Obtaining Poly(3-Hexylthiophene) (P3HT) by Electropolymerization as an Alternative for the Substitution of Initiators with Free Radicals
Abstract
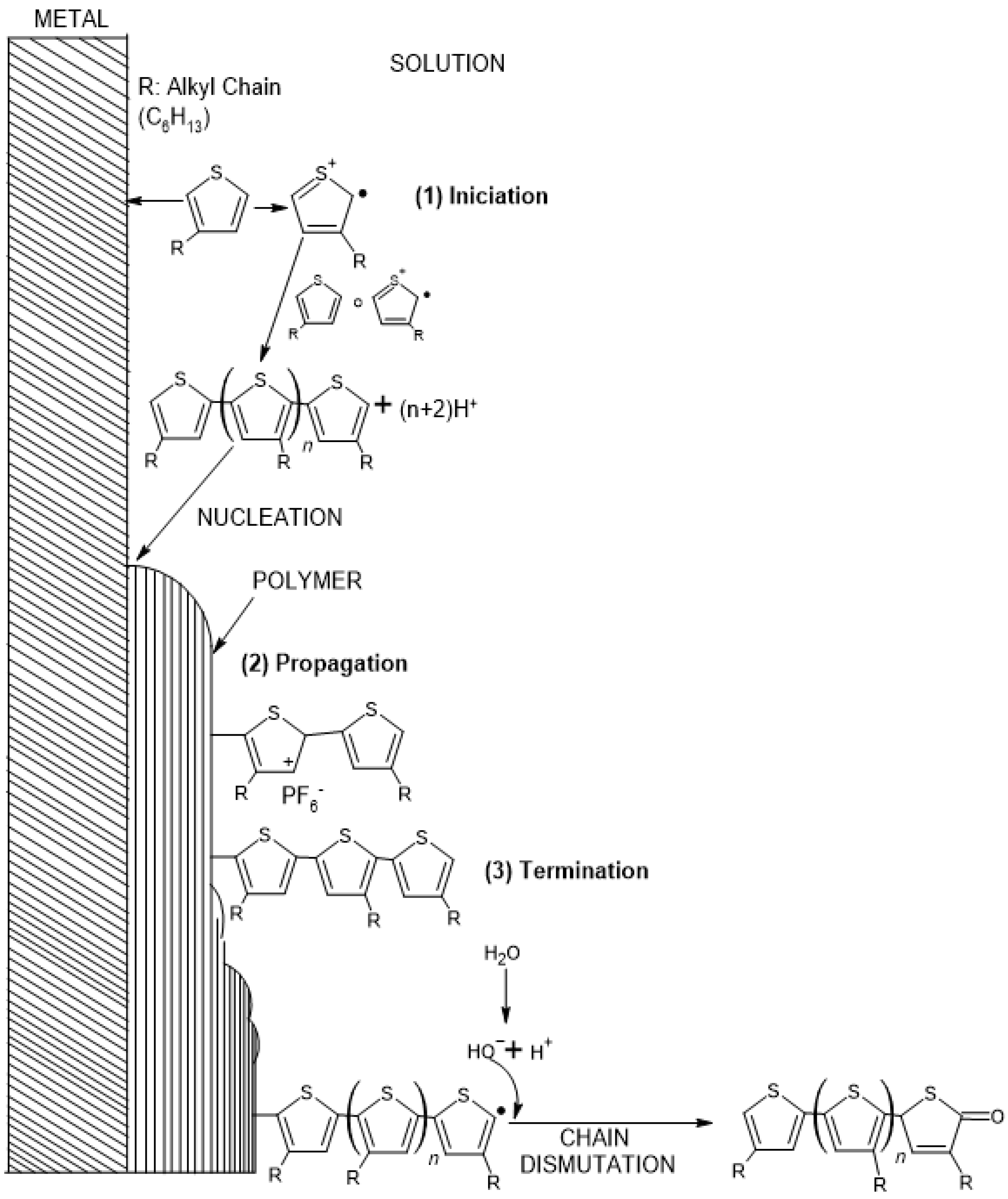
1. Introduction
2. Materials and Methods
2.1. Working Electrodes
2.2. Solvent and Monomer Solution
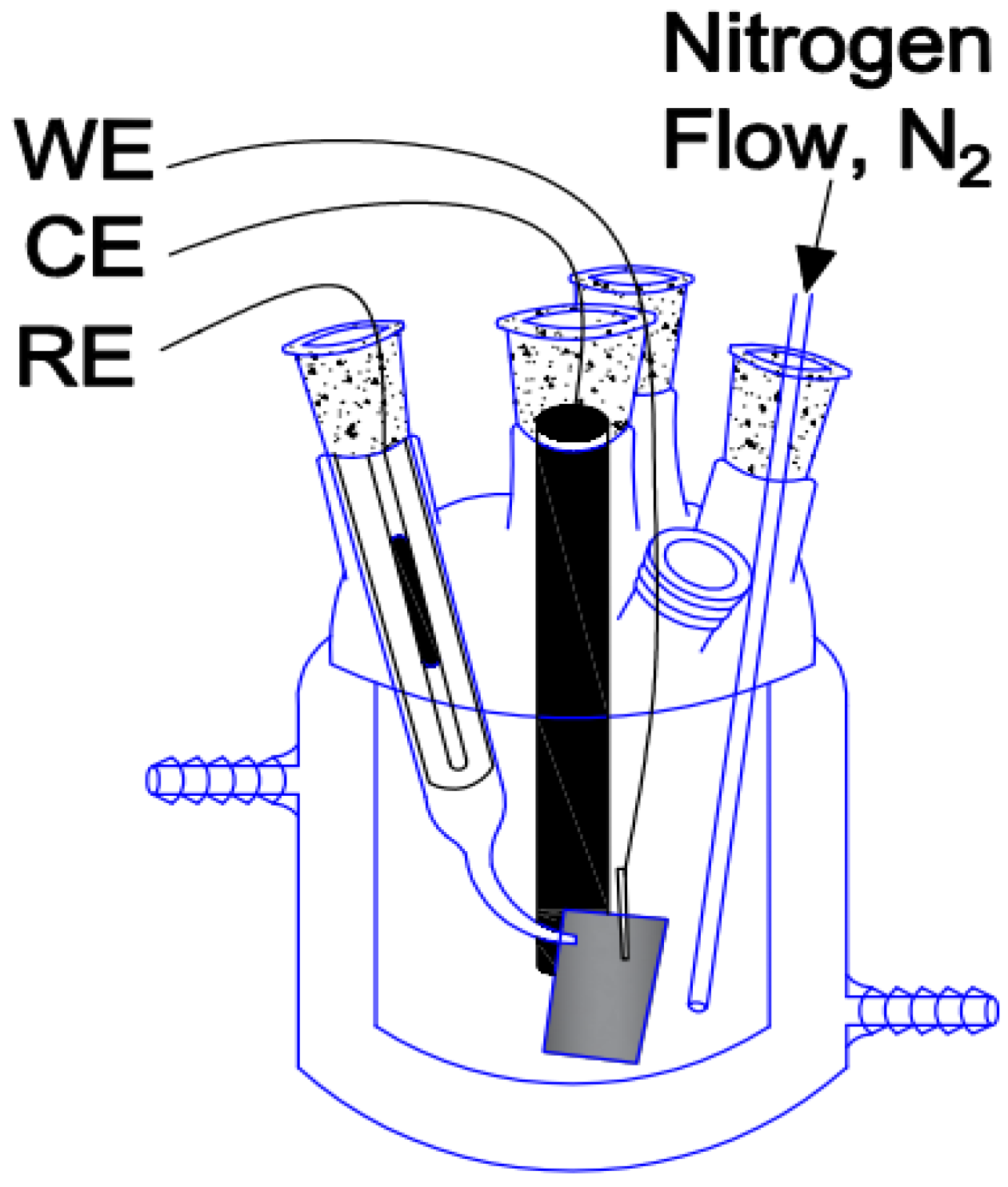
2.3. Tests Under Atmospheric Conditions and in an Inert Atmosphere
2.4. Electropolymerization
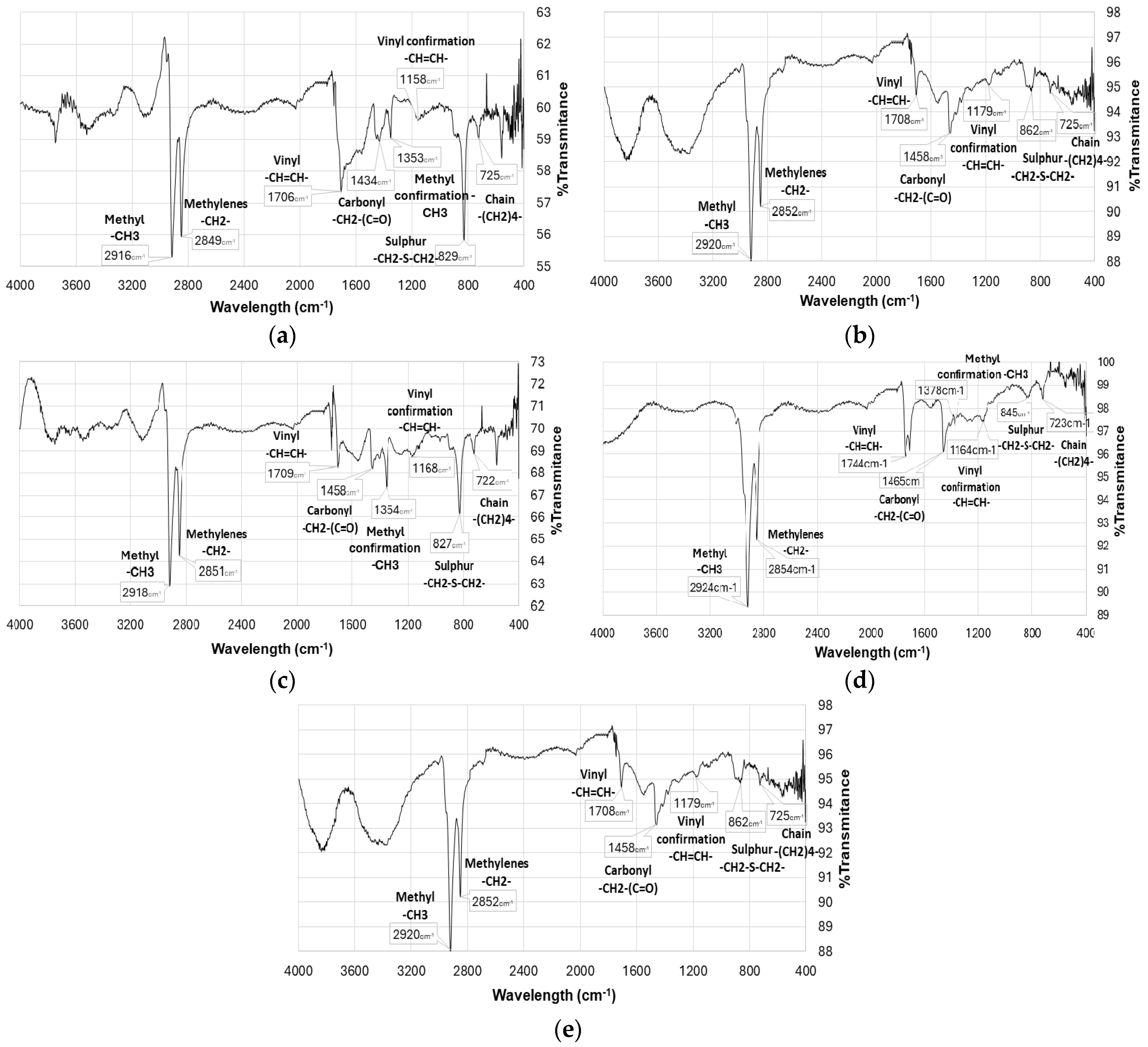
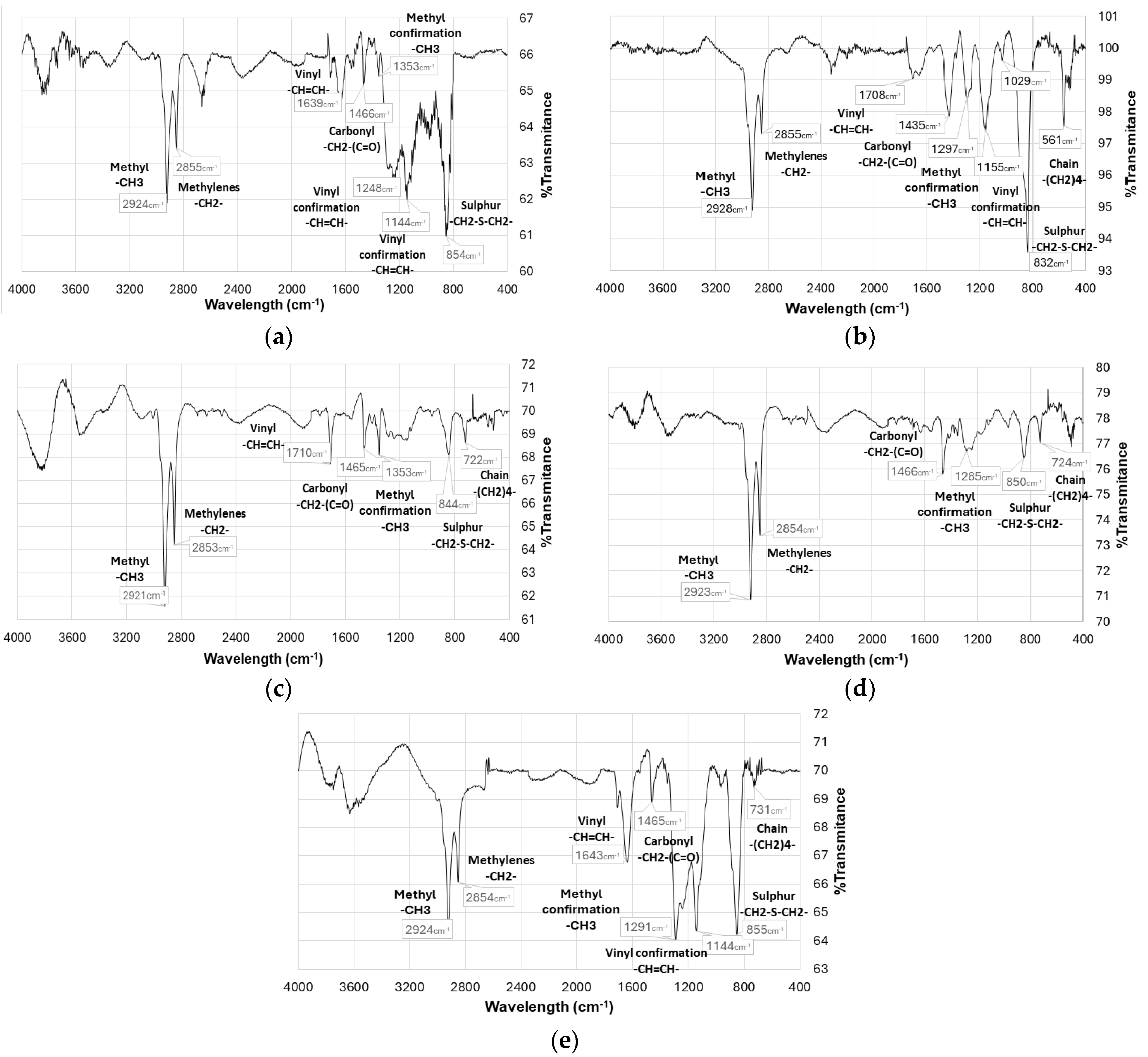
2.5. Fourier Transform Infrared Spectroscopy
3. Results
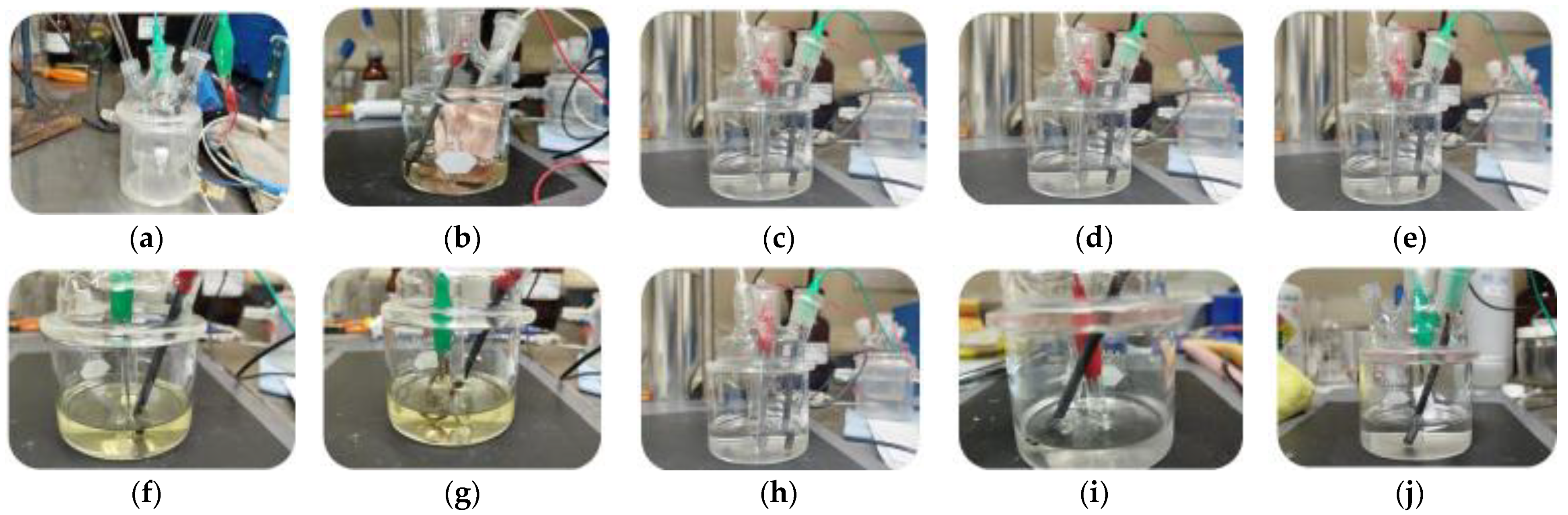
3.1. Tests Under Atmospheric Conditions
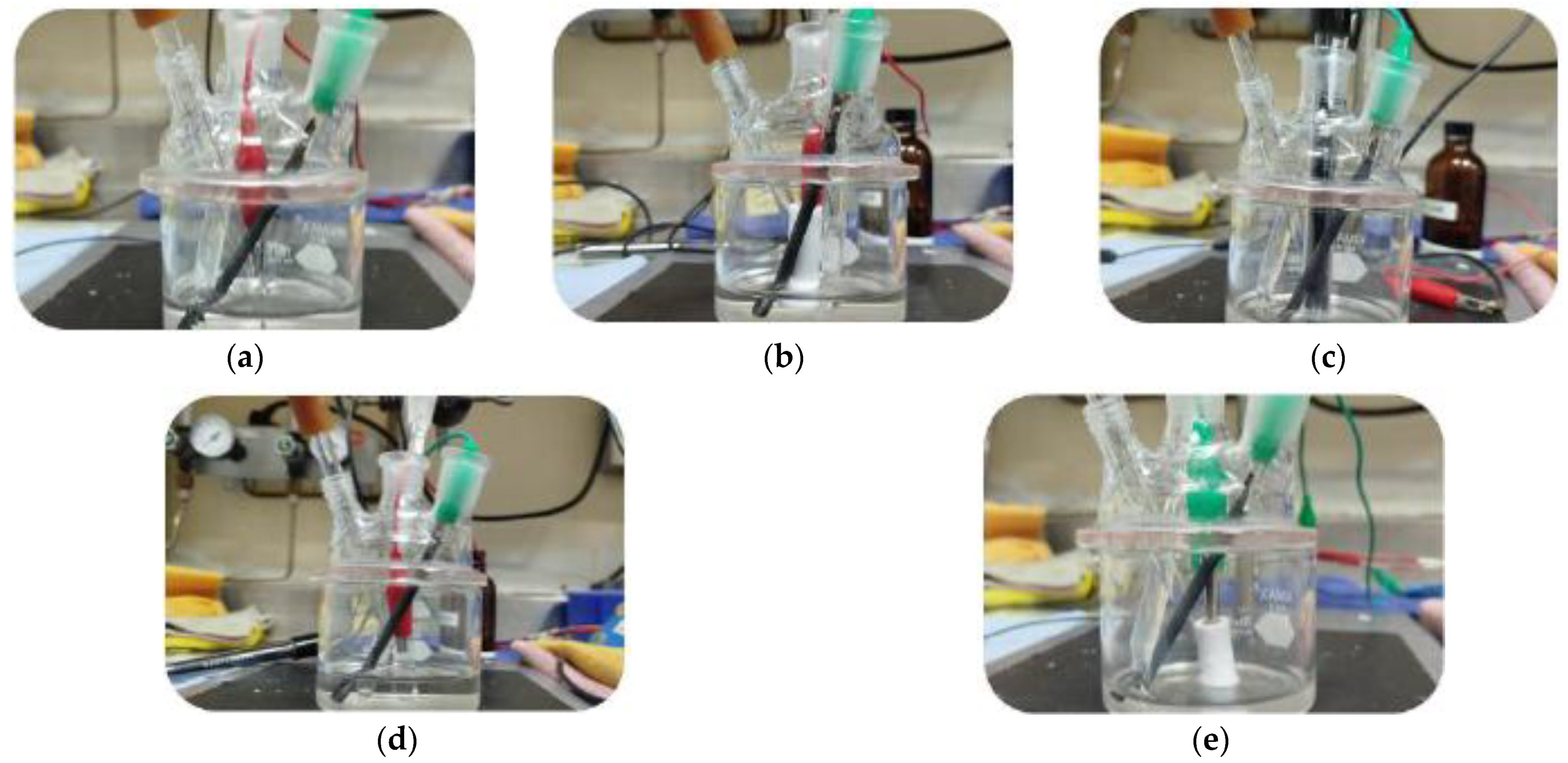
3.2. Tests in Inert Atmosphere (Controlled Conditions)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P3HT | Poly(3-hexylthiophene) |
| FT-IR | Fourier transform infrared spectroscopy |
| ICPs | intrinsically conducting organic polymers |
| PPP | poly(p-phenylene) |
| PA | polyacetylene |
| PT | polythiophene |
| PPV | poly(p-phenylene-vinylene) |
| PANI | polyaniline |
| PPy | polypyrrole |
| P3ATs | poly(3-alkylthiophene) |
| FETs | Field Effect Transmiter |
| OLEDs | Light-Emitting Diodes |
| LiPF6 | lithium hexafluorophosphate |
| FTO | fluorine-doped tin oxide |
| 3HT | 3-hexylthioene |
| AcN | acetonitrile |
| THF | Tetrahydrifuran |
| WE | working electrode |
| CE | counter electrode |
| N2 | nitrogen flow |
| ATR | Atenuated Total Reflectance |
| MIR | mid-range |
| LiPF6 | Heaflourofosfate |
References
- Cotarelo Méndez, M.D.L.Á. Síntesis de Polímeros Conductores Obtenidos a Partir de Dímeros de Anilina; Universidad de Alicante: San Vicente del Raspeig, Spain, 2008. [Google Scholar]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M.J.P. Polymerization reactions and modifications of polymers by ionizing radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Otero, T.F.; España, C.J.P. Polímeros conductores: Síntesis, propiedades y aplicaciones electro-químicas. Polimeros 2015, 4. [Google Scholar]
- Salido, F.P.; Fernández, J.J.R. Influencia de los radicales libres en el envejecimiento celular. OFFARM 2002, 21, 96–100. [Google Scholar]
- Das, I.; NAMITA, R.A.; Ansari, S.A.; GUPTA, S.K. Pattern formation and oscillatory electropolymerization of thiophene. Indian J. Chem. 2008, 47, 1798–1803. [Google Scholar]
- Ch, L.B.; Alvarez, L.X. Polímeros conductores: Aplicaciones en celdas fotovoltaicas y dispositivos electrónicos. Rev. Cienc. Tecnol. 2018, 34. [Google Scholar]
- Östergård, T.; Paloheimo, J.; Pal, A.; Stubb, H. Langmuir—Blodgett light-emitting diodes of poly (3-hexylthiophene): Electro-optical characteristics related to structure. Synth. Met. 1997, 88, 171–177. [Google Scholar] [CrossRef]
- Serna, V.M. Nanocompósitos Obtenidos de la síntesis In-Situ de Quantum Dots de CdSe en Películas Compuestas de Quitosano y Polímeros Conductores; Pontificia Universidad Catolica de Chile: Santiago, Chile, 2020. [Google Scholar]
- Kesornsit, S.; Direksilp, C.; Phasuksom, K.; Thummarungsan, N.; Sakunpongpitiporn, P.; Rotjanasuworapong, K.; Sirivat, A.; Niamlang, S.J.P. Synthesis of highly conductive poly (3-hexylthiophene) by chemical oxidative polymerization using surfactant templates. Polymers 2022, 14, 3860. [Google Scholar] [CrossRef]
- Ramoroka, M.E.; Tesfay, H.H.; Ekwere, P.; Mokwebo, K.V.; John-Denk, V.S.; Modibane, K.D.; Douman, S.F.; Iwuoha, E.I. Electro-photovoltaics of grignard metathesis-derived poly (propylene imine) tetra (salicylaldimine)-co-poly (3-hexylthiophene-2, 5-diyl) copolymer. Mater. Chem. Phys. 2025, 336, 130549. [Google Scholar] [CrossRef]
- Fournier, P.É. Radicales libres y Toxicología. In Anales de Medicina y Cirugía; Reial Academia de Medicina de Catalunya: Barcelona, Spain, 1973; pp. 195–214. [Google Scholar]
- Wang, M.; Baek, P.; Akbarinejad, A.; Barker, D.; Travas-Sejdic, J. Conjugated polymers and composites for stretchable organic electronics. J. Mater. Chem. C 2019, 7, 5534–5552. [Google Scholar] [CrossRef]
- Sun, C. Optical and Morphology Properties of Supramolecularly Controlled Conjugated Polymers; Universidad Complutense de Madrid: Madrid, Spain, 2020. [Google Scholar]
- Yoshioka, N.A.; Faraco, T.A.; Barud, H.S.; Ribeiro, S.J.; Cremona, M.; Fragneaud, B.; Maciel, I.O.; Quirino, W.G.; Legnani, C.J.P. Synthesis of Organic Semiconductor Nanoparticles with Different Conformations Using the Nanoprecipitation Method. Polymers 2022, 14, 5336. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, Y.; Zhang, R.; Wang, H.; Sun, C.; Wang, T.J.S. Recent progress in gas sensors based on P3HT polymer field-effect transistors. Sensors 2023, 23, 8309. [Google Scholar] [CrossRef]
- Flores Argáez, B.A. Desarrollo y análisis de películas compuestas de poli-3 hexiltiofeno y grafenos para su uso en celdas solares. In Tesis de Ingeniería en Sistemas de Energía; Universisdad de Quintana Roo: Chetumal, Mexico, 2013. [Google Scholar]
- Machui, F.; Langner, S.; Zhu, X.; Abbott, S.; Brabec, C.J. Determination of the P3HT: PCBM solubility parameters via a binary solvent gradient method: Impact of solubility on the photovoltaic performance. Sol. Energy Mater. 2012, 100, 138–146. [Google Scholar] [CrossRef]
- Hajduk, B. Nanostructure-Dependent Electrical Conductivity Model Within the Framework of the Generalized Effective Medium Theory Applied to Poly (3-hexyl) thiophene Thin Films. Polymers 2024, 16, 3227. [Google Scholar]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Zeng, W.; Song, G.; Li, Y.; Yuan, C.; Fan, T.; Tang, W.; Wang, J.; Zhao, C.; Lai, W.; Zhang, H. Enhanced performance of poly (3-hexylthiophene-2, 5-diyl):[6, 6]-phenyl-C61-butyric acid methyl ester solar cells by UV irradiation. Thin Solid Film. 2016, 600, 136–141. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT: PCBM, best seller in polymer photovoltaic research. Adv. Mater. 2011, 23, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Dadmun, M.D.J.P. Important thermodynamic characteristics of poly (3-hexyl thiophene). Polymer 2014, 55, 4–7. [Google Scholar] [CrossRef]
- Søndergaard, R.; Hösel, M.; Angmo, D.; Larsen-Olsen, T.T.; Krebs, F.C. Roll-to-roll fabrication of polymer solar cells. Mater. Today 2012, 15, 36–49. [Google Scholar] [CrossRef]
- Pantawane, S.; Gekle, S.J.P. Temperature-dependent conformation behavior of isolated poly (3-hexylthiopene) chains. Polymers 2022, 14, 550. [Google Scholar] [CrossRef]
- Rojas Fuentes, V.J. Electropolimerización de Alquil y Alquiloxi derivados de tiofeno vinileno en líquidos iónicos y su potencial uso en celdas solares. 2016. Available online: https://repositorio.uchile.cl/handle/2250/139877 (accessed on 25 September 2025).
- Ludwigs, S. P3HT Revisited-from Molecular Scale to Solar Cell Devices; Springer: Berlin/Heidelberg, Germany, 2014; Volume 265. [Google Scholar]
- Tanaka, K.; Shichiri, T.; Wang, S.; Yamabe, T. A study of the electropolymerization of thiophene. Synth. Met. 1988, 24, 203–215. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Tkach, V.; Nechyporuk, V.; Yagodynets, P. Descripción matemática de la síntesis electroquímica de polímeros conductores en la presencia de surfactantes. Av. Química 2013, 8, 9–15. [Google Scholar]
- Wu, G.; Dong, X.; Cui, G.; Sun, R.; Wu, X.; Gu, M.; Zuo, Z.; Liu, Y. A facile strategy for high performance air-processed perovskite solar cells with dopant-free poly (3-hexylthiophene) hole transporter. Sol. Energy 2022, 237, 153–160. [Google Scholar] [CrossRef]
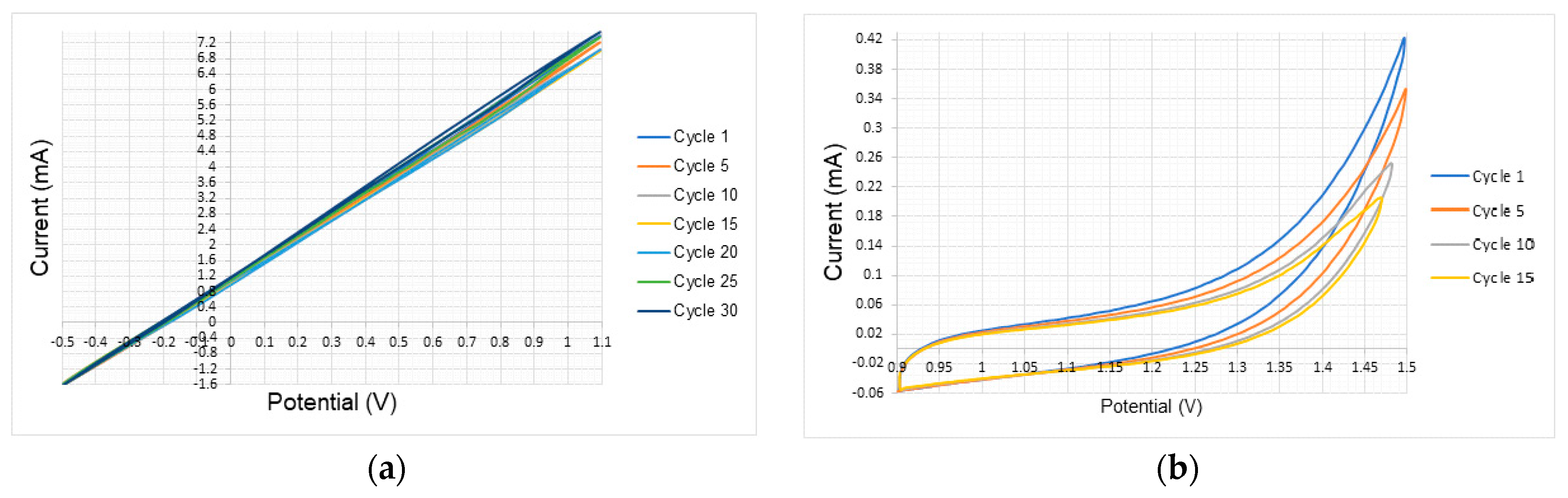
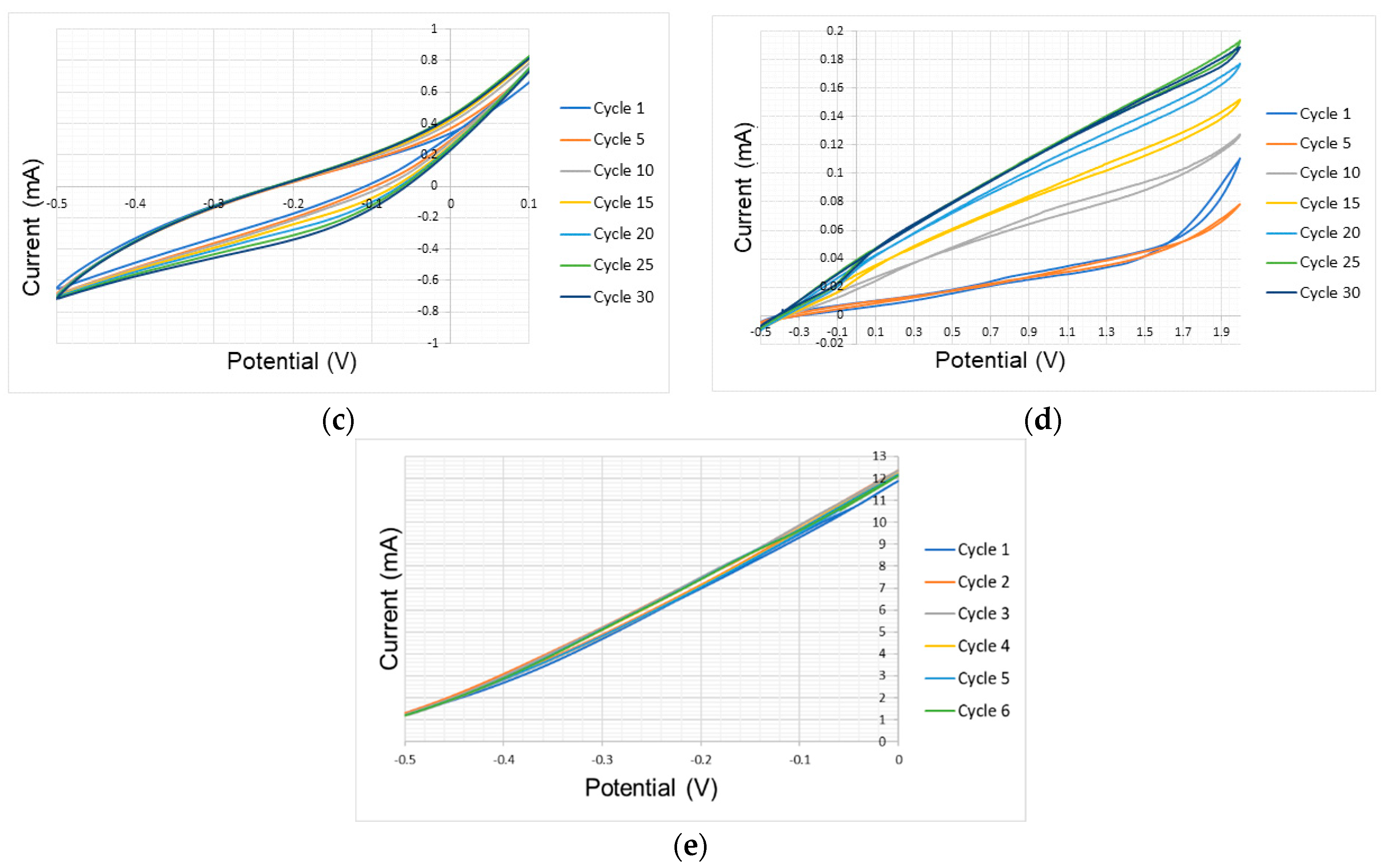
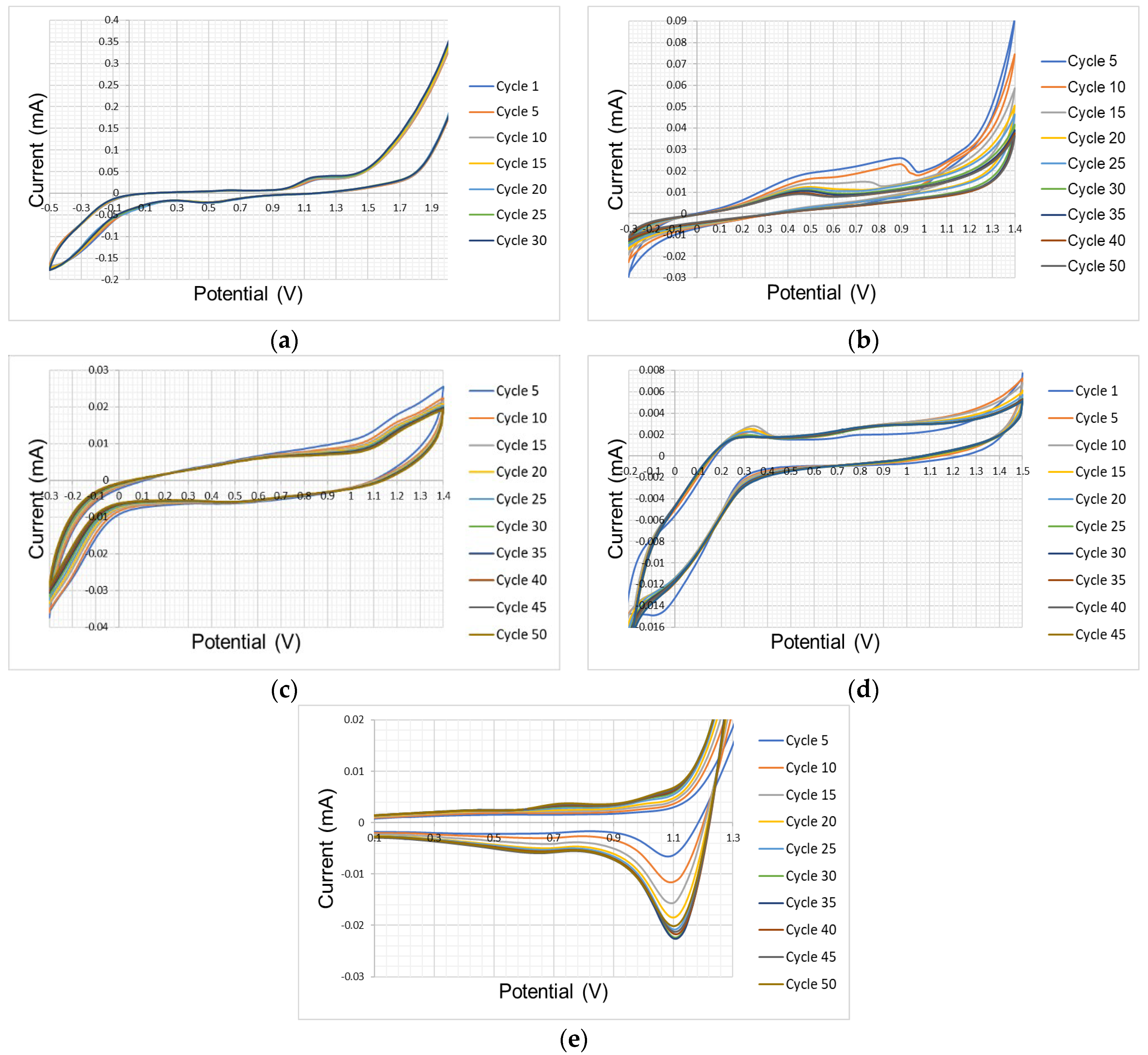

| Atmospheric Conditions Test Results | ||||
|---|---|---|---|---|
| Electrode | Potential Range (V) | Oxidation Peaks (V) | Current Range (mA) | Polymer Growth |
| Copper | −0.5–1.1 | - | −1.6–7.4 | - |
| Zinc | −0.5–0 | - | 1–12 | |
| Tin | −0.5–0.1 | - | −0.7–0.8 | |
| 316 Stainless Steel | 0.9–1.5 | - | −0.06–0.38 | |
| Aluminum | −0.5–2 | - | −0.02–0.2 | |
| Nickel | −0.3–1.4 | 0.44 | −0.03–0.09 | ✔ |
| Glassy Carbon | −0.3–1.4 | 0.47 and 0.7 | −0.04–0.02 | |
| Gold | 0–1.3 | 0.74 and 1.12 | −0.023–0.02 | |
| Platinum | −0.5–2 | 0.5 and 1.2 | −0.17–0.3 | |
| FTO | −0.2–1.5 | 0.33 | −0.02–0.008 | |
| Test results in inert atmosphere | ||||
| Electrode | Potential Range (V) | Oxidation Peaks (V) | Current Range (mA) | Polymer growth |
| Nickel | −0.3–1.4 | 0.5 | −0.01–0.025 | ✔ |
| FTO | −0.3–1.4 | 0.35 | −0.005–0.006 | |
| Platinum | −0.3–1.4 | 0.5 | −0.012–0.018 | |
| Glassy Carbon | −0.3–1.4 | 0.55 and 0.8 | −0.005–0.005 | |
| Gold | −0.3–1.3 | 0.3 and 0.45 | −0.005–0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landa Valdivia, C.U.; Cabrera Sierra, R.; Guzmán Castañeda, J.I.; Lozano Rojas, K.J.; Barraza Madrigal, J.A. Obtaining Poly(3-Hexylthiophene) (P3HT) by Electropolymerization as an Alternative for the Substitution of Initiators with Free Radicals. Polymers 2025, 17, 2656. https://doi.org/10.3390/polym17192656
Landa Valdivia CU, Cabrera Sierra R, Guzmán Castañeda JI, Lozano Rojas KJ, Barraza Madrigal JA. Obtaining Poly(3-Hexylthiophene) (P3HT) by Electropolymerization as an Alternative for the Substitution of Initiators with Free Radicals. Polymers. 2025; 17(19):2656. https://doi.org/10.3390/polym17192656
Chicago/Turabian StyleLanda Valdivia, Christopher Uriel, Román Cabrera Sierra, Jesús Israel Guzmán Castañeda, Karla Jenny Lozano Rojas, and José Antonio Barraza Madrigal. 2025. "Obtaining Poly(3-Hexylthiophene) (P3HT) by Electropolymerization as an Alternative for the Substitution of Initiators with Free Radicals" Polymers 17, no. 19: 2656. https://doi.org/10.3390/polym17192656
APA StyleLanda Valdivia, C. U., Cabrera Sierra, R., Guzmán Castañeda, J. I., Lozano Rojas, K. J., & Barraza Madrigal, J. A. (2025). Obtaining Poly(3-Hexylthiophene) (P3HT) by Electropolymerization as an Alternative for the Substitution of Initiators with Free Radicals. Polymers, 17(19), 2656. https://doi.org/10.3390/polym17192656






