Effects of Wharton’s Jelly Mesenchymal Stem Cells and Its-Derived Small Extracellular Vesicles Loaded into Injectable Genipin-Crosslinked Gelatin Hydrogel on Vocal Fold Fibroblast
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Processing
2.1.1. WJMSC Isolation and Cryopreservation
2.1.2. VFFs Isolation
2.2. WJMSC-sEV Isolation and Characterization
2.2.1. Concentration of WJMSCs Lysate, WJMSC-sEVs, and WJMSC-sEVs Lysate
2.2.2. BCA
2.2.3. Nanoparticle Tracking Analysis (NTA)
2.2.4. Western Blot
2.2.5. Transmission Electron Microscopy (TEM)
2.3. Fabrication of Hydrogel
2.3.1. Construction of GCGH
2.3.2. Encapsulation of WJMSCs and sEVs in GCGH
2.4. Biocompatibility of WJMSC-sEVs and Selection Optimal Dosage
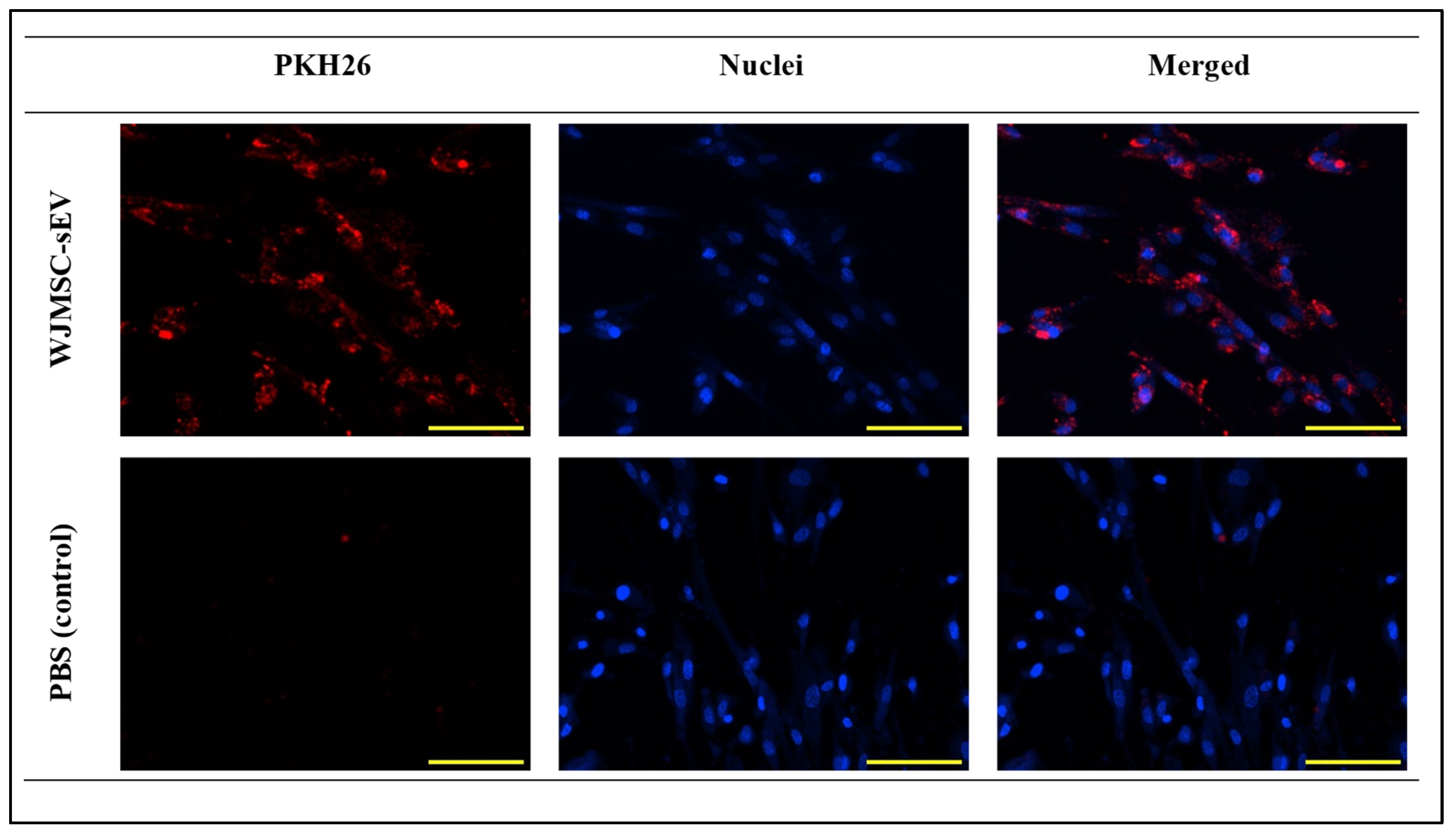
2.4.1. Internalization of WJSMC-EVs in VFFs
2.4.2. VFFs Proliferation Assay via MTT Assay
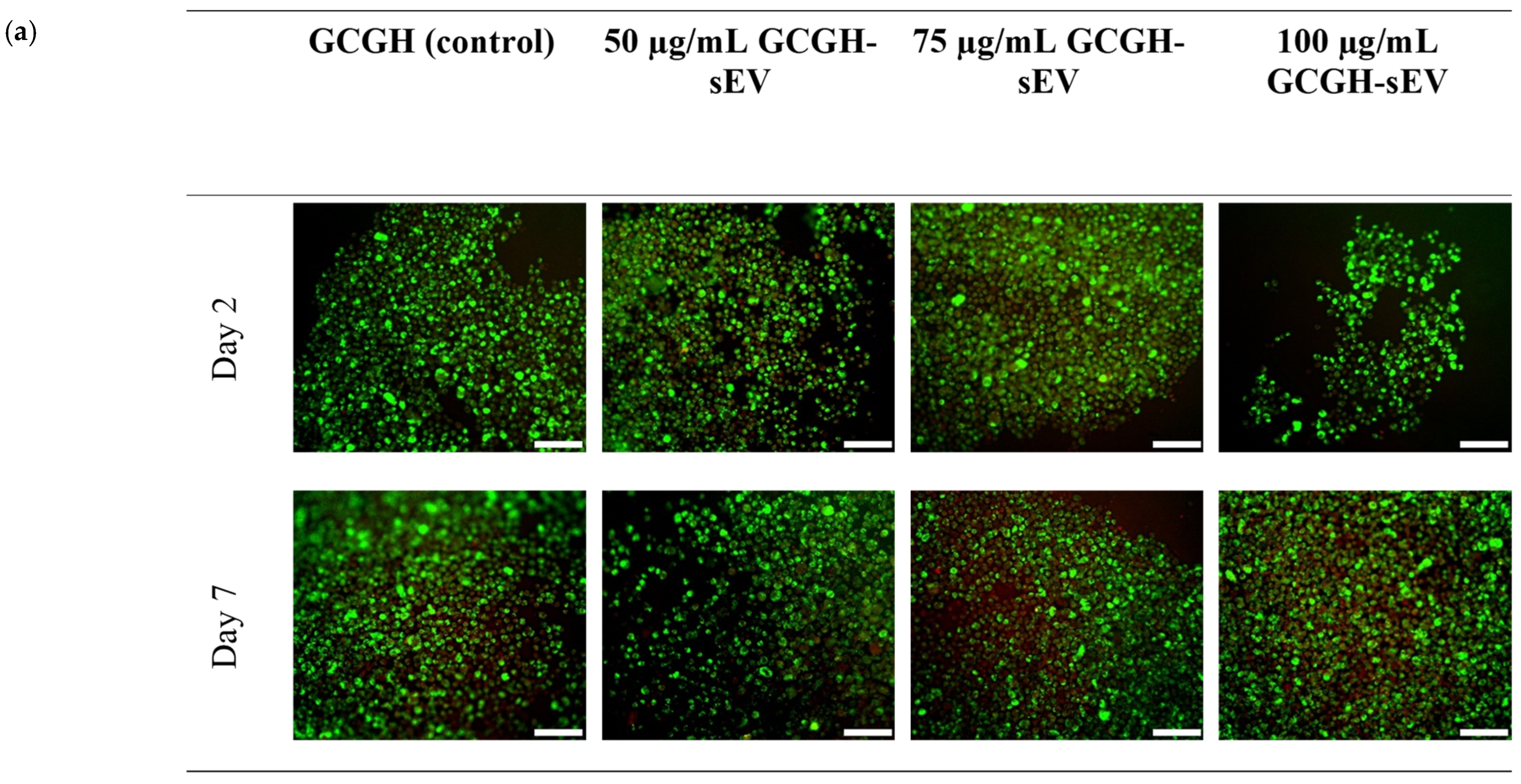
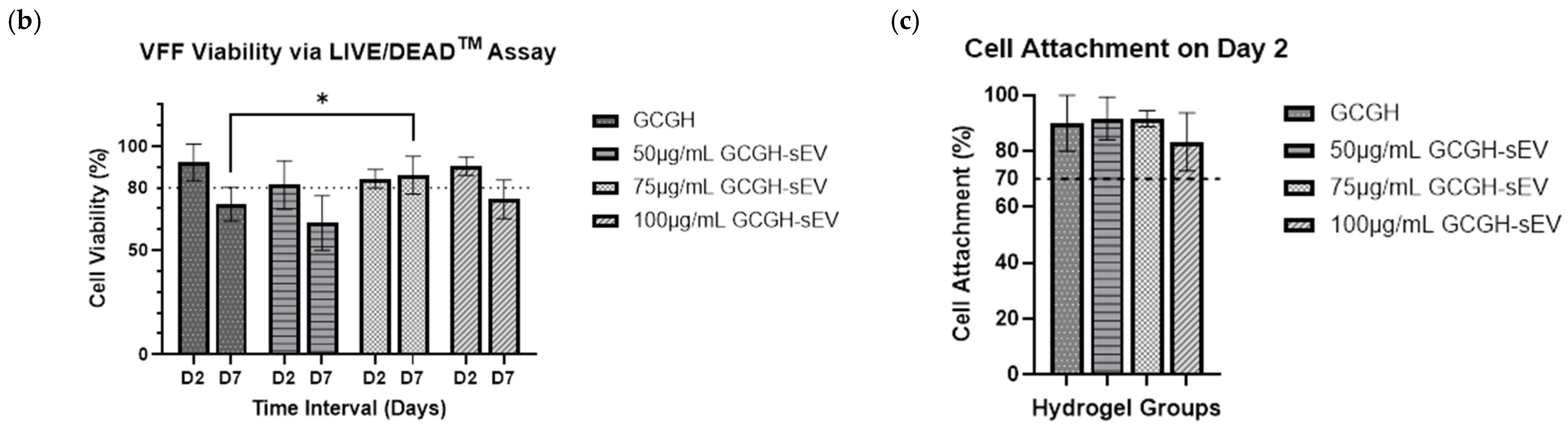
2.4.3. Biocompatibility of VFFs with GCGH-sEVs via LIVE/DEADTM Assay
2.5. Physicochemical Properties of GCGH, GCGH-MSC, and GCGH-sEVs
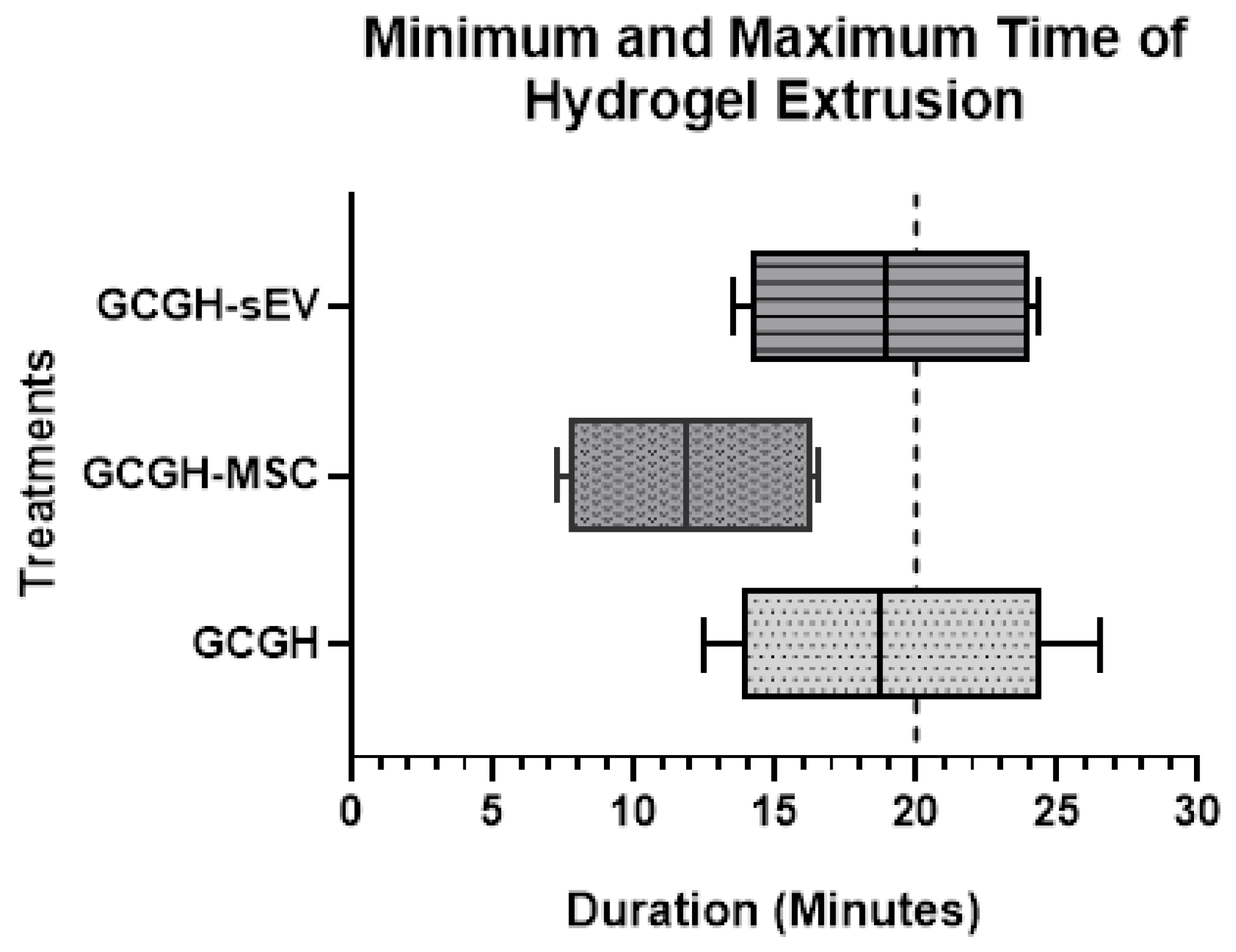
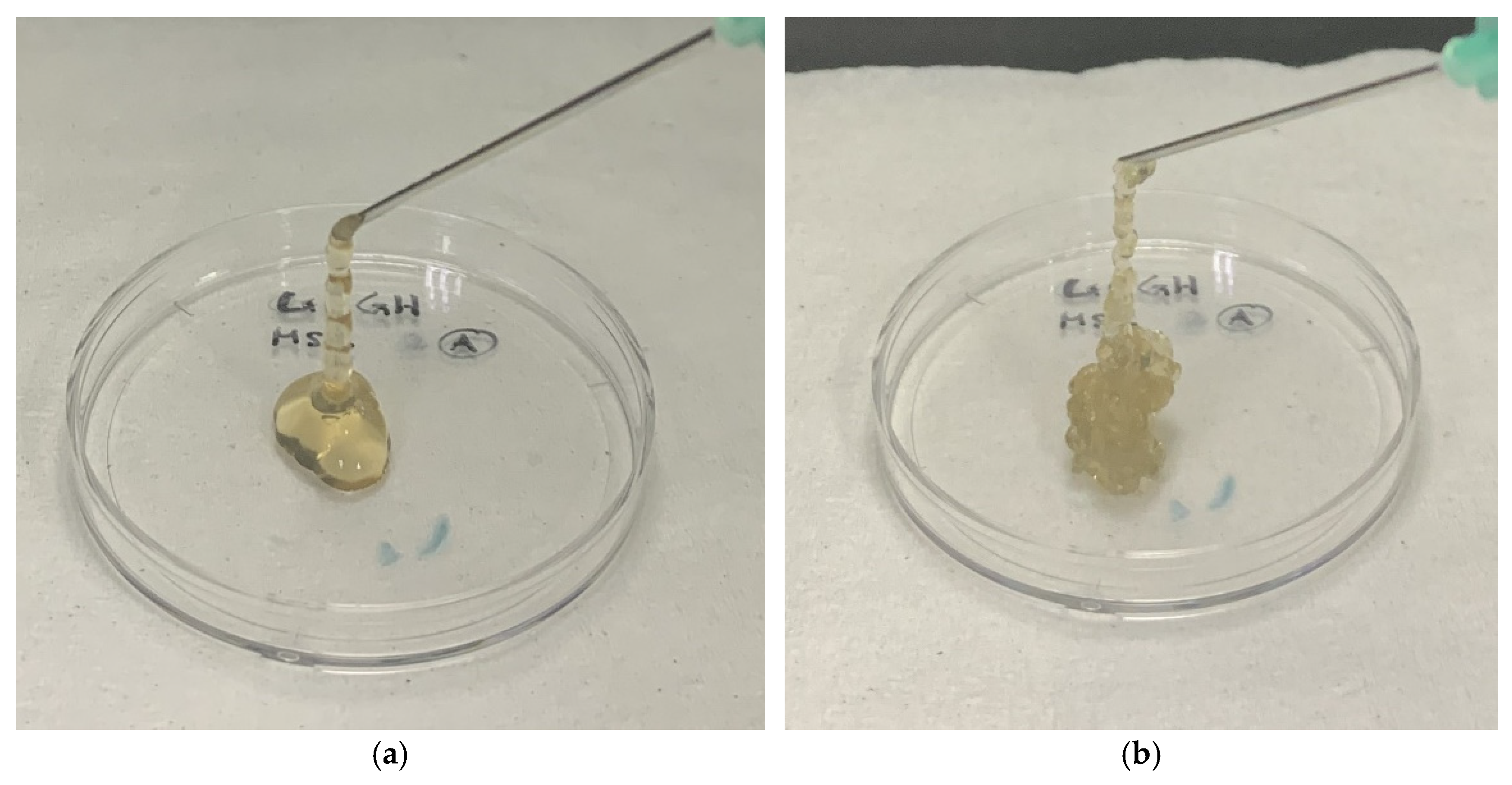
2.5.1. Injectability Study
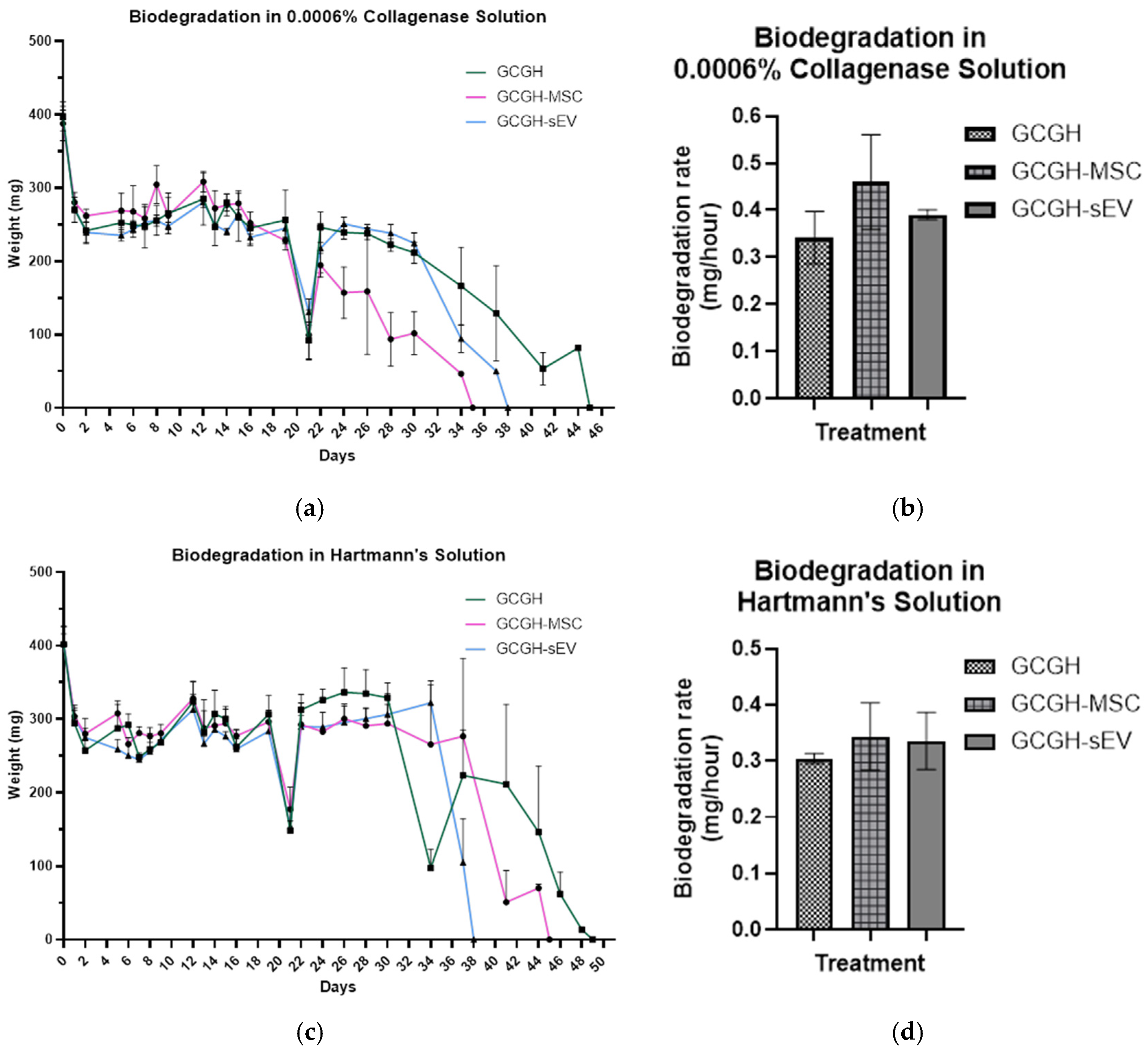
2.5.2. Biodegradation Study
2.6. Biocompatibility and Mechanistic Effect with GCGH Treatments
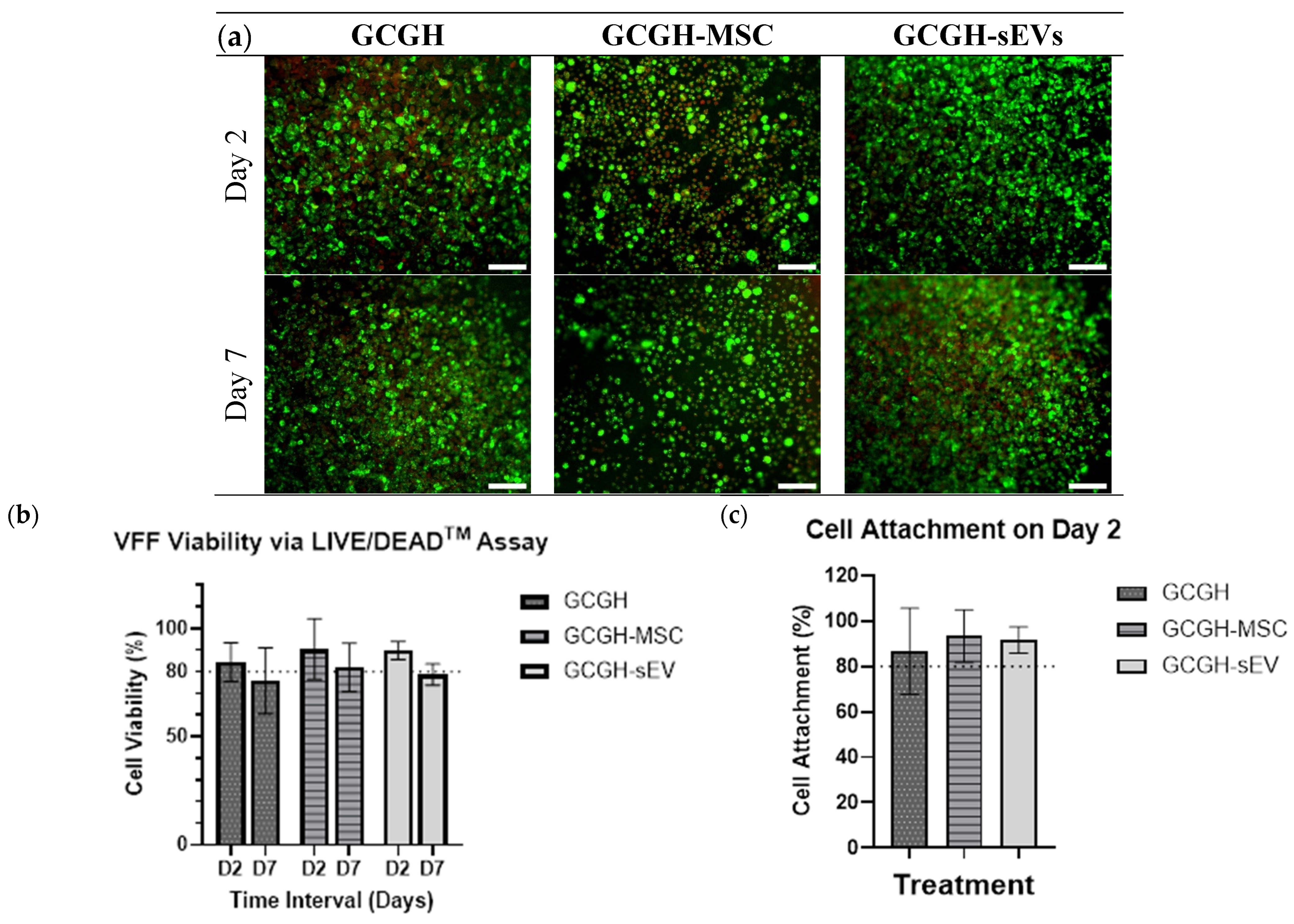
2.6.1. Biocompatibility of VFFs via LIVE/DEADTM Assay
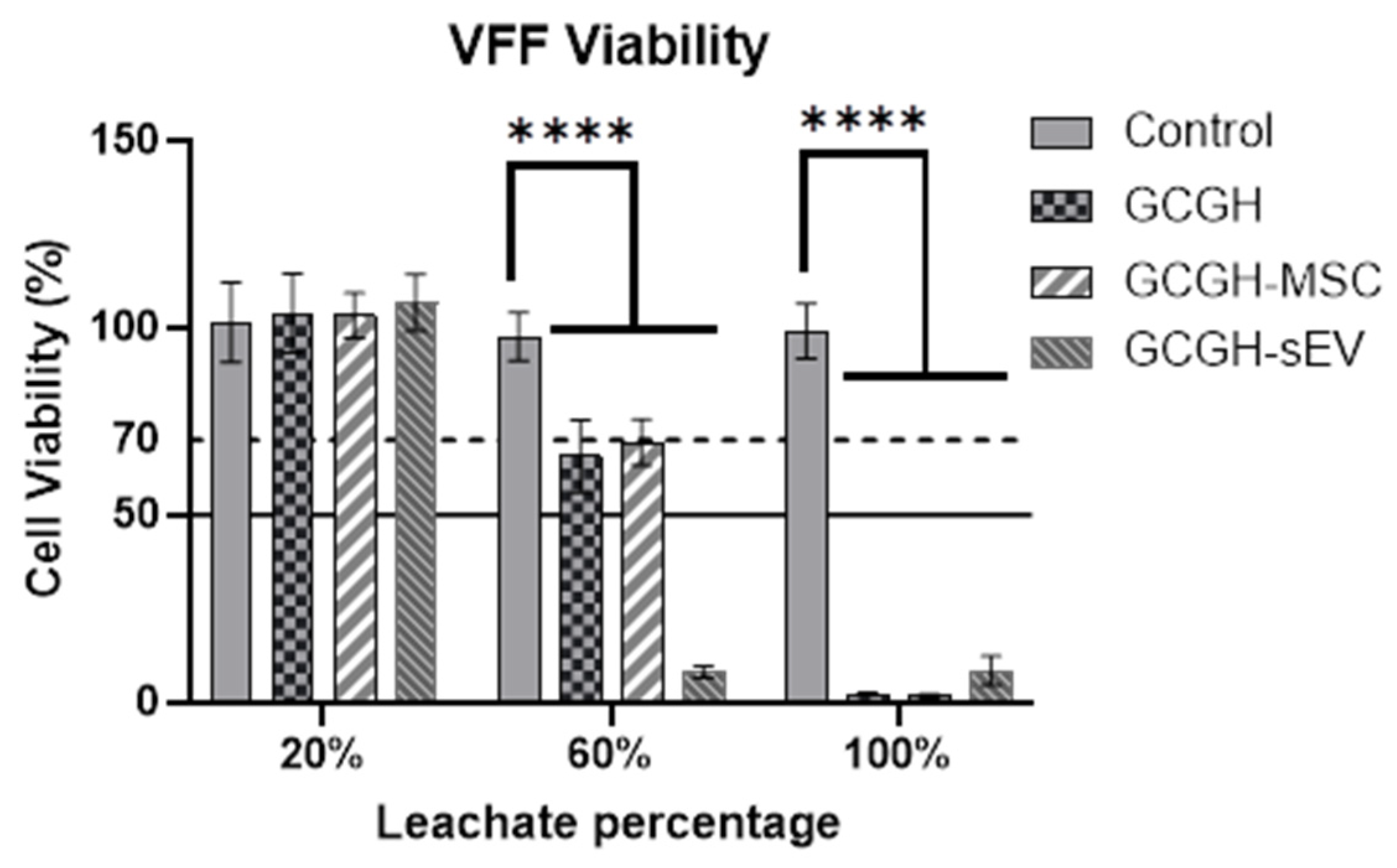
2.6.2. Cytotoxicity of Hydrogel Leachate
2.6.3. Collagen Gel Contraction Assay
2.6.4. PBMCs Collection and Cell Culture
2.6.5. PBMC Proliferation Using MTT Assay
2.7. Statistical Analysis
3. Results
3.1. Characterization of WJMSCs and WJMSC-sEVs
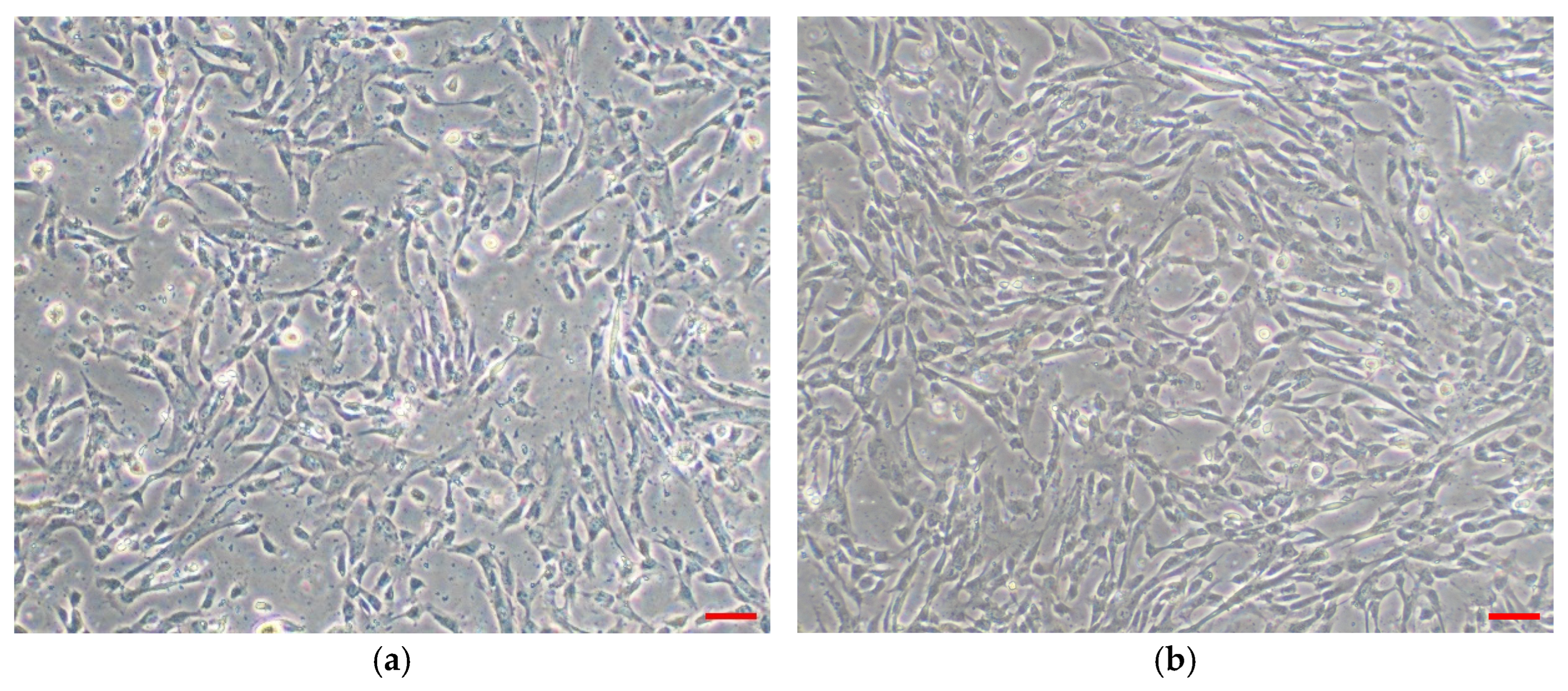
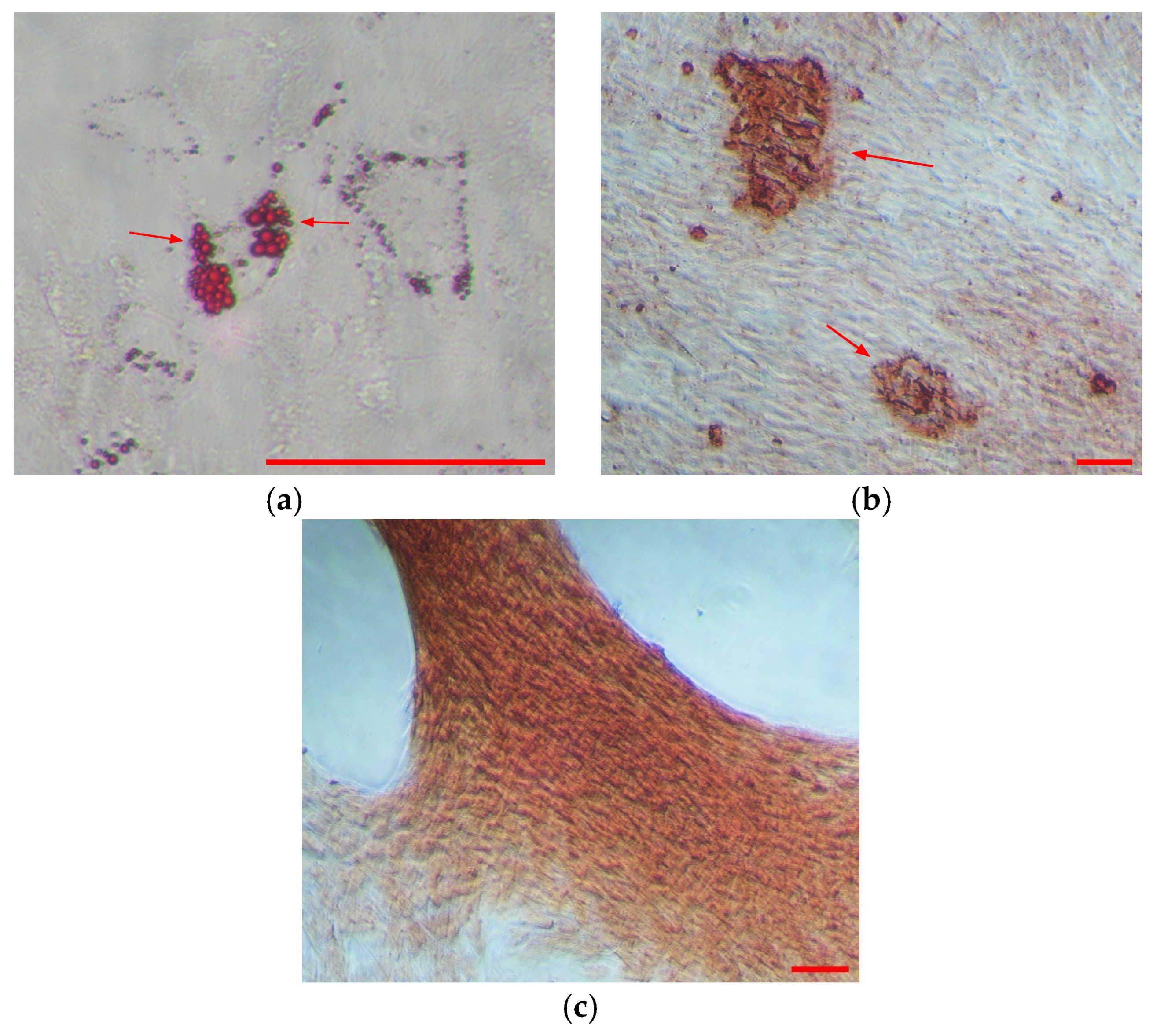
3.1.1. WJMSCs Characterization
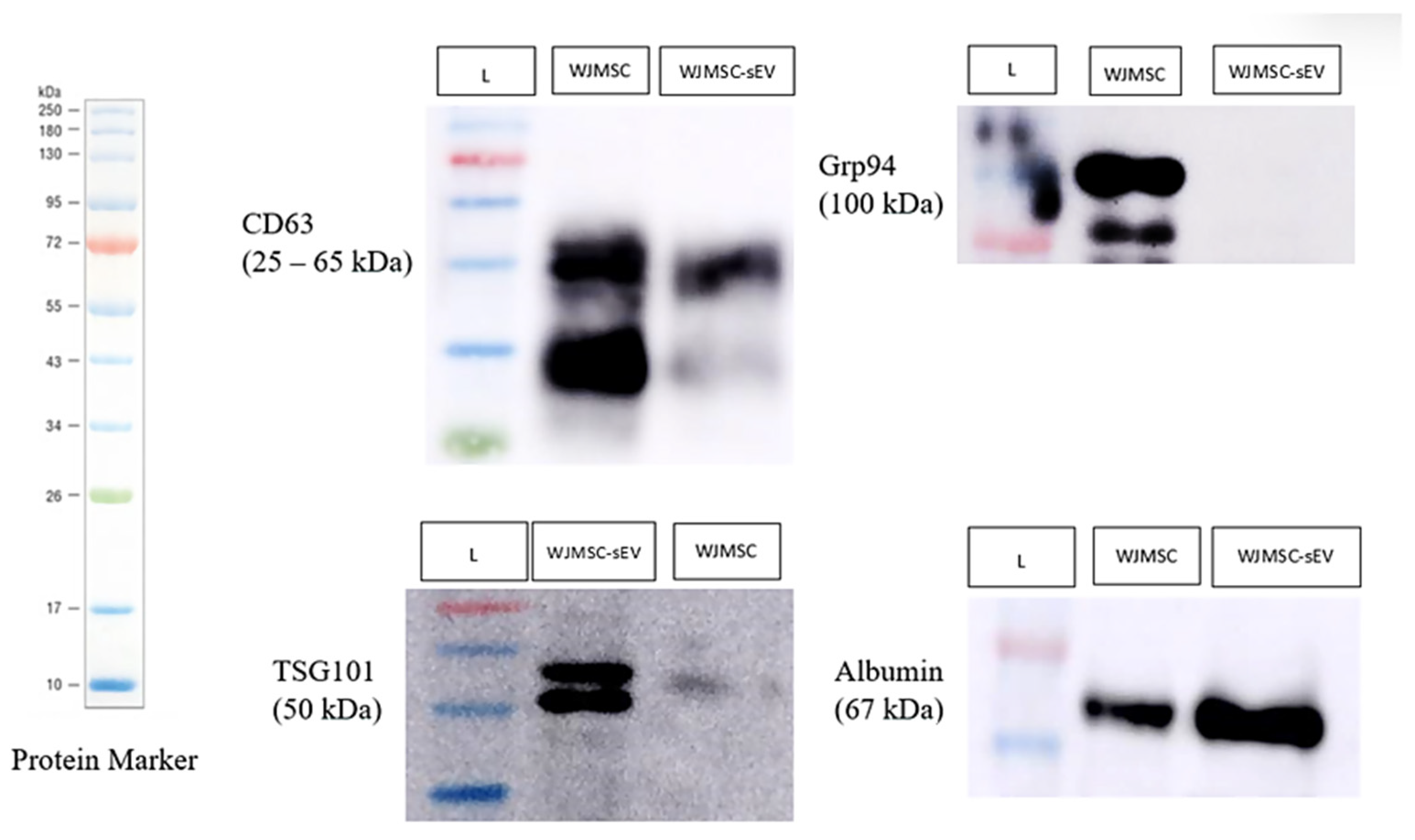
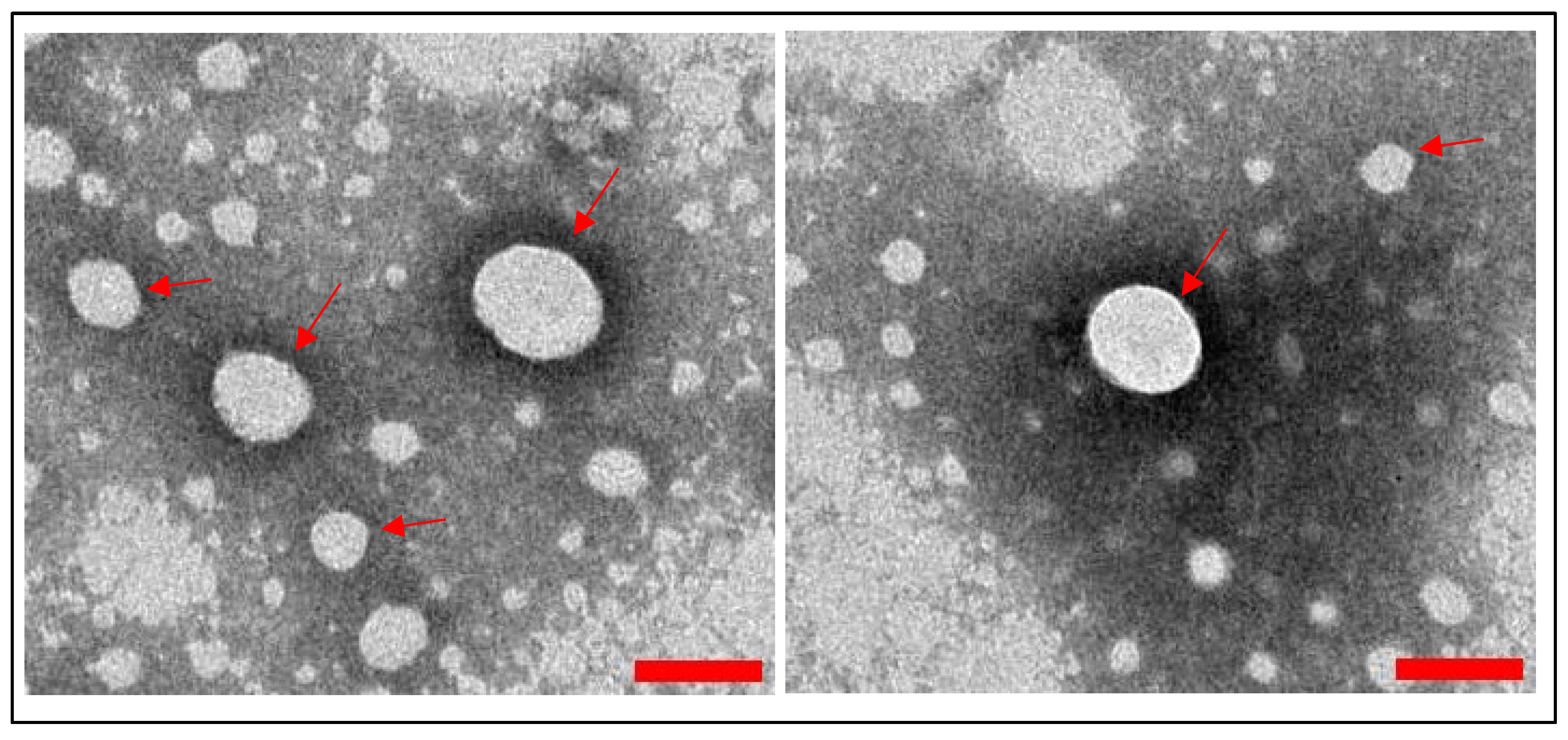
3.1.2. WJMSC-sEV Characterization
3.2. Dose Selection of WJMSC-sEVs
3.2.1. VFFs’ Ability to Uptake WJMSC-sEVs
3.2.2. Proliferation of VFFs Cultured with Different Concentration of WJMSC-sEVs
3.2.3. VFFs’ Compatibility with GCGH-sEVs
3.3. Physicochemical Properties of GCGH, GCGH-MSCs, and GCGH-sEVs
3.3.1. Injectability Study
3.3.2. In Vitro Biodegradation
3.4. Biocompatibility of GCGH, GCGH-MSC, and GCGH-sEVs
3.4.1. VFFs Compatibility by LIVE/DEAD Assay
3.4.2. Cytotoxicity of Hydrogel Leachate to VFFs
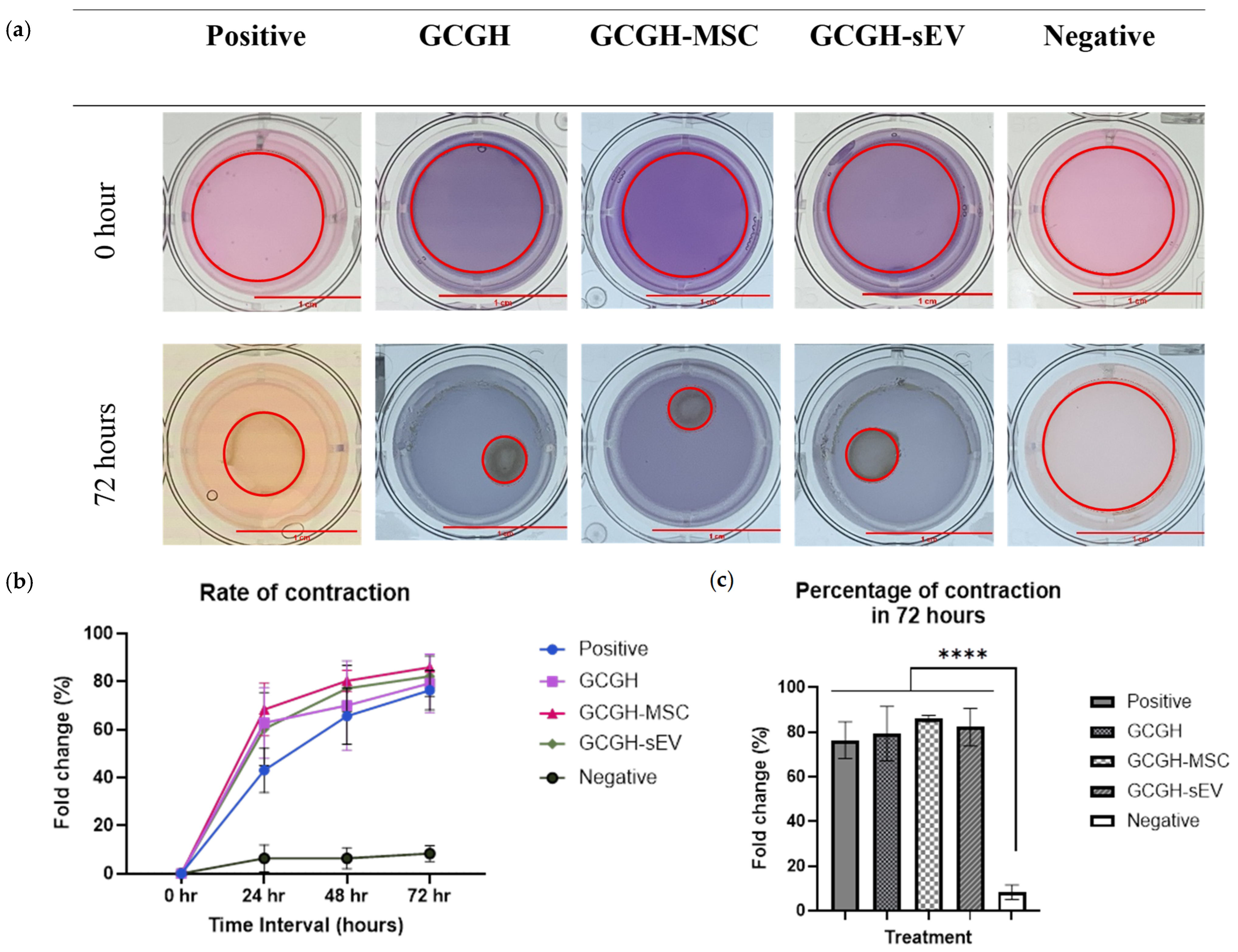
3.4.3. Collagen Gel Contraction Assay
3.4.4. Immunomodulatory Response of PBMCs
4. Discussion
4.1. Characterization of WJMSCs and WJMSC-sEVs
4.2. Selection of WJMSC-sEV Concentration
4.3. Incorporation of Biological Components in GCGH on VFFs Compatibility
4.4. Biocompatibility of VFFs with GCGH Groups
5. Conclusions and Future Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Antibiotic-Antimycotic |
| ANOVA | Two-Way Analysis of Variance |
| BSA | Bovine Serum Albumin |
| BCA | Bicinchoninic Acid Assay |
| CaHA | Calcium Hydroxyapatite |
| Col-Gel | Collagen Contraction |
| DAPI | 6-diamidino-2-phenylindole |
| DMEM F-12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| DMSO | Dimethyl sulfoxide |
| DPBS | Dulbecco’s phosphate-buffered saline |
| ECM | Extracellular Matrix |
| ECL | Chemiluminescence |
| EV | Extracellular Vesicles |
| FBS | Fetal Bovine Serum |
| F-12 DMEM | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| FRGS | Fundamental Research Grant Scheme |
| GCGH | Genipin Crosslinked Gelatin Hydrogel |
| GCGH-MSC | Genipin Crosslinked Gelatin Hydrogel-Mesenchymal Stem Cells |
| GCGH-sEVs | Genipin Crosslinked Gelatin Hydrogel-Small Extracellular Vesicles |
| GelMA | Gelatin Methacrylate |
| HA | Hyaluronic Acid |
| HPL | Human Platelet Lysate |
| ISCT | International Society for Cellular Therapy |
| LG-DMEM | Dulbecco’s Modified Eagle’s Medium-Low Glucose |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| miRNA | Micro Ribonucleic Acid |
| MoHE | Minister of Higher Education |
| MSCs | Mesenchymal Stem Cells |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NaOH | Sodium hydroxide |
| NTA | Nanoparticle Tracking Analysis |
| OMF | Oral Mucosa Fibroblasts |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PBS | Phosphate-Buffered Saline |
| Pen-Strep | Pennicilin-Streptomycin |
| PFA | Paraformaldehyde |
| PHA-M | Phytohemagglutinin M-form |
| PTA | Phosphotungstic Acid |
| RIPA | Radio Immunoprecipitation Assay |
| RNA | Ribonucleic Acid |
| RPMI | Roswell Park Memorial Institute-1640 medium |
| SDS | Sodium dodecyl Sulphate |
| SDS-PAGE | Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis |
| SEC | Size Exclusion Chromatography |
| sEV | Small Extracellular Vesicles |
| TBS-T | Tris-Buffered Saline with Tween 20 |
| TEM | Transmission Electron Microscopy |
| TFF | Tangential Flow Filtration |
| UCMSC | Umbilical Cord-Derived Mesenchymal Stem Cells |
| UKM | Universiti Kebangsaan Malaysia |
| VFFs | Vocal Fold Fibroblasts |
| WJMSCs | Wharton’s Jelly mesenchymal stem cells |
| WJMSC-sEVs | WJMSC-Derived Small EV |
| α-MEM | Minimum Essential Medium Eagle-Alpha Modification |
References
- Giraldez-Rodriguez, L.A.; Johns, M. Glottal Insufficiency with Aspiration Risk in Dysphagia. Otolaryngol. Clin. N. Am. 2013, 46, 1113–1121. [Google Scholar] [CrossRef]
- Hu, H.-C.; Hung, Y.-T.; Lin, S.-Y.; Tung, T.-H.; Chang, S.-Y. Office-Based Autologous Fat Injection Laryngoplasty for Glottic Insufficiency in Patients Under 50 Years Old. J. Voice 2019, 33, 747–750. [Google Scholar] [CrossRef]
- Onwordi, L.N.; Al Yaghchi, C. Airway Glottic Insufficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- de Araújo Pernambuco, L.; Espelt, A.; Balata, P.M.M.; de Lima, K.C. Prevalence of voice disorders in the elderly: A systematic review of population-based studies. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 2601–2609. [Google Scholar] [CrossRef]
- Lyberg-Åhlander, V.; Rydell, R.; Fredlund, P.; Magnusson, C.; Wilén, S. Prevalence of Voice Disorders in the General Population, Based on the Stockholm Public Health Cohort. J. Voice 2019, 33, 900–905. [Google Scholar] [CrossRef]
- Rosow, D.E.; Pan, D.R. Presbyphonia and Minimal Glottic Insufficiency. Otolaryngol. Clin. N. Am. 2019, 52, 617–625. [Google Scholar] [CrossRef]
- Heman-Ackah, Y.D.; Ivey, C.M.; Alexander, R. Options for treatment of a small glottic gap. Laryngoscope Investig. Otolaryngol. 2023, 8, 720–729. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 31, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Allensworth, J.J.; O’Dell, K.; Ziegler, A.; Bryans, L.; Flint, P.; Schindler, J. Treatment Outcomes of Bilateral Medialization Thyroplasty for Presbylaryngis. J. Voice 2019, 33, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Ab Rani, A.; Azman, M.; Ubaidah, M.A.; Mohamad Yunus, M.R.; Sani, A.; Mat Baki, M. Nonselective Laryngeal Reinnervation versus Type 1 Thyroplasty in Patients with Unilateral Vocal Fold Paralysis: A Single Tertiary Centre Experience. J. Voice 2021, 35, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Y.; Shi, X.; Shen, H.; Ning, H.; Liu, H. Bioscaffolds embedded with regulatory modules for cell growth and tissue formation: A review. Bioact. Mater. 2021, 6, 1283–1307. [Google Scholar] [CrossRef]
- Wan-Chiew, N.; Baki, M.M.; Lokanathan, Y.; Fauzi, M.B.; Azman, M. Genipin cross-linked gelatin hydrogel for encapsulating wharton jelly mesenchymal stem cells and basic fibroblast growth factor delivery in vocal fold regeneration. Front. Cell Dev. Biol. 2024, 12, 1489901. [Google Scholar] [CrossRef]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef]
- Ding, D.-C.; Chang, Y.-H.; Shyu, W.-C.; Lin, S.-Z. Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Wong, J.K.U.; Mehta, A.; Vũ, T.T.; Yeo, G.C. Cellular modifications and biomaterial design to improve mesenchymal stem cell transplantation. Biomater. Sci. 2023, 11, 4752–4773. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, X.; Nong, B.; Luo, M.; Niu, Y.; Huang, S.; Zhang, J.; Wei, A.; Huang, J. Transplantation of Wharton’s jelly mesenchymal stem cells encapsulated with Hydroactive® Gel promotes diabetic wound antifibrotic healing in type 2 diabetic rats. J. Mater. Chem. B 2022, 10, 8330–8346. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.-J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Zhao, A.G.; Shah, K.; Cromer, B.; Sumer, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Potential. Stem Cells Int. 2020, 2020, 8825771. [Google Scholar] [CrossRef]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lee, B.H.; Irvine, S.A.; Wong, Y.S.; Bianco Peled, H.; Venkatraman, S. Inclusion of Cross-Linked Elastin in Gelatin/PEG Hydrogels Favourably Influences Fibroblast Phenotype. Polymers 2020, 12, 670. [Google Scholar] [CrossRef] [PubMed]
- Utami Nike, D.; Md Fadilah, N.I.; Sallehuddin, N.; Nor Azlan, A.Y.H.; Imran, F.H.; Maarof, M.; Fauzi, M.B. Genipin-Crosslinking Effects on Biomatrix Development for Cutaneous Wound Healing: A Concise Review. Front. Bioeng. Biotechnol. 2022, 10, 865014. [Google Scholar] [CrossRef]
- Ng, W.-C.; Lokanathan, Y.; Fauzi, M.B.; Baki, M.M.; Zainuddin, A.A.; Phang, S.J.; Azman, M. In vitro evaluation of genipin-crosslinked gelatin hydrogels for vocal fold injection. Sci. Rep. 2023, 13, 5128. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Zulkiflee, I.; Mohd Razip Wee, M.F.; Masood, A.; Siow, K.S.; Motta, A.; Fauzi, M.B. Plasma-Polymerised Antibacterial Coating of Ovine Tendon Collagen Type I (OTC) Crosslinked with Genipin (GNP) and Dehydrothermal-Crosslinked (DHT) as a Cutaneous Substitute for Wound Healing. Materials 2023, 16, 2739. [Google Scholar] [CrossRef]
- Zawawi, N.A.; Maarof, M.; Fadilah, N.I.M.; Hao, D.L.Q.; Tabata, Y.; Fauzi, M.B. Hybrid Adhesive Hydrogel Patch Containing Genipin-Crosslinked Gelatin–Hyaluronic Acid for Future Use in Atopic Dermatitis. J. Funct. Biomater. 2025, 16, 195. [Google Scholar] [CrossRef]
- Wahba, M.I. A comprehensive review on genipin: An efficient natural cross-linker for biopolymers. Polym. Bull. 2024, 81, 14251–14305. [Google Scholar] [CrossRef]
- Kahoush, M.; Behary, N.; Guan, J.; Cayla, A.; Mutel, B.; Nierstrasz, V. Genipin-mediated immobilization of glucose oxidase enzyme on carbon felt for use as heterogeneous catalyst in sustainable wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 105633. [Google Scholar] [CrossRef]
- Lim, J.; Razi, Z.R.M.; Law, J.; Nawi, A.M.; Idrus, R.B.H.; Ng, M.H. MSCs can be differentially isolated from maternal, middle and fetal segments of the human umbilical cord. Cytotherapy 2016, 18, 1493–1502. [Google Scholar] [CrossRef]
- Hassan, M.N.F.; Yap, Z.; Tang, Y.; Ng, M.H.; Law, J.X. Expired Platelet Concentrate as a Source of Human Platelet Lysate for Xenogeneic-Free Culture of Human Dermal Fibroblasts. Sains Malays. 2021, 50, 2355–2365. [Google Scholar] [CrossRef]
- Pincha, N.; Saha, D.; Bhatt, T.; Zirmire, R.K.; Jamora, C. Activation of Fibroblast Contractility via Cell-Cell Interactions and Soluble Signals. Bio-Protocol 2018, 8, e3021. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Teresa, M.; Antonio, L.; Angela, B.; Rosario, P.; Giovanni, P. Tangential Flow Filtration Technique: An Overview on Nanomedicine Applications. Pharm. Nanotechnol. 2018, 6, 48–60. [Google Scholar] [CrossRef]
- Kawai-Harada, Y.; Nimmagadda, V.; Harada, M. Scalable isolation of surface-engineered extracellular vesicles and separation of free proteins via tangential flow filtration and size exclusion chromatography (TFF-SEC). BMC Methods 2024, 1, 9. [Google Scholar] [CrossRef]
- Singh, P.; Szigyarto, I.C.; Ricci, M.; Gaal, A.; Queme-Pena, M.M.; Kitka, D.; Fulop, L.; Turiak, L.; Drahos, L.; Varga, Z.; et al. Removal and identification of external protein corona members from RBC-derived extracellular vesicles by surface manipulating antimicrobial peptides. J. Extracell. Biol 2023, 2, e78. [Google Scholar] [CrossRef]
- Dietz, L.; Oberlander, J.; Mateos-Maroto, A.; Schunke, J.; Fichter, M.; Kramer-Albers, E.M.; Landfester, K.; Mailander, V. Uptake of extracellular vesicles into immune cells is enhanced by the protein corona. J. Extracell. Vesicles 2023, 12, e12399. [Google Scholar] [CrossRef]
- Liam-Or, R.; Faruqu, F.N.; Walters, A.; Han, S.; Xu, L.; Wang, J.T.-W.; Oberlaender, J.; Sanchez-Fueyo, A.; Lombardi, G.; Dazzi, F.; et al. Cellular uptake and in vivo distribution of mesenchymal-stem-cell-derived extracellular vesicles are protein corona dependent. Nat. Nanotechnol. 2024, 19, 846–855. [Google Scholar] [CrossRef]
- Forteza-Genestra, M.A.; Antich-Rosselló, M.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Purity Determines the Effect of Extracellular Vesicles Derived from Mesenchymal Stromal Cells. Cells 2020, 9, 422. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xu, H.; Mao, C. Transmission electron microscopy as a tool to image bioinorganic nanohybrids: The case of phage-gold nanocomposites. Microsc. Res. Tech. 2011, 74, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Snyder, O.L.; Christenson, L.K.; He, H.; Weiss, M.L. Effect of Pre-Processing Storage Condition of Cell Culture-Conditioned Medium on Extracellular Vesicles Derived from Human Umbilical Cord-Derived Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2022, 23, 7716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hao, M.; Jiang, F.; Li, J.; Yang, K.; Li, C.; Ma, L.; Liu, S.; Kou, X.; Shi, S.; et al. Lyophilized apoptotic vesicle-encapsulated adhesive hydrogel sponge as a rapid hemostat for traumatic hemorrhage in coagulopathy. J. Nanobiotechnol. 2023, 21, 407. [Google Scholar] [CrossRef]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Pužar Dominkuš, P.; Stenovec, M.; Sitar, S.; Lasič, E.; Zorec, R.; Plemenitaš, A.; Žagar, E.; Kreft, M.; Lenassi, M. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim. Biophys. Acta (BBA)–Biomembr. 2018, 1860, 1350–1361. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Xia, J.; Liang, Y.; Li, G. Tracking tools of extracellular vesicles for biomedical research. Front. Bioeng. Biotechnol. 2022, 10, 943712. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chang, T.-M.; Tsai, W.-C.; Hsieh, Y.-J.; Wang, L.-T.; Huang, H.-C. Human Umbilical Cord Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Reduce Skin Inflammation In Vitro. Int. J. Mol. Sci. 2023, 24, 17109. [Google Scholar] [CrossRef]
- Tognoli, M.L.; Dancourt, J.; Bonsergent, E.; Palmulli, R.; de Jong, O.G.; Van Niel, G.; Rubinstein, E.; Vader, P.; Lavieu, G. Lack of involvement of CD63 and CD9 tetraspanins in the extracellular vesicle content delivery process. Commun. Biol. 2023, 6, 532. [Google Scholar] [CrossRef]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113961. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, C.; Liu, Y.; Zhou, J.; Dong, Y.; Huang, J.; Yang, T.; Xiao, H.; Liu, D.; Wang, S.; et al. GelMA Hydrogel Loaded with Extracellular Vesicles Derived from Umbilical Cord Mesenchymal Stem Cells for Promoting Cutaneous Diabetic Wound Healing. ACS Omega 2023, 8, 10030–10039. [Google Scholar] [CrossRef]
- Chen, P.; Tang, S.; Gao, H.; Zhang, H.; Chen, C.; Fang, Z.; Peng, G.; Weng, H.; Chen, A.; Zhang, C.; et al. Wharton’s jelly mesenchymal stem cell-derived small extracellular vesicles as natural nanoparticles to attenuate cartilage injury via microRNA regulation. Int. J. Pharm. 2022, 623, 121952. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef] [PubMed]
- Inthamat, P.; Boonsiriwit, A.; Lee, Y.S.; Siripatrawan, U. Effects of genipin as natural crosslinker on barrier and mechanical properties of chitosan-astaxanthin film. J. Food Process. Preserv. 2022, 46, e15707. [Google Scholar] [CrossRef]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.-H.; Atluri, P.; Burdick, J.A. Methods To Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef] [PubMed]
- Raja, I.S.; Fathima, N.N. Gelatin–Cerium Oxide Nanocomposite for Enhanced Excisional Wound Healing. ACS Appl. Bio Mater. 2018, 1, 487–495. [Google Scholar] [CrossRef]
- Pan, Z.; Brassart, L. A reaction-diffusion framework for hydrolytic degradation of amorphous polymers based on a discrete chain scission model. Acta Biomater. 2023, 167, 361–373. [Google Scholar] [CrossRef]
- Shuai, Q.; Liang, Y.; Xu, X.; Halbiyat, Z.; Wang, X.; Cheng, J.; Liu, J.; Huang, T.; Peng, Z.; Wang, L.; et al. Sodium alginate hydrogel integrated with type III collagen and mesenchymal stem cell to promote endometrium regeneration and fertility restoration. Int. J. Biol. Macromol. 2023, 253, 127314. [Google Scholar] [CrossRef]
- Pangjantuk, A.; Kaokaen, P.; Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. 3D culture of alginate-hyaluronic acid hydrogel supports the stemness of human mesenchymal stem cells. Sci. Rep. 2024, 14, 4436. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Yang, L. Hydrogel Encapsulation: Taking the Therapy of Mesenchymal Stem Cells and Their Derived Secretome to the Next Level. Front. Bioeng. Biotechnol. 2022, 10, 859927. [Google Scholar] [CrossRef]
- Karbiener, M.; Darnhofer, B.; Frisch, M.-T.; Rinner, B.; Birner-Gruenberger, R.; Gugatschka, M. Comparative proteomics of paired vocal fold and oral mucosa fibroblasts. J. Proteom. 2017, 155, 11–21. [Google Scholar] [CrossRef]
- Kodama, H.; Kumai, Y.; Nishimoto, K.; Toya, Y.; Miyamaru, S.; Furushima, S.; Yumoto, E. Potential treatment for vocal fold scar with pirfenidone. Laryngoscope 2018, 128, E171–E177. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, P.; Fang, X.; Lin, F.; Fang, J.; Xiong, C. Collagen gel contraction assays: From modelling wound healing to quantifying cellular interactions with three-dimensional extracellular matrices. Eur. J. Cell Biol. 2022, 101, 151253. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, N.; Bing, R.; Kraja, I.; Branski, R.C. Stem Cell–Mediated Paracrine Signaling Alters Fibroplasia in Human Vocal Fold Fibroblasts in Vitro. Ann. Otol. Rhinol. Laryngol. 2017, 126, 581–588. [Google Scholar] [CrossRef]
- Molaee, N.; Mosayebi, G.; Pishdadian, A.; Ejtehadifar, M.; Ganji, A. Evaluating the Proliferation of Human Peripheral Blood Mononuclear Cells Using MTT Assay. Int. J. Basic Sci. Med. 2017, 22, 25–2825. [Google Scholar] [CrossRef]
- Longhini, A.L.F.; Salazar, T.E.; Vieira, C.; Trinh, T.; Duan, Y.; Pay, L.M.; Li Calzi, S.; Losh, M.; Johnston, N.A.; Xie, H.; et al. Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS ONE 2019, 14, e0212642. [Google Scholar] [CrossRef] [PubMed]
- Baharlooi, H.; Nouraei, Z.; Azimi, M.; Moghadasi, A.N.; Tavassolifar, M.J.; Moradi, B.; Sahraian, M.A.; Izad, M. Umbilical cord mesenchymal stem cells as well as their released exosomes suppress proliferation of activated PBMCs in multiple sclerosis. Scand. J. Immunol. 2021, 93, e13013. [Google Scholar] [CrossRef]


| Markers | Events (%) | ||
|---|---|---|---|
| Pool 1 | Pool 2 | Pool 3 | |
| CD90+ | 100 | 99.95 | 99.95 |
| CD105+ | 99.67 | 96.9 | 96.19 |
| CD73+ | 97.45 | 99.59 | 99.55 |
| Negative | 2.63 | 0.6 | 0.19 |
| Pool | 1 | 2 | 3 | Mean ± SD |
|---|---|---|---|---|
| Total cell number | 44 × 106 | 75 × 106 | 68 × 106 | 62.3 × 106 ± 13.23 × 106 |
| Cell viability (%) | 95% | 98.2% | 96.3% | 97% ± 0.013 |
| Protein concentration of cell lysate (µg/mL) | 1779.6 | 2025.78 | 1745.07 | 1850.14 ± 124.99 |
| Initial total CM volume (mL) | 200 | 200 | 200 | n/a |
| Pool | 1 | 2 | 3 | Mean ± SD |
|---|---|---|---|---|
| Final concentrated volume (mL) | 9 | 7.5 | 8 | 8.17 ± 0.62 |
| Protein concentration of sEVs (µg/mL) | 208.97 | 940.159 | 608.545 | 585.89 ± 298.94 |
| Protein concentration of sEV lysate (μg/mL) | 425.4 | 1044.31 | 673.64 | 714.45 ± 254.31 |
| Total protein of sEV lysate (mg) | 3.84 | 7.83 | 5.39 | 5.68 ± 1.64 |
| Total protein per 106 cells (μg/106 cells) | 87.01 | 104.43 | 79.25 | 90.23 ± 10.53 |
| Pool | 1 | 2 | 3 | Mean ± SD |
|---|---|---|---|---|
| Concentration (particles/mL) | 3.9 × 1010 ± 6.01 × 109 | 5.55 × 1010 ± 5.81 × 109 | 4.95 × 1010 ± 1.09 × 1010 | 4.8 × 1010 ± 0.68 × 1010 |
| Total particle count | 3.51 × 1011 | 4.16 × 1011 | 3.96 × 1011 | 38.76 × 1010 ± 2.71 × 1010 |
| Mode size (nm) | 65.1 ± 2.7 | 63.4 ± 4.4 | 72.7 ± 2.7 | 67.07 ± 4.04 |
| Mean size (nm) | 89.2 ± 2.0 | 84.0 ± 2.5 | 91.2 ± 1.1 | 88.13 ± 3.03 |
| Number of particles secreted per cell | 7.98 × 103 | 5.55 × 103 | 5.82 × 103 | 6.45 × 103 ± 1.09 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamlus, Z.I.; Azman, M.; Lokanathan, Y.; Fauzi, M.B.; Baki, M.M. Effects of Wharton’s Jelly Mesenchymal Stem Cells and Its-Derived Small Extracellular Vesicles Loaded into Injectable Genipin-Crosslinked Gelatin Hydrogel on Vocal Fold Fibroblast. Polymers 2025, 17, 2653. https://doi.org/10.3390/polym17192653
Zamlus ZI, Azman M, Lokanathan Y, Fauzi MB, Baki MM. Effects of Wharton’s Jelly Mesenchymal Stem Cells and Its-Derived Small Extracellular Vesicles Loaded into Injectable Genipin-Crosslinked Gelatin Hydrogel on Vocal Fold Fibroblast. Polymers. 2025; 17(19):2653. https://doi.org/10.3390/polym17192653
Chicago/Turabian StyleZamlus, Zarqa Iffah, Mawaddah Azman, Yogeswaran Lokanathan, Mh Busra Fauzi, and Marina Mat Baki. 2025. "Effects of Wharton’s Jelly Mesenchymal Stem Cells and Its-Derived Small Extracellular Vesicles Loaded into Injectable Genipin-Crosslinked Gelatin Hydrogel on Vocal Fold Fibroblast" Polymers 17, no. 19: 2653. https://doi.org/10.3390/polym17192653
APA StyleZamlus, Z. I., Azman, M., Lokanathan, Y., Fauzi, M. B., & Baki, M. M. (2025). Effects of Wharton’s Jelly Mesenchymal Stem Cells and Its-Derived Small Extracellular Vesicles Loaded into Injectable Genipin-Crosslinked Gelatin Hydrogel on Vocal Fold Fibroblast. Polymers, 17(19), 2653. https://doi.org/10.3390/polym17192653









