Study on the Effect of the Nucleophilicity of Amine Accelerators on the Process and Dielectric Properties of Epoxy Materials for Dry Bushing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Gel Time
2.2.3. Crosslinking Density
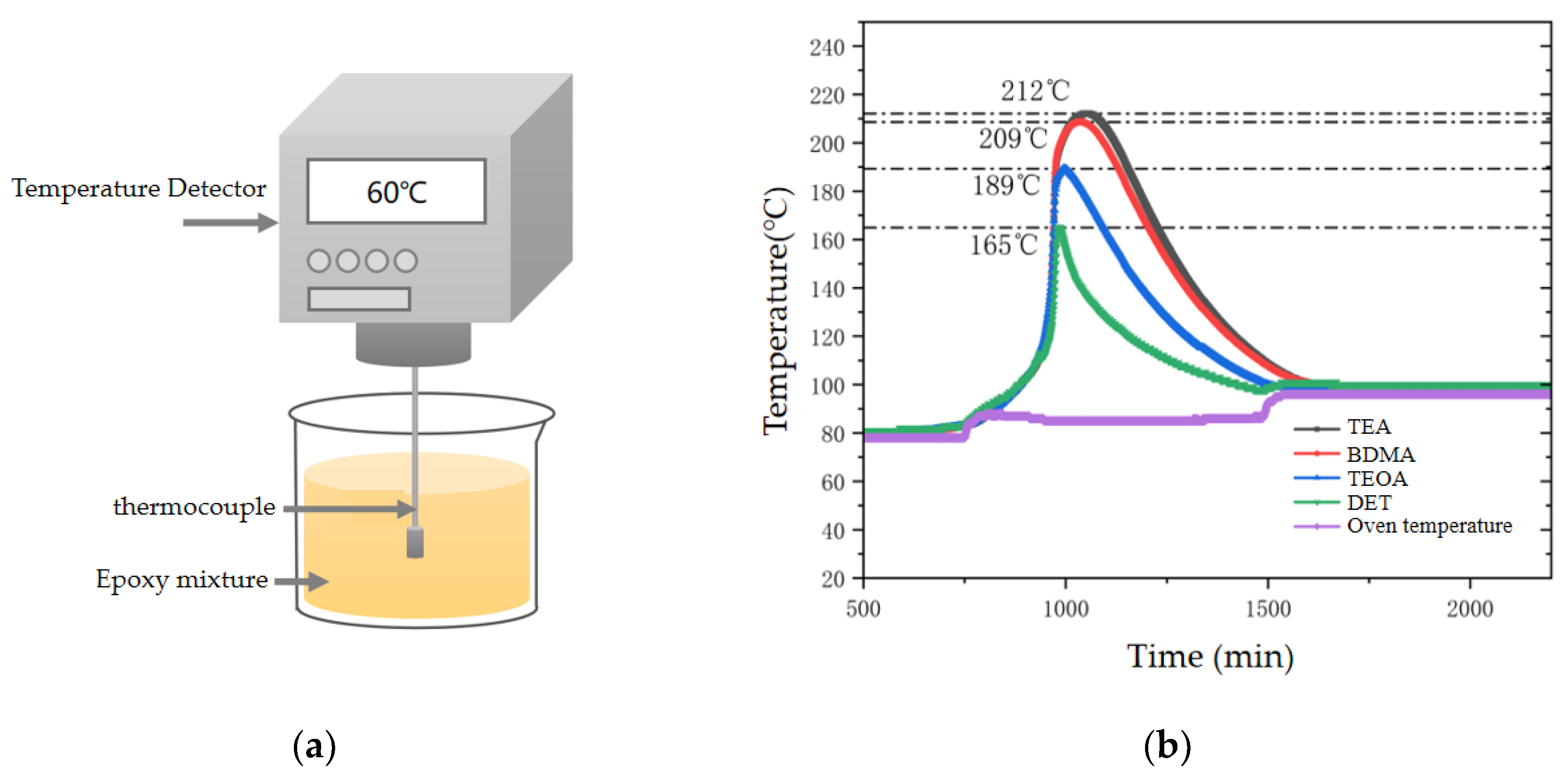
2.2.4. Curing Exothermic Temperature Rise
2.2.5. Dielectric Constant and Dielectric Loss
2.2.6. Breakdown Strength
2.2.7. Molecular Dynamics Simulation
3. Results and Discussion
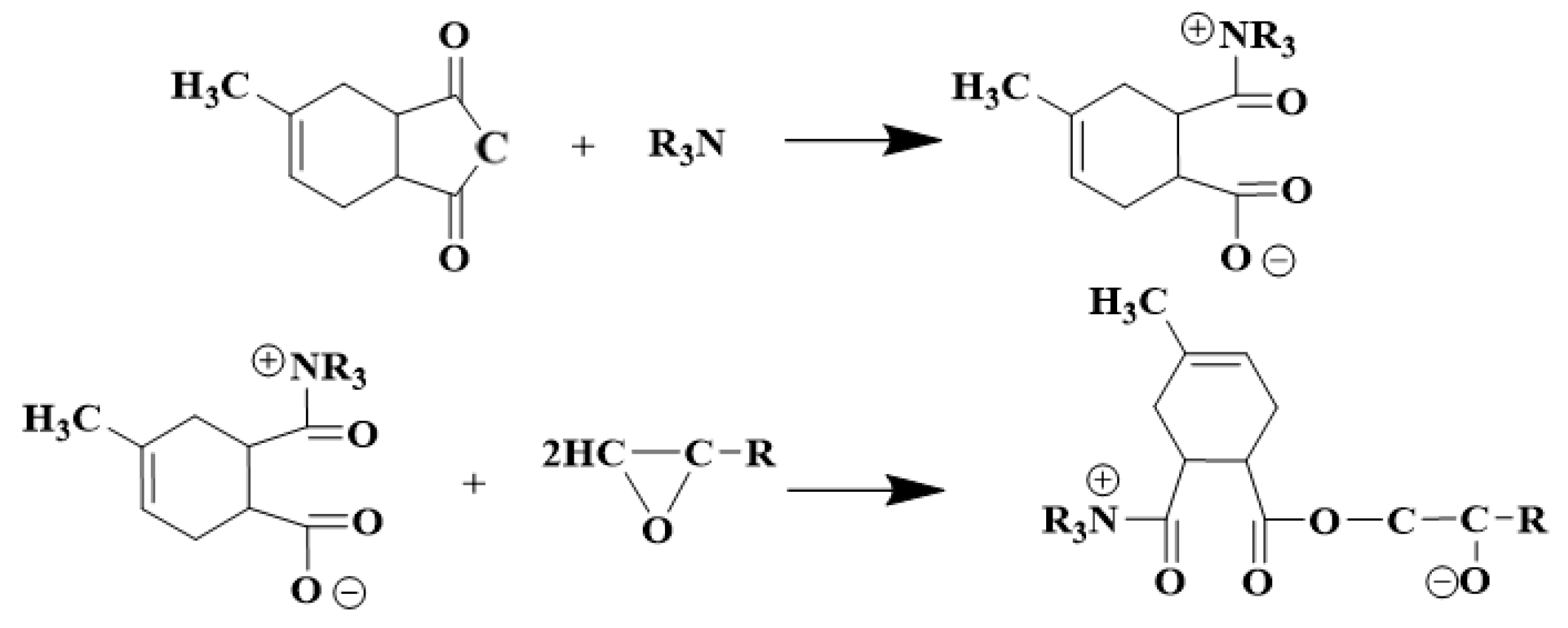
3.1. Influence of Nucleophilicity of Accelerators on the Crosslinking Reaction of Epoxy Materials
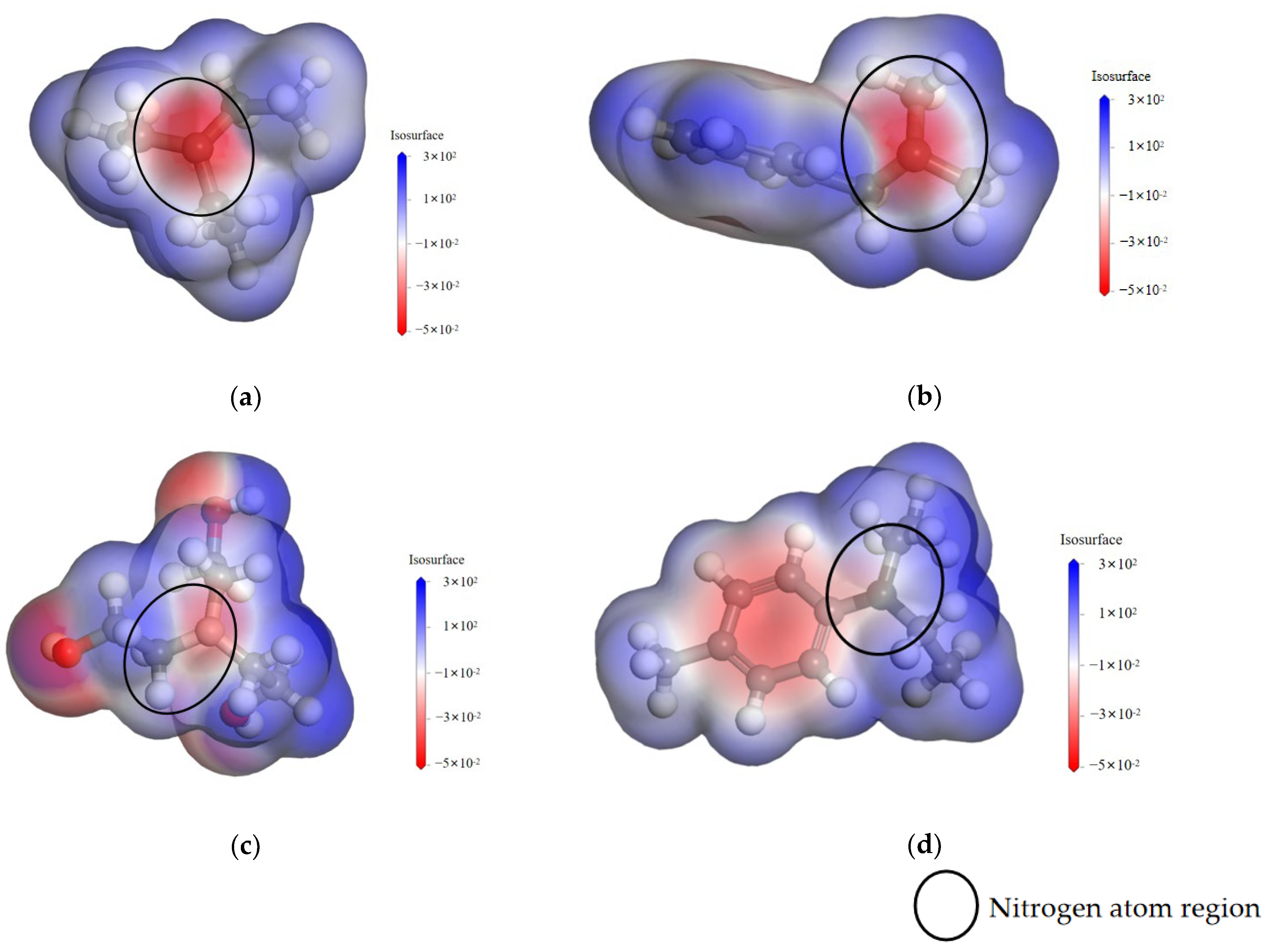
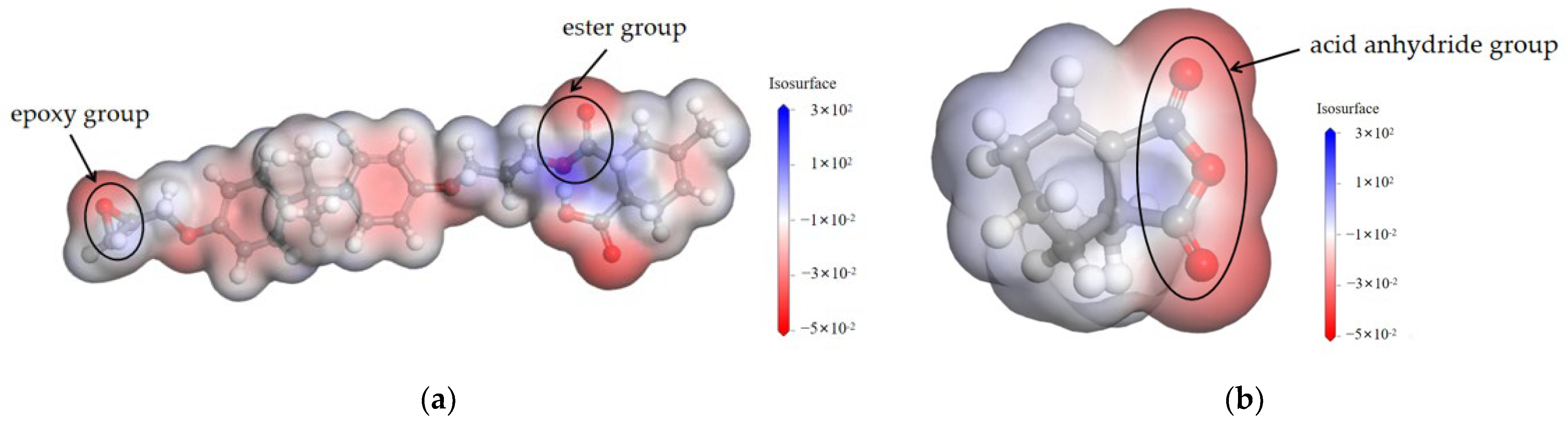
3.1.1. Nucleophilicity of Amine Accelerators
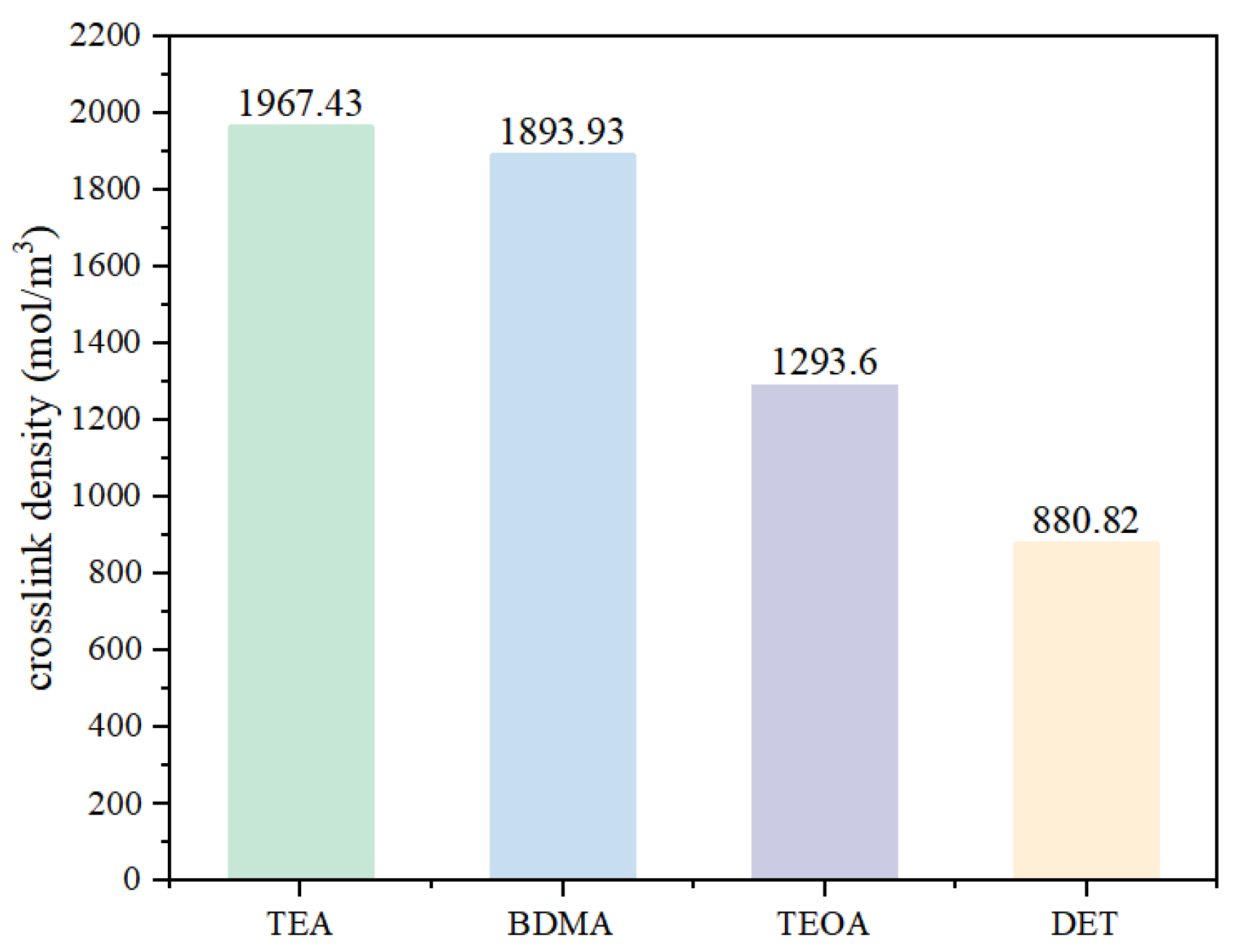
3.1.2. Crosslinking Density
3.2. Process Performance
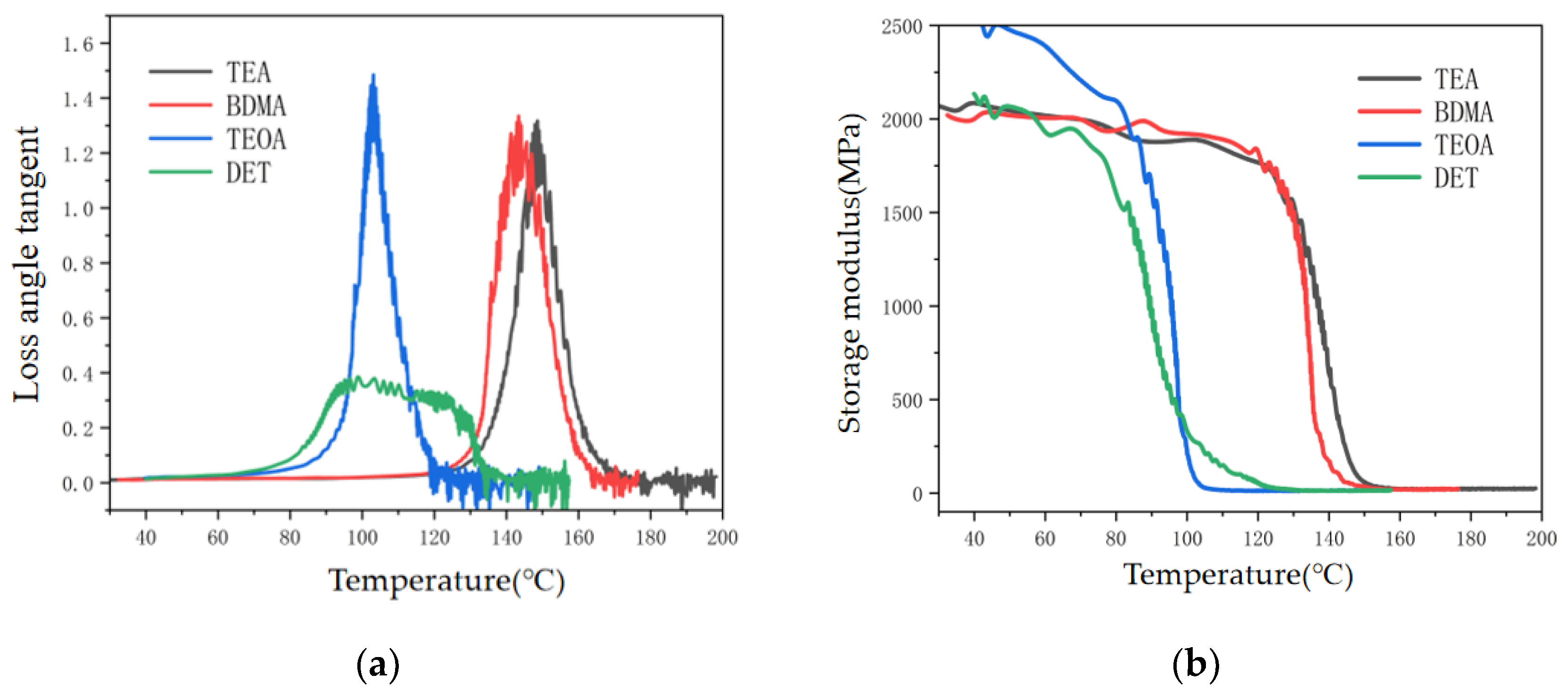
3.2.1. Gel Time Test Results
3.2.2. Exothermic Temperature Rise for Curing
3.3. Dielectric Properties
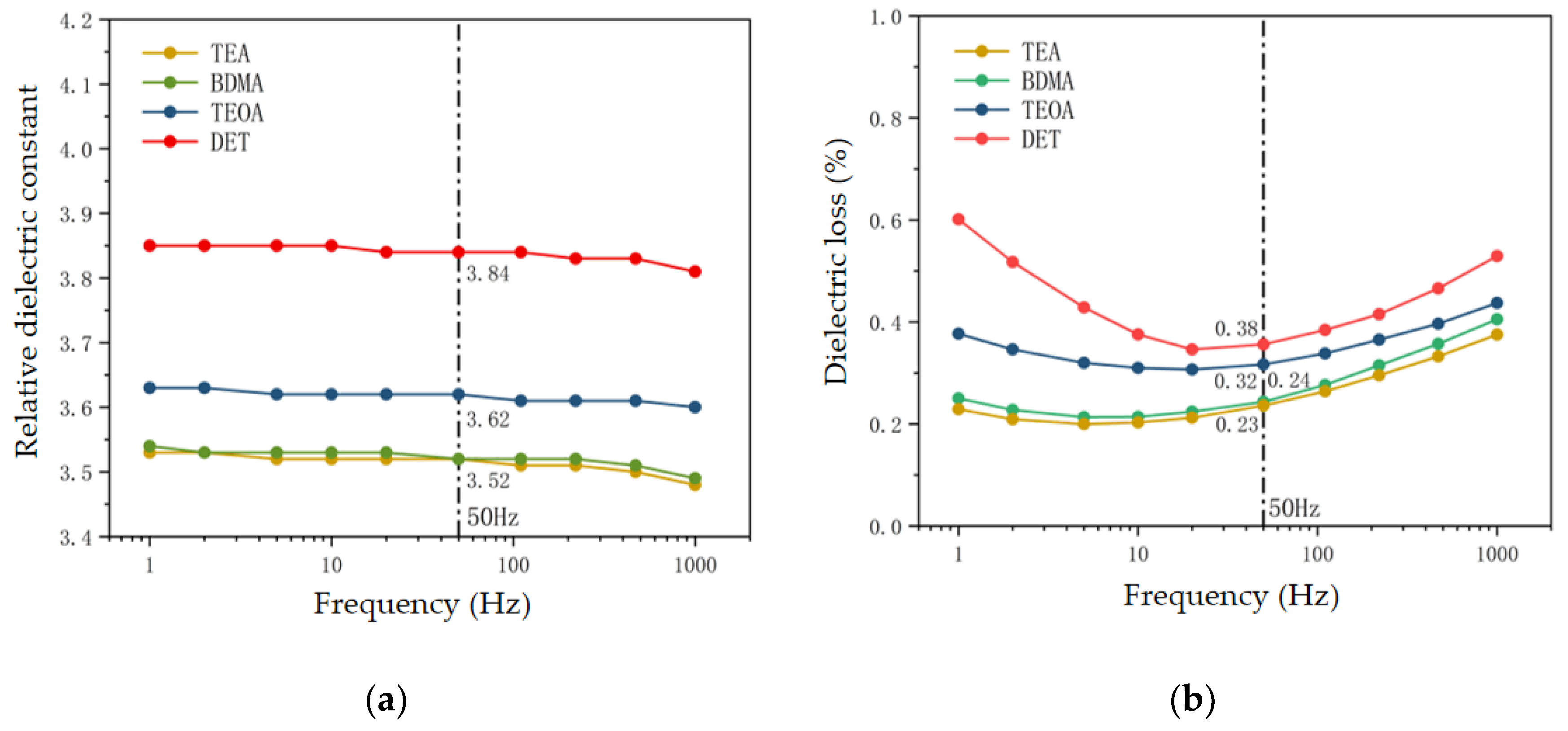
3.3.1. Dielectric Loss and Dielectric Constant
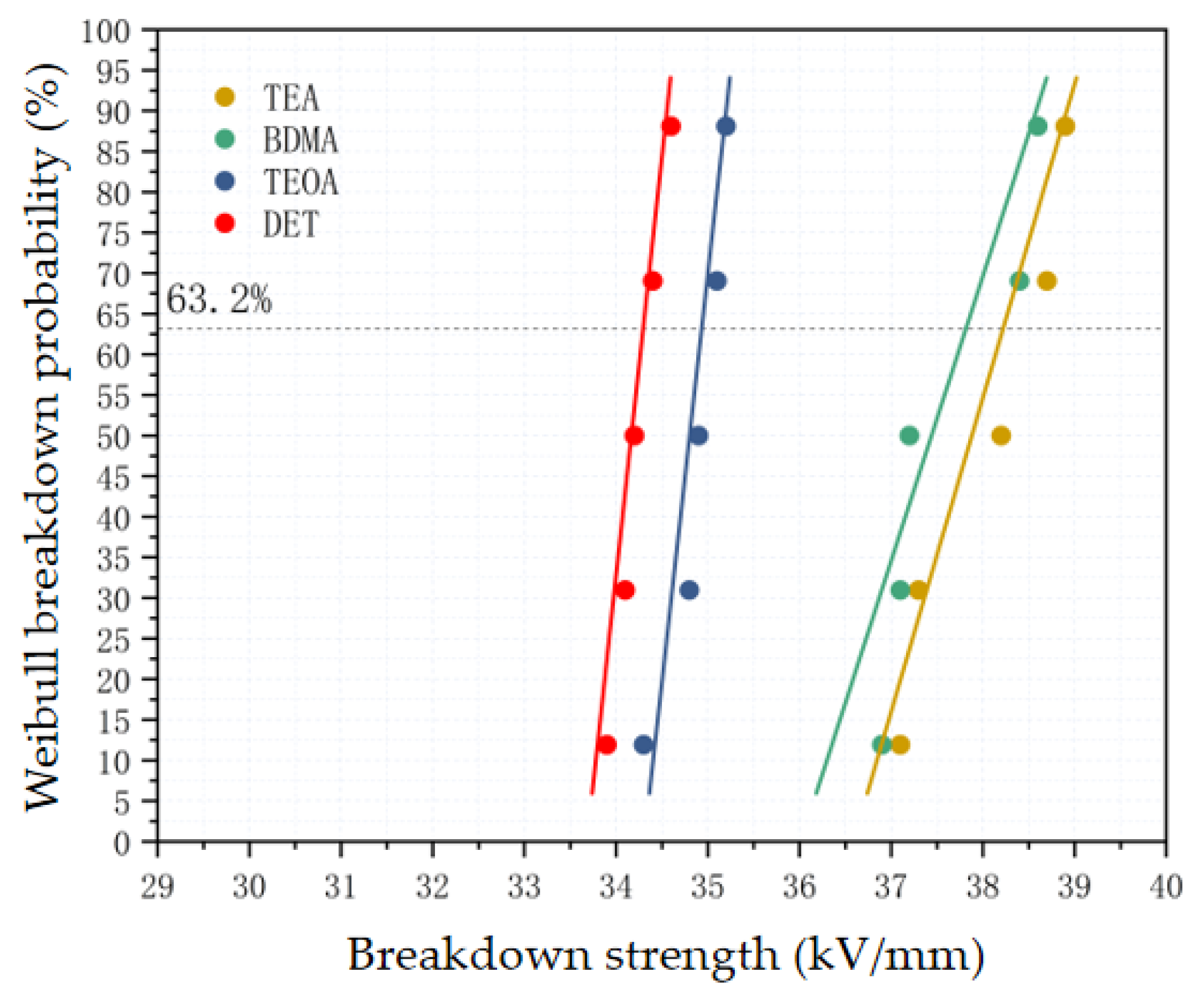
3.3.2. Effect of Amine Accelerators on Breakdown Strength of Epoxy Materials
4. Conclusions
- (1)
- In terms of process properties, the stronger the nucleophilic ability of the amine accelerators, the shorter the gel time of the epoxy materials and the higher the exothermic temperature rise of curing. Specifically, the gel time of the TEA system was 50% shorter than that of the DET system, and the curing exothermic temperature rise was 55.3% higher. This is due to the fact that the stronger nucleophilic ability of the accelerator enables easier anhydride curing agent ring-opening, accelerating the epoxy resin and curing agent crosslinking reaction rate.
- (2)
- From the dielectric properties, the stronger the nucleophilic ability of the amine accelerators, the lower the dielectric loss and dielectric constant of the epoxy material, and the higher the breakdown strength. The dielectric constant and dielectric loss of the TEA system were reduced by 8.3% and 39.5% compared with those of the DET system, and the breakdown strength was improved by 11.4%. This is attributed to the enhanced nucleophilicity of the accelerator which reduces the number of polar dipoles and at the same time restricts the movement of carriers in the crosslinked network, thus improving the dielectric properties of the epoxy material.
- (3)
- The selection of amine accelerators with appropriate nucleophilic ability can, on the one hand, meet the process performance requirements in the manufacturing process of capacitor cores—that is, facilitate the molding operation and reduce the defects caused by insufficient impregnation. On the other hand, it can ensure that the cured capacitor core has excellent dielectric properties, thereby effectively enhancing the insulation strength of the dry bushing and making it more in line with the requirements of practical engineering applications.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Du, H. Research on the Seismic Performance of ±800 kV UHV DC Dry Through-Wall Bushing and Its Damping Measures. Master’s Thesis, Chongqing University, Chongqing, China, 2022. [Google Scholar]
- Du, Y. Evaluation of Moisture and Aging State of Oil-Paper Capacitor Type Bushing Based on Frequency Domain Dielectric Response. Master’s Thesis, Chongqing University, Chongqing, China, 2017. [Google Scholar]
- Chen, M.; Liu, X.; Shao, Y.; Shang, G.; Zhang, Q.; Tang, H.; Liu, L. Degradation characteristics of insulation near aluminium foil edges inside dry-type bushing cores under electrothermal compound stress. High Volt. 2022, 7, 1153–1164. [Google Scholar] [CrossRef]
- Lan, Z.; Song, Y.; Deng, J.; Nie, D.; Peng, Z.; Xu, Z. Research status of extra-high voltage AC/DC bushing in China. Electr. Porcelain Surge Arrester 2021, 2, 1–6+14. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Q.; Wang, X.; Chi, M.; Liu, H.; Ning, X. Dielectric and Thermal Conductivity of Epoxy Resin Impregnated Nano-h-BN Modified Insulating Paper. Polymers 2019, 11, 1359. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Zhu, X.; Lu, Y.; Gao, Y. Influence of low temperature environment on the electrical properties of epoxy resin. High Volt. Technol. 2023, 49, 2891–2899. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, X.; Li, X.; He, S.; Zeng, X.; Yue, Y.; Yu, X.; Li, Z. Molecular dynamics simulation of pyrolysis of 4-methylhexahydrophthalic anhydride-cured bisphenol A-type epoxy resin. Insul. Mater. 2020, 53, 24–29. [Google Scholar] [CrossRef]
- Namazian, Z.; Yaghoubi, M. An innovative approach for resin infusion and impregnation processes of a commercial resin-impregnated paper bushing. Int. J. Adv. Manuf. Technol. 2020, 110, 593–604. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, X.; Yang, Z.; Xu, H.; Jiang, J.; Mu, H.; Zhang, G.-J. Study on water absorption and dielectric response of epoxy resin impregnated paper bushing. IET Gener. Transm. Distrib. 2023, 17, 3789–3800. [Google Scholar] [CrossRef]
- Ding, J.; Jia, Q.; Li, F.; Chen, N.; Gao, P.; Guo, L. Research progress on epoxy resin formulation system for high voltage insulation. Insul. Mater. 2024, 57, 9–19. [Google Scholar] [CrossRef]
- Fan, X.; Sun, Z.; Zhang, X.; Bin, H.; Xiao, H.; Lv, F. Impregnation Model and Experimental Study of Mineral Oil/Meta-Aramid Paper Combination Insulation. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 2003–2012. [Google Scholar] [CrossRef]
- Liu, P.; Feng, H.; Zhang, H.; Ning, X.; Li, D.; Peng, Z. Characteristics of space charge distribution in epoxy-paper composite insulation system. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2385–2392. [Google Scholar] [CrossRef]
- Li, J.; Guo, P.; Kong, X.; Wang, Y.; Yang, Y.; Fang, L.; Du, B. Curing Kinetics and Dielectric Properties of Anhydride Cured Epoxy Resin With Different Accelerator Contents. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 20–30. [Google Scholar] [CrossRef]
- ISO 2555:2018; Plastics–Resins in the Liquid State or as Emulsions or Dispersions–Determination of Apparent Viscosity Using a Single Cylinder Type Rotational Viscometer Method. International Organization for Standardization: Geneva, Switzerland, 2018.
- IEC 62631-2-1:2018; Dielectric and Resistive Properties of Solid Insulating Materials—Part 2-1: Relative Permittivity and Dissipation Factor—Technical Frequencies (0,1 Hz–10 MHz)—AC Methods. International Electrotechnical Commission: Geneva, Switzerland, 2018.
- IEC 60243-1:2013; Electric Strength of Insulating Materials—Test Methods—Part 1: Tests at Power Frequencies. International Electrotechnical Commission: Geneva, Switzerland, 2013.
- Wu, X. Preparation and Properties of Nanohybrid Materials for Waterborne Epoxy-Alkyd Resins. Master’s Thesis, Hebei University, Baoding, China, 2014. [Google Scholar]
- Fu, K.; Xie, Q.; Lü, F.; Duan, Q.; Wang, X.; Zhu, Q.; Huang, Z. Molecular dynamics simulation and experimental studies on the thermomechanical properties of epoxy resin with different anhydride curing agents. Polymers 2019, 11, 975. [Google Scholar] [CrossRef]
- Cheng, X.; Jia, X.; Ba, S. Research on accelerators for dicyandiamide epoxy resin curing system. Bonding 2019, 40, 32–35. [Google Scholar]
- Hamlin, T.A.; Swart, M.; Bickelhaupt, F.M. Nucleophilic Substitution (SN2): Dependence on Nucleophile, Leaving Group, Central Atom, Substituents, and Solvent. ChemPhysChem 2018, 19, 1315. [Google Scholar] [CrossRef]
- Fu, G.C. Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes. ACS Cent. Sci. 2017, 3, 692–700. [Google Scholar] [CrossRef]
- Scheer, A.M.; Gallup, G.A.; Burrow, P.D. Unoccupied orbital energies of 1,4-benzenedithiol and the HOMO-LUMO gap. Chem. Phys. Lett. 2008, 466, 131–135. [Google Scholar] [CrossRef]
- De Vylder, A.; Lauwaert, J.; Sabbe, M.K.; Reyniers, M.-F.; De Clercq, J.; Van Der Voort, P.; Thybaut, J.W. Rational design of nucleophilic amine sites via computational probing of steric and electronic effects. Catal. Today 2019, 334, 96–103. [Google Scholar] [CrossRef]
- Chen, K.; Shi, H. Nucleophilic Aromatic Substitution of Halobenzenes and Phenols with Catalysis by Arenophilic π Acids. ACC Chem. Res. 2024, 57, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ma, X.; Zheng, F.; Lu, X.; Lu, Q. High performance transparent polyimides by controlling steric hindrance of methyl side groups. Eur. Polym. J. 2019, 120, 109235. [Google Scholar] [CrossRef]
- Zhang, T.; Nagel-Steger, L.; Willbold, D. Solution-Based Determination of Dissociation Constants for the Binding of Aβ42 to Antibodies. ChemistryOpen 2019, 7, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Png, Z.M.; Ng, S.H.; Tham, G.X.; Ye, E.; Goh, S.S.; Loh, X.J.; Li, Z. Vitrimers: Current research trends and their emerging applications. Mater. Today 2021, 51, 586–625. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. WIREs Comput. Mol. Sci. 2017, 6, e1326. [Google Scholar] [CrossRef]
- Begum, S.; Ullah, H.; Kausar, A.; Siddiq, M.; Aleem, M.A. Fabrication of epoxy functionalized MWCNTs reinforced PVDF nanocomposites with high dielectric permittivity, low dielectric loss and high electrical conductivity. Compos. Sci. Technol. 2018, 167, 497–506. [Google Scholar] [CrossRef]
- Chakradhary, V.K.; Juneja, S.; Akhtar, M.J. Correlation between EMI shielding and reflection loss mechanism for carbon nanofiber/epoxy nanocomposite. Mater. Today Commun. 2020, 25, 101386. [Google Scholar] [CrossRef]
- Wang, H.; Lin, C.; Li, S.; Li, J. Research on the Relaxation Loss Mechanism of CaCu3Ti4O12 Ceramic. Phys. Lett. 2013, 62, 480–485. [Google Scholar]
- Shi, L.; Zhao, Z.; Zong, L.; Wang, J.; Jian, X. Reducing the dielectric loss of poly(aryl ether)s by grafting. Polym. Adv. Technol. 2023, 34, 3125–3136. [Google Scholar] [CrossRef]
- Li, J.; Guo, P.; Kong, X.; Wang, Y.; Li, F.; Du, B. Curing Degree Dependence of Dielectric Properties of Bisphenol-A-Based Epoxy Resin Cured With Methyl Hexahydrophthalic Anhydride. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 2072–2079. [Google Scholar] [CrossRef]
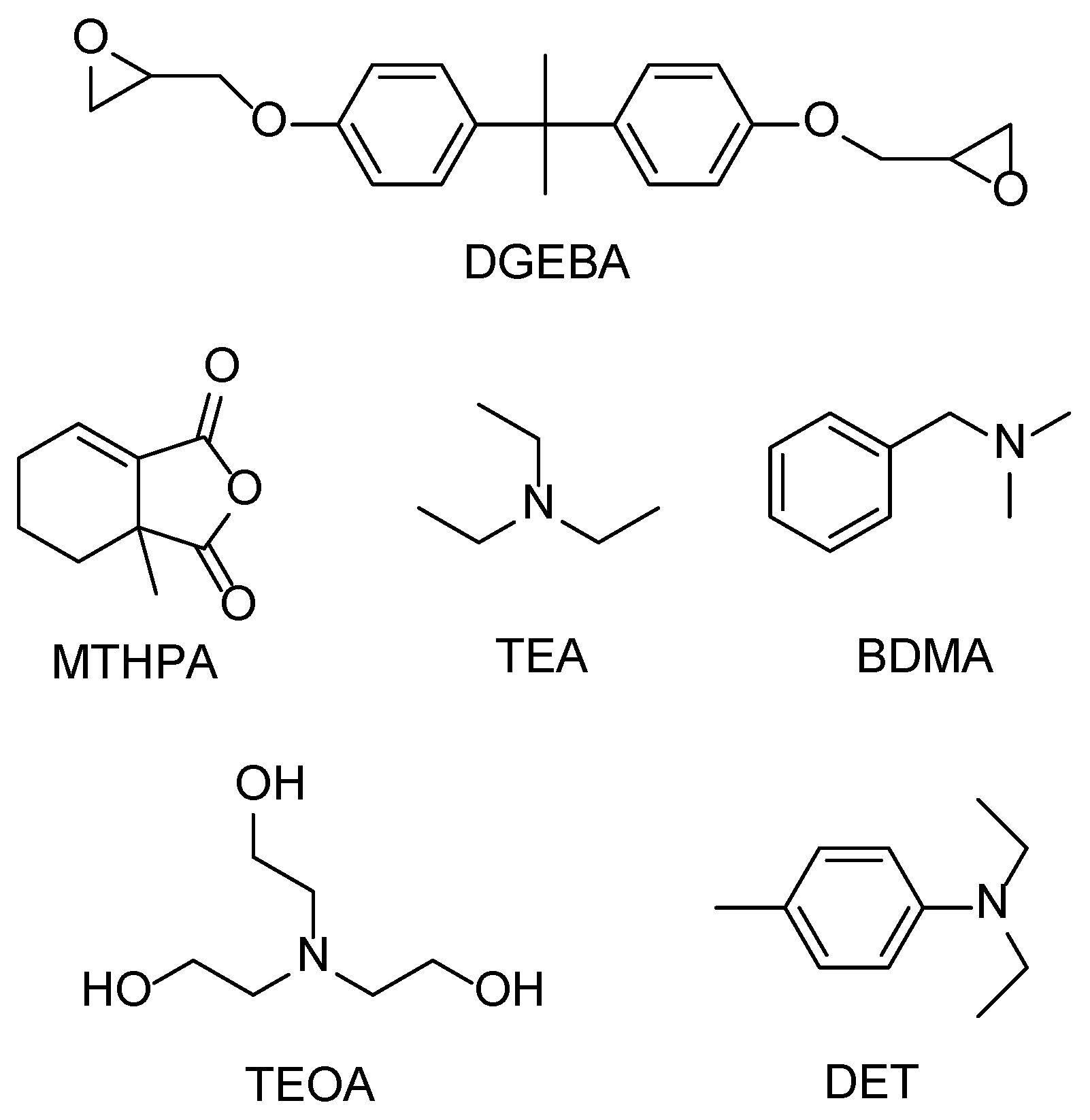


| Group | P-1 | P-2 | P-3 | P-4 |
|---|---|---|---|---|
| DRE331 | 100 g | 100 g | 100 g | 100 g |
| MTHPA | 88.91 g | 88.91 g | 88.91 g | 88.91 g |
| TEA | 0.6 g | 0 g | 0 g | 0 g |
| BDMA | 0 g | 0.6 g | 0 g | 0 g |
| TEOA | 0 g | 0 g | 0.6 g | 0 g |
| DET | 0 g | 0 g | 0 g | 0.6 g |
| Group | TEA | BDMA | TEOA | DET |
|---|---|---|---|---|
| pKb | 3.28 | 4.98 | 6.20 | 9.44 |
| TEA | BDMA | TEOA | DET | |
|---|---|---|---|---|
| Tg(K) | 420.55 | 415.35 | 385.85 | 381.95 |
| Energy storage modulus E (MPa) | 22.60 | 21.51 | 13.74 | 9.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Chen, S.; Guo, R.; Luo, C.; Zhang, C.; Li, W.; Zhao, Y.; Lu, T.; Zhao, Y. Study on the Effect of the Nucleophilicity of Amine Accelerators on the Process and Dielectric Properties of Epoxy Materials for Dry Bushing. Polymers 2025, 17, 2655. https://doi.org/10.3390/polym17192655
Cui H, Chen S, Guo R, Luo C, Zhang C, Li W, Zhao Y, Lu T, Zhao Y. Study on the Effect of the Nucleophilicity of Amine Accelerators on the Process and Dielectric Properties of Epoxy Materials for Dry Bushing. Polymers. 2025; 17(19):2655. https://doi.org/10.3390/polym17192655
Chicago/Turabian StyleCui, Huize, Shuo Chen, Ruilu Guo, Chumeng Luo, Chong Zhang, Wenpeng Li, Yushun Zhao, Taisen Lu, and Yanning Zhao. 2025. "Study on the Effect of the Nucleophilicity of Amine Accelerators on the Process and Dielectric Properties of Epoxy Materials for Dry Bushing" Polymers 17, no. 19: 2655. https://doi.org/10.3390/polym17192655
APA StyleCui, H., Chen, S., Guo, R., Luo, C., Zhang, C., Li, W., Zhao, Y., Lu, T., & Zhao, Y. (2025). Study on the Effect of the Nucleophilicity of Amine Accelerators on the Process and Dielectric Properties of Epoxy Materials for Dry Bushing. Polymers, 17(19), 2655. https://doi.org/10.3390/polym17192655





