Hyaluronan-Based Hydrogel Hybrid Insulin Carriers—Preformulation Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Hybrid Hydrogels
Preparation of a Hydrogel Matrix Based on Hydroxypropyl Methylcellulose and Sodium Hyaluronate (HPMC/HA)
Preparation of a Hydrogel Matrix Based on Sodium Alginate and Sodium Hyaluronate (ALG/HA)
2.2.2. Study of the Pharmaceutical Availability of Insulin from Hydrogels in Vitro
2.2.3. Rheological Analysis
Rotational Test Measurements
Oscillatory Test Measurements
2.2.4. Texture Analysis
2.2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sopata, M.; Jawień, A.; Mrozikiewicz-Rakowska, B.; Augusewicz, Z.; Bakowska, M.; Samson, I.; Gabriel, M.; Grzela, T.; Karpiński, T.; Kuberka, I.; et al. Wytyczne postępowania miejscowego w ranach niezakażonych, zagrożonych infekcją oraz zakażonych—Przegląd dostępnych substancji przeciwdrobnoustrojowych stosowanych w leczeniu ran. Zalecenia Polskiego Towarzystwa Leczenia Ran. Leczenie Ran 2020, 17, 1–21. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 10th ed.; EDQM Council of Europe: Strasbourg, France, 2022.
- Kumar, A.; Sah, D.K. A calcium and zinc composite alginate hydrogel for pre-hospital hemostasis and wound care. Carbohydr. Polym. 2023, 299, 120186. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; Esposito, V.D.; Acierno, S.; Ambrosio, M.R.; Caro, C.D.; Avagliano, C.; Russo, P.; Russo, R.; Miro, A.; Ungaro, F.; et al. Alginate—Hyaluronan composite hydrogels accelerate wound healing process. Carbohydr. Polym. 2015, 131, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From Extracellular Glue to Pericellular Cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Petrey, A.C.; De La Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Zarei, N.; Hassanzadeh-Tabrizi, S. Alginate/hyaluronic acid-based systems as a new generation of wound dressings: A review. Int. J. Biol. Macromol. 2023, 253, 127249. [Google Scholar] [CrossRef]
- Voigt, J.; Driver, V.R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2012, 20, 317–331. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Dinu-Pîrvu, C.-E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef] [PubMed]
- Al-Bazzaz, F.Y.; Ismail, S.T. Topical HPMC/Carbopol 934 gel for wound healing: Formulation and in-vivo evaluation. Pharmakeftiki 2024, 36, 42–54. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Przybyła, M.; Wójcik, W.; Birówka, K.; Majczyna, M.; Dolińska, B. Review of Research in Developing Hydrogels with Insulin to Promote Wound Healing. Med. Sci. Forum 2023, 21, 17. [Google Scholar] [CrossRef]
- Przybyła, M.; Dolińska, B.; Ostróżka-Cieślik, A. Research Progress on Insulin Dressings to Promote Wound Healing. Eng. Proc. 2023, 56, 21. [Google Scholar] [CrossRef]
- Przybyła, M.; Dolińska, B.; Ostróżka-Cieślik, A. Progress of knowledge in the development of chitosan formulations with insulin to promote skin wound healing. Prog. Chem. Appl. Chitin Deriv. 2024, 29, 44–59. [Google Scholar] [CrossRef]
- Vatankhah, N.; Jahangiri, Y.; Landry, G.J.; Moneta, G.L.; Azarbal, A.F. Effect of systemic insulin treatment on diabetic wound healing. Wound Repair Regen. 2017, 25, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Chinkes, D.L.; Wolf, S.E.; Wolfe, R.R. Insulin but not growth hormone stimulates protein anabolism in skin wound and muscle. Am. J. Physiol. 1999, 276, E712–E720. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, O.; Shabbak, E.; Aslani, A.; Bidar, R.; Jafari, M.; Safarnezhad, S. A randomized, double-blind, placebo-controlled trial to determine the effects of topical insulin on wound healing. Ostomy/Wound Manag. 2009, 55, 22. [Google Scholar]
- Wilson, J.M.; Baines, R.; Babu, E.D.; Kelley, C.J. A role for topical insulin in the management problematic surgical wounds. Ann. R. Coll. Surg. Engl. 2008, 90, 160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, X.; Zhang, X.; Liu, Y. Effect of topical insulin application on wound neutrophil function. Wounds 2012, 24, 178–184. [Google Scholar]
- Hrynyk, M.; Neufeld, R.J. Insulin and wound healing. Burns 2014, 40, 433–446. [Google Scholar] [CrossRef]
- Mumuni, A.M.; Calister, E.U.; Aminu, N.; Franklin, C.K.; Musiliu Oluseun, A.; Usman, M.; Abdulmumuni, B.; James, Y.O.; Ofokansi, C.K.; Anthony, A.A.; et al. Mucin-Grafted Polyethylene Glycol Microparticles Enable Oral Insulin Delivery for Improving Diabetic Treatment. Appl. Sci. 2020, 10, 2649. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Strasser, C.; Dolińska, B. Insulin-Loaded Chitosan–Cellulose-Derivative Hydrogels: In Vitro Permeation of Hormone through Strat-M® Membrane and Rheological and Textural Analysis. Polymers 2024, 16, 2619. [Google Scholar] [CrossRef]
- Muselík, J.; Komersová, A.; Kubová, K.; Matzick, K.; Skalická, B. A Critical Overview of FDA and EMA Statistical Methods to Compare In Vitro Drug Dissolution Profiles of Pharmaceutical Products. Pharmaceutics 2021, 13, 1703. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Wilczyński, S.; Dolińska, B. Hydrogel Formulations for Topical Insulin Application: Preparation, Characterization and In Vitro Permeation across the Strat-M® Membrane. Polymers 2023, 15, 3639. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Maciążek-Jurczyk, M.; Pożycka, J.; Dolińska, B. Pre-Formulation Studies: Physicochemical Characteristics and In Vitro Release Kinetics of Insulin from Selected Hydrogels. Pharmaceutics 2021, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Lee, D.; Zhang, H.; Ryu, S. Elastic modulus measurement of hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Yılmaz Usta, D.; Teksin, Z.S.; Tugcu-Demiroz, F. Evaluation of Emulgel and Nanostructured Lipid Carrier-Based Gel Formulations for Transdermal Administration of Ibuprofen: Characterization, Mechanical Properties, and Ex-Vivo Skin Permeation. AAPS PharmSciTech 2024, 25, 124. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, A.; Mozafari, N.; Fekri, N.; Memarpour, M.; Azadi, A. Preparation and Characterization of a Nanohydroxyapatite and Sodium Fluoride Loaded Chitosan-Based in Situ Forming Gel for Enamel Biomineralization. Heliyon 2024, 10, e24217. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A. The Potential of Pharmaceutical Hydrogels in the Formulation of Topical Administration Hormone Drugs. Polymers 2022, 14, 3307. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Hering, A.; Cal, K.; Kowalczyk, M.; Kastsevich, A.; Ivashchanka, Y.; Ochocka, J.R.; Stefanowicz-Hajduk, J. Bufadienolide Penetration Through the Skin Membrane and Antiaging Properties of Kalanchoe spp. Juices in Dermal Applications. Molecules 2025, 30, 802. [Google Scholar] [CrossRef]
- Khadka, A.; Giri, B.R.; Baral, R.; Shakya, S.; Shrestha, A.K. Formulation and In Vitro Characterization of Cellulose-Based Propranolol Hydrochloride Sustained Release Matrix Tablets. BioChem 2025, 5, 14. [Google Scholar] [CrossRef]
- Kshirsagar, M.M.; Chatale, B.C.; Dyawanapelly, S.; Vora, L.K.; Amin, P.D. Continuous Processing Strategies for Amorphous Solid Dispersions of Itraconazole: Impact of Polymer Selection and Manufacturing Techniques. Pharmaceutics 2025, 17, 1090. [Google Scholar] [CrossRef]
- Kokol, V.; Pottathara, Y.B.; Mihelčič, M.; Perše, L.S. Rheological properties of gelatine hydrogels affected by flow-and horizontally-induced cooling rates during 3D cryo-printing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126356. [Google Scholar] [CrossRef]
- Angar, N.E.; Aliouche, D. Rheological behavior and reversible swelling of pH sensitive poly(acrylamide-co-itaconic acid) hydrogels. Polym. Sci. Ser. A 2016, 58, 541–549. [Google Scholar] [CrossRef]
- Kadian, V.; Rao, R. Enhancing anti-inflammatory effect of brucine nanohydrogel using rosemary oil: A promising strategy for dermal delivery in arthritic inflammation. 3 Biotech 2024, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci. World J. 2016, 2016, e7394685–e7394689. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Sharma, G.; Singh, B.; Nirbhavane, P.; Tyagi, R.K.; Shukla, R.; Katare, O.P. Quality by Design (QbD)-enabled development of aceclofenac loaded-nano structured lipid carriers (NLCs): An improved dermatokinetic profile for inflammatory disorder(s). Int. J. Pharm. 2017, 517, 413–431. [Google Scholar] [CrossRef]
- Huang, N.; Sun, J.; Liu, J.; Lv, K.; Deng, X.; Zhang, T.; Sun, Y.; Yan, H.; Hou, D. Enhancing Rheology and Wettability of Drilling Fluids at Ultra-Low Temperatures Using a Novel Amide Material. Gels 2025, 11, 687. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef]
- Eskens, O.; Villani, G.; Amin, S. Rheological Investigation of Thermoresponsive Alginate-Methylcellulose Gels for Epidermal Growth Factor Formulation. Cosmetics 2021, 8, 3. [Google Scholar] [CrossRef]
- Liparoti, S.; Speranza, V.; Marra, F. Alginate hydrogel: The influence of the hardening on the rheological behaviour. J. Mech. Behav. Biomed. Mater. 2021, 116, 104341. [Google Scholar] [CrossRef]
- Özgüney, I.; Kardhiqi, A. Properties of bioadhesive ketoprofen liquid suppositories: Preparation, determination of gelation temperature, viscosity studies and evaluation of mechanical properties using texture analyzer by 4 × 4 factorial design. Pharm. Dev. Technol. 2014, 19, 968–975. [Google Scholar] [CrossRef]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Hilmioglu-Polat, S.; Metin, D.Y.; Zekioglu, O.; Guneri, T.; Jones, D.S. In-situ gel formulations of econazole nitrate: Preparation and in-vitro and in-vivo evaluation. J. Pharm. Pharmacol. 2011, 63, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int. J. Pharm. 1997, 151, 223–233. [Google Scholar] [CrossRef]
- Woolfson, A.D.; McCafferty, D.F.; Gorman, S.P.; McCarron, P.A.; Price, J.H. Design of an Apparatus Incorporating a Linear Variable Differential Transformer for the Measurement of Type III Bioadhesion to Cervical Tissue. Int. J. Pharm. 1992, 84, 69–76. [Google Scholar] [CrossRef]
- Rani, P.; Verma, V.; Kumar, S.; Bhatia, M. Isolation, characterization and evaluation of pineapple crown waste nanofiber gel entrapping ampicillin in topical bacterial infections. Iran. Polym. J. 2024, 33, 687–698. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião, M.P.; De Freitas, O.; Lara, E.H. Semisolid systems containing propolis for the treatment of periodontal disease: In vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J. Pharm. Sci. 2007, 96, 2074–2089. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm. Res. 1997, 14, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Alves, L.; Antunes, F.E.; Sousa, J.J.; Pais, A.A.C.C. Design of a Dual Nanostructured Lipid Carrier Formulation Based on Physicochemical, Rheological, and Mechanical Properties. J. Nanopart. Res. 2013, 15, 1993. [Google Scholar] [CrossRef]
- Sudhakar, K.; Ji, S.m.; Kummara, M.R.; Han, S.S. Recent Progress on Hyaluronan-Based Products for Wound Healing Applications. Pharmaceutics 2022, 14, 2235. [Google Scholar] [CrossRef]
- Ishfaq, B.; Khan, I.U.; Khalid, S.H.; Asghar, S. Design and evaluation of sodium alginate-based hydrogel dressings containing Betula utilis extract for cutaneous wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1042077. [Google Scholar] [CrossRef]
- Mishra, R.; Jain, N.; Kaul, S.; Nagaich, U. Central composite design-based optimization, fabrication, and pharmacodynamic assessment of sulfasalazine-loaded lipoidal nanoparticle-based hydrogel for the management of rheumatoid arthritis. Drug Deliv. Transl. Res. 2023, 13, 994–1011. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef]
- Ruan, H.; Shen, L.; Hou, X.; Li, J.; Guo, T.; Zhu, C.; Feng, N.; Zhang, Y. Phytosterol-Mediated Glycerosomes Combined with Peppermint Oil Enhance Transdermal Delivery of Lappaconitine by Modulating the Lipid Composition of the Stratum Corneum. Drug Deliv. Transl. Res. 2023, 13, 3014–3029. [Google Scholar] [CrossRef]
- Maret, W. Inhibitory zinc sites in enzymes. Biometals 2013, 26, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Tuğcu-Demiröz, F. Vaginal delivery of benzydamine hydrochloride through liposomes dispersed in mucoadhesive gels. Chem. Pharm. Bull. 2017, 65, 660–667. [Google Scholar] [CrossRef]
- Sanad, W.G.; Bader, Q.A.; Mahdi, F.M.S.; Kabbani, F. Formulation and in Vitro Evaluation of Moxifloxacin-Lidocaine Base as A Topical Hydrogel Dressing. J. Nat. Sci. Biol. Med. 2023, 14, 152. [Google Scholar]
- Kulkarni, R.V.; Biswanath, D.S. Electrically responsive smart hydrogels in drug delivery: A review. J. Appl. Biomater. Biomech. 2018, 5, 125–139. [Google Scholar]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Cofelice, M.; Lopez, F. Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion. Polymers 2019, 11, 259. [Google Scholar] [CrossRef] [PubMed]
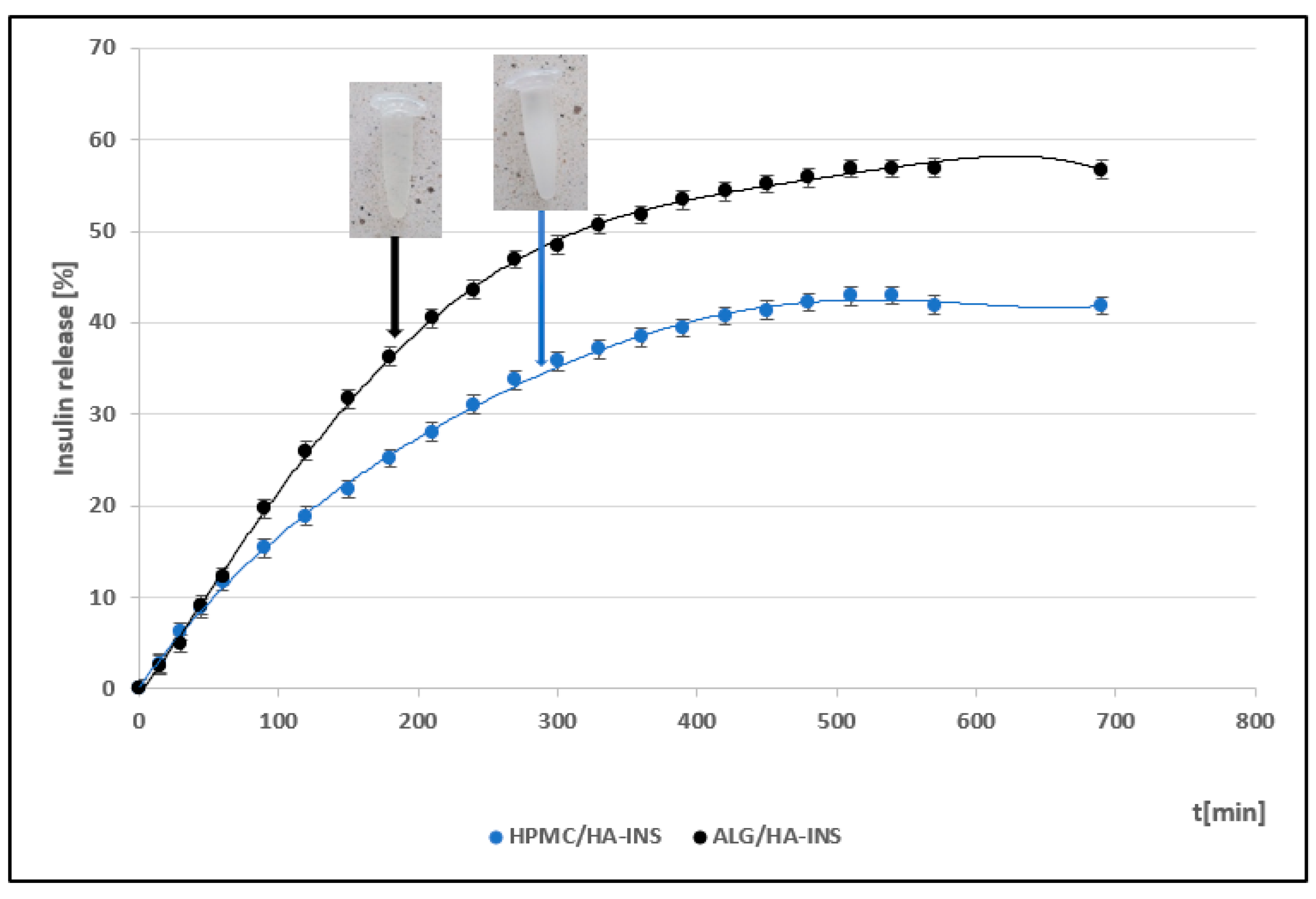
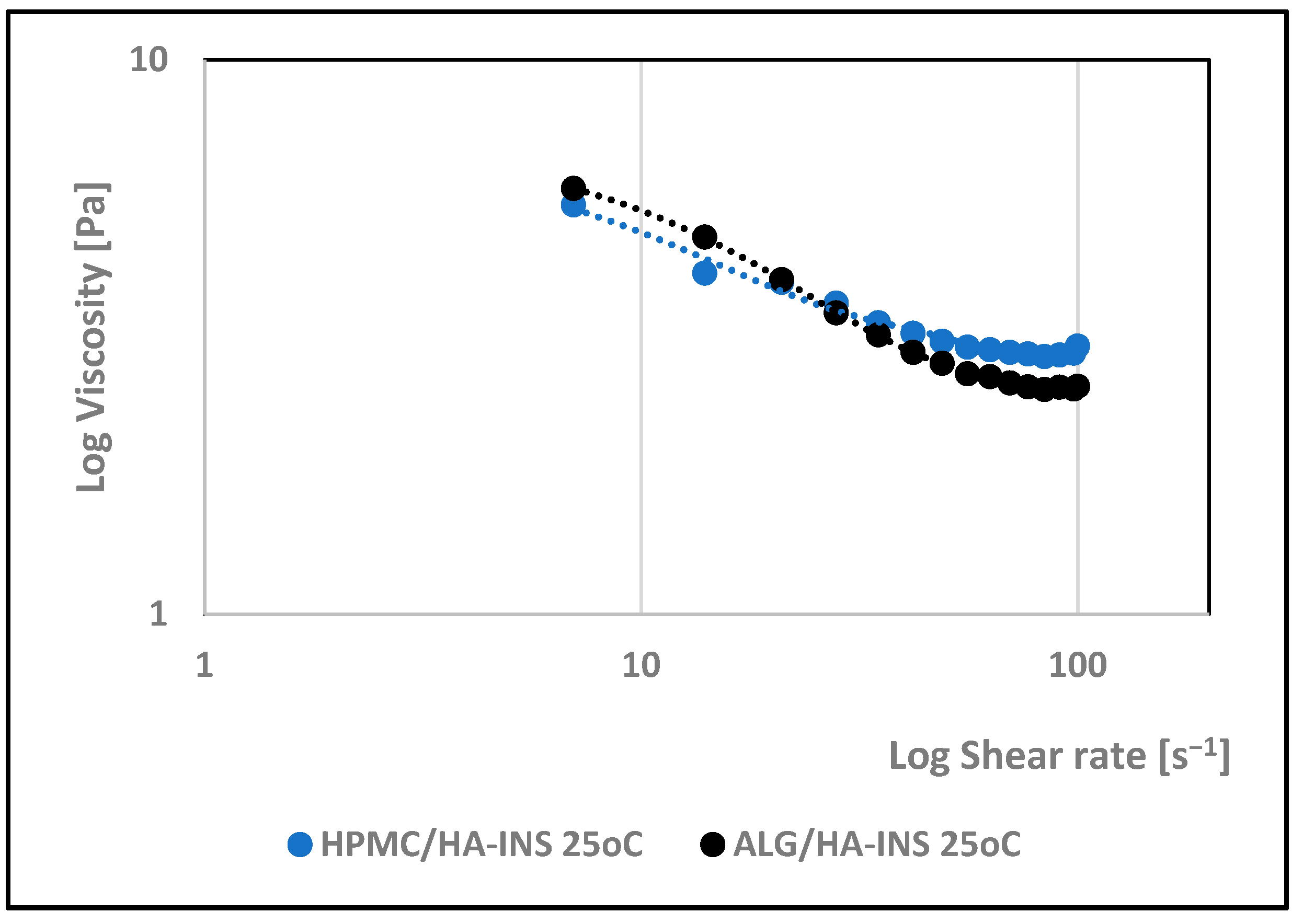
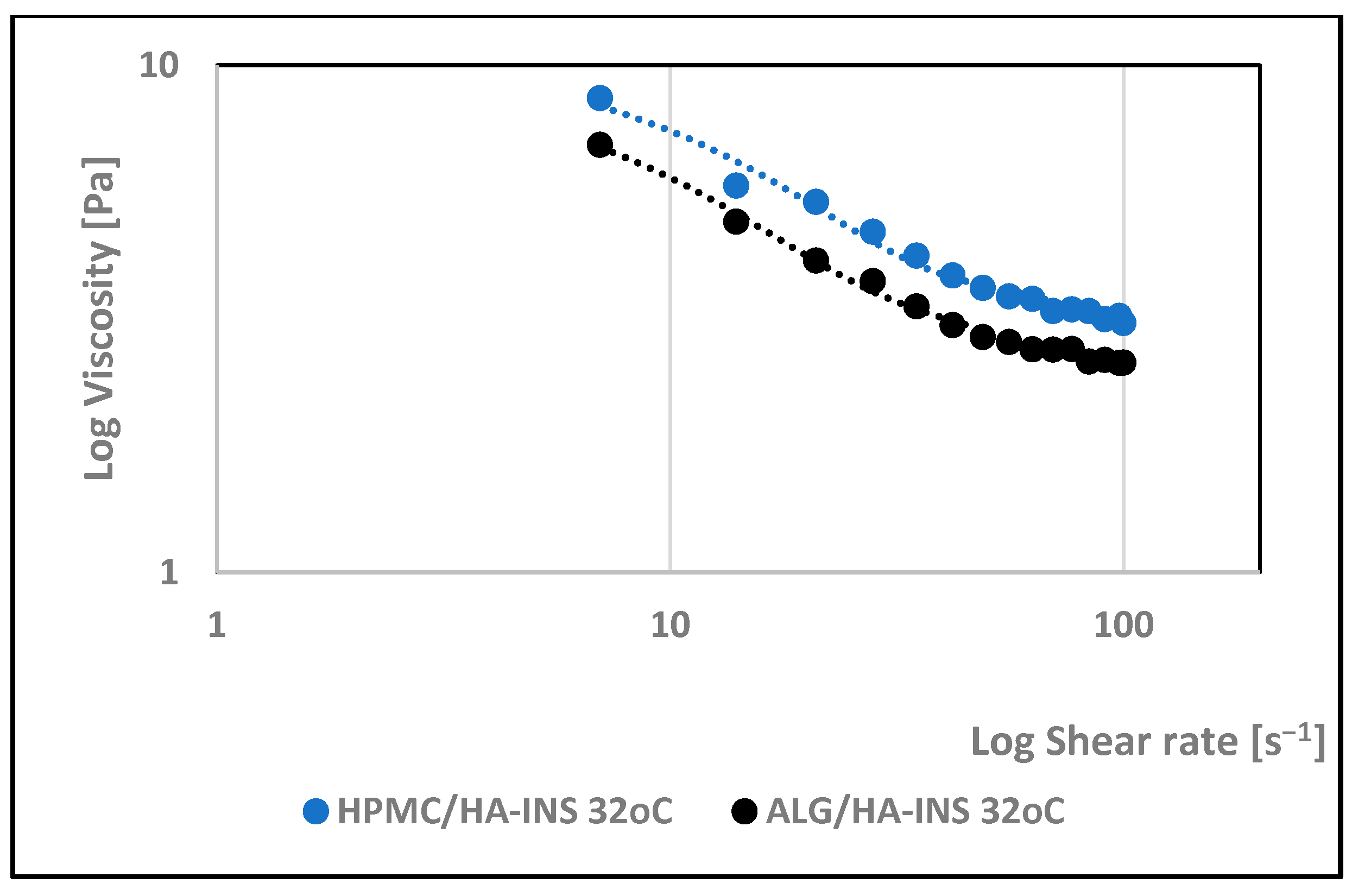
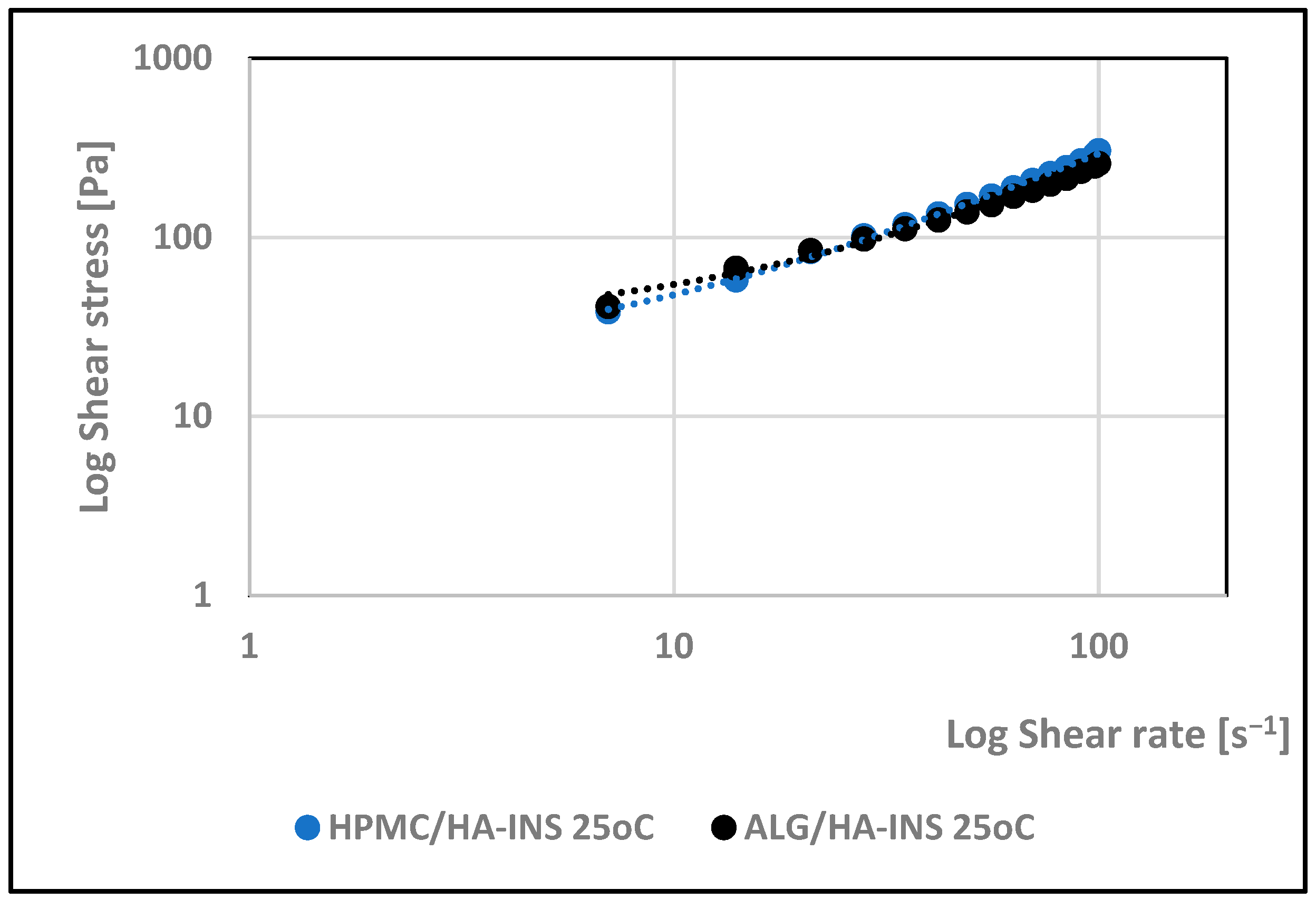
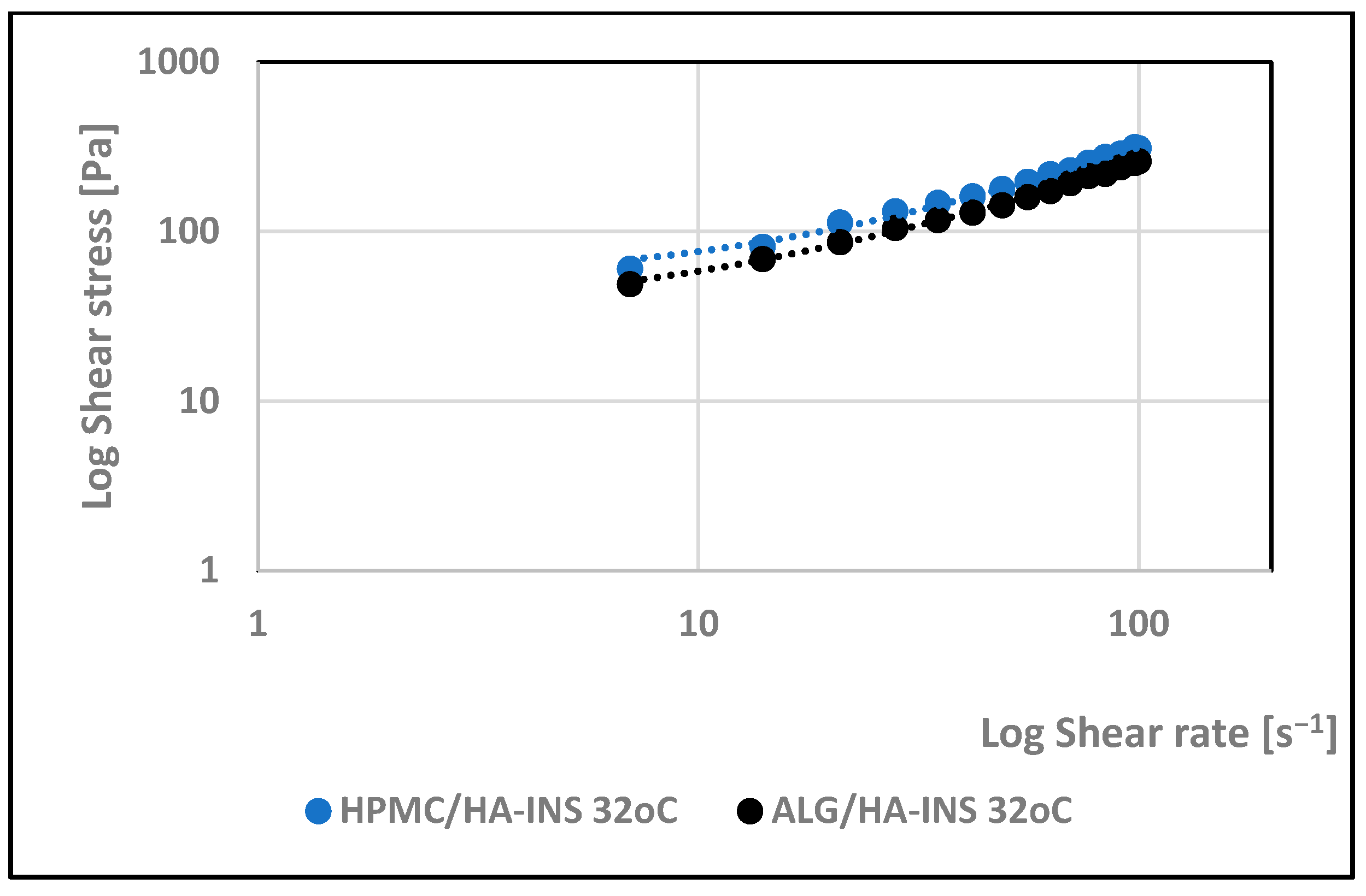
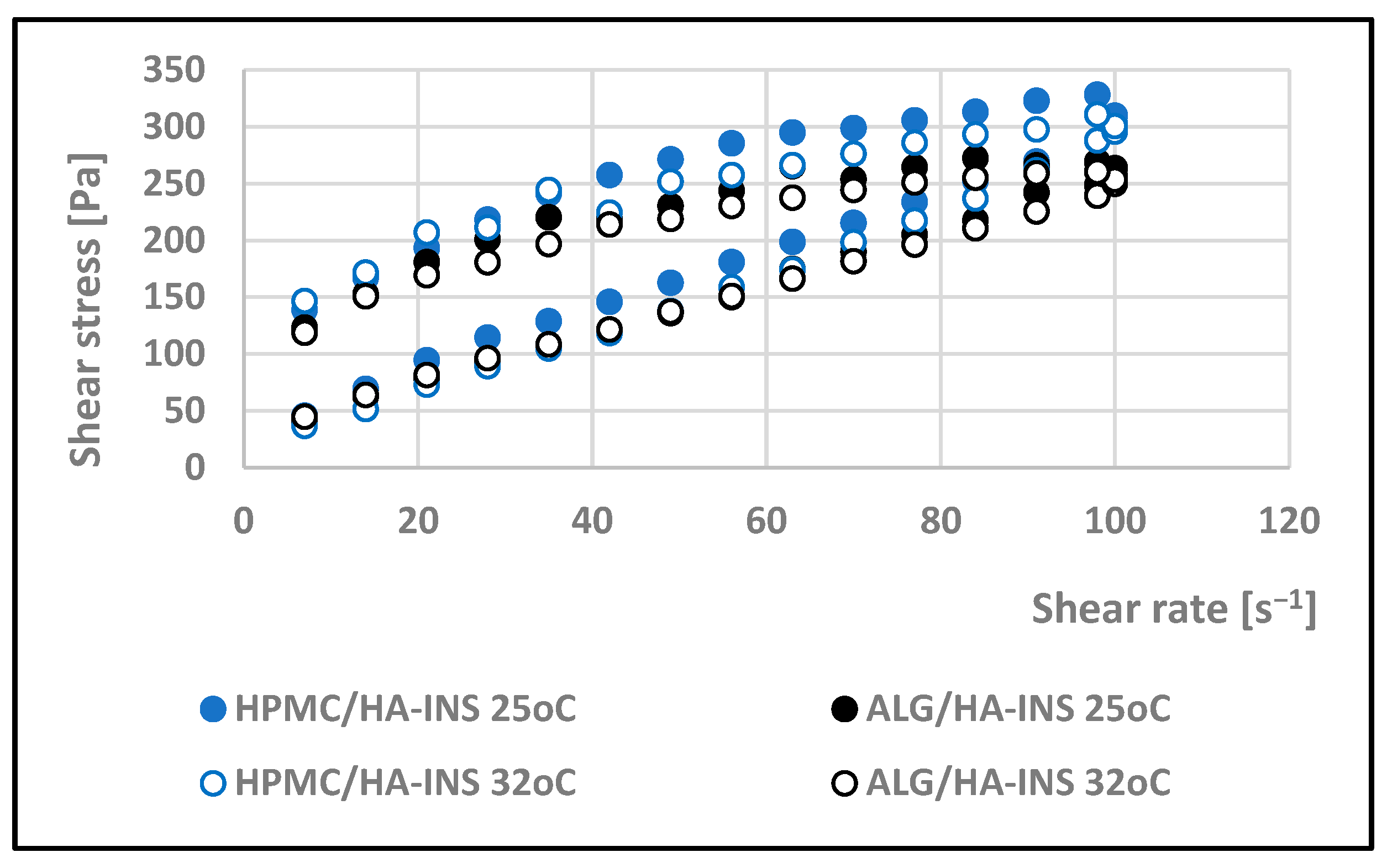
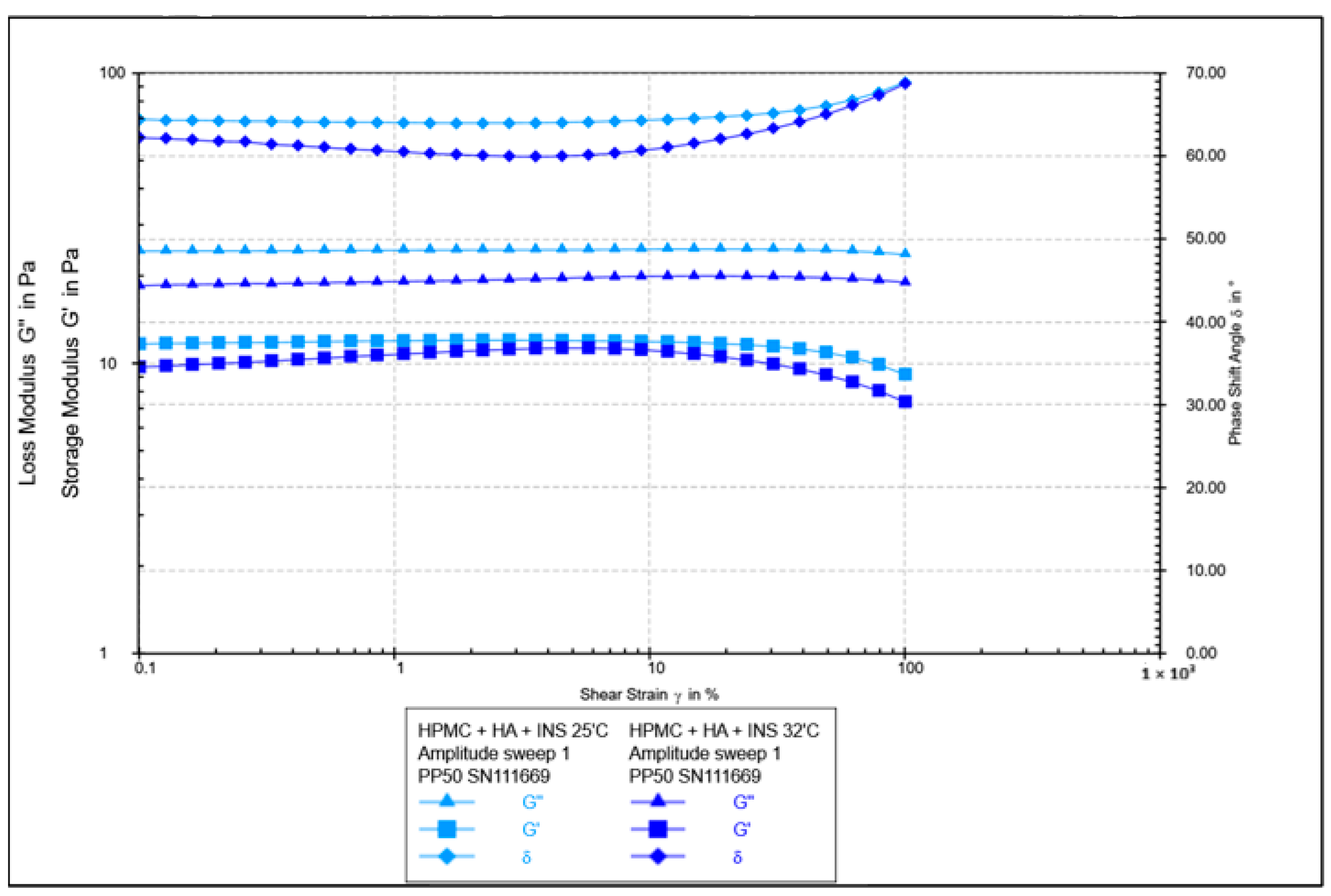
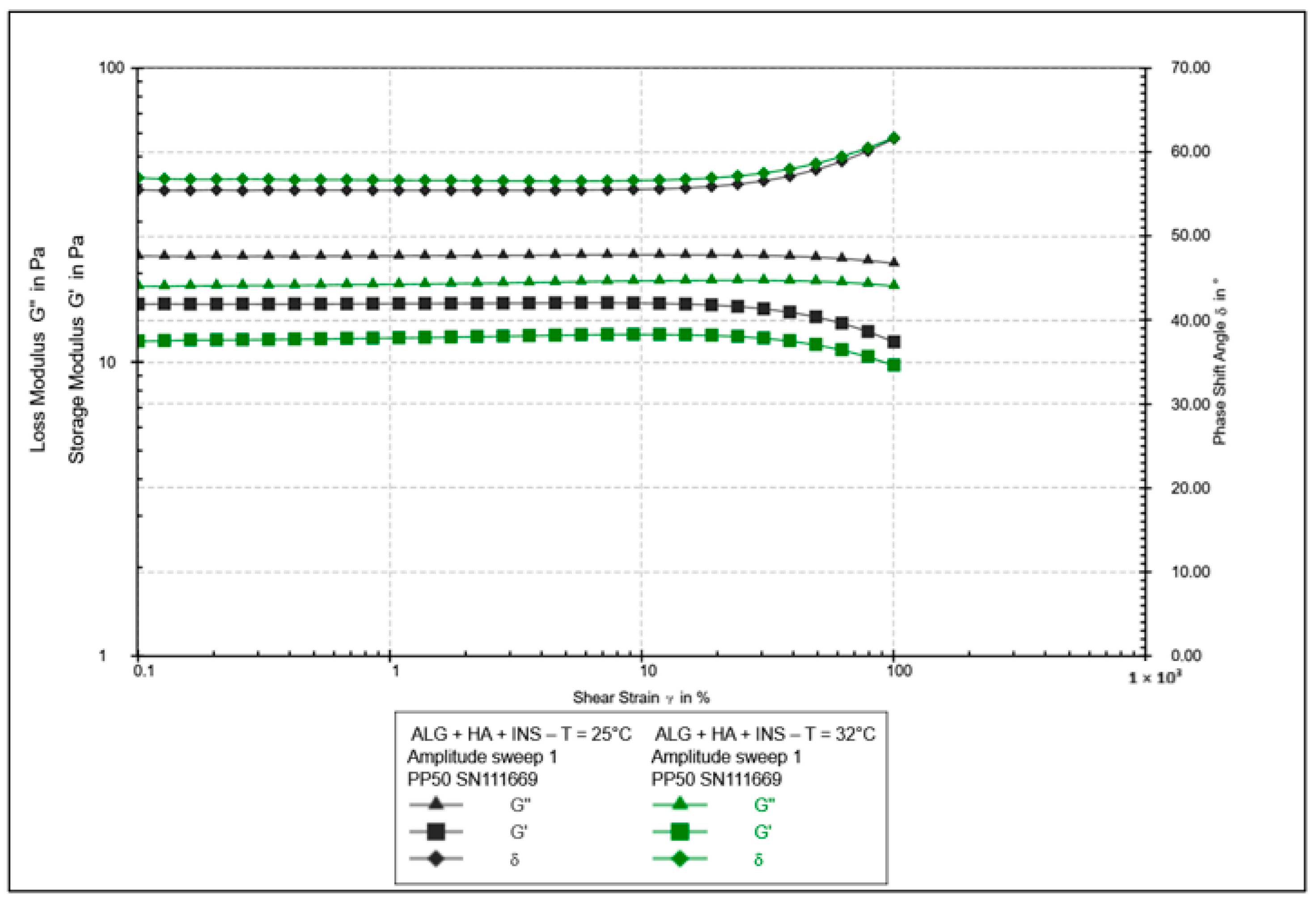
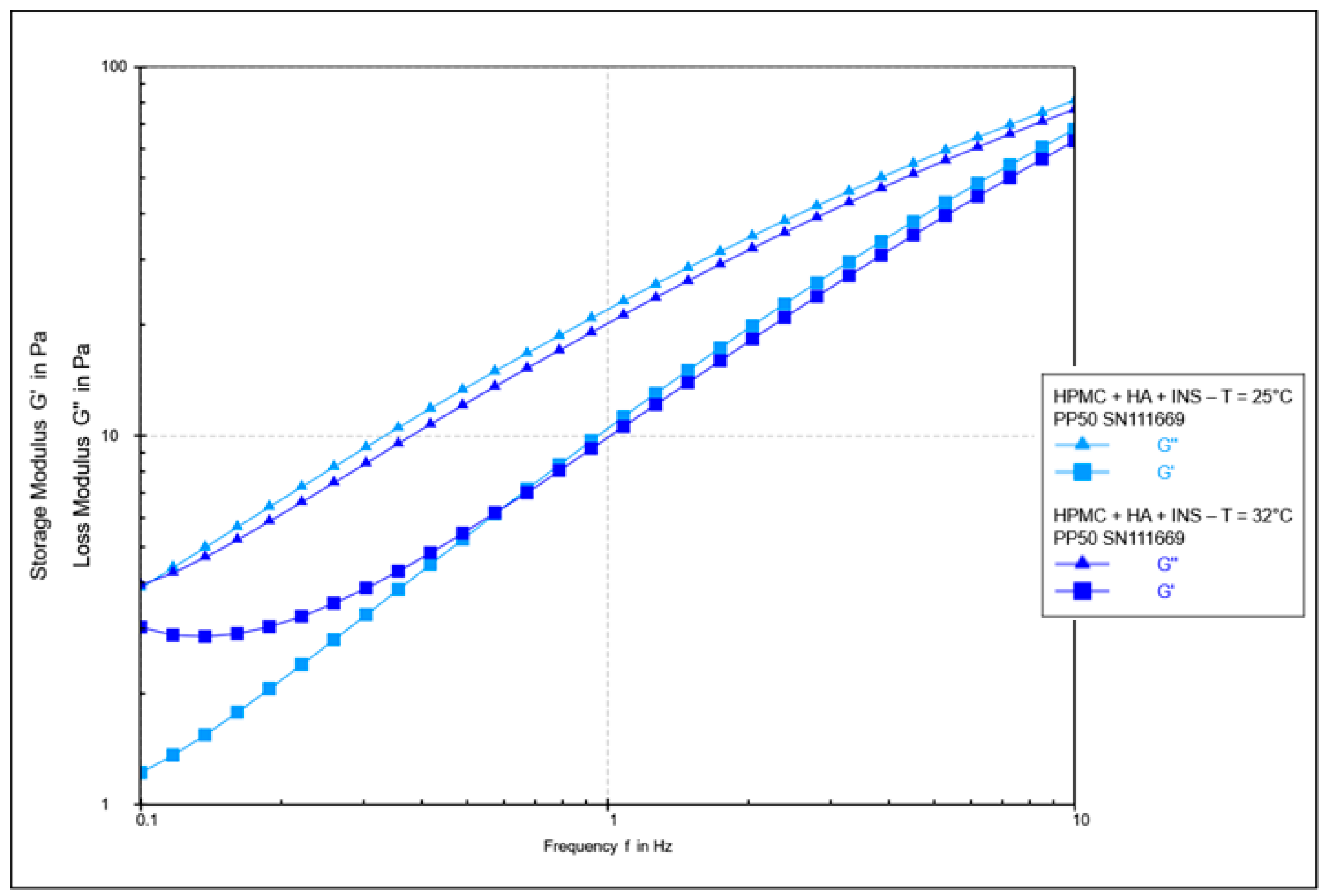
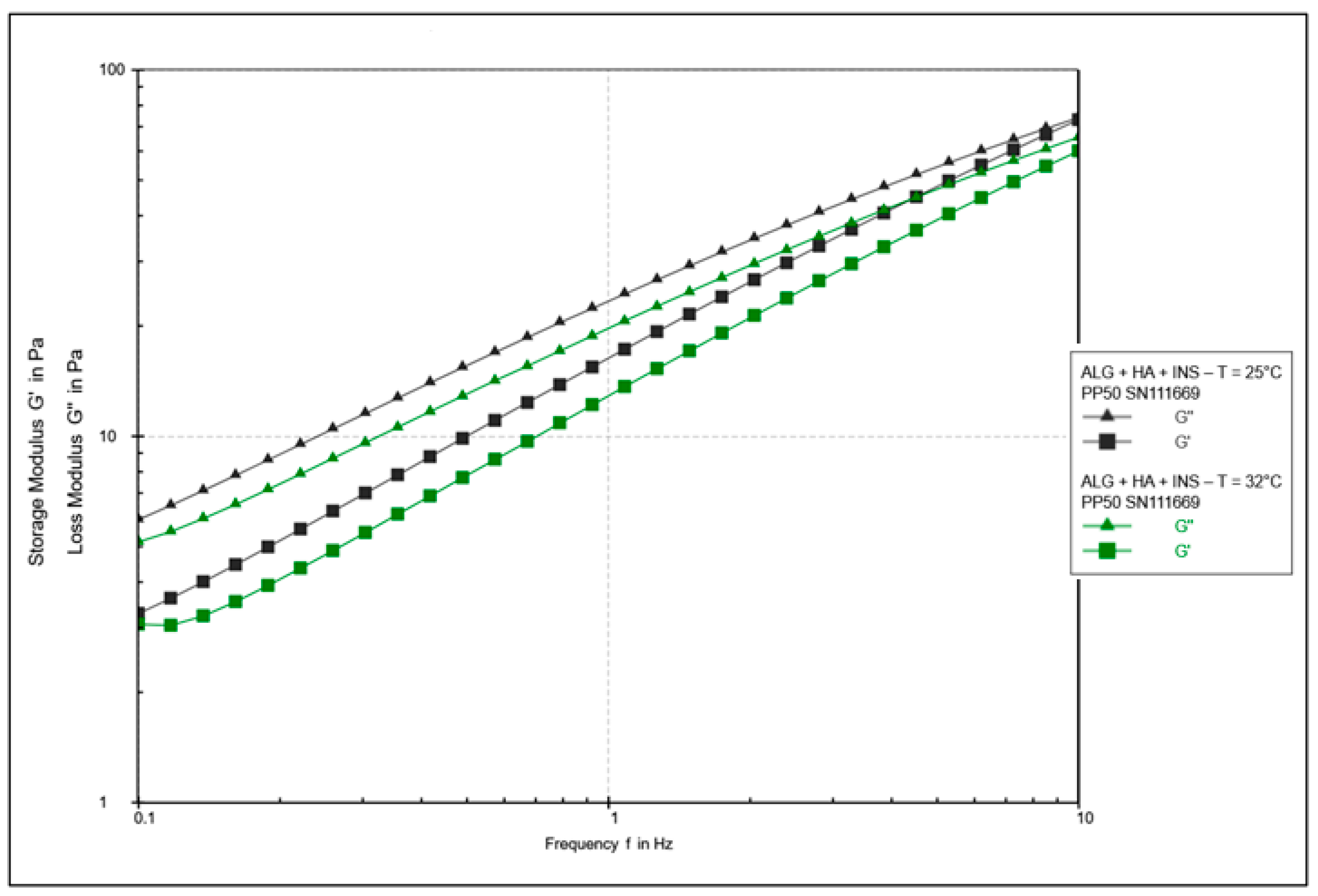
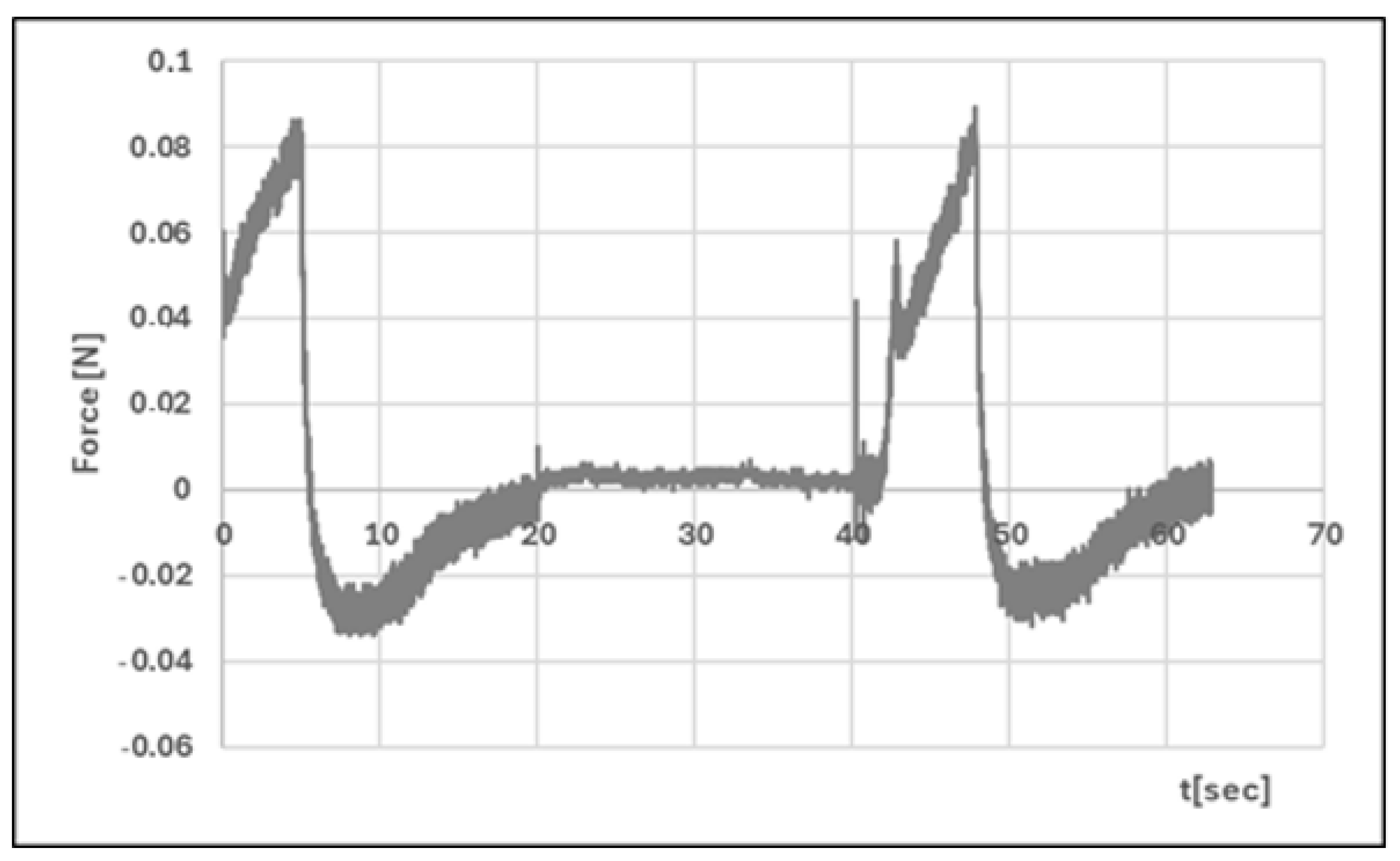
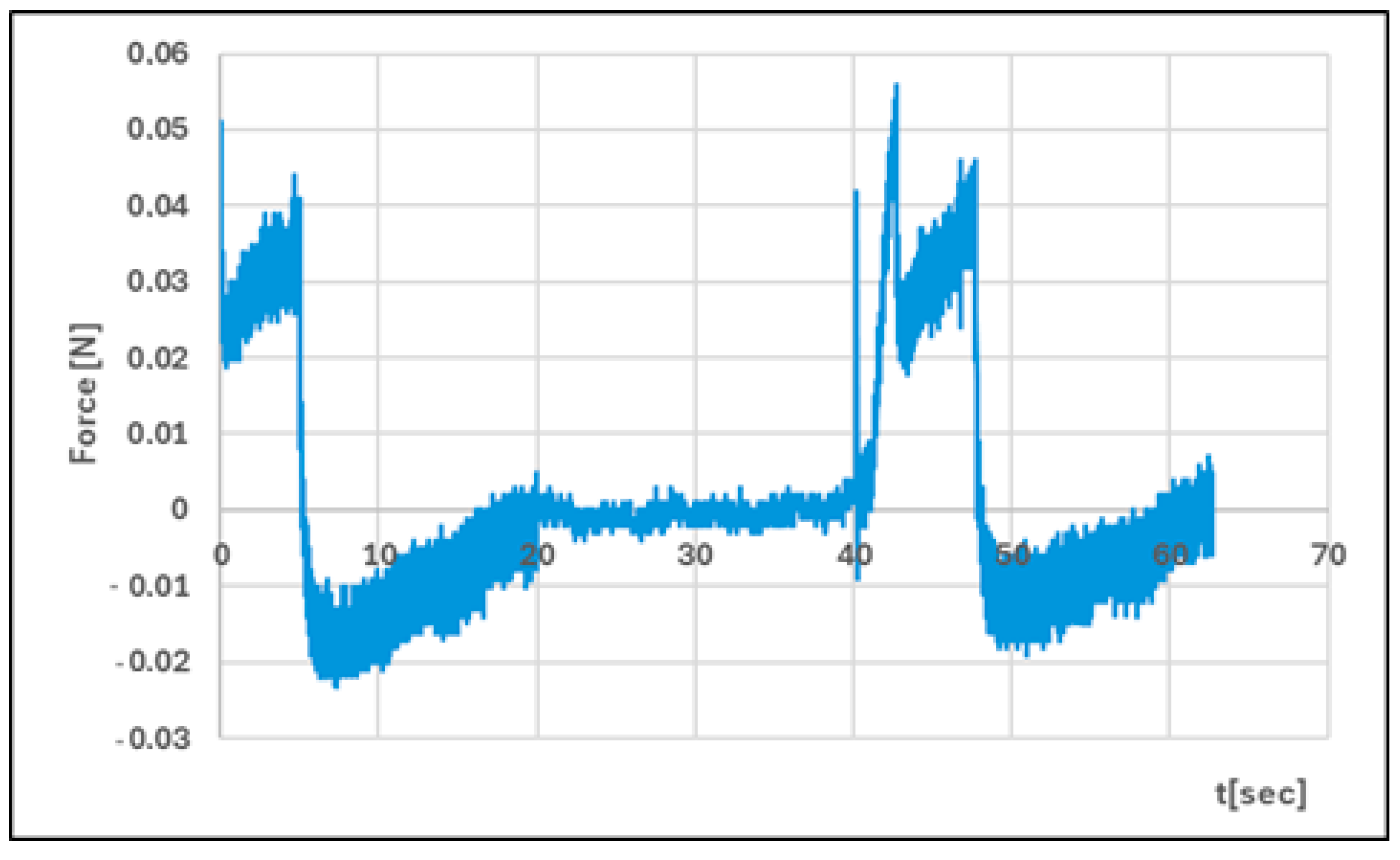
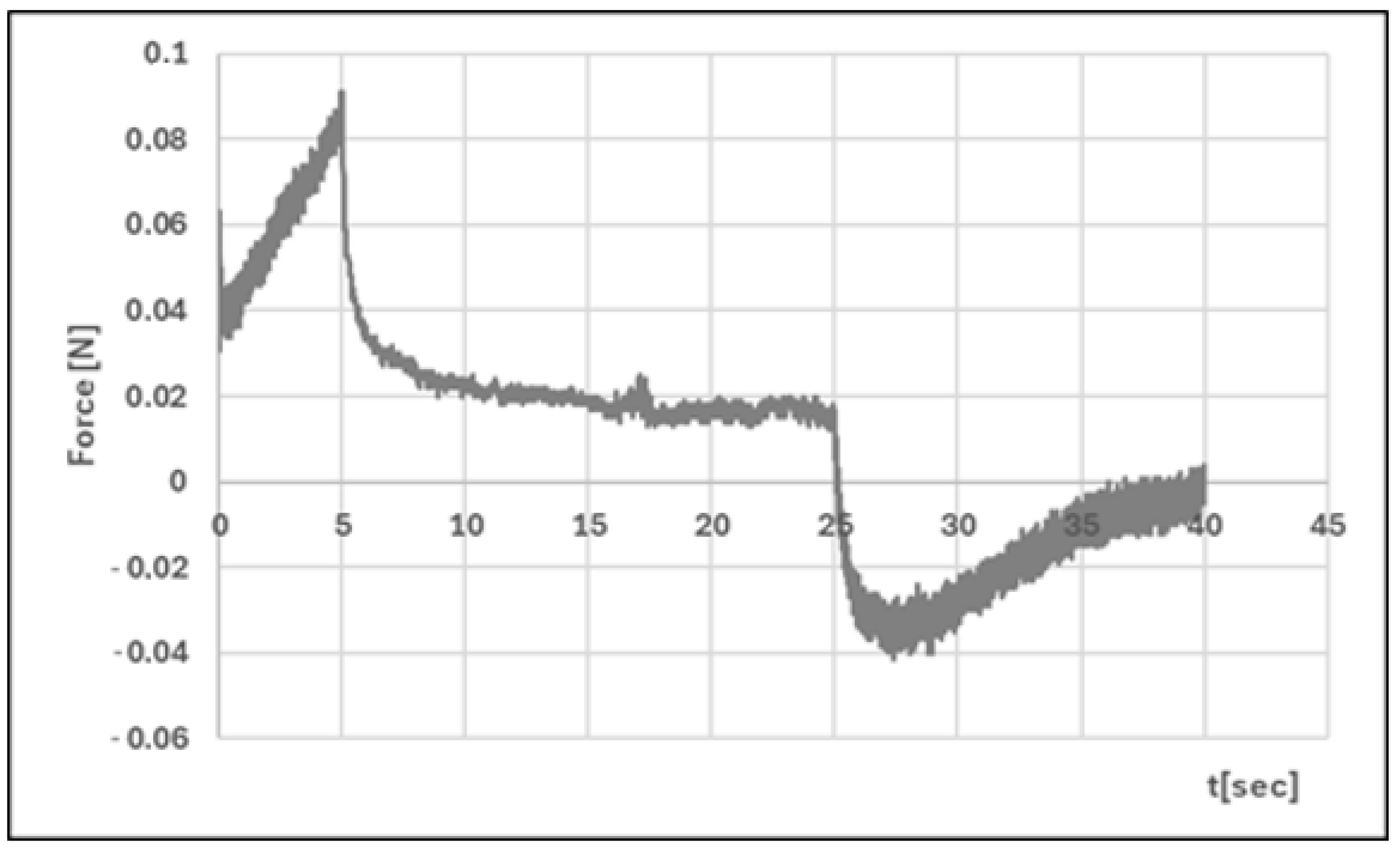
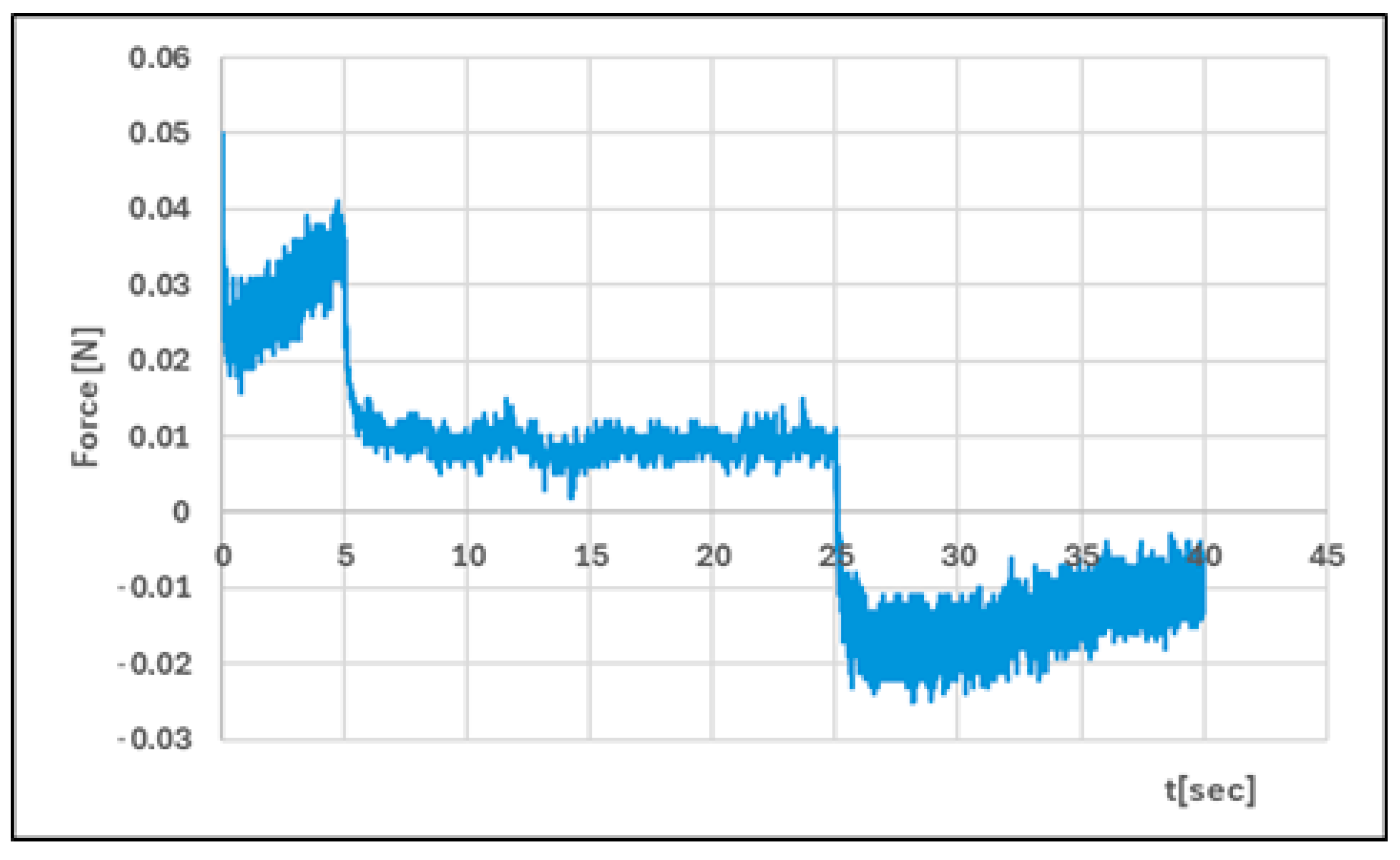
| Formula Code | Equation | Result | Interpretation |
|---|---|---|---|
| f1 HPMC/HA-INS vs. ALG/HA-INS | f1 = | 34.63 | Dissimilar |
| f2 HPMC/HA-INS vs. ALG/HA-INS | f2 = 50 log | 48.23 | Dissimilar |
| Model | Equation | Hydrogel HPMC/HA-INS (Parameters, R2 adj, AIC, MSC) | Hydrogel ALG/HA- INS (Parameters, R2 adj, AIC, MSC) |
|---|---|---|---|
| Zero-order | F = k0 t | k0 = 0.099 R2 adj = 0.8371 AIC = 139.1119 MSC = 1.5305 | k0 = 0.139 R2 adj = 0.8458 AIC = 143.5498 MSC = 1.5959 |
| First-order | F = 1−e−k1t | k1 = 0.001 R2 adj = 0.9302 AIC = 121.3200 MSC = 2.3778 | k1 = 0.002 R2 adj = 0.9592 AIC = 116.9775 MSC = 2.9245 |
| Higuchi | F= kH t0.5 | kH = 1.927 R2 adj = 0.9735 AIC = 100.9586 MSC=3.3474 | kH = 2.616 R2 adj = 0.9503 AIC = 120.9035 MSC = 2.7282 |
| Korsmeyer–Peppas | F = kKP tn | kKP = 1.181 n = 0.584 R2 adj = 0.9825 AIC = 93.2225 MSC = 3.7158 | kKP = 1.381 n = 0.611 R2 adj = 0.9644 AIC = 115.1723 MSC = 3.0148 |
| Hixson–Crowell | F = 1−(1−kHC t)3] | kHC = 0.001 R2 adj = 0.9195 AIC = 138.1944 MSC = 2.2501 | kHC = 0.001 R2 adj = 0.9330 AIC = 126.8927 MSC = 2.4288 |
| Peppas–Sahlin | F = kPS1 tm + kPS2 t2m | kPS1 = 0.308 kPS2 = −0.001 m = 0.890 R2 adj = 0.9993 AIC = 27.3617 MSC = 6.8520 | kPS1 = 0.244 kPS2 = 0.000 m = 0.998 R2 adj = 0.9967 AIC = 68.2465 MSC = 5.3611 |
| Weibull | F = 100 (1−e−(t^β)/α) | α = 133.388 β = 0.701 R2 adj = 0.9894 AIC = 82.6533 MSC = 4.2190 | α = 155.449 β = 0.801 R2 adj = 0.9801 AIC = 103.4961 MSC = 3.5986 |
| Hydrogel | Herschel–Bulkley | Ostwald–de Waele | Bingham | Casson | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| τ0 | n | K | R2 | n | K | R2 | τ0 | R2 | τ0 | R2 | |
| 25 °C | |||||||||||
| HPMC/HA-INS | 16.000 | 0.94 | 3.60 | 0.998 | 0.780 | 7.66 | 0.994 | 20.533 | 0.997 | 4.309 | 0.997 |
| ALG/HA-INS | 14.400 | 0.794 | 5.91 | 0.997 | 0.674 | 10.7 | 0.992 | 32.627 | 0.995 | 10.236 | 0.996 |
| 32 °C | |||||||||||
| HPMC/HA-INS | 28.800 | 0.822 | 6.34 | 0.997 | 0.633 | 16.1 | 0.991 | 49.837 | 0.996 | 17.353 | 0.996 |
| ALG/HA-INS | 27.00 | 0.873 | 4.06 | 0.998 | 0.639 | 12.7 | 0.988 | 37.722 | 0.997 | 12.920 | 0.997 |
| Parameter | Hydrogel HPMC/HA-INS (Mean ± SD) | Hydrogel ALG/HA-INS (Mean ± SD) | p |
|---|---|---|---|
| Relaxation [%] | 86.9 ± 0.88 | 81.8 ± 0.97 | p < 0.01 |
| Hardness 1 [N] | 0.051 ± 0.01 | 0.086 ± 0.02 | p < 0.05 |
| Hardness 2 [N] | 0.056 ± 0.01 | 0.089 ± 0.01 | p < 0.05 |
| Cohesiveness [-] | 1.088 ± 0.08 | 0.997 ± 0.20 | NS |
| Adhesiveness [mJ] | 0.2 ± 0.05 | 0.2 ± 0.10 | NS |
| Elasticity [-] | 1.016 ± 0.05 | 1.141 ± 0.11 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostróżka-Cieślik, A. Hyaluronan-Based Hydrogel Hybrid Insulin Carriers—Preformulation Studies. Polymers 2025, 17, 2661. https://doi.org/10.3390/polym17192661
Ostróżka-Cieślik A. Hyaluronan-Based Hydrogel Hybrid Insulin Carriers—Preformulation Studies. Polymers. 2025; 17(19):2661. https://doi.org/10.3390/polym17192661
Chicago/Turabian StyleOstróżka-Cieślik, Aneta. 2025. "Hyaluronan-Based Hydrogel Hybrid Insulin Carriers—Preformulation Studies" Polymers 17, no. 19: 2661. https://doi.org/10.3390/polym17192661
APA StyleOstróżka-Cieślik, A. (2025). Hyaluronan-Based Hydrogel Hybrid Insulin Carriers—Preformulation Studies. Polymers, 17(19), 2661. https://doi.org/10.3390/polym17192661






