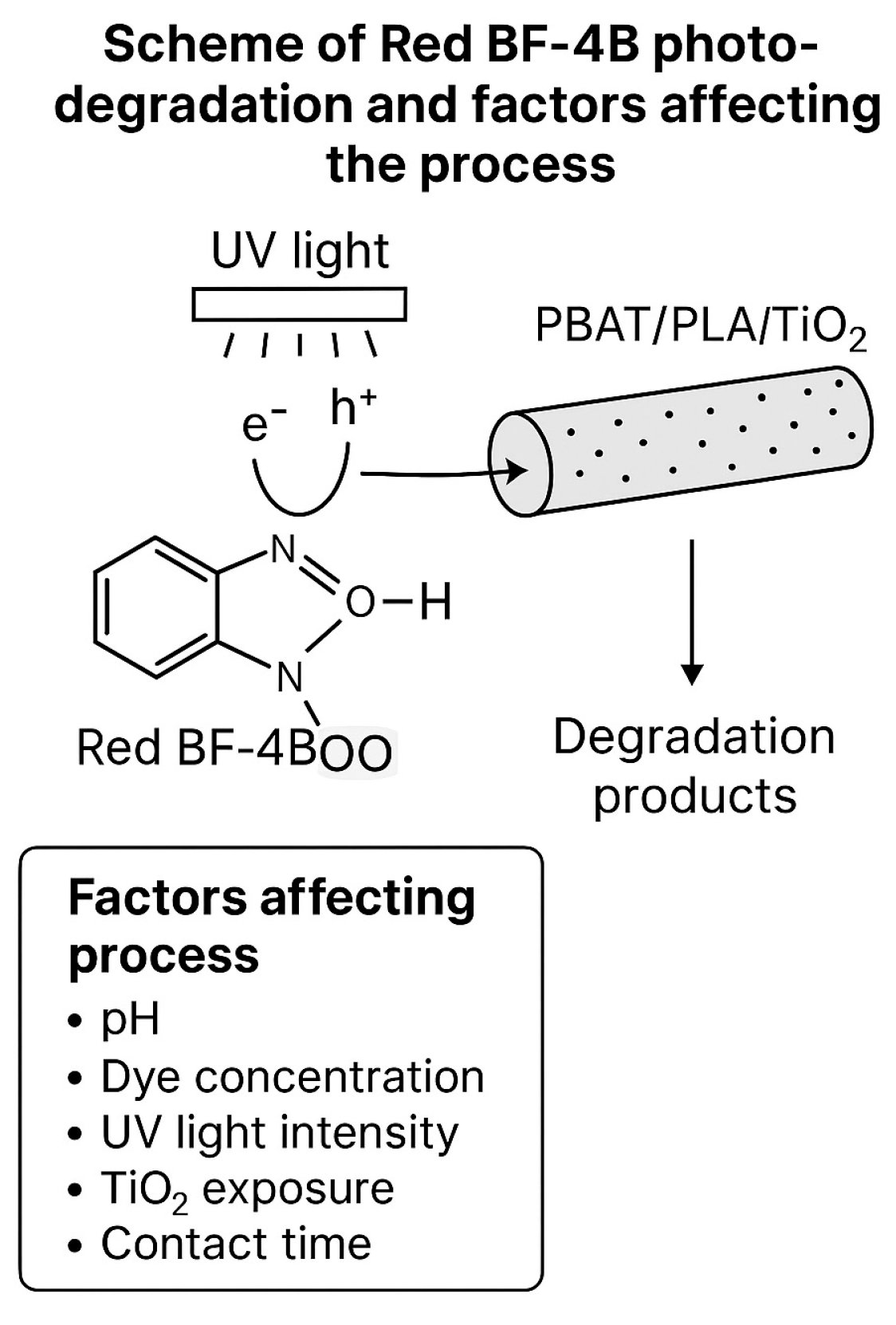
2.2.2. Heterogeneous Photocatalysis Experiments
2.2.3. Characterization of the PBAT/PLA/TiO2 Polymeric Membrane After the Photocatalytic Process
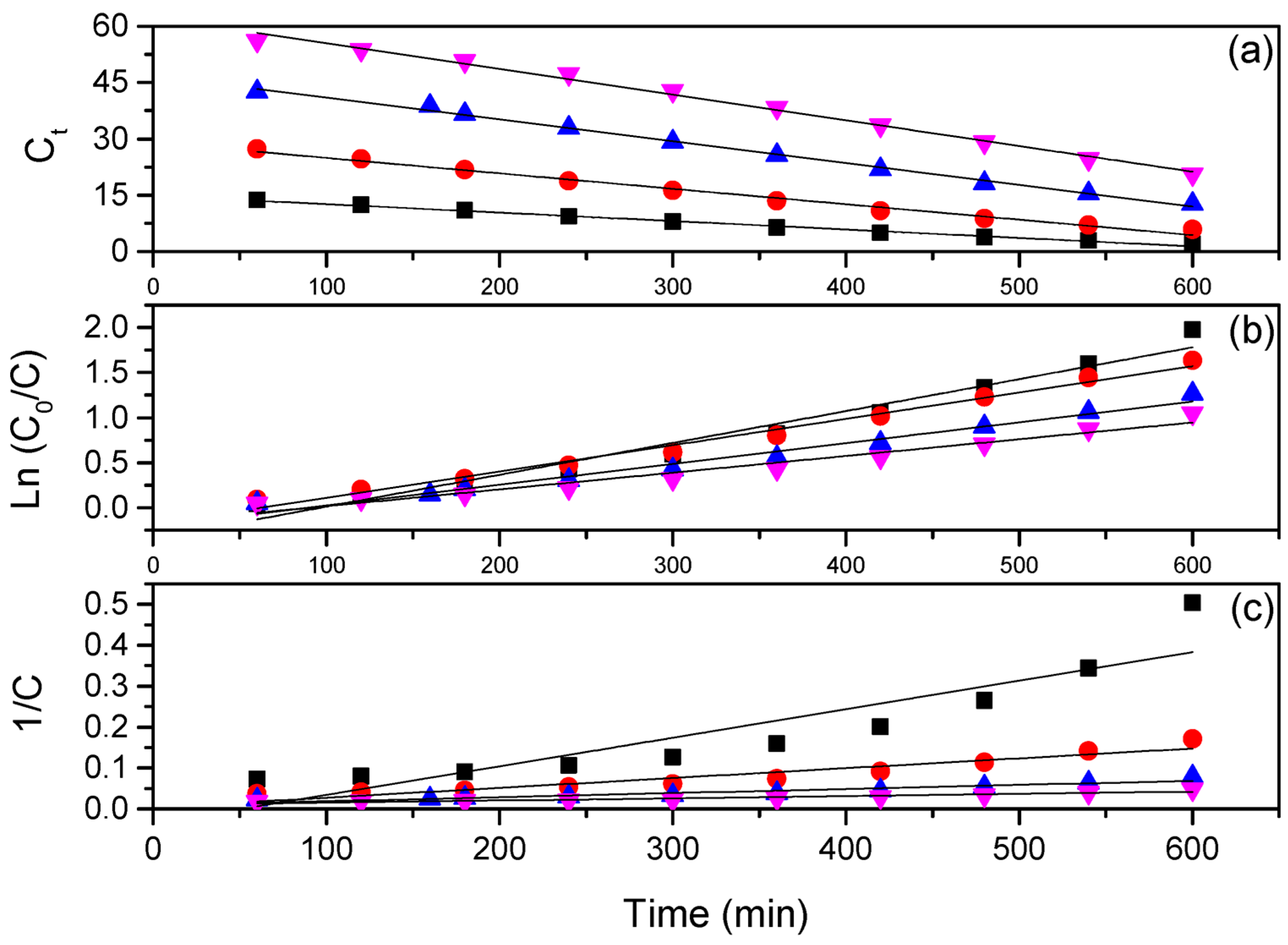
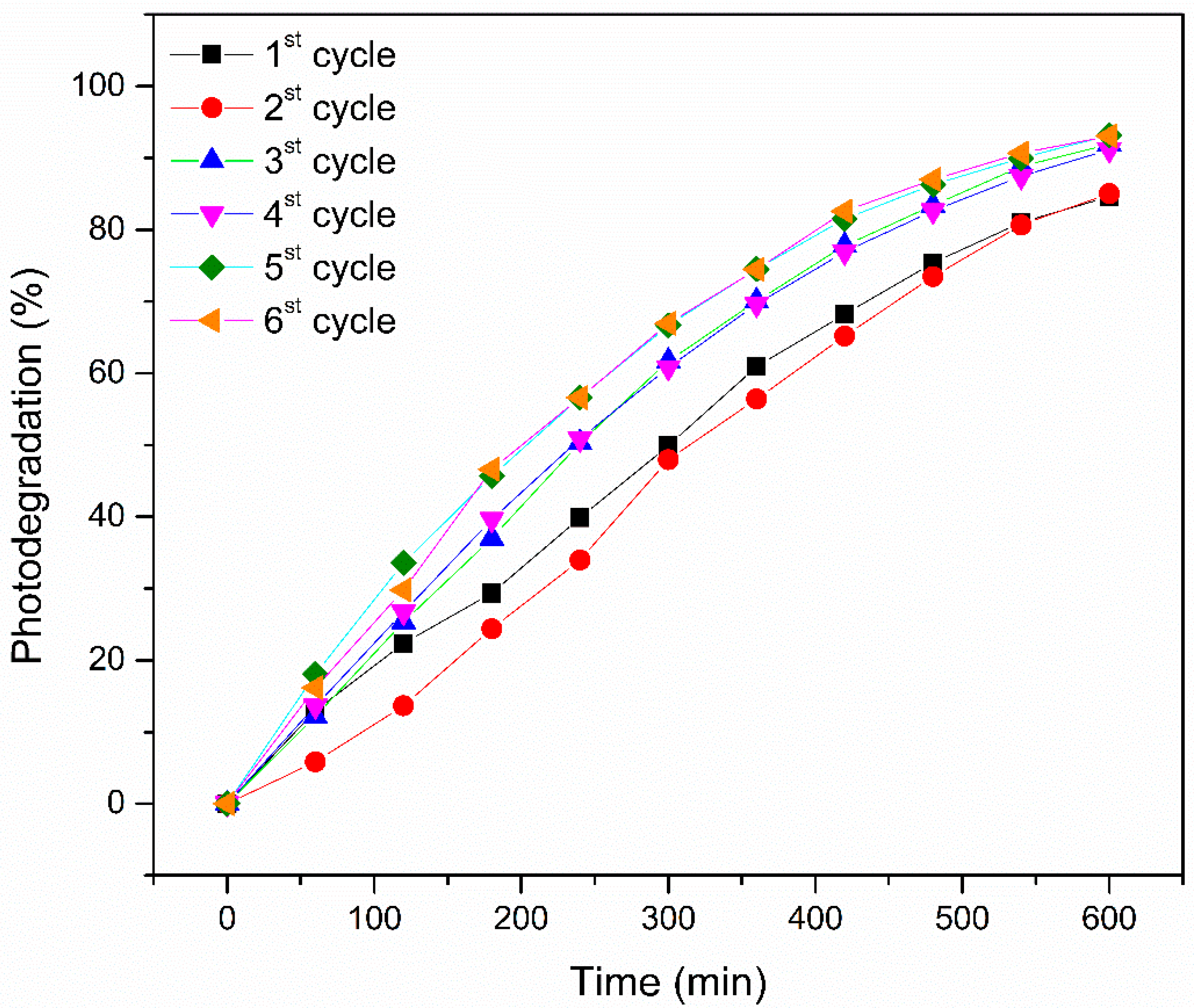
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Industry Analysts, Inc. (GIA). Textiles. Global Market Trajectory & Analytics. Available online: https://www.strategyr.com/market-report-textiles-forecasts-global-industry-analysts-inc.asp (accessed on 20 January 2025).
- Bick, R.; Halsey, E.; Ekenga, C.C. The Global Environmental Injustice of Fast Fashion. Environ. Health 2018, 17, 92. [Google Scholar] [CrossRef]
- Amaral, M.C.D.; Zonatti, W.F.; da Silva, K.L.; Junior, D.K.; Neto, J.A.; Baruque-Ramos, J. Industrial Textile Recycling and Reuse in Brazil: Case Study and Considerations Concerning the Circular Economy Reciclagem Industrial e Reuso Têxtil No Brasil: Estudo de Caso e Considerações Referentes à Economia Circular. Gest. Prod. 2018, 25, 431–443. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Hossain, L.; Sarker, S.K.; Khan, M.S. Evaluation of Present and Future Wastewater Impacts of Textile Dyeing Industries in Bangladesh. Environ. Dev. 2018, 26, 23–33. [Google Scholar] [CrossRef]
- Lum, P.T.; Foo, K.Y.; Zakaria, N.A.; Palaniandy, P. Ash Based Nanocomposites for Photocatalytic Degradation of Textile Dye Pollutants: A Review. Mater. Chem. Phys. 2020, 241, 122405. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of Different Coagulants in the Coagulation/Flocculation Process of Textile Wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Al Arni, S.; Ghareba, S.; Solisio, C.; Palma, M.S.A.; Converti, A. Methods of Reactive Red 141 Dye Decolorization, Treatment, and Removal from Industrial Wastewaters: A Critical Review. Environ. Eng. Sci. 2020, 38, 577–591. [Google Scholar] [CrossRef]
- Mate, M.S.; Pathade, G. Biodegradation of C.I. Reactive Red 195 by Enterococcus Faecalis Strain YZ66. World J. Microbiol. Biotechnol. 2012, 28, 815–826. [Google Scholar] [CrossRef]
- Gundogdu, A.; Ozdes, D.; Duran, C.; Bulut, V.N.; Soylak, M.; Senturk, H.B. Biosorption of Pb(II) Ions from Aqueous Solution by Pine Bark (Pinus Brutia Ten.). Chem. Eng. J. 2009, 153, 62–69. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A Critical Review on Recent Advancements of the Removal of Reactive Dyes from Dyehouse Effluent by Ion-Exchange Adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Lourenco, N.D.; Novais, J.M.; Pinheiro, H.M. Reactive Textile Dye Colour Removal in a Sequencing Batch Reactor. Water Sci. Technol. 2000, 42, 321–328. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid. Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Macossay, J.; Cantu, T.; Zhang, X.; Shamshi Hassan, M.; Esther Salinas, M.; Farhangi, C.S.; Ahmad, H.; Kim, H.; Bowlin, G.L. Imaging, Spectroscopy, Mechanical, Alignment and Biocompatibility Studies of Electrospun Medical Grade Polyurethane (CarbothaneTM 3575A) Nanofibers and Composite Nanofibers Containing Multiwalled Carbon Nanotubes. J. Mech. Behav. Biomed. Mater. 2015, 41, 189–198. [Google Scholar] [CrossRef]
- Vranić, E.; Sirbubalo, M.; Tucak, A.; Hadžiabdić, J.; Rahić, O.; Elezović, A. Development of Inhalable Dry Gene Powders for Pulmonary Drug Delivery by Spray-Freeze-Drying. In IFMBE Proceedings; Springer: Cham, Switzerland, 2020; pp. 533–537. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A Review of Textile Industry: Wet Processing, Environmental Impacts, and Effluent Treatment Methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The Utilization of Leaf-Based Adsorbents for Dyes Removal: A Review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater Treatment by Means of Advanced Oxidation Processes at Basic PH Conditions: A Review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Donkadokula, N.Y.; Kola, A.K.; Naz, I.; Saroj, D. A Review on Advanced Physico-Chemical and Biological Textile Dye Wastewater Treatment Techniques. Rev. Environ. Sci. Biotechnol. 2020, 19, 543–560. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L. The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials 2020, 10, 1766. [Google Scholar] [CrossRef]
- Parrino, F.; Bellardita, M.; García-López, E.I.; Marcì, G.; Loddo, V.; Palmisano, L. Heterogeneous Photocatalysis for Selective Formation of High-Value-Added Molecules: Some Chemical and Engineering Aspects. ACS Catal. 2018, 8, 11191–11225. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Márquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; Ramírez del Solar, M.; Levchuk, I. Photocatalytic Degradation of Pharmaceutically Active Compounds (PhACs) in Urban Wastewater Treatment Plants Effluents under Controlled and Natural Solar Irradiation Using Immobilized TiO2. Sol. Energy 2020, 208, 480–492. [Google Scholar] [CrossRef]
- Melinte, V.; Stroea, L.; Chibac-Scutaru, A.L. Polymer Nanocomposites for Photocatalytic Applications. Catalysts 2019, 9, 986. [Google Scholar] [CrossRef]
- Georgiopoulos, P.; Kontou, E.; Christopoulos, A. Short-Term Creep Behavior of a Biodegradable Polymer Reinforced with Wood-Fibers. Compos. B Eng. 2015, 80, 134–144. [Google Scholar] [CrossRef]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend under Soil Conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Rosenberger, A.G.; Dragunski, D.C.; Muniz, E.C.; Módenes, A.N.; Alves, H.J.; Tarley, C.R.T.; Machado, S.A.S.; Caetano, J. Electrospinning in the Preparation of an Electrochemical Sensor Based on Carbon Nanotubes. J. Mol. Liq. 2020, 298, 112068. [Google Scholar] [CrossRef]
- Goes, A.M.; Carvalho, S.; Oréfice, R.L.; Avérous, L.; Custódio, T.A.; Pimenta, J.G.; Souza, M.d.B.; Branciforti, M.C.; Bretas, R.E.S. Viabilidade Celular de Nanofibras de Polímeros Biodegradáveis e Seus Nanocompósitos Com Argila Montmorilonita. Polímeros 2012, 22, 34–41. [Google Scholar] [CrossRef]
- Mimura, A.M.S.; Vieira, T.V.d.A.; Martelli, P.B.; Gorgulho, H.d.F. Aplicação Da Casca de Arroz Na Adsorção Dos Íons Cu2+, Al3+, Ni2+ e Zn2+. Quim. Nova 2010, 33, 1279–1284. [Google Scholar] [CrossRef]
- Kumar, A. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumran, S.; Jegatheesan, V. Factors Affecting the Degradation of Remazol Turquoise Blue (RTB) Dye by Titanium Dioxide (TiO2) Entrapped Photocatalytic Membrane. J. Environ. Manag. 2020, 272, 111090. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Pathways of Solar Light-Induced Photocatalytic Degradation of Azo Dyes in Aqueous TiO2 Suspensions. Appl. Catal. B 2003, 40, 271–286. [Google Scholar] [CrossRef]
- Pizato, E.; Lopes, A.C.; Rocha, R.D.C.; Barbosa, A.d.M.; da Cunha, M.A.A. Caracterização de Efluente Têxtil e Avaliação Da Capacidade de Remoção de Cor Utilizando o Fungo Lasiodiplodia Theobromae MMPI. Eng. Sanit. E Ambient. 2017, 22, 1027–1035. [Google Scholar] [CrossRef]
- BRASIL. Conselho Nacional do Meio Ambiente—CONAMA. RESOLUÇÃO No 430, DE 13 DE MAIO DE 2011. Diário Of. Da União 2011, 92, 89. [Google Scholar]
- Qi, Y.; Ma, H.L.; Du, Z.H.; Yang, B.; Wu, J.; Wang, R.; Zhang, X.Q. Hydrophilic and Antibacterial Modification of Poly(Lactic Acid) Films by γ-Ray Irradiation. ACS Omega 2019, 4, 21439–21445. [Google Scholar] [CrossRef] [PubMed]
- Lertphirun, K.; Srikulkit, K. Properties of Poly(Lactic Acid) Filled with Hydrophobic Cellulose/SiO2 Composites. Int. J. Polym. Sci. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Brandelero, R.P.H.; Grossmann, M.V.; Yamashita, F. Films of Starch and Poly(Butylene Adipate Co-Terephthalate) Added of Soybean Oil (SO) and Tween 80. Carbohydr. Polym. 2012, 90, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, K.O.; Künkel, A.; Skupin, G.; Yamamoto, M. Ecoflex® and Ecovio®: Biodegradable, Performance-Enabling Plastics. In Biological Research; Springer: Berlin, Heidelberg, 2011; Volume 5, pp. 91–136. [Google Scholar] [CrossRef]
- Lee, E.J.; An, A.K.; He, T.; Woo, Y.C.; Shon, H.K. Electrospun Nanofiber Membranes Incorporating Fluorosilane-Coated TiO2 Nanocomposite for Direct Contact Membrane Distillation. J. Memb. Sci. 2016, 520, 145–154. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2: A Review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Saggioro, E.M.; Oliveira, A.S.; Pavesi, T.; Maia, C.G.; Ferreira, L.F.V.; Moreira, J.C. Use of Titanium Dioxide Photocatalysis on the Remediation of Model Textile Wastewaters Containing Azo Dyes. Molecules 2011, 16, 10370–10386. [Google Scholar] [CrossRef]
- Segne, T. Studies on Characterization and Photocatalytic Activities of Visible Light Sensitive TiO2 Nano Catalysts Co-Doped with Magnesium and Copper. Int. Res. J. Pure Appl. Chem. 2011, 1, 84–103. [Google Scholar] [CrossRef]
- Davis, R.J.; Gainer, J.L.; O’Neal, G.; Wu, I.-W. Photocatalytic Decolorization of Wastewater Dyes. Water Environ. Res. 1994, 66, 50–53. [Google Scholar] [CrossRef]
- Rodrigues, M.H.d.M.; dos Santos, L.M.; Gonçalves, R.d.F.; Santos, M.R.d.C.; Gurgel, M.F.D.C.; Godinho, M.J. Estudo Da Cinética De Degradação Do Corante Azul De Metileno Utilizando Catalisadores De Céria Dopada Com Índio. J. Eng. Exact. Sci. 2017, 4, 0028–0034. [Google Scholar] [CrossRef]
- Ball, D.W. Físico-Química; Pioneira Thomson Learn: São Paulo, Brazil, 2006; Volume 2, pp. 732–742. [Google Scholar]
- Chládková, B.; Evgenidou, E.; Kvítek, L.; Panáček, A.; Zbořil, R.; Kovář, P.; Lambropoulou, D. Adsorption and Photocatalysis of Nanocrystalline TiO2 Particles for Reactive Red 195 Removal: Effect of Humic Acids, Anions and Scavengers. Environ. Sci. Pollut. Res. 2015, 22, 16514–16524. [Google Scholar] [CrossRef] [PubMed]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N,Pd Co-Doped TiO2 Composite Membranes for Photocatalytic Dye Degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Aoudjit, L.; Martins, P.M.; Madjene, F.; Petrovykh, D.Y.; Lanceros-Mendez, S. Photocatalytic Reusable Membranes for the Effective Degradation of Tartrazine with a Solar Photoreactor. J. Hazard. Mater. 2018, 344, 408–416. [Google Scholar] [CrossRef]
- Pant, H.R.; Pant, B.; Pokharel, P.; Kim, H.J.; Tijing, L.D.; Park, C.H.; Lee, D.S.; Kim, H.Y.; Kim, C.S. Photocatalytic TiO2-RGO/Nylon-6 Spider-Wave-like Nano-Nets via Electrospinning and Hydrothermal Treatment. J. Memb. Sci. 2013, 429, 225–234. [Google Scholar] [CrossRef]
- Teixeira, S.; Martins, P.M.; Lanceros-Méndez, S.; Kühn, K.; Cuniberti, G. Reusability of Photocatalytic TiO2 and ZnO Nanoparticles Immobilized in Poly(Vinylidene Difluoride)-Co-Trifluoroethylene. Appl. Surf. Sci. 2016, 384, 497–504. [Google Scholar] [CrossRef]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Sol-Gel Membrane Modification for Enhanced Photocatalytic Activity. Sep. Purif. Technol. 2017, 180, 69–81. [Google Scholar] [CrossRef]
- Virág, Á.D.; Tóth, C.; Molnár, K. Photodegradation of Polylactic Acid: Characterisation of Glassy and Melt Behaviour as a Function of Molecular Weight. Int. J. Biol. Macromol. 2023, 252, 126336. [Google Scholar] [CrossRef] [PubMed]
- Phetwarotai, W.; Tanrattanakul, V.; Phusunti, N. Mechanical Characteristics and Thermal Behaviours of Polylactide Blend Films: Influence of Nucleating Agent and Poly(Butylenes Adipate-Co-Terephthalate). Plast. Rubber Compos. 2016, 45, 333–345. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W.; Won, K. Preparation of Poly(Lactide)/Lignin/Silver Nanoparticles Composite Films with UV Light Barrier and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 107, 1724–1731. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT Bionanocomposites with Antimicrobial Natural Rosin for Green Packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Canevarolo, S.V., Jr. Ciência Dos Polímeros—Um Texto Básico Para Tecnólogos e Engenheiros; Artliber: São Paulo, Brazil, 2006; pp. 139–168. [Google Scholar]
- Çoban, O.; Bora, M.Ö.; Kutluk, T.; Özkoç, G. Mechanical and Thermal Properties of Volcanic Particle Filled PLA/PBAT Composites. Polym. Compos. 2018, 39, E1500–E1511. [Google Scholar] [CrossRef]
- Ren, Y.; Hu, J.; Yang, M.; Weng, Y. Biodegradation Behavior of Poly (Lactic Acid) (PLA), Poly (Butylene Adipate-Co-Terephthalate) (PBAT), and Their Blends Under Digested Sludge Conditions. J. Polym. Environ. 2019, 27, 2784–2792. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Olmedo-Martínez, J.L.; Sabino-Gutiérrez, M.A.; Collinson, E.; López, J.V.; Irusta, L.; González, A.; de Ilarduya, A.M.; Guerrica-Echevarria, G.; Aranburu, N.; et al. Mechanical, Barrier, and Photodegradation Properties of Biodegradable PLA-Based Blend Films. RSC Sustain. 2025, 1, 1–10. [Google Scholar] [CrossRef]
- del Campo, A.; de Lucas-Gil, E.; Rubio-Marcos, F.; Arrieta, M.P.; Fernández-García, M.; Fernández, J.F.; Muñoz-Bonilla, A. Accelerated Disintegration of Compostable Ecovio Polymer by Using ZnO Particles as Filler. Polym. Degrad. Stab. 2021, 185, 109501. [Google Scholar] [CrossRef]
- Basf. Ecovio®. Available online: https://plastics-rubber.basf.com/global/en/performance_polymers/products/ecovio.html (accessed on 13 March 2021).
- Fojt, J.; David, J.; Přikryl, R.; Řezáčová, V.; Kučerík, J. A Critical Review of the Overlooked Challenge of Determining Micro-Bioplastics in Soil. Sci. Total Environ. 2020, 745, 140975. [Google Scholar] [CrossRef]
- Hui, K.C.; Suhaimi, H.; Sambudi, N.S. Electrospun-based TiO2 nanofibers for organic pollutant photodegradation: A comprehensive review. Rev. Chem. Eng. 2022, 38, 641–668. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.H.; Li, R.; Cui, Y.Q.; Wang, X.X.; Yu, J.Q.; Long, Y.Z. Efficient under visible catalysts from electrospun flexible Ag2S/TiO2 composite fiber membrane. J. Mater. Sci. 2021, 56, 7966–7981. [Google Scholar] [CrossRef]





| pHinitial | pHfinal |
|---|---|
| 4.00 | 3.67 |
| 6.00 | 4.88 |
| 8.00 | 6.33 |
| 10.00 | 7.02 |
| Kinetic Model | 15 mg L−1 | 30 mg L−1 | 45 mg L−1 | 60 mg L−1 | |
|---|---|---|---|---|---|
| Zero-order | Linear equation | ||||
| Rate constant k (min−1) | |||||
| R2 | |||||
| RMSE | |||||
| Pseudo-first-order | Linear equation | ||||
| Rate constant k (min−1) | |||||
| R2 | |||||
| RMSE | |||||
| Pseudo-second-order | Linear equation | ||||
| Rate constant k (min−1) | |||||
| R2 | |||||
| RMSE | |||||
| Cycle | Photodegradation Percentage |
|---|---|
| 1st | 84.60 |
| 2nd | 85.04 |
| 3rd | 91.94 |
| 4th | 91.18 |
| 5th | 93.22 |
| 6th | 93.07 |
| PBAT/PLA/TiO2 Before Use | PBAT/PLA/TiO2 After 1st Cycle | PBAT/PLA/TiO2 After 6th Cycle | |
|---|---|---|---|
| Average diameter | 1.12 | 0.77 | 0.71 |
| Standard deviation | 0.26 | 0.22 | 0.14 |
| Samples | C (%) | O (%) | Ti (%) | Total (%) |
|---|---|---|---|---|
| PBAT/PLA/TiO2 | 31.99 ± 0.97 | 40.07 ± 1.06 | 27.94 ± 1.51 | 100.00 |
| After 1st cycle | 38.04 ± 0.87 | 33.20 ± 1.18 | 28.76 ± 0.32 | 100.00 |
| After 6th cycle | 23.38 ± 1.58 | 33.68 ± 2.90 | 42.94 ± 4.49 | 100.00 |
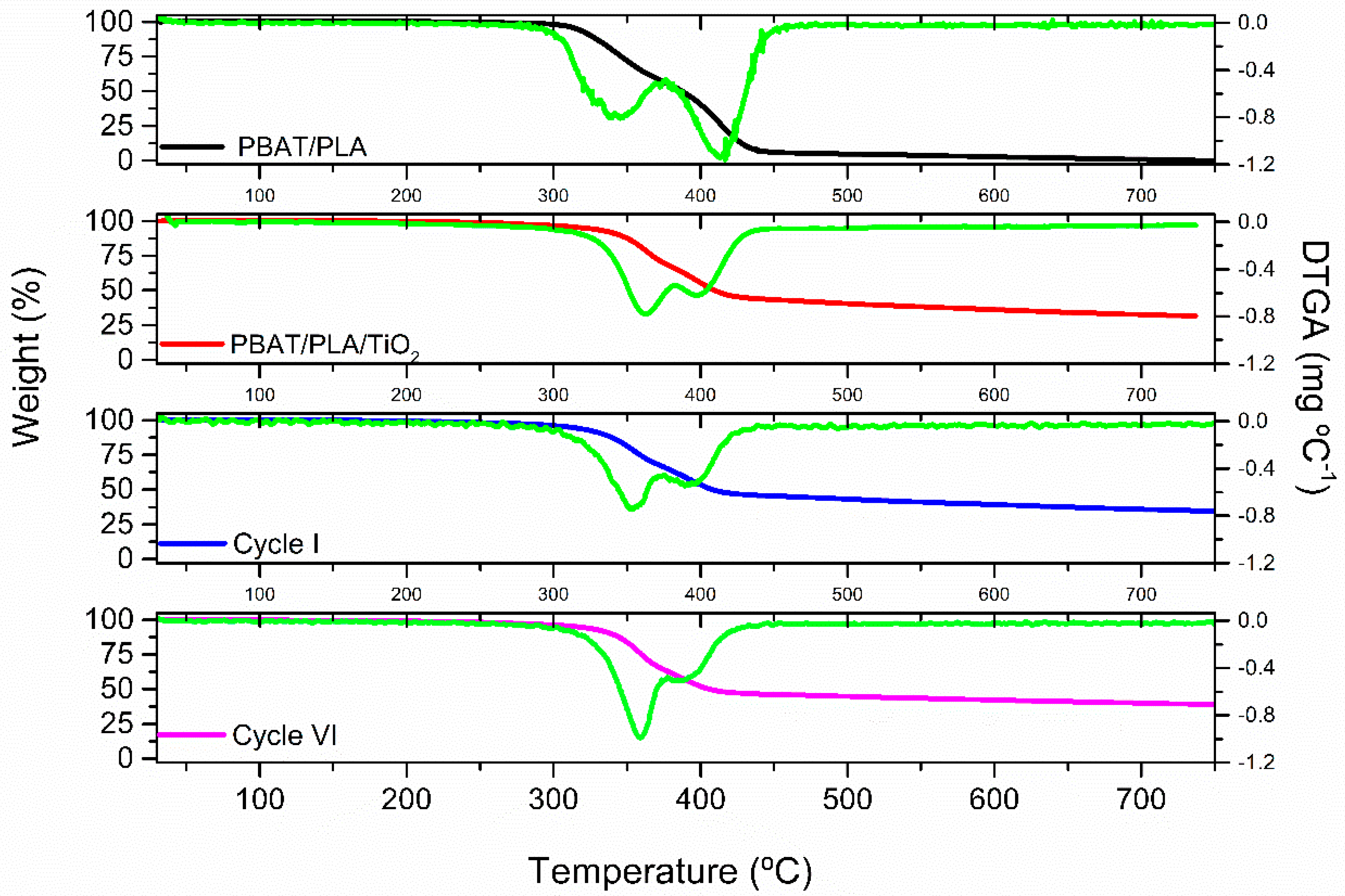
| Samples | Tm PLA (°C) | Tm PBAT (°C) | % Degradation T475.8 °C |
|---|---|---|---|
| PBAT/PLA | 345.0 | 413.3 | 95.0 |
| PBAT/PLA/TiO2 | 362.5 | 397.1 | 41.8 |
| 1st cycle | 351.4 | 389.1 | 44.3 |
| 6th cycle | 359.1 | 386.4 | 45.5 |
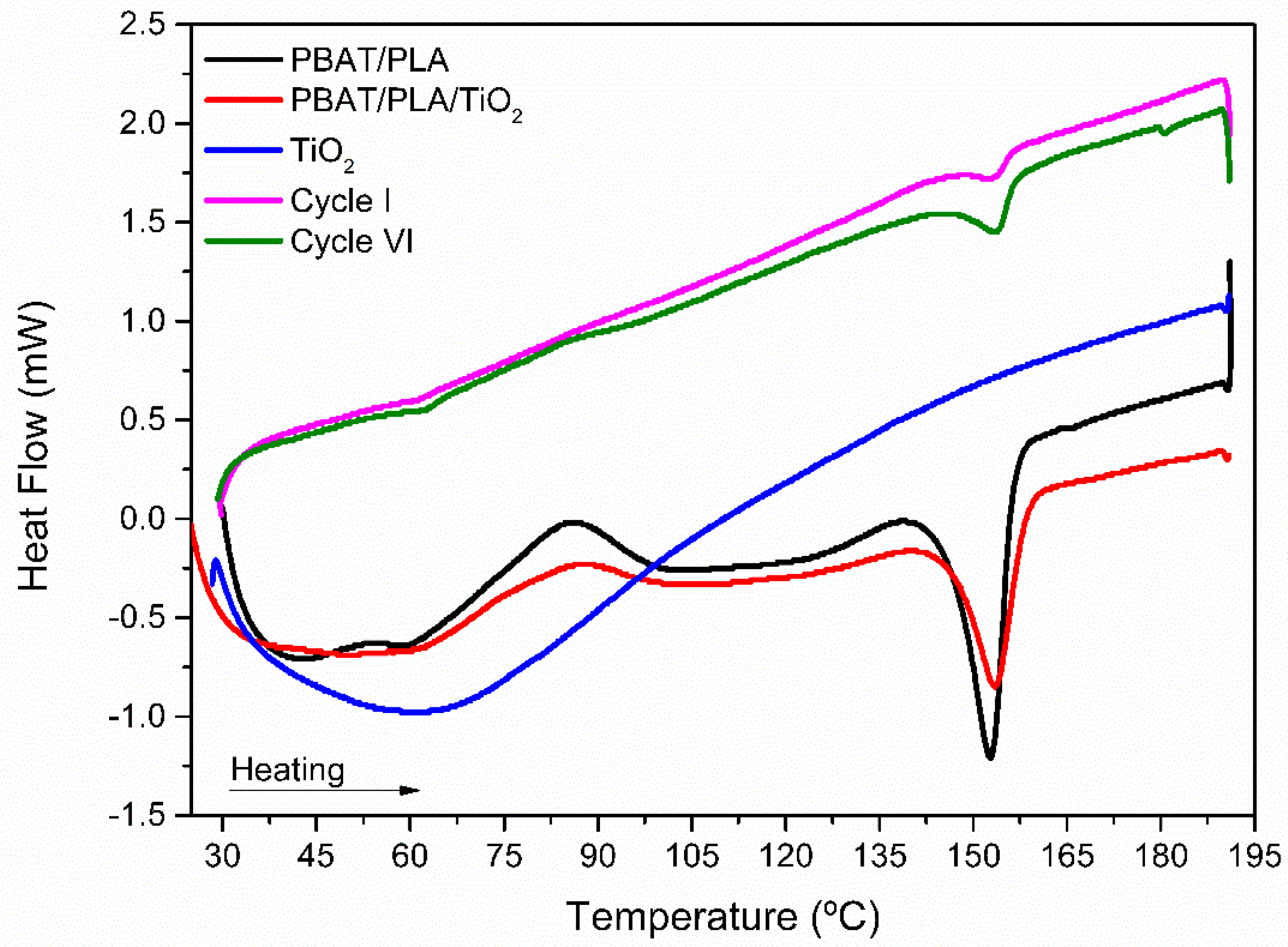
| Sample | TMelting (°C) | Heat (J/g) |
|---|---|---|
| PBAT/PLA | 152.5 | 12.76 |
| PBAT/PLA/TiO2 | 153.9 | 7.58 |
| 1° | 159.0 | 0.61 |
| 6° | 153.6 | 1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenberger, A.G.; Ballmann, E.; Lima, F.d.S.; Caetano, J.; Dragunski, D.C.; Muniz, E.C.; Módenes, A.N. Electrospun Polymeric Film in Red BF-4B Dye Degradation. Polymers 2025, 17, 2669. https://doi.org/10.3390/polym17192669
Rosenberger AG, Ballmann E, Lima FdS, Caetano J, Dragunski DC, Muniz EC, Módenes AN. Electrospun Polymeric Film in Red BF-4B Dye Degradation. Polymers. 2025; 17(19):2669. https://doi.org/10.3390/polym17192669
Chicago/Turabian StyleRosenberger, Andressa Giombelli, Eduarda Ballmann, Fabiana da Silva Lima, Josiane Caetano, Douglas Cardoso Dragunski, Edvani Curti Muniz, and Aparecido Nivaldo Módenes. 2025. "Electrospun Polymeric Film in Red BF-4B Dye Degradation" Polymers 17, no. 19: 2669. https://doi.org/10.3390/polym17192669
APA StyleRosenberger, A. G., Ballmann, E., Lima, F. d. S., Caetano, J., Dragunski, D. C., Muniz, E. C., & Módenes, A. N. (2025). Electrospun Polymeric Film in Red BF-4B Dye Degradation. Polymers, 17(19), 2669. https://doi.org/10.3390/polym17192669





