Physicochemical and Mechanical Performance of Dental Resins Formulated from Dimethacrylated Oligoesters Derived from PET Recycling via Glycolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Light-Curable Dental Resin Formulations
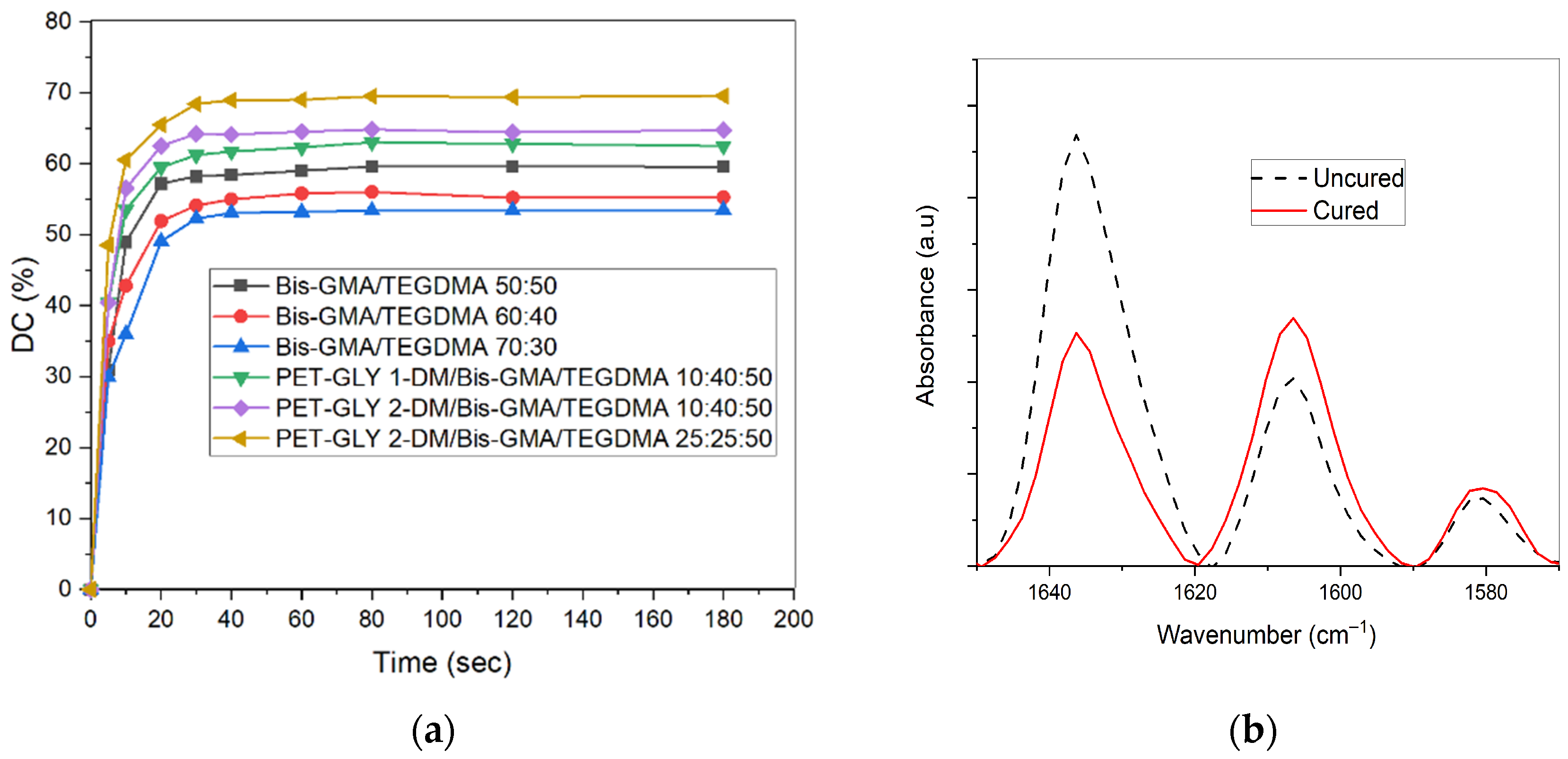
2.3. Degree of Conversion
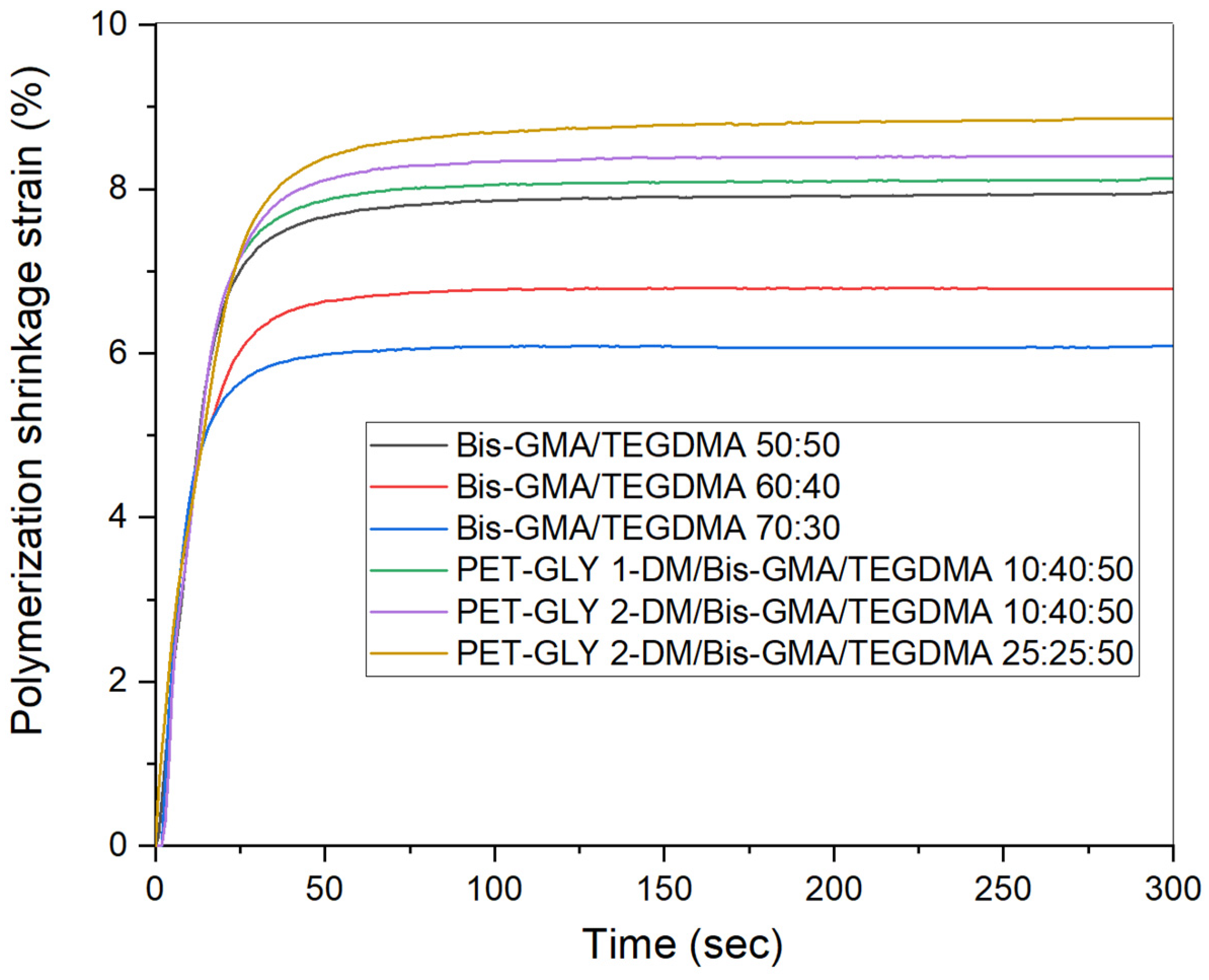
2.4. Polymerization Shrinkage
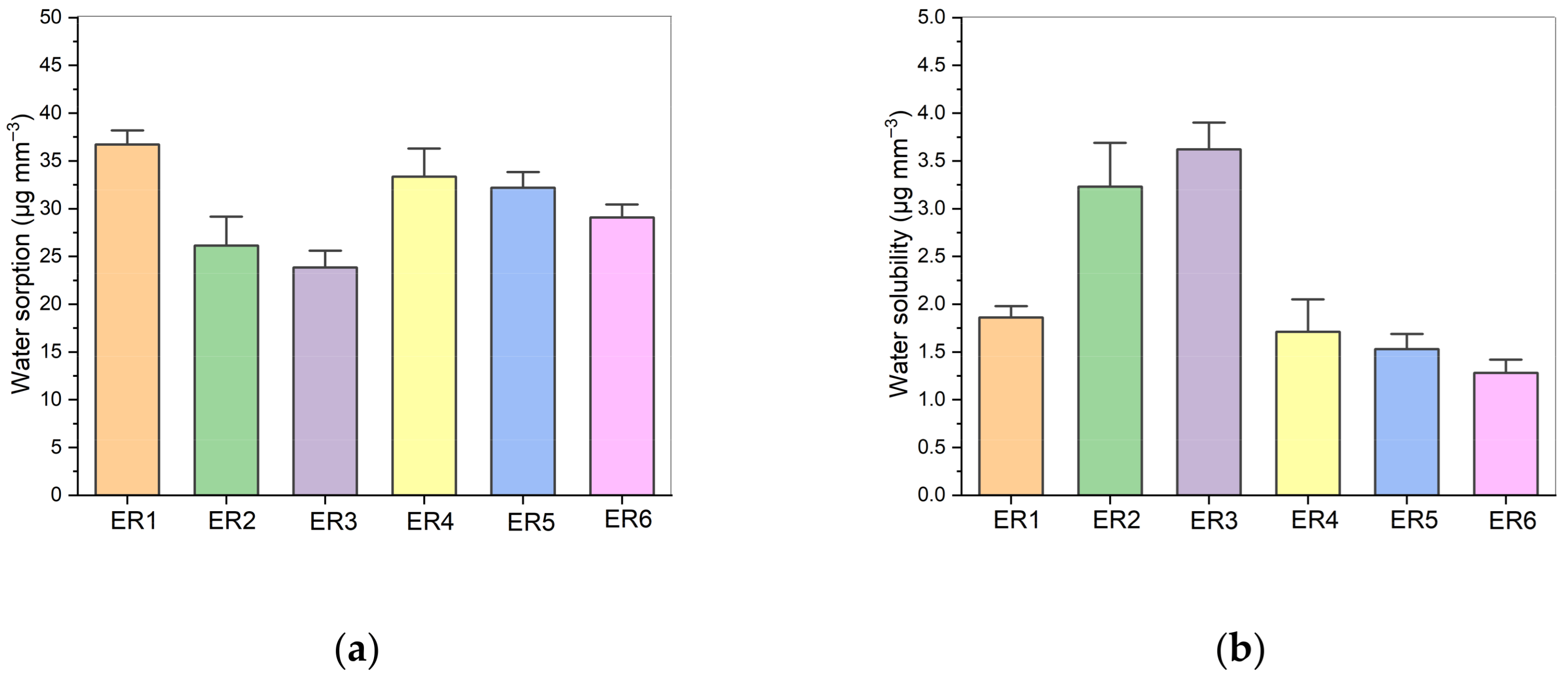
2.5. Water Sorption and Solubility
2.6. Measurement of Mechanical Properties
2.7. Analysis of Organic Eluates from Dental Resins
2.7.1. Sample Preparation
2.7.2. Liquid Chromatography Analysis
2.8. Statistical Analysis
3. Results
3.1. Evaluation of Polymerization Kinetics
3.2. Determination of Polymerization Shrinkage Kinetics
3.3. Water Sorption and Solubility Results
3.4. Mechanical Properties
3.5. Detection and Quantification of Resins’ Organic Eluates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bis-GMA | 2,2-Bis[p-(2′-hydroxy-3′-methacryloxypropoxy)phenylene]propane/bisphenol A glycidyl methacrylate |
| BPA | Bisphenol-A |
| CQ | Camphorquinone |
| DC | degree of conversion |
| DMAEMA | 2-(dimethylamino)ethyl methacrylate |
| EM | elastic modulus |
| FS | flexural strength |
| GPC | gel permeation chromatography |
| LVDT | linear variable displacement transducer |
| PET | poly(ethylene terephthalate) |
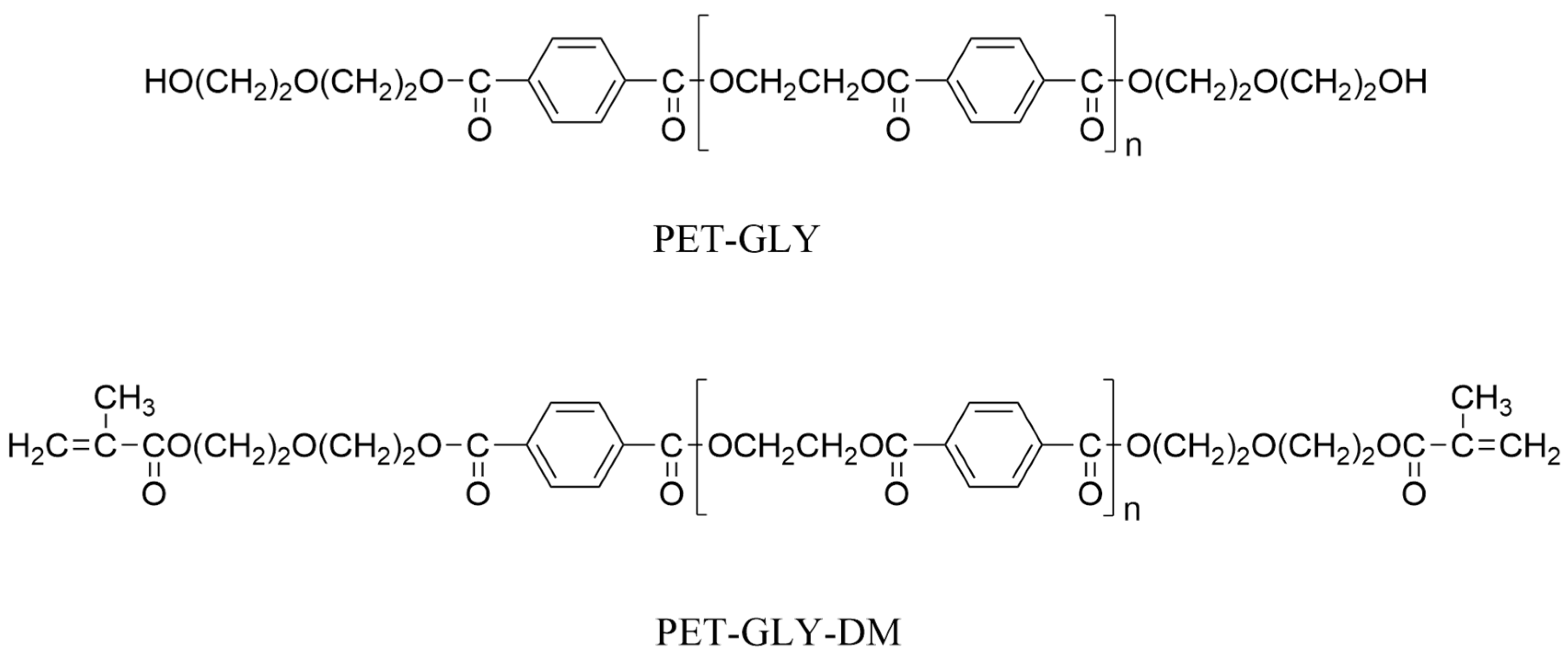
| PET-GLY-DMs | dimethacrylated oligoesters |
| TEGDMA | triethylene glycol dimethacrylate |
| THF | Tetrahydrofuran |
| UDMA | urethane dimethacrylate |
References
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Sarosi, C.; Moldovan, M.; Soanca, A.; Roman, A.; Gherman, T.; Trifoi, A.; Chisnoiu, A.M.; Cuc, S.; Filip, M.; Gheorghe, G.F.; et al. Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties. Polymers 2021, 13, 4415. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Yoshihara, K.; Yoshida, Y. Development of new diacrylate monomers as substitutes for Bis-GMA and UDMA. Dent. Mater. 2021, 37, e391–e398. [Google Scholar] [CrossRef]
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, S.; Zhang, M.; Hou, J. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109275. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, K.-J.; Mariotti, A. Bis-Gma–Based Resins in Dentistry: Are They Safe? J. Am. Dent. Assoc. 1999, 130, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Szczesio-Wlodarczyk, A.; Polikowski, A.; Krasowski, M.; Fronczek, M.; Sokolowski, J.; Bociong, K. The Influence of Low-Molecular-Weight Monomers (TEGDMA, HDDMA, HEMA) on the Properties of Selected Matrices and Composites Based on Bis-GMA and UDMA. Materials 2022, 15, 2649. [Google Scholar] [CrossRef]
- Gonçalves, F.; Azevedo, C.L.N.; Ferracane, J.L.; Braga, R.R. BisGMA/TEGDMA ratio and filler content effects on shrinkage stress. Dent. Mater. 2011, 27, 520–526. [Google Scholar] [CrossRef]
- Martim, G.C.; Pfeifer, C.S.; Girotto, E.M. Novel urethane-based polymer for dental applications with decreased monomer leaching. Mater. Sci. Eng. C 2017, 72, 192–201. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. Characterization of urethane-dimethacrylate derivatives as alternative monomers for the restorative composite matrix. Dent. Mater. 2014, 30, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.M.; Chrószcz, M.W.; Chladek, G. Novel Urethane-Dimethacrylate Monomers and Compositions for Use as Matrices in Dental Restorative Materials. Int. J. Mol. Sci. 2020, 21, 2644. [Google Scholar] [CrossRef]
- Lazouzi, G.A.; Vuksanović, M.M.; Tomić, N.; Petrović, M.; Spasojević, P.; Radojević, V.; Jančić Heinemann, R. Dimethyl Itaconate Modified PMMA—Alumina Fillers Composites With Improved Mechanical Properties. Polym. Compos. 2019, 40, 1691–1701. [Google Scholar] [CrossRef]
- Spasojevic, P.; Zrilic, M.; Panic, V.; Stamenkovic, D.; Seslija, S.; Velickovic, S. The Mechanical Properties of a Poly(methyl methacrylate) Denture Base Material Modified with Dimethyl Itaconate and Di- n -butyl Itaconate. Int. J. Polym. Sci. 2015, 2015, 561012. [Google Scholar] [CrossRef]
- Berlanga Duarte, M.L.; Reyna Medina, L.A.; Reyes, P.T.; González Pérez, S.E.; Herrera González, A.M. Biobased isosorbide methacrylate monomer as an alternative to bisphenol A glycerolate dimethacrylate for dental restorative applications. J. Appl. Polym. Sci. 2017, 134, app.44591. [Google Scholar] [CrossRef]
- Herrera-González, A.M.; Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E. Evaluation of bio-based monomers from isosorbide used in the formulation of dental composite resins. J. Mech. Behav. Biomed. Mater. 2019, 100, 103371. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, H.W.; Lee, J.-H.; Lee, S.-H.; Cho, J.K.; Shin, S. Synthesis of a novel isosorbide-based dental material with improved water sorption. Eur. Polym. J. 2019, 112, 629–635. [Google Scholar] [CrossRef]
- Wang, X.; Huyang, G.; Palagummi, S.V.; Liu, X.; Skrtic, D.; Beauchamp, C.; Bowen, R.; Sun, J. High performance dental resin composites with hydrolytically stable monomers. Dent. Mater. 2018, 34, 228–237. [Google Scholar] [CrossRef]
- Tauscher, S.; Catel, Y.; Fässler, P.; Fischer, U.; Moszner, N. Development of low-shrinkage composites based on novel crosslinking vinyl cyclopropanes. J. Appl. Polym. Sci. 2017, 134, 45577. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhao, C.; Bu, W.; Zhang, Y.; Na, H. Synthesis, characterization and evaluation of a fluorinated resin monomer with low water sorption. J. Mech. Behav. Biomed. Mater. 2018, 77, 446–454. [Google Scholar] [CrossRef]
- Moreira, M.M.; Da Silva, A.L.; Pereira, R.D.C.S.; Da Silva, L.R.R.; Feitosa, V.P.; Lomonaco, D. Effect of Replacing Bis-GMA with a Biobased Trimethacrylate on the Physicochemical and Mechanical Properties of Experimental Resin Composites. Clin. Oral Investig. 2024, 28, 578. [Google Scholar] [CrossRef]
- Krasowski, M.; Ciesielska, S.; Śmielak, B.; Kopacz, K.; Bociong, K. Preparation of an Experimental Dental Composite with Different Bis-GMA/UDMA Proportions. Mater. Manuf. Process. 2024, 39, 1044–1051. [Google Scholar] [CrossRef]
- Mahmoudi Meimand, N.; Tsoi, J.K.H.; Burrow, M.F.; He, J.; Cho, K. A Comparative Study on the Mechanical and Antibacterial Properties of BPA-Free Dental Resin Composites. Dent. Mater. 2024, 40, 31–39. [Google Scholar] [CrossRef]
- Karkanis, S.F.; Nikolaidis, A.K.; Koulaouzidou, E.A.; Achilias, D.S. Production of Novel Dental Resin Monomers Using Dimethacrylated Oligoesters Derived from Chemically Recycling PET Waste. ChemSusChem 2025, 18, e202402371. [Google Scholar] [CrossRef]
- Rueggeberg, F.A.; Hashinger, D.T.; Fairhurst, C.W. Calibration of FTIR conversion analysis of contemporary dental resin composites. Dent. Mater. 1990, 6, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.C.; Marouf, A.S. Optimal specimen geometry in bonded-disk shrinkage-strain measurements on light-cured biomaterials. Dent. Mater. 2000, 16, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Watts, D. Photo-polymerization shrinkage-stress kinetics in resin-composites: Methods development. Dent. Mater. 2003, 19, 1–11. [Google Scholar] [CrossRef]
- Al Sunbul, H.; Silikas, N.; Watts, D.C. Polymerization shrinkage kinetics and shrinkage-stress in dental resin-composites. Dent. Mater. 2016, 32, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- ISO 4049:2019; Dentistry-Polymer Based Restorative Materials. ISO: Geneva, Switzerland, 2019; pp. 1–29.
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Gonçalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef]
- Achilias, D.S. A Review of Modeling of Diffusion Controlled Polymerization Reactions. Macromol. Theory Simul. 2007, 16, 319–347. [Google Scholar] [CrossRef]
- Achilias, D.S.; Verros, G.D. Modeling of diffusion-controlled reactions in free radical solution and bulk polymerization: Model validation by DSC experiments. J. Appl. Polym. Sci. 2010, 116, 1842–1856. [Google Scholar] [CrossRef]
- Verros, G.D.; Achilias, D.S. Modeling gel effect in branched polymer systems: Free-radical solution homopolymerization of vinyl acetate. J. Appl. Polym. Sci. 2009, 111, 2171–2185. [Google Scholar] [CrossRef]
- Verros, G.D.; Latsos, T.; Achilias, D.S. Development of a unified framework for calculating molecular weight distribution in diffusion controlled free radical bulk homo-polymerization. Polymer 2005, 46, 539–552. [Google Scholar] [CrossRef]
- Luo, S.; Liu, F.; He, J. Preparation of low shrinkage stress dental composite with synthesized dimethacrylate oligomers. J. Mech. Behav. Biomed. Mater. 2019, 94, 222–228. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Mitchem, J.C. Relationship between composite contraction stress and leakage in Class V cavities. Am. J. Dent. 2003, 16, 239–243. [Google Scholar]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef]
- Fonseca, A.S.Q.S.; Labruna Moreira, A.D.; De Albuquerque, P.P.A.C.; De Menezes, L.R.; Pfeifer, C.S.; Schneider, L.F.J. Effect of monomer type on the CC degree of conversion, water sorption and solubility, and color stability of model dental composites. Dent. Mater. 2017, 33, 394–401. [Google Scholar] [CrossRef]
- Atai, M.; Watts, D.; Atai, Z. Shrinkage strain-rates of dental resin-monomer and composite systems. Biomaterials 2005, 26, 5015–5020. [Google Scholar] [CrossRef]
- Davidson, C.L.; Feilzer, A.J. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J. Dent. 1997, 25, 435–440. [Google Scholar] [CrossRef]
- Dauvillier, B.S.; Aarnts, M.P.; Feilzer, A.J. Developments in Shrinkage Control of Adhesive Restoratives. J. Esthet. Restor. Dent. 2000, 12, 291–299. [Google Scholar] [CrossRef]
- Barnes, D.M.; Thompson, V.P.; Blank, L.W.; McDonald, N.J. Microleakage of Class 5 composite resin restorations: A comparison between in vivo and in vitro. Oper. Dent. 1993, 18, 237–245. [Google Scholar]
- Kemp-Scholte, C.M.; Davidson, C.L. Marginal Sealing of Curing Contraction Gaps in Class V Composite Resin Restorations. J. Dent. Res. 1988, 67, 841–845. [Google Scholar] [CrossRef]
- Camps, J.; Déjou, J.; Rémusat, M.; About, I. Factors influencing pulpal response to cavity restorations. Dent. Mater. 2000, 16, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Henderson, L.J. Dentin adhesives and microleakage in cervical resin composites. Am. J. Dent. 1992, 5, 240–244. [Google Scholar]
- Atai, M.; Watts, D. A new kinetic model for the photopolymerization shrinkage-strain of dental composites and resin-monomers. Dent. Mater. 2006, 22, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ferracane, J.L. Contraction Stress Related to Degree of Conversion and Reaction Kinetics. J. Dent. Res. 2002, 81, 114–118. [Google Scholar] [CrossRef]
- Calheiros, F. Relationship between contraction stress and degree of conversion in restorative composites. Dent. Mater. 2004, 20, 939–946. [Google Scholar] [CrossRef]
- Charton, C.; Falk, V.; Marchal, P.; Pla, F.; Colon, P. Influence of Tg, viscosity and chemical structure of monomers on shrinkage stress in light-cured dimethacrylate-based dental resins. Dent. Mater. 2007, 23, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Floyd, C.J.E.; Dickens, S.H. Network structure of Bis-GMA- and UDMA-based resin systems. Dent. Mater. 2006, 22, 1143–1149. [Google Scholar] [CrossRef]
- Dewaele, M.; Truffier-Boutry, D.; Devaux, J.; Leloup, G. Volume contraction in photocured dental resins: The shrinkage-conversion relationship revisited. Dent. Mater. 2006, 22, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ren, L.; Cheng, Y.; Xu, M.; Luo, W.; Zhan, D.; Sano, H.; Fu, J. Evaluation of the Color Stability, Water Sorption, and Solubility of Current Resin Composites. Materials 2022, 15, 6710. [Google Scholar] [CrossRef]
- Ozera, E.H.; Pascon, F.M.; Correr, A.B.; Puppin-Rontani, R.M.; Castilho, A.R.D.; Correr-Sobrinho, L.; Paula, A.B.D. Color Stability and Gloss of Esthetic Restorative Materials after Chemical Challenges. Braz. Dent. J. 2019, 30, 52–57. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. A Guide through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef]
- Turner, D.T.; Abell, A.K. Water sorption of poly(methyl methacrylate): 2. Effects of crosslinks. Polymer 1987, 28, 297–302. [Google Scholar] [CrossRef]
- Arima, T.; Hamada, T.; McCabe, J.F. The Effects of Cross-linking Agents on Some Properties of HEMA-based Resins. J. Dent. Res. 1995, 74, 1597–1601. [Google Scholar] [CrossRef]
- Yang, C.; Xing, X.; Li, Z.; Zhang, S. A Comprehensive Review on Water Diffusion in Polymers Focusing on the Polymer–Metal Interface Combination. Polymers 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Ajithkumar, S.; Patel, N.K.; Kansara, S.S. Sorption and diffusion of organic solvents through interpenetrating polymer networks IPNs) based on polyurethane and unsaturated polyester. Eur. Polym. J. 2000, 36, 2387–2393. [Google Scholar] [CrossRef]
- Elliott, J.E.; Lovell, L.G.; Bowman, C.N. Primary cyclization in the polymerization of bis-GMA and TEGDMA: A modeling approach to understanding the cure of dental resins. Dent. Mater. 2001, 17, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Lovell, L.G.; Berchtold, K.A.; Elliott, J.E.; Lu, H.; Bowman, C.N. Understanding the kinetics and network formation of dimethacrylate dental resins. Polym. Adv. Technol. 2001, 12, 335–345. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Malacarne, J.; Carvalho, R.M.; De Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R.D.O. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Moy, P.; Karasz, F.E. Epoxy-water interactions. Polym. Eng. Sci. 1980, 20, 315–319. [Google Scholar] [CrossRef]
- Kumar, N.; Sangi, L. Water sorption, solubility, and resultant change in strength among three resin-based dental composites. J. Investig. Clin. Dent. 2014, 5, 144–150. [Google Scholar] [CrossRef] [PubMed]
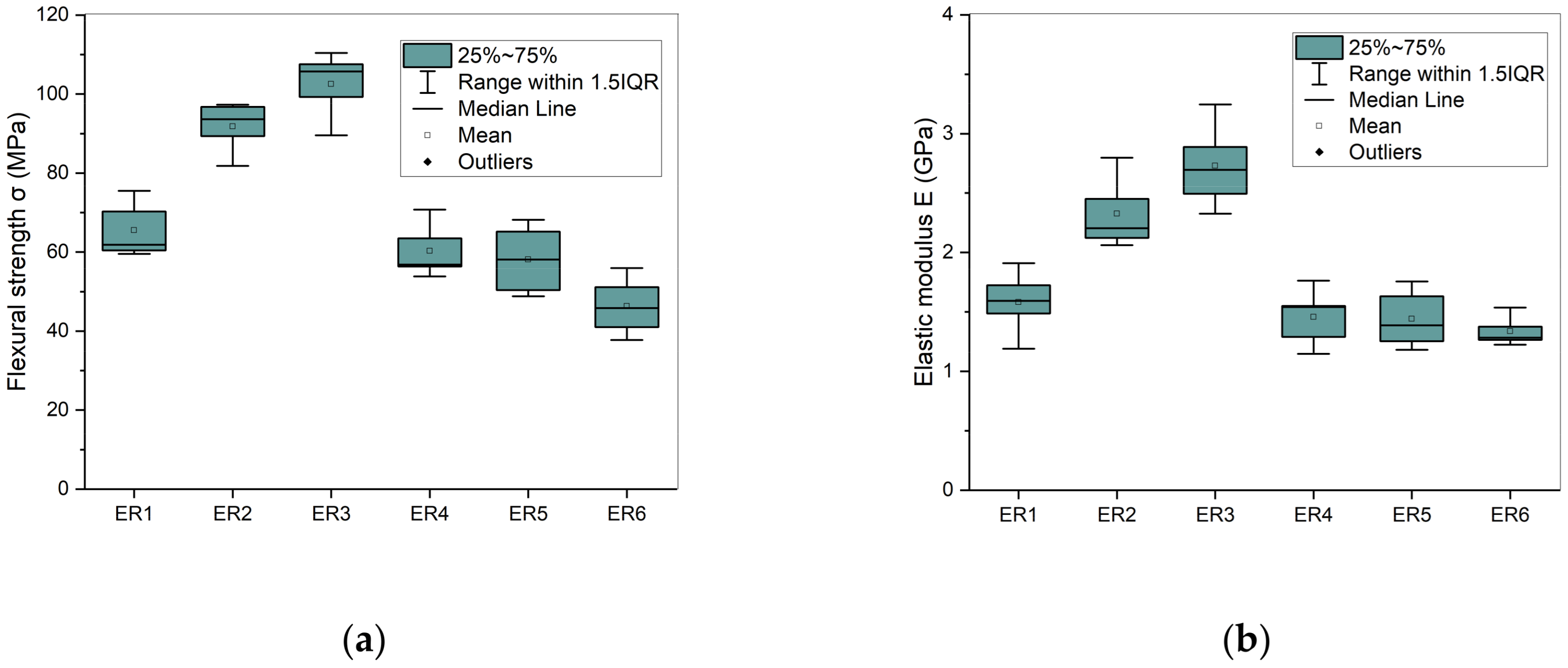
 [25] and after
[25] and after  immersion in distilled water for 1 week.
immersion in distilled water for 1 week.
 [25] and after
[25] and after  immersion in distilled water for 1 week.
immersion in distilled water for 1 week.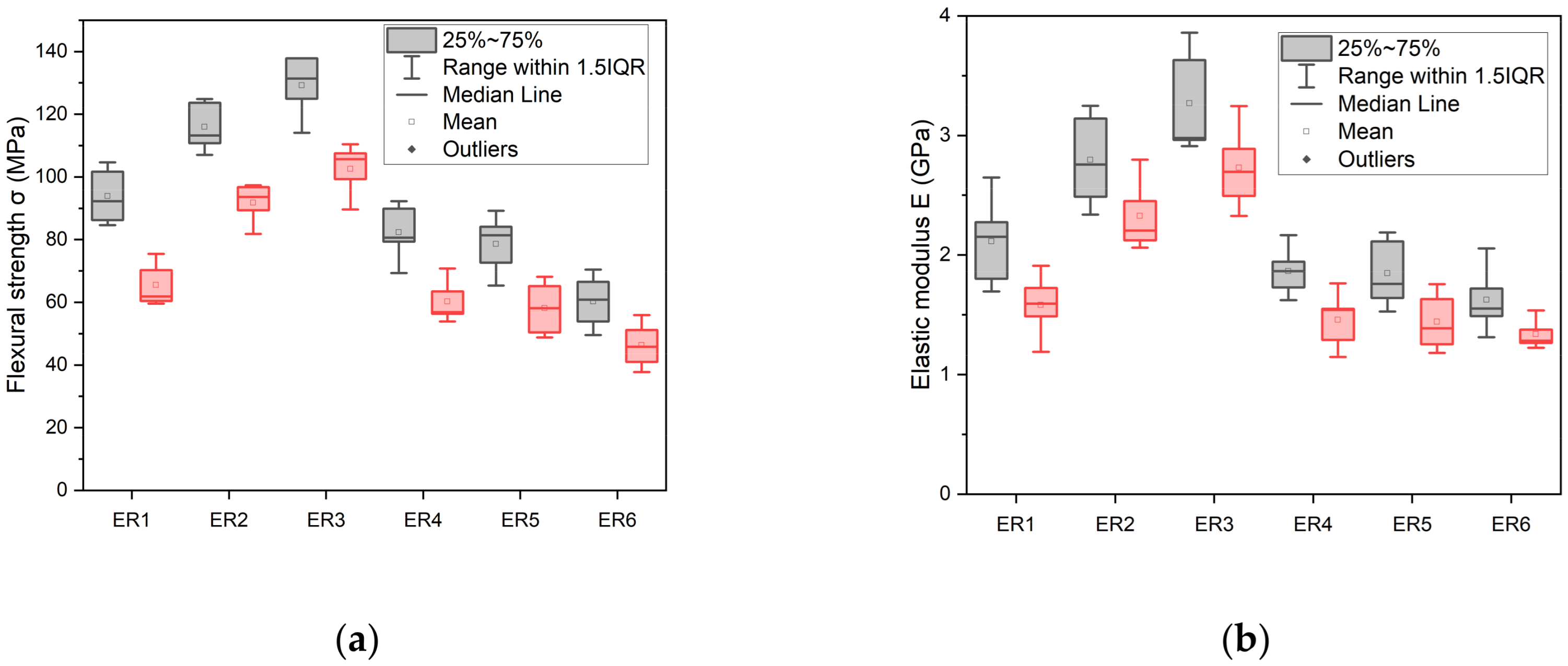
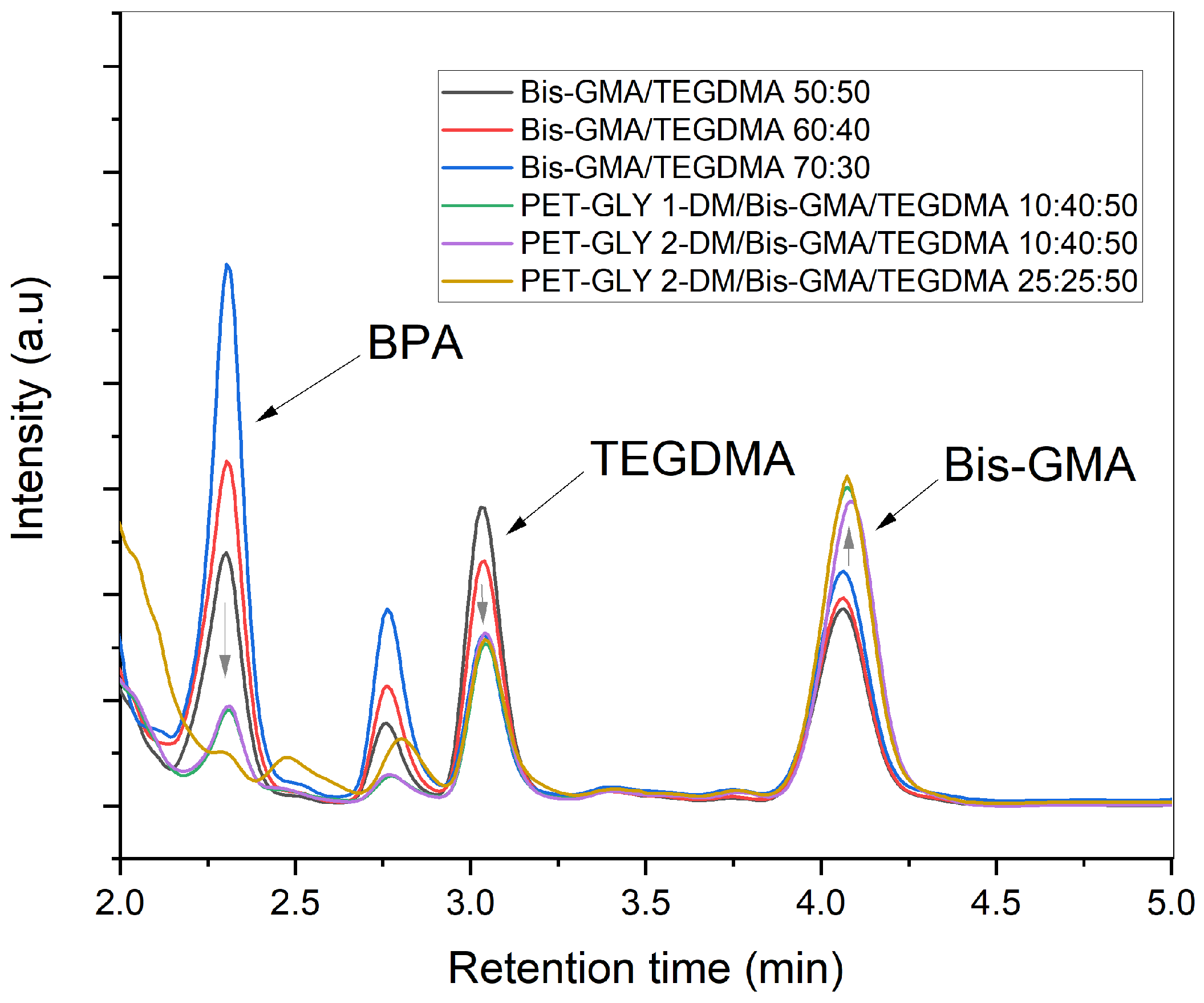
| Experimental Resins (ER) | Bis-GMA (%) | TEGDMA (%) | PET-GLY 1-DM (%) | PET-GLY 2-DM (%) |
|---|---|---|---|---|
| 1 | 50 | 50 | ||
| 2 | 60 | 40 | ||
| 3 | 70 | 30 | ||
| 4 | 40 | 50 | 10 | |
| 5 | 40 | 50 | 10 | |
| 6 | 25 | 50 | 25 |
| Experimental Resins (ER) | Final DC (%) | Total Strain (%) | Water Sorption (μg mm−3) | Water Solubility (μg mm−3) |
|---|---|---|---|---|
| 1 | 59.56 ± 1.13 a | 7.96 ± 0.26 a | 36.72 ± 1.21 a | 1.86 ± 0.12 a |
| 2 | 55.26 ± 1.11 b | 6.79 ± 0.50 b | 26.13 ± 2.39 b | 3.23 ± 0.49 b |
| 3 | 53.46 ± 1.25 c,b | 6.09 ± 0.45 c | 23.85 ± 1.56 c,b | 3.62 ± 0.30 c,b |
| 4 | 62.5 ± 1.32 d | 8.13 ± 0.26 a | 33.36 ± 2.14 a,f | 1.71 ± 0.25 a |
| 5 | 64.72 ± 1.08 e,d | 8.4 ± 0.22 a,e | 32.19 ± 1.23 d,f | 1.53 ± 0.14 a |
| 6 | 69.54 ± 1.76 f | 8.85 ± 0.23 d,e | 29.09 ± 1.02 e,b | 1.28 ± 0.11 a |
| Experimental Resins (ER) | FS Before Immersion (MPa) | EM Before Immersion (GPa) | FS After Immersion (MPa) | EM After Immersion (GPa) |
|---|---|---|---|---|
| 1 | 93.89 ± 9.01 a A | 2.11 ± 0.38 a A | 65.53 ± 7.01 a A | 1.58 ± 0.27 a A |
| 2 | 115.90 ± 7.96 b B | 2.79 ± 0.39 a Β | 91.79 ± 6.4 b B | 2.33 ± 0.3 b Β |
| 3 | 129.21 ± 9.99 c,b C | 3.26 ± 0.44 b C | 102.5 ± 8.3 c,b C | 2.73 ± 0.36 c,b D |
| 4 | 82.28 ± 9.15 a,e D | 1.86 ± 0.21 a,d E | 60.26 ± 6.86 a,e D | 1.45 ± 0.24 a,d E |
| 5 | 78.54 ± 9.53 a,e E | 1.84 ± 0.29 a,d,e F | 58.14 ± 8.63 a,e E | 1.44 ± 0.25 a,d G |
| 6 | 60.24 ± 8.65 d F | 1.62 ± 0.28 c,d,e H | 46.33 ± 7.38 d,e G | 1.33 ± 0.13 a,d I |
| Experimental Resins (ER) | BPA (mg/mgresin) | TEGDMA (mg/mgresin) | Bis-GMA (mg/mgresin) |
|---|---|---|---|
| 1 | 0.124 ± 0.021 a | 0.038 ± 0.002 a | 0.192 ± 0.017 a |
| 2 | 0.261 ± 0.016 b | 0.027 ± 0.001 b | 0.214 ± 0.025 a |
| 3 | 0.357 ± 0.045 c | 0.017 ± 0.002 c | 0.246 ± 0.030 a |
| 4 | 0.028 ± 0.004 d | 0.016 ± 0.001 d,c | 0.281 ± 0.019 b,e |
| 5 | 0.028 ± 0.004 e,d | 0.017 ± 0.001 e,d | 0.273 ± 0.045 c,e |
| 6 | 0.000 ± 0.000 f,d | 0.015 ± 0.002 f,c | 0.290 ± 0.032 d,e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkanis, S.; Nikolaidis, A.K.; Koulaouzidou, E.A.; Achilias, D.S. Physicochemical and Mechanical Performance of Dental Resins Formulated from Dimethacrylated Oligoesters Derived from PET Recycling via Glycolysis. Polymers 2025, 17, 2660. https://doi.org/10.3390/polym17192660
Karkanis S, Nikolaidis AK, Koulaouzidou EA, Achilias DS. Physicochemical and Mechanical Performance of Dental Resins Formulated from Dimethacrylated Oligoesters Derived from PET Recycling via Glycolysis. Polymers. 2025; 17(19):2660. https://doi.org/10.3390/polym17192660
Chicago/Turabian StyleKarkanis, Stefanos, Alexandros K. Nikolaidis, Elisabeth A. Koulaouzidou, and Dimitris S. Achilias. 2025. "Physicochemical and Mechanical Performance of Dental Resins Formulated from Dimethacrylated Oligoesters Derived from PET Recycling via Glycolysis" Polymers 17, no. 19: 2660. https://doi.org/10.3390/polym17192660
APA StyleKarkanis, S., Nikolaidis, A. K., Koulaouzidou, E. A., & Achilias, D. S. (2025). Physicochemical and Mechanical Performance of Dental Resins Formulated from Dimethacrylated Oligoesters Derived from PET Recycling via Glycolysis. Polymers, 17(19), 2660. https://doi.org/10.3390/polym17192660










